Note: Descriptions are shown in the official language in which they were submitted.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
1
CLEANSING COMPOSITIONS
TECHNICAL FIELD
The present invention relates to cleansing compositions. In particular it
relates to mild personal cleansing compositions which display improved
rinse feel in combination with good skin feel attributes, and foaming
properties which are suitable for simultaneously cleansing and
conditioning the skin and/or the hair and which may be used, for example,
in the form of foam bath preparations, shower products, skin cleansers,
hand, face and body cleansers, shampoos, etc.
Background Of The Invention
Mild cosmetic compositions must satisfy a number of criteria including
cleansing power, foaming properties and mildness/low irritancy/good feel
with respect to the skin, hair and the ocular mucosae. Skin is made up of
several layers of cells which coat and protect the underlying tissue. The
keratin and collagen fibrous proteins that form the skeleton of its structure.
The outermost of these layers is referred to as the stratum corneum. Hair
similarly has a protective outer coating enclosing the hair fibre which is
called the cuticle. Anionic surfactants can penetrate the stratum corneum
membrane and the cuticle and, by delipidization destroy membrane
integrity and loss of barrier and water retention functions.
This interference with skin and hair protective membranes can lead to a
rough skin feel and eye irritation and may eventually permit the surfactant
to trigger immune response creating irritation.
Ideal cosmetic cleansers should cleanse the skin or hair gently, without
defatting and/or drying the hair and skin and without irritating the ocular
mucosae or leaving skin taut after frequent use. Most lathering soaps,
shower and bath products, shampoos and bars fail in this respect.
CA 02300108 2000-02-14
WO 99/09948 PCTNS98/10648
2
Certain synthetic surfactants are known to be mild. However, a major
drawback of some mild synthetic surfactant systems when formulated for
shampooing or personal cleansing is that they have what can be described
as a "slippy" or "non-draggy" rinse feel which is not liked by some
consumers. The use of certain surfactants such as potassium laurate, on
the other hand, can yield a "draggy" rinse feel but at the expense of clinical
skin mildness. These two facts make the selection of suitable surfactants
in the rinse feel and mildness benefit formulation process a delicate
balancing act.
Thus a need exists for personal cleansing compositions which deliver a
"draggy" rinse feel while at the same time having excellent skin mildness,
in addition to excellent product characteristics such as lather, cleansing,
stability, thickening, rheology and in-use skin feel attributes.
Certain polyalphaolefin oils are known for use in personal cleansing
compositions for the skin and hair. References to the use of such oils in
personal cleansing formulations are to be found in WO 97/09031, US-A-
5441730, WO 94/27574, EP-A-0692244, WO 96/32092 and W096/06596.
Hydrophobically modified silicones oils are also known for use in personal
cleansing compositions and are disclosed for example in JP OS-310540.
Surprisingly, it has now been found that personal cleansing compositions
having a "draggy" rinse feel at the same time as having excellent mildness
characteristics are provided by the combination of certain water-insoluble
oils, such as certain polyalphaolefin oils or hydrophobically modified
silicones oils, in combination with a mild, water-soluble surfactant system.
Whilst not wishing to be bound by theory, the "draggy" rinse feel is
considered to be associated with an increase in wet skin friction. An
important mechanism for action of such oils is considered to be their
ability to deposit and change the surface energy of the skin, i.e. making the
skin surface more hydrophobic. During rinsing, the water film is
considered to be the lubricant for the skin, and as surface hydrophobicity
increases so the water film is destabilised and the surface de-wetted. As a
result the water film is at first thinned and then displaced, allowing some
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
3
direct contact between the surfaces. Both changes increase friction and
produce "draggy rinsing".
Summary Of The Invention
According to the present invention there is provided a rinse-off liquid
personal cleansing composition comprising water, from about I% to
about 60% by weight of a water-soluble surfactant, 0.5% or greater of a
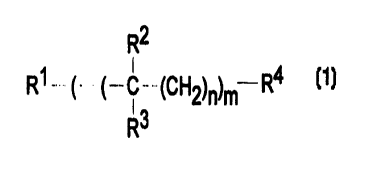
water-insoluble oil having selected from peralk(en)yl materials of type (b)
which have the following formula:
R2
R 1 __ ~ - ( _ C _ ~CHZ)n)m R4
R3
wherein RI is H or C1-C4 alkyl, R4 is C1-C4 alkyl, R2 is H or C1-Cq.
alkyl or C2-C4 alkenyl, and R3 is H or C I-C4 alkyl or C2-C4 alkenyl, n is
an integer from 0 to 3 and m is an integer of from 1 to 1000 and having a
number average molecular weight of from about 600 to about 1000,
preferably from about 750 to about 1000, especially from about 800 to
about 1000.
The compositions of the present invention provide an improvement in
rinse feel while at the same time being exceptionally mild to the skin.
Ali concentrations and ratios herein are by weight of the cleansing
composition, unless otherwise specified. Surfactant chain lengths are also
on a weight average chain length basis, unless otherwise specified.
Detailed Descrit~tion of the Invention
The liquid cleansing compositions herein comprise water, surfactant and a
certain water-insoluble oil which will be described below.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
4
As used herein the term "rinse feel" means the feeling of the skin during
the process of rinsing lather from the skin after cleansing with a cleansing
composition. The type of rinse feel which is provided by the compositions
of the present invention can be described by terms such as a "draggy" rinse
feel, a "soap-like" rinse feel and a "non-slippery" or "non-slimy" rinse feel.
Such a "draggy", "soap-like", "non-slippery" or "non-slimy" rinse feel can
be detected by an increase in friction between the hand and skin during the
process of rinsing lather from the skin.
As used herein the term "water-insoluble" in relation to oils as used herein
is a material which is substantially insoluble in distilled water at room
temperature without the addition of other adjuncts and/or ingredients such
as described herein.
The water-insoluble oils for use herein are those of type (b) which are
peralk(en)yl materials having the following formula:
Rz
R1-~-~-~._~CH2)n)m R4
R3
wherein R1 is H or C1-C4 alkyl, R4 is C1-C4 alkyl, R2 is H or C1-C4
alkyl or C2-C4 alkenyl, and R3 is H or C1-C4 alkyl or C2-C4 alkenyl, n is
an integer from 0 to 3 and m is an integer of from 1 to 1000 and having a
number average molecular weight of from about 600 to about 1000,
preferably from about 750 to about 1000, especially from about 800 to
about 1000. Preferably the hydrocarbon materials of type (b) have a
viscosity in the range of from about SOOcst to about SO,OOOcst, preferably
from about 1000cst to about 10,000cst measured at 40°C using the ASTM
method D-445 for measuring viscosity.
Suitable hydrocarbon materials of type (b) for use herein are polymers of
butene, isoprene, terpene, styrene or isobutene, preferably butene or
isobutene.
