Note: Descriptions are shown in the official language in which they were submitted.
CA 02301570 2000-02-23
1
Annular chromatograph
The present invention relates to an annular chromatograph with charging in the
form
of a particulate bed.
Annular chromatography is a variant of preparative chromatographic separation
which over the past few years has been recognised and used to an ever-
increasing
extent. Annular chromatography is used mainly when large quantities of mixed
substances have to be separated, since this type of chromatography can be
operated
continuously and with a relatively high degree of resolution.
A typical P-CAC unit ("P-CAC" = preparative continuous annular chromatography)
consists of an annular particulate bed which is packed into the space (annular
gap)
between two concentric cylinders. Whilst the particulate bed is being rotated
around
its axis, feed solution and one or more eluents are introduced continuously at
the top
end. Such methods are known under prior art and are widespread (see for
example
EP-A-371.648).
Apart from the advantage of the large throughputs in annular chromatography
this
method is also characterised by high resolution and specificity. Nonetheless,
for
various specific separation problems it is necessary for further stages of
annular
chromatography to be added - for example, using different separating gels from
those in the first stage - in order to achieve the required degree of
separation. On
the one hand this can be done relatively easily by comparison with
conventional
column chromatography, since with annular chromatographic separation it is
known
at what point on the circumference the required products) will be discharged;
on the
other hand further annular chromatographs are necessary, which does of course
mean a sharp increase in the amount of equipment, and thus the cost.
The aim of the present invention is therefore to provide equipment for annular
chromatography which can be used to efficiently increase the resolution and
CA 02301570 2000-02-23
2
specificity of this method of chromatographic separation without giving rise
to an
undue increase in the amount of equipment and thereby the cost of the method.
The invention achieves this aim by means of an annular chromatograph with
charging in the form of a particulate bed, wherein said chromatograph is
characterised by the fact that the particulate bed comprises at least two
zones,
arranged one on top of the other, that contain different bed material and that
are
preferably separated from each other by at least one separation layer.
In this way both the preliminary and the main separation stages, or several
separations based on different methods-like for example exclusion
chromatography
or affinity chromatography and ion-exchange chromatography, etc.-can be
carried
out one after the other, continuously and with high resolution and specificity
within
the same separating equipment. In addition, the amount of time, money and
equipment needed to change columns, including for example concentration and
conditioning of the mixture for the next stage of separation, regeneration or
re-
equilibration of the columns, etc., as well as the danger of sample impairment
during
handling (e.g. in the case of substances which are sensitive to oxidation or
moisture)
are minimised or completely eliminated.
In a particularly preferred embodiment at least one of the zones can be
adapted to
create an electrophoresis zone by equipping it with electrical connections,
preferably
in the form of sliding contacts; electrophoretic separations can then also be
carried
out in the beneficial manner described above. This electrophoresis zone can
preferably be kept separate from the other zones) in each case by at least one
electrically non-conducting separation layer in order to increase the
efficiency of the
electrophoresis.
The bed material for the two or more zones can generally be selected from
anion-
exchange resins, cation-exchange resins, exclusion gels, gel permeation gels,
affinity
gels, hydrophobic interaction chromatography (HIC) gels, displacement resins,
CA 02301570 2000-02-23
3
reversed-phase gels, electrophoretic gels and other separating media used in
practice, thereby offering a wide range of possibilities for combined
separations, as
described in more detail below. If one of the zones in the chromatograph
according
to the invention is an electrophoresis zone an electrophoretic gel will of
course be
chosen as particulate bed for this zone.
According to the invention the separation layer or separation layers are
selected
preferably from membranes, non-porous, inert particulate material, especially
glass
beads, and - specifically for any electrophoresis zones - electrically non-
conducting
material.
In preferred embodiments of the invention the topmost zone of the particulate
bed is
covered with a covering layer and/or has a base layer beneath it; both the
covering
and base layer should preferably be of the same material as the separation
layer(s).
It is particularly preferred that a chromatograph in accordance with the
invention
should have at least one electrophoresis zone or at least one exclusion gel
zone and
at least one adsorber resin zone, with, especially, at least one adsorber
resin zone
containing an ion-exchange resin, with which for example proteins (one after
the
other according to their size and their charge) and numerous products of
biotechnology processes-like for example enzymes, h-EGF (human epidermal
growth factor) and immunoglobulins-can be separated and purified with an
extremely
high degree of selectivity and high resolution.
