Note: Descriptions are shown in the official language in which they were submitted.
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
INHIBITORS OF ANDROGEN-INDEPENDENT
ACTIVATION OF ANDROGEN RECEPTOR
BACKGROUND OF THE INVENTION -
Various diseases have an androgen mediated aspect, including prostate cancer,
benign prostatic hyperplasia, hirsutism, androgenic alopecia, acne, breast
cancer and
10 Kennedy disease.
Androgen deprivation, aimed at inducing the death of androgen-dependent
tumor cells is a potential treatment for such "androgen mediated diseases" and
is the
only useful systemic therapy available for prostate cancer in humans. The
treatment
involves surgical castration of the patient or treatment with anti-androgen
drugs or
15 drugs that inhibit production of androgens. The inability of androgen
deprivation to
eliminate all prostate cancer cell populations in a patient is manifested by a
predictable pattern of response to the treatment. Initial therapy-induced
regression of
the malignancy is usually followed by a relapse with ultimate progression to
complete
resistance to further hormonal manipulations. This progression is the result
of
20 adaptation of surviving tumor cells to diminishing levels of androgen or
outgrowth of
subpopulations of androgen-independent tumor cells. Androgen independence
appears
to involve changes in androgen receptor regulated gene transcription.
The androgen receptor (AR) as a transcription factor forms the basis of a
signal transduction pathway that regulates the expression of certain androgen
25 responsive genes. Control of androgen responsive gene activity may be
agonistic
(stimulated) or antagonistic (repressed) and results from binding of androgen-
activated AR to upstream flanking nucleotide sequences, termed androgen
response
elements (A.REs). The AR is activated (transformed) by androgens, including
the
naturally occurring androgenic hormones testosterone and dihydrotestosterone,
as
30 well as synthetic androgens such as 81881.
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99100604
AR from various mammalian sources has been isolated and characterized. As
well, various assays for AR are known. The deduced amino acid sequence of
human
AR has been reported (1) as have DNA sequences encoding human and rat AR and
cells transformed with such sequences (2, 3 10).
Androgen activation of AR is mediated by ligand (androgen) binding to a
ligand binding domain (LBD) located in the carboxy-terminus of the AR. In
human
AR, the LBD is in the region of amino acids 671-919. Certain anti-androgen
compounds are also known to bind to the LBD. Androgen induced activation of
the
10 AR is associated with conformation of the AR becoming more compact upon
ligand
binding, heatshock proteins being dissociated, and dimerization and
phosphorylation
occurnng prior to DNA-binding.
The DNA-binding domain (DBD) is centrally located in AR. In human AR,
the DBD is in the region of amino acids 557-622. The N-terminus region
preceding
15 the DBD contributes to DNA-binding as well as transcriptional activation or
repression ( 1 I ). Expression of various N-terminal AR deletion and insertion
mutants
lacking a LBD but comprising the AR DBD as well as co-expression of various AR
N-terminal fragments fused to a heterologous DBD (GAL4) with a GAL4 responsive
reporter, demonstrates the presence of transcription activation domains in the
first 485
20 amino acids and in the region of amino acids 360-528 of human AR (12).
AR belongs to the superfamily of nuclear receptors that mediate actions of
lipophilic ligands, including steroids, retinoids, vitamin D3, and thyroid
hormones.
These receptors have distinct functional domains that include a carboxy-
terminal
LBD, a highly conserved DBD comprised of two zinc finger motifs, and a poorly
25 conserved amino-terminal domain that may contain several transcriptional
activation
or repression domains. In all cases, binding of ligand to the receptor results
in
activation, such that the receptor can effectively bind to its specific DNA
element.
Estrogen and progesterone receptors belong to the same family of
ligand-activated transcription factors as the AR. The former receptors can be
activated
30 in the absence of their respective ligands. The estrogen receptor, for
example, can be
2
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
activated through three different signaling events, i.e. not only by
estrogens, but also
by cAMP or by a growth factor (e.g., epidermal growth factor), each of these
compounds acting via a different domain of the receptor. The AR can also be
activated in the absence of androgen by elevation of cyclic AMP (CAMP) levels
and
5 by growth factors (4-6, 8). Phosphorylation has been implicated in the
ligand-
independent activation of the progesterone, estrogen, and retinoic acid
receptors.
While there are three identified phosphorylation sites on the AR,
phosphorylation
does not appear to be essential for the induction of androgen-regulated genes
(7) and
phosphorylation does not alter activity.
