Note: Descriptions are shown in the official language in which they were submitted.
CA 02300008 2000-03-06
- NSC-6470-CA
- 1 -
STAINLESS STEEL AND TITANIUM FOR SOLID
POLYMER ELECTROLYTE FUEL CELL MEMBERS
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a material for
solid polymer electrolyte fuel cell members used for
automobiles that use electric power as a source of drive
and for small-scale generating systems. More
specifically, the invention relates to a surface-treated
low-contact-resistance material for solid polymer
electrolyte fuel cell members.
2. Description of the Related Art
In recent years, study of the fuel cells for
electric cars has rapidly developed and has been
supported by success in the development of a solid
polymer electrolyte material.
The solid polymer electrolyte fuel cell is a
fuel cell which features the use of an organic membrane
of the type selectively transmitting hydrogen ions as an
electrolyte, and is different from the conventional
alkali fuel cell, phosphorus fuel cell, molten carbonate
fuel cell or solid electrolyte fuel cell.
The solid polymer electrolyte fuel cell is a
system which uses, as a fuel, pure hydrogen as well as
hydrogen gas obtained by reforming alcohols, and produces
electric power by electrochemically controlling the
reaction thereof with oxygen in the air.
Despite of its small thickness, the solid
polymer electrolyte membrane exhibits its function to a
sufficient degree by having electrolyte secured in the
membrane. Upon controlling the dew point in the cell,
therefore, the solid polymer electrolyte membrane works
as an electrolyte, and makes it possible to design the
cell itself in a compact size and in a simplified manner
without using a medium having fluidity, such as an
CA 02300008 2000-03-06
- 2 -
aqueous solution-type electrolyte or a molten salt-type
electrolyte.
As the stainless steels for fuel cells, there
have heretofore been known a corrosion-resistant
stainless steel for molten carbonate fuel cells as
disclosed in Japanese Unexamined Patent Publication
(Kokai) No. 4-247852, a highly corrosion-resistant steel
plate for molten carbonate fuel cell separators as
disclosed in Japanese Unexamined Patent Publication
(Kokai) No. 4-358044, a stainless steel having excellent
corrosion resistance against molten salts and a method of
producing the same as disclosed in Japanese Unexamined
Patent Publication (Kokai) No. 7-188870, a stainless
steel having excellent resistance against molten
carbonate as disclosed in Japanese Unexamined Patent
Publication (Kokai) No. 8-165546, a stainless steel
having excellent corrosion resistance against molten
carbonate as disclosed in Japanese Unexamined Patent
Publication (Kokai) No. 8-225892, and a stainless steel
that can be favorably hot worked and exhibits excellent
corrosion resistance against molten salts as disclosed in
Japanese Unexamined Patent Publication (Kokai) No. 8-
3114620.
Further, as stainless steels for fuel cells
that operate in a molten carbonate environment where a
high degree of corrosion resistance is required, there
have been developed solid electrolyte fuel cell materials
used at temperatures of several hundreds of degrees
Celsius as represented by metal materials for solid
electrolyte fuel cells disclosed in Japanese Unexamined
Patent Publications (Kokai) Nos. 6-264193 and 6-293941
and a ferrite-type stainless steel disclosed in Japanese
Unexamined Patent Publication (Kokai) No. 9-67672.
As a material constituting the solid polymer
electrolyte fuel cell that operates in a region of not
higher than 100°C, on the other hand, there has been used
a carbon-type material since the temperature is not so
CA 02300008 2000-03-06
- 3 -
high and corrosion resistance and durability can be
exhibited to a sufficient degree in the environment in
which it is used. No study has heretofore been conducted
concerning applying a metal-type material such as
stainless steel or titanium to the fuel cells of this
type.
As for applying a metal material to a separator
which is one of the important members of the fuel cell,
all that is used as a separator for the phosphoric acid
type fuel cell is an amorphous alloy of a nickel group as
taught in Japanese Unexamined Patent Publications (Kokai)
No. 63-277734, 63-277735, 63-277736 and 63-277737. No
study has at all been conducted concerning applying a
stainless steel to the separator for solid polymer
electrolyte fuel cells nor concerning the concrete shapes
thereof.
Use of carbon as a material for constituting
the solid polymer electrolyte fuel cell involves problems
such as increased cost and increased cell size, hindering
the widespread use of the solid polymer electrolyte fuel
cells.
The solid polymer electrolyte fuel cell is
constituted by a laminate of catalytic electrode units of
fine carbon particles and ultrafine noble metal particles
on both surfaces of a solid polymer electrolyte membrane
that serves as an electrolyte, a current collector of an
aggregate of a felt-like carbon fiber (usually called
carbon paper) having functions of taking out electric
power generated therein as an electric current and of
feeding, at the same time, a reaction gas to the
catalytic electrode units, and separators for receiving
the electric current therefrom and for separating two
kinds of reaction gases comprising chiefly oxygen and
hydrogen, as well as a cooling medium.
A carbon material has heretofore been used even
for the separators. When the fuel cell is to be mounted
on an automobile, however, there arise problems such as
CA 02300008 2000-03-06
- 4 -
an increased cost and an increased cell size. Therefore,
a study has been conducted concerning applying a
stainless steel to the members such as separators.
The present inventors have already disclosed
concrete shapes and components for using a stainless
steel as members for the solid polymer electrolyte fuel
cell, such as separators, in Japanese Unexamined Patent
Publications (Kokai) Nos. 11-61146 and 11-62813. When
the separators are made of a stainless steel or titanium,
however, it has been pointed out that the energy
efficiency of the fuel cell is greatly lowered due to a
large contact resistance to the carbon paper which serves
as a current collector.
SUMMARY OF THE INVENTION
According to a first aspect of the invention, there
is provided a low-contact-resistance material for solid
polymer electrolyte fuel cell members for enabling the
solid polymer electrolyte fuel cell to exhibit its energy
conversion efficiency to a maximum degree. The material
was found by studying the contact resistance among the
materials that are used.
According to a second aspect of the invention, there
is provided a component system of a low cost withstanding
the environment of use. The material was found by
studying stainless steels to substitute for a carbon
material in order to satisfy the demand for decreasing
the size and decreasing the cost of the solid polymer
electrolyte fuel cell.
According to a third aspect of the invention, there
is provided concrete technology for substituting a
stainless steel for the carbon material that is used for
constituting the members, in order to mass-produce the
solid polymer electrolyte fuel cells in compact sizes.
In order to accomplish the above-mentioned objects,
the present invention provides the following (1) to (16).
(1) A low-contact-resistance stainless steel plate
for solid polymer electrolyte fuel cell members, wherein
CA 02300008 2000-03-06
- 5 -
a noble metal or an alloy of a noble metal is deposited
on the surface of a portion that comes in contact with
other member developing a contact resistance and from
where an oxide film has been removed.
(2) A stainless steel plate as described in (1),
wherein the noble metal or the alloy of the noble metal
that is deposited has an average thickness of not smaller
than 5 nm.
(3) A low-contact-resistance stainless steel plate
for solid polymer electrolyte fuel cell members described
in (1) or (2), wherein the stainless steel plate contains
the following components in following amounts by weight:
Cr: not more than 30~,
10 - 0.3 x ((Cry) + 3 (Mo$) + 0.05(Ni~)} s_
5, and
the remainder being chiefly Fe.
(4) A low-contact-resistance stainless steel plate
for solid polymer electrolyte fuel cell members described
in (3), further containing one or more of:
Mo: not more than 10$, and
Ni: not more than 25~,
by weight.
(5) Low-contact-resistance titanium for solid
polymer electrolyte fuel cell members, wherein a noble
metal or an alloy of a noble metal is deposited on the
surface of a portion that comes in contact with other
member developing a contact resistance and from where an
oxide film has been removed.
(6) Titanium described in (5), wherein the noble
metal or the alloy of the noble metal that is deposited
has an average thickness of not smaller than 5 nm.
(7) A method of producing a low-contact-resistance
stainless steel plate for solid polymer electrolyte fuel
cell members by depositing a noble metal or an alloy of a
noble metal on the surface of a portion that comes into
contact with another member developing a contact
resistance and from where an oxide film has been removed
CA 02300008 2000-03-06
, - 6 -
by blasting the surface of the plate with particles
coated with the noble metal or the alloy of the noble
metal.
(8) A method of producing a low-contact-resistance
stainless steel plate for solid polymer electrolyte fuel
cell members by depositing a noble metal or an alloy of a
noble metal on the surface of a portion that comes in
contact with another member developing a contact
resistance and from where an oxide film has been removed
by wet-plating with the noble metal or the alloy of the
noble metal while effecting the polishing.
(9) A method of producing a low-contact-resistance
titanium for solid polymer electrolyte fuel cell members
by depositing a noble metal or an alloy of a noble metal
on the surface of a portion that comes in contact with
another member developing a contact resistance and from
where an oxide film has been removed by blasting the
surface of the plate with particles coated with the noble
metal or the alloy of the noble metal.
(10) A method of producing a low-contact-resistance
titanium for solid polymer electrolyte fuel cell members
by depositing a noble metal or an alloy of a noble metal
on the surface of a portion that comes into contact with
another member developing a contact resistance and from
where an oxide film has been removed by wet-plating the
noble metal or the alloy of the noble metal while
effecting the polishing.
(11) A stainless steel separator for a solid polymer
electrolyte fuel cell, wherein the separator has, in the
central portion thereof, a corrugated plate structure
constituted by a plurality of grooves of which both ends
are coupled together by coupling portions, and has, in
the peripheral flat portions thereof, holes serving as
gas passages for the one reaction gas, holes serving as
gas passages for the other reaction gas and holes serving
as passages for the coolant, each in a number of two or
more.
CA 02300008 2000-03-06
(12) A stainless steel separator for a solid polymer
electrolyte fuel cell described in (11) by using the
stainless steel of any one of (1) to (4).
