Note: Descriptions are shown in the official language in which they were submitted.
CA 02300246 2000-03-09
1
TITLE OF THE INVENTION
Local Delivery of 17 - beta Estradiol Decreases
Neointimal Hyperplasia Following Coronary Angioplasty in Porcine Model
FIELD OF THE INVENTION
The present invention relates to the local use of estradiol
for preventing restenosis. More specifically, the present invention is
concerned with the local use of estradiol for decreasing neointimal
hyperplasia that occurs during restenosis.
BACKGROUND OF THE INVENTION
Restenosis is currently the major limitation of percutaneous transluminal
coronary angioplasty (PTCA), and is seen in up to 30-40 % of
patients.'The most important mechanisms contributing to restenosis are
neointimal proliferation, vascular remodelling, and elastic recoil.2 Elastic
recoil and vascular remodelling can be reduced to a large extent by
stenting.3 Although radiation therapy has been reported to show beneficial
effeets,4~5 no effective therapy exists yet for neointimal proliferation.
Vascular smooth muscle cell (SMC) migration and proliferation have been
documented to occur as early as 36 hours following arterial injury.s In cell
culture assays, 17 - beta estradiol inhibited migration and proliferation of
rat vascular SMC.'~8 Similar effects have also been shown with human
vascular SMC from saphenous vein.9 Prolonged systemic administration
of estrogen has been shown to inhibit intimal hyperplasia in animal
CA 02300246 2000-03-09
2
studies.'°'" In the present experiment, we tested the hypothesis that
local
administration of 17 - beta estradiol during PTCA could effectively inhibit
neointimal proliferation.
SUMMARY OF THE INVENTION
An object of the present invention is therefore to provide an efficient
method by which 17-~i estradiol is used locally during PTCA to prevent
restenosis. Compositions for executing this method are also a further
object of this invention.
Other objects, advantages and features of the present
invention will become more apparent upon reading of the following non
restrictive description of preferred embodiments thereof, given by way of
example only with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
Figure 1 Representative light micrographs (x 40 magnification) of arterial
segments from the same animal, stained with Verhoeffs stain. 17 - beta
estradiol (a) treated segment shows markedly less neointimal hyperplasia
compared to PTCA only (b), or vehicle alone (c) groups. The extent of
injury is similar in all 3 segments.;
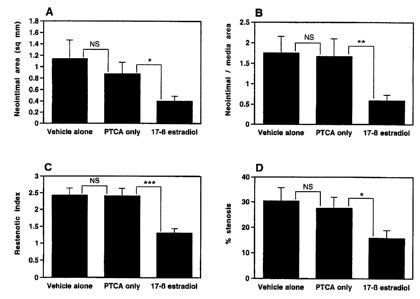
Figure 2 Comparison of (A) neointimal area, (B) neointimallmedia area,
(C) restenotic index, and (D) % stenosis between PTCA alone vs vehicle
CA 02300246 2000-03-09
3
only, and PTCA only vs 17 -beta estradiol groups; * p < 0.05, ** p < 0,01
*** p < 0.002. Values are expressed as mean t SEM.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Methods
Animal preparation
Eighteen juvenile farm pigs (9 female, and 9 castrated male) weighing 20
- 25 kg were studied. The study was approved by, and conducted in
accordance with, the guidelines of the Animal Care and Ethical Research
Committee of the Montreal Heart Institute. Before the procedure, animals
were given 650 mg of acetylsalicylic acid and 30 mg of nifedipine orally,
premedicated with intramuscular injection of 6 mg/kg of a mixture of
tiletamine hydrochloride and zolazepam hydrochloride, and given 0.05 mg
of atropine. The invasive procedure was performed under general
anesthesia with a mixture of isoflurane (1 to 1.5 %) and oxygen enriched
air. The right femoral artery was cannulated percutaneously, and an 8 Fr
arterial sheath was introduced. After arterial access had been obtained,
100 mg of lidocaine and 250 Ulkg of heparin were administered
intra-arterially via the sheath. Activated coagulation time was maintained
at > 300 seconds throughout the procedure.