10 Androgen-independent activation of the AR involving cAMP-dependent
protein kinase A (PKA) signal transduction pathways has been observed {6). One
of
the inventors named herein has determined that exposing prostate cells to
forskolin
(an activator of PKA) in the absence of androgen, leads to activation of the
promoter
of the prostate specific antigen (PSA) gene, a process shown to be mediated by
the
15 AR (9).
PSA is a clinically important androgen-stimulated gene which is used to
monitor treatment responses, prognosis and progression in patients with
prostate
cancer. Expression of PSA is initially androgen-regulated and undergoes a
sharp
decline following medical or surgical castration. When the tumor becomes
androgen-
20 independent, PSA mRNA is constitutively up-regulated (9). The promoter and
enhancer regions of the PSA gene have been sequenced as far as -5824 from the
start
site of transcription and the following DNA response elements have been
characterized: TATA box , -28 to -23; androgen response elements {AREs), -170
to
156, -4148 to -4134; and, androgen response region (ARR), -395 to -376.
25
SUMMARY OF THE INVENTION
The inventors have found that interaction of the AR with the PKA pathway
involves a discrete region of the N-terminal domain of the AR (amino acid
region
30 234-391). This region is also involved in androgen-independent activation
of the AR
3
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
resulting from treatment with a differentiating agent (butyrate). This
invention now
provides the means for generation of small molecules (including peptides and
peptide
mimetics) for inhibiting androgen-independent activation of the human AR. Such
inhibitory compounds, used in combination with androgen deprivation would more
5 effectively limit androgen mediated disease progression.
This invention provides a non-androgen ligand binding peptide comprising
one or more tracts of amino acids derived from at least 10 contiguous amino
acids of
amino acids 234-391 of human AR, providing that said peptide does not contain
a
DNA-binding domain.
10 Peptides of this invention will comprise one or more tracts of amino acids
derived from preferably at least 20, and more preferably at least 30, even
more
preferably at least 50 and even more preferably at least 100 contiguous amino
acids
derived from amino acids 234-391 of human AR. The tract of amino acids may be
substantially identical to amino acids 234-391 of human AR. Peptides of this
15 invention may comprise AR derived amino acids substantially consisting of
only the
aforesaid tracts of amino acids:
Peptides of this invention may substantially consist of the aforesaid tracts
of
amino acids or may comprise additional amino acids. Where a peptide comprises
AR
derived amino acids substantially consisting of one or more of the aforesaid
tracts, the
20 peptide may additionally comprise a DNA-binding domain.
This invention also provides nucleic acid constructs encoding peptides of this
invention as well as said constructs in an expression vector. This invention
also
provides cells and pharmaceutical compositions comprising the peptides,
nucleic acid
constructs and expression vectors of this invention.
25 This invention also provides a method of inhibiting androgen-independent
activation of AR by introducing into said cell, a peptide, nucleic acid
construct or
expression vector of this invention.
This invention also provides the use of a peptide, nucleic acid construct,
expression vector or cell of this invention for the preparation of a
medicament for the
30 treatment of androgen mediated diseases including prostate tumors. This
invention
4
CA 02302169 2000-02-28
WO 00101813 PCT/CA99/00604
also provides the use of a peptide, nucleic acid construct, expression vector
or
pharmaceutical composition of this invention for treatment of androgen
mediated
diseases including prostate tumors, particularly in patients undergoing
androgen
deprivation therapy.
5 This invention also provides a method of determining whether a compound or
a mixture of compounds affects androgen-independent activation of androgen
receptor (AR) comprising the steps of:
( 1 ) contacting a compound or a mixture of compounds with a peptide
comprising one or more tracts of amino acids derived from at least 10
contiguous amino acids of amino acids 234-391 of human AR; and,
(2) detecting whether a compound of said compound or mixture of
compounds binds to said one or more tracts, or whether said compound
15 or mixture of compounds affects a transcription activation or
repression function of said peptide, with the proviso that when said
detecting is of an effect on transcription activation or repression, said
peptide will not comprise a ligand binding domain for androgen
activation of AR and will comprise a DNA-binding domain.
In the preceding method, said one or more tracts of amino acids may be derived
from
differing lengths of contiguous amino acids of amino acids 234-391, as
described
above for the peptides of this invention.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic showing a general scheme for preparation of a reporter,
as well as expression vectors encoding AR proteins fused to the GAL4 DBD.