(13) A titanium separator for a solid polymer
electrolyte fuel cell, wherein the separator has, in the
central portions thereof, a corrugated plate structure
constituted by a plurality of grooves of which both ends
are coupled together by coupling portions, and has, in
the peripheral flat portions thereof, holes serving as
gas passages for the one reaction gas, holes serving as
gas passages for the other reaction gas and holes serving
as passages for the coolant, each in a number of two or
more.
(14) A titanium separator for a solid polymer
electrolyte fuel cell described in (13) by using titanium
of (5) or (6).
(15) A laminated module for a solid polymer
electrolyte fuel cell comprising:
A) a stainless steel separator having, in the
central portion thereof, a corrugated plate structure
constituted by a plurality of grooves of which both ends
are coupled together by coupling portions, and having, in
the peripheral flat portions thereof, holes serving as
gas passages for the one reaction gas, holes serving as
gas passages for the other reaction gas and holes serving
as passages for the coolant, each in a number of two or
more;
B) a spacer having a cut-away portion of a
shape corresponding to the central portion of the
separator, and having, in the peripheral flat portions
thereof, holes serving as gas passages for the one
reaction gas, holes serving as gas passages for the other
reaction gas and holes serving as passages for the
coolant, each in a number of two or more so as to be
overlapped on the holes in the separator, the holes
serving as passages for the reaction gases and the holes
serving as passages for the coolant being communicated
CA 02300008 2000-03-06
. _ 8 _
with the cut-away portion;
C) a solid polymer electrolyte membrane
having, in the peripheral flat portions thereof, holes
serving as passages for the one reaction gas, holes
serving as passages for the other reaction gas and holes
serving as passages for the coolant, each in a number of
two or more so as to be overlapped on the holes of the
separator and on the holes of the spacer; and
D) a stainless steel terminating plate having
reaction gas feed/drain ports and coolant feed/drain
ports at positions corresponding to the reaction gas
passages and the coolant passages formed by laminating
the separator, the spacer and the solid polymer
electrolyte membrane at an end where the separator, the
spacer and the solid polymer electrolyte membrane are
laminated.
(16) A laminated module for a solid polymer
electrolyte fuel cell comprising:
A) a titanium separator having, in the
central portion thereof, a corrugated plate structure
constituted by a plurality of grooves of which both ends
are coupled together by coupling portions, and having, in
the peripheral flat portions thereof, holes serving as
gas passages for one reaction gas, holes serving as gas
passages for the other reaction gas and holes serving as
passages for the coolant, each in a number or two or
more;
B) a spacer having a cut-away portion of a
shape corresponding to the central portion of the
separator, and having, in the peripheral flat portions
thereof, holes serving as gas passages for the one
reaction gas, holes serving as gas passages for the other
reaction gas and holes serving as passages for the
coolant, each in a number of two or more so as to be
overlapped on the holes in the separator, the holes
serving as passages for the reaction gases and the holes
serving as passages for the coolant being communicated
CA 02300008 2000-03-06
_ g _
with the cut-away portion;
C) a solid polymer electrolyte membrane
having, in the peripheral flat portions thereof, holes
serving as passages for the one reaction gas, holes
serving as passages for the other reaction gas and holes
serving as passages for the coolant, each in a number of
two or more so as to be overlapped on the holes of the
separator and on the holes of the spacer; and
D) a titanium terminating plate having
reaction gas feed/drain ports and coolant feed/drain
ports at positions corresponding to the reaction gas
passages and the coolant passages formed by laminating
the separator, the spacer and the solid polymer
electrolyte membrane at an end where the separator, the
spacer and the solid polymer electrolyte membrane are
laminated.
The separator used in the solid polymer electrolyte
fuel cell has heretofore been obtained by forming grooves
in both surfaces of a carbon plate having a thickness of
about 5 mm so that the reaction gases and the coolant may
flow into predetermined portions in the fuel cell. In
this case, however, the carbon material itself is
expensive and, besides, laborious work is required by the
cutting operation further driving up the cost.
In an attempt to cheaply produce the parts through a
mass production system, therefore, the present inventors
have conducted a study to supply parts of a predetermined
shape by press-molding and punching a thin stainless
steel plate, and have succeeded in determining the shapes
of the stainless steel parts and of the accompanying
constituent parts as well as the laminated module
constituted thereby, and have thus completed the
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic view illustrating a stainless
steel separator for a solid polymer electrolyte fuel cell
according to the present invention;
CA 02300008 2000-03-06
- 10 -
Fig. 2 is a schematic view illustrating a spacer A
for the solid polymer electrolyte fuel cell according to
the present invention;
Fig. 3 is a schematic view illustrating a spacer B
for the solid polymer electrolyte fuel cell according to
the present invention;
Fig. 4 is a schematic view illustrating a~spacer C
for the solid polymer electrolyte fuel cell according to
the present invention;
Fig. 5 is a schematic view illustrating a spacer D
for the solid polymer electrolyte fuel cell according to
the present invention;
Fig. 6 is a schematic view illustrating a solid
polymer electrolyte membrane for the solid polymer
electrolyte fuel cell according to the present invention
to which a catalytic electrode is imparted;
Fig. 7 is a schematic view illustrating a current
collector used in the present invention;
Fig. 8 is a schematic view illustrating a stainless
steel terminating plate for the solid polymer electrolyte
fuel cell according to the present invention; and
Fig. 9 is a schematic view illustrating a laminated
module for the solid polymer electrolyte fuel cell
according to the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
A preferred embodiment according to a first aspect
of the invention will now be described.
The contact resistance was measured by arranging
jigs having disk-like current-feeding surfaces of a
diameter of 30 mm above and below, placing, therebetween,
two disk-like metallic test pieces or carbon papers
having a diameter of 30 mm and a thickness of 4 mm,
placing a weight on the upper part thereof such that the
surface pressure of the contacting surfaces was 2.79
kg/cm2, feeding a constant current of a current density
of 1.13 A/cm2, and measuring the potential difference
across the two disk-like metallic test pieces.
CA 02300008 2000-03-06
- 11 -
As the materials of the disk-like metallic test
pieces, there were selected copper plated with gold to a
thickness of 30 Vim, commercially available stainless
steels SUS316L, YUS270, YUS260, YUS190L, and class 1 pure
titanium for industrial use (YUS stands for a standard
specified by Shin-Nihon Seitetsu Co.)
As the carbon paper, there was selected a test
product of a thickness of 0.6 mm regarded to be most
suited for the fuel cells from the standpoint of gas
permeability and an electrical conducting property. The
carbon paper was cut into a square of a side of 30 mm and
was subjected to testing. In order to obtain standard
values, first, the test pieces of the stainless steels
and titanium were mirror-surface polished on their
contact surfaces to measure the contact resistance.
As a result, the contact resistances (unit in
mS2~cm2) were gold/gold: 0.02, gold/carbon paper: 5.30,
gold/SUS316L: 22.43, SUS316L/SUS316L: 54.90,
titanium/carbon paper: 655.83, YUS270/carbon paper:
696.50, YUS260/carbon paper: 679.87, YUS190L/carbon
paper: 819.40, SUS316L/carbon paper: 614.52.
The contact resistance of the gold/carbon paper was
found by holding the carbon paper by two disk-like test
pieces of gold-plated copper, dividing the potential
difference between the disk-like test pieces of gold-
plated copper by the current density and, then, dividing
the value by 2 and, hence, includes the resistance of
one-half the thickness of the carbon paper. Further, the
contact resistance of the stainless steel or titanium and
the carbon paper was found by measuring the potential
difference across both ends of a combination of a
stainless steel or titanium/carbon paper/gold-plated
copper, dividing the potential difference by the current
density to obtain the total resistance, and subtracting
the contact resistance of gold/carbon paper from the
total resistance.
CA 02300008 2000-03-06
- 12 -
By rearranging the results, it was found that:
O There is almost no resistance on the contact
surfaces of gold/gold and gold/carbon paper;
O An oxide film exists on the stainless steel or
titanium developing a contact resistance of about several
tens of mS2 ~ cm2; and
OO A large contact resistance that cannot be
expected from the Ohm s law develops at the contact
surface between the stainless steel or titanium and the
carbon paper.
This phenomenon is deeply related to the fact that
the electric conduction of graphite constituting the
carbon paper varies depending upon the ~-electrons that
are conjugation double-bonded, and a so-called Schottky
barrier is formed on the contact interface relative to
the stainless steel or titanium having a work function
value greatly different from that of graphite, developing
a large contact resistance.
Upon considering the contact resistance from the
standpoint of semiconductor physics, the data measured
this time can be explained consistently. Thus, the
contact resistance could be decreased by holding a noble
metal (which may be gold, platinum, palladium, silver,
copper, tin, lead or the like metal) having a work
function value equivalent to that of graphite or by
holding an alloy of such a metal between the contact
surfaces of the stainless steel or titanium and the
carbon paper.
It has been known that a very thin oxide film exists
on the stainless steel or titanium, and it has been
learned through experiment that this film, too, is a
cause of increasing the contact resistance. Concerning
this, the noble metal is deposited while removing the
film to decrease the contact resistance to the carbon
paper.
Therefore, the contact surface of the carbon paper
CA 02300008 2000-03-06
- 13 -
was coated with gold by the ion-plating method to measure
the effect of decreasing the contact resistance to a
mirror-surface-polished stainless steel, from which it
was learned that the contact resistance starts decreasing
when gold is deposited in an average thickness of not
smaller than 5 nm as calculated from the vaporization
rate and vaporization time.
Further, a stainless steel was lightly polished by
using a water-resistant silicon carbide paper in a 1N
hydrochloric acid aqueous solution containing 0.5~ of
chloroplatinic acid, in order to precipitate a trace
amount of platinum on the surface of the metal by the
corrosion reaction while mechanically removing the
passive film, and the contact resistance to the untreated
carbon paper was measured. It was learned that the
contact resistance decreases when platinum is deposited
in an average thickness of not smaller than 5 nm.
Example 1.