In one embodiment of the invention a tempering (i.e. heating or cooling)
jacket can
be provided for on the inner andlor outer circumference of the annular
separating
column (11) so that the optimum temperature can be set for the particular
separation.
The invention is described below with examples and with reference to the
enclosed
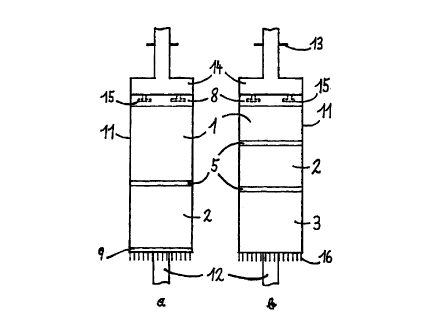
drawings, where Fig. 1 a) and 1 b) are schematic representations of an annular
chromatograph with 2 and 3 separation zones, respectively, which can be used
in
accordance with the present invention, Fig 2a) is a schematic partial view of
a section
CA 02301570 2000-02-23
4
through another annular chromatograph in accordance with the invention with an
electrophoresis zone and Fig. 2b) is a schematic detail enlargement of the
broken-
line box from Fig. 2a).
Two variants of the annular chromatograph of the present invention are shown
in
schematic form in Fig. 1 a) and 1 b). Fig. 1 a) shows an embodiment with two
separation zones in the particulate bed; Fig 1 b shows an embodiment with
three
separation zones. These zones 1 and 2, or 1, 2 and 3, consist preferably of
separation resins, selected for example from anion-exchange resins, cation-
exchange resins, exclusion gels, affinity gels, hydrophobic interaction
chromatography (HIC) gels, displacement resins, electrophoretic gels, and
possibly
other stationary phases which are usual in the field, like for example silica
gel,
aluminium oxide, etc. This broad range of possibilities provides a large
variety of
combinations of two or more of these separating agents.
Thus the invention encompasses for example the following combinations of two
stationary phases:
~ cation exchanger - anion exchanger
~ cation exchanger - exclusion gel
~ anion exchanger - exclusion gel
~ cation exchanger - affinity gel
~ anion exchanger - affinity gel
~ cation exchanger - hydrophobic interaction chromatography gel
~ anion exchanger - hydrophobic interaction chromatography gel
~ exclusion gel - affinity gel
~ exclusion gel - hydrophobic interaction chromatography gel
~ hydrophobic interaction chromatography gel - affinity gel
as well as such combinations with an electrophoretic gel instead of one of the
two
gels referred to above or in addition to them (three-zone chromatography). The
CA 02301570 2000-02-23
above list of possible binary combinations does not describe how the gels are
arranged in the column relative to one another, i.e. which is the higher gel
and which
is the lower one. This depends solely on the separating problem.
5 Examples of the separating techniques which can be used in the invention
include:
~ ion-exchange chromatography: e.g. Mono S (Pharmacia) or Toyopearl DEAE
650S (TosoHaas);
~ reversed-phase chromatography: e.g. Amberchrom CG-161 cd (Rohm & Haas)
or Sephasil C8 (Pharmacia);
~ hydrophobic interaction chromatography: Phenyl Sepharose (Pharmacia) or
TSK Gel Phenyl 5PW (TosoHaas);
~ size exclusion chromatography and gel permeation chromatography: e.g.
Sephadex G15 (Pharmacia) or Toyopearl HW 40F (TosoHaas);
~ affinity chromatography: e.g. Toyopearl AF Tresyl-650M (TosoHaas) or EAH
Sepharose-4B (Pharmacia);
~ adsorption chromatography: e.g. Amberlite XAD (Rohm & Haas) or Purolite
MN200 (Purolite).
The scope of protection of the invention also includes combinations of two or
more
gels of the same type, which may however come for example from different
manufacturers.. For example, two cation-exchange gels or two exclusion gels
may
also be arranged one over the other (e.g. Sephadex G15 above Toyopearl HW 40F
or similar).
According to the invention there is a particular preference for annular
chromatographs with a particulate bed consisting of at least one exclusion gel
zone
and at least one adsorber resin zone, where in particular at least one
adsorber resin
zone contains an ion-exchange resin; and for annular chromatographs with at
least
one electrophoretic gel in one of the zones.