Angioplasty and Local Delivery
Standard PTCA equipment was used. An 8 Fr right Amplatz guiding
catheter and right Judkins guiding catheter were used for cannulation of
CA 02300246 2000-03-09
4
the left and right coronary arteries, respectively. PTCA was performed with
a balloon size chosen to correspond to a balloonlartery ratio of 1.1-1.3.
Three 30-second inflations at 10 atm pressure were performed with a
30-second interval between each inflation. Inflations were performed
adjacent to major side branches to facilitate identification during
harvesting, taking precaution not to include any side branch in the
intended PTCA site. The left anterior descending, left circumflex, and right
coronary arteries of each animal were subjected to PTCA. After PTCA,
each coronary artery of an animal was randomized to receive either 600
Ng of 17 - beta estradiol locally, or vehicle alone locally, or PTCA only. The
chemicals 17 - beta estradiol and its vehicle 2-hydroxypropyl-beta-
cyclodextrin (HPCD) were purchased from Sigma Chemical Co. The
InfusaSleeve catheter (Local Med, Inc.) was used for local delivery.'2 Five
ml of the designated substance was delivered at a driving pressure of 10
atm and support balloon pressure of 6 atm.
Of the 18 animals, 2 died a few days after PTCA, and were excluded;
thus, 16 animals were analyzed. Twelve animals were euthanised at 28
days, and 4 at 7 days. After premedication and anesthesia, the right
internal jugular vein and common carotid artery were cannulated.
Following cross-clamping of the descending thoracic aorta exposed via a
left lateral thoracotomy, exsanguination was performed, with simultaneous
administration of 1 I of 0.9 % NaCI solution. The heart was perfusion-fixed
in vivo with 2 1 of 10 % buffered formalin at 200 mm Hg pressure,
removed from the animal, and placed in 10 % buffered formalin solution.
Coronary arteries were then dissected free from surrounding tissues. The
site of PTCA was identified in relation to adjacent side branches, which
CA 02300246 2000-03-09
served as landmarks. The injured segment was harvested with a 1 cm
normal segment proximal and distal to the injured site. Serial sections 3
to 5 mm long were made from the harvested segment, with a minimum of
at least 3 sections (maximum 5) from each PTCA site. Sections were
5 stored in buffered 10 % formalin and subjected to dehydration with
increasing concentrations of alcohol, followed by treatment with xylene
and paraffin. Each section was then cut to slices of 6 pm thickness with a
microtome (Olympus cut 4060 E), and stained with VerhoefPs stain for
morphometric analysis.
Morphometric analysis
Measurements were made with a video microscope (Leitz Diaplan,
equipped with a Sony DXC 970 MD color video camera) linked to a 486
personal computer and customized software. A minimum of 3 sections for
each injured segment were analyzed and results averaged. Analyses were
made by a single observer unaware of the treatment group to which each
segment had bee allocated. Randomly selected sections were viewed by
a second observer (also blinded to protocol) independently; inter-observer
variability was < 5 %. The areas of external elastic lamina (EEL), internal
elastic lamina (IEL), and lumen were measured by digital planimetry;
neointimal (I) area (IEL - lumen area) and media (M) area (EEL - IEL area)
were obtained. The % neointima was defined as the % of total vessel area
occupied by neointima (% neointima = [I/EEL] x 100). Morphologic
stenosis was calculated as 100 (1 - IumenIlEL area).'3 The restenotic
index was defined as [II(I + M)]I(FIIEL circumference), where F is the
CA 02300246 2000-03-09
6
fracture length of internal elastic lamina. '4 Histologic injury score was
determined as previously defined.'S
Immunohistochemistry
Following slicing with a microtome and blocking of non-specific antibodies,
the sections were treated with mouse anti - proliferating cell nuclear
antigen (PCNA) antibodies and diluted biotynilated goat anti - mouse
antibodies. They were then incubated with avidin -biotin (Elite ABC Kit,
Vector Laboratories), and developed with 3, 3'- diaminobenzidine (Vector
Laboratories). They were finally counter-stained with hematoxylin. Porcine
liver cells were used as a positive control. For each section, a 6 Nm slice
counter-stained with hematoxylin without treatment with the primary
antibody (mouse anti - PCNA) served as a negative control.