Examples of hydrocarbon materials of type (b) include polybutene oils
such as those commercially available from Amoco under the tradename
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
Indopol 40 and Indopol 100 and polyisobutene oils such as those
commercially available from Presperse Inc. under the tradename Permethyl
104A.
Mixtures of the above water-insoluble oil are also suitable for use herein.
In preferred embodiments the number average particle size for the water-
insoluble oil used herein lies in the range of from about 1 micron to about
500 microns, preferably from about 5 to about 200 microns, more
preferably from about 5 to 50 microns, especially from about 5 to about 20
microns.
The compositions herein preferably comprise from about 0.5% to about
20%, more preferably from about 0.5% to about 10%, especially from
about 1% to about 5% by weight of water-insoluble oil.
Surfactant Svstem
As a further essential feature the compositions of the present invention
comprise a surfactant system of water-soluble surfactants. Water-soluble,
as defined herein, means a surfactant having a molecular weight of less
than about 20,000 wherein the surfactant is capable of forming a clear
isotropic solution when dissolved in water at 0.2 % w/w under ambient
conditions. Surfactants suitable for inclusion in compositions according to
the present invention generally have a lipophilic chain length of from
about 6 to about 22 carbon atoms and can be selected from anionic,
nonionic, zwitterionic and amphoteric surfactants and mixtures thereof.
The total level of surfactant is preferably from about 2% to about 40%,
more preferably from about 3% to about 20% by weight, and especially
from about 5% to about 15% by weight. The compositions preferably
comprise a mixture of anionic with zwitterionic and/or amphoteric
surfactants. The weight ratio of anionic surfactant: zwitterionic and/or
amphoteric surfactant is in the range from about 1:10 to about 10:1,
preferably from about 1:5 to about 5:1, more preferably from about 1:3 to
about 3:1. Other suitable compositions within the scope of the invention
comprise mixtures of anionic, zwitterionic and/or amphoteric surfactants
with one or more nonionic surfactants.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
6
The compositions of the invention can comprise a water-soluble anionic
surfactant at levels from about 0.1 % to about 20%, more preferably from
about 0.1 % to about 15%, and especially from about 1 % to about 10% by
weight.
Water soluble anionic surfactants suitable for inclusion in the
compositions of the invention include alkyl sulfates, ethoxylated alkyl
sulfates, alkyl ethoxy carboxylates, alkyl glyceryl ether sulfonates, ethoxy
ether sulfonates, methyl acyl taurates, fatty acyl glycinates, N-acyl
glutamates, acyl isethionates, alkyl sulfosuccinates, alkyl
ethoxysulfosuccinates, alpha-sulfonated fatty acids, their salts and/or their
esters, alkyl phosphate esters, ethoxylated alkyl phosphate esters, acyl
sarcosinates and fatty acid/protein condensates, soaps such as ammonium,
magnesium, potassium, triethanolamine and sodium salts of lauric acid,
myristic acid and palmitic acid, acyl aspartates, alkoxy cocamide
carboxylates, (ethoxylated) alkanolamide sulfosuccinates, ethoxylated
alkyl citrate sulfosuccinates, acyl ethylene diamine triacetates, acyl
hydroxyethyl isethionates, acyl amide alkoxy sulfates, linear alkyl benzene
sulfonates, paraffin sulfonates, alpha olefin sulfonates, alkyl alkoxy
sulfates, and mixtures thereof. Alkyl and/or acyl chain lengths for these
surfactants are C6-C22, preferably C 12-C 1 g more preferably C 12_C 14~
Additional water-soluble anionic surfactants suitable for use in the
compositions according to the present invention are the salts of sulfuric
acid esters of the reaction product of 1 mole of a higher fatty alcohol and
from about 1 to about 12 moles of ethylene oxide, with sodium,
ammonium and magnesium being the preferred counterions. Particularly
preferred are the alkyl ethoxy sulfates containing from about 2 to 6,
preferably 2 to 4 moles of ethylene oxide, such as sodium laureth-2 sulfate,
sodium laureth-3 sulfate, ammonium laureth-3 sulfate and magnesium
sodium laureth-3.6 sulfate. In preferred embodiments, the anionic
surfactant contains at least about 50% especially at least about 75% by
weight of ethoxylated alkyl sulfate.
In addition to the broad range ethoxylated alkyl sulfates obtained via
conventional sodium catalysed ethoxylation techniques and subsequent
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/i0648
7
sulphation processes, ethoxylated alkyl sulfates obtained from narrow
range ethoxylates {NREs) are also suitable water-soluble anionic
surfactants for use in the present compositions. Narrow range ethoxylated
alkyl sulfates suitable for use herein are selected from sulphated alkyl
ethoxylates containing on average from about 1 to about 6, preferably from
about 2 to about 4 and especially about 3 moles of ethylene oxide such as
NRE sodium laureth-3 sulfate. NRE materials suitable for use herein
contain distributions of the desired ethylene oxide (EOn) in the ranges of
from 15% to about 30% by weight of EOn, from about 10% to about 20%
by weight of EOn+1 and from about 10% to about 20% by weight of EOn_
1. Highly preferred NRE materials contain less than about 9% by weight
of ethoxylated alkyl sulfate having 7 or more moles of ethylene oxide and
less than about 13% by weight of non-ethoxylated alkyl sulfate. Suitable
laureth 3 sulfate NRE materials are available from Hoechst under the trade
names GENAPOL ZRO Narrow Range and GENAPOL Narrow Range.
The compositions of the present invention may contain, as a water-soluble
anionic surfactant alkyl ethoxy carboxylate surfactant at a level of from
about 0.5% to about 15%, preferably from about 1 % to about 10%, more
preferably from about 1 % to about 6% and especially from about 1 % to
about 4% by weight. Alkyl ethoxy carboxylate surfactant is particularly
valuable in the compositions according to the present invention for the
delivery of excellent skin mildness attributes in combination with excellent
rinsing performance and desirable lather characteristics.
Alkyl ethoxy carboxylates suitable for use herein have the general formula
(I):
R30(CH2CH20)kCH2C00-M+
wherein R3 is a C 1 p to C 15 alkyl or alkenyl group, preferably a C 11 _C 15
more preferably a C 12-C 14 alkyl or C 12-C 13 alkyl group, k is an average
value of ethoxylation ranging from 2 to about 7, preferably from about 3 to
about 6, more preferably from about 3.5 to about 5.5, especially from
about 4 to about 5, most preferably from about 4 to about 4.5, and M is a
water-solubilizing cation, preferably an alkali metal, alkaline earth metal,
ammonium, lower alkanol ammonium, and mono-, di-, and tri-ethanol
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
8
ammonium, more preferably sodium, potassium and ammonium, most
preferably sodium and ammonium and mixtures thereof with magnesium
and calcium ions.
Particularly preferred as water-soluble anionic surfactants suitable for use
herein are alkyl ethoxy carboxylate surfactants having a selected
distribution of alkyl chain length and/or ethoxylate. Thus, the alkyl ethoxy
carboxylate surfactants suitable for use in the compositions according to
the present invention may comprise a distribution of alkyl ethoxy
caxboxylates having different average values of R3 and/or k.