CA 02301570 2000-02-23
6
The two or three gels are introduced one after the other into the annular gap
of an
annular column 11 made of a material which is inert in relation to the
components of
the separating solutions, preferably of glass; each of the gel zones is
covered with a
separation layer 5 before the next is added to prevent mixing of the various
materials.
The particulate bed is closed off at the top in both cases by a covering layer
8. Fig.
1 a) also shows a base layer 9 which (in addition to a porous base (not
shown), e.g.
frit, membrane disc, etc.) serves to prevent particulate material from
escaping at the
bottom of the column. The material for the separation, covering and base
layers 5, 8
and 9 is selected from membranes as well as non-porous particulate material
which
is inert in relation to all the components of the particular separating
solutions and
may be the same or different for all three layers, though specifically for
electrophoretic separations it must not be electrically conductive. According
to the
invention there is a preference for glass beads since these are inert in
practically all
the usual applications and are easily introduced.
Charging for the annular chromatograph of Fig. 1a) is thus in the order 9-2-5-
1-8 and
for that of Fig. 1 b) in the order 3-5-2-5-1-8.
The remaining components of the annular chromatograph can be designed in a
conventional manner. Thus column 11 (driven by a motor which is not shown) is
rotatable and mounted on a shaft 12, and it is fed via connections 13 for feed
and
eluent and a distributor head 14 fixed to the shaft; the feed ducts 15 of the
distributor
head 14 should preferably be immersed in the covering layer 8 to ensure an
even
feed. The ducts may be of the usual designs, i.e. single, multiple or slit-
shaped
nozzles or similar, though bent slit-shaped nozzles of different widths,
adapted to the
circumference of the column, are preferred for the invention, so that the
flows of feed
and eluent can be matched as precisely as possible to each other.
Outlet ducts and tubes 16 are provided at the bottom end of the column to
collect the
eluates. These outlets 16 may either be connected to column 11 (i.e. they
rotate with
CA 02301570 2000-02-23
7
the column around shaft 12) or be fixed to shaft 12 and be in contact - e.g.
by way of
a slip-ring - with the column which rotates relative to it; the latter design
is preferred.
Fig. 2a shows a partial view of an embodiment of an annular chromatograph in
accordance with the invention with an electrophoretic separating stage, namely
the
electrophoretic separation zone 4 which is separated off from a zone above it
and a
zone below it (neither of which is shown) by means of a separation layer 5 of
electrically non-conducting material. Both at the top and the bottom end of
the
electrophoresis zone 4, preferably directly adjacent to the relevant
separation
layer(s), there are sliding contacts consisting of a conductive ring 6,
mounted on the
outside of shaft 12 and supplied with electricity from a mains connection 10,
and an
annular current pick-up 7 which is provided for on the inside of column 11,
lies close
to the conductive ring 6 and is in electrical contact with this ring. The pick-
up is
passed through the column casing close to the sliding contact - either at a
certain
point or over an area - to build up the electrical voltage necessary for
electrophoretic
separation inside the column.
Fig. 2b) represents an enlarged view of the broken-line box from Fig. 2a and
shows
the sliding contact in a more detailed form.
Specific examples of applications for the present invention follow. The
invention is
however by no means restricted to these possible uses. Preferred areas of use
for
annular chromatographs according to this invention include for example
separations
of base metals and noble metals as well as numerous types of separation and
purification stages in biotechnology.
Example 1: Cation exchanger - exclusion giel:
Ion-exchange I size-exclusion chromatogiraphy
Separation of platinum group metals by liquid chromatography with
simultaneous removal of base metals
CA 02301570 2000-02-23
8
The platinum-group metals rhodium, palladium, platinum and iridium can be
separated from each other continuously by means of preparative annular
chromatography. Base metals present at the same time, like for example iron,
copper, nickel or cobalt, can also be separated continuously from the noble
metals.
Chromatographic separation of the platinum-group metals using exchanger gels
(e.g.
Sephadex, Toyopearl or Biogel) has already been described in US-A-4.885.143.
Concentrated noble-metal solutions from the leaching processes of separator
units
normally also contain base metals such as iron, copper or nickel at different
concentrations.
