Note: Descriptions are shown in the official language in which they were submitted.
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
1
DESCRIPTION
ANTI-INFLAMMATORY AGENT
TECHNICAL FIELD
5 The present invention relates to an anti-inflammatory
agent which is useful as an agent for prophylaxis and
treatment of a TNF(Tumor Necrosis Factor)-a mediated
inflammatory disease.
10 BACKGROUND ART
Regarding a relationship between TNF-a and a
thiazolidine derivative, the following references 1) to 4)
are known.
1) JP-A H7(1995)-285864 describes that a thiazolidine
15 derivative inhibits production and response reaction of
TNF.
2) Saishin-Igaku,Vo1.52,No.6,pp.95-102 (1997) describes
that a thiazolidine derivative reduces expression of TNF-
a and improves insulin-resistance caused by TNF-a.
20 3) Endocrinology, Vol. 134, No. 1, pp.264-270 (1994)
describes that the overexpression of mRNA for TNF-a and
both of its receptors are at least partly normalized by
treatment of the diabetic animals with the insulin-
sensitizing agent pioglitazone.
25 4) Endocrinology, Vol. 136, No. 4, pp.1474-1481 (1995)
describes that insulin-sensitizing agents exert their
antidiabetic activities by antagonizing the inhibitory
effects of TNF- a .
While, regarding a relationship between an
30 inflammatory disease and a thiazolidine deivative, the
following references 5) and 6) are known.
5) WO 96/34943 describes a method for treating a cytokine
mediated autoimmune, inflammatory or atherosclerotic
disorder with a human 12-lipoxygenase inhibitor. The
35 human 12-lipoxygenase inhibitor is exemplified by
pioglitazone, namely 5-[4-[2-(5-ethyl-2-
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98/03692
2
pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione.
6) The Journal of Biological Chemistry, Vo1.271, No.23,
pp.13515-13522 (1996) describes that a thiazolidinedione
related compound such as 1-(3-allyl-4-oxothiazolidine-
5 2-yliden)-4-methylthiosemicarbazone exhibits
antiarthritic activity.
However, none of the above references describes that
a thiazolidine derivative is useful as an agent for
prophylaxis and treatment of a TNF- a mediated inflammatory
disease.
An inflammatory reaction includes various acute and
chronic reactions which occur when stimulation was added
to the living body. Such reactions include unfavorable
reactions which cause destruction of the living tissues as
well as favorable reactions to the living body with the
purpose of excluding the alien substance. So far,
inflammatory diseases are treated with steroid or a
nonsteroidal anti-inflammatory agent, an
immunosuppressive agent, and the like. However, such
agents have problems that they inhibit favorable reactions
as well as unfavorable reactions at the time of inflammation.
Therefore, agents which inhibit only unfavorable
reactions to the living body are desired.
25 It is thought that various cytokines are produced to
regulate inflammation reactions at the time of inflammation.
TNF-a which is one of such cytokines is thought to play
an important role in expansion and delay of inflammation.
For instance, it is thought that production of TNF-c~
increased to cause destruction of articular tissues in
rheumatoid arthritis which belongs to an inflammatory
disease.
Based on the above situations, agents which
specifically inhibit TNF- a mediated inflammation
35 reactions are expected to be an anti-inflammatory agent
with reduced side effects, therefore development of such
CA 02300813 2000-02-17
WO 99/09965 PCT/.1P98/03692
3
agents are desired.
DISCLOSURE OF INVENTION
The present invention relates to
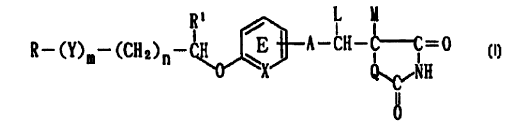
(1) An anti-inflammatory agent which affects by way of a
TNF-a inhibitory action and comprises a compound of the
formula:
/ I
R- (Y)m- (CH2)~- H ~~'A-CH-C-C=0
1 o Q~~~IH
II
0
_ wherein R represents a hydrocarbon group that may be
substituted or a heterocyclic group that may be
substituted; Y represents a group of the formula -CO-,
-CH (OH) -, or -NR3- where R3 represents an alkyl group that
may be substituted; m is 0 or 1; n is 0, 1 or 2; X represents
CH or N; A represents a chemical bond or a bivalent aliphatic
2 0 hydrocarbon group having 1 to 7 carbon atoms ; Q represents
oxygen or sulfur; Rl represents hydrogen or an alkyl group;
ring E may have further 1 to 4 subs tituents, which may form
a ring in combination wi th Rl ; L and M respectively represent
hydrogen or may be combined with each other to form a
chemical bond; or a salt thereof (hereinafter referred to
simply as Compound (I));
(2 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein the heterocyclic group represented by R is a 5- to
7-membered monocyclic and heterocyclic group containing 1
to 4 hetero-atoms selected from oxygen, sulfur and nitrogen
in addition to carbon as ring members or a condensed
heterocyclic group;
(3) An anti-inflammatory agent according to the above (1) ,
wherein R represents a heterocyclic group that may be
substituted;
(4) An anti-inflammatory agent according to the above (3) ,
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
4
wherein the heterocyclic group is pyridyl, oxazolyl or
thiazolyl;
( 5 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein the partial structural formula:
/ /
is the
formula:
( 6 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein X represents CH;
( 7 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein R1 represents hydrogen;
( 8 ) An anti-inflammatory agent according to the above ( 1 } ,
wherein L and M respectively represent hydrogen;
15 ( 9 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein the compound is 5-[4-[2-(5-ethyl-2-
pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione;
( 10 ) An anti-inflammatory agent according to the above ( 1 ) ,
wherein the compound is (R)-(+)-5-[3-[4-(2-(2-furyl)-5-
methyl-4-oxazolylmethoxy]-3-methoxyphenyl]propyl]-2,4-
oxazolidinedione;
(11} Method for treating or preventing a TNF-c~ mediated
inflammatory disease in a mammal in need thereof, which
comprises administering to said mammal an effective amount
25 of a compound as defined in the above (1) or a
pharmacologically acceptable salt thereof; and
(12) Use of a compound as defined in the above (1) or a
pharmacologically acceptable salt thereof for the
manufacture of an agent for prophylaxis or treatment of a
TNF- c~ mediated inflammatory disease.
Referring to the hydrocarbon group that may be
substituted for R,the hydrocarbon group includes aliphatic,
alicyclic, alicyclic-aliphatic, aromatic-aliphatic, and
35 aromatic hydrocarbon groups. The number of carbon atoms
constituting such hydrocarbon groups is preferably 1 to 14 .