The average value of k will generally fall in the range of from about 3 to
about 6 when the average R3 is C 11, C 12~ C 13 or C 14. Preferred water-
soiuble anionic alkyl ethoxy carboxylate surfactants suitable for use herein
are the C 12 to C 14 (average EO 3-6) ethoxy carboxylates and the C 12 to
C 13 (average EO 3-6) ethoxy carboxylates. Suitable materials include
salts of NEODOX 23-4 (RTM) available from Shell Inc. (Houston, Texas,
USA) and EMPICOL (RTM) CBCS (Albright & Wilson). Highly
preferred for use herein are alkyl ethoxy carboxylate surfactants wherein,
when R3 is a C 12-C 14 or C 12-C 13 alkyl group and the average value of k
is in the range of from about 3 to about 6, more preferably from about 3.5
to about 5.5, especially from about 4 to about 5 and most preferably from
about 4 to about 4.5.
In preferred embodiments, the compositions are substantially free of soap,
i.e. they contain less than about 5%, preferably less than about 1 %,
preferably 0% by weight of soap.
The compositions according to the present invention may additionally
comprise water-soluble nonionic surfactant at levels from about 0.1 % to
about 20%, more preferably from about 0.1% to about 10%, and especially
from about 1% to about 8% by weight. Surfactants of this class include
sucrose polyester surfactants, C 10-C 1 g alkyl polyglycosides and
polyhydroxy fatty acid amide surfactants having the general formula (III).
O R9
R8-C-N-Z2
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
9
The preferred N-alkyl, N-alkoxy or N-aryloxy, polyhydroxy fatty acid
amide surfactants according to formula (III) are those in which Rg is CS-
C31 hydrocarbyl, preferably C6-C 19 hydrocarbyl, including straight-chain
and branched chain alkyl and alkenyl, or mixtures thereof and Rg is
typically, hydrogen, C 1-Cg alkyl or hydroxyalkyl, preferably methyl, or a
group of formula -R1-O-RZ wherein Rl is C2-Cg hydrocarbyl including
straight-chain, branched-chain and cyclic (including aryl), and is
preferably C2-C4 alkylene, R2 is Cl-Cg straight-chain, branched-chain
and cyclic hydrocarbyl including aryl and oxyhydrocarbyl, and is
preferably C1-C4 alkyl, especially methyl, or phenyl. Z2 is a
polyhydroxyhydrocarbyl moiety having a linear hydrocarbyl chain with at
least 2 (in the case of glyceraldehyde) or at least 3 hydroxyls (in the case
of other reducing sugars) directly connected to the chain, or an alkoxylated
derivative (preferably ethoxylated or propoxylated) thereof. ZZ preferably
will be derived from a reducing sugar in a reductive ammination reaction,
most preferably Z2 is a glycityl moiety. Suitable reducing sugars include
glucose, fructose, maltose, lactose, galactose, mannose, and xylose, as well
as glyceraldehyde. As raw materials, high dextrose corn syrup, high
fructose corn syrup, and high maltose corn syrup can be utilised as well as
the individual sugars listed above. These corn syrups may yield a mix of
sugar components for Z2. It should be understood that it is by no means
intended to exclude other suitable raw materials. Z2 preferably will be
selected from the group consisting of CH2-(CHOH)n-CH20H,
CH(CH20H)-(CHOH)n_1-CH20H, CH2(CHOH)2{CHOR')CHOH)-
CH20H, where n is an integer from 1 to S, inclusive, and R' is H or a
cyclic mono- or poly-saccharide, and alkoxylated derivatives thereof. As
noted, most preferred are glycityls wherein n is 4, particularly CH2-
(CHOH)4-CH20H.
The most preferred polyhydroxy fatty acid amide has the formula
Rg(CO)N(CH3)CH2(CHOH)4CH20H wherein Rg is a C6-C 19 straight
chain alkyl or alkenyl group. In compounds of the above formula, Rg-CO-
N< can be, for example, cocoamide, stearamide, oleamide, lauramide,
myristamide, capricamide, caprylicamide, palmitamide, tallowamide, etc.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
Exemplary non-ionic surfactants suitable for use in the compositions
according to the present invention include primary amines such as
cocamine (available as Adagen IbOD (TM) from Witco) and
alkanolamides such as cocamide MEA (available as Empilan CME (TM)
from Albright and Wilson), PEG-3 cocamide, cocamide DEA (available as
Empilan CDE (TM) from Albright and Wilson), lauramide MEA (available
as Empilan LME (TM) from Albright and Wilson), lauramide MIPA,
lauramide DEA, and mixtures thereof.
Suitable amphoteric surfactants for use herein include (a) ammonium
derivatives of formula [V]:
R~ CON(CH2)2N CHZC02M
R3 R2
wherein R1 is CS-C22 alkyl or alkenyl, Rz is CHZCH20H or
CH2C02M, M is H, alkali metal, alkaline earth metal, ammonium or
alkanolammonium and R3 is CH2CH20H or H;
(b) aminoalkanoates of formula [VI]
R1NH(CH2)nCO2M
iminodialkanoates of formula [VII]
R 1 N[(CH2)mC02M]2
and iminopolyalkanoates of formula (VIII)
R1 ~(CH2)p]q - N [CH2C02M]2
CH2C02M
wherein n, m, p, and q are numbers from 1 to 4, and R1 and M are
independently selected from the groups specified above; and
(c) mixtures thereof.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
11
Suitable amphoteric surfactants of type (a) include compounds of formula
(V) in which Rl is C8H17 (especially isocapryl), C9H19 and C11H23
alkyl. Suitable amphoteric surfactants of type {a) are marketed under the
trade name Miranol and Empigen.
In CTFA nomenclature, materials suitable for use in the present invention
include cocoamphocarboxypropionate, cocoamphocarboxy propionic acid,
cocoamphoacetate, cocoamphodiacetate (otherwise referred to as
cocoamphocarboxyglycinate), sodium lauroamphoacetate (otherwise
referred to as sodium lauroamphocarboxyglycinate). Specific commercial
products include those sold under the trade names of Ampholak 7TX
(sodium carboxy methyl tallow polypropyl amine), Empigen CDL60 and
CDR 60 (Albright & Wilson), Miranol H2M Conc. Miranol C2M Conc.
N.P., Miranol C2M Conc. O.P., Miranol C2M SF, Miranol CM Special,
Miranol Ultra L32 and C32 (Rhone-Poulenc); Alkateric 2CIB (Alkaril
Chemicals); Amphoterge W-2 (Lonza, Inc.); Monateric CDX-38,
Monateric CSH-32 (Mona Industries); Rewoteric AM-2C (Rewo Chemical
Group); and Schercotic MS-2 (Scher Chemicals).