The proliferative response to injury was studied by immunohistochemical
analysis of samples from animals euthanised at 7 days. The
proliferating SMC was obtained by dividing the number of PCNA - positive
SMC by the total number of SMC in each field; separate measurements
were made for neointimal and media layers. The proliferating cells were
identified as SMC by positive staining of parallel sections with a-smooth
muscle actin antibody. To standardize comparison among treatment
groups, measurements were obtained at 4 fixed locations separated by
90° sites for each section, and the results averaged. For each segment,
two sections demonstrating maximal neointimal response were analyzed,
and the results averaged.
CA 02300246 2000-03-09
7
Statistical Analysis
Values are expressed as mean t standard deviation, except as otherwise
indicated. Kruskal - Wallis analysis was used for comparison of data
among the 3 groups; subsequently, 17 - beta estradiol and vehicle alone
groups were separately compared with the PTCA only group using the
Mann - Whitney rank sum test. Chi - square analysis was used for
comparison of proportions. The Mann - Whitney rank sum test was also
used for comparison of data between male and female animals within the
17 - beta estradiol treated group. Values were considered statistically
significant if p < 0.05.
Res a Its
Following PTCA and local delivery, animals were allowed to recover, and
gained weight steadily. Two animals died 48 and 72 hours after procedure
respectively, and were not included; thus 16 animals were studied.
Autopsy of the 2 animals revealed occlusive thrombus at the site of PTCA
(in the 17 - beta estradiol treated vessel in one pig, and in the vessel
treated with PTCA only in the other pig).
Injured segments
Balloonlartery ratio and artery diameter were not significantly different
among the 3 treatment groups (Table 1). Segments with intact IEL in
which discernible injury was absent were excluded from analysis (2 from
PTCA only group, and 1 from vehicle alone group). Two segments were
CA 02300246 2000-03-09
8
lost during harvesting and processing (1 of vehicle alone, and 1 of PTCA
only group).
Morphometric analysis
Of the 12 animals that underwent morphometric analysis at 28 days,
arterial segments treated with local delivery of 17 - beta estradiol showed
significantly less neointimal hyperplasia (Figure 1). This beneficial effect
was noted in all parameters of neointimal response to injury that were
analyzed (Table 1 ). Of note, the extent of morphologic injury was similar
among the 3 groups, suggesting that the use of the InfusaSleeve catheter
was not associated with an enhanced risk of injury.
It was important to exclude an inhibitory effect on intimal proliferation due
to the vehicle, and, to confirm that the effect noted was in response to
treatment with 17 - beta estradiol. Analyses comparing segments treated
with vehicle alone and PTCA only showed a similar response in terms of
the extent of neointimal proliferation. On the other hand, significantly less
intimal hyperplasia was observed in 17 - beta estradiol treated segments
as compared to segments treated with PTCA only (Figure 2). Compared
to PTCA only, or vehicle alone, 17 - beta estradiol decreased neointima
formation by 54.6 % and 64.9 % respectively.
To exclude the possibility of influence of sex on response to estrogen, the
7 segments obtained from male pigs treated with 17 - beta estradiol, and
5 segments obtained from female pigs treated with 17 - beta estradiol
CA 02300246 2000-03-09
9
were analyzed. No statistically significant differences were evident (Table
2).
Immunohistochemistry
The number of PCNA - positive SMC was low overall; sacrifice at an
earlier time might have yielded a higher number. However, a statistically
significant decrease in the proliferative response was seen in animals
treated with 17 - beta estradiol. Among the different groups, the % of
PCNA - positive SMC in the neointima were 0.43 t 0.52 % in 17 - beta
estradiol, 4.26 t 2.33 % in PTCA only, and 4.27 t 2.73 % in vehicle alone
groups respectively (p < 0.05 for 17 - beta estradiol vs other 2 groups).