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
The aliphatic hydrocarbon group is preferably a C1_8
aliphatic hydrocarbon group. The aliphatic hydrocarbon
group includes saturated C1_$ aliphatic hydrocarbon groups
(e. g. alkyl groups) such as methyl, ethyl, propyl,
5 isopropyl, butyl, isobutyl, sec-butyl, t-butyl, pentyl,
isopentyl, neopentyl, t-pentyl, hexyl, isohexyl, heptyl,
and octyl; and unsaturated CZ_8 aliphatic hydrocarbon groups
(e.g. alkenyl, alkadienyl, alkynyl, and alkadiynyl groups)
such as ethenyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-
10 butenyl, 3-butenyl, 2-methyl-1-propenyl, 1-pentenyl, 2-
pentenyl, 3-pentenyl, 4-pentenyl, 3-methyl-2-butenyl,
1-hexenyl, 3-hexenyl, 2,4-hexadienyl, 5-hexenyl, 1-
heptenyl, 1-octenyl, ethynyl, 1-propynyl, 2-propynyl,
1-butynyl, 2-butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl,
15 3-pentynyl, 4-pentynyl, 1-hexynyl, 3-hexynyl, 2,4-
hexadiynyl, 5-hexynyl, 1-heptynyl, and 1-octynyl.
The alicyclic hydrocarbon group is preferably a C3_,
alicyclic hydrocarbon group. The alicyclic hydrocarbon
group includes saturated C3_, alicyclic hydrocarbon groups
20 (e. g. cycloalkyl groups) such as cyclopropyl, cyclobutyl,
cyclopentyl,cyclohexyl,cycloheptyl,etc. and unsaturated
CS_, alicyclic hydrocarbon groups ( a . g . cycloalkenyl groups
and cycloalkadienyl groups) such as 1-cyclopentenyl, 2-
cyclopentenyl, 3-cyclopentenyl, 1-cyclohexenyl, 2-
25 cyclohexenyl, 3-cyclohexenyl, 1-cycloheptenyl, 2-
cycloheptenyl,3-cycloheptenyl,and 2,4-cycloheptadienyl.
The alicyclic-aliphatic hydrocarbon group is a group
consisting of the above-described alicyclic hydrocarbon
30 group and aliphatic hydrocarbon group (e. g. cycloalkyl-
alkyl and cycloalkenyl-alkyl groups) and is preferably a
C,_9alicyclic-aliphatic hydrocarbon group. Specifically,
the alicyclic-aliphatic hydrocarbon group includes
cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyi,
35 cyclopentylmethyl, 2-cyclopentenylmethyl, 3-
cyclopentenylmethyl, cyclohexylmethyl, 2-
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
6
cyclohexenylmethyl, 3-cyclohexenylmethyl,
cyclohexylethyl, cyclohexylpropyl, cycloheptylmethyl,
cycloheptylethyl, etc.
The aromatic-aliphatic hydrocarbon group is
5 preferably a C,_13 aromatic-aliphatic hydrocarbon group ( e. g.
aralkyl and aryl-alkenyl groups). The aromatic-aliphatic
hydrocarbon group includes C,_9 phenylalkyl such as benzyl,
phenethyl, 1-phenylethyl, 3-phenylpropyl, 2-phenylpropyl
and 1-phenylpropyl; C~~-13 naphthylalkyl such as cx -
10 naphthylmethyl, (x-naphthylethyl, a-naphthylmethyl, and
a-naphthylethyl; CB_lo phenylalkenyl such as styryl and
4-phenyl-1,3-butadienyl; and ClZ_13 naphthylalkenyl such as
2-(2-naphthyl)vinyl.
The aromatic hydrocarbon group is preferably a C6_14
15 aromatic hydrocarbon group (e.g. aryl groups). The
aromatic hydrocarbon group includes phenyl and naphthyl ( a
-naphthyl, a-naphthyl).
Referring to the formula ( I ) , the heterocyclic group
in a heterocyclic group that may be substituted for R is
20 a 5- to 7-membered monocyclic and heterocyclic group
containing 1 to 4 hetero-atoms selected from oxygen, sulfur,
and nitrogen in addition to carbon as ring members or a
condensed heterocyclic group. The condensed heterocyclic
group may for example be one consisting of such a 5- to
25 7-membered monocyclic and heterocyclic group and a 6-
membered ring containing 1 or 2 nitrogen atoms , a benzene
ring, or a 5-membered ring containing one sulfur atom.
Specifically the heterocyclic group includes 2-
pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-
30 pyrimidinyl,5-pyrimidinyl,6-pyrimidinyl,3-pyridazinyl,
4-pyridazinyl, 2-pyrazinyl, 2-pyrrolyl, 3-pyrrolyl, 2-
imidazolyl, 4-imidazolyl, 5-imidazolyl, 3-pyrazolyl, 4-
pyrazolyl, isothiazolyl, isoxazolyl, 2-thiazolyl, 4-
thiazolyl, 5-thiazolyl, 2-oxazolyl, 4-oxazolyl, 5-
35 oxazolyl, 1,2,4-oxadiazol-5-yl, 1,2,4-triazol-3-yl,
1,2,3-triazol-4-yl, tetrazol-5-yl, benzimidazol-2-yl,
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
7
indol-3-yl, 1H-indazol-3-yl, 1H-pyrrolo[2,3-b]pyrazin-
2-yl, 1H-pyrrolo[2,3-b]pyridin-6-yl, 1H-imidazo[4,5-
b]pyridin-2-yl, 1H-imidazo[4,5-c]pyridin-2-yl, 1H-
imidazo[4,5-b]pyrazin-2-yl, benzopyranyl and 3,4-
dihydrobenzopyran-2-yl. The preferred heterocyclic group
is pyridyl, oxazolyl, or thiazolyl.
Referring to the formula (I), the hydrocarbon group
and heterocyclic group for R may respectively have 1 to 5 ,
preferably 1 to 3 substituents at substitutable positions .
Such substituents include for example aliphatic
hydrocarbon groups, alicyclic hydrocarbon groups, aryl
groups, aromatic heterocyclic groups, non-aromatic
heterocyclic groups , halogen , nitro , amino group that may
be substituted, acyl groups that may be substituted,
hydroxy group that may be substituted, thiol that may be
substituted, and carboxyl group that may be esterified.
The aliphatic hydrocarbon group includes straight
chain or branched aliphatic hydrocarbon groups having 1 to
15 carbon atoms , such as alkyl groups , alkenyl groups , and
alkynyl groups.
The preferred alkyl group is a Cl_lo alkyl group, such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl,t-butyl,pentyl,isopentyl,neopentyl,t-pentyl,
1-ethylpropyl, hexyl, isohexyl, 1,1-dimethylbutyl, 2,2-
dimethylbutyl, 3,3-dimethylbutyl, 2-ethylbutyl, hexyl,
pentyl, octyl, nonyl, and decyl.