It will be understood that a number of commercially-available amphoteric
surfactants of this type are manufactured and sold in the form of
electroneutral complexes with, for example, hydroxide counterions or with
anionic sulfate or sulfonate surfactants, especially those of the sulfated Cg-
C 1 g alcohol, Cg-C 1 g ethoxylated alcohol or Cg-C 18 acyl glyceride types.
Preferred from the viewpoint of mildness and product stability, however,
are compositions which are essentially free of (non-ethoxylated) sulfated
alcohol surfactants. Note also that the concentrations and weight ratios of
the amphoteric surfactants are based herein on the uncomplexed forms of
the surfactants, any anionic surfactant counterions being considered as part
of the overall anionic surfactant component content.
Examples of suitable amphoteric surfactants of type (b) include N-alkyl
polytrimethylene poly-, carboxymethylamines sold under the trade names
Ampholak X07 and Ampholak 7CX by Berol Nobel and also salts,
especially the triethanolammonium salts and salts of N-lauryl-beta-amino
propionic acid and N-lauryl-imino-dipropionic acid. Such materials are
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
12
sold under the trade name Deriphat by Henkel and Mirataine by Rhone-
Poulenc.
The compositions herein can also contain from about 0.1 % to about 20%,
more preferably from about 0.1 % to about 10%, and especially from about
1 % to about $% by weight of a zwitterionic surfactant.
Water-soluble betaine surfactants suitable for inclusion in the
compositions of the present invention include alkyl betaines of the formula
RSR6R~N+ (CH2)nC02M and amido betaines of the formula (IX)
R6
R5 CON ( CH2 ) mN ( CH2 ) nC02M
R~
wherein RS is CS-C22 alkyl or alkenyl, R6 and R~ are independently Cl-
C3 alkyl, M is H, alkali metal, alkaline earth metal, ammonium or
alkanolammonium, and n, m are each numbers from 1 to 4. Preferred
betaines include cocoamidopropyldimethylcarboxymethyl betaine,
commercially available from TH Goldschmidt under the tradename Tego
betaine, and laurylamidopropyldimethylcarboxymethyl betaine,
commercially available from Albright and Wilson under the tradename
Empigen BR and from TH Goldschmidt under the tradename Tegobetaine
LIOS. .
Water-soluble sultaine surfactants suitable for inclusion in the
compositions of the present invention include alkylarnido sultaines of the
formul a;
R2
R~ CON(CH2)mN+(CH2)nCH(OH)CH2S03 M+
R3
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
13
wherein R1 is C~ to C22 alkyl or alkenyl, R2 and R3 are independently C1
to C3 alkyl, M is H, alkali metal, alkaline earth metal, ammonium or
alkanolammonium and m and n are numbers from 1 to 4. Suitable for use
herein is coco amido propylhydroxy suitaine which is commercially
available under the tradename Mirataine CBS from Rhone-Poulenc.
Water-soluble amine oxide surfactants suitable for inclusion in the
compositions of the present invention include alkyl amine oxide
RSR6R~N0 and amido amine oxides of the formula
R6
R5CON(CH2)mN ~ O
R7
wherein RS is C 11 to C22 alkyl or alkenyl, R6 and R~ are independently
C 1 to C3 alkyl, M is H, alkali metal, alkaline earth metal, ammonium or
alkanolammonium and m is a number from 1 to 4. Preferred amine oxides
include cocoamidopropylamine oxide, lauryl dimethyl amine oxide and
myristyl dimethyl amine oxide.
Polymeric Cationic Conditioning_Agent
The compositions according to the present invention can optionally
include a polymeric cationic conditioning agent. Polymeric cationic
conditioning agents are valuable in the compositions according to the
present invention for provision of desirable skin feel attributes. The
polymeric skin conditioning agent is preferably present at a level from
about 0.01% to about 5%, preferably from about 0.01% to about 3% and
especially from about 0.01 % to about 2% by weight.
Suitable polymers are high molecular weight materials (mass-average
molecular weight determined, for instance, by light scattering, being
generally from about 2,000 to about 5,000,000, preferably from about
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
l4
5,000 to about 3,000,000 more preferably from 100,000 to about
1,000,000).
Representative classes of polymers include cationic guar gums, cationic
polysaccharides; cationic homopolymers and copolymers derived from
acrylic and/or methacrylic acid; cationic cellulose resins, quaternized
hydroxy ethyl cellulose ethers; cationic copolymers of
dimethyldiallylammonium chloride and acrylamide and/or acrylic acid;
cationic homopolymers of dimethyldiallylammonium chloride;
copolymers of dimethyl aminoethylmethacrylate and acrylamide, acrylic
acid/dimethyldiallylammonium chloride/acrylamide copolymers,
quaternised vinyl pyrrolidone acrylate or methacrylate copolymers of
amino alcohol, quaternized copolymers of vinyl pyrrolidone and
dimethylaminoethylmethacrylamide, vinyl pyrollidone/vinyl imidazolium
methochloride copolymers and polyalkylene and ethoxypolyalkylene
imines; quaternized silicones, terpolymers of acrylic acid,
methacrylamidopropyl trimethyl ammonium chloride and methyl acrylate
and mixtures thereof.
By way of exemplification, cationic polymers suitable for use herein
include cationic guar gums such as hydroxypropyl trimethyl ammonium
guar gum (d.s. of from 0.11 to 0.22) available commercially under the
trade names Jaguar C-14-S(RTM) and Jaguar C-17(RTM) and also Jaguar
C-16(RTM), which contains hydroxypropyl substituents (d.s. of from 0.8-
1.1 ) in addition to the above-specified cationic groups, and quaternized
hydroxy ethyl cellulose ethers available commercially under the trade
names Ucare Polymer JR-30M, JR-400, LR400, Catanal (RTM) and
Celquat. Other suitable cationic polymers are homopolymers of
dimethyldiallylammonium chloride available commercially under the trade
name Merquat 100, copolymers of dimethyl aminoethylmethacrylate and
acrylamide, copolymers of dimethyldiallylammonium chloride and
acrylamide, available commercially under the trade names Merquat 550
and Merquat S, acrylic acid/dimethyldiallylammonium
chloride/acrylamide copolymers available under the trade name Merquat
3330, terpolymers of acrylic acid, methacrylamidopropyl trimethyl
ammonium chloride and methyl acrylate commercially available under the
tradename Merquat 2001, quaternized vinyl pyrrolidone acrylate or
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
methacrylate copolymers of amino alcohol available commercially under
the trade name Gafquat, for example Polyquaternium 11, 23 and 28
(quaternized copolymers of vinyl pyrrolidone and dimethyl
aminoethylmethacrylate - Gafquat 755N and quaternized copolymers of
vinyl pyrrolidone and dimethyl aminoethylmethacrylamide - HS-100),
vinyl pyrrolidone/vinyl imidazolium methochloride copolymers available
under the trade names Luviquat FC370, Polyquaternium 2, and
polyalkyleneimines such as polyethylenimine and ethoxyiated
polyethylenimine. Also suitable for use herein are those cationic polymers
commercially available under the tradename Aqualon N-Hance.
