Note: Descriptions are shown in the official language in which they were submitted.
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Pyrrolopyrrolone derivatives as inhibitors of neutrophil elastase
The present invention relates to therapeutically active bicyciic compounds,
processes for their manufacture, pharmaceutical formulations containing them
and their use in chemotherapy. In particular, we have found a group of novel
bicyclic compounds which are effective in treating inflammatory diseases.
Inflammation is a primary response to tissue injury or microbial invasion and
is
characterised by circulating leukocytes binding to and extravasation through
vascular endothelium. Circulating leukocytes include neutrophils, eosinophils,
basophils, monocytes and lymphocytes. Different forms of inflammation involve
different types of infiltrating leukocytes.
The inflammatory process can be triggered in a number of ways, including by
infection, tissue damage and autoimmune reactions. As part of the
inflammatory process, neutrophils move from the bloodstream into the tissue at
the site of tissue lesion. The neutrophils contain large numbers of different
intracellular granules and when activated at the site of inflammation the
contents
of these granules are secreted into the tissue. The different granules contain
a
variety of enzymes and other proteins, many of which have antibacterial
properties.
One of the enzymes found in the azurophiiic granules is neutrophil elastase.
Neutrophil eiastase has a wide spectrum of activities in the body. For
example,
within the lung the enzyme increases mucus production and changes the
cellular composition of the epithelium The enzyme also causes vascular
permeability changes within the microcirculation of many tissues and it is a
potent destructive agent against a number of connective tissue components.
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Although there are within the body endogenous inhibitors of elastase,
including
the anti-trypsin and the leukocyte protease inhibitor, elastase activity has
been
implicated in the pathogenesis of a number of disease states including
inflammatory diseases of the airways, the joints and the skin. The enzyme is
also responsible for some or most of the symptoms of acute respiratory
distress
syndrome CARDS) and other acute inflammatory states brought about by trauma
and/or sepsis.
We have now found a group of novel compounds which inhibit neutrophil
elastase. These compounds are therefore of potential therapeutic benefit in
the
treatment and amelioration of symptoms of diseases where elastase activity is
implicated.
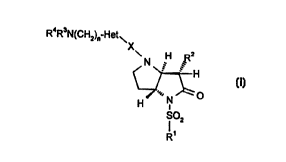
Thus, according to one aspect of this invention, we provide a compound of the
formula (I)
R4R3N(CHz)~ Het~
z
N H R
H
'~,, ~ O (I,
H N
SOz
R'
(relative stereochemistry indicated)
wherein:
R' represents C~.~alkyl;
R2 represents C2.~alkyl or C2~alkenyl;
X represents CO or S02;
Het represents an optionally substituted 5 to 10 membered monocylic or
bicyclic
aromatic ring system containing 1 to 4 heteroatoms selected from O, N and S;
2
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
n represents an integer 0 to 4;
R3 and R4 independently represent hydrogen, C~.~alkyl, -(CH2)~~CONRSRg,
COC~.~alkyl or (CH2)~.Z Ph where Ph represents phenyl optionally substituted
by
one or more C~.~alkyl or halogen groups or NR3 R4 together represents
azetidinyl, pyrrolidinyl, piperidinyl, azepinyl, morpholinyl, piperazinyl
optionally N-
substituted by C~.~alkyl, phenyl (optionally substituted by halogen or
C~~alkyl) or
benzyl (optionally substituted on the benzene ring by halogen or Ci.~alkyl) or
NR3R4 together represents a ring as just described save that it is substituted
on
carbon by one or more C~.~alkyl, CONR5R6 or COOR6 groups;
R5 and R6 independently represent hydrogen or C~.~alkyl;
and salts and solvates thereof (hereinafter ucompounds of the invention").
Formula (I) shows the retative stereochemistry of the chiral centres.
The invention embraces compounds of the invention in racemic form as well as
in a form in which one enantiomer predominates or is present exclusively.
Generally, we prefer to provide a compound of formula (I) in enantiomerically
pure form, most particularly the enantiomer having the absolute
stereochemistry
illustrated in formula (I).
The present invention also covers the physiologically acxeptable salts of the
compounds of formula (I). Suitable physiologically acceptable salts of the
compounds of formula (I) include inorganic and organic acid salts such as
hydrochloride and tartrate.
When used herein "alkyl" includes branched as well as straight chain alkyl and
may also include cycloalkyl when 3 or more carbon atoms are present.
Suitable R~ alkyl groups include methyl, ,ethyl and propyl.
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Examples of Het groups include furanyl, imidazolyl, thiophenyl, pyrrolyl,
thiazolyt, isoxazolyl, pyrazolyl, pyridinyl and pyrazinyl.
Het may be connected to the pyrrolidine ring via X in any position. Examples
of
connectivities include furan-2-yl, furan-3-yl, imidazol-2-yl, imidazol-4-yl,
thiophen-2-yl, pyrrol-2-yl, thiazol-~-yl, isoxazol-3-yl; pyrazol-3-yl, pyrazol-
5-yl,
pyridin-3-yl, 1-methyl-pyrrol-2-yl, 1-methyl-pyrazol-3-yl, 1-methyl-pyrazol-5-
yl
and pyrazin-2-yl.
Examples of substituents for Het include C~.~alkyl (e.g. methyl, ethyl),
C~~alkoxy
(e.g. methoxy), vitro and halogen (e.g. chlorine, bromine, fluorine, iodine).
The
substituent(s) may be on carbon or nitrogen.
Examples of substituted Het include 1-methyl pyrrolyl, 1-methyl pyrazolyl.
Examples of positions for the sidechain shown in formula (I) include for furan-
2-
yl the 5 position, for furan-3-yl the 2 position, for thiophen-2-yl the 5.
position, for
pyrrol-2-yl the 4 or 5 position, for 1-methyl pyrrol-2-yl the 5 position, for
thiazol-4-
yl the 2 position, for isoxazol-3-yf the 5 position, for 1-methyl-pyrazol-3-yl
the 5
position, for 1-methyl-pyrazol-5-yl the 3 position, for pyridin-3-yl the 6
position
and for pyrazin-2-yl the 5 position.
When R3 and R4 independently represent C~.~alkyl, examples include methyl,
ethyl, cyclopropyl, n-propyl, isopropyl, n-butyl, CH(iPr)2 and cyclohexyl.
When R3 and R4 independently represent (CH2)o-2Ph, examples include phenyl,
benzyl and (4-F-phenyl)methyl.
4
CA 02303176 2000-03-07
WO 99/11933 PCT/EP98/05609
When NR3R4 together represents N-substituted piperazinyl, examples include N-
phenyl-piperazinyl and N-methyl-piperazinyl.
When NR3R4 together represents a ring substituted on carbon, examples of
substituents include methyl, CONH2 and COOMe. Examples of such NR3R4
include 4-methyl piperidin-1-yl.