The preferred alkenyl group is a C2_lo alkenyl group,
such as vinyl, allyl, isopropenyl, 1-propenyl, 2-
methyl-1-propenyl, 1-butenyl, 2-butenyl, 3-butenyl, 2-
ethyl-1-butenyl, 3-methyl-2-butenyl, 1-pentenyl, 2-
pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-pentenyl,
1-hexenyl,2-hexenyl, 3-hexenyl,4-hexenyl, ands-hexenyl.
The preferred alkynyl group is a Cz_lo alkynyl group,
such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-
butynyl, 3-butynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl,
4-pentynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl,
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
8
and 5-hexynyl.
The alicyclic hydrocarbon group includes saturated
and unsaturated alicyclic hydrocarbon groups having 3 to
12 carbon atoms , such as cycloalkyl groups , cycloalkenyl
groups, and cycloalkadienyl groups.
The preferred cycloalkyl group is a C3_io cycloalkyl
group, such as cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl,
bicyclo[2.2.1]heptyl, bicyclo(2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.1]nonyl, bicyclo[4.2.1]nonyl, and
bicyclo[4.3.1]decyl.
The preferred cycloalkenyl group is a C3_lo
cycloalkenyl group, such as 2-cyclopenten-1-yl, 3-
cyclopenten-1-yl, 2-cyclohexen-1-yl, and 3-cyclohexen-
1-yl.
The preferred cycloalkadienyl group is a C,_lo
cycloalkadienyl group, such as 2,4-cyclopentadien-1-yl,
2,4-cyclohexadien-1-yl, 2,5-cyclohexadien-1-yl.
The term "aryl group" means a monocyclic or condensed
polycyclic aromatic hydrocarbon group. As preferred
examples , C6_14 aryl groups such as phenyl , naphthyl , anthryl ,
phenanthryl, acenaphthylenyl can be mentioned.
Particularly preferred are phenyl, 1-naphthyl, and 2-
naphthyl.
The preferred aromatic heterocyclic group includes 5-
to 7-membered monocyclic aromatic heterocyclic groups
containing 1 to 4 hetero-atoms selected from oxygen, sulfur,
and nitrogen in addition to carbon as ring members, such
as furyl, thienyl, pyrrolyl, oxazolyl, isoxazolyl,
thiazolyl, isothiazolyl, imidazolyl, pyrazolyl, 1,2,3-
oxadiazolyl, 1,2,4-oxadiazolyl, 1,3,4-oxadiazolyl,
furazanyl, 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl,
1,3,4-thiadiazolyl, 1,2,3-triazolyl, 1,2,4-triazolyl,
tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl,
and triazinyl; and bicyclic or tricyclic condensed aromatic
CA 02300813 2000-02-17
WO 99/09965 PCTL1P98/03692
9
heterocyclic groups containinglto 5hetero-atoms selected
from oxygen, sulfur, and nitrogen in addition to carbon as
ring members, such as benzofuranyl, isobenzofuranyl,
benzo[b]thienyl, indolyl, isoindolyl, 1H-indazolyl,
benzimidazolyl, benzoxazolyl, 1,2-benzisoxazolyl,
benzothiazolyl, 1,2-benzisothiazolyl, 1H-benzotriazolyl,
quinolyl, isoquinolyl, cinnolinyl, quinazolinyl,
quinoxalinyl, phthalazinyl, naphthyridinyl, purinyl,
pteridinyl, carbazolyl, (x-carbolinyl, l3-carbolinyl, ?'
-carbolinyl, acridinyl, phenoxazinyl, phenothiazinyl,
phenazinyl, phenoxathiinyl, thianthrenyl,
phenanthridinyl, phenanthrolinyl, indolizinyl,
pyrrolo[1,2-b]pyridazinyl, pyrazolo[1,5-a]pyridyl,
imidazo[1,2-a]pyridyl, imidazo[1,5-a]pyridyl,
imidazo[1,2-b]pyridazinyl, imidazo[1,2-a]pyrimidinyl,
1,2,4-triazolo[4,3-a]pyridyl, and 1,2,4-triazolo[4,3-
b]pyridazinyl.
The preferred non-aromatic heterocyclic group
includes oxiranyl, azetidinyl, oxetanyl, thietanyl,
pyrrolidinyl, tetrahydrofuryl, thiolanyl, piperidyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl,
piperazinyl, pyrrolidino, piperidino, and morpholino.
The halogen includes fluorine, chlorine, bromine, and
iodine, and is preferably fluorine or chlorine.
The amino group that may be substituted includes amino
(-NHZ) that may be mono- or di-substituted by, for example,
Cl_to alkyl groups , C3_~o cycloalkyl groups , CZ_lo alkenyl
groups , C3_lo cycloalkenyl groups , C1_13 acyl groups ( a . g . CZ_,o
alkanoyl groups , C,_13 arylcarbonyl groups ) , or C6_lz aryl
groups. As examples of the substituted amino group, there
can be mentioned methylamino, dimethylamino, ethylamino,
diethylamino, dibutylamino, diallylamino,
cyclohexylamino, acetylamino, propionylamino,
benzoylamino, phenylamino, and N-methyl-N-phenylamino.
The acyl group in the acyl groups that may be
substituted includes Cl_13 acyl groups. For example, formyl
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
and groups formed between carbonyl and C1_lo alkyl groups ,
C3_lo cycloalkyl groups , CZ_lo alkenyl groups , C3_lo
cycloalkenyl groups, C6_12 aryl groups, or aromatic
heterocyclic groups (e.g. thienyl, furyl, pyridyl). The
5 preferred acyl group includes acetyl, propionyl, butyryl,
isobutyryl, valeryl, isovaleryl, pivaloyl, hexanoyl,
heptanoyl, octanoyl, cyclobutanecarbonyl,
cyclopentanecarbonyl, cyclohexanecarbonyl,
cycloheptanecarbonyl, crotonyl, 2-cyclohexenecarbonyl,
10 benzoyl, and nicotinoyl. The substitutent in the
substituted acyl groups includes C1_3 alkyl , C1_3 alkoxy
groups, halogen (e. g. chlorine, fluorine, bromine, etc.),
nitro, hydroxy, and amino.
Referring to the hydroxy group that may be substituted,
the substituted hydroxy includes alkoxy, alkenyloxy,
aralkyloxy, acyloxy, and aryloxy groups.
The preferred alkoxy group includes C1_lo alkoxy groups ,
such as methoxy, ethoxy, propoxy, isopropoxy, butoxy,
isobutoxy, sec-butoxy, t-butoxy, pentyloxy, isopentyloxy,
20 neopentyloxy, hexyloxy, heptyloxy, nonyloxy, cyclobutoxy,
cyclopentyloxy, and cyclohexyloxy.