After the leaching process the noble-metal solution is usually present in
concentrated
hydrochloric acid. Because of the high chloride concentration the noble metals
are
all present as anionic chlorocomplexes. Base metals have the characteristic of
binding themselves to a cation exchanger under certain conditions, whereas the
noble metals run through the cation exchanger as anionic complexes without any
retention. This characteristic can be used to separate the accompanying base
metals from the noble metals in an initial process stage and then to separate
out the
various noble metals in a second process stage. With the P-CAC system of the
present invention this can be done using a very simple set-up of equipment.
A column, as shown in Fig. 1a), is filled up to a certain height (exact test
conditions in
Tables 1 and 2 below) with an exclusion gel 2 (e.g. Sephadex, Toyopearl or
Biogel)
and glass beads 5 (d = 150-240 Vim) are arranged in a layer on top of it; a
layer of
cation exchanger 1 (e.g. Dowex, Purolite, Amberlite) is placed over this layer
of glass
beads 5 and is in turn finally covered with a layer of glass beads 8.
The feed solution is pumped into the annular column at the radial position
0°. The
feed duct 15 is immersed in the top layer of glass beads 8. 0.5-1 m HCI is
used as
main eluent. The noble metals run through the layer of cation exchanger 1
without
any interaction and are then separated from one another in the layer of
exclusion gel
CA 02301570 2000-02-23
9
2. The various fractions of rhodium (Rh), palladium (Pd), platinum (Pt) and
iridium
(Ir) can be collected separately at the end of the column.
The base metals adsorb on the cation exchanger and are stripped with a step
eluent
(2 m or higher HCI) at the radial position, at which the last noble metal (Ir)
elutes.
The 2 m HCI desorbs the base metals from the cation exchanger and drives them
to
the layer of exclusion gel. There the base metals do not have any interaction
at all
with the stationary phase. The base metals can now we recovered as a combined
fraction.
Specifically a solution (6 m HCI) with the following concentration is
separated
continuously into the various noble metals and into a fraction containing the
base
metals.
Table 1
Concentration of metals in the feed solution
Pt: 30 g/l Pd: 15 gll Rh: 3 g/l Ir: 1 gll
Fe: 4 gll Co: 4 gll Ni: 4 gll Cu: 4 g/l
A 26 cm high layer of Sephadex G-15 was covered with a 5 cm layer of glass
beads.
A 5 cm thick layer of Dowex 50-W X8 was added on top of this layer and was in
turn
covered with a layer of glass beads. The solution was eluted with 0.5 m HCI.
The
noble metals passed through the layer of cation exchanger 1 without any
interaction
and became separated on the layer of Sephadex 2. Four bands of different
colours
formed: Rh band (red), Pd band (brown), Pt band (yellow) and Ir band (brown).
The step eluent (2 m HCI) for stripping the base metals was added at a radial
position of 270° relative to the feed inlet. The step eluent desorbed
the base metals
from the cation exchanger and a greenish-yellow band formed and ran through
the
column without any interaction. The base metals were recovered as a combined
fraction after the iridium fraction.
CA 02301570 2000-02-23
Table 2
Test conditions
Flow-rate: Flow-rate: Flow-rate: Rate of Cross-sectional
top eluent step eluent feed rotation area of annular
gap
18 mllmin 4 mllmin 0.4 mllmin 125Ih 24.4 cm2
Height of column: 41 cm of which
26 cm Sephadex G-15
5 cm glass beads (d = 150-240 cam)
5 cm Dowex 50 W-XB in the H+ form
5 cm glass beads (d = 150-240 Vim)
Top - Eluent
Feed I I I '
Gta.ss beds
. . .
:.,~:. ~ "vrT.~;'
: ~, i ~
: .v~! '
~ i
~
~ '
; ~
a
f ~
~
~
'
.: ~ .., . ~ ,
r . .
r r, . , ..
S " ,
.r ~ r
. :r
. '
'
';'
l
; '.
. .
.
'
. ,
- ' ;", ., ~ ., ~ . .
G~i , :'.::; . , , ,
. ~~';t;~ ry,
a ~..,. . ." '~.. '~~'r'
;
, s i .
:
;
;
,
a
~;
''.
on. " ~ .:;
,, . , ." , ".
, . , , w " ,
., ,
~~..~,. r
.s
; .,
:;,...