We prefer R' to represent methyl or ethyl, especially methyl.
We prefer R2 to represent isopropyl or propyl, especially isopropyl.
We prefer X to represent CO.
We prefer Het to represent a 5 or 6 membered monocyclic aromatic ring
containing 1 or 2 heteroatoms selected from O, N and S which is more
preferably thiazolyl, isoxazolyl, pyrazolyl or pyrazinyl, especially thiazolyl
(particularly thiazol-4-yl) or pyrazinyl.
We also prefer Het to represent pyridinyl, especially 3-pyridinyl.
We most especially prefer Het to represent pyrazinyl. Ideally Het represents
pyrazin-2-yl in which the sidechain is in the 5-position.
We also prefer Het to represent oxazolyl, particularly oxazol-4-yl.
We prefer n to represent 1 to 3, particularly 1 or 2, especially 1.
We prefer R3 and R° independently to represent hydrogen or C~_8alkyl
or for
NR3R4 to represent pyrrolidinyl, piperidinyl, morpholinyl or piperazinyl
optionally
CA 02303176 2000-03-07
WO 99/12933 PCf/EP98/05609
N-substituted by C~.~alkyl or phenyl (optionally substituted by halogen or
C~.~alkyl). When R3 and R4 independently represent hydrogen or C~.~alkyl,
preferred NR3R4 groups are NMe2, N(n-butyl)2, NHMe, NH(cyclopropyl),
NHCH(iPr)2 and N(cyclohexyl)2.
A set of compounds of formula (I) of particular interest are compounds of
formula (IA) in which Het represents oxazol-4-yl with the sidechain in the 2-
position:
R4R3N(CHZ)n
O N
X~ N H R2
H
(IA)
O
H ~~~ N
SOZ
R'
(relative stereochemistry indicated)
wherein R4, R3, n, X, R2 and R' are as defined above.
We prefer X to represent CO. We prefer R2 to represent isopropyl or propyl,
especially isopropyl. We prefer R' to represent methyl or ethyl, especially
methyl. We prefer n to represent 1 to 3, particularly 1 or 2, especially 1. We
prefer R3 and R4 independently to represent hydrogen or C~~alkyl or for NR3R4
to represent pyrrolidinyl, piperidinyl, morpholinyl or piperazinyl optionally
N-
substituted by C~~alkyl or phenyl (optionally substituted by halogen or
C~.~alkyl). We particularly prefer -NR3R4 to represent pyrrolidinyl,
piperidinyl, N-
phenylpiperazinyl, N(butyl)2, NMe(cyclopropyl) or N(cyclohexyl)2, most
particularly pyrrolidinyl.
6
CA 02303176 2000-03-07
WO 99/12933 ' PCT/EP98105609
The potential for compounds of the invention to inhibit neutrophil elastase
activity may be demonstrated, for example, using the following in vitro and in
vivo assays:
In vitro assays of human neutrophil elastase
Assay contents:
50mM Tris/HCl (pH 8.6)
150mM NaCI
11.8nM purified human neutrophil elastase
Suitable concentrations of compound under test diluted with water from a 1 OmM
stock solution in dimethylsulphoxide. Values above are final concentrations
after the addition of substrate solution (see below).
The mixture above is incubated for fifteen minutes at 30~C at which time the
remaining elastase activity is measured for 10 minutes in a BioTek 3401 plate-
reader, after the addition of 0.6mM Me0-succinyl-alanyl-alanyl-prolyl-valyl-p-
nitroanilide. The rate of increase in absorbance at 405nm is proportional to
elastase activity. Enzyme activity is plotted against concentration of
inhibitor and
an IC50 determined using curve fitting software.
In vivo activity of inhibitors of human neutrophil elastase~
An oral in vivo model using IL-8 induced lun infiltrates for the assessment of
intracellular elastase inhibition
Adult hamsters (100-150g} are randomised into groups (n=4) and fasted
overnight. Under gaseous anaesthetic (3% isofluorane} animals are dosed orally
with 1mU100g water as vehicle or containing predissolved compounds. Either
7
CA 02303176 2000-03-07
WO 99/12933 pC'iy~p9g/p5609
at the same time, or subsequently under anaesthetic, animals are dosed
intratracheally with 1 ug recombinant human IL-8 in 100uL sterile saline. Six
hours after IL-8 dosing animals are sacrificed using intraperitoneal
pentobarbitone. The lungs are lavaged with 2 x 2.5 mL sterile saline and
femurs
are removed by dissection.
Intracellular elastase is prepared from neutrophils collected by lavage and
from
femoral bone marrow . This is achieved by sonication of the neutrophils and
centrifugation to yield intracellular granules. These are disrupted by
freeze/thawing and sonication. Elastase and myeloperoxidase assays are then
performed on these samples to assess the efficacy of the compounds and to
normalise for neutrophil recovery.
Human whole blood elastase inhibition assay
Triplicate aliquots of fresh, heparinised human whole blood (200p,1) are added
to
appropriately diluted samples (10p,1) of compounds under test. Control samples
(6 replicates) contain water in place of compound. Samples are mixed
thoroughly by pipette, and are then incubated for 30 minutes at 37°C.
Cold red
cell lysis buffer (750p,1 of 155mM ammonium chloride, 0.12mM EDTA, 10mM
potassium bicarbonate, pH 7.4) is then added. Tubes are capped, inverted
several times, and maintained at 4°C for 15 minutes, inverting every 5
minutes.
After centrifugation at 2508 for 10 minutes, at 4°C, the resulting
pelleted cells
are washed. The wash is with saline (300,1), followed by centrifugation at
1008
for 10 minutes at 4°C. Pellets are washed twice more, before
resuspension of
the final cell pellet in buffer (200w1 of 1 OOmM Tris, 300mM NaCI, 1 % (w/v)
HTAB,
pH 8.6). Samples are stored at -20 °C. After freeze thawing of the
samples four
times, elastase activity is determined by a coiorimetric assay in 50mM Tris,
8
CA 02303176 2000-03-07
WO 99/IZ933 PGT/EP98/05609
150mM NaCI, 0.6mM Me0-Succ-Ala-Ala-Ala-Pro-Val-pNA at pH 8.6, measuring
the rate of increase in absorbance at 405nm.
Acxordingly, the compounds of the invention are of potential therapeutic
benefit
in the treatment and amelioration of symptoms of diseases where elastase
activity is implicated. Such diseases particularly include bronchitis,
including
chronic bronchitis. Also any chronic obstructive pulmonary disease (COPD).
Examples of disease states in which the compounds of the invention have
potentially beneficial effects include inflammatory diseases of the
respiratory
tract such as bronchitis (including chronic bronchitis), bronchiectasis,
asthma
and hyper-reactivity states of the lung, acute respiratory distress syndrome
and
septic shock, inflammatory or destructive conditions of the lung such as
emphysema and cystic fibrosis and inflammatory or destructive conditions of
external tissue such as skin diseases (e.g. lupus and psoriasis) and
periodontal
disease including gingivitis.