There were no statistically significant differences in % PCNA - positive
SMC in the media among the 3 groups: 0.4 t 0.3 %, 1.38 t 1.74 %, and
1.24 t 1.57 % for 17 - beta estradiol, PTCA only, and vehicle alone groups
respectively (p = NS).
Vascular remodeling
To determine the effect on vascular remodeling of the agents used, the
EEL area of the injured segment and of the normal vessel proximal to site
of PTCA were obtained, and their ratio calculated. '3 No significant
difference among the groups was noted: 1.01 t 0.16, 1.16 t 0.28, 1.31 t
0.37 respectively for 17 - beta estradiol, PTCA only, and vehicle alone
groups respectively (p = NS).
CA 02300246 2000-03-09
Discussion
The present study demonstrates, for the first time, that locally delivered 17
- beta estradiol decreases neointimal proliferation following PTCA in pigs.
5 The study also shows that the InfusaSleeve catheter can be used to
deliver effectively 17 - beta estradiol intramurally in coronary arteries.
Several previous experiments in animals have demonstrated that estrogen
administered subcutaneously for up to 3 weeks inhibited the myointimal
10 response to arterial injury. ,°." Recently, short-term subcutaneous
estrogen therapy (6 to 17 days) was also shown to be effective in reducing
the injury response in rat carotid artery. '6 Estrogen administered
intramuscularly for at least 3 weeks has also demonstrated the potential
to inhibit vascular smooth muscle cell proliferation and neointimal
hyperplasia in rabbits. " However, the efficacy of local delivery of 17 - beta
estradiol to inhibit intimal hyperplasia has not been previously studied.
The biologic effects of estrogen, like other steroid hormones, involve
intracellular receptors. The first estrogen receptor (ER) to be discovered
was ERa,'e~'9 which was thought to mediate the beneficial effects of
estrogen following vascular injury. ERa was also present in coronary
arteries obtained from autopsy specimens in both pre- and post-
menopausal women,z° and in cell cultures of human saphenous vein and
internal mammary artery specimens.2' Recently, a second estrogen
receptor, ER(3, has been identified in animals and humans.22,2s The role of
ER(3 in response to vascular injury was subsequently demonstrated in
experiments with ERa deficient mice. 24 Normal and ERa deficient mice
treated with estrogen, when subjected to arterial injury, showed the same
CA 02300246 2000-03-09
11
extent of inhibition of neointimal proliferation compared to control mice;
thereby demonstrating that inhibition of vascular injury response by
estrogen is independent of ERa. Although the present experiment was not
designed to study the mechanism of action of 17 - beta estradiol, evidence
exists for multiple potential mechanisms by which 17 - beta estradiol can
inhibit the vascular response to injury. Of importance may be the effect of
17 - beta estradiol on nitric oxide (NO) synthesis. In cell culture studies
with human and bovine endothelial cells, treatment with 17 - beta estradiol
stimulated NO synthase and increased NO production.25,zs
Postmenopausal women treated with transdermal 17 - beta estradiol
showed enhanced in vivo NO synthesis.2' NO has demonstrated inhibitory
effects on both migration 28 and proliferation 29 of vascular SMC, and
decreased neointima formation after PTCA.'3 Preliminary reports have
shown that therapy with 17 - beta estradiol decreases intercellular and
vascular cell adhesion molecule expression by human coronary SMC.3o
Cellular adhesion molecules are expressed by SMC following arterial
injury3' and their suppression with the use of monoclonal antibodies
inhibited intimal hyperplasia after arterial injury in rats.3z The regulatory
effect of 17 - beta estradiol on vascular endothelial growth factor
expression may also be partly responsible.33-s5 perhaps the most important
mechanism may be a direct inhibitory effect of 17 - beta estradiol on
vascular SMC proliferation.36 The binding of 17 - beta estradiol to its
intracellular receptor activates DNA containing "estrogen responsive
elements", leading to altered gene expression. 17 - beta estradiol also
reduces platelet derived growth factor - induced migration and proliferation
of vascular SMC.9
CA 02300246 2000-03-09
12
The beneficial effects of 17 - beta estradiol, the predominant circulating
estrogen in premenopausal women, on vascular injury response may not
be replicated by other kinds of estrogens; for example, conjugated equine
estrogen was found to have no effect on neointimal proliferation in
non-human primate models.3' Simultaneous administration of
progesterone may attenuate the vascular injury response to 17 - beta
estradiol.38 A sexually dimorphic response to estrogen in intact rats has
been reported following arterial injury, with male rats deriving no benefit
with estrogen therapy .39 This sexually dimorphic effect was, however, not
observed in another experiment with gonadeetomized rats." I In the
present study, too, no significant difference in neointimal proliferative
response to 17 - beta estradiol was noted between the sexes. Increased
expression of ER~3 mRNA (ER~i is directly associated with inhibition of
vascular SMC proliferation) following arterial injury has been demonstrated
in intact male rats;4° of additional interest in the study is that no
increase
in ERa was seen following arterial injury.
