Note: Descriptions are shown in the official language in which they were submitted.
CA 02301959 2000-03-22
SMB
Peptide Havinq~ Affinity for Coagulation Factor VIII
The present invention relates to a peptide having binding affinity for factor
VIII, a support material to which the peptide according to the invention is
bound, a diagnostic agent containing the peptide according to the invention,
the use of the peptide for the labeling, identification or purification of
factor
VIII, . and a process for the preparation of the peptide according to the
invention synthesized in a liquid or solid phase system.
A method perfectly Established in the prior art for the purification of, in
particular, complex structures such as proteins is affinity chromatography
(Yannis Chonis; ProcEas Affinity Chromatography, in: Separation Processes
in Biotechnology, Ed. J.A. Asenjo, pages 401 to 494, Marcel Dekker, New
York 1990; S.R. Narayanon, Preparative affinity chromatography of pro-
teins, J. Chromatogr. A, 658 (1994), 237 to 258). In "Bioorganic & Medici-
nal Chemistry", Vol. 4, No. 5, pp. 699 to 708, 1996, Huang, Ping Y. et al.
describe the use of peptides which can be employed for the affinity purifica-
tion of von Willebrancl factor. Von Willebrand factor is a component of blood
plasma and plays an important role in the stabilization of factor VIII.
Factor VIII itself is also a valuable plasma component which is of thera-
peutical interest, in particular, namely for repairing disorders in the blood
clotting cascade in which factor VIII plays an important role. In addition to
factor VIII preparation by recombinant methods, preparation of factor VIII
from blood plasma still plays an important role.
CA 02301959 2000-03-22
_2_
A wide variety of methods for the preparation of factor VIII by chromato-
graphic methods are known. This includes the use of immunoaffinity
chromatographic methods wherein antibodies against factor VIII are
immobilized on a support and samples containing factor VIII are contacted
with this support material so that factor VIII can be isolated from the
respective samples by immunoaffinity reactions.
However, in the latter method, there is a disadvantage, in particular, in that
the antibodies are relatively large molecules which occupy a large portion of
the surface of the support material and thus lead to a limitation of capacity.
Moreover, it is not trivial to bind the antibodies to the surface of this
material and yet retaun and present the specific binding sites; thus, in the
immobilization of antibodies, additional capacity is lost by inactivation of
the
affinity ligand. In addition, contaminations may occur by bleeding of the
antibodies from the separation column.
It has been the object: of the present invention to provide a material which
facilitates the purification of factor VIII.
According to the invention, this object is achieved by peptides having the
features of claim 1.
The peptides according to the invention have the structure
Rl-X-R2,
wherein Rl is NHZ or a peptide, preferably containing not more than 20
amino acid units, R2 is COOH or a peptide, and X is a peptide which is at
least a tripeptide, preferably a hepta- to dodecapeptide, which undergoes
affinity binding to factor VIII.
CA 02301959 2000-03-22
-3-
Thus, in the structural element according to the invention, R1 represents
the N-terminal end of a peptide represented by the structural element X,
whereas RZ represents the carboxy terminal end of a peptide defined by X.
It will be understood by one skilled in the art that the structural elements
include their respective salts and derivatives, such as N-modified deriva-
tives or derivatives modified at the carboxy terminus.
Amino acids which can be employed for the preparation of the peptide
according to the invention include both the naturally occurring amino acids
and non-proteinogeniic amino acids. As a rule, they are a-amino acids.
Structural variants of the amino acids may be those which have modifica-
tions in the side chain, cyclic peptides, branched or mixotropic peptides.
The structural element X comprises at least three amino acids which are
connected by peptide bonds; preferably, the structural element X is a
hepta-, octa- or nonapeptide, more preferably an octapeptide to decapep-
tide, which is characterized by binding to factor VIII. Particularly preferred
are the octapeptides explicitly stated in the sequence listing under the
sequence identification numbers SEQ ID N0. 1 to SEQ ID NO. 94. The
particularly preferred ~octapeptides having the stated structures according to
SEQ ID NOS. 1 to 94 are capable of specifically binding to factor VIII.