The preferred alkenyloxy group includes CZ_lo
alkenyloxy groups, such as allyloxy, crotyloxy, 2-
pentenyloxy, 3-hexenyloxy, 2-cyclopentenylmethoxy, and
2-cyclohexenylmethoxy.
The preferred aralkyloxy group includes C,_lo
aralkyloxy groups, such as phenyl-C1_, alkyloxy (e. g.
benzyloxy, phenethyloxy, etc.).
The preferred acyloxy group includes CZ_13 acyloxy
groups, more preferably CZ_4 alkanoyloxy (e. g. acetyloxy,
propionyloxy, butyryloxy, isobutyryloxy, etc.).
The preferred aryloxy group includes C6_14 aryloxy
groups, such as phenoxy, and naphthyloxy. This aryloxy
group may have 1 or 2 substituents such as halogen (e. g.
35 chlorine, fluorine, bromine, etc.). The substituted
aryloxy group includes 4-chlorophenoxy.
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
11
Referring to the thiol group that may be substituted,
the substituted thiol group includes alkylthio,
cycloalkylthio, aralkylthio, and acylthio groups.
The preferred alkylthio group includes C1_lo alkylthio
groups, such as methylthio, ethylthio, propylthio,
isopropylthio, butylthio, isobutylthio, sec-butylthio,
t-butylthio, pentylthio, isopentylthio, neopentylthio,
hexylthio, heptylthio, and nonylthio. The preferred
cycloalkylthio group includes C3_lo cycloalkylthio groups
10 such as cyclobutylthio, cyclopentylthio, and
cyclohexylthio.
The preferred aralkylthio group includes C,_lo
aralkylthio groups, such as phenyl-C1_, alkylthio (e. g.
benzylthio, phenethylthio, etc.).
15 The acylthio group is preferably a CZ_13 acylthio group,
more preferably a CZ_, alkanoylthio group ( a . g . acetylthio,
propionylthio, butyrylthio, isobutyrylthio, etc.).
The carboxyl group that may be esterified includes
alkoxycarbonyl, aralkyloxycarbonyl, and aryloxycarbonyl
20 groups.
The preferred alkoxycarbonyl group includes CZ_5
alkoxycarbonyl groups, such as methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, and butoxycarbonyl.
The preferred aralkyloxycarbonyl group includes CB_lo
25 aralkyloxycarbonyl groups, such as benzyloxycarbonyl.
The preferred aryloxycarbonyl group includes C,_ls
aryloxycarbonyl groups, such as phenoxycarbonyl, and p-
tolyloxycarbonyl.
The preferred substituent on the hydrocarbon or
30 heterocyclic group for R includes C1_lo alkyl groups,
aromatic heterocyclic groups, and C6_14 aryl groups.
Particularly preferred is C1_3 alkyl, furyl, thienyl, phenyl,
or naphthyl.
Referring to the formula ( I ) , when the substituent on
35 the hydrocarbon or heterocyclic group for R is an alicyclic
hydrocarbon group, an aryl group, an aromatic heterocyclic
CA 02300813 2000-02-17
WO 99/09965 PCTlJP98/03692
12
group, or a non-aromatic heterocyclic group, this
substituent may be further substituted by one or more,
preferably 1 to 3 suitable substituents. As such
substituents, there can be mentioned Cl_6 alkyl groups, CZ_s
5 alkenyl groups , C2_6 alkynyl groups , C3_, cycloalkyl groups ,
C6-14 ar'Yl groups (e. g. phenyl, naphthyl, etc.), aromatic
heterocyclic groups (e. g. thienyl, furyl, pyridyl,
oxazolyl, thiazolyl, etc.), non-aromatic heterocyclic
groups (e.g. tetrahydrofuryl, morpholino, thiomorpholino,
10 piperidino, pyrrolidino, piperazino, etc.), C,_9 aralkyl
groups, amino, N-mono(C1_4)alkylamino groups, N,N-di(C1_
4 ) alkylamino groups , C2_8 acylamino groups ( a . g . acetylamino ,
propionylamino, benzoylamino, etc.), amidino, CZ_e acyl
groups (e.g. CZ_8 alkanoyl groups, etc.), carbamoyl, N-
15 mono ( C1_4 ) alkylcarbamoyl groups , N, N-di ( Cl_
4)alkylcarbamoyl groups, sulfamoyl, N-mono(C1_
4 ) alkylsulfamoyl groups , N, N-di ( Cl_, ) alkylsulfamoyl groups ,
carboxyl, CZ_e alkoxycarbonyl groups, hydroxy, Cl_4 alkoxy
groups , C2_5 alkenyloxy groups , C3_, cycloalkyloxy groups ,
20 C,_9 aralkyloxy groups , C6_l, aryloxy groups ( a . g. phenyloxy,
naphthyloxy, etc.), mercapto, C1_q alkylthio groups, C,_9
aralkylthio groups , C6_14 arylthio groups ( a . g . phenylthio ,
naphthylthio , etc . ) , sulf o , cyano , azido , nitro , nitroso ,
and halogen ( a . g . fluorine , chlorine , bromine , iodine ) .
25 In the formula (I), R is preferably a heterocyclic
group that may be substituted. More preferably, R is
pyridyl, oxazolyl, or thiazolyl group, which may have 1 to
3 substituents selected from C1_3 alkyl, furyl, thienyl,
phenyl, and naphthyl.
30 Referring to the formula (I), Y represents -CO-, -
CH ( OH ) - or -NR3- . Y is preferably -CH ( OH ) - or -NR'- and more
preferably -CH( OH) - . Referring to an alkyl group that may
be substituted for R', the alkyl group includes Cl_4 alkyl
groups, such as methyl, ethyl, propyl, isopropyl, butyl,
35 isobutyl, sec-butyl, and t-butyl. The substituent
includes halogen (e. g. fluorine, chlorine, bromine,
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98!03692
13
iodine ) , Cl_4 alkoxy groups ( a . g . methoxy, ethoxy, propoxy,
butoxy, isobutoxy, sec-butoxy, t-butoxy), hydroxy, nitro,
and C1_, acyl groups ( a . g . f ormyl , acetyl , propionyl , et c . ) .
The symbol m represents 0 or 1 and is preferably 0.
The symbol n represents 0, 1 or 2 and is preferably
0 or 1.
The symbol X represents CH or N and is preferably CH.