The compositions of the invention may also contain from about 0.1 % to
about 20%, preferably from about 1% to about 15%, and more preferably
from about 2% to about 10% by weight of an oil derived nonionic
surfactant or mixture of oil derived nonionic surfactants. Oil derived
nonionic surfactants are valuable in compositions according to the
invention for the provision of skin feel benefits both in use and after use.
Suitable oil derived nonionic surfactants for use herein include water
soluble vegetable and animal-derived emollients such as triglycerides with
a polyethyleneglycol chain inserted; ethoxylated mono and di-glycerides,
polyethoxylated lanolins and ethoxyiated butter derivatives. One preferred
class of oil-derived nonionic surfactants for use herein have the general
formula (XII)
0
RCOCH2CH(OH)CH2(OCH2CH2)nOH
wherein n is from about S to about 200, preferably from about 20 to about
100, more preferably from about 30 to about 85, and wherein R comprises
an aliphatic radical having on average from about 5 to 20 carbon atoms,
preferably from about 7 to 18 carbon atoms.
Suitable ethoxylated oils and fats of this class include polyethyleneglycol
derivatives of glyceryl cocoate, glyceryl caproate, glyceryl caprylate,
glyceryl tallowate, glyceryl palmate, glyceryl stearate, glyceryl laurate,
glyceryl oleate, glyceryl ricinoleate, and glyceryl fatty esters derived from
CA 02300108 2000-02-14
WO 99/09948 PCTNS98/10648 _
16
triglycerides, such as palm oil, almond oil, and corn oil, preferably
glyceryl tallowate and glyceryl cocoate.
Suitable oil derived nonionic surfactants of this class are available from
Croda Inc. (New York, USA) under their Crovol line of materials such as
Crovol EP40 (PEG 20 evening primrose glyceride), Crovol EP 70 {PEG 60
evening primrose glyceride) Crovol A-40 (PEG 20 almond glyceride),
Crovol A-70 (PEG 60 almond glyceride), Crovol M-40 {PEG 20 maize
glyceride), Crovol M-70 (PEG 60 maize glyceride), Crovol PK-40 (PEG
12 palm kernel glyceride), and Crovol PK-70 (PEG 45 palm kernel
glyceride) and under their Solan range of materials such as Solan E, E50
and X polyethoxylated lanolins and Aqualose L-20 (PEG 24 lanolin
alcohol) and Aqualose W 15 (PEG 15 lanolin alcohol) available from
Westbrook Lanolin. Further suitable surfactants of this class are
commercially available from Sherex Chemical Co. (Dublin, Ohio, USA)
under their Varonic LI line of surfactants and from Rewo under their
Rewoderm line of surfactants. These include, for example, Varonic LI 48
(polyethylene glycol {n=80) glyceryl tallowate, alternatively referred to as
PEG 80 glyceryl tallowate), Varonic LI 2 (PEG 28 glyceryl tallowate),
Varonic LI 420 (PEG 200 glyceryl tallowate), and Varonic LI 63 and 67
(PEG 30 and PEG 80 glyceryl cocoates), Rewoderm LIS-20 (PEG-200
palmitate), Rewoderm LIS-80 (PEG-200 palmitate with PEG-7 glyceryi
cocoate) and Rewoderm LIS-75 (PEG-200 palmitate with PEG-7 glyceryl
cocoate) and mixtures thereof. Other oil-derived emollients suitable for
use are PEG derivatives of corn, avocado, and babassu oil, as well as
Softigen 767 (PEG(6) caprylic/capric glycerides).
Also suitable for use herein are nonionic surfactants derived from
composite vegetable fats extracted from the fruit of the Shea Tree
(Butyrospermum Karkii Kotschy) and derivatives thereof. This vegetable
fat, known as Shea Butter is widely used in Central Africa for a variety of
means such as soap making and as a barrier cream, it is marketed by
Sederma (78610 Le Perray En Yvelines, France). Particularly suitable are
ethoxylated derivatives of Shea butter available from Karlshamn Chemical
Co. (Columbos, Ohio, USA) under their Lipex range of chemicals, such as
Lipex 102 E-75 and Lipex 102 E-3 (ethoxylated mono, di-glycerides of
Shea butter) and from Croda Inc. (New York, USA) under their Crovol line
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
17
of materials such as Crovol SB-70 (ethoxylated mono, di-glycerides of
Shea butter). Similarly, ethoxylated derivatives of Mango, Cocoa and
Illipe butter may be used in compositions according to the invention.
Although these are classified as ethoxylated nonionic surfactants it is
understood that a certain proportion may remain as non-ethoxylated
vegetable oil or fat.
Other suitable oil-derived nonionic surfactants include ethoxylated
derivatives of almond oil, peanut oil, rice bran oil, wheat germ oil, linseed
oil, jojoba oil, oil of apricot pits, walnuts, palm nuts, pistachio nuts,
sesame seeds, rapeseed, cade oil, corn oil, peach pit oil, poppyseed oil,
pine oil, castor oil, soybean oil, avocado oil, safflower oil, coconut oil,
hazelnut oil, olive oil, grapeseed oil, and sunflower seed oil.
Oil derived nonionic surfactants highly preferred for use herein from the
viewpoint of optimum mildness and skin feel characteristics are Lipex
102-3 (RTM) (PEG-3 ethoxylated derivatives of Shea Butter) and Softigen
767 (RTM) (PEG-6 caprylic/capric glycerides).
The compositions according to the present invention can also comprise
lipophilic emulsifiers as skin care actives. Suitable lipophilic skin care
actives include anionic food grade emulsifiers which comprise a di-acid
mixed with a monoglyceride such as succinylated monoglycerides,
monostearyl citrate, glyceryl monostearate diacetyl tartrate and mixtures
thereof.
Optional Ingredients
The compositions herein can additionally comprise a wide variety of
optional ingredients. Non-limiting examples of such ingredients are
described below.
In addition to the water-insoluble alk(en)yl oils described above other
water-insoluble oils can be used in the compositions of the present
invetion. Additional water-insoluble oils for use in the personal cleansing
compositions of the present invention include (a) highly branched
polyalphaolefins having the following formula:
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
18
R2
R~ -(-(-C-(CH2)n)m R4
R3
wherein R 1 is H or C 1-C2p alkyl, R4 is C 1-C2p alkyl, R2 is H or C 1-C20,
and R3 is C 1-C2p preferably from CS-C2p, n is an integer from 0 to 3 and
m is an integer of from 1 to 1000 and having a number average molecular
weight of from about 1000 to about 25,000, preferably from about 2000 to
about 6000, more preferably from about 2500 to about 4000. Preferably
the polyalphaolefins of type (a) use herein have a viscosity of from about
300cst to about SO,OOOcst, preferably from about 1000cst to about 12,000
cst, more preferably from about 1000cst to about 4000cst at 40°C using
the
ASTM D-445 method for measuring viscosity. The oils of type (a) may
also have a degree of unsaturation.