According to the invention, those peptides are preferred which contain the
structural element X :_ -R3-R4-RS-R6-R'-R8-R9-Ri°- wherein R3 = V or E,
R4
=M,WorY,RS=IorK,R6=KorS,R'=C,EorW,R8=EorF,R9=Yor
E, Rl° = C or F, and Rl and R2 have the indicated meanings. The
latter
peptides will bind to human or recombinant factor VIII. Especially preferred
are peptides in which R' = W, R8 = E, R9 = E or Y, Rl° = F or C, and
Ri, R2
and R3 to R6 have i:he indicated meanings. This includes the peptides
according to the invention having the SEQ ID NOS: 66, 72, 78, 84, 89 and
CA 02301959 2000-03-22
-4-
90. The latter peptides have a prominent affinity for both recombinant
factor VIII and factor VIII prepared from plasma.
The octapeptides having the SEQ ID NOS. 61, 66, 71, 72, 76, 77, 78, 81 to
84, 89 and 90 are characterized by binding to pdFVIII (plasma-derived
factor VIII). The peptide having the SEQ ID NO. 61 does not bind to
recombinant factor VIII. The octapeptides having the SEQ ID NOS. 56 to
58, 66, 68, 71, 72, 76 to 78, 81 to 84 and 87 to 90 do bind to recombinant
factor VIII; of these, the octapeptides having the SEQ ID NOS. 66, 71, 72,
76 to 78, 81 to 84, 89 and 90 also bind to plasma-derived factor VIII.
According to the invE~ntion, the octapeptides having the SEQ ID NOS. 66,
72, 78, 84, 89 and 90 are particularly preferred.
The peptides having the SEQ ID NOS. 66 and 90 to 94 are particularly
suitable for the affinity chromatography of rFVIII (recombinant factor VIII),
and the peptides 66, 72 and 91 to 94 are particularly suitable for the
affinity chromatography of pdFVIII.
According to the invention, these peptides may also be used for the prepa-
ration of a support material according to the invention. This support mate-
rial has a surtace with peptides according to the invention bound thereto; it
is the subject matter of claims 8 to 9.
The peptides according to the invention are bound to the surtace of the
support material according to the invention, preferably by chemical bond-
ing. "Chemical bonding" as used herein includes both covalent and ionic,
hydrophobic and/or complex interactions.
The support material preferably consists of inorganic or organic, especially
polymeric, material. llseful materials include the polymeric materiais which
usually can be employed per se in the chromatography of biopolymers.
CA 02301959 2000-03-22
-5-
The peptides according to the invention are preferably bound to the surtace
of the support maternal through spacers (arms). Such spacers are known to
those skilled in the art. The spacers are preferably those which will not
interfere with the afr~inity binding between factor VIII and the peptides in
the chromatographic separation process.
Spacers usually consnsts of linear low-molecular weight hydrocarbon chains
provided with functional groups at both ends. A summary of the state of the
art is found in G.T. Hermanson, A.K. Mallia and P.K. Smith; Immobilized
Affinity Ligand Techniques, Academic Press, Inc., San Diego, New York;
Boston, London, Sydney, Tokyo, Toronto, 1992. One end is bound to the
solid support while the other end serves for binding the peptide ligand. The
hydrocarbon chains may also be modified. In particular, useful spacers
include diaminodipropylamine (DADPA) (3,3'-iminobis(propylamine)),
succinic acid (as the anhydride), 6-aminocaproic acid, 1,3-diamino-2-
propanol, 1,6-diaminohexane (DH) and/or ethylenediamine (EDA). Further,
amino acids, peptides., sugars and other polyols may also serve as spacers.
