Note: Descriptions are shown in the official language in which they were submitted.
CA 02300596 2000-03-17
1
IMPROVED SAMPLE CARD
FIELD OF THE INVENTION
The invention relates to an improved sample for analyzing biological or other
samples.
BACKGROUND OF THE INVENTION
Biocards have been used to analyze blood or other biological samples in a
spectroscopic or other automated reading machine. Such machines receive a
small biocard,
roughly the size of a playing card, in which biological reagents, nutrients or
other materials
is deposited and sealed, prior to injection of patient samples.
The biocard contains the reagents and receives the patient samples in a series
of small
wells, formed in the card in rows and columns and sealed, typically with tape
on both sides.
The biocards are filled with patient sample material through fine hydraulic
channels formed
in the card. The microorganisms in the samples may then be permitted to grow
or reactions
to proceed, generally over a period of up to a few hours, although the period
varies with the
type of bacteria or other substance analyzed and sample used.
After the incubation, the samples contained in the walls are placed in front
of a laser,
fluorescent light or other illumination source. The content of the sample in a
given well can
then be deduced according to readings on the spectrum, intensity or other
characteristics of
the transmitted or reflected radiation, since the culture of different
bacteria or other agents
leave distinctive signatures related to turbidity, density, byproducts,
coloration, fluorescence
and so forth. Biocards and machines for reading them of this general type for
use in these
biomedical applications can for example be seen in U.S. Patent Nos. 4,318,994;
4,118,280;
4,116,775; 4,038,151; 4,018,652; and 3,957,583.
Despite the general success of biocards in this area, there is an ongoing
desire to
improve the performance of the cards and reading on their samples. It is for
example an
advantage to impress more reaction wells in a given card, so that a greater
variety of reactions
and therefore discrimination of samples can be realized. A given facility may
have only one
such machine, or be pressed for continuous analysis of samples of many
patients, as at a large
CA 02300596 2000-03-17
2
hospital. Conducting as many identifying reactions on each sample as possible
is frequently
desirable, yielding greater overall throughput.
However, biocards that have been exploited commercially have often been
limited
to a total of 30 sample wells (or 45 wells in some designs). For compatibility
with existing
reading machines, the cards generally cannot be enlarged from a certain
standard profile
(roughly 3'/i" by 2'/4"). Total well capacity has accordingly not grown beyond
these levels,
limiting the throughput on the machines.
It has also been the case that as the total number of reaction wells on a
given card has
increaseil, while the card size has remained constant, the wells have
necessarily been formed
increasingly close together. With the sample wells crowding each other on the
card, it has
become more likely that the sample contained in one well can travel to the
next well, to
contaminate the second well. The threat of increased contamination comes into
play
especially as card well capacity increases above 30 wells.
SUMMARY OF THE INVENTION
It is accordingly an object of the invention to provide a biocard having an
increased
number of sample wells.
It is another object of the invention to provide a biocard with increased
capacity, yet
retaining overall standard card sizes.
It is another object of the invention to provide a biocard which can be loaded
with
samples quickly, easily and with a minimum of sample corruption.
It is another object of the invention to provide a biocard with improved
disposal of
injection bubbles arising during loading of the samples.
It is another object of the invention to provide a biocard which increase the
effective
fluid flow distance between adjacent wells, reducing well-to-well
contamination.
It is another object of the invention to provide a biocard with better,
smoother, more
reliable fluid flow throughout the card.
The invention achieving these and other objects is an improved biocard having
a
signiFcantly improved sample well capacity, easily achieving 45 wells, and
reaching 64 wells
and feasibly more. The biocard of the invention likewise provides carefully
structured fluid
CA 02300596 2000-03-17
76909-138D
3
channels which improve fluid flow, reduce bubbling yet improve
disposal of any bubbles which do form through specially
designed bubble traps.
The biocard of the invention provides, as well,
improved security against well-to-well contamination, in part
by increasing the effective distance that the samples in
adjacent sample wells must travel to corrupt neighboring sites.