Referring to the formula ( I ) , the symbol A represents
a chemical bond or a bivalent aliphatic hydrocarbon group
having 1 to 7 carbon atoms. This aliphatic hydrocarbon
group may be straight-chain or branched and may further be
saturated or unsaturated. Thus, for example, -CHz-, -
CH(CH3)-, -(CHZ)z-, -CH{CZHS)-, -(CHz)3-, -(CHZ),-, -(CHz)5-,
- ( CHz ) 6- , - ( CHz ),- , etc . can be mentioned for the saturated
15 bivalent aliphatic hydrocarbon group, while -CH=CH-, -
C ( CH3 ) =CH- , -CH=CH-CHz- , -C ( CzHs ) =CH- , -CHZ-CH=CH-CHZ- , -
CHz-CHZ-CH=CH-CHz- , -CH=CH-CH=CH-CHz- , -CH=CH-CH=CH-
CH=CH-CHz-, etc. can be mentioned for the unsaturated
bivalent aliphatic hydrocarbon group. The symbol A
20 preferably represents a chemical bond or a bivalent
aliphatic hydrocarbon group having 1 to 4 carbon atoms,
which is preferably a saturated group. More preferably,
A represents a chemical bond, -CHz- or - ( CHz ) z- . Still more
preferably, A represents a chemical bond or -(CHz)z-
25 The alkyl group for R1 includes C1_4 alkyl groups such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl, and t-butyl. Preferably, R1 represents
hydrogen.
Referring to the formula ( I ) , the partial structural
30 formula:
is preferably
\X the formula: \x
35 wherein each symbols has the same meanings as defined above.
Furthermore, ring E may optionally have 1 to 4
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
14
substituents at substitutable positions. Such
substituents include an alkyl group, a hydroxy group that
may be substituted, halogen, an acyl group that may be
substituted, nitro, and an amino group that may be
5 substituted. These substituents may be the same as the
substituents mentioned for the hydrocarbon or heterocyclic
group for R.
Ring E, the partial structural formula:
~2
is preferably
the formula:
wherein RZ represents hydrogen, an alkyl group, a hydroxy
group that may be substituted, halogen, an acyl group that
15 may be substituted, nitro, or an amino group that may be
substituted.
The alkyl group, hydroxy group that may be substituted,
halogen, acyl group that may be substituted, and amino group
that may be substituted, for R2, may each be the same as
20 the substituents mentioned for the hydrocarbon or
heterocyclic group for R. Preferably, RZ is hydrogen,
hydroxy group that may be substituted, or halogen. More
preferably, RZ is hydrogen or hydroxy group that may be
substituted. Particularly preferred is hydrogen or a C1_4
25 alkoxy group.
L and M respectively represent hydrogen or may be
combined with each other to form a chemical bond, and
preferably they are hydrogen.
Referring to the formula (I), the compound in which
30 L and M are combined with each other to form a chemical bond:
R~
R-CY)~-CCH2)n- ~H ~ ~A'CH=C--C=0 -
~~NH C I A 1 )
0
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98l03692
wherein each symbols has the same meanings as defined above,
may exist as ( E ) - and ( Z ) - isomers , owing to the double bond
at 5-position of the azolidinedione ring.
The compound in which L and M respectively represent
5 hydrogen:
R'
R-(Y)~-(CHz)n- H E~A-'CH2-CH--C=0
~X Q~iNH ( I - A 2 )
0
to
wherein each symbols has the meanings as defined above, may
exist as optical isomers, i.e. (R)- and (S)-forms, with
respect to the asymmetric carbon at 5-position of the
15 azolidinedione ring. This compound includes those
optically active compounds, i.e. (R)- and (S)-forms, as
well as the racemic form.
The preferred compound of the formula ( I ) is the
compound in which R represents pyridyl, oxazolyl, or
20 thiazolyl group, optionally having 1 to 3 substituents
selected from the group consisting of C1_3 alkyl, furyl,
thienyl, phenyl, and naphthyl; Y represents -CH(OH)- or
-NR3- wherein R' is methyl; n is 0 or 1; X represents CH;
A represents a chemical bond or -(CHZ)2-; R1 represents
hydrogen; ring E, namely the partial structural formula:
Rz
is the
formula:
30 wherein RZ is hydrogen or a C1_, alkoxy group; and L and M
respectively represent hydrogen.
As preferred species of the compound of the formula
(I), the following compounds are mentioned.
1) 5-[4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]-2,4-
thiazolidinedione;
2) 5-[4-[2-hydroxy-2-(5-methyl-2-phenyl-4-
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
16
oxazolyl)ethoxy]benzyl]-2,4-thiazolidinedione;
3) (R)-(+)-5-[3-[4-(2-(2-furyl)-5-methyl-4-
oxazolylmethoxy]-3-methoxyphenyl]propyl]-2,4-
oxazolidinedione;
5 4) (S}-(-)-5-(3-[4-[2-(2-furyl)-5-methyl-4-
oxazolylmethoxy]-3-methoxyphenyl]propyl]-2,4-
oxazolidinedione;
5) 5-[3-[3-fluoro-4-(5-methyl-2-phenyl-4-
oxazolylmethoxy)phenyl]propyl]-2,4-oxazolidinedione;
6) 5-[5-[3-methoxy-4-(5-methyl-2-phenyl-4-
oxazolylmethoxy)phenyl]pentyl]-2,4-oxazolidinedione;
7) 5-[3-(3,5-dimethoxy-4-[2-[(E)-styryl]-4-
oxazolylmethoxy]phenyl]propyl]-2,4-oxazolidinedione;
8) 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-
2H-1-benzopyran-2-yl)methoxy]phenyl]methyl]-2,4-
thiazolidinedione;
9) 5-[[4-[2-(methyl-2-
pyridylamino)ethoxy]phenyl]methyl]-2,4-
thiazolidinedione.
20 Hereafter, these compounds are sometimes simply
ref erred to as compound No .1, compound No . 2 , and the like .
Among the above compounds , compound Nos . 1, 3 , 8 and
9 are preferred, and compound Nos .1 and 3 are particularly
preferred.
25 The salt of compound (I) of the present invention is
preferably a pharmacologically acceptable salt, which
includes salts with inorganic bases, salts with organic
bases , salts with inorganic acids , salts with organic acids ,
and salts with basic or acidic amino acids.
30 The preferred salt with an inorganic base includes
alkali metal salts such as sodium salt, potassium salt,
etc.; alkaline earth metal salts such as calcium salt,
magnesium salt, etc.; aluminum salt, and ammonium salts.
The preferred salt with an organic base includes salts
35 with trimethylamine, triethylamine, pyridine, picoline,
ethanolamine, diethanolamine, triethanolamine,
CA 02300813 2000-02-17
WO 99!09965 PCflJP98/03692
17
dicyclohexylamine, N,N'-dibenzylethylenediamine, etc.