Iminodiacetic acid or' lysine may also be used as spacers. For example,
lysine is bound to a aupport as Fmoc-Lys(Fmoc)-OH or Fmoc-(Lys)-(Boc)-
OH. Due to the introduction of the protective groups, lysine is bound to the
support at the carboxy terminus. By selectively cleaving the protective
groups, free amino acids are obtained to which at least one peptide can be
bound. The amino groups of lysine may also be blocked by other protective
groups.
As chromatographic supports, there may be used, in particular, polymers
having a hydrophilic surface, e.g., so-called tentacle materials. It is also
advantageous to imrnobilize the peptides according to the invention on
compact porous disks and/or membranes, both preferably having a hydro-
philic surface. Corresponding devices are described in WO-A-96/06158.
CA 02301959 2000-03-22
-6-
Due to the fact that the peptides according to the invention will interact
with factor VIII, they peptide compounds according to the invention are
suitable as diagnostic agents for factor VIII. Thus, the diagnostic agent
according to the invention contains at least one peptide according to the
invention or a support material according to the invention to which at least
one peptide according to the invention is bound, and optionally auxiliary
agents.
To recognize that a reaction has occurred between a peptide according to
the invention and factor VIII, the peptide according to the invention is
labeled. As the label, there may be used, for example, a radioactive label or
a label using fluorescent ligands, the avidin/streptavidin system, or en-
zymes which can cause color reactions, as generally known in the ELISA
technique.
The peptides according to the invention are suitable for the labeling,
identii=ICation or purification of factor VIII.
The peptides according to the invention can be prepared by methods well
established in the peptide chemistry, especially according to the Merrifield
solid-phase synthesis or in liquid phase.
Examples
Exams 1~ a 1
Affinity chromatography
From membranes on which the respective peptides have been immobilized,
30 disks each having a diameter of 11 mm were punched out. They were
packed in an HR 10 column (Pharmacia Biotechnology, Uppsala, Sweden)
and fixed between two plugs.
CA 02301959 2000-03-22
_ 7 _
For all chromatographic experiments, the chromatographic system ProSys
(Biosepra Inc., Marlborough, USA) was used. PBS buffer, pH 7.0, was used
as an equilibration and washing buffer. For elution, 0.1 M glycine buffer, pH
3.0, was used. In all experiments, the flow rate was 0.1 ml/min.
pdFVIII
Lyophilized factor VIII from plasma was dissolved in 11 ml of distilled water
to a concentration of 90 units/ml. Of this, 4 ml each was charged onto the
column with a flow rate of 0.1 ml/min. Subsequently, the affinity supports
were washed with PBS buffer, pH 7.0, until the UV signal (280 nm) again
reached the baseline" The bound material was eluted with 0.1 M glycine, pH
3Ø The collected fractions were analyzed by SDS PAGE under reducing
conditions and by WEatern blotting. Thus, peptides and the ~iAla-~iAla blank
were examined for their capability of binding factor VIII from the Octavi
preparation.
rFVIII
The binding of recombinant factor VIII was examined using Kogenate~
(Bayer, Germany). Four hundred microliters each of the preparation (200
units) was diluted with 1,400 NI of distilled water and then charged onto the
affinity support with a flow rate of 0.1 ml/min: Washing was again per- .
formed with PBS, pH 7.0, and the following elution was performed with
0.1 M glycine, pH 3Ø The fractions were analyzed by SDS PAGE under
reducing conditions and by Western blotting.
SDS PAGE
The percolate and the eluates of all runs were collected and analyzed by
SDS PAGE under reducing conditions and by Western blotting.
CA 02301959 2000-03-22
_ 8 _
For the separation, 4 to 20% tris-glycine gels from Novex were used. SDS
sample buffer (1.5 M Tris-HCI, pH 8.45, 1.2 ml of glycerol, 0.4 g of SDS,
0.1% Coomassie blue, 0.1% phenol red per 10 ml) and 5% (3-mercapto-
ethanol were added to the samples, followed by incubation over night at
4 °C. After the separation, the gels were subjected to silver staining
and
recorded by scanning.