Further examples of disease states and conditions in which compounds of the
invention have potentially beneficial effects include wound healing and
treatment of burns, cardiovascular diseases such as myocardial infarction and
stroke, peripheral vascular disease including intermittent claudication,
atherosclerosis, reperfusion injury, cardiovascular changes occurring during
cardiopulmonary bypass surgery and septicemia.
Compounds of the invention may also be useful in the treatment of connective
tissue disorders such as rheumatoid arthritis, osteoarthritis and spondylitis
and
inflammatory conditions of the kidney such as glomerulonephritis.
CA 02303176 2000-03-07
WO 99/12933 ~ PCT/EP98/05609
They may also be useful in the treatment of certain leukemias including acute
myelogenous leukemia, acute myelomonocytic leukemia and the chronic
monocytic leukemias and in prevention or inhibition of metastasis of solid
tumours e.g. lung, breast, prostate and stomach cancers and melanomas.
A particular aspect of the present invention is the use of compounds of
formula
(I) in the treatment of chronic bronchitis. Chronic bronchitis is a condition
which
results from the exposure of the airway surface to noxious chemicals or agents
or is secondary to another disease. The symptoms of the condition are caused
by the excessive secretion of mucus onto the surface of the airways. This
excess mucus cannot be cleared effectively and the result is reduced gas
exchange within the lungs resulting in laboured breathing and hypoxemia,
recurrent microbial infections and persistent cough associated with the
expectoration of mucoid material. The proposed mechanism for the excessive
secretion of mucus involves the recruitment of neutrophils into the airways
following the exposure of the epithelium to irritant materials; the
neutrophils
secrete elastase onto the surface of the airways and the enzyme brings about
both an increase in the amount of mucus secreted onto the airway surfaces and
a dramatic change in the cellular composition of the airway epithelium.
Inhibition
of elastase activity by the administration of compounds of this invention is
therefore an approach to the treatment of chronic bronchitis. Reduced lung
function in COPD (eg in chronic bronchitics with airflow obstruction) is also
due
to elastase mediated lung damage leading to airway narrowing and
inflammation. Thus an elastase inhibitor will improve lung function.
As indicated above, compounds of the invention are useful in human or
veterinary medicine, in particular as inhibitors of the enzyme neutrophil
elastase.
CA 02303176 2000-03-07
WO 99/12933. PCT/EP98/05609
Thus, there is provided as a further aspect of the present invention a
compound
of fom~ula (I) or a physiologically acceptable salt or solvate thereof for use
in
human or veterinary medicine, particularly in the treatment of conditions
where
elastase activity is implicated such as chronic bronchitis.
It will be appreciated that references herein to treatment extend to
prophylaxis
as well as the treatment of established conditions.
According to another aspect of the invention, there is provided the use of a
compound of formula (I) or a physiologically acceptable salt or solvate
thereof
for the manufacture of a medicament for the treatment of conditions where
elastase activity is implicated, particularly in chronic bronchitis.
In a further or alternative aspect there is provided a method for the
treatment of
a human or animal subject with a condition caused or mediated by elastase
activity which method comprises administering to said human or animal subject
an effective amount of a compound of formula (I) or a physiologically
acceptable
salt or solvate thereof.
The compounds according to the invention may be formulated for administration
in any convenient way, and the invention therefore also includes within its
scope
pham~aceutical compositions for use in therapy, comprising a compound of
formula (I) or a physiologically acceptable salt or solvate thereof in
admixture
with one or more physiologically acceptable diluents or carriers.
There is also provided according to the invention a process for preparation of
such a pharmaceutical composition which comprises mixing the ingredients.
x1
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
The compounds according to the invention may, for example, be formulated for
oral, buccal, parenteral, topical or rectal administration.
Tablets and capsules for oral administration may contain conventional
excipienfis
such as binding agents, for example syrup, acacia, gelatin, sorbitol,
tragacanth,
mucilage of starch or polyvinyl pyrrolidone; fillers, for example, lactose,
microcrystalline cellulose, sugar, maize- starch, calcium phosphate or
sorbitol;
lubricants, for example, magnesium stearate, stearic acid, talc, polyethylene
glycol or silica; disintegrants, for example, potato starch, croscarmellose
sodium
or sodium starch glycollate; or wetting agents such as sodium lauryl sulphate.
The tablets may be coated according to methods well known in the art. Oral
liquid preparations may be in the form of, for example, aqueous or oily
suspensions, solutions, emulsions, syrups or elixirs, or may be presented as a
dry product for constitution with water or other suitable vehicle before use.
Such
liquid preparations may contain conventional additives such as suspending
agents, for example, sorbitol syrup, methyl cellulose, glucose/sugar syrup,
gelatin, hydroxymethyl cellulose, carboxymethyl cellulose, aluminium stearate
gel or hydrogenated edible fats; emulsifying agents, for example, lecithin,
sorbitan mono-oleate or acacia; non-aqueous vehicles (which may include
edible oils), for example almond oil, fractionated coconut oil, oily esters,
propylene glycol or ethyl alcohol; or preservatives, for example, methyl or
propyl
p hydroxybenzoates or sorbic acid. The preparations may also contain buffer
salts, flavouring, colouring and/or sweetening agents (e.g. mannitol) as
appropriate.
For buccal administration the compositions may take the form of tablets or
lozenges formulated in conventional manner.
12
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
The compounds may also be formulated as suppositories, e.g. containing
conventional suppository bases such as cocoa buffer or other glycerides.
The compounds according to the invention may also be formulated for
parenteral administration by bolus injection or continuous infusion and may be
presented in unit dose form, for instance as ampoules, vials, small volume
infusions or pre-filled syringes, or in multi-dose containers with an added
preservative. The compositions may take such forms as solutions, suspensions,
or emulsions in aqueous or non-aqueous vehicles, and may contain formulatory
agents such as anti-oxidants, buffers, antimicrobial agents and/or toxicity
adjusting agents. Alternatively, the active ingredient may be in powder form
for
constitution with a suitable vehicle, e.g. sterile, pyrogen-free water, before
use.
The dry solid presentation may be prepared by frlling a sterile powder
aseptically
into individual sterile containers or by filling a sterile solution
aseptically into
each container and freeze-drying.
By topical administration as used herein, we include administration by
insufflation and inhalation. Examples of various types of preparation for
topical
administration include ointments, creams, lotions, powders, pessaries, sprays,
aerosols, capsules or cartridges for use in an inhaler or insufflator or drops
(e.g.
eye or nose drops).