The preferred salt with an inorganic acid includes
saltswith hydrochloric acid,hydrobromic acid,nitric acid,
sulfuric acid, phosphoric acid, etc.
The preferred salt with an organic acid includes salts
with formic acid, acetic acid, trifluoroacetic acid,
fumaric acid, oxalic acid, tartaric acid, malefic acid,
citric acid, succinic acid, malic acid, methanesulfonic
acid, benzenesulfonic acid, p-toluenesulfonic acid, etc.
The preferred salt with a basic amino acid includes
salts with arginine, lysine, ornithine, etc. The
preferred salt with an acidic amino acid includes salts with
aspartic acid, glutamic acid, etc.
The most preferred of all the above-mentioned salts
is hydrochloride, sodium salt or potassium salt.
Compound ( I ) or a salt thereof of the present invention
can be produced in accordance with methods described in JP-A
S55(1980)-22636 (EP-A-8203), JP-A S60(1985)-208980 (EP-
A-155845}, JP-A 561(1986)-286376 (EP-A-208420), JP-A
561(1986)-085372 (EP-A-177353), JP-A S61(1986)-267580
(EP-A-193256), JP-A H5(1993)-86057 (WO-A-9218501), JP-A
H7(1995)-82269 (EP-A-605228), JP-A H7(1995)-101945 {EP-
A-612743), EP-A-643050, EP-A-710659 (JP-A H9(1997)-
194467), etc, or methods analogous thereto.
Compound ( I ) or a salt thereof of the present invention
(hereinafter simply referred to as compound of the present
invention) is useful as an anti-inflammatory agent which
affects by way of a TNF- a inhibitory action. In addition,
the toxic potential of the compound of the present invention
is low. The TNF-cx inhibitory action means reduction in
the production amount of TNF- lx in the living tissues ( a . g . ,
skeletal muscles, monocytes, macrophages, neutrophils,
fibroblasts, epithelial cells, astrocytes, etc.} and
reduction in the activity of TNF-cx.
CA 02300813 2000-02-17
WO 99/09965 PC1~/JP98/03692
18
The anti-inflammatory agent of the present invention
can be used as an agent for prophylaxis and treatment of
TNF- cx mediated inflammatory diseases in mammals ( a . g . , man,
mouse , rat , rabbit , dog , cat , bovine , equine , swine , monkey ,
5 etc.). The TNF-c~ mediated inflammatory diseases mean
inflammatory diseases which occur in the presence of TNF
cx and can be treated by way of a TNF- cx inhibitory action .
Examples of such inflammatory diseases include
diabetic complications (e. g., retinopathy, nephropathy,
10 neutropathy, disorders in the great arteries, etc.),
rheumatoid arthritis, osteoarthritis of the spine,
osteoarthritis, low back pain, gout, postoperative or
traumatic inflammation, remission of swelling, neuralgia,
laryngopharyngitis, cystitis, hepatitis, pneumonia, etc.
15
As the anti-inflammatory agent of the present
invention, the compound of the present invention as such
can be used. Usually, the anti-inflammatory agent is used
in the form of a pharmaceutical composition obtained by
20 formulating the compound of the invention with per se known
pharmaceutically acceptable carriers.
As the pharmaceutically acceptable carrier, a variety
of organic and inorganic carriers in common use as raw
materials for pharmaceutical preparations are employed.
25 The carrier is formulated in the form of the excipient,
lubricant, binder, and disintegrator for a solid dosage
form; and the solvent, solubilizer, suspending agent,
isotonizing agent, buffering agent and local analgesic for
a liquid dosage form. When necessary, pharmaceutical
30 additives such as the preservative, antioxidant, coloring
agent, sweetener, etc. can also be used.
The preferred excipient includes lactose, sucrose,
D-mannitol, starch, crystalline cellulose, light silicic
anhydride, etc.
35 The preferred lubricant includes magnesium stearate,
calcium stearate, talc, colloidal silica, etc.
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
19
The preferred binder includes crystalline cellulose,
sucrose, D-mannitol, trehalose, dextrin,
hydroxypropylcellulose, hydroxypropylmethylcellulose,
polyvinylpyrrolidone, etc.
The preferred disintegrator includes starch,
carboxymethylcellulose, carboxymethylcellulose calcium,
croscarmellose sodium, carboxymethylstarch sodium, etc.
The preferred solvent includes water for injection,
alcohol, propylene glycol, macrogol, sesame oil, corn oil,
tricaprylin, etc.
The preferred solubilizer includes polyethylene
glycol, propylene glycol, D-mannitol, trehalose, benzyl
benzoate, ethanol, trisaminomethane, cholesterol,
triethanolamine, sodium carbonate, sodium citrate, etc.
The preferred suspending agent includes surfactants
such as stearyltriethanolamine, sodium lauryl sulfate,
laurylaminopropionic acid, lecithin, benzalkonium
chloride, benzethonium chloride, glyceryl monostearate,
etc. and hydrophilic polymers such as polyvinyl alcohol,
ZO polyvinylpyrrolidone, carboxymethylcellulose sodium,
methylcellulose, hydroxymethylcellulose,
hydroxyethylcellulose, hydroxypropylcellulose, etc.
The preferred isotonizing agent includes sodium
chloride, glycerin, D-mannitol, etc.
The preferred buffering agent includes buffer
solutions such as phosphate, acetate, carbonate, citrate,
etc.
The preferred local anesthetic includes benzyl
alcohol, etc.
The preferred antiseptic includes p-hydroxybenzoic
esters, chlorobutanol, benzyl alcohol, phenethyl alcohol,
dehydroacetic acid, sorbic acid, etc.
The preferred antioxidant includes salts of sulfurous
acid, ascorbic acid, etc.
The above pharmaceutical composition can be
manufactured by conventional methods in the pharmaceutical
CA 02300813 2000-02-17
WO 99/09965 PCT/:IP98/03692
preparation techniques, for example methods described in
the Japanese Pharmacopoeia.
Examples of dosage forms of the pharmaceutical
composition include oral dosage forms such as tablets,
5 capsules (inclusive of soft capsules and microcapsules),
powders, granules, and syrups; and non-oral dosage forms
such as injections, suppositories, pellets, and drip
infusions . These dosage forms can be safely administered
either orally or non-orally.
10 The dosage of the anti-inflammatory agent of the
present invention differs depending on the subject, route
of administration, clinical condition, etc. For oral
administration to an adult patient, for instance, the usual
unit dose is about 0.1 mg/kg to about 30 mg/kg, preferably
15 about 2 mg/kg to about 20 mg/kg, as the compound of the
invention which is an active ingredient, which dose is
preferably administered once to 3 times a day.
BEST MODE FOR CARRYING OUT THE INVENTION
20 The following examples and test examples are intended
to describe the present invention in further detail and
should by no means be construed as defining the scope of
the invention.