Western Blot
After completion of the electrophoretic separation, the gels were electro-
blotted to a nitrocellulose membrane at a constant current of 200 mA for
two hours. Then, the membrane was blocked with PBS + 3% BSA for two
hours. After washing with PBS buffer, addition of the anti-FVIII antibody
mixture (Chemicon International Inc., MAB038, Sera-Lab MAS 530, MAS
531, MAS 532) was performed for two hours. After washing with PBS,
incubation with anti-mouse IgG/alkaline phosphatase conjugate (1:1000;
Sigma Chemicals, A-3438) was pertormed for one hour. The blot was
developed with 0.1 M tris, pH 9.2, NBT and BCIP.
The peptides SEQ ID~ NOS. 55 to 90 were immobilized on a membrane.
After equilibration with PBS buffer, pH 7.0, one membrane was incubated
with factor VIII Octavi~ (240 units in 10 ml PBS, pH 7.0, with 1% BSA),
another membrane was incubated with Kogenate~ (250 units in 10 ml of
PBS, pH 7.0, with 1°io BSA), and one membrane was incubated only
with
PBS, pH 7.0, with 1% BSA, for two hours. The membranes occupied by the
peptides were carefully washed with PBS buffer, pH 7.0, and subsequently
contacted with the anti-FVIII antibodies (Chemicon International Inca,
MAB038, Sera-Lab MAS 530, MAS 531, MAS 532) for one hour. After
washing and incubation with anti-mouse-IgG/alkaline phosphatase conju-
gate (1:1000, Sigma Chemicals, A-4338), the membranes were developed
using NBT and BCIP iin 0.1 M tris buffer, pH 9.2. Factor VIII binding pep-
CA 02301959 2000-03-22
_g_
tides appeared as colored spots. The control membrane (only with PBS, pH
7.0, without factor VI:fI) was compared with the two other membranes.
ExamJ~le 2
Affinity chromatography with the peptides
The affinity chromatography was performed as described in Example 1.
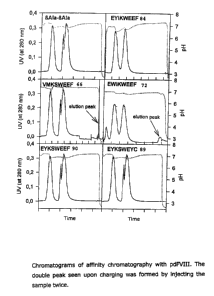
Affinity chromatography with pdFVIII
When the (~3Ala)Z coni:rol membrane is used, only a very small elution peak
can be detected (see Figure 1). Also in the silver staining of the SDS PAGE
under reducing conditions, only rather weakly colored bands appear. The
Western blot of the eluate is slightly positive.
The peptides SEQ ID NOS. 66 (VMKSWEEF) and 72 (EWIKWEEF) (Figure 2)
exhibit larger elution peaks and more intensely colored bands in the SDS
PAGE under reducing conditions (see Figure 3) and silver staining. In both
cases, the Western blot is positive.
Binding of recombinant factor VIII from Kogenate~ from Bayer
As can be seen from the chromatogram (Figure 4), the control membrane
with immobilized ~iAla-~iAla binds recombinant factor VIII but very slightly.
The Western blot of the eluate was positive.
The elution peaks from the peptides VMKSWEEF (SEQ ID NO: 66),
EYKSWEEF (peptide 90) in Figure 4 are clearly larger than that of the ~Ala-
~iAla control membrane. In both cases, the Western blot of the eluates is
positive (Figure 4).