Suitable polyalphaolefins of type (a) as described above can be derived
from 1-alkene monomers having from about 4 to about 20 carbon atoms,
preferably from about 6 to about 12 carbon atoms, especially from about 8
to about 12 carbon atoms. The polyalphaolefins useful herein are
preferably hydrogenated polyalphaolefin polymers.
Non-limiting examples of 1-alkene monomers for use in preparing the
polyalphaolefin polymers herein include 1-hexene, 1-octene, 1-decene, 1-
dodecene, 1-tetradecene, branched chain isomers such as 4-methyl-1-
pentene, and combinations thereof. Also suitable for preparing the
polyolefin liquids are 1-hexene to 1-hexadecenes and combinations
thereof, more preferably 1-octene to 1-dodecene or combinations thereof.
Examples of such oils include polydecene oils such as those commercially
available from Mobil Chemical Company, P.O. Box 3140, Edison, New
3ersey 08818, USA, under the tradename Puresyn 40 and Puresyn 100.
Also suitable for use herein are hydrophobically modified silicones having
the following formula: hydrophobically modified silicones having the
following formula:
CA 02300108 2000-02-14
WO 99/09948 PCT/US98l10648
19
R' R R R'
R'-Si-O Si-O Si-O Si-R'
R' (CH2)Z R R'
CH3 x y
wherein R is C1-C4 alkyl or phenyl, R' is C1-C20 alkyl or phenyl, z is 5 to
21, and x has a number average value in the range of from about 20 to 100,
y has a number average value in the range of from about 0 to about 10 and
x + y lies in the rarige of 30 to 100. Preferred materials have values for x
of from 60 to 100, values for y of from 0 to 5, preferably 0, and values for
the sum of x and y of from 60 to 100. The alkylene chain z may be linear
or branched. In addition, the silicone backbone may contain a small
degree of branching to yield a resin (eg. MDQ or MDT resins).
Examples of such oils include those hydrophobically modified silicones
available from GE Silicones under the tradename SF1632 (C 16-C 18 alkyl
methicone), and octyl and decyl methicone.
In preferred embodiments the number average particle size for the
additional water-insoluble oil used herein lies in the range of from about 1
micron to about 500 microns, preferably from about 5 to about 200
microns, more preferably from about 5 to SO microns, especially from
about 5 to about 20 microns.
.Another water-insoluble, skin/hair care ingredient suitable for use in the
foaming compositions herein is a liquid, polyol carboxylic acid ester.
The polyol ester preferred for use herein is a nonocclusive liquid or
liquifiable polyol carboxylic acid ester. These polyol esters are derived
from a polyol radical or moiety and one or more carboxylic acid radicals or
moieties. In other words, these esters contain a moiety derived from a
polyol and one or more moieties derived from a carboxylic acid. These
carboxylic acid esters can also be derived from a carboxylic acid. These
carboxylic acid esters can also be described as liquid polyol fatty acid
esters, because the terms carboxylic acid and fatty acid are often used
interchangeably by those skilled in the art.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
The preferred liquid polyol polyesters employed in this invention comprise
certain polyols, especially sugars or sugar alcohols, esterified with at least
four fatty acid groups. Accordingly, the polyol starting material must have
at least four esterif able hydroxyl groups. Examples of preferred polyols
are sugars, including monosaccharaides and disaccharides, and sugar
alcohols. Examples of monosaccharides containing four hydroxyl groups
are xylose and arabinose and the sugar alcohol derived from xylose, which
has five hydroxyl groups, i.e., xylitol. The monosaccharide, erythrose, is
not suitable in the practice of this invention since it only contains three
hydroxyl groups, but the sugar alcohol derived from erythrose, i.e.,
erythritol, contains four hydroxyl groups and accordingly can be used.
Suitable five hydroxyl group-containing monosaccharides are galactose,
fructose, and sorbose. Sugar alcohols containing six -OH groups derived
from the hydrolysis products of sucrose, as well as glucose and sorbose,
e.g., sorbitol, are also suitable. Examples of disaccharide polyols which
can be used include maltose, lactose, and sucrose, alI of which contain
eight hydroxyl groups.
Preferred polyols for preparing the polyesters for use in the present
invention are selected from the group consisting of erythritol, xylitol,
sorbitol, glucose, and sucrose. Sucrose is especially preferred.
The polyol starting material having at least four hydroxyl groups is
esterified on at least four of the -OH groups with a fatty acid containing
from about 8 to about 22 carbon atoms. Examples of such fatty acids
include caprylic, capric, lauric, myristic, myristoleic, palmitic,
palmitoleic,
stearic, oleic, ricinoleic, linoleic, iinolenic, eleostearic, arachidic,
arachidonic, behenic, and erucic acid. The fatty acids can be derived from
naturally occurring or synthetic fatty acids; they can be saturated or
unsaturated, including positional and geometrical isomers. However, in
order to provide liquid polyesters preferred for use herein, at least about
50% by weight of the fatty acid incorporated into the polyester molecule
should be unsaturated. Oleic and linoieic acids, and mixtures thereof, are
especially preferred.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
21
The polyol fatty acid polyesters useful in this invention should contain at
least four fatty acid ester groups. It is not necessary that all of the
hydroxyl groups of the polyol be esterified with fatty acid, but it is
preferable that the polyester contain no more than two unesterified
hydroxyl groups. Most preferably, substantially all of the hydroxyl groups
of the polyol are esterified with fatty acid, i.e., the polyol moiety is
substantially completely esterified. The fatty acids esterified to the polyol
molecule can be the same or mixed, but as noted above, a substantial
amount of the unsaturated acid ester groups must be present to provide
liquidity.
To illustrate the above points, a sucrose fatty triester would not be suitable
for use herein because it does not contain the required four fatty acid ester
groups. A sucrose tetra-fatty acid ester would be suitable, but is not
preferred because it has more than two unesterified hydroxyl groups. A
sucrose hexa-fatty acid ester would be preferred because it has no more
than two unesterified hydroxyl groups. Highly preferred compounds in
which all the hydroxyl groups are esterified with fatty acids include the
liquid sucrose octa-substituted fatty acid esters.
The following are non-limiting examples of specific polyol fatty acid
polyesters containing at least four fatty acid ester groups suitable for use
in
the present invention: glucose tetraoleate, the glucose tetraesters of
soybean oiI fatty acids (unsaturated), the mannose tetraesters of mixed
soybean oil fatty acids, the galactose tetraesters of oleic acid, the
arabinose
tetraesters of linoleic acid, xylose tetralinoleate, galactose pentaoleate,
sorbitol tetraoleate, the sorbitol hexaesters of unsaturated soybean oil fatty
acids, xylitol pentaoleate, sucrose tetraoleate, sucrose pentaoletate, sucrose
hexaoleate, sucrose hepatoleate, sucrose octaoleate, and mixtures thereof.