.;s,
. ~.. ~
. . yr
. ; ~..,
-'
v
~
~.~ , ~ ~l, .
GrlGIV1V.IV.. .. . ~ .w../T ,
~ ... ,
9 ~ '..lo 'a. ,.~:,'j ~1 ...~.1~
jW . . V.. r ... I. , y ,"
~ 1 ... X7,.17 - ,
~
, ; 1
. .. :w ;:
' . ~
' ~ ~'
;;: ;
=
:
~~ '
'~
:
~~
. ., .~; .
.; , ,
: , .,
. .
.,
:
.
.
.., ;;...,,
~:~:
. ' .'w
.. .. W..:, ..'1''.. .,.... - _.. ,1 'JW~
~t.. .... .. , . ' . ~ . r _-~ W
Gta.ss beads
.i. ..'V: h".y~'wr'. i.~~4
>,:,. 4..i.:~": ~.~
. .~r
; '
' .~:''w~'~
N
M
',r.
',
'
I
,~; .. ,
v ' ,
N ' . ,
. .
' a
, ~ ~ ",
' ~t
,
.vY,l
Yw
, .
w .
~w
i., .
.:.. '
'4
~1.~
'
r . !
. . "
.,~ . .
j:r~ ..c i.j,
.y ~ ~. . . ~r , i
,
"
v-n.': , . .r'
~'
. . ,
-,''~.. < . , ,., 'i
. .
5.
. . .
. . , . . .n.s .i
. ~ , ~' .j
,J . ... ~ ~~~ ~ .; .1 ~I. , h' .
' :
~ "
EXCLU .; '. .. q
LOR ..sa' " y
' ~~'
, .A
>: ~; ,,. '
.
S , , :N',
, f~ ' ,
r,; ::'~ ~,
<-'v " ,, '~
: ::
:
~;;
'
E.( .;, , , ,
. . ' ,.
. , ' ,
< ..
' , ,
'"'
~
I
, :. v
: ' ,:i, :' ,
'~ ,~r
,;s';;;.;
~
;:'M.
~
.,." , - . ..~
. , w, .. ,.
. ,,,;,~, ,.>.'. _ '
' , . ~
' '
.. . ;.. ..
~ ~_ .. .v
~~ ': s::'r' '" ' ~
;
;' :. .. ~ ,
. ' . u7r , .
f ; 1.-r~
l ' . '
~J r
.' t, . , ,
~ I' ' ~ ~
.w4'
~ , ,
,.
~
,
. ' ., ., ,
' rr '.t .... .. . -'
.
' '
~...
0 1 1 ~ ~ i 360
Rh Pd Pt Ir ~cx,se ncctaLs
If there are also Ru and Os in the mixture, Ru elutes between Rh and Pd and Os
after Ir (see Example 2).
CA 02301570 2000-02-23
11
Example 2: Adsorption resin - Exclusion gel
Adsorption I size-exclusion chromatographY
Selective separation of gold from a noble metal solution and simultaneous
separation of platinum-group metals
The exclusion gel is filled into the P-CAC (at least up to half the height); a
separation
layer (glass beads or membrane) is placed over the exclusion get, and the
adsorption
resin (Amberlite XAD7 or Purolite MN 200) is added on top of this layer.
A noble metal solution containing all platinum-group metals (or at least two
of them)
and gold is used as feed for the P-CAC. Gold is adsorbed selectively by the
adsorber resin, whilst the other platinum-group metals flow through the layer
of
adsorber without being retarded and are separated out on the exclusion gel.
Following elution of the last component the gold is stripped from the adsorber
resin
with a step eluent.
~,~SSd rbU~ r'G S ~ rt
exc(,useon gel
ExamJ~le 3: Cation exchanger - Anion exchanger
Ion-exchange chromatography
30 Separation of base metals from a Rhllr solution and subsequent separation
of
rhodium and iridium
Feed Efuent Step-Eluent
CA 02301570 2000-02-23
12
The anion exchanger is filled into the P-CAC up to at least half the height. A
separation layer (membrane or glass beads of appropriate size) is added on top
of
the anion exchanger; the ration exchanger is added on top of the separation
layer up
to the maximum filling height. A metal solution containing rhodium and iridium
as
well as base metals such as iron, nickel, cobalt or copper is pumped into the
annular
column as feed. The base metals adsorb on the ration exchanger, whereas
rhodium
and iridium pass through as far as the anion exchanger. Ir (IV) adsorbs on the
anion
exchanger and rhodium passes through. After all the rhodium has been eluted
the
base metals are eluted at this angle position with step eluent 1. Following
this elution
Ir (I~ is reduced to Ir (III) with step eluent 2.