CA 02301959 2000-OS-OS
-13-
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
(A) NAME: Octapharma AG
(B) STREET: Seidenstrasse 2 / Postfach
(C) CITY: Lachen
(E) COUNTRY: Switzerland
(F) POSTAL CODE (ZIP): CH-8853
(ii) TITTLE OF INVENTION: Peptide having affinity for
coagulation factor VIII
(iii) NUMBER OF SEQUENCES: 94
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Swabey Ogilvy Renault
(B) STREET: 1981 McGill College, Suite 1600
(C) CITY: Montreal
(D) STATE: Quebec
(E) COUNTRY: Canada
(F) ZIP: H3A 2Y3
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.30 (EPO)
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: 2,301,959
(B) FILING DATE: 22-MAR-2000
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Kevin P. Murphy
(B) REGISTRATION NUMBER: 3302
(C) REFERENCE/DOCKET NUMBER: 6603-144 KPM/ld
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 514-845-7126
(B) TELEFAX: 514-288-8389
(C) TELEX:
(2) INFORMATION FOR SEQ ID N0: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
Gly Cys Val Ser Gly Cys Leu Cys
1 5
CA 02301959 2000-OS-OS
-14-
(2) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 2:
Cys Val Ser Gly Cys Leu Cys Pro
1 5
(2) INFORMATION FOR SEQ ID N0: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: S Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 3:
Val Ser Gly Cys Leu Cys Pro Pro
1 5
(2) INFORMATION FOR SEQ ID N0: 4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOG't: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 4:
Ser Gly Cys Leu Cys Pro Pro Gly
1 5
(2) INFORMATION FOR SEQ ID N0: 5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
Gly Cys Leu Cys Pro Pro Gly Met
1 5
CA 02301959 2000-OS-OS
-15-
(2) INFORMATION FOR SEQ ID NO: 6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 6:
Cys Leu Cys Pro Pro Gly Met Val
1 5
(2) INFORMATION FOR SEQ ID NO: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 7:
Leu Cys Pro Pro Gly Met Val Arg
1 5
(2) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 8:
Cys Pro Pro Gly Met Val Arg His
1 5
(2) INFORMATION FOR SEQ ID N0: 9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
Pro Pro Gly Met Val Arg His Glu
1 5
CA 02301959 2000-OS-OS
-16-
(2) INFORMATION FOR SEQ ID N0: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 10:
Pro Gly Met Val Arg His Glu Asn
1 5
(2) INFORMATION FOR SEQ ID N0: 11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 11:
Gly Met Val Arg His Glu Asn Arg
1 5
(2) INFORMATION FOR SEQ ID N0: 12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Arnino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 12:
Met Val Arg His Glu Asn Arg Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 13:
Val Arg His Glu Asn Arg Cys Val
1 5
CA 02301959 2000-OS-OS
-17-
(2) INFORMATION FOR SEQ ID NO: 14:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 14:
Arg His Glu Asn Arg Cys Val Ala
1 5
(2) INFORMATION FOR SEQ ID NO: 15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 15:
His Glu Asn Arg Cys Val Ala Leu
1 5
(2) INFORMATION FOR SEQ ID N0: 16:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: S Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 16:
Glu Asn Arg Cys Val Ala Leu Glu
1 5
(2) INFORMATION FOR SEQ ID N0: 17:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 17:
Asn Arg Cys Val Ala Leu Glu Arg
1 5
CA 02301959 2000-OS-OS
-18-
(2) INFORMATION FOR SEQ ID NO: 18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 18:
Arg Cys Val Ala Leu Glu Arg Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 19:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 19:
Cys Val Ala Leu Glu Arg Cys Pro
1 5
(2) INFORMATION FOR SEQ ID NO: 20:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Atnino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 20:
Val Ala Leu Glu Arg Cys Pro Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 21:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 21:
Ala Leu Glu Arg Cys Pro Cys Phe
1 5
CA 02301959 2000-OS-OS
-19-
(2) INFORMATION FOR SEQ ID NO: 22:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 22:
Leu Glu Arg Cys Pro Cys Phe His
1 5
(2) INFORMATION FOR SEQ ID NO: 23:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 23:
Glu Arg Cys Pro Cys Phe His Gln
1 5
(2) INFORMATION FOR SEQ ID NO: 24:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 24:
Arg Cys Pro Cys Phe His Gln Gly
1 5
(2) INFORMATION FOR SEQ ID NO: 25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 25:
Cys Pro Cys Phe His Gln Gly Lys
1 5
CA 02301959 2000-OS-OS
-20-
(2) INFORMATION FOR SEQ ID N0: 26:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 26:
Pro Cys Phe His Gln Gly Lys Glu
1 5
(2) INFORMATION FOR SEQ ID NO: 27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 27:
Cys Phe His Gln Gly Lys Glu Tyr
Z J
(2) INFORMATION FOR SEQ ID NO: 28:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 28:
Phe His Gln Gly Lys Glu Tyr Ala
1 5
(2) INFORMATION FOR SEQ ID NO: 29:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 29:
His Gln Gly Lys Glu Tyr Ala Pro
1 5
CA 02301959 2000-OS-OS
-21 -
(2) INFORMATION FOR SEQ ID N0: 30:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 30:
Gln Gly Lys Glu Tyr Ala Pro Gly
1 5
(2) INFORMATION FOR SEQ ID NO: 31:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: S Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIP~PION: SEQ ID NO: 31:
Gly Lys Glu Tyr Ala Pro Gly Glu
1 5
(2) INFORMATION FOR SEQ ID N0: 32:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 32:
Lys Glu Tyr Ala Pro Gly Glu Thr
1 5
(2) INFORMATION FOR SEQ ID NO: 33:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 33:
Glu Tyr Ala Pro Gly Glu Thr Val
1 5
CA 02301959 2000-OS-OS
-22-
(2) INFORMATION FOR SEQ ID N0: 34:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 34:
Tyr Ala Pro Gly Glu Thr Val Lys
1 5
(2) INFORMATION FOR SEQ ID N0: 35:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 35:
Ala Pro Gly Glu Thr Val Lys Ile
1 5
(2) INFORMATION FOR SEQ ID NO: 36:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 36:
Pro Gly Glu Thr Val Lys Ile Gly
1 5
(2) INFORMATION FOR SEQ ID N0: 37:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 37:
Gly Glu Thr Val Lys Ile Gly Cys
1 5
CA 02301959 2000-OS-OS
-23-
(2) INFORMATION FOR SEQ ID N0: 38:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 38:
Glu Thr Val Lys Ile Gly Cys Asn
1 5
(2) INFORMATION FOR SEQ ID N0: 39:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 39:
Thr Val Lys Ile Gly Cys Asn Thr
1 5
(2) INFORMATION FOR SEQ ID N0: 40:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 40:
Val Lys Ile Gly Cys Asn Thr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 41:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 41:
Lys Ile Gly Cys Asn Thr Cys Val
1 5
CA 02301959 2000-OS-OS
-24-
(2) INFORMATION FOR SEQ ID N0: 42:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 42:
Ile Gly Cys Asn Thr Cys Val Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 43:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 43:
Gly Cys Asn Thr Cys Val Cys Arg
1 5
(2) INFORMATION FOR SEQ ID NO: 44:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 44:
Cys Asn Thr Cys Val Cys Arg Asp
1 5
(2) INFORMATION FOR SEQ ID N0: 45:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 45:
Asn Thr Cys Val Cys Arg Asp Arg
1 5
(2) INFORMATION FOR SEQ ID NO: 46:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
CA 02301959 2000-OS-OS
-25-
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 46:
Thr Cys Val Cys Arg Asp Arg Lys
1 5
(2) INFORMATION FOR SEQ ID N0: 47:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 47:
Cys Val Cys Arg Asp Arg Lys Trp
1 5
(2) INFORMATION FOR SEQ ID NO: 48:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 48:
Val Cys Arg Asp Arg Lys Trp Asn
1 5
(2) INFORMATION FOR SEQ ID NO: 49:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 49:
Cys Arg Asp Arg Lys Trp Asn Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 50:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
CA 02301959 2000-OS-OS
-26-
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 50:
Arg Asp Arg Lys Trp Asn Cys Thr
1 5
(2) INFORMATION FOR SEQ ID N0: 51:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 51:
Asp Arg Lys Trp Asn Cys Thr Asp
1 5
(2) INFORMATION FOR SEQ ID N0: 52:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 52:
Arg Lys Trp Asn Cys Thr Asp His
1 5
(2) INFORMATION FOR SEQ ID NO: 53:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 53:
Lys Trp Asn Cys Thr Asp His Val
1 5
(2) INFORMATION FOR SEQ ID NO: 54:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
CA 02301959 2000-OS-OS
-27-
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 54:
Trp Asn Cys Thr Asp His Val Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 55:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 55:
Val Met Ile Lys Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 56:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 56:
Val Met Ile Lys Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 57:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 57:
Val Met Ile Lys Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 58:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-28-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 58:
Val Met Ile Lys Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 59:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 59:
Val Met Ile Lys Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 60:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 60:
Val Met Ile Lys Trp Glu Glu Phe
1 S
(2) INFORMATION FOR SEQ ID NO: 61:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 61:
Val Met Lys Ser Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 62:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-29-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 62:
Val Met Lys Ser Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 63:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 63:
Val Met Lys Ser Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 64:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 64:
Val Met Lys Ser Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 65:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 65:
Val Met Lys Ser Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 66:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-30-
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 66:
Val Met Lys Ser Trp Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 67:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 67:
Glu Trp Ile Lys Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 68:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 68:
Glu Trp Ile Lys Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 69:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 69:
Glu Trp Ile Lys Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 70:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-31 -
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 70:
Glu Trp Ile Lys Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 71:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOG'~: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 71:
Glu Trp Ile Lys Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 72:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 72:
Glu Trp Ile Lys Trp Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 73:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 73:
Glu Trp Lys Ser Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 74:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-32-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 74:
Glu Trp Lys Ser Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 75:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 75:
Glu Trp Lys Ser Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 76:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 76:
Glu Trp Lys Ser Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 77:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 77:
Glu Trp Lys Ser Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 78:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-33-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 78:
Glu Trp Lys Ser Trp Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 79:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 79:
Glu Tyr Ile Lys Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 80:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 80:
Glu Tyr Ile Lys Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 81:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 81:
Glu Tyr Ile Lys Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 82:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-34-
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 82:
Glu Tyr Ile Lys Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 83:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 83:
Glu Tyr Ile Lys Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 84:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 84:
Glu Tyr Ile Lys Trp Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 85:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 85:
Glu Tyr Lys Ser Cys Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID N0: 86:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-35-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 86:
Glu Tyr Lys Ser Cys Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 87:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 87:
Glu Tyr Lys Ser Glu Phe Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 88:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 88:
Glu Tyr Lys Ser Glu Phe Glu Phe
1 5
(2) INFORMATION FOR SEQ ID N0: 89:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 89:
Glu Tyr Lys Ser Trp Glu Tyr Cys
1 5
(2) INFORMATION FOR SEQ ID NO: 90:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-36-
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 90:
Glu Tyr Lys Ser Trp Glu Glu Phe
1 5
(2) INFORMATION FOR SEQ ID NO: 91:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 91:
Leu Cys Pro Pro Gly Met Val Arg His Glu
1 5 10
(2) INFORMATION FOR SEQ ID NO: 92:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 9 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 92:
Arg Cys Pro Cys Phe His Gln Gly Lys
1 5
(2) INFORMATION FOR SEQ ID N0: 93:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 9 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 93:
Cys Phe His Gln Gly Lys Glu Tyr Ala
1 5
(2) INFORMATION FOR SEQ ID NO: 94:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 Amino acids
(B) TYPE: Amino acid
(C) STRANDEDNESS: Single strand
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: Peptide
CA 02301959 2000-OS-OS
-37-
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 94:
Arg Asp Arg Lys Trp Asn Cys Thr Asp His Val Cys
1 5 10