As noted above, highly preferred polyol fatty acid esters are those wherein
the fatty acids contain from about 14 to about 18 carbon atoms.
The preferred liquid polyol polyesters preferred for use herein have
complete melting points below about 30oC, preferably below about
27.SoC, more preferably below about 25oC. Complete melting points
reported herein are measured by Differential Scanning Calorimetry (DSC).
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
22
The polyol fatty acid polyesters suitable for use herein can be prepared by
a variety of methods well known to those skilled in the art. These methods
include: transesterification of the polyol with methyl, ethyl or glycerol
fatty acid esters using a variety of catalysts; acylation of the polyol with a
fatty acid chloride; acylation of the polyol with a fatty acid anhydride; and
acylation of the polyol with a fatty acid, per se. See U.S. Patent No.
2,831,854; U.S. Patent No. 4,005,196, to Jandacek, issued January 25,
1977; U.S. Patent No. 4,005,196, to Jandacek, issued January 25, 1977.
The present compositions can also comprise an auxiliary nonionic or
anionic polymeric thickening component, especially a water-soluble
polymeric materials, having a molecular weight greater than about 20,000.
By "water-soluble polymer" is meant that the material will form a
substantially clear solution in water at a 1% concentration at 25°C and
the
material will increase the viscosity of the water. Examples of water-
soluble polymers which may desirably be used as an additional thickening
component in the present compositions, axe hydroxyethylcellulose,
hydroxypropyl cellulose, hydroxypropyl methylcellulose, polyethylene
glycol, polyacrylamide, polyacrylic acid, polyvinyl alcohol (examples
include PVA 217 from Kurary Chemical Co., Japan), polyvinyl
pyrrolidone K-120, dextrans, for example Dextran purified crude Grade
2P, available from D&O Chemicals, carboxymethyl cellulose, plant
exudates such as acacia, ghatti, and tragacanth, seaweed extracts such as
sodium alginate, propylene glycol alginate and sodium carrageenan.
Preferred as the additional thickeners for the present compositions axe
natural polysaccharide materials. Examples of such materials are guar
gum, locust bean gum, and xanthan gum. Also suitable herein preferred is
hydroxyethyl cellulose having a molecular weight of about 700,000.
H,~trope
The compositions according to the present invention may contain as an
optional feature a hydrotrope. Suitable for use herein as hydrotropes are
those well known in the art, including sodium xylene sulphonate,
ammonium xylene sulphonate, sodium cumene sulphonate, short chain
alkyl sulphate and mixtures thereof. Hydrotrope may be present in the
CA 02300108 2000-02-14
WO 99!09948 PCT/US98/10648
23
compositions according to the invention at a level of from about 0.01% to
about S%, preferably from about 0.1% to about 4%, more preferably from
about O.S% to about 3% by weight. Hydrotrope, as defined herein, means,
a material which, when added to a non-dilute, water-soluble surfactant
system can modify its viscosity and rheological profile.
In addition to the water-insoluble oil described above, the compositions of
the invention may also include an insoluble perfume or cosmetic oil or
wax or a mixture thereof at a level up to about 10%, preferably up to about
3% by weight wherein the oil or wax is insoluble in the sense of being
insoluble in the product matrix at a temperature of 2S°C.
Suitable insoluble cosmetic oils and waxes for use herein can be selected
from water-insoluble silicones inclusive of non-volatile polyalkyl and
polyaryl siloxane gums and fluids, volatile cyclic polydimethylsiloxanes,
polyalkoxylated silicones, amino and quaternary ammonium modified
silicones, rigid cross-linked and reinforced silicones and mixtures thereof,
C1-C24 esters of Cg-C3p fatty acids such as isopropyl myristate, myristyl
myristate and cetyl ricinoleate, Cg-C3p esters of benzoic acid, beeswax,
saturated and unsaturated fatty alcohols such as behenyl alcohol,
hydrocarbons such as mineral oils, petrolatum, squalane and squalene,
fatty sorbitan esters (see US-A-3988255, Seiden, issued October 26th
1976), lanolin and oil-like lanolin derivatives, animal and vegetable
triglycerides such as almond oil, peanut oil, wheat germ oil, rice bran oil,
linseed oil, jojoba oil, oil of apricot pits, walnuts, palm nuts, pistachio
nuts,
sesame seeds, rapeseed, cade oil, corn oil, peach pit oil, poppyseed oil,
pine oil, castor oil, soyabean oil, avocado oil, safflower oil, coconut oil,
hazelnut oil, olive oil, grapeseed oil, and sunflower seed oil, and C 1-C24
esters of dimer and trimer acids such as diisopropyl dimerate,
diisostearylmalate, diisostearyldimerate and triisostearyltrimerate.
The viscosity of the final composition (Brookfield DV II, with Cone CP41
or CPS2, 2S°C, neat) is preferably at least about 500 cps, more
preferably
from about 1,000 to about 50,000 cps, especially from about 1,000 to
about 30,000 cps, more especially from about 1,000 to about 15,000 cps.
CA 02300108 2000-02-14
WO 99/09948 PCTNS98/10648
24
The cleansing compositions can optionally include other hair or skin
moisturizers which are soluble in the cleansing composition matrix. The
preferred level of such moisturizers is from about 0.5% to about 20% by
weight. In preferred embodiments, the moisturizer is selected from
essential amino acid compounds found naturally occurring in the stratum
corneum of the skin and water-soluble nonpolyol nonocclusives and
mixtures thereof.
Some examples of more preferred nonocclusive moisturizers are squalarze,
sodium pyrrolidone carboxylic acid, D-panthenol, lactic acid, L-proline,
guanidine, pyrrolidone, hydrolyzed protein and other collagen-derived
proteins, aloe vera gel, acetamide MEA and lactamide MEA and mixtures
thereof.
The compositions herein may also include one or more suspending agents.
Suitable suspending agents for use herein include any of several long chain
acyl derivative materials or mixtures of such materials. Included are
ethylene glycol esters of fatty acids having from about I6 to about 22
carbon atoms. Preferred are the ethylene glycol stearates, both mono and
distearate, but particularly the distearate containing less than about 7% of
the mono stearate. Other suspending agents found useful are alkanol
amides of fatty acids, having from about 16 to 22 carbon atoms, preferably
from about 16 to 18 carbon atoms. Preferred alkanol amides are stearic
monoethanolamide, stearic diethanolamide, stearic monoisopropanolamide
and stearic monoethanolamide stearate.
Still other suitable suspending agents are alkyl (C 16-C22) dimethyl amine
oxides such as stearyl dimethyl amino oxide and trihydroxystearin
commercially available under the tradename Thixcin (RTM) from Rheox.
The suspending agent is preferably present at a level of from about 0.5% to
about 5%, preferably from about 0.5% to about 3%. The suspending
agents serves to assist in suspending the water-insoluble oil and may give
pearlescence to the product. Mixtures of suspending agents are also
suitable for use in the compositions of this invention.