Feed Eluent Step1 Step2
Ga t i o n arch.e~r~.se c
aatior~ exchart,~er
Example 4: Anion exchanger - Exclusion gel
Ion-exchange I size-exclusion chromato rq, aphy
Separation of gold cyanide from a noble metal solution with simultaneous
separation of the noble metals
CA 02301570 2000-02-23
13
The P-CAC is filled with an exclusion gel up to at least half the filling
height, then
covered with a separation layer, which is in turn covered with a layer of
anion
exchanger. A noble metal solution containing both gold as a cyanide complex
and
platinum-group metals is fed onto the annular gap as feed solution. The gold
cyanide
binds itself to the anion exchanger as an anionic complex; the remaining
platinum-
group metals pass through the anion exchanger without interaction and are
separated on the exclusion gel. After the angle at which the last platinum-
group
metal (iridium) leaves the column a stripping solution is introduced into the
annular
gap to desorb the gold.
A combination of anion exchangers and exclusion gels is also conceivable in
biotechnology, e.g. to obtain and purify monoclonal antibodies. Here
pyrogenes,
nucleic acids and proteases can be removed by the anion exchanger; the salts
of the
buffer solution can then be removed in the exclusion gel.
a,ni,dn. excjtert~er
exC~usi,o2 ~e~
Feed Eluent Strip
CA 02301570 2000-02-23
14
Example 5: Affinit~gel - Exclusion qel
Affinity I size-exclusion chromatograehy
Separation of base metals from a noble metal solution with subsequent
separation of the noble metals
A P-CAC column is filled with an exclusion gel up to at least 50% of the
filling height,
covered with a separation layer and charged with Superlig gels up to the final
filling
height (Superlig gels are gels with crown ether as functional group; trademark
of IBC
Advanced Technology). A noble metal solution containing a high proportion of
base
metals is introduced as feed onto the annular gap of the P-CAC. The base
metals do
not have any affinity to the Superlig gel and therefore elute throughout the
entire
column and can be collected as a combined fraction. The noble metals are
stripped
by the Superlig gel and become separated on the exclusion gel in the sequence
Rh -
Pd-Pt-Ir.
Similar applications are conceivable with stationary phases which complexing
agents
(such as dithizones, dehydrodithizones, hydroxyquinolinates, pyridylazo-
naphtholates
etc.) have united as reactive groups. With these stationary phases, however,
it is
30 only possible to separate the noble metals qualitatively from one another
under
difficult conditions (a strip eluent must be used for each metal). It is
therefore not
Feed Eluent Strip
CA 02301570 2000-02-23
practicable to cover these stationary phases with yet another phase, since
this would
make the elution conditions more difficult and the process uneconomical.
Example 6: Cation exchanger - Anion exchanger
5 Ion-exchange chromatogiraphy
Two-stage purification of a cellulase
The anion exchanger as top layer (Pharmacia Mono Q) is used for preliminary
purification of the cellulase; a tris HCI buffer is used for elution. The
fraction obtained
with the cellulase in it is then fractionated further in a cation exchanger as
bottom
10 layer (Pharmacia Mono S) with an acetate buffer as eluent. A very high
resolution
can be achieved with the cation exchanger, so the cellulase can be obtained in
a
pure condition.
15 Example 7: Cation exchanger - Exclusion c~el
Ion-exchangie / size-exclusion chromatography
Obtaining of monoclonal IgG2B from a fermentation broth
Preliminary purification and concentration of the antibody is achieved with
the cation
exchanger as top phase (Pharmacia S Sepharose High Performance). For this MES
is used with NaCI as buffer. The pool (fraction which also contains the
antibody) is
then separated from dimersloligomers and from transferrin by means of an
exclusion
gel (Superdex 200) as bottom phase with a sterile sodium chloride buffer.