In order to confirm the effect according to the
first aspect of the invention, the surfaces of the
stainless steel and titanium were treated, and the
contact resistances of the contact surfaces of the
combinations thereof were measured. The results were as
shown in Tables 1 to 8.
A combination number 1 represents a case when both
the carbon paper and the stainless steel were not treated
(reference 1).
Combination numbers 2 to 28 represent the measured
results of contact resistances of when the surfaces of
various stainless steels and titanium on which a noble
metal was precipitated or deposited while mechanically
removing the oxide film, are contacted to the surface of
the carbon paper on which gold has been deposited
maintaining a thickness of 1000 nm by ionic vaporization.
The results show that the contact resistances of the
stainless steels and titanium decrease after the
treatment.
CA 02300008 2000-03-06
- 14 -
In order to precipitate or deposit the noble metal
while removing the film, various methods were carried
out, such as a method of lightly polishing with the
water-resistant silicon carbide paper in a 1N
hydrochloric acid aqueous solution containing 0.5~ of
chloroplatinic acid, so that platinum or the like was
precipitated in trace amounts by the corrosion reaction,
a method of precipitating platinum in larger amounts by
the cathodic electrolysis after the above polishing has
been effected, and a method of blasting with glass beads
which are nonelectrolytically plated with gold or silver.
The effect of decreasing the contact resistance could be
observed by any method.
Combination numbers 29 to 45 represent the results
of contact resistances on the contact surfaces between
stainless steels, between titanium, and between stainless
steel and titanium. A decrease in the contact resistance
was calculated with the contact resistance of the
untreated surfaces of a combination No. 29 as a reference
(reference 2). It was proved that the contact
resistances could be decreased in all cases.
Combination numbers 46 to 57 represent the measured
results of contact resistance between the untreated
carbon paper and various stainless steel plates or
titanium plate lightly polished with a water-resistant
silicon carbide paper #320 in a mixture solution of 0.5$
of potassium chloroplatinic acid and 1N of hydrochloric
acid, and leaving them for a predetermined period of time
or cathodically treating the surfaces, as a method of
depositing a noble metal while removing the film. The
effect of greatly decreasing the contact resistance could
be observed in all cases.
Combination numbers 58 to 69 represent the measured
results of contact resistance between the untreated
carbon paper and various stainless steel plates or
titanium plate lightly polished with the water-resistant
silicon carbide paper #320 in a gold-plating bath, and
CA 02300008 2000-03-06
- 15 -
leaving them for a predetermined period of time or
treating the surfaces by cathodic electrolysis, as a
method of depositing a noble metal while removing the
film. The effect of greatly decreasing the contact
resistance could be observed in all cases.
Combination numbers 70 to 81 represent the measured
results of contact resistance between the untreated
carbon paper and various stainless steel plates or
titanium plate lightly polished with the water-resistant
silicon carbide paper #320 in a palladium-plating bath,
and leaving them for a predetermined period of time or
treating the surfaces by cathodic electrolysis, as a
method of depositing a noble metal while removing the
film. The effect of greatly decreasing the contact
resistance could be observed in all cases.
Combination numbers 82 to 93 represent the measured
results of contact resistance between the untreated
carbon paper and various stainless steel plates or
titanium plate lightly polished with the water-resistant
silicon carbide paper #320 in a silver-plating bath, and
leaving them for a predetermined period of time or
treating the surfaces by cathodic electrolysis, as a
method of depositing a noble metal while removing the
film. The effect of greatly decreasing the contact
resistance could be observed in all cases.
Combination numbers 94 to 105 represent the measured
results of contact resistance between the untreated
carbon paper and various stainless steel plates or
titanium plate of which the surfaces are treated by being
blasted with glass beads plated with gold, silver or
platinum, as a method of depositing a noble metal while
removing the film. The effect of greatly decreasing the
contact resistance could be observed in all cases.
It is desired that a noble metal or an alloy of the
noble metal is deposited on both surfaces that come in
contact with each other. The noble metals and the method
of depositing the noble metals are not limited to the
CA 02300008 2000-03-06
- 16 -
above-mentioned examples only, but any conventionally
employed method or combinations thereof may be used.
CA 02300008 2000-03-06
' - 17 -
0 0 G G 0 0 0 0 ~ ~ 0 0
O 0 O 0 0 0 O 0 O 0 0 O
a +~ +~ ~ +~w w ~ ~ ~ w w ~ .-,ui
ro ~; a a a a a a a a a a a a
v v v v v v v v v v v v v
~
~ ~ ~ > ~ > > 5 > > 0 0
a
x a a a a a a a a ~ a a a
H H H H H H H H H H H H ~ w
NG
J~O oW oW op oW oWoW op oW dP oW uW dP oW "d
~
ue"
O ~ 01 O 0001 O~ OD 01 01 W 01 O
01 01 O 0101 01 O~ 01 01 O~ 01 O (a ~ .O
H H
~OG U7 U~ .~',
'd
'b1~ p O
I
N
ro
N N N 1l1M N 41 m 1f1 111 OD 01 .~1.~1 U
tn O 1f7 N y 0 M ~ In M ~ Il7 N ~ O
.
'
~r ,- H ~ +~
' ~
~ ~c ~ ~
ro
~
v
N
v
vb
N
b~ "'d'b
U
N
p
,t~
.
ro o m o o m o o m o o m o 0
ro
.u
,~c
tr
v
~ i '-~ ~ "-I ~ '1 ~ '~ ~ U U
~
O
N
N
N
.- b
1
~
>auxNro V
~
~
+~
~
''"~.'..~
U
v p G G p G >~ p G ~ C ~ ~ G ~ .
.,.
u
~
b
. p +~ +~ w +~.u ~ +~ +~ +~ w .u +~
'
mN~~' z ro ro ro roro ro ro ro ro ro ro ro
w w w w w n~ w w w w w w
~ I p O
I
0
rov
N a ~ o 0 o
~vv v ~ V
p ~C PO U ~C00 U ~ W U ~C al U V
v
z
~ ~N x x
~ cn a4
y~
da dP
N ~ tl1tll dP
O ro ~ w ~ ~ 0
0 0 0 0 0 0 0 o
H U ~ .-i .-I ,-.~ ~ r w o vo w
~ M M M M N N N N N N
N ~ H H H I W ~r-I L:
l ~
p N ~ 1~
N
'b
N
tT
U o 0 0 0 0 0 0 0 0 0 0 0 0
v
G
.~
.~.
ro
ro+~ae
tTV
Nw o 0 0 0 0 0 0 0 0 0 0 o V V o
ro
U
p
O
p o 0 0 0 0 0 0 0 0 0 0 0
a
p
.,~
O
,_.I
~ .~ ~, ,~
~ V
a
x
a
ro
~ r~ rl
~n~+~~>
I x x U
b~U
vaa v b v b b b b b b b b b b
ro a ~ .~ ,~ ~ ~ ~ ~ ~ ~ . .~ ~ ~ z
:~ I , , ~
~
Z ~ 7 o ~ o ~ p 0 o p o .-Ir-i
~ ~ ~ ~ ~ ~ ~ ~ ~ ~
c c ~
9
' ,
~c~ny , ~ ~
~
. .
p p ~ p >~ G ~ p p p C >~
~ 0 0 0 O 0 0 O O O O 0 O
~ ~
p p .rl ~rl n-1~ri~ri ~rl -rl ~ra ~.i ~rl ~rl ~rl O
lIl
N J..1 ,a.1 1~ yJ1~ 11 1.1 a.l .a.l .IJ ~ 1.7 N N N
U v ro ro ro roro ro ro ro ro ro ro ro M M
I ro ~ N N N N N N N N N N N N
a z U U U U U U U U U U U U
v a a a H a a a a a a H a ~' ~'
a ~.1 ~rl wl ~rl~rl ~rl w"I ~.I ~.~1 ~rl wl ml
0 0 0 0 O 0 O O 0 O 0
~ d 1~ ~ p 1~ p C G p G ~ G ~ ~1~ ~ U7
+~
o~o oid oroorooid oro oro oro oro oro oro oro .~ -.
H H H H H H H H H H H H 3
,'~ ',7 ,~a 'J'> ,'! ,'a ,'7,'a ',! ',7 '>
~
I ro a a ~ a a a a a a a a a a b
,~ ou ouo uou ouou ou ou ou ou ou ou ou
vv n cm v ~ v ~ >a v ~
v v v
a .v .~v,~ ~ . . . . . . . .~
a a a
i v v aroaroa roa a a roa roa a H roro ro ro roro ro
ro+~ ro ro ro rororororo ro roro rororo
ro U U U U U U U U U U U U U
~ ~
.
I ' +~ ~ ~ ~ ~
0 Q, Oa
.~
O ~-I N d' 1l'1~O l~ aD O~ O r1 N M O
.,~ I
M
I ~, ~ .~ ~-I z .. ..
~ Gfa U
CA 02300008 2000-03-06
- 18 -
~ G ~ G C ~ G C G G ~ ~f~C~>~G~
N O O 0 0 O 0 0 0 0 0 0 _ 00000 00
.rl .rl~rl.(", 'rl wl f.'wl rl Ci ~ri rl f.." w~ wl ' rirlrl l
rl r~ rl
r
~-I 1~ l~1~O .LI l~ 0 11 a.1O 1.1 11 0 a..t 11 . 1.1.N1~~ ~
1i.l1~
1
ro ~ G C'.N G CI N C; i.."N G ~ N C." G W C.~~ C.
~ >.~',.'...
~ a~ ar
m ar
d a~arI a~ a~ ~ v v I m a~ I m m ~, m
.~I .~I .~.I .~ a
a ~ oroG a oro a a oro ~ a '~ aaaaa aa
~ a ob a a
H H H U H H U H H U H H U H H HHHH HH
N
C
. ~
O dP owdPdP dP dr dpus op aP dP as ow as op dP
yJ OD O~O N 01 Om .-IQ1 01 e-1 01 01 .~ 01 01
N 01 010 01 01 Ot O~CW 1 O~ 01 O~ O~ 01 01
I
U
NN~
a
~','b
~
J-I .-1.-IN aD Ill Lf1N lf1 ri N l0 N N Il7 lf1 M
I
N
~
U+1
W
uroN U
~ O 111N y f1 aD ~ ~D aD ~ l0 f~ ~ 10
ro ~
0
0
O
~UUro
' N
U G
v
b
j ~ .~.~.
roroW?~b'N u1 0 o O O o O o O o O o 0 o O
EIWroU~~ ,-~o o O O O O O O o
.~
o~
a~
a
v~
.