Ointments and creams may, for example, be formulated with an aqueous or oily
base with the addition of suitable thickening and/or gelling agents andlor
solvents. Such bases may thus, for example, include water and/or an oil such
as
liquid paraffin or a vegetable oil such as arachis oil or castor oil or a
solvent such
as a polyethylene glycol. Thickening agents which may be used include soft
paraffin, aluminium stearate, cetostearyl alcohol, polyethylene glycols,
microcrystalline wax and beeswax.
13
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Lotions may be formulated with an aqueous or oily base and will in general
also
contain one or more emulsifying agents, stabilising agents, dispersing agents,
suspending agents or thickening agents.
Powders for external application may be formed with the aid of any suitable
powder base, for example, talc, lactose or starch. Drops may be formulated
with
an aqueous or non-aqueous base also comprising one or more dispersing
agents, solubilising agents or suspending agents.
Spray compositions may be formulated, for example, as aqueous solutions or
suspensions or as aerosols delivered from pressurised packs, with the use of a
suitable propellant, e.g. dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane, 1,1,1,2-
tetrafluorethane, carbon dioxide or other suitable gas.
Capsules and cartridges for use in an inhaler or insufflator, of for example
gelatin, may be formulated containing a powder mix of a compound of the
invention and a suitable powder base such as lactose or starch.
The pharmaceutical compositions according to the invention may also be used
in combination with other therapeutic agents, for example anti-inflammatory
agents such as corticosteroids or NSAIps, bronchodilators such as beta-2
adrenergic agonists and xanthines (e.g. theophylline), mucolytic agents, anti-
muscarinics, anti-leukotrienes, inhibitors of cell adhesion (e.g. ICAM
antagonists), anti-oxidants (eg N-acetylcysteine), lung surfactants andlor
antimicrobial and anti-viral agents. The compositions according to the
invention
may also be used in combination with gene replacement therapy.
14
CA 02303176 2000-03-07
WO 99/12933 . PCT/EP98/OS609
The invention thus provides, in a further aspect, a combination comprising a
compound of formula (I) or a physiologically acceptable salt or solvate
thereof
together with another therapeutically active agent.
The combination referred to above may conveniently be presented for use in the
form of a pharmaceutical formulation and thus pharmaceutical formulations
comprising a combination as defined above together with a pharmaceutically
acceptable carrier thereof represent a further aspect of the invention.
The individual components of such combinations may be administered either
sequentially or simultaneously in separate or combined pharmaceutical
formulations. Appropriate doses of known therapeutic agents will be readily
appreciated by those skilled in the art.
The compound of the invention may conveniently be administered in amounts
of, for example, 0.01 to 50mglkg body weight, suitably 0.05 to 25mg/kg body
weight orally, one or more times a day. The precise dose will of course depend
on the age and condition of the patient, the particular route of
administration
chosen, and the disease being treated. The compound is preferably
administered orally for the treatment of bronchitis. Other routes of
administration may be needed for other indications, for instance i.v. for
ARDS.
The compounds of the invention have useful duration of action.
The compounds of formula (I) and salts and solvates thereof may be prepared
by the methodology described hereinafter, constituting a further aspect of
this
invention.
is
CA 02303176 2000-03-07
WO 99/12933
PG"f/EP98/05609
A process according to the invention for preparing a compound of formula (I)
comprises:
(i) condensation of a compound of formula (II):
H s
,~~R
H
(II)
''~ O
H N
SOZ
R'
(relative stereochemistry indicated)
with a compound R4R3N(CH~"HetCOOH or an acid derivative thereof such as
an acid chloride, activated ester, acid anhydride, or a mixed anhydride or
with a
compound R4R3N(CH2)"HetXY, where Y is a reactive group such as halogen,
e.g. chlorine, or a protected derivative thereof; or
(ii) sulphonylation of a compound of formula (III):
R4R3N(CH2)~ Het-X~
N H
H
'~, ~ (III)
H p 0
(relative stereochemistry indicated)
or a protected derivative thereof
with a compound Y02SR' wherein Y is a reactive group such as halogen, e.g.
chlorine; or
(iii) cyciising a compound of formula (I~:
16
CA 02303176 2000-03-07
WO 99/12933 , PCT/EP98/05609
R°R3N-(CH~)"Het-X, a R2
(relative stereochemistry indicated)
or a carboxylic acid ester thereof; or
(iv) oxidation of a corresponding compound of formula (V)
R4R3N-(CHZj~ Het-X~ H
N
H
.,, ~ M
N O
H I
Xe
R'
(relative stereochemistry indicated)
wherein Xe is sulphur or SO; or
(v) reaction of a corresponding compound of formula (VI)
L(CH2j~ Het-X~ H
N ;R
H
N~)
.,. N O
H I
SOZ
R'
(relative stereochemistry indicated)
wherein L is a leaving group
with a compound of formula R4R3NH; or
(vi) preparation of a compound of formula f in which n represents an
integer 1 to 4 by reduction of the product of reaction of a corresponding
compound of formula (VII)
17
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
CHO-(CHz)~_~-Het-X~ H z
N R
H
wn)
H .,. N O
I
SOz
R'
(relative stereochemistry indicated)
with a compound of formula R4R3NH; or
(vii) preparation of a compound of formula I in which n represents 1 by
reaction of a corresponding compound of formula (VIII)
Het-X~ H z
N ;R
H
-., ~ "'rn
H N O
I
SOz
R'
(relative stereochemistry indicated)
with formaldehyde or paraformaldehyde together with a compound of formula
R4R3NH under acidic conditions; or
(viii) deprotecting a compound of formula (I) which is protected; or
(ix) purifying one enantiomer of the compound of formula (I) ftom a
mixture of enantiomers;
and where desired or necessary converting a resultant free base compound of
formula I into a physiologically acceptable salt form or vice versa or
converting
one salt form into another physiologically acceptable salt form.
Process i
The condensation reaction with R4R3N(CHz)nHetCOOH is suitably carried out in
the presence of a coupling agent such as 1-(3-N,N-dimethylaminopropyl)-3-
ethylcarbodiimide, preferably also in the presence of HOBT, and a solvent such
as dichloromethane, DMF, MeCN or tetrahydrofuran at a temperature of suitably
1s
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
between O°C and ambient. When an acid derivative such as the acid
chloride,
activated ester, acid anhydride, or a mixed anhydride is used, reaction
conditions will be modified accordingly, for instance by inclusion of a base.
If
one or both of R3, R'' represents hydrogen, it will generally be preferred to
protect the nitrogen, e.g. with BOC.
With R4R3N(CH2)"HetSOZY, the reaction is suitably carried out in the presence
of a base such as triethylamine and a solvent such as DCM, suitably at
0°C-
ambient.
Process ii
The sulphonylation reaction is suitably carried out in the presence of LHMDS
or
IdaH, in a solvent such as tetrahydrofuran at a temperature of suitably
between
-78°C to O°C.
When one or both of R3 and R4 represents hydrogen, it may be necessary to
protect the nitrogen, e.g. with BOC.