Example 8: Anion exchanger - Exclusion gel
Ion-exchange / size-exclusion chromatography
Purification of Human Epidermal Growth Factor (h-EGF), expressed as
extracellular product through Saccharomyces cerevisiae
The anion exchanger (Pharmacia Q - Sepharose) as top phase is used for
preliminary purification (purification based on charge) of the growth factor;
the bottom
CA 02301570 2000-02-23
16
phase, the exclusion gel (Pharmacia Superdex 75), is used for final
purification of the
growth factor.
Example 9: Hydrophobic interaction chromatography CHIC) giel - Exclusion gel
Affinity / size-exclusion chromatography
Obtaining of human pituitary prolactin
The upper stationary phase (HIC gel, Pharmacia Phenyl Sepharose CL) is used
for
initial purification and concentration of the prolactin (concentration by a
factor of 7).
In the second stage the prolactin undergoes final purification on an exclusion
gel
(e.g. Pharmacia Sephadex G100).
Example 10: Cation exchanger- Hydrophobic interaction chromatography (HIC) gel
Ion-exchange / affinity chromatography
Obtaining of a2-macroglobulin
With the cation exchanger (upper stationary phase, e.g. Pharmacia Mono S) it
is
possible to obtain a protein pool containing proteins with the same charge,
including
the macroglobulin. The macroglobulin is purified in the second layer, the HIC
gel
(e.g. Pharmacia Phenyl Sepharose), until there is homogeneity.
Example 11: Anion exchanger- Hydrophobic interaction chromato~raQ,hy (HIC) gel
Ion-exchange I affinity chromato rq aphy
Purification of a recombinant reverse transcriptase of HIV
The fermentation broth is separated according to charge in the upper phase,
the
anion exchanger (e.g. Pharmacia DEAE Sepharose). The pool containing the
transcriptase passes through the second layer, the HIC gel (e.g. Pharmacia
Phenyl
Sepharose High Performance). The transcriptase is obtained in pure form as a
result
of the differences in hydrophobicity after this layer.
CA 02301570 2000-02-23
17
Example 12: Cation exchanger - Anion exchanger, with one of the stationary
phases
in displacement mode
Ion-exchange I displacement chromatography
Protein separation
An anion exchanger (e.g. Pharmacia Mono Q) is filled into an annular
chromatograph. An inert layer, e.g. glass beads or a membrane, is placed on
top of
this anion exchanger; a cation exchanger (e.g. Pharmacia Mono S) is then added
in
turn on top of this. A mixture of different proteins is separated on the
cation
exchanger because of the different charges; the separated fractions are
concentrated
on the anion exchanger using a displacement reagent (displacer) (e.g.
Nalcolyte). In
this way separation can be combined with simultaneous concentration.
Example 13: Exclusion gel - Electrophoretic gel
Size-exclusion chromatography / electrophoresis
Protein separation
The electrophoretic gel forms the lower layer in the P-CAC and is covered with
an
exclusion gel. If proteins with different charges and different pl values are
now
brought onto the exclusion gel they separate according to size. The
electrophoretic
layer then takes care of further separation of the proteins according to their
charge.
Example 14: Exclusion gel - Cation exchanger with the cation exchanger in
displacement mode
Size-exclusion / ion-exchange chromatography
Working-up of an amino-acid mixture with simultaneous separating-off of
proteins
The upper stationary phase, an exclusion gel (e.g. Pharmacia Sephadex G25) is
used to separate off proteins (e.g. albumins) from a feed solution containing
the
amino acids L-glutamic acid, L-valine and L-leucine. Separation in the
exclusion gel
yields a fraction containing the proteins and a fraction containing the amino
acids. In
CA 02301570 2000-02-23
18
the second layer the amino acids are separated on a cation exchanger (e.g.
Dowex
50 W-X8) with a displacer (e.g. 0.1 n NaOH) and simultaneously concentrated.
The
amino acids are obtained in the order glutamic acid - valine - leucine. A
regenerating
solution (e.g. dilute H2S04) has to follow the displacer in order to bring the
gel bed to
the initial state.
The example of displacement elution is not limited to use in cation exchange,
As can be seen from the above description, the present invention provides a
new
annular chromatograph with a particulate bed comprising several different
zones
which can be used to carry out several types of chromatographic separation
continuously in a single stage and thereby more quickly and more economically
than
has previously been possible according to the state of the art.