FIG. 2 is a graph showing that the amino-terminus of the AR is activated by
forskolin as manifested by Gal4-luciferase activity. Transactivation assays
were
5
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99I00604
performed in LNCaP cells transfected with a SxGaI4UAS-TATA-luciferase
reporter,
GaI4DBD, and ARN-Gal4 DBD and exposed to 81881 or forskolin (aPKA). Lanes: 1-
2, control (DMSO, 0.5%); 3-4, 81881 (10 nM); 5-6, forskolin (50 ~M); 1, 3, and
5,
Gal4 DBD (0.5 p.g); 2, 4, and 6, ARl_ss9-Gal4 DBD (0.5 ~,g); 1-6, SxGaI4UAS-
TATA-luciferase reporter (1.0 pg).
FIG. 3 is a schematic showing chimeric expression vectors consisting -of
GAL4-DBD DNA sequences fused to DNA sequences encoding various sections of
the N-terminal domain of AR.
FIG. 4 is a graph showing GAL4 luciferase activity in cells transfected with
the expression vectors shown in Fig. 3 and a plasmid containing the reporter
shown in
Fig. 1. The cells were exposed to the PKA catalytic domain {A) and butyrate
{B).
FIG. 5 is a schematic showing identification of a proteins) interacting with
the 234-391 amino acid region of the AR during androgen-independent activation
of
the human AR.
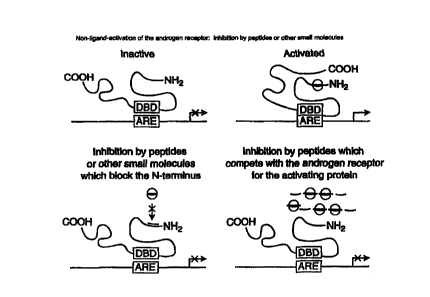
15 FIG. 6 is a schematic showing two ways by which small molecules inhibit the
interaction of the 234-391 amino acid region of AR with activating agents
involved in
androgen-independent activation of AR.
FIG. 7 is a graph showing inhibition of forskolin (FSK) mediated stimulation
of PSA luciferase activity by a vector expressing a N-tenminal domain of AR.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
The amino acid sequence of the human AR, as determined by Lubahn et al.
(1), is given below. The section of the N-terminal domain, (amino acids 234-
391,
SEQ ID NO:1 ) which mediates androgen-independent activation of the AR, is
printed
in bold letters.
6
CA 02302169 2000-02-28
WO 00101813 PCTICA99100604
1 mevqlglgrv yprppsktyr gafqnlfqsv reviqnpgpr hpeaasaapp gasllllqqq
61 qqqqqqqqqq qqqqqqqqet sprqqqqqqg edgspq~' gptgylvlde eq9Ps9Pqsa
121 lechpergcv pepgaavaas kglpqqlpap pdeddsaaps tlsllgptfp glsscsadlk
181 dilseastmq llqqqqqeav segsssgrar easgaptssk dnylggtsti sdnakelcka
241 vsvsmglgve alehlspgeq lrgdcmyapl lgvppavrpt pcaplaeckg sllddsagks
301 tedtaeyspf kggytkgleg eslgcsgsaa agssgtlelp stlslyksga ldeaaayqsr
361 dyynfplala gpppppppph phariklenp ldygsawaaa aaqcrygdla slhgagaagp
421 gsgspsaaas sswhtlftae egqlygpcgg gggggggggg gggggggggg ggeagavapy
481 gytrppqgla gqesdftapd vwypggmvsr vpypsptcvk semgpwmdsy sgpygdmrle
541 tardhvlpid yyfppqktcl icgdeasgch ygaltcgsck vffkraaegk qkylcasrnd
601 ctidkfrrkn cpscrlrkcy eagmtlgark lkklgnlklq eegeasstts pteettqklt
661 vshiegyecq piflnvleai epgwcaghd nnqpdsfaal lsslnelger qlvhwkwak
721 alpgfrnlhv ddqmaviqys wmglmvfamg wrsftnvnsr mlyfapdlvf neyrmhksrm
781 ysqcvrmrhl sqefgwlqit pqeflcmkal llfsiipvdg lknqkffdel nnrlyikeldr
841 iiackrknpt scsrrfyqlt klldsvqpia relhqftfdl likshmvsvd fpemmaeiis
901 vqvpkilsgk vkpiyfhtq (SEQ ID N0:2).
The following definitions apply to terms used in this specification.
The term "non-androgen ligand binding peptide" means a peptide capable of
binding to a ligand other than androgen.
The term "tract(s) of amino acids" is a sequence of two or more amino acids
joined by peptide bonds.
The term "peptide" means a compound comprising at least two amino acids
joined by peptide bonds and includes a polypeptide and a protein, and peptides
thaf
comprise non-naturally occuring amino acids and peptide linkages.