Process (iii)
The cyclisation reaction is suitably carried out in the presence of 2-chloro-1-
methylpyridinium iodide, or EDC, in a solvent such as dichloromethane, at a
temperature of suitably 0°C - reflux. This reaction may also be
performed
using a carboxylic acid thioester derivative of the compound of formula (IV).
Alternatively, another acid derivative such as an acid halide (e.g. acid
chloride)
may be used.
Process iv
This oxidation reaction may be carried out in conventional manner such as by
peracid oxidation.
Process v
I9
CA 02303176 2000-03-07
WO 99/iZ933 PC'T/EP98/05609
Preferred leaving groups include halogen (such as chlorine, bromine or
iodine),
mesylate and tosylate. The reaction may be performed by combining the
reactants optionally in the presence of a base such as triethytamine or
potassium carbonate in an inert solvent such as DMF or MeCN.
Process (vi)
This reaction will take place on combining the reagents in an inert solvent,
e.g.
DCM at ambient or elevated temperature.
Reduction can be performed in situ using a conventional mild reducing agent
such as NaBH3CN or NaBH(OAc)3.
Process (vii)
Reaction of the heterocyclic compound of formula (VIII) with formaldehyde or
paraformaldehyde and the amine will take place under standard Mannich
conditions, e.g. reflux under acid conditions, typically in acetic
acid/ethanol. If
the amine is used as an acid salt (e.g. the hydrochloride) the acetic acid may
be
omitted.
Process (viii)
Protecting groups, especially nitrogen protecting groups,. and means for
deprotection are described in T W Greene "Protective Groups in Organic
Synthesis", 2nd Ed (1991) J Wiley & Sons.
Process (ix)
Purification of a single enantiomer may be achieved by conventional methods
such as chiral chromatography (e.g. chiral HPLC) and crystallisation with a
homochtral acid (e.g. tartaric acid).
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
Physiologically acceptable acid salts of the compound of formula (I) such as
the
hydrochloride or tartrate may be prepared by treating a basic compound of
formula (I) with the desired acid.
Intermediate compounds of formula (II) may conveniently be prepared according
to the methodology in Scheme I below:
21
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Scheme 1
SMe ~~~
(a) (b)
~H PsNH COOH PzNH CONHZ
methlonine (IX) (X)
(c)
SMe
NHPT ~--~- ..
N
PzNH CONHP~ P2NH CONHPT
(X111) (XII)
(XI)
O
O PT ~ NHCOCF~
N~ (9) N
N~ ----~
(X~
(XI~
(h)
RZ~o~n
z
R CO~alkyl OSf(dkyl~ P, ~ ~~k~- NHCOCF
) N
P' ~ N NHCOCF~
(XVill)
ref-(2S, 3R)
RZ ~~ R2 0
NH
P \N 'o~'N~ ~ PT\
(X~) (k) N, '[ (XX)
~..J rel-(3S,3aS,6aR)
(I)
0
2
(m) R ~'"~ N-so,-r:'
(II) : P'\N
(XXI)
22
CA 02303176 2000-03-07
WO 99/12933 PC'T/EP98/05609
Ste a
This is a conventional protection reaction which, in the case when P2
represents
BOC, may be performed by reacting with (BOC)20 in the presence of base (e.g.
NaOH) in a polar solvent system such as dioxanlwater.
Ste b
This conversion may be performed on treatment with ammonium bicarbonate in
the presence of a suitable solvent such as pyridine/DMF and in the presence of
(BOC)20 or suitable equivalent.
Ste c
This is a conventional protection reaction which, in the case when PZ
represents
CBZ, may be performed by reaction with nBuLi followed by CBZ-CI in the
presence of an inert solvent such as THF below -50 °C.
Ste d
This reaction may be performed by treatment with RX where RX is a compound
(e.g. Mel, benzyl iodide or Me2S04) capable of converting sulphur in the SMe
moiety to sulphonium in a suitable solvent, e.g. propanone or acetonitrile.
Generally R will represent alkyl or aralkyl and X will represent halide,
especiaNy
iodide, or sulphate. Protection of the amide is convenient, although not
essential, for this reaction.
Ste a
This ring closure reaction may be performed by treatment with Dowex 2 x 8 400
mesh OH' resin in a suitable solvent, e.g. MeCN. Alternatively, the ring
closure
may be performed by treatment with potassium carbonate in a suitable solvent,
e.g. MeCN.
23
CA 02303176 2000-03-07
WO 99/12933
PGT/EP98/05609
Ste
Deprotection may be performed in a conventional manner, for example, a BOC
protecting group may be removed by treatment with HCI, e.g. in dioxan.
Ste
This reaction may be performed by treatment with a trifluoroacetic acid alkyl
ester (e.g. the methyl ester) or trifluoroacetic anhydride in the presence of
a
suitable base e.g. N-methylmorpholine.
Ste h
This conversion will take place on treating the compound of formula (XV) with
a
reducing agent e.g. lithium borohydride, followed by treatment with
concentrated
sulphuric acid in the presence of an alkyl alcohol e.g. ethanol solvent.
Ste i
The reaction of compounds of formula (XVI) and (XVII) takes place in the
presence of a Lewis acid e.g. boron trifluoride dietherate and an inert
solvent
e.g. dichloromethane or MeCN. The group "alkyl" in Oalkyl and OSi(alkyl)3
generally represents C~,~alkyl. In the compound of formula (XVII), suitable
alkyl
groups in the siiyl alkyl moiety include methyl, isopropyl and t-butyl.
Preferred
Oalkyl is OEt and prefer-ed OSi(alkyl)3 is OSi(i-Pr)3 or OSi(Me)2(t-Bu). The
use
of variants of compounds of formula (XVII) in which Oalkyl is replaced by
OSi(alkyl)3 is also envisaged.
Compounds of formula (XVII) may be prepared by treatment of the
corresponding carboxylic acid ester (R2CH2COOEt or another alkyl ester, which
compounds are either known or may be prepared by known methods) with a
strong base (eg LHMDS) followed by a trialkylsilylchloride (such as
trimethylsilylchloride) or a trialkylsilyltriflate. Typically the reaction
will be
24
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
performed at low temperature (less than 0 °C) in an inert solvent (such
as THF)
in the presence of DMPU.
Ste '
This deprotection reaction will take place on treatment with base, such as
potassium carbonate.
Step (k)
This ring closure reaction may be performed on treatment with an alkyl
Grignard
reagent (e.g. t-butylmagnesium choride) in an inert solvent such as THF in the
presence of tetramethytethylenediamine at a temperature of -20°C to
25°C.
Step (I)
This is a lactam sulphonylation reaction. It is suitably carried out by
reaction
with R~S02-Y, wherein Y is a reactive group, preferably chloro, in the
presence
of LHMDS, NaH or KH, in a solvent such as THF, at a temperature of suitably
-78° to 0°C.