N
N~~
~
.
D
Q1
U N
~ Ul 'd N N 'd N GI 'b N N 'd O Gl
!
y .~, ..~.~a ~I ~ a ~ > a .~ ~ a ~ ~ a ~ .~.~ ~I.~I
~,. ~I
ro roroz ~ ~ x ~ ~ z ~ '~ o o ~I o +~~~ +~
.uro+~ +r
ro rorororo
m c . x ~ ~~Ix ro
~I
N ~ U1 ~
i ~~ ~ W W WO~WWCLWW
~ ro
N
>~a ~ ~N 1.1 N ' N ~ W N
' '
I y~ . ""O ..~ro i~b N ro N d ro '~ N ro
N N ~ ,b ~.~b .~ d
. ..~ ~ a~~ N
b ~ b''~
a~b
'~
~ ~ 3b y ~~ 3ro~ 3 ~ 3ro ~ ~ ~ 3 ~
~ ro ~ 3 3 ro3
ro~ ' ro~ ' bd ' d ro ar
~ ~ ~ ~ ~ ~ ~ ~
W ~ t~U N N NN'dN N N'd~ QI N'dN N N"dO '~U~C U
0 G tl ~ ~ fJ7W
' N N 11
ro I ~ 1 , I ~ 1 ~ NClNx
~ ~ ~ 1 ~
~ ~ I
N1
I
.1. I .~NNN.1-I NNNa .~ I NG1N . ~
N N LI'dN NJ. p N I 1 1. N'Ci 1~'L~
N 1- 9 N 1 1~'b N N.4 ~ N N
'd N N'd N N'b ~ N N
N N N J
N N
N~,~ N N . rororo~ro ro.~roro roro ro.~roro . roro
ro~lroro ~lroro
N roro ro,-Iro N ~roro
U1+~ ro~roro
H H . H H H H ~ r-I r-I ~
H H ' -I n-I O rl.-i
. H r-I G)
Nro ,La CT N CT N L~ b l7 CT N
G~ ~ ~ Cl.c7
.
a a a a a a a a a a aaaaa a
o .,~ 0 0 0 ~ ~ ~ 0 0 0 0 0 0
a, o,a~,-r ,~ rI r r~ ~ o~ o, v, ~
E"IU ~ H -1 -I
~bN~ rl W -iM M M N N N ~ . . ~ p~~
v ~ ~ ~ ~ ~ o p ~ p ~ O
' ro
ro m in tIlH H H t/1 U1V~ tl~
a) H
~ '
N N
N
b
tTU p,p..
Q1 .xtra~0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
roro+~
uWroU~ ~ O o o o O o o O o o o o o O o
NuN .~o~ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
N
'b 'd'd'b 'd 'b 'd'd 'b 'd 'G 'd 'd 'd 'riN CIN~ ~
m ~ ~
' r1 rlr-Ir~ rl r1 r-Irl rl rl rl r~ r~ r~ rl ~' ~iGir) rl
~ rr rl r)
c~ z xzzb o
ro b
ww a~a~
a a a a a a a a a a a a a a a
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
a~ .~, .~,.~
~
~U ro rororo ro ro roro ro ro ro ro ro ro ro v a~a~v
~ N N N N N N N N N N N N N N N C. f'G~.UU UW
ro G."
W .,, .,~.u.~, .,~ .~,.,~.,~ .,~.,~ .~, .,~.,~ .,~ .,~0 0 0
ro 0
uN Ufa UUUUUU Uu UN UNUu Uu Uu UN UN Uu UN UN x xxx
'a .r-I rirl~1 .rl .rl~.'1~.I ~.~1.rl ..~ rl .rl .n-I w~
H O O 0 O 0 O O O 0 0 0 0 0 O O
~~, a a a a as as a as as a as as a as as
H H H H H H H H N H H N H ,~a H
,~a ,'~,'a ",r5 ",s,'7~ ,'7y ,'a ,'! H y
y
o c ~ ~ ~ ~ c o ~ ~ ~ ~ ~ ~ ~ a aaaaa a
Ou 0u0u ~0u ~o~ ~ouou~ou ~o~~u ,o~u~ .$u~yo u '$u~ u~ '" - --- _
NN N H N N N ~ tiN ~ H N a H H a H '
a
Uro rororororo U~ Ua UroU ~ Up Uro UG Up U ro UG Up ~
~ ~ ~~
~ ~
, . H , H. f u tU ~H
J~ 7tl~I
l~
..i
O m n ~ r ao o~ o rI N M a Iw o r ao a~ V'1
'
y~ ,-~ rI,-1,-1 H rl N N N N N N N N N
.
~ i '
~
z
c
CA 02300008 2000-03-06
- 19 -
A: polished with #320 in 1N HC1 containing 0.5~ K
chloroplatinic acid, left to stand for 1 min.
B: polished with #320 in 1N HC1 containing 0.5~ K
chloroplatinic acid, left to stand for 5 min.
C: polished with #320 in 1N HC1 containing 0.5$ K
chloroplatinic acid, put to cathodic electrolysis for
5 min.
CA 02300008 2000-03-06
- 2~ -
G>~~ GGG
000 000 0 0
.s! ~~I.~I~.I~~I~I~~I~ W
~ N NNN N d N
I NN i
~
v ~~ ~~ ~ > > ~I
a
H roH Q
HHH HH U H 4a
NGI
~o '~ U
-~~.I dpdadadPoadpdPdP dP
' '
1~ lW~O~lO~O ~ N
~oo,o,~o,-Io, ~ ~
~ o,
, . ,+
.~ O
arx b
bJ.I I N
aro N S t11NN OWnW ~-1N N ~ U
H.-1N NrIO LOf'~ d' O
U N N
N
v ab ar ~~~ ~~~ ~ ~ ~ . 4.
I
~o v ax..
ro ro~,~e 000 000 0 0 0
b~v "c~
a w ro
U ~ ~
vuv.uo~ ,-i,-I.-I.-~00 0 o U
aoao -i -r .
I
D ~ a x . . b
N ro ~
U
~ u~ + rd
~
~~~ ~~~
N ~ ~ ~ 'd
~~+'~~~~+0 0 ~ -,-I
ro .'~I' ~
I. +.~ , +~
N u r z '~
N ~ ~~~ ~~~ i
w,w ,ww ~ it
w w
--1
C ~ ~ ~ Q
~ N
4
" v a 3v a 0
~ v roro rn
O
w~ ro~ b b~~ b
~ I
. U
f~GOCGaIUU
NN~ ~> U
' b
N
o7 x
du
l
M " cn.u rororo~roro~roro
.~~~I ~~~~
o~ v
ro .n.n .o
rn N
a.ra
I I
a ro a . m
b o .~, 000 0 0 ; 0 0
" 0
U N I~lCT r I~l' I~ O
~
N~
~ '~~~ ~ ~ v
~ HH G
,
v v ar b
a~
~ b~U v Gx... p +~
U ~ ~ 000 000 O O O O
NW
ro '1-i-I-~oo 0 o U O
N a v ~.~
0 ~
~N~~v> U
i I bI U a x U
I U
~ b
~ ~ .~.~.~.~.~.~p b
"
~I n ro~~ ro~roz c~ ~ z ~
wror
,~ ,
~ www ww~
l ~n~ p
'Iro
i x ~ .
I'rl N
v ~ l ~ O
i l
ro
'r. r O
b Q/
I~' vv 3ro3+~ro3 M ct
~
~ ~
f~lP4GOCOUU ~ ~ ~ ~ ~ O
a, N'b
N N ~ +~a~ +~
m I v
N N
N
~u I NN(!l'bNUlDI.I'd
rororo~roro~roro+.~
' ~ ~ y
~ ~ ~
b ~N
p
~
, I ,
b o~~ oo0 0 0 0
N NNN N N N
N NrI
rtro ~~~ ~~~ ~ ~ ~ ~
~ v
.
. .
oo ~ ~ ,~ ~ ,~ a~
a
~~ w a
l~dD01O.-IN f"1V~ ~ Q
z ..
v ~ -- ~ c~
z
CA 02300008 2000-03-06
- 21 -
G 0 C 0 C ~ 0 ~ ~ C G 0
N ,10 0 0 O 0 0 O O 0 O O O
N ~ +~+~ +~.~ +~~ .rJ+~~ +~+~
ro ' a a a a a a a a a a a a
v v v v v v v v v v v v
v > > a 5 ~ > a > > > > > mn
~ G C C C 0 G G ~ ~ G
H H H H H N H H H H H H
0 0
dada dado dadP dada dP dpdP daaP W W U
o t~ aoa~ r ao o>'c~ v~ rnt~ a~o rl
NI U ~ o~41 O~01 01a1 O~ o~o~ 01O .~ 'O .O
s~ t~ O
Nrx b c0 c~i
bJ~ I
N
~roN w coN .-Irn u~a~ om n o~ m ~ U
N ~ ~ O~ f~110 OD t0 tnN ~
) O
~ Ul U ,-IH N ~ ,
Cn .1-
~-'
o ro ~ ' ~ ~
c
w
w
vurbaNr
b~U v G.C..
ro roa-'x o m o o m o o m o o m o o ' ' '
tnm
NW ro U ~-IO .-1O .-iO riO 'O 'O 'L3
~ ~
v N N ~~1 pp OD oD a0 .-I n-I ri
0 .-I
a ~ N .;0
N ro
,~ rn ~
+> rd rd td
U U
v G 0 G G 0 G s~ ~ t~G 0 0 U
.,~ .,~ .rl
p
~ ~ t~
I o +~ .aJ+~ ~ +~ +~+~ a ~ .u +~r, w w w
'
uo~ro~' j z ro roro roro roro ro roro roro
N v ,~ .~ H ~I .~~I ,~.~ ~ .~~ .~~ .~ +~ +~
a w w w w w w w w w >~ c
irocn a u~ w w r
v .--I
~r
O O O
~ ~vv o 0 0
v
aIU ACf7pU ~ W U ~C f~U ~
.
x U U U
x x x
U
ro
dP da da
a ~ a a a a
0 .,~ ~ ~o ~o~0 0 0 0 0 0 0
U N .~.~ ,~,~ ~ ~ ~ w o vo 0 0 0
H ~ N
~J ~ M M f C N N N N N
1 1
~ N ~ ~ ~ ~ ~ ~ ~ N
V0V~ H H H
1 7 ~ ~ W
N
v va~bv ~ ~ j ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
~ ~
c
i b~ U v a
~ G
C
.~.