The term "DNA-binding domain" (DBD) is a tract of amino acids capable of
binding to DNA in a regulatory element of a gene. Examples include the
androgen
receptor DBD which bind to regulatory elements of androgen sensitive genes. In
the
case of PSA, the DBD binds to an ARE region of the PSA promoter. The DBD of
GAL4 is an example of a yeast DBD used in the commercial reporter systems in
7
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
which expression of the reporter is activated or repressed by a moiety joined
to the
GAL4 DBD and brought into close proximity with a regulatory element (eg.
promoter) of the reporter when the DBD binds to the reporter.
The term "nucleic acid" means DNA or RNA. A nucleic acid encoding a
peptide is a nucleic acid containing or corresponding to a sequence of
nucleotides,
which sequence when transcribed and translated (or simply translated in the
case~of
RNA) would result in the peptide. The term is meant to include any such
sequence of
nucleotides which, because of the degeneracy of the nucleic acid code, will
encode (or
corresponds to a sequence which will encode) the peptide as described above. A
10 nucleic acid which corresponds to a sequence encoding a peptide is a
nucleic acid that
is complementary to a coding sequence. Nucleic acids of this invention may
include
anti-sense molecules where inhibition of expression of the peptides is
desired.
The term 'expression vector" is a nucleic acid construct including a plasmid,
phage genome or other nucleic acid which is able to replicate in a host cell
and which
15 contains a nucleic acid sequence encoding a desired peptide product. The
nucleic acid
sequence encoding the peptide will be expressed in the host cell. The vector
may
include elements necessary for incorporation of the encoding sequence into a
host cell
genome and the vector will contain regulatory elements operably linked to the
coding
sequence to permit expression of the gene product in the host cell.
20 The term "derived from" with reference to an amino acid sequence means that
a first sequence of amino acids is substantially identical to a reference
sequence of
amino acids. The term "androgen receptor derived amino acids" means those
parts of
a peptide consisting of at least 10 contiguous amino acids which are
substantially
identical to a tract of 10 or more amino acids in androgen receptor. Thus, a
peptide
25 that comprises androgen receptor derived amino acids substantially
consisting of a
specified tract of amino acids, is a peptide in which the only tract
substantially
identical to a tract of amino acids in androgen receptor is the specified
tract. In the
latter case, the peptide may also contain tracts of amino acids not
substantially
identical to tracts of amino acids in androgen receptor or the peptide may
consist
30 substantially of only of tracts substantially identical to androgen
receptor.
8
CA 02302169 2000-02-28
WO 00/01813 PCTICA99/00604
The term "substantially identical" with reference to peptides of this
invention
or used in this invention refers to first and reference sequences which have
at least
one function of a peptide of this invention in common and having at least 75%
identity (more preferably at least 90% identity) as determined by comparing
positions
5 in each sequence. Sequence identity may be determined by the BLAST algorithm
described in Altschul et al. (1990) ,l. Mol. Biol. 215:403-410, using the
published
default settings. When a position in the compared sequence is occupied by the
same
amino acid, the molecules are considered to have shared identity at that
position. The
degree of identity between sequences is a function of the number of matching
10 positions shared by the sequence.
The term "derived from" is meant to include situations where conservative
substitutions, deletions or additions are made in a first sequence of amino
acids
relative to a reference sequence. Examples of such conservative changes are
the
substitution of amino acids having similar "side" or "functional" groups,
preferably
15 similar in charge and ligand reactivity; most preferably also similar in
size. Thus, a
tract of amino acids derived from a specified number of contiguous amino acids
of a
reference sequence may or may not have the same number of and exact sequence
of
the specified contiguous amino acids of the reference, providing the tract and
the
specified amino acids of the reference are substantially identical as defined
herein.
20 Nucleic acids of this invention may be synthesized when convenient or may
be
produced by recombinant techniques known in the art. Nucleic acids within the
scope
of this invention may contain linkers, modified or unmodified restriction
endonuclease sites and other sequences of nucleotides useful for cloning,
expression,
or purification. Nucleic acids within the scope of this invention may be
incorporated
25 in a larger sequence of nucleotides, including plasmids and vectors useful
for
manipulation or expression of nucleic acids.
Peptides of this invention may be synthesized when convenient by any number
of known peptide synthesis techniques. Alternatively, the peptides may be
expressed
in any suitable host cell into which an expression vector for the peptide has
been
30 introduced. The host cell and expression vector components will typically
be selected
9
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
to allow for expression of the peptide in the host. Any peptide of this
invention may
comprise multiple (eg. repeat) tracts of amino acids derived from SEQ ID NO:1
which may enhance the activity of the peptide.