Example 1
25 A fluidized-bed granulating and drying machine
(produced by powerex, Japan) was charged with 2479.5 g of
hydrochloride of Compound No.l (2250 g in terms of Compound
No.l), 13930.5 g of lactose and 540 g of
carboxymethylcellulose calcium (carmellose calcium),
30 followed by mixing at the preheating temperature and
spraying 7500 g of an aqueous solution containing 450 g of
hydroxypropylcellulose to yield granules. 16820 g of the
granules were processed with cutter-mill (produced by Showa
Kagaku Kikai Kousakusho, Japan) to yield milled granules.
35 16530 g of the milled granules, 513 g of carmellose calcium
and 57 g of magnesium stearate were mixed to yield
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
21
lubricated powders by using tumbling mixer (produced by
Showa Kagaku Kikai Kousakusho, Japan). 16800 g of the
lubricated powders were tabletted by using tabletting
machine (produced by Kikusui Seisakusho, Japan) to yield
140000 tablets having the following formula and each
containing 15 mg of Compound No. 1.
Formula per tablet (Unit: mg):
1) Hydrochloride of Compound No.l 16.53
2) Lactose 92.87
3) Carmellose calcium 7.2
4) Hydroxypropylcellulose 3.0
5) Maq~nesium stearate 0.4
Total: 120.0
Example 2
In substantially the same manner as in Example 1,
140000 tablets having the following formula and each
containing 30 mg of Compound No.l were obtained.
Formula per tablet (Unit: mg):
1) Hydrochloride of Compound No.1 33.06
2) Lactose 76.34
3) Carmellose calcium 7.2
4) Hydroxypropylcellulose 3.0
5) Magnesium stearate 0.4
Total: 120.0
Example 3
In substantially the same manner as in Example 2,
140000 tablets having the following formula and each
containing 45 mg of Compound No.l were obtained.
Formula per tablet (Unit: mg):
1) Hydrochloride of Compound No.l 49.59
2) Lactose 114.51
3) Carmellose calcium 10.8
4) Hydroxypropylcellulose 4.5
5) Maq~nesium stearate 0.6
CA 02300813 2000-02-17
WO 99/09965 PCT/,TP98/03692
22
Total: 180.0
Test Example 1 (Reduction of plasma TNF-cx level in mice)
The plasma TNF- a level was determined by using KKA''
mice which are genetically obese, diabetic models, and a
TNF-(x inhibitory action of the compound of the present
invention was evaluated.
Namely, eighteen male KKA" mice (10 week old),
genetically obese, diabetic models, were divided into two
groups each of which consists of nine mice. A powdered
commercial diet (CE-2, produced by Japan Clea) was given
to one group ( control group ) , and the above powdered diet
also containing 0 . 001 ~ (w/w ) of hydrochloride of Compound
No. 1 was given to the other group (drug administration
group) ad libitum. Mice in these groups were bred for 4
days. The average dosage of drug per mouse was 16 mg/kg
body weight/day. On the fourth day, mice were sacrificed
and blood was collected in tubes containing heparin.
The collected blood was centrifuged and the plasma
TNF- c~ level was determined by the enzyme immunoassay based
on the biotin-streptavidin method. Namely, 5 a 1 of a
solution of an anti-TNF-a antibody IgG (produced by
Genzyme, USA] (100 ,c.~g/ml) diluted with 0.05 M Tris-HC1
buffer (pH 8.0) was added to each wells of a 96-well
polystyrene microtiter plate [produced by Falcon, USA],
followed by standing at the room temperature for 2 hours
to adhere the anti-TNF- cx antibody IgG to the plate . After
removal of an excess antibody solution, each wells was
washed with 0.1 M Tris-HC1 buffer (pH 7.6) containing 0.4
M NaCl, 0.1 ~(w/w) bovine serum albumin, 0.1 ~(w/w) NaN,
and 1 mM MgCl2 (hereafter referred to as a washing buffer) .
Ten ,u 1 of plasma or standard solution of TNF- (x
[ Serotec , Great Britain ] was added to each wells , followed
by standing for 2.5 hours at the room temperature. After
each wells was washed with a washing buffer, 200 !~ 1 of a
solution of a biotinylated anti-TNF-a antibody IgG (35
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98/03692
23
ng/ml) diluted with a washing buffer was added, followed
by standing over night at 4 '~ . After each wells was washed
with a washing buffer, 20 ,ct 1 of a solution of a a -D-
galactosidase-linked streptavidin [produced by Boehringer
Mannheim GmbH, Germany] diluted 6000 fold with a washing
buffer was added, followed by standing for one hour at the
room temperature.
Then, each wells was washed with a washing buffer, and
a -D-galactosidase activity of an immune complex fixed at
a solid phase was assayed. Namely, 30 ~cl of a substrate
[ 60 mM of 4-methylumbelliferyl- a -D-galactoside, produced
by Sigma, USA] was added to each wells to start an enzyme
reaction. After the reaction was conducted at the room
temperature for 4 hours, the enzyme reaction was stopped
by addition of 0 .13 ml of 0 .1 M glycine-NaOH buffer ( pH 10 . 3 ) .
The fluorescence intensity of the produced 4-
methylumbelliferone was determined using a fluorescence
spectrometer [Cyto Fluor II, PerSeptive Biosystems, USA]
at the wavelengths of 350 and 460 nm for excitation and
emission, respectively.
Then, the amount of TNF- c~ was calculated from the
obtained fluorescence intensity using a separately
prepared dose-response curve.
The results are shown in Table 1.
Table 1. Plasma TNF-~ level (pg/ml)
Control Drug administration group
croup (Present invention)
4 . 97'x' 1. 75 1. 52 t 1. 08**
Mean ~ Standard Deviation; Significantly different from
Control group (**:p<0.01)
It is apparent from Table 1 that the compound of the
present invention significantly reduced plasma TNF-cx
level in mice.
Test Example 2 ( Reduction of plasma TNF- cx level in rats )
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98/03692
24
The plasma TNF- cx level was determined by using Wistar
fatty rats which are genetically obese, diabetic models,
and a TNF-cx inhibitory action of the compound of the
present invention was evaluated.
5 Namely, hydrochloride of Compound No. 1 was orally
administered to sixteen male Wistar tatty rats ( 16 week old} ,
genetically obese, diabetic models, via gastric tube at a
dose of 3 mg/kg body weight/day. Ten rats were sacrificed
before drug administration, and the first, second, third
10 and fourth day after drug administration, respectively.
Then, blood was collected.
As the normal group, ten Wistar lean rats ( 16 week old)
were sacrificed without drug administration and blood was
collected.