In accordance with the present invention, there is
provided a test sample card having plurality of sample wells
in fluid communication with a fluid sample intake port, said
test sample card for containing a fluid sample subject to
analysis by an optical system, the improvement comprising: at
least one aperture comprising an optical sensor stop hole in
said card positioned in registry with said sample wells,
whereby optical detection of said sensor stop hole by said
optical system permits accurate alignment of said wells with
said optical system as said test sample card moves relative
to said optical system for reading of said wells.
Brief Description of the Drawings
The invention will be described with reference to
the drawings, in which like parts are labeled with like
numbers. The drawings are briefly described below.
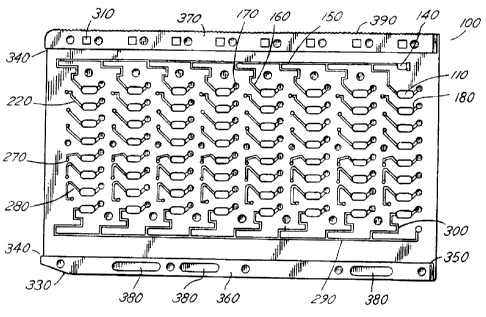
Figure 1 illustrates an improved biocard according
to the invention, in a front planar view.
Figure 2 illustrates the improved biocard according
to the invention, in a back planar view.
Figure 3 illustrates the improved biocard according
to the invention, in a top edge view.
Figure 4 illustrates the improved biocard according
to the invention, in a bottom edge view.
76909-138 CA 02300596 2000-03-17
3a
Figure 5 illustrates the improved biocard according
to the invention, in a side edge view.
Figure 6 illustrates the improved biocard according
to the invention, in an opposite side edge view.
Figure 7 illustrates a sample well with associated
fill channel and bubble trap, according to the improved
biocard to the invention.
Detailed Description of the Drawings
A preferred embodiment of the invention is
illustrated in Figures 1-7. This embodiment provides an
improved biocard 100, having a generally rectangular shape and
in standard dimensions. Biocard 100 in the illustrated
embodiment contains a total of 64 separate sample wells 110,
each of which receives a sample, for example a biological
sample extracted from blood, other fluids, tissue or other
material of a patient, for spectroscopic or
CA 02300596 2000-03-17
4
other automated analysis. The biological sample may be a direct sample from
the patient,
or be patient sample which is extracted, diluted, suspended, or otherwise
treated, in solution
or otherwise. Other types of samples, including antibiotic dosages or other
material, can also
be introduced for analysis. It will be understood that well capacities other
than 64 can be
used. Biocard 100 is generally used in a landscape orientation.
In terms of materials, biocard 100 may be made of polystyrene, PET, or any
other
suitable plastic or other material. Biocard 100 may be tempered during
manufacture with a
softening material, so that crystalline rigidity, and resultant tendency to
crack or chip, is
reduced. Biocard 100 for instance may be manufactured out of a blend of
polystyrene,
approximately 90% or more, along with an additive of butyl rubber to render
the card slightly
more flexible and resistant to damage. Biocard 100 may also be doped with
coloring agents,
for instance titanium oxide to produce a white color, when desired.
The biocard 100 of the invention may be of use in identifying and/or
enumerating any
number of microorganisms, such as bacterial and/or other biological agents.
Many bacteria
lend themselves to automated spectroscopic, fluorescent and similar analysis
after incubation,
as is known in the art. The transmission and absorption of light is affected
by the turbidity,
density and color metric properties of the sample. Fluorescent reactions may
performed as
well, independently or along with spectroscopic or other measurements. If
fluorescent data
are gathered, use of coloring agent in biocard 100 is preferable, since an
opaque card reduces
or eliminates the scattering of fluorescent emissions throughout the card, as
can occur with
a translucent material. Other types of detection and analysis can be done on
biocard 100,
including testing of susceptibility of microorganisms to antibiotics of
different types, and at
different concentrations, so that biocard 100 is a general-purpose instrument.