5 Peptides of this invention are useful as having one or more of the following
functions: an agent for inhibiting androgen dependent activation of the AR; an
activation domain; xnd, a tool for screening compounds which affect androgen-
dependent activation of AR. Examples whereby peptides of this invention are
tested
or used for such functions are set out below. In the former case, the peptides
will
10 typically lack an intact or functional DBD, in particular a DBD that is
capable of
binding to an androgen sensitive gene. In such a case, the peptide might
include other
portions of the AR including the LBD (which will not be effective absent a
functional
androgen receptor DBD). Preferably, peptides of this invention used for
inhibiting
androgen independent activation of AR will substantially consist of androgen
receptor
1 S derived amino acids which are derived only from SEQ ID NO:1. In the case
of
screening for compounds that affect androgen-dependent activation of AR,
peptides
of this invention may include a DBD if DNA-binding is desirable in the chosen
mode
of screening. However, where the mode of screening involves monitoring a
change in
pattern of activation or repression of a gene by the peptide when affected by
a test
20 compound (thereby necessitating the inclusion of DBD on the peptide), the
peptide
will not have an AR LBD so as to avoid an erroneous response brought about by
ligand binding to the LBD.
Methods of this invention for determining whether a compound affects
androgen-dependent activation of the AR may be used to detect compounds that
25 potentially inhibit or bring about activation of the AR. In one embodiment,
this
method involves determining whether a compound binds to amino acid tracts
derived
from SEQ ID NO:1. This may be accomplished by a variety of known methods
which may also facilitate the separation and recovery of the binding compound.
Detection of an apparent change in conformation or molecular weight of a
peptide
30 when bound to a compound may be carried out, for example by gradient
CA 02302169 2000-02-28
WO 00/01813 PCTICA99/00604
ultra-centrifugation or by SDS PAGE. The peptide could be labeled (for example
by
a fluorescent compound) to facilitate separation. Alternatively, the peptide
could be
immobilized to facilitate separation of binding compounds. An example of such
immobilization is attachment of the peptide to a suitable activated substrate
(eg.
5 beads of a chromatography gel), or by immunological techniques. Antibodies
to AR
and methods of producing anti-AR antibodies have been described (2, 12). _
Alternate methods of screening which are part of this invention involve
monitoring a change in the function of a peptide of this invention. In such
embodiments, one may take advantage of the transactivation/repression
characteristics
10 of the N-terminal region of the AR and employ methods whereby enhancement
or
repression of such characteristics are observed as being indicative of the
presence of a
compound that interact with the peptide in such a way as to affect such
characteristics.
For example, cells may be prepared which express a chimeric protein consisting
of an
AR N-terminal portion including a tract derived from SEQ ID NO:1, joined to a
DBD.
15 In this example, the DBD is a "label" as the term is used herein. Following
treatment
of the cell with a test compound, one observes whether the function of the
chimeric
protein is enhanced or inhibited with respect to activation or repression of a
selected
"reporter". The reporter could be an androgen sensitive gene whose product is
measurable or the reporter could be part of a construct intended to produce a
readily
20 detectable signal (such as the luciferase reporter used in the examples
herein). A
measurable androgen sensitive gene product is PSA and methods for detecting
expression of PSA immunologically or by monitoring PSA mRNA are known. In the
latter case, the protein "labeled" with the DBD will either not contain an AR
LBD or
the cells will be deprived of androgen (the cells will not contain endogenous
androgen
25 and no androgen will be applied to the cells).
Pharmaceutical compositions of this invention will typically comprise a
peptide of this invention as an active ingredient and a pharmaceutically
acceptable
carrier. The carrier will be one which will facilitate delivery of the peptide
into
afflicted cells such as those of a prostate tumor. Suitable carriers fox
injection or
30 diffusion of the peptide into a tumor would include traditional vehicles
such as saline,
11
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
as well as more complex formulations such as liposome formulations and
nonrecombinant viral vectors such as was described by Nazareth, et aL (6).
Further,
pharmaceutical compositions of this invention may include "gene therapy"
vectors
such as recombinant adenovirus for delivery and replication of an expression
vector
capable of producing a peptide of this invention.
EXAMPLES
Role of Amino Acid Region 234-391 in
Androgen-Independent Activation of the Human AR
To identify the particular region of the AR involved in androgen-independent
activation, use was made of a yeast transcription factor, GAL4, which consists
of two
well-defined domains, i.e. an activation domain and a DNA-binding domain.
Chimeric proteins were generated by substituting the activation domain of GAL4
by
one of a number of sections of the N-terminal region of the human AR. While
these
chimeric proteins had a DNA-binding site located in their GAL4 segment, they
did
not have the LBD of AR.