: O O ~ 0 0 0 0 0 0 0 0 0 0
ro ro.E.Ix 0
C~v
~ O 0 O
I tnU U U U
v v v v v v v v v v v v v x x x
a a a a a a a a a a a a a
0 0 0 0 0 0 0 0 0 0 0 0 0
aN~ x x x x x z z x x x z x x
~ ,~
Ny
vG O O O If1
p N N v G! GIQ1 CI01 v N N v v M M M ~
-I
ro a a a a a a a a a a a a
~
ro o 0 0 0 0 0 0 0 0 0 0 0 0
z z x x z z x z I, z x z z w
x
i~N '
~~ I
.r.,
3 3 3
~'
ro a a a a a a a a a a a a a
.~ 0 O N 0 0 O O N O 0 N H H
N H N .~N N N N .~ N N
.>a~ ,~ .a.~ ,~ ~a
v
v v N . H N N H H fa N N H s'1N
N
~+~ rororororororororororororororororororororororororo
ro ro U U U U U U U U U U U U U rl ri ~ U
G4 t~ ~ rl C, rl 'f..,"
r~ N
~
..a o
.
~,
.,~ ,-Itp t'aD O~O ~-iN f~'1V'1l1~OI~ O
~ oo U
~
c
v
x
CA 02300008 2000-03-06
- 22 -
a a a a a a a a a a a a-
N O 0 O O O 0 O O 0 O 0 O
N 1J 1.~.1.~.1~aJ 1.~.1Ji~ l~~.1l~1t
ro w a a a a a a a a a a a a
v v v v v v v v v v v v
v
v > > > ~ > > > >
~
PG O C ~ G 0 C G C G G 0 G
H H H H H H H H H H H H
l~0
op op dPop opop d~op dP doop dpop
H,~ O OD ODOt OD01 O I'~00 01CO O~O
01.1
N~ U ~ O~o~ o~01 O o1 01 0~o1 0~O
NN O
aPG 'd
tn ~ N c'~101 Il1I~ 1n ~ ~ d001
Hb N
~ M ~ N O vD tl1N
~ ~' ~ .-1 ~ ~ ~
N0 -1
U
4 t0
1
I ~' W
N
U v ~ .'
b
~
.~ .
~
ro ro+ o ~ o o ~ o o m o o m o 0
x b~N
a w ro r,o ,~ o ,-io .~o ?,
o ~ ~
N N v m a0 ao ao .-1
~.~ O
r1
o
y~ N
l
l
i ~
I
0 r
.
..
~i V 0 'b b b ~ b b b b b 'T~b b ~-1 u1 N
~ ~
. 'fr'ra rirl r~H r-Ir~ ri r1n-1rirl
td 1.1 ~ ~ ~ O
J.1 '
N
~N N x O ~
'~
~ v~
u
'
'
U .~I
"d .O .O
ro v~
v
N U v ' +~ ~ +~
L1 W Gu L1W Ls,fa W Gufa W G4 fly U7 td
U
'' '' '
.
~ ~ N .
o
~r, " +~
~~
w w
.
~
~
p
~~o
~ 0 0 0 0 0 0 ~
~ -I
,
U y .-1H ~-.W l' I'~l~10 t0t0
N ,~ '~ ' -1
' '~~'
0 v M f ( f N N N N N N
1 1 1
~ O N
~
tl~~ c a H H H
I7
~ ~ ~
c n N .L~ .~ .Ll
~ ~.
b~ U v
~
, .~ .G
ro roux
trv
U 0 0 0 0 0 0 0 0 0 0 0 0 0
O
NNN
O
.-
I
~.
i
~ ~
N
~Q', r-I W -i
l~
D
_ i
U
i ~ I I I
N N v v v N v N v N v N Q~ '0 'b 'b
a a a a a a a a a a a a a
0 0 0 0 0 0 0 0 0 0 0 0 0
a ~~N x x x x x z z z z x z z z
,
a a
,~
v ~ 0 0 0
N v v N v v N N v N N N v M M c~7
b O G G G O ~ G G O O C 0
w ro O O O 0 0 0 O O 0 O 0 O 0
uv z x z x z z x z x x z z x
3 3 3
b G G IOC O C C G C G G ~'GO
; ~~i O 0 0 0 O 0 O O 0 0 0 0 O
a a a N a a a N a a N a a N N O
, N .t7,~ ~7.p .4.A l711 .f7.A.L1,p,f~
N v N v Q1N v N
I v N a N N a a a N a a H a ~ a
I N~ rororororororororororo~rororororororobrorororororo
roro U U U U U U U U U U U U U
~
~
i G
.~ O
O
.,~ ~-1OD 01O H N ~'1d' If1l0t" ~ 01
I-1 ~f1tf1~D ~O~0 10t0 lD t0t0 ~Dt0 -~ .. ..
-- A w w
o
c
z
CA 02300008 2000-03-06
- 23 -
~ o C o ~
N ~ 0 0 O O 0 O O O O O O O
,y~
N ~1 1.~i~ 1~ i~ .1.) 11 1~ 1.1 JJ i~.I-~
ro w a o a a a a a a a a a a
v v v v v v v v v v v v
a ~ a a G ~ a ~ ~ ~ a a a a
H H H H H H H H H H H H
NG G".
oW oW oWoW cW oY~ oY~ cVo cW dP oW dPdP .
O l~ ODQ1 l'~ ~ 01 OD ~ O~ OD OfO
OyJ 01 01O~ O~ Of 01 O~ O1 01 01 O~O
NI
NU
v
~
Np4
'd
bJ.1 ~ O
I
N
H~
tUd t17 DDN d' O~ N 01 f~1IW f1 ~ 01 4-1
N
. ~ ~7 CD Qt cr1 to ~ ll'1N
roNU ~ ~ .,
G~
O
U ~ U7
N
N
~
N . ,--I
vv'dQl ~ ~ O
r1r1 i..l
b~ '
U
v
>~
.~
.~.
N o ~ o O u1 O o ~r1 o o u1 o 0
w ~
b
U
~
~
~ o .-r o ~ o ,-Io U
v W ao ao ,mn
N
v
~
~
0
~
a
~
~
.~
s~
ro
N
J..I U
1~
"
D
U ~ ~
I b b b
tT
C
U
N
~r ~ b b b b
I
ro o ro roro ro ro ro ro ro ro ro roro b b b
+~
+~
~w x ,~ ~ ~I ~ ~ ~I ,~ ~ ~ .~ ~ ~I
ro o ro
N
Nv ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ c
' o
N ~osa ro roro ro ro ro ro ro ro ro roro
~ w a w w w w w w w w w w
v rox"N I
U
rtN
~ ~ +~ O
N v
~~ v i +
'' ro o c~ x H c~ x H c~ x H ~ x H ~''w
N ~
~ +~
v N ..,
~
z a~a~
ro ~ ~
~ ~ 0 0 0 0 0 0 ~ rd cd
~-1 v-1 v-1r-1 l'~ L'~L~ t0 101D ~ .Q
N U M f~'1 f'~1f~'1 N N N N N N
0
N N ~ U ~ ~ .~ .,~ .,~ ~~ c~ tn N v~m
W
(n tn U7U7 N H H
~ ~
~
' ~
N '-I~--I
' N
d
N
~.. b~ W ~.,Or
U
N
~
.G
~.
ro O O O O O O O O O O O O O I I I
roW,l~
p~v
HWroUD~
,..Ir-1r-I
N
v v v v v v v v v v v v v
a a a a a a a a a a a a a
0 0 0 0 0 0 0 0 0 0 0 0 0
~
~N z z x z z z x x x x z x z
~ ~
~
~, fn - .!"',>~"t~..
ro L~
N
. .r-I.~i.r-I
i
I N
G O O O
U v N 01v v N v v GI N GI v d
~
M M M
ro,~ a >~ a a a a a a a a a a a
z z
uv z x z z x z x x x x x
3 3 3
ro a a a a a ~ a a a a a a a
.~ N O O O O O O O 0 0 0 N0 O N
~ N H N N N N H H i~ N
N v.L7N p .L7N~1 l7 .Ll ,~ .0 .p .>a .4.L1
v ~I H N N H N H H N H N N N
v
N ~ rororororororororororororororororororo ~rorororororo rn~n m
ro ro U U U U U U U U U U U U U
i ~ ~
I ~ ~ ~ d
G ~
.~ O O
.,~ ,-1 O .-IN M C~ In t0 I' aD Ot O r-i
I '~ y . r. r r ~ r r ~ ~ r ~ aoa~ Z .... ..
o ro o .--C7x I-i
U
ax
CA 02300008 2000-03-06
~ - 24 -
- O O C O O C p G:O G C .f.