To receive sample fluid, the biocard 100 includes a sample intake plenum or
port 120
at an upper right corner of the card 100, located on a perimeter edge of the
card. The sample
wells of card 100 contain dry biological reagents which are previously put in
place in the
wells, by evaporative, freeze-drying or other means, prior to being dissolved
in solution with
the injected patient sample for analysis. Each well can hold a deposit of a
different reagent,
for identifying different biological agents, if desired.
Intake port 120 receives a fluid injection tip and related assembly
(schematically
CA 02300596 2000-03-17
S
illustrated as 130), through which the sample fluid or other solution which
arrives to dissolve
the biological reagent is injected, under a vacuum pulled on biocard 100
(typically .7-.9
PSIA), then released to atmospheric pressure. Injection port 120 includes a
small intake
reservoir 140 formed as a roughly rectangular hole through the card 100, which
receives
incoming fluid, and acts as a fluid buffer.
The fluid (patient sample or other solution) enters intake port 120, collects
in intake
reservoir 140 and travels along first distribution channel 150, located on the
front or facing
side of card 100. First distribution channel 150 consists of a relatively long
channel formed
in the surface of card 100, which extends substantially across the width of
the card, and may
have a cross section of approximately .1-.2mm2. First distribution channel 150
is tapped at
intervals along its length by a series of parallel distribution legs or fill
channels 160, which
generally descend from channel 1 ~0 toward the sample wells 110 in each of the
eight
illustrated columns. When the sample is injected into the card, a short
segment of the sample
tip can be pinched off or heat sealed and left in place in intake port 120,
acting as a sealing
plug.
Fill channels 160 are relatively short channels (which may be kinked) which
extend
down from first distribution channel 140 into respective sample wells 110
located in the first
row of card 100, and having a cross section of approximately .1-.2mm'-.
It will be appreciated that each of fill channels 160 descend to and enter
sample wells
W o at an angle, which results in the natural flow of the sample fluid down
through the fill
channels 160 by gravity, and resistance to small pieces of undissolved
material flowing back
up into the fluid circuitry. When the sample fluid actually enters the well
110, the fluid fills
the well by action of both gravity and a vortex-type of flow effect into that
well. Also, any
of the fill channels 160, as schematically illustrated in Figure 7, as well as
other connecting
fluid channels in the invention may be preferably formed in fill-radius style,
that is, as a
semicircular conduit, rather than a squared-off channel as in some older
designs. The full-
radius feature has been found by the inventors to reduce friction and fluid
turbulence, further
enhancing the performance of biocard 100.
Each of sample wells 110 in the first and other rows includes an associated
bubble
trap 170, connected to sample well 110 at an upper comer of the well, and
located at a height
CA 02300596 2000-03-17
6
slightly above the well on the card surface. As illustrated in Figure 7, each
bubble trap 170
is connected to its respective well by a short trap connecting conduit 180,
formed as a hollow
passage part-way into the card surface and forming a short conducting path for
trapped
gaseous bubbles which have been formed in, or communicated to, the well 110
during the
injection operation, by bacterial or other biological reaction, or otherwise.
Bubble trap 170
does not cut through the card completely, instead consisting of a depression
or well of
roughly cylindrical shape, with a rounded bottom contour, and a volume of
approximately
4.2 cubic mm in the illustrated embodiment.
Because the bubble trap 170 is located at an elevated position above each
respective
well 110, any gaseous bubbles will tend to rise and be trapped in the
depression of trap 170.
With gaseous remnants led off to the bubble trap 170, analytical readings on
the biological
sample can be made more reliably, since scattering and other corruption of the
microbial
radiation reading by gas is reduced or eliminated.
As will also be understood from the following, the two-sided nature of biocard
100
permits fluid channels to be formed opposite to non-penetrating bubble traps
170, on the
other side of the card. Some older card designs have employed bubble traps
which penetrate
through the card, eliminating the possibility of surface channels being routed
in their vicinity.