To generate chimeric proteins, expression vectors were constructed as
described below, consisting of DNA encoding the DNA-binding domain (DBD) of
GAL4 (supplied by Clontech) fused to DNA encoding various regions of the AR:
i.e.
a region including the N-terminal region of AR [amino acids (aa) 1 to 558],
and
regions as 392 to 558, as 1 to 233, as 234 to 391 and as 1 to 391 (Figures 1
and 3). A
luciferase reporter vector (Clontech), consisting of the luciferase gene and
the GAL4
DNA response element located upstream of the luciferase gene, was used for
assessing activation of transcriptional activity of the chimeric proteins.
Another
vector, containing the catalytic domain of PICA (Clontech), was used to induce
PKA
signaling. Following co-transfection of the various vectors into human
prostate cancer
cells and incubation with stimuli, luciferase activity in cell lysates was
assayed as
described below.
12
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
All chemicals were purchased from Sigma (St. Louis, MO), unless stated
otherwise. PC3 cells between the 30'" and 45'" generation, were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine
serum (FBS) (GIBCOBRL, Burlington, Ontario, Canada). LNCaP cells between the
5 44'" and 55'" generation were maintained in RPMI 1640 supplemented with S%
FBS.
When the plates or wells were 60-70% confluent with cells, the culture medium
was
changed to serum-free medium containing vehicle (DMSO), 81881, or forskolin.
The ARNGaI4 plasmid was constructed by PCR amplification of the
nucleotides 363 to 2039 of human AR cDNA using primers 5'-AAA GGA TCC GGA
10 TGG AAG TGC AGT TAG GGC T (SEQ ID NO: 3) and 5'-AAA AGG ATC CTT
CAG GTC TTC TGG GGT GGA AAG TAA TAG (SEQ ID N0:4}. The amplified
DNA fragment was purified, blunt-end ligated into the EcoRV site of Bluescript
SK(-) excised with BamHI and cloned into the BamHI site of pFA-CMV plasmid
(Stratagene Cloning System, La 7olla, CA). Similarly, constructs containing
different
1 S AR N-terminal domains were prepared. The orientation and sequences were
confirmed by DNA sequence analysis and expressed protein was detected by
Western
blotting. An expression vector for the catalytic subunit of protein kinase A
(PKAc)
was purchased from Stratagene (PathDetect CREB trans-Reporting System).
LNCaP cells (3 x 105) were plated on 6-well plates and incubated in RPMI
20 1640 with 5% FBS prior to transfection. Total amount of plasmid DNA used
was
typically normalized to 3 ltg/well by the addition of empty plasmid. Medium
was
replaced after 24 h by serum-free RPMI 1640 containing variously, DMSO, 81881,
or
forskolin. Cells were collected after 48 h incubation.
PC3 cells (3 x 105) between the 30t" and 45'" generation, were maintained irr
25 Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine
serum (FBS) (GIBCOBRL, Burlington, Ontario, Canada). Total amount of plasmid
DNA used was typically normalized to 3 ,ug/well by the addition of empty
plasmid.
Cells were transfected in serum-free conditions for S h and then incubated
with
forskolin (FSK, SO,uM), 81881 (10 nM), or vehicle (DMSO, 0.5%), for an
additional
30 16 h under serum-free conditions before harvesting.
13
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99100604
Luciferase activities in cell lysates were measured using the Dual Luciferase
Assay System (Promega, Madison, WI). Luciferase activities were normalized by
the
Renilla activities and protein concentrations of the samples. The results were
converted to fold-induction, which is the relative luciferase activity of the
treated cells
over that of the control cells, or activity in the presence of a stimulus over
activity
absent stimuli.
Amino terminal fragments containing approximately amino acids 1-559 (ARN)
of the human AR were cloned into the carboxyl terminus of Gal4 DBD. Expression
vectors for these chimeric proteins were cotransfected into LNCaP cells with
the
10 above-described reporter gene which contains the Gal4-binding site as a cis-
acting
element (p5xGa14UAS-TATA-luciferase). LNCaP cells express endogenous AR and
PSA. As shown in Fig. 2, the synthetic androgen 81881 did not significantly
change
the activity of the reporter when comparing ARNGaI4DBD (lane 4) to GaI4DBD
lacking the amino-terminus of the AR (lane 3). Upon the addition of forskolin,
the
15 Gal4 DBD fused to the N-terminal fragment of human AR activated the
Gal4-luciferase reporter 60-fold (lane 6) over levels achieved with the Gal4
DBD
lacking the amino-terminus of the AR (lane 5). These results show that the
amino-terminal domain of the AR is targeted by the PKA pathway of androgen
independent activation of AR.