N O O 0 O 0 0 O 0 0 0 0 O
~
x .,~ .~, .,~
+~ .H +~ +t .H+~ ~ 1~
ro w a a a a a a a a a a a a
v a~ ar w m v a~ o a~ w a~ a
a a a a c a a a a a a
a a a
H H H H H H H H H H H H
NO
0
o r. o~ o~ ~ 00 0~ ao ao~ ao o~ o
a, c, o, o, o, o, o, o,o, a, o, o
NE
b
P
G
b~ N
i
N
b g ~ O~ 01 tl1 01 N 01 M ~1 If1 W 01
W
'
N0 Ci-i-1 W 'd' ~ 01 f t0 in N
N ~Y
U
U ~ ~ . w
N ~
b
b UI
U
N
G
~ I '
.~
.,
ro O m ~ o m o o u~ o o m '' o
ro o o
.H
x
b~
N
f.l I O .-1 O W o ' O ~ C.'O
w ,1 -i r1
ro
U
0
O
N pp a~ ao ao
I N
N
.~
O.-~
D
~
N.C
N
ro
, ~C
j N
.i~
y
U
H N N s-I N H N N LI N H N
r
V ro GlQ1 GI GI Ql N CI 01 N N Gl N N
C
, ~ y,1Q
~ o > y ~ a ~ a ~ ~ > a ~ >
ro~+ p o
0
0
~U 'Q 'Q'Q
7
~
N
O
N
o h x a h x a h x a h x a ~ ~ o
H z
v
H ~,
+~
w w .~.i
a ~ w
,
,a a b a a a a
~ .y o me ~ 0 0 0 0 0 0
o
i N .-1~-I .-i '-1 l~ h t~ ~O t0 t0 .~ . ~"..
U -t"'.
M N m N ~ ~ ~ N N
t (~ U t i U U
ll~ ~ J H E H /l ~ ~ 1 7
i ~ ~
N ~ t u t I .I1.~7.L1
~ ll 7
O ,
Q
~ b~ ..
U 0 0 ' .
N
~
.~
~ o O o 0 o O , o 0 0 0
O
N r-ir-1r-1
N
~
~rl
O
~
>oHxs~ro
~
N
1)
l~
,"a
U HI S-Ib-1
i
b~
U ~ v a~ v a~ a~ v d a~a~ a~ v a~ ~ D D
~
ro
", a a a a a a a a a a a a a
~~ x x z x x x z x
v
N
z x x x x
-1~
N
~ rotn
+.1
N
~ ~ rI r-Ir1
Ny
O
O
N N N
U N N Gl Ol N N d N N N N N N
ro O O O O 0 O O O O ~ G ~ O M M M
I wro o 0 0 0 0 0 0 0 0 0 0 0 0
,
x z z x x x x x x x x x x
3 3 3
ro a a a a a a a a a a a a a .a .d.n
~~I O O O O O O 0 O N O NOH0 HOH Q U U
H N N N N N H N H
~ .4 ,p .~ Q .L7 .4 .4 ,4 N.4 .p .A
Q1 H N N N H N N Ul H H
QI N N H
N N N
rorororo rorororororororororo robroro roro roro roro roro m cnu~
N U U U U U U U U U U U U U
~
~
i U O O O
G
~.1 .1~Cl~Cl.~~1.,
O
.1N f'~1 W 17 l0 l~ Ca 01O r1 N Ih Q
o ao 0o ao ao ao ao ao aoo, o, a, o,
Uax -- h x a
CA 02300008 2000-03-06
- 25 -
o c ~ o o ~ ~ o
0 0 0 0 0 0 0 0 0 0 0 0
a , y w w .~ +~ w w +~ w .~ w w
ro w a a o a a a a a a a a a
v v v v v v v v v v v v v
v
a > > > > > ~ > > > a
x ~ a
a a a a a a a a a a a
H H H H H H H H H H H H
N C
11 0 dP dP oW op oW op aW dP cW oW oW dP oW
O O~ O~ 01 O O O O ~ O~ 01 O O
N I ~ ~ ~ O O O O O~ 01 C1 O O
U
v N .1 ..a .1 .-a .-r r-I
~
a !x'd
N
v ow
0 ~ N N N In 1f'1 01 t0 V~ In ~ ~f1 t0
ro O (~,''.~1.~~7 cp M N rl e-t N M sf Il1 N N
N
~
~ U ,
a WO
ro
I I
vNbN I
b~
U o 0 0 0 0 0 0 0 0 0 0 0 0
N
G
.~
..
ro
roux
tnv
uwroU~~
0 0 0 0 0 0 0 0 0 0 0 0
'~ -I 1 a -
N . . . ~ .1 rl ..a .-a .1 ~I
~ I
~
N
p
~ ~ ~ u
v ro ~ b ~ b ~ b o
r
0 0 ,~ .~ '~ ~ ~ '~ ~ ~ '~ ,~ ~.u
~ ~
~ uv+~ z ~ ~ ~ i ~ ~
~
, n c
~~ w c~ n w
~
v a.
ro
U x .~ ,~ ,~ N ~ UNl ~ ~
. Ul W ~ UN ~
rl
ro
v ~ ~ ~ a ~ ~ ~b l i ro ro
vb ro ro .~ ro .
~
N~ vN 3+~ro 3 31~ vb .. ..~ .~ ..~ .,~ro ~~b.a rl.~,~
..~ 3~ro3 ro 3y~ro ro 3 1.~ 3 uro3 31
rl 31~ 3
rl
w~ ~ ~ v~ bl ro~~ ~~ o' ~~ ~ ~' ~~ , ~' ~ ~
~ ' b s ~ ~
~
N w . . l b b bl b . bl b
~ . b
ro o va vub vab va vub vab va vuv v ab va vub vab
v z w ~
uv I .uvvN w..~vN I wvvN w.~vN.r , +~vvN.u. .,vN.u
.uvvN +~..~vN
a N N I I
+ N m
~ Nb N N.~+~b Nb N N+~+~b wd N f ~Nb N N+~b
a N b N ~a~b N >.u2 m+~.u m alb
~, ~w ro~,ro ro~roro rorororo ro~roro~roro rorororo ro~ro
ro~rororo rororo ro~ro ro~roro rorororo
~ ~l.,~~, ~~I~ ~ ~l.~l.~ ~~I.~ o~ ~.~~ ~~ ~ ~.~,~ .~~I~
o.~ ~ ~ o~ n ~ ~ .~ ~ o~l ~ v
v N a ~ N n ~
cr a a a~ a~' a
~ ~ a~'
ro . . . . . . .~ ~ .~ .a ,~ .~
. , N a o~ N a
a. a. a. a.a
H
SD t0 ~O l0 O O O O O O
N U ~ .-~.-1 rl '-1 Ice. I~ I~ t0 t0 10
' I
teaa7 tea t~ H H H
N
~ CPU
N
G
C--
.
ro
rowx
crv
uwroU~~ 0 0 0 0 0 0 0 0 0 0 0 0 0
vuv~~o~
uxuro
~'
UJ1~1~~,~
I
v v v v v v v v v v v v v
a a a a a a a a a a a a a
0 0 0 0 0 0 0 0 0 0 0 0 0
z z z z z z z z z
z x z z
~~
~
ro
a
y~
v
g N v N v N N v GI v N v v GI
U
ro.N G ~ ~ G G ~ G E C ~ ~ >~
wro o 0 0 0 0 0 0 0 0 0 0 0 0
z z z z z z z z z z z z z
~w
ro a a a a a a a a a a a ~ a
N ~ ~ v 4
a
. ~~ .~ . . .o~ ,n ~ . ~ .~ ~ . ~ . ~. ~ .$
N N a N a 4 a ~ a a c~ c ~ 4 c~
N a H N
a ~ 1 a N N a
UroUA U a U p UQ U
N~ , , , , p,U p,U p,U a,U Q, U ~U p,U 0~
~
i I
I .~ .-1W f7 t0 I~ OD O~ O .-a N M sf ll1
, O~ 01 O~ O~ 01 01 O O O O O O
oroo .~ ~, ,
U
az
CA 02300008 2000-03-06
- 26 -
The baths for plating gold, palladium and silver
used for the combination numbers 58 to 93 possessed the
compositions as described below.
Composition of a Qold-platincr bath
An aqueous solution in which are mixed:
Potassium gold cyanide:KAu(CN)2:4 g/L
Sodium cyanide:NaCN:30 g/L
Soda ash:Na2C03:40 g/L
and maintained at a temperature of 65°C.
Composition of a palladium~lating bath
An aqueous solution in which are mixed:
Palladium chloride:PdC13:5 g/L
Hydrochloric acid:HC1:250 ml/L
and maintained at normal temperature.
Composition of a silver-plating bath
An aqueous solution in which are mixed:
Silver cyanide:AgCN:5 g/L
Potassium cyanide:KCN:75 g/L
and maintained at normal temperature.
A stainless steel plate was machined into a
separator, a paste of a fine carbon powder containing
platinum was applied onto a commercially available solid
polymer electrolyte membrane and was dried, and a
nonwoven fabric of carbon fiber was used as a current
collector to obtain a fuel cell.
A pure hydrogen gas or a simulated methanol-cracked
gas (25~ of C02, 75$ of Hz) was fed as a fuel gas to the
side of the hydrogen electrode, a simulated air gas (20~
of O2, 80~ of NZ) was fed to the side of the oxygen
electrode under the atmospheric pressure, the whole cell
was held in a high-temperature chamber at a temperature
of 90°C, and a short-circuit current flowing into the
external unit from the positive electrode to the negative
electrode was measured to confirm the performance of the
fuel cell.
The electrodes used for the test possessed a size of
100 x 100 mm. By taking the corrosion resistance into
CA 02300008 2000-03-06
_ 27 _
consideration, the separator was formed by press-molding
a stainless steel plate YUS270 having a thickness of 0.4
mm so as to possess grooves and holes that sereve as gas
passages and coolant passages.