In addition to the introduction of fluid through the path of first
distribution channel
150, fluid also travels to wells below the first row of wells tluough other
directions. More
specifically, intake port 120 also connects to a second distribution channel
190 formed on the
opposite or back surface of the biocard 100, second distribution channel 190
also leading
away from the intake reservoir 140. Second distribution channel 190 also
extends
substantially along the width of card 100, but on the rear surface of the
card. Second
distribution channel 190 has a cross-sectional area of approximately .2-.3mm'-
.
Second distribution channel 190 is tapped above each of the eight illustrated
columns
of sample wells by a triplet of additional distribution legs or channels 200.
Each of triplet
legs 200 contains three relatively short connecting channels leading down from
second
distribution channel to a set of three respective through-channels 210 formed
through the
body of card 100.
Through-channels 210 are small apertures, approximately 1 mm in diameter,
formed
CA 02300596 2000-03-17
7
cleanly through the body of biocard 100, forming conduits or vias from one
surface of the
card to the other. The channels of triplet legs 200 connect to the respective
through-channels
210, which in turn are connected to additional well fill channels 220, forming
a short link to
three additional respective samples wells 110.
However, the fill channels 220 deliver the fluid to the sample wells from the
opposite,
that is rear, side of the card 100, creating a different fluid flow circuit
which extends from
intake port 120. That is, this path involves the second distribution channel
on the rear surface
of the card, through the body of the card by way of through-channels 210, then
out to
connecting fill channels 220 which deliver the sample to the well 110 (again
at an inclined
angle, providing gravity resistance to debris uptake).
The sample wells which receive the fluid from the second distribution through-
channel circuit, like the sample wells which receive the fluid through the
(front-planar) first
distribution channel, also have bubble traps 170 associated with them, in the
same general
above-well configuration.
The biocard 100 therefore includes four rows by eight columns of sample wells
built
up by connecting channels through the first and second distribution channels.
This provides
a set of 32 sample wells. In addition, another contiguous set of samples
wells, making up the
remaining 32 wells for the total of 64, is also deployed along the bottom of
the card body
using through-channels.
More specifically, a third distribution channel 230 is in fluid connection
with intake
port 120, but traces a generally vertical path downward from the port to a
third distribution
through-channel 240, located at a lower right section of the card 100. Third
distribution
channel 230 and its corresponding third distribution through-channel 240 have
slightly larger
cross-sections than the first two distribution channels and their through-
channels 210, to
accommodate larger fluid flow to a greater total number of destination wells
(32, versus 8
and 24 wells, respectively).
The fluid flows down through the third distribution channel 230, into third
distribution through-channel 240, and then splits into two subchannels. The
first subchannel
250 on third distribution channel 230, located on the rear of card 100, is a
widthwise channel
extending along the lower base of the card, having a cross-section of
approximately .2-
CA 02300596 2000-03-17
8
.3mmz. Rising up from first subchannel 250 are another set of triplet legs
260, which
generally resemble first triplet 200 but which extend upward from first
subchannel 230,
rather than downward.
However, triplegs 260 perform the same basic function, delivering the fluid to
another
set of through-channels 270, identical to through-channels 210. Through-
channels 270 in
turn lead through the card body, that is, to the front of the card, to
connecting fill channels
280, which are generally short concave links (which may be kinked) to
respective additional
sample wells 110. Fill channels 280 likewise enter the sample wells 110 at an
inclined angle,
from above.
The last fluid flow path is second subchannel 290, leading off of third
distribution
through-channel 240 along the front of card 100, in a generally horizontal or
widthwise
manner. Second subchannel 290 is connected to the last (eighth), bottom row of
eighth
sample wells 110 by another set of vertical connecting conduits 300, single
conduits
connecting to single wells. Conduits 300 are generally dog-legged in
structure, enter the well
at a slightly inclined angle, and the associated wells each also include an
associated bubble
trap 170.