Ste m
This is a N-deprotection reaction, which can suitably be carried out in
conventional manner. Thus when P~ is CBZ, it is suitably carried out by
hydrogenation over Pd (OH)2 catalyst in solvents such as ethyl acetate or THF.
Compounds of formula (X~ may also be prepared by following a route
described in Scheme 2:
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Scheme 2
(XXIII)
(a)
2HG ----.~ ~~ 2HG
N~ H=N Hz
(XXII)
1 (b)
0
NHCOCF3
HN
H N NHZ
(d)
0
P~ N NHCOCF~
Ste a
The reaction will proceed under standard conditions for forming alkyl esters,
for
example by treatment with an alcohol eg methanol in the presence of SOCl2.
R~3 is suitably a C~~alkyl group, preferably methyl.
Step (b)
The cyclisation reaction will take place on stirring in water with Dowex 2X8
(preferably 400 mesh).
Ste c
The TFA protected amine is formed by treating the compound of formula (XXI~
with methyl trifluoroacetate in a polar erotic solvent, eg MeOH.
Ste d
Suitable protecting groups P~ include CBZ. In this case, the compound of
formula (XX~ may be treated with a strong base such as LHMDS or nBuLi in an
inert solvent such as THF, followed by treatment with CBZ-CI.
26
CA 02303176 2000-03-07
WO 99/t2933 PCT/EP98/05609
An alternative route for preparation of compounds of formula (XX) from Scheme
1 is given in Scheme 3:
Scheme 3
OEt Et
P~_H . PI_N
I (XXVII)
OEt Pz OEt
(XXVI)
OH
P' N ~ COzR~3 ~ P~ _N CHO (XXVIII}
Pz (XXIX) ~c~ P,
1«
O N O (e) O N ~O (XXXI)
C02R~3 P~ 'N COzR~a
Pz H
(,
P~
H
N
H C02R~3
N ~ O
v
COzR~a (g) H N
(XXXII)
H N-P
gel-(2S,3R)
H (XXXIII) O
1 ~h~
27
CA 02303176 2000-03-07
WO 99/12933 pC"T/Eip98/p5609
Scheme 3 continued
Rz P~ Rz
N H , H ~ H H
~C''O2Rt3 -~~ --1 N Cp2H
H H'Ps
H NHZ .HCI
Ste a
The compounds of formula (XXVI) are either known compounds or may be
made in analogous manner to known compounds. P~ is a N-protecting group,
preferably CBZ. Step (a) is a further N-protection reaction. P2 in formula
(XXVII) is a different N-protecting group, preferably BOC. When P2 is BOC, the
reaction is suitably carried out using BOC2O.
Suitably the reaction is carried out in the presence of a base such as
triethylamine or 4-dimethylaminopyridine in a solvent such as ethyl acetate,
at
temperature of suitably 0°-25° C.
Step (b)
This conversion is suitably carried out with pyridinium p-toluenesulfonate, in
a
solvent such as acetonelwater, at a temperature suitably between 25°-
75° C.
Ste c
28
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/OS609
This is a condensation rearrangement reaction suitably carried out using a 2-
phenylsulfinyl acetic acid ester (PhSOCH2 COZR~3) and piperidine, in a solvent
such as acetonitrile, suitably at ambient temperature.
R~3 is suitably a C~.~alkyi group, preferably methyl.
Ste d
This is a Mitsunobu substitution reaction, using phthalimide, PPh3 and a
dialkylazodicarboxylate such as DEAD, in the presence of a solvent such as
THF, at a temperature of suitably 0°-40° C.
Ste a
This is a deprotection reaction, preferably using a strong acid such as TFA
in a solvent such as DCM, at a temperature of suitably 0°-40° C.
R~3 is suitably C~.~alkyl, preferably ethyl.
Ste
This is a cyclisation reaction, suitably carried out as an intramolecular
Michael
reaction. Suitably NaH is used, in a solvent such as THF, at a temperature
such
as 0° - 25° C.
Step (g)
In this step two reactions occur: N-deprotection and re-protection. The
phthalimido group is removed suitably with hydrazine hydrate in a solvent such
as ethanol at a temperature between 0°C and reflux. Protecting group P3
is
incorporated in a conventional manner. When P3 is BOC, this is suitably
achieved with BOC20.
Ste h
29
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
The R2 side chain may be introduced by alkylation, using as reactant R2-Y,
wherein Y is a reactive group such as bromo or iodo. Thus the reaction is
carried out using a base, preferably a strong base such as LHMDS. With
LHMDS suitably a cosolvent DMPU in THF is used. Suitable reaction
temperatures are -78° to 50°C. Under these conditions the
reaction generally
takes place with good stereochemicai control.
Ste i
This is an ester hydrolysis reaction, followed by a N-deprotection reaction.
The former is carried out in a conventional manner, for example by using KOH
in
aqueous ethanol, at a temperature of suitably 25°-80°C . The
latter is carried
out in a conventional manner, for example by using HCI in dioxan, at a
temperature of suitably 0°-50°C or, if the protecting group is
trifluoroacetate, by
treatment with base.
Step U)
This is a cyclocondensation reaction, suitably carried out in the presence of
2-chloro-1-methylpyridinium iodide and a suitable base such as N, N-
diisopropyl
ethylamine in a solvent such as dichloromethane, at a temperature of suitably
O°C-reflux: We have also found that it is possible to use the
compound of
formula (XXX~ as a carboxylic acid ester in which case the ester hydrolysis of
step (i) is not necessary. In this case the preferred conditions for the
cyclocondensation reaction involve the use of an alkyl Grignard reagent eg t
BuMgCI in THF at a temperature between -20°C and 25°C.
An alternative process for preparation of compounds of formula (XXX111) is
shown in Scheme 4:
Scheme 4
CA 02303176 2000-03-07
WO 99/IZ933 PCT/EP98/05609
diaminobutyric acid
(b')
O
~N COzH COzH
C02H
NHPs ~ ~ P~HN
H N NHPs
) (XXXVII) NHPs (XXXVIII)
(C)
CO=R,s
CHO
CH20H
8
PIHN ~ P HN ~~ (~ _ P~HN
NHPs ~ NHP ~ NHPs
s
I) (XXXX) (XXXIx)
1(~
P~.H
~zR~s
(xxxul)
H NHPs rel-(2S,3R)
Ste a
The compounds of formula (XXXVI) are either known compounds or may be
prepared in analogous manner to known compounds. P3 is a protecting group
as discussed above, and is suitably BOC. The reaction is suitably carried out
using PIFA (phenyl iodosylbis(trifluoroacetate)) and a base such as pyridine
in
an aqueous solvent, such as aqueous THF, dioxan or acetonitrile. This is the
method of Stansfield, C.F. Organic Preparations and Procedures Int., 1990,
22(5), 593-603.