The stainless steel separator possessed a contact
surface that was lightly polished by using a silicon
carbide paper #320 in a 1N hydrochloric acid containing
0.5$ of potassium chloroplatinic acid by making reference
to the results of table 1 and on which surface platinum
was precipitated by the cathodic electrolysis conducted
for 5 minutes. For the purpose of comparison, further,
another stainless steel separator was prepared having a
contact surface that was not treated. There were further
prepared carbon papers having a contact surface on which
gold was deposited maintaining a thickness of about 1000
nm by the ionic vaporization and having a contact surface
that was not treated for comparison.
As a result, the solid polymer electrolyte fuel cell
that was not treated generated a short-circuit current of
only about 15 A, whereas the solid polymer electrolyte
fuel cell that was treated to decrease the contact
resistance generated a short-circuit current of about 85
A, from which it is obvious that a decrease in the
internal contact resistance contributes to greatly
increasing the power efficiency.
According to the first aspect of the present
invention as described above, it is possible to greatly
decrease the contact resistance of the member that so far
created a problem in using a stainless steel material,
which is cheaper than the traditional carbon materials
and makes it possible to decrease the size, as a material
of the separator for the solid polymer electrolyte fuel
cell that is promising as a generator for cars and as a
portable generator. This contributes greatly to putting
the solid polymer electrolyte fuel cell into practical
use.
Next, described below is a preferred embodiment
CA 02300008 2000-03-06
- 28 -
according to the second aspect of the invention.
In the solid polymer electrolyte fuel cell, the
solid polymer electrolyte membrane that selectively
transmits hydrogen ions is held by the catalytic
electrodes comprising fine particles of carbon and a
noble metal, and the electric power is generated by
taking out electrons from the oxidation reaction of
hydrogen and by taking out electrons from the reducing
reaction of oxygen that takes place on the respective
electrodes. The electrons are collected by the current
collector constituted by a nonwoven fabric having an
electrical conducting property, such as carbon fibers,
and are guided to the electrically conducting separator.
Single cells having such a basic structure are stacked in
series to obtain a cell which as a whole generates a
required electromotive power.
The separator requires functions such as one for
conducting electricity, as well as a separation function
so that a hydrogen gas or a hydrogen-containing gas which
is a reaction gas and a gas such as the air containing
oxygen will not be mixed together, and, as required, a
structural function which permits a coolant such as water
to flow inside the cell structure but circulates the
coolant and the reaction gases separated from each other.
The carbon material has heretofore been chiefly used as a
member for the solid polymer electrolyte fuel cell, such
as the separator. However, the carbon material requires
an increased production cost for forming grooves and
imposes a limitation on decreasing the thickness, making
it difficult to decrease the cost of the fuel cell as a
whole and to decrease the size. In order to solve this
problem, therefore, the present inventors have attempted
to use a stainless steel instead of the carbon material
and, at the same time, have studied combinations of the
additives and their amounts to withstand the environment
in which the solid polymer electrolyte fuel cell is used.
The reaction gas, which is a fuel flowing in the
CA 02300008 2000-03-06
- 29 -
solid polymer electrolyte fuel cell, is pure hydrogen,
hydrogen containing some impurities, alcohol such as
methanol, or a cracked gas of hydrocarbons
(representative composition: 25~ of carbon dioxide gas,
75~ of hydrogen and several tens of ppm of carbon
monoxide). On the other hand, the reaction gas for
controlling the combustion is an oxygen-containing gas
and, generally, air from the atmosphere. In order for
the solid polymer electrolyte membrane to work as an
electrolyte, an amount of water is necessary, and these
gases are controlled to have a dew point of about 80°C.
The operation temperature is generally about 90°C.
In this system, the fuel cell starts operating and
stops repetitively. It needs not be pointed out, first,
that what is most important is that the separator itself
does not corrode. In particular, when a cracked gas of
methanol is used, the carbon dioxide gas contained
therein is absorbed by the condensed water in the fuel
cell to form an acidic solution. Besides, the solid
polymer electrolyte membrane itself is an acidic solid
electrolyte. Therefore, the environment in which the
separator is exposed is an acidic aqueous environment at
a temperature of from normal temperature up to a boiling
point of the coolant such as water (usually, up to about
150°C), and it has been pointed out that the pH drops
down to about 2 depending upon the conditions in which it
is used. Once the corrosion takes place, the metal ions
eluted from the corroded portion contaminates the solid
polymer electrolyte membrane even though the corrosion
may be of a slight degree, impairing the function for
selectively transmitting hydrogen and seriously affecting
the cell performance. Thus, the corrosion causes a
problem even when it is eluting out trace amounts of
ions.
The present inventors have estimated that the
elements that impart corrosion resistance in an acidic
environment of relatively low temperatures are chiefly
CA 02300008 2000-03-06
- 30 -
Cr, Mo and Ni, have prepared thin plates of stainless
steels while changing the amounts of addition and
combination of the elements, machined the steel plates
into separators, applied a paste of a fine carbon powder
containing platinum onto the commercially available solid
polymer electrolyte membrane followed by drying, and
employed a nonwoven fabric of carbon fiber as a current
collector to constitute a fuel cell. A pure hydrogen gas
or a simulated methanol-cracked gas (25~ of C02, 75~ of
Hz) was fed as a fuel gas to the side of the hydrogen
electrode, a simulated air gas (20~ of O2, 80$ of NZ) was
fed to the side of the oxygen electrode under the
atmospheric pressure, the whole cell was held in a high-
temperature chamber maintained at 90°C, and a change in
the short-circuit current flowing into the external unit
from the positive electrode to the negative electrode was
measured with the passage of time to confirm the
endurance and reliability of the fuel cell performance
(endurance generation testing).
The electrode units used for the testing possessed a
size of 100 mm x 100 mm, and the separators were prepared
by cutting 4 mm-thick stainless steel plates to form
grooves that serve as gas passages. After 100 days have
passed from the start of the testing, the external
current was measured and a ratio to the current initially
generated was found to evaluate the endurance and
reliability. Here, it was so judged that the fuel cell
could be practically used provided the ratio was not
smaller than 0.9. The conditions such as cell size,
reaction gas, temperatures for use and the like were
selected from the practical point of view, and severe
testing was conducted by continuously passing the current
for 2400 hours (100 days). Therefore, the stainless
steels for practical use could be selected very reliably.
After the endurance generation testing, those having
a ratio of the current after the continuous use of 2400
hours/the initial current of not smaller than 0.9 were
CA 02300008 2000-03-06
- 31 -
rearranged for their components, and it was found that a
numerical value calculated according to 10 - 0.3 x ([Cr$]
+ 3 x [Mod] + 0.05 x [Nib])~ stands for $ by weight of
the elements) served as an effective index provided Cr
was contained as an essential component and Mo and Ni
were preferably contained. According to the study by the
inventors, it was found that when the numerical value
calculated according to the above formula is not larger
than 5, the separator exhibits suitable properties when
pure hydrogen is used as the fuel gas and when the
numerical value calculated according to the above formula
is not larger than 4, the separator exhibits suitable
properties even when a reformed gas of alcohols is used
as the fuel gas. That is, the important point of the
invention is that the lower limits of the contents of Cr,
Mo and Ni, that are basic elements associated with
imparting corrosion resistance to the stainless steels,
can be expressed by the above-mentioned formula under the
environmental conditions in which the separator of the
solid polymer electrolyte fuel cell is exposed.
Therefore, the above index makes it possible to specify a
stainless steel having a performance sufficient for the
solid polymer electrolyte fuel cell and, hence, to
provide a material, of low cost, which avoids unnecessary
or excess amounts of addition of the elements. As
described above, the testing was conducted under the
environmental conditions in which the separator was
exposed. Since the severest environmental conditions
were employed, the invention can be applied to other
constituent members made of stainless steel, such as a
terminating plate used for the terminating portion of the
laminate.
In the present invention, it is important that, in
an environment where pure hydrogen is used as the fuel
gas, 10 - 0.3 x ([Cr$] + 3 x [Mod] + 0.05 x [Nib]) s 5 is
satisfied and in an environment where a reformed gas of
alcohols is used as the fuel gas, 10 - 0.3 x ([Cr$] + 3 x
CA 02300008 2000-03-06
- 32 -
[Mod] + 0.05 x [Nib]) s 4 is satisfied. Though the roles
of the individual elements have not yet been clarified in
detail yet, described below are the elements that are
added.
Chromium is a major element that imparts corrosion
resistance by establishing a passive state in a corrosive
environment to which the invention is related, and
exhibits a effect even when it is added alone. The
effect is exhibited if the lower limit value of the
addition complies with the condition of the above-
mentioned formula. The effect saturates when the
addition exceeds 30~. Therefore, the upper limit is 30~.
From the standpoint of sufficiently lowering the cost,
however, the addition is so adjusted as to satisfy the
above formula in a range of not exceeding 23~.
It is desired to add molybdenum since it is
considered that molybdenum exhibits the effect of
suppressing, particularly, local corrosion in the
corrosive environment with which the invention is
concerned. The effect is exhibited so far as the lower
limit value of addition complies with the condition of
the above formula. The effect, however, saturates when
the addition exceeds 10$. Therefore, the upper limit is
10~. From the standpoint of sufficiently lowering the
cost, however, the addition is so adjusted as to satisfy
the above formula in a range of not larger than 7~ and,
particularly, not larger than 3~ in a pure hydrogen
environment.
It is desired to add nickel since it is considered
that nickel further increases the corrosion resistance of
the steel material by increasing the austenite phase in
the corrosive environment with which the invention deals.
The effect is exhibited so far as the lower limit value
of addition complies with the condition of the above
formula. The effect, however, saturates when the
addition exceeds 25~. Therefore, the upper limit is 25~.
From the standpoint of sufficiently lowering the cost,
CA 02300008 2000-03-06
- 33 -
however, the addition is so adjusted as to satisfy the
above formula in a range of not larger than 20~ and,
particularly, not larger than 15~ in a pure hydrogen
environment.
Though not specified by the above-mentioned formula,
copper, which is effective in imparting corrosion
resistance, may be suitably added in an amount of not
larger than 2.5~ provided it does not sharply drive up
the cost, without departing from the scope of the
invention. Within a scope investigated by the present
inventors, the corrosion resistance in an environment
with which the invention is concerned is not affected by
the method of production. Therefore, any conventional
production method may be employed provided it does not
cause any extreme production problem.