It may thus be seen that through the use of through-channels penetrating the
card
body 110, along with carefully distributed links through a plurality of
distribution channels,
in the invention valuable surface area is freed up on the card, by allowing
the necessary
connecting channels to be split up between the front and rear surfaces of the
card.
The fluid flow paths thoroughly dispersed over card 100, including both front
and rear
surfaces, also result in a longer total linear travel of the flowing fluid
than conventional cards.
This leads to the significant advantage that the possibility of inter-well
contamination is
reduced. The well-to-well distance in fact in the illustrated embodiment comes
to
approximately 35 mm, significantly more than the l2mm or so on many older card
designs.
The inventors have also observed that the rate of inter-well contamination
varies with
the square of the linear distance, so the elongated fluid paths significantly
enhance the
integrity of readings on the card. Contamination itself is a function of
sample mixing
(density of solution falling out of wells) and liquid molecular diffusion,
both of which are
discouraged by the relatively find channel cross-sections in many sections of
the overall fluid
CA 02300596 2000-03-17
9
circuit, as well as overall path length.
The contamination rate is also reduced by the fact that the volume of the
channels
along the fluid circuit varies slightly along the overall circuit traveled by
a given sample.
That is, the through-channels, the three main distribution channels and other
segments of the
paths have cross-sectional areas which, although all relatively fine, may
differ slightly. The
change in volume over the path tends to retard the progression of
contamination, as do dog-
legged or kinked-sections of connecting conduits.
All these structural adaptations cooperate in reducing the rate of inter-well
contamination in the biocard 100. The inventors have, as one indication of
contamination
management, measured the time required for test dye to infiltrate a
neighboring well in
conventional biocards and the card of the invention. Contamination in a
conventional, low-
capacity, non-through-body card has been observed in approximately 2-4 hours.
In the
biocard of the invention under similar conditions, in contrast, the
contamination time has
been observed at 16-18 hours.
Besides contamination kinematics, the upper-placed bubble traps 170 also more
efficiently scrub the samples wells 110 of gas bubbles which form after the
sample injection.
Samples are typically injected as noted by evacuating the card, introducing
the fluid at the
intake and then releasing the vacuum pull, so that the whole fluid circuitry
returns to
atmospheric pressure. Vacuum filling of the card may typically be done over a
period of 3-
60 seconds, slower rates helping to reduce the tendency of bubbles to form.
Those bubbles
can ruin sample readings, so reducing them results in a smoother, more
efficient, higher-
capacity yet more reliable biocard.
In addition, the improved fluid circuitry of biocard 100, including full-
radius fill and
other channels, generally narrower channels than older card designs, width-
variation and
other features result in a high capture percentage of sample intake actually
reaching the
sample wells 110, which the inventors have calculated at as high as 90-95%.
This compares
with a capture percent in the 80s for older card designs.
For mechanical interaction with the automated reading machine, biocard 100 may
also be provided with a series of sensor stop holes 310, located along the
bottommost edge
of the card. Sensor stop holes 310, illustrated as regularly spaced,
rectangular through-holes,
CA 02300596 2000-03-17
permit associated photo detectors to detect when a biocard 100 mounted in a
reading machine
has come into proper alignment for optical reading. The sensor stop holes 310
are arranged
in vertical register with the vertical columns of wells 110, so that the
optical detection of the
stop hole 310 corresponds exactly to positioning of the sample wells 110
before optical
reading devices. Older biocards have been aligned by sensor holes which are
formed not
integrally with he card itself, but in carriages or other supports which are
attached to the card
at some point in the reading process, as for instance disclosed in U.S. Patent
No. 4,118,280.
These structures have however been prone to time-consuming maintenance,
particularly
requiring the mechanical calibration and lining up of the carriage with the
cards, and
photodetectors. Integral sensor stop holes 310 eliminate that type of
difficulty.