17 - beta estradiol is a lipophilic compound with poor solubility in aqueous
solutions, thereby needing a vehicle for parenteral administration. HPCD
is a starch derivative that has been successfully tested as an effective
excipient for protein drugs. 4' The pharmacokinetics of HPCD are similar
to that of inulin, and the toxic dose (nephrotoxicity) has been estimated to
be 200 mglkg in rats.42 The dose of HPCD used to dissolve 17 - beta
estradiol in the present study was 0.63 mglkg, far below the toxic dose.
Furthermore, HPCD has been used for administration of ophthalmic
preparations and intravenous anaesthetic agents in humans.4s,44 HPCD
complexed to 17 - beta estradiol has been used to enhance bioavailability
CA 02300246 2000-03-09
13
of orally, or, sublingually administered 17 - beta estradiol with no untoward
effects in humans.45
Retrospective studies in humans have shown no benefit of hormonal
replacement therapy on angiographic restenosis following PTCA;4s
although one study did show a beneficial effect after directional
atherectomy.4' However, it should be noted that conjugated estrogen (and
not 17 - beta estradiol) was the predominant form of estrogen used in
many of these patients, and, no information about concommittent use of
progesterone is available.
In conclusion, we have shown that, a single dose of 17 - beta estradiol
delivered locally during PTCA has the potential to inhibit neointimal
proliferation effectively. The delivery of 17 - beta estradiol can be
performed easily with the InfusaSleeve catheter, without risk of additional
injury. With this approach, it may be possible to avoid potential
undesirable effects of long term systemic administration of estrogen. ER~i
has been identified in humans, and inhibition of proliferation of human
vascular SMC by 17 - beta estradiol has been demonstrated in cell culture
assays. The local administration of 17 - beta estradiol is therefore a
promising new approach, which might be useful in preventing the
proliferative response after PTCA in humans. Its usefulness in preventing
restenosis after PTCA merits further investigation.
CA 02300246 2000-03-09
14
References
1. Dangas G, Fuster V. Management of restenosis after coronary
intervention. Am Heart J 1996 ;132 :428-36.
2. Post MJ, Borst C, Kuntz RE. The relative importance of arterial
remodelling compared with intimal hyperplasia in lumen narrowing after
balloon angioplasty. Circulation 1994; 89: 2816-21.
3. Currier JW, Faxon DP. Restenosis after percutaneous transluminal
coronary angioplasty: Have we been aiming at the wrong target? J Am
Coll Cardiol 1995; 25: 516-20.
4. Teirstein PS, Massullo V, Jani S, Popma JJ, Mintz GS, Russo RJ,
Schatz RA, Guarneri EM, Steuterman S, Morris NB, Leon MB, Tripuraneni
P. Catheter-based radiotherapy to inhibit restenosis after coronary
stenting. N Engl J Med 1997; 336: 1697-703.
5. King SBIII, Williams DO, Chougule P, Klein JL, Waksman R, Hilstead
R, Macdonald J, Anderberg K, Crocker IR. Endovascular beta-radiation to
reduce restenosis after coronary balloon angioplasty: results of the Beta
Energy Restenosis Trial (BERT). Circulation 1998; 97: 2025-30.
6. Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation
after arterial injury: smooth muscle cell growth in the absence of
endothelium. Lab Invest 1983; 49: 327-3 3 .
CA 02300246 2000-03-09
7. Akishita M, Ouchi Y, Miyoshi H, Kozaki K, Inoue S, Ishikawa M, Eto M,
Toba K, Orimo H. Estrogen inhibits cuff- induced intimal thickening of rat
femoral artery: effects on migration and proliferation of vascular smooth
muscle cells. Atherosclerosis 1997; 130: 1-10.
5
8. Kolodgie FD, Jacob A, Wilson PS, Carlson GC, Farb A, Verma A,
Virmani R. Estradiol attenuates directed migration of vascular smooth
muscle cells in vitro. Am J Pathol 1996; 148: 969-76.
10 9. Dai - Do D, Espinosa E, Liu G, Rabelink TJ, Julmy F, Yang Z, Mahler
F, Luscher TF. 17 - beta estradiol inhibits proliferation and migration of
human vascular smooth muscle cells: similar effects in cells from
postmenopausal females and in males. Cardiovascular Research 1996;
32: 980-5.
15 10. Sullivan Jr TR, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ,
O'Donnell Jr TF, Mendelsohn ME. Estrogen inhibits the response - to -
injury in a mouse carotid artery model. J Clin Invest 1995; 96: 2482-8.
11. Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces
myointimal proliferation after balloon injury of rat carotid artery.
Circulation
1996; 93: 577-84.
12. Moura A, Lam JYT, Hebert D, Kermode JR, Grant GW, Robitaille D,
Klein EJ, Yock PG, Simpson JB, Kaplan AV. Intramural delivery of agent
via a novel drug - delivery sleeve: histological and functional evaluation.
Circulation 1995; 92: 2299-2305.
CA 02300246 2000-03-09
16
13. Varenne O, Pislaru S, Gillijns H, Pelt NV, Gerard RD, Zoldhelyi P, Van
de Werf F, Collen D, Janssens S. Local adenovirus - mediated transfer of
human endothelial nitric oxide synthase reduces luminal narrowing after
coronary angioplasty in pigs. Circulation 1998; 98: 916-26.
14. Bonan R, Paiement P, Scortichini D, Cloutier MJ, Leung TK. Coronary
restenosis: evaluation of a restenosis injury index in a swine model. Am
Heart J 1993; 126: 1334-40.
15. Karas SP, Gravanis MB, Santoian EC, Robinson KA, Anderberg KA,
King III SB. Coronary intimal proliferation after balloon injury and stenting
in swine: an animal model of restenosis. J Am Coll Cardiol 1992; 20:
467-74.
16. Mori T, Durand J, Chen YF, Thompson JA, Oparil S. Short term
estrogen treatment prior to and following balloon injury of rat carotid artery
effectively blunts the vascular injury response. J Am Coll Cardiol 1999; 33
(2 suppl A): 259A (abstract).
17. Foegh ML, Asotra S, Howell MH, Ramwell PW. Estradiol inhibition of
arterial neointimal hyperplasia after balloon injury. J Vasc Surg 1994;
19(4): 722-6.
18. Colburn P, Buonassis V. Estrogen - binding sites in endothelial cell
cultures. Science 1978; 201: 817-9.
CA 02300246 2000-03-09
17
19. Venkov CD, Rankin AB, Vaughan DE. Identification of authentic
estrogen receptor in cultured endothelial cells: a potential mechanism for
steroid hormone regulation of endothelial function. Circulation 1996; 94:
727-33.
20. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable
expression of the estrogen receptor in normal and atherosclerotic coronary
arteries of premenopausal women. Circulation 1994; 89: 1501-10.
21. Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth
muscle cells contain functional estrogen receptor. Circulation 1994; 89:
1943-50.
22. Kuiper CiGMJ, Enmark E, Pelto - Huikko M, Nilsson S, Gustafsson JA.
Cloning of a novel estrogen receptor expressed in rat prostrate and ovary.
Proc Natl Acad Sci USA 1996; 93: 5925-5930.