Example 2.
The invention will be further described in detail
based on the results of the above endurance generation
testing over 100 days. Details of the testing conditions
are as described above. As a result of putting the
stainless steels containing components listed in Table 9
to the test, it was confirmed that the current decreased
little after 100 days and the ratio of the current after
the testing/the initial current was not smaller than 0.9
when stainless steel materials satisfying a relationship
10 - 0.3 x ([Cry] + 3 x [Mod] + 0.05 x [Nib]) s 5 in the
pure hydrogen environment and a relationship 10 - 0.3 x
([Cr$] + 3 x [Mod] + 0.05 x [Nib]) 5 4 in the methanol-
reformed gas environment were used. Thus, the separators
exhibited their functions to a sufficient degree in the
solid polymer electrolyte fuel cell in their respective
gaseous environments. When these requirements were not
satisfied, the ratios were not larger than 0.9 and the
separators failed to exhibit their functions to a
sufficient degree as shown in the dotted areas in Table
9.
Image
CA 02300008 2000-03-06
- 35 -
According to the second aspect of the invention as
described above, optimum component ranges are specified
for the separator materials for the solid polymer
electrolyte fuel cell which is promising as a generator
for cars and as a portable generator, making it possible
to provide stainless steel materials at a low cost and in
a compact size compared with the traditional carbon
materials.
Next, described below is a preferred embodiment
according to the third aspect of the present invention.
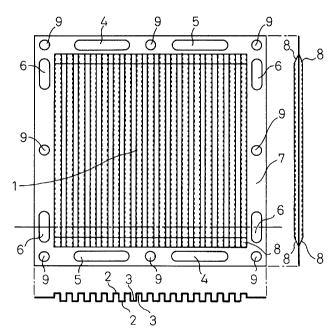
First, Fig. 1 illustrates a stainless steel
separator for the solid polymer electrolyte fuel cell.
The separator has, at its central portion 1, a corrugated
structure comprising a plurality of grooves, including
electrically conducting portions 2 of convex surfaces and
passage portions 3 of concave surfaces for the gas or the
coolant as shown in a sectional view thereof. The
grooves are coupled together at their both ends by
coupling portions 8, and the passages are secured
permitting the reaction gas or the coolant to flow
through the grooves at the central portion. Peripheral
portions 7 are provided with holes 4 that serve as
passages for the one reaction gas and with holes 5 that
serve as passages for the other reaction gas, enabling
the reaction gases to be fed or drained. The peripheral
portions 7 further have holes 6 serving as passages for
the coolant, enabling the coolant to be fed or drained.
The stainless steel plate which is a blank material has a
thickness of not larger than 2 mm. Though an optimum
thickness is determined from the corrosion resistance and
the strength, the stainless steel plate having a small
thickness is desired from the standpoint of productivity
and cost. From the standpoint of production cost, it is
desired that the part is produced by press-molding and
punching.
Next, described below are spacers A and B for the
solid polymer electrolyte fuel cell. The spacers A and 8
CA 02300008 2000-03-06
- 36 -
have shapes for securing passages for the one reaction
gas and for securing passages for the other reaction gas.
The spacer A is shown in Fig. 2 and the spacer B is shown
in Fig. 3. The spacer A has in the peripheral portions 7
thereof holes 10 communicated with the central portion,
and the spacer B has in the peripheral portions 7 thereof
holes 11 communicated with the central portion, to secure
the passages for the one reaction gas and the passages
for the other reaction gas, so as to be guided to the
groove-coupling portions 8 of the separator. To the
central portions are fitted the central portion 1 of the
separator. Therefore, the spacers should have a
thickness corresponding to the height of concave grooves
or convex portions of the separator. Any material may be
employed provided the gas does not leak. From the
standpoint of cost, however, it is desired to use a resin
that is not deformed or does not undergo a chemical
change up to 100°C.
Fig. 4 illustrates a spacer C for the solid polymer
electrolyte fuel cell. The spacer C secures passages for
the coolant and has, in the peripheral portions 7
thereof, holes 12 serving as passages for the coolant,
and communicated with the central portion, so that the
coolant is guided to the groove-coupling portion of the
separator. The spacer C may be sandwiched by two
separators, or may be used at an end and may be
sandwiched by the separator and the terminating plate,
without any difference in the structure except the
thickness. Any material may be employed provided the gas
does not leak. From the standpoint of cost, however, it
is desired to use a resin that is not deformed or does
not undergo a chemical change up to 100°C.
Fig. 5 illustrates a spacer D for the solid polymer
electrolyte fuel cell. The spacer D is inserted between
the solid polymer electrolyte membrane to which a
catalytic electrode is imparted as shown in Fig. 6 and
the spacer A or B. The spacer D serves as a frame for
CA 02300008 2000-03-06
- 37 -
the catalytic electrode unit 14 formed on the solid
polymer electrolyte membrane 13 shown in Fig. 6 and for a
current collector 15 shown in Fig. 7, and has a thickness
as close to the sum of the thickness of the catalytic
electrode unit 14 and the thickness of the current
collector 15 as possible. Any material may be employed
provided the gas does not leak. From the standpoint of
cost, however, it is desired to use a resin that is not
deformed or does not undergo a chemical change up to
100°C.
In order to further decrease the number of parts and
to further lower the cost, it is desired to employ a
spacer E constituted by the spacers A and D as a unitary
structure and a spacer F constituted by the spacers B and
D as a unitary structure from the standpoint of
productivity.
Fig. 8 illustrates a terminating plate for the solid
polymer electrolyte fuel cell. The terminating plate has
ports 16 and 17 for feeding and draining the reaction
gases and ports 18 for feeding and draining the coolant.
The terminating plate works to take out the electric
power to the external unit from the solid polymer
electrolyte fuel cell that is laminated in series, feeds
the reaction gases and the coolant, and applies a
suitable pressure to both ends of the laminated module so
that no gas leaks or no coolant leaks from the whole
laminated structure.
The separators, spacers, terminating plates and the
solid polymer electrolyte membranes are laminated and
secured, desirably, by using bolts. It is therefore
desired that these members have bolt holes 9 formed in
the peripheral portions thereof. Thus, the fastening
means is contained in the laminate, which is very
desirable for decreasing the size. Besides, a reduction
in the number of the fastening members makes it possible
to lower the cost.
Fig. 9 illustrates a laminated module for the solid
CA 02300008 2000-03-06
- 38 -
polymer electrolyte fuel cell that is constituted in a
laminated manner so that separate passages are secured
for the one reaction gas, for the other reaction gas and
for the coolant. The solid polymer electrolyte membrane
13 to which the catalytic electrode 14 is imparted,
current collector 15, spacer A19, spacer B20, spacers
C21, 22, spacer D23, stainless steel separator 24, and
stainless steel terminating plate 25 are laminated to
secure passages 26 for the coolant, passages 27 for the
one reaction gas, and passages 28 for the other reaction
gas. The reaction gases and the coolant are fed through
the reaction gas feed/drain ports 16, 17 and the coolant
feed/drain ports 18. Though not diagramed, the spacer E
combines the spacers 19 and 23 integrally together, and
the spacer F combines the spacers 20 and 23 integrally
together. The shapes of these parts and the structure of
the fuel cell are only some examples, and it need not be
pointed out that shapes and sizes may be changed provided
the same fundamentals apply.
In the solid polymer electrolyte fuel cell, the one
reaction gas is usually a fuel gas such as a hydrogen-
containing gas or a methanol-reformed gas, and the other
reaction gas is usually a combustion-assisting gas such
as oxygen-containing gas for controlling the combustion.
As the coolant, water is usually used from the standpoint
of cost and safety. That is, the cooling is effected
relying on the cooling water and, hence, the solid
polymer electrolyte fuel cell is used at temperatures of
not higher than the boiling point thereof and, typically,
at about 90°C.
The separator and the terminating plate used for the
solid polymer electrolyte fuel cell are made of a
stainless steel, and are used each in a number of one or
more.
Further, the cell may be constituted by combining
one or more of the laminated modules.
Example 3.
CA 02300008 2000-03-06
- 39 -
The members shown in Figs. 1 to 8 and having a
square shape of a side of 240 mm were prepared, i.e.,
stainless steel separators were prepared by using a
stainless steel plate having a thickness of 0.5 mm and
containing 20~ Cr - 18~ Ni - 6~ Mo - 0.2~ N, and spacers
were prepared by using a fluorine-contained resin.
A solid polymer electrolyte membrane available on
the market was used, and the catalytic electrode was
applied thereon and dried, and was cut into a
predetermined shape and was laminated. Further, a
nonwoven fabric of carbon fiber was cut into a
predetermined shape and was used as the current
collector.
A laminated module shown in Fig. 9 was prepared by
using the above members as a basic structure. In order
to prevent the leakage of gases and coolant, the
contacting surfaces of the parts that do not require
electric conduction were coated with a thin silicone
resin film as a sealing material, and the whole laminate
was pressurized by fastening the bolts. The cell
consisted of a laminate of 10 stages. After having
confirmed that there is no leakage of reaction gases or
coolant, pure hydrogen and the air gas were used as the
reaction gas, and electric power was generated while so
controlling the temperature and the flow rate of the
cooling water that the whole cell was maintained at 90°C.
The electromotive force of 5 to 6 V and a short-circuit
current of a maximum of 400 A were observed, from which
it was proved that the solid polymer electrolyte fuel
cell of this system can be constituted to a sufficient
degree.
According to the third aspect of the invention,
there is provided concrete technical means for using a
cheap stainless steel as members of the solid polymer
electrolyte fuel cell instead of using the conventional
expensive carbon material. It is therefore made possible
to enhance the productivity and to greatly decrease the
CA 02300008 2000-03-06
- 40 -
cost, contributing to the widespread use of the solid
polymer electrolyte fuel cell.