The biocard 100 of the invention is formed in the illustrated embodiment, as
shown
in Figure 7, with a mold parting line 320 which is formed most of the way down
into a
sample well 110, toward the bottom of the card as opposing mold dies meet
during
manufacture. Older card designs often had the mold parting line, which forms a
tiny lip in
a fluid cavity, at an upper point (above midway) of the card. The upper mold
parting lines
could tend to induce annular bubble rings to form during filling, as well as
reduce the
efficiency of drying of antibiotics or other material during manufacturing.
The use of a
downward offset mold parting line 320 avoids these difficulties, as well as
improving the
efficiency of chemical or antibiotic dehydration during incubation, and may
act as a slight
aperture during light and fluorescence reading operations. As illustrated in
Figure 7, the
walls of the sample well, and other features, are usually formed at a slight
angle or incline
(typically 1-4°), as an artifact or conventional molding processes in
which separating the
molded part from opposing molding pieces is made easier with slight surface
inclinations.
The shifting of the mold parting line 320 to the bottom area of biocard 100
likewise results
in a smaller inclined (roughly speaking, trapezoidal) area in the bottom of
the sample well
which can tend to trap material, slightly.
Another advantage of biocard 100 of the invention is that patient sample and
other
markings are not introduced directly on the card itself, in pre-formed
segments, as for
example shown in aforementioned U.S. Patent No. 4,116,775 and others. Those on-
card
stipplings and markings can contribute to debris, mishandling and other
problems. In the
CA 02300596 2000-03-17
11
invention, instead, the card may be provided with bar-coding or other data
markings by
adhesive media, but markings or pre-formed information segments are not
necessary (though
some could be imprinted if desired) and debris, mishandling, loss of surface
area and other
problems can be avoided.
Biocard 100 furthermore includes, at the lower left corner of the card as
illustrated
in Figure 1, a tapered bezel edge 330. Tapered bezel edge 330 provides an
inclinod surface
for easier insertion of biocard 100 into, carrousels or cassettes, into slots
or bins for card
reading, and other loading points in the processing of the card. Tapered bezel
edge 330
provides a gently inclined surface, which relieves the need for tight
tolerances during loading
operations.
Biocard 100 also includes a lower rail 360 and an upper rail 370, which are
slight
structural "bulges" at along the top and bottom areas of the card to reinforce
the strength and
eWance handling and loading of the biocard 100. The extra width of lower and
upper rails
360 and 370 also exceeds the thickness of sealing material, such as adhesive
tape, that is
affixed to the front and back surfaces of biocard 100 for sealing during
manufacture and
impregnation with reagents. The raised rails therefore protect the tape,
especially edges from
peeling, during the making of the biocard 100, as well as during handling of
the card,
including during reading operations.
Upper rail 370 may have serrations 390 formed along its top edge, to provide
greater
friction when biocard 100 is transported in card reading machines or otherwise
using belt
drive mechanisms. Lower card rail 360 may also have formed in it reduction
cavities 380,
which are small elongated depressions which reduce the material, weight and
expense of the
card by carving out space where extra material is not necessary in the
reinforcing rail 360.
In terms of sealing of biocard 100 to contain reagents and other material, it
has been
noted that sealing tapes are typically used to seal flush against biocard 100
from either side,
with rail protection. Biocard 100 also includes a leading lip 340 on lower
card rail 360, and
on upper card rail 370 which projects slightly over the leading edge of the
card. Conversely,
at the opposite end of the biocard 100 there is a trailing tnmcation 350 in
both rails. This
structure permits sealing tape to be applied in the card preparation process
in a continuous
manner, with card after card having tape applied, then the tape cut between
successive cards
CA 02300596 2000-03-17
12
without the tape from successive cards getting stuck together. The leading lip
340 and
trailing truncation 350 provides a clearance to separate cards and their
applied tape, which
may be cut at the trailing truncation 350 and wrapped back around the card
edge, for
increased security against interference between abutting cards.
***
The foregoing description of the improved biocard of the invention is
illustrative, and
variations on certain aspects of the inventive system will occur to persons
skilled in the art.
The scope of the invention is accordingly intended to be limited only by the
following
claims.