Ste b
P~ is a protecting group eg CBZ. This protection reaction may be carried out
in
a conventional manner: For instance it is suitably carried out in a water
miscible
31
CA 02303176 2000-03-07
WO 99/12933 p~yEp9g/p5609
solvent such as THF, DMF or dioxan using N-
(benryloxycarbonyloxy)succinamide, benzyloxycarbonyl chloride, or any suitable
source of the benzyloxycarbonyl group, with pH adjustment to alkaline with
sodium carbonate.
As an alternative step (b'), the compound of formula (XXXVIII) can be prepared
in conventional manner from diaminobutyric acid.
Ste c
This reaction is suitably carried out in two stages. The first stage involves
reacting the compound of formula (XXXVIII) at reduced temperature with N-
m~thylmorpholine and then an alkyl chloroformate such as ethyl chloroformate,
in an organic solvent such as DCM, dioxan or THF. In the second stage the
product is reduced, suitably with sodium borohydride at reduced temperature,
such as -20° to 10°C, in a solvent such as THF.
Step (d)
This oxidation reaction may be carried out in any suitable manner, for
instance
using oxalyl chloride in DMSO and methylene dichloride under nitrogen at
reduced temperature, such as -30° to -70°C, followed by
triethylamine. The
intermediate (XXXX) suitably is not isolated.
Ste a
This reaction is suitably carried out using a Wittig reagent such as a
triphenylphosphorane R~~02CCH=PPh3, or may also be carried out using a
phosphonate in a Wadsworth-Emmons reaction.
Step (f)
32
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
This Michael addition reaction is suitably carried out using LHMDS or other
strong base in an organic solvent such as THF, ether or toluene, and
preferably
a complexing agent such as TMEDA is also present.
The intermediate compounds of formula (III) may be prepared by reacting a
deprotected compound of formula (XX) from Scheme 1 with
R4R3N(CH2)"HetCOOH or R4R3N(CH~"HetXY in the manner described above in
relation to main process (i) above.
(The initial N- deprotection may be carried out as described above under
Scheme 1 Step (m).
The intermediate compounds of formula (IV) may be prepared from a
compound of formula (XIX) (with the primary amine suitably protected) in an
analogous manner to that described above for preparing a compound of formula
(III) from a compound of formula (XX) together with main process (ii) above.
Compounds of formula (~ wherein Xa represents S may be prepared by
reaction of a corresponding compound of formula (III) with a compound of
formula R~SSR~ under standard conditions for nucleophilic displacement.
Compounds of formula (~ wherein Xa represents SO may be prepared by
peracid oxidation of a corresponding compound wherein Xe represents S.
Compounds of formula (VI), (VII) and (VIII) may be prepared from compounds of
formula (II) following conventional methods known per se. Mesylate and
tosylate derivatives may be prepared from the corresponding alcohol following
treatment with MeS02Cl or paramethylbenzenesulphonylchloride.
33
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Compounds of formula R''R3N(CHZ)"HetCOOH or an acid derivative thereof, and
R4R3N(CH2)~HetXY are either known or may be prepared by conventional
methods known per se.
It will be apparent that Schemes 1, 2, 3 and 4 may be modified to produce
homochiral products by using homochiral starting materials (e.g. S-methionine
in
Scheme 1 or S-diaminobutyric acid in Scheme 4) or by performing an additional
chiral resolution step.
If compounds of formula (XI~ in racemic form are prepared following Scheme 1
from racemic methionine, we have found that the isomers of the compounds of
formula (XIIn may be resolved by a dynamic resolution procedure. Thus a
raoemic compound of formula (XI~/j may be treated with homochiral di-p-toluoyl
tartaric acid in the presence of 3,5-dichloro-2-hydroxybenzaldehyde as
catalyst
in an inert solvent, e.g. THF. A homochiral salt of the compound of formula
(XI~ results. A compound of formula (X~ may then be produced by
subsequent treatment with trifluoroacetic acid methyl ester in the presence of
N-
methylmorpholine.
Both enantiomers of the compound of formula (XI~ may also be produced from
a synthesis based on S-rnethionine or R-methionine following similar
procedures.
It will be apparent to a person skilled in the art that the above synthetic
processes for the preparation of compounds of formula (I) may be modified so
as to include or omit protecting groups or so as to use alternative protecting
groups (for example those described in T W Greene "Protective Groups in
Organic Synthesis", 2nd Ed (1991) J Wiley & Sons) in the course of routine
optimisation of experimental conditions.
34
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/OSb09
Novel chiral intermediates in the above described chiral and resolution
sections
also form an important aspect of this invention.
Processes for preparation of intermediates are also provided as an aspect of
this invention.
The compounds of the invention have the advantage that they may be more
efficacious, show greater selectivity, have fewer side effects, have a longer
duration of action, be more bioavailable by the preferred route have more
attractive pharmacodynamic or pharmacokinetic properties or have other more
desirable properties than similar known compounds.
The following non-limiting Examples illustrate the present invention.
ABBREVIATIONS
BOC t-butyloxycarbonyl
CBZ Benzyloxycarbonyl
(BOC)20 Di-tert-butyldicarbonate
THF Tetrahydrofuran
LHMDS Lithium bis (trimethylsilyl)amide
DMPU 1,3-dimethyl-3,4,5,6-tetrahydro
2 (1H)-
pyrimidinone
DMAP 4-dimethylaminopyridine
DMF Dimethylformamide
EDC 1-(3-N, N-d imethylam inopropyl)-3-
ethylcarbodiimide
DEAD diethylazodicarboxylate
DCM dichloromethane
CA 02303176 2000-03-07
WO 99/12933
TMEDA tetramethylethylenediamine
DMSO dimethylsulphoxide
HOBT 1-hydroxybenzotriazole
NaBH(OAc)3 sodium triacetoxyborohydride
In the foregoing Intermediates and Examples, all T.Lc. experiments were
performed on silica plates.
Intermediates
Intermediate 1
2,4-Diamino-butyric acid methyl ester dihydrochloride
To D,L-diaminobutyric acid dihydrochloride (350g) in methanol (1.61) at
0°C was
added thionyl chloride (200m1) over %h. After reflux for 3h, the solvent was
removed in vacuo and the residue tritrurated with toluene (650m1) to give the
title compound as a white solid (385g).
Mass spec. of free base MH+ (found) 133 MH+ (calculated) 133
Intermediate 2
3-Amino-pyrrolidin-2-one
Intermediate 1 (1g), water (70m1) and Dowex 2x8-400 mesh (16.4m1) were
stirred for 1 h. The resin was then filtered and the filtrate concentrated _in
vacuo
to give the title compound as a white solid (0.40g), T.Lc (6:1 ethyl acetate:
methanol) Rf 0.07.