23. Mosselman S, Polman J, Dijkema R. ERI3: identification and
characterization of a novel human estrogen receptor. FEBS Left 1996;
392:49-53.
24. lafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan Jr TR, Lubahn DB,
O'Donnell Jr TF, Korach KS, Mendelsohn ME. Estrogen inhibits the
vascular injury response in estrogen receptor a-deficient mice.
25. Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up -
regulation of nitric oxide synthase by estradiol in human aortic endothelial
cells. FEBS Lett 1995; 360: 291-3.
CA 02300246 2000-03-09
18
26. Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T,
Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a
receptor - mediated system. Biochem Biophys Res Commun 1995; 214(3):
847-55.
27. Rosselli M, Imthurn B, Keller PJ, Jackson EK, Dubey RK. Circulating
nitric oxide (nitritelnitrate) levels in postmenopausal women substituted
with 170-estradiol and norethisterone acetate: a two-year follow-up study.
Hypertension 1995; 25(part 2): 848-53.
28. Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide
reversibly inhibits the migration of cultured vascular smooth muscle cells.
Circ Res 1996; 78: 225-230.
29. Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth
muscle cell growth by nitric oxide and activation of cAMP-dependent
protein kinase by cGMP. Am J Physiol 1994; 267: C1405-13.
30. Speir E, Yu ZX, Ferrans VJ, Cannon III RO. Estrogen inhibits
transcription factor and cell adhesion molecule activation in
cytokine-stimulated human coronary smooth muscle cell via antioxidant
effects. Circulation 1998; suppl I: I-220 (abstract).
31. Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky
MI, Libby P. Sustained activation of vascular cells and leucocytes in the
rabbit aorta after balloon injury. Circulation 1993; 88: 1788-1803.
CA 02300246 2000-03-09
19
32. Yasukawa H, Imaizumi T, Matsuoka H, Nakashima A, Morimatsu M.
Inhibition of intimal hyperplasia after balloon injury by antibodies to
intercellular adhesion molecule-1 and lymphocyte function - associated
antigen-1. Circulation 1997; 95: 1515-22.
33. Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL,
Makela S. Uterine expression of vascular endothelial growth factor is
increased by estradiol and tamoxifen. Cancer Res 1996; 56(17): 3954-60.
34. McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH,
Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by
peritoneal fluid macrophages in endometriosis and is regulated by ovarian
steroids. J Clin Invest 1996; 98: 482-9.
35. Asahara T, Banters C, Pastore C, Kearney M, Rossow S, Bunting S,
Ferrara N, Symes JF, Isner JM. Local delivery of vascular endothelial
growth factor accelerates reendothelialization and attenuates intimal
hyperplasia in balloon-injured rat carotid artery. Circulation 1995; 91:
2793-2801.
36. Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall.
Current Opinion in Cardiology 1994; 9: 619-26.
37. Geary RL, Adams MR, Benjamin ME, Williams JK. Conjugated equine
estrogens inhibit progression of atherosclerosis but have no effect an
intimal hyperplasia or arterial remodelling induced by balloon catheter
injury in monkeys. J Am Coll Cardiol 1998; 31: 1158-64.
I I
CA 02300246 2000-03-09
38. Levine RL, Chen SJ, Durand J, Chen YF, Oparil S.
Medroxyprogesterone attenuates estrogen-mediated inhibition of
neointima formation after balloon injury of the rat carotid artery.
Circulation
1996; 94: 2221-7.
5
39. Oparil S, Levine RL, Chen SJ, Durand J, Chen YF. Sexually dimorphic
response of the balloon-injured rat carotid artery to hormone treatment.
Circulation 1997; 95: 1301-7.
10 40. Lindner V, Kim SK, Karas RH, Kuiper GGJM, Gustafsson JA,
Mendelsohn ME. Increased expression of estrogen receptor-f3 mRNA in
male blood vessels after vascular injury. Circ Res 1998; 83: 224-9.