20 Figure 4 shows that non-androgen activation of transcriptional activity of
the
chimeric proteins can be achieved in response to two different stimuli, i.e.
PKA
signaling and butyrate, a differentiating agent. Construct 1-558 (which
includes the
complete amino terminal region of the AR) substantially enhanced GAL4
induction of
the luciferase reporter in PC3 cells. PC3 cells are of human prostate cancer
origin but
25 are poorly differentiated and do not express AR or PSA. This activation was
localized to amino acid region 234-391, since only constructs containing this
amino
acid region (1-558, 234-391 and 1-391) showed enhanced induction. Furthermore,
constructs not containing amino acid region 234-391 (i.e. 1-233 and 392-558}
did not
increase induction above that obtained with a construct containing only the
GAL4
14
CA 02302169 2000-02-28
WO 00/01813 - PCT/CA99100604
DNA-binding site (GAL4-DBD). Thus, non-androgen activation is confined to
amino
acid region 234-391 of the AR.
Identification of Proteins Interacting with
Amino Acid Region 234-391 of the Human AR
Figure 5 shows an example procedure for identification of proteins
interactixlg
with amino acid region 234-391 of the AR during its androgen-independent
activation. As indicated, cells are transfected with e.g., a GAL4-AR234-391
plasmid
10 construct, and then exposed to stimuli. The cells are then lysed and the
GAL4-AR
fusion protein is precipitated with an antibody specific for the GAL4 segment
of the
chimeric protein. If, as a result of stimulation, a proteins) binds to the
expressed
234-391 region of the AR, it will be co-precipitated along with the GAL4-AR
fusion
protein and may be detected as an additional protein band on an SDS
polyacrylamide
gel. Recovery and characterization of the protein could then be done using
standard
procedures.
Development of Inhibitors of
Androgen-Independent Activation of the Human AR
Figure 6 shows two mechanisms by which small molecules (e.g., peptides,
peptide mimetics) would inhibit the interaction of the 234-391 amino acid
region of
the AR with activating agents (proteins). As shown in the Left bottom corner,
a small
molecule binds to the 234-391 region of the AR and as a result, prevents
activation.
25 Alternatively, as shown in the right bottom corner and in the example
below,
compounds mimicking the 234-391 region of the AR (in terms of amino acid
sequences or active sites) compete with the AR for the activating protein and
hence
prevent activation of the AR.
The first category of inhibitory compounds may be developed, for example,
using a phage display method based on the binding of small peptides from a
combinatorial phage library to a recombined "bait" protein, containing the 234-
391
amino acid region of the human AR or sections thereof (eg. 10-20 mer
sections).
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
Phage display protein ligand screening systems are described by Lowman, H
B. et al., Biochem. 30:10832-10838 (1991); Markland, W. et al., Gene 109:13-19
(1991); Roberts, B. L. et al., Proc. Natl. Acad. Sci. (LJ.S.A.) 89:2429-2433
(1992);
Smith, G. P., Science 228:1315-1317 (1985); and, Smith, R. P. et al., Science
5 248:1126-1128 (1990). In general, this method involves expressing a fusion
protein
in which a putative ligand for the "bait" is fused to the N-terminus of a
viral coat
protein {such as the M13 Gene III coat protein, or a lambda coat protein). A
library of
phage are engineered to display putative peptide ligands on the coat protein.
The
phage are placed in contact with the bait. Phage that display coat protein
having
10 peptides that are capable of binding to the bait are immobilized by such
treatment,
whereas all other phage can be washed away. After the removal of unbound
phage,
the bound phage can be amplified, and the DNA encoding their respective coat
proteins is sequenced. In this manner, the amino acid sequence of peptides
that bind to
the "bait" can be deduced.
15 T'he second category of inhibitors could be developed by examining the
inhibitory effect on non-ligand activation of the GAL4-AR234-391 chimeric
protein
of short peptides (10-20 mer length) which encode fragments of amino acid
region
234-391 of the human AR. To overcome possible cellular uptake problems,
potentially inhibitory peptides could be introduced into the cells in the form
of
20 expression vectors encoding the sequences of the peptides. Animal tumor
models are
available for in vivo testing of identified inhibitory compounds.