15 The collected blood was centrifuged, and the plasma
TNF-a level was determined in substantially the same
manner as in Test Example 1.
The results are shown in Table 2.
Table 2. Plasma TNF-lx level (pg/ml)
Days after drug TNF-c~
administration level (oa/ml)
Normal 0 56 . 9 ~ 47 . 5
group
Control 0 139.5~50.0
group
Present 1 109.9161.0
invention 2 115.1~59.0
3 69.9164.3
4 67.2~70.6*
Mean t Standard Deviation; Significantly different from
Control group (*:p<0.05)
It is apparent from Table 2 that the compound of the
present invention reduced the plasma TNF- a level in rats
35 time-dependently.
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
Test Example 3 (Reduction of TNF-a content in skeletal
muscle of rats)
The TNF- a content in skeletal muscle was determined
by using Wistar fatty rats which are genetically obese,
5 diabetic models, and a TNF-cx inhibitory action of the
compound of the present invention was evaluated.
Namely, hydrochloride of Compound No. 1 was
administered to male Wistar fatty rats (16 week old),
genetically obese, diabetic models in substantially the
10 same manner as in Test Example 2. Ten rats were sacrificed
before drug administration, and the first, second, third
and fourth day after drug administration, respectively.
Then, skeletal muscle was collected.
As the normal group, ten Wistar lean rats ( 16 week old)
15 were sacrificed without drug administration and skeletal
muscle was collected.
To the collected skeletal muscle, 0.1 M Tris-HC1
buffer (pH 7.6) containing 1 M NaCl, 2 %(w/w) bovine serum
albumin, 2 mM ethylenediaminetetraacetic acid disodium
20 salt(EDTA),aprotinin(80 tripsin-inhibitory units/liter)
and 0.02 %(w/w) NaN3 was added in an amount of 20 weight
times of the weight of the wet skeletal muscle. After
ultrasonic disintegration, the mixture was centrifuged at
15000 rpm for 30 minutes to obtain a supernatant.
25 The amount of TNF- cx in the obtained supernatant was
determined in substantially the same manner as in Test
Example 1.
The results are shown in Table 3.
Table 3. TNF-(x content in skeletal muscle (pg/g wet
weight)
Days after drug Amount of
administration TNF-a (pg/g wet weight)
Normal 0 156.7~ 61.9
groug
Control 0 356.6~105.6
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98/03692
26
9~P
Present 1 200.1~165.1*
invention 2 181.4~108.2**
3 105.1~ 96.4**
4 107.3~ 95.7**
Mean t Standard Deviation; Significantly different from
Control group (*:p<0.05, **:p<0.01)
It is apparent from Table 3 that the compound of the
present invention reduced the TNF-cx content in skeletal
10 muscle of rats significantly and almost time-dependently.
Test Example 4 (Suppression of the active oxygen
production in neutrophils)
The in vitro effect of the compound of the present
15 invention on suppression of the active oxygen production
in neutrophils was evaluated by determining the amount of
peroxides in cells.
Namely, venous blood was collected from male Wistar
rats ( 6 week old ) while adding heparin . To the collected
20 blood, the same volume of an aqueous solution of 3 ~(w/w)
dextran was added for separation of blood cells . After the
mixture was allowed to stand for 30 minutes, precipitates
obtained by centrifugation was suspended with saline. The
suspension was piled on Ficoll-Hypaque solution (Sigma,
25 USA), followed by centrifugation.
From the obtained precipitates, erythrocytes were
removed by hemolysis to separate neutrophils.
The hemolysis Was conducted in the following manner.
Namely, 4 ml of an ice-cooled 0.2 ~(w/w) aqueous solution
30 of NaCl was added to the above precipitates, which was
suspended quickly, followed by standing for 20 to 30 seconds
to puncture the erythrocytes. Then, 4 ml of an ice-cooled
1.6 $(w/w) aqueous solution of NaCl was added to the
obtained suspension, which was mixed to yield a mixed
35 solution having the same osmotic pressure with the
erythrocytes before puncture. The mixed solution was
CA 02300813 2000-02-17
WO 99/09965 PCT!JP98/03692
27
centrifuged at 4 ~ at 150 X g for 5 minutes . After the
supernatants were removed, the precipitates were washed
with PBS (phosphate buffer saline).
The thus obtained erythrocytes were washed with saline,
followed by addition of a minimum essential medium to
prepare a neutrophils floating solution. The obtained
neutrophils floating solution was fractionated into tubes
so that the number of neutrophiles per tube is 106.
Then , hydrochloride of Compound No . 1 or Compound No .
8 was added to the obtained tubes at the concentration of
1 uM. After incubation for one hour, a fluorescent pigment
[DCFH-DA (2,7-dichlorofluoresceine diacetic acid)] was
added, which was subjected to determination of the
fluorescence intensity by FACScan (Becton Dickinton, USA).
15 As the control group, the fluorescence intensity in
the case of adding no drug was determined.
The relative values of the fluorescence intensity in
the drug addition group when the fluorescence intensity in
the control group was 100 were calculated. These values
20 ware defined as the amount of peroxides caused by active
oxygen derived from neutrophils.
The results are shown in Table 4.
Table 4. Fluorescence intensity and peroxide level
25 Fluorescence Peroxide
intensitv level
Control group 707 100
Hydrochloride of 466 66
Compound No. 1
30 (Present invention)
Control group 377 100
Hydrochloride of 242 64
Compound No. 8
(Present invention)
35 It is apparent from Table 4 that the compound of the
present invention suppressed the active oxygen production
CA 02300813 2000-02-17
WO 99/09965 PCT/JP98/03692
28
in neutrophils.
TNF- a is produced by various cells such as monocytes,
macrophages, neutrophils, fibroblasts, epithelial cells,
astrocytes, and etc. TNF- a increases production of
active oxygen in neutrophils, which are suggested to have
a close relation with occurrence of rheumatoid arthritis
[Clinical and Experimental Rheumatology, vol. 15,
pp.233-237 (1997); Inflammation, vol. 20, pp.427-438
(1996) ] .
Therefore, it is considered that the compound of the
present invention exhibited suppressive effects on the
active oxygen production by reducing TNF- a production or
TNF-a sensitivity in neutrophils based on the results of
Test Example 4.
Industrial Applicability
The anti-inflammatory agent of the present invention
is used as an agent for prophylaxis and treatment of TNF
20 a mediated inflammatory diseases such as diabetic
complications (e. g., retinopathy, nephropathy,
neutropathy, disorders in the great arteries, etc.),
rheumatoid arthritis, osteoarthritis of the spine,
osteoarthritis, low back pain, gout, postoperative or
traumatic inflammation, remission of swelling, neuralgia,
sore throat, cystitis, hepatitis, pneumonia, and etc.