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
Compositions according to the present invention may also include an
opacifier or pearlescing agent. Such materials may be included at a level
of from about 0.01 % to about 5%, preferably from about 0.2% to about
1.3% by weight.
Opacifiers/pearlescers suitable for inclusion in the compositions of the
present invention include: titanium dioxide, Ti02; EUPERLAN 810
(RTM); TEGO-PEARL (RTM); long chain (C 16 - C22) acyl derivatives
such as glycol or polyethylene glycol esters of fatty acid having from
about 16 to about 22 carbon atoms and up to 7 ethyleneoxy units;
alkanolamides of fatty acids, having from about 16 to about 22 carbon
atoms, preferably about 16 to 18 carbon atoms such as stearic
monoethanolamide, stearic diethanolamide, stearic monoisopropanolamide
and stearic monoethanolamide and alkyl (C 16 - C22) dimethyl amine
oxides such as stearyl dimethyl amine oxide.
In preferred compositions the opacifier/pearlescer is present in the form of
crystals. In highly preferred compositions the opacifier/pearlescer is a
particulate polystyrene dispersion having a particle size of from about 0.05
microns to about 0.45 microns, preferably from about 0.17 microns to
about 0.3 microns, such dispersions being preferred from the viewpoint of
providing optimum rheology and shear-thinning behaviour. Highly
preferred is styrene acrylate copolymer and OPACFIER 680 (RTM)
commercially available from Morton International.
A number of additional optional materials can be added to the cleansing
compositions each at a level of from about 0.1 % to about 2% by weight.
Such materials include proteins and polypeptides and derivatives thereof;
water-soluble or solubilizable preservatives such as DMDM Hydantoin,
Germall 115, methyl, ethyl, propyl and butyl esters of hydroxybenzoic
acid, EDTA, Euxyl (RTM) K400, natural preservatives such as benzyl
alcohol, potassium sorbate and bisabalol; sodium benzoate and 2-
phenoxyethanol; other moisturizing agents such as hyaluronic acid, chitin
and starch-grafted sodium polyacrylates such as Sanwet (RTM) IM-1000,
IM-1500 and iM-2500 available from Celanese Superabsorbent Materials,
Portsmith, VA, USA and described in US-A-4,076,663; solvents ; suitable
anti-bacterial agents such as Oxeco (phenoxy isopropanol),
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
26
Trichlorocarbanilide (TCC) and Triclosan and; low temperature phase
modifiers such as ammonium ion sources (e.g. NH4 CI); viscosity control
agents such as magnesium sulfate and other electrolytes; colouring agents;
Ti02 and Ti02-coated mica; perfumes and perfume solubilizers; and
zeolites such as Valfour BV400 and derivatives thereof and Ca2+/Mg~+
sequestrants such as polycarboxylates, amino polycarboxylates,
polyphosphonates, amino polyphosphonates, EDTA etc, water softening
agents such as sodium citrate and insoluble particulates such as zinc
stearate and fumed silica. Water is also present at a level preferably of
from about 20% to about 99.89%, preferably from about 40% to about
90%, more preferably at least about 7S% by weight of the compositions
herein.
The pH of the compositions is preferably from about 3 to about 10, more
preferably from about S to about 9, especially from about S to about 8 and
most preferably from about S to 7.
The compositions of the present invention may be applied with the hand or
preferably with a personal cleansing implement such as a puff. Suitable
personal cleansing implements for use with the compositions of the present
invention include those disclosed in the following patent documents which
are incorporated herein by reference: US-A-5,144,744 to Campagnoli,
issued September 8,1992, US-A-3,343,196 to Barnhouse, W09S/26671 to
The Procter & Gamble Compa.-~y, W09S/00116 to The Procter & Gamble
Company and W095/26670 to The Procter & Gamble Company.
The compositions of the present invention can be used for a variety of skin
and hair care applications such as shower gels, body washes, hair shampoos,
and the like.
The compositions according to the present invention are illustrated bythe
following non-limiting examples.
U% II/% IIl//o IV/% V/%
Ammonium laureth-38.4 8.4 I S 15 15
sulphate (Empicol
EAC.TP)'- .
SUBSTITUTE SHEET (RULE 26)
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
27
Ammonium Lauryl - - - 5 5
Sulphate
(Empicol AL30
Na Lauroamphoacetate3.6 3.6 IO . _,
(Empigen CDL60P>Z
Na Lauroyl Sarcosinate0.5 0.5 _
(Hamposvl L30)3
cocamido MEA - _ 1.0 1.0 1.0
(Empilan
CME)2
Ethylene Glycol 2.0 2.0 2.0 2.0 2,0
Distearate
Dobanol234 0.4 0.4 _ _
Polybutene (Indopol0.5 0 6.0 4.0 0
H 10015
Polvbutene (Indopol0 10 0 0
H40)5
Perfume 0.5 0.5 1.0 1.0 1.0
EDTA 0.11 0.11 0.11 0.11 0.11
Sodium Benzoate 0.25 0.25 0.25 0.25 0.25
DMDM Hydantoin 0.138 0.138 0.138 0.138 0.138
NaCI 0.5 0.5 0.5 0.5 0.5
Citric acid 0.7 0.7 I.5 0.4 0.4
Water _ . _ _
. _ _.___..____________..
. . _
_ _ _
_ . _
_ _ to
100 -
_ _ .
. _ .
_ _ _
_ _ __________
_ _ _
_ .
1. Supplied by Hoechst
?. Supplied by Albright & Wilson
3. Supplied by Hampshire Chemicals
4. Supplied by Shell Chemicals
5. Supplied by Amoco Chemical Co.
Method of Manufacture
Compositions can be prepared by firstly making a premix of surfactants
and a suspending agent. This premix should contain no more than 15%, by
weight of total composition, of surfactant. This is done by combining the
surfactants (except sarcosinate), a portion of the water, powder
preservatives and the pH adjuster with mild agitation. This mixture is then
heated up to about 90°C during which time, fatty alcohol/fatty acid,
the
suspending agent and sodium chloride are added with agitation.
SUBSTITUTE SHEET (RULE 26)
CA 02300108 2000-02-14
WO 99/09948 PCT/US98/10648
2$
The mixture is held at high temperatures for five minutes to one hour
before being cooled at a controlled rate to approximately 30° to
40°C via a
heat exchanger causing the suspending agent to crystallize out.
To this premix the remaining water is then added followed by the water-
insoluble oil, remaining surfactant, liquid preservatives and perfume. This
part of the process is done at room temperature using mild agitation to
yield the preferred droplet diameter of 5 to 20 microns.
The products provide excellent rinse feel and mildness benefits together
with excellent rheological attributes in storage, in dispensing and in-use, in
combination with good efficacy benefits including skin conditioning, skin
moisturising, good product stability, cleansing and lathering.