Inhibition of Androgen-Independent
Activation of the Human AR with N-terminal AR Fragments
25 _
Androgen-independent AR expression of the prostate specific antigen (PSA)
gene can be obtained in cultured LNCaP human prostate cancer cells by
incubating
the cells with forskolin, an activator of PKA (9). In this example, a
luciferase reporter
vector consisting of the PSA promoter located upstream of the luciferase gene,
was
30 used for assessing androgen-independent activation of the PSA gene. In
order to
construct the PSA promoter plasmid (PSA), PSA 5' flanking DNA (-6301+12) was
16
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
obtained by PCR amplification of human genomic DNA using oligonucleotide
primers corresponding to the PSA gene and ligated with EcoRv-digested
pBluescriptTM (pBS) sk(-) (Stratagene, La Jolla, CA, USA) according to a
method
previously described (10).
5 A chimeric protein was generated by inserting the N-terminal region [amino
acids 1 to 558] of the human AR into a His-tag expression vector (In
Vitrogen). The
expression vector (1-558) or the vector with no N-terminal domain (HisTag)
were
co-transfected with the PSA-luciferase reporter into LNCaP human prostate
cancer
cells and incubated without androgens in the presence or absence of forskolin.
As an
additional control, the luciferase reporter without the PSA promoter (pGL2)
was also
co-transfected into LNCaP cells.
Figure 7 shows non-ligand activation of AR by forskolin (PKA stimulation)
(HisTag+PSA). In the control (non-treated LNCaP cells), there is very little
luciferase
activity showing that the PSA promoter is not being stimulated. Upon addition
of
15 forskolin (FSK), there is a dramatic increase in luciferase activity
indicating
non-androgen stimulation of PSA. The empty vector (HisTag) had no effect on
forskolin stimulation of PSA. Expression of the pGL2 vector does not increase
upon
addition of forskolin showing that activation is dependent upon the PSA
promoter
(HisTag+pGL2). There is a significant drop in PSA luciferase activity when
HisTag
20 is replaced with vector expressing the complete amino terminus of the AR,
(compare
1-558+PSA in the presence of FSK to HisTag+pSA in the presence of FSK). The
decrease in luciferase activity shows that the AR N-terminal peptide fragment
is
suppressing forskolin stimulation of the PSA promoter. Thus, a N-terminal
fragment
can inhibit androgen-independent activation of the human AR through
competition. -
25 Although the foregoing invention has been described in some detail by way
of
illustration and example for purposes of clarity of understanding, it will be
readily
apparent to those of skill in the art in light of the teachings of this
invention that
changes and modification may be made thereto without departing from the spirit
or
scope of the appended claims. All patents, patent applications and
publications
30 referred to herein are hereby incorporated by reference.
17
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
REFERENCES
1. Lubahn, D.B. et al., (1988) The Human Androgen Receptor: Complementary
Deoxyribonucleic Acid Cloning, Sequence Analysis and Gene Expression in
Prostate. Mol. Endocrinol. 2:1265-1275.
2. International patent application PCT/LTS89/01548 of University of North
Carolina at Chapel Hill, published October 19, 1989 under No. W08909791.
3. Liao, et al., United States Patent No. 5,614,620 issued March 25, 1997.
4. Ikonen, T. et al., (1994) Stimulation of Androgen-Regulated Transactivation
by Modulators of Protein Phosphorylation. Endocrinology 135:1359-1366.
1 S 5. Culig, Z. et al., ( 1994) Androgen Receptor Activation in Prostatic
Tumor Cell
Lines by Insulin-Like Growth Factor-I, Keratinocyte Growth Factor, and
Epidermal Growth Factor. Cancer. Res. 54:5474-5478.
6. Nazareth, L.V. and Weigel, N.L. (1996) Activation of the Human Androgen
Receptor Through a Protein Kinase A Signaling Pathway. J. Biol. Chem.
271:19900-19907.
7. Zhou, Z.X et al., (1995) Mol. Endocrinol. 9:605-615.
8. Culig, Z. et al., (1997) Synergistic Activation of Androgen Receptor by
Androgen and Luteinizing Hormone-Releasing Hormone in Prostatic
Carcinoma Cells. Prostate 32:106-114.
18
i
CA 02302169 2000-02-28
WO 00/01813 PCT/CA99/00604
9. Sadar, M.D. (1999) Androgen-Independent Induction of Prostate-specific
Antigen Gene Expression Via Cross-talk between the Androgen Receptor and
Protein Kinase A Signal Transduction Pathways. J. Biol. Chem.
274:7777-7783.
10. Sato, N. et al., (1997) .l. Biol. Chem. 272:17485-17494. _
11. Gast, A. et al., (1998} J. Steroid Biochem. Molec. Biol. 65:117-123.
12. Jenster; G. et al., (1995) J. Biol. Chem. 270:7341-7346.
19