41. Brewster ME, Hora MS, Simpkins JW, Bodor N. Use of
15 2-hydroxypropyl-betacyclodextrin as a solubilizing and stabilizing
excipient
for protein drugs. Pharm Res 1991; 8(6): 792-5.
42. Frijlink HW, Visser J, Hefting NR, Oosting R, Meijer DKF, Lerk CF. The
pharmacokinetics of beta-cyclodextrin and 2-hydroxypropyl-beta-
20 cyclodextrin in the rat. Pharm Res 1990; 7(12): 1248-52.
43. Kristinsson JK, Fridriksdottir H, Thorisdottir S, Sigurdardottir AM,
Stefansson E, Loftsson T. Dexamethasone-cyclodextrin-polymer
co-complexes in aqueous eye drops: aqueous humor pharmacokinetics
in humans. Invest Ophthalmol Vis Sci 1996; 37: 1199-1203.
CA 02300246 2000-03-09
21
44. Doenicke A, Roizen MF, Nebauer AE, Kugler A, Hoernecke R,
Beger-Hintzen H. A comparison of two formulations for etomidate,
2-hydroxypropyl-beta-cyclodextrin (HPCD) and propylene glycol. Anesth
Analg 1994; 79: 933-9.
45. Hoon TJ, Dawood Y, Khan-Dawood FS, Ramos J, Batenhorst RL.
Bioequivalence of a 17 - beta estradiol hydroxypropyl-beta-cyclodextrin
complex in postmenopausal women. J Clin Pharmacol 1993; 33: 1116-21.
46. O'Keefe JH, Kim SC, Hall RR, Cochran VC, Lawhorn SL, McCallister
BD. Estrogen replacement therapy after coronary angioplasty in women.
J Am Coll Cardiol 1997; 29: 1-5.
47. O'Brien JE, Peterson ED, Keeler GP, Berdan LG, Ohman EM, Faxon
DP, Jacobs AK, Topol EJ, CaliffRM. Relation between estrogen
replacement therapy and restenosis after percutaneous coronary
interventions. J Am Coll Cardiol 1996; 28: 1111-8.
i lil
CA 02300246 2000-03-09
22
Table 1. Morphometric Analysis
Characteristics 17 - beta estradiolPTCA only Vehicle p value*
alone
Segments analyzed12 9 10 NS
Artery size (mm) 2.86 t 0.35 2.94 t 0.242.94 t NS
0.41
BalloonlArtery 1.22 t 0.09 1.2 t 0.06 1.17 t NS
ratio 0.11
EEL~e,/EEL;~~ 1.01 t 0.16 1.31 t 0.371.16 t NS
t 0.28
Neointimal area 0.4 t 0.3 0.88 t 0.611.14 t < 0.05
(mmz) 1.03
neointima 12.16 t 8.89 23.02 t 25.46 t < 0.025
11.91 14.96
NeointimalMedia 0.59 t 0.48 1.67 t 1.291.75 t < 0.01
area 1.29
stenosis 15.67 t 11.13 27.51 t 30.34 t < 0.025
13.17 17.05
Restenotic index 1.3 t 0.5 2.4 t 0.68 2.42 t < 0.005
0.71
Injury score 1.64 t 0.34 1.7 t 0.43 1.77 t NS
0.47
* 17 - beta estradiol vs other 2 groups; tEEL~ef = proximal reference
segment external elastic lamina area, EEL;~~ = injured segment external
elastic lamina area (averaged).
Table 2. Response to 17 - beta estradiol According to Sex of the Animal
Characteristics Male Female p value
Restenotic index 1.2 t 0.59 1.37 t 0.45 > 0.1
Neointimal area 0.51 t 0.34 0.25 t 0.15 > 0.1
(mm2)
NeointimalMedia 0.78 t 0.55 0.32 t 0.16 > 0.1
area
neointima 14.93 t 10.688.29 t 3.72 > 0.1
stenosis 18.93 t 13.3911.09 t 5.16> 0. I
CA 02300246 2000-03-09
23
Although the present invention has been described
hereinabove by way of preferred embodiments thereof, it can be modified,
without departing from the spirit and nature of the subject invention as
defined in the appended claims.