Intermediate 3
2,2,2-Trifluoro-N-(2-oxo-pyrrolidin-3-yl)-acetamide
A suspension of Intermediate 2 (181g), methyl trifluoroacetate (218m1) and
methanol (2.61) was suspended for 2h. The solvent was then removed in vacuo
to afford the title compound as a cream solid {355g).
36
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Mass spec. MNH4'' (found) 214 MNH4+ (calculated) 214
Intermediate 4
2-Oxo-3-(2,2,2-trifluoro-acetylamino)-pyrrofidine-1-carboxylic acid benzyl
ester
To intermediate 3 (3.5g) and tetrahydrofuran (100m1) at -70°C was
added
LHMDS (20m1). After'/<h, benzyl chloroformate (2.8m1) was added. The
mixture was warmed to room temperature for 1 h and 1 M hydrochloric acid
(25m1) added. After extraction with ethyl acetate (3x25m1), the combined
extracts were washed with 2% ammonia solution, 2M hydrochloric acid and
brine then dried (MgS04 ). After solvent removal, the white solid was
recrystallised from ethyl acetate: hexane 5:1 to give the title compound as
white
crystals (4.2g), T.Lc. (9:1 ethyl acetate: methanol) Rf 0.7.
Intermediate 5
2-Ethoxy-3-(2,2,2-trifluoro-acetylamino)-p rrolidine-1-carboxylic acid benzyl
ester
To Intermediate 4 (34g) in ethanol (1070m1) at -5°C was added
sodium
borohydride (9.86g). A solution of 4M hydrogen chloride in 1,4-dioxan (20m1)
was then added dropwise. Periodically further portions of 4M hydrogen chloride
in 1,4-dioxan (2x5m1, 1x10m1) and sodium borohydride (2g) were added. After
3h, concentrated sulphuric acid (11m1) was added and the mixture warmed to
room temperature for 2h. Saturated aqueous sodium bicarbonate (300m1) was
then added and the ethanol and dioxan removed in vacuo. The residue was
diluted with wafer (500m1) and extracted with ethyl acetate (3x500m1). The
combined extracts were washed with brine and dried (MgS04). The solvent was
removed in vacuo and the residue purled by flash chromatography on silica gel
9385 eluting with ether, to give the title compound as a solid (21g). Mass
spec.
MNH4+ (found) 378 MNH4' (calculated) 378
37
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Intermediate 6
trans-2-(1-Ethoxycarbonyl-2-methyl-propyl)-3-(2,2,2-trifluoro-acetylamino)
pyrrolidine-1-carboxylic acid benzyl ester
Intermediate 5 (10g), ethyl trimethylsilyl isopropylketene acetal (11m1) and
dichloromethane (250m1) were cooled to 5°C and boron trifluoride
dietherate
(17m1) added over'/.h. After 1 h, further boron trifluoride dietherate (3.4m1)
and
ketene acetal (11 ml) were added. After a further 1 h, 1 M hydrochloric acid
(200m1) was added and the organic layer separated and washed with brine and
dried (MgS04). Solvent removal in vacuo gave the title compound as an oil
(16.7g), T.Lc.(2:1 ether: cyclohexane) Rf 0.18 and 0.27.
Intermediate 7
trans-3-Amino-2-(1-ethoxycarbonyl-2-methyl-propyl)-pyn-olidine-1 carboxylic
acid benzyl ester
Intermediate 6 (31g), potassium carbonate (71g), water (930m1) and ethanol
(930m1) were warmed at 60°C for 3h. The ethanol was removed _in vacuo
and
the aqueous residue extracted with ethyl acetate (3x300m1). The combined
extracts were washed with brine and dried (MgS04) and concentrated _in vacuo
to give the title compound as a brown oil (17.5g).
Mass spec. MH'' (found)) 349 MH+ (calculated) 349
Intermediate 8
rel-(3R,3aR,6aS)-6-Isopropyl-5-oxo-hexah dro-pyrrolo[3,2-b]pyrrole 1-
carboxylic
acid benzyl ester
Intermediate 7 (17.5g) in tetrahydrofuran (1,800m1) was cooled to -5°C
and 1M t-
butylmagnesium chloride in tetrahydrofuran(204m1) was added over %Zh. After
2h, 1 M hydrochloric acid (250m1) and brine (300m1) were added and the mixture
was extracted with ethyl acetate (250m1). After concentrating the extracts to
half
the volume in vacuo, the extracts were washed with brine and dried (MgS04).
38
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Solvent removal in vacuo followed by trituration with diethyl ether (60m1)
gave a
white solid. This was recrystallised from ethyl acetate to give the title
compound
(3.4g). Mass spec. MH+ (found) 303 MH+ (calculated) 303
Intermediate 9
rel-(3R,3aR,6aS)-6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-pyrrolo[3,2-
b]pyrrole-1-carboxylic acid benzyl ester
To a stirred solution of Intermediate 8 (15.01g) in anhydrous tetrahydrofuran
(950m1) at -74°C under nitrogen, was added 1.OM LHMDS in
tetrahydrofuran
(69.5m1) dropwise. After stirring at -74°C for 10 min, the mixture was
allowed to
warm up to 0°C over 45 min, then left at this temperature for 20 min.
It was then
cooled to -76°C, treated dropwise with methanesulfonyl chloride (9.61
ml) and
left to stir at this temperature for 1.5h. It was then warmed to -50°C,
quenched
with saturated ammonium chloride solution (480m1) and allowed to warm up to
room temperature. The mixture was partitioned between water (300m1) and
ethyl acetate (750m1), the aqueous layer extracted with further ethyl acetate
(750m1), then the combined organic extracts washed with brine (450m1), dried
(Na2S04) and concentrated in vacuo to a cream solid. Purification by flash
column chromatography on silica (Merck 9385) eluting with ethyl acetate:
cyclohexane (1:3, 1:2, 1:1 then 3:1) gave the title compound as a white
crystalline solid (13.65g). T.Lc. (dichloromethane) Rf 0.22 Mass spec MNH4+
(found) = 398 MNH4+ (calculated) = 398
Intermediate 10
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b~pyrrol-
2-one
A suspension of Intermediate 9 (13.63g) in ethyl acetate (900m1) was added to
20% palladium hydroxide (moist) on carbon (3.16g) and the resulting black
suspension stirred vigorously under hydrogen at room temperature for 90 min.
39
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
The mixture was then filtered through Harborlite J2 and concentrated in vacuo
to
give the title compound as a ftne white powder (8.63g). Tlc
(Methanol:dichloromethane 1:9) Rf 0.50 Mass spec MH+ (found) = 247 MH+
(calculated) = 247
Intermediate 11
rel-5-(6R-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-(3aS,6aR)-pyrrolo[3,2-
b]pyrrole-1-carbonyl)-furan-2-carbaldehyde
To a stirred solution of Intermediate 10 (100mg) in acetonitrile (5ml) was
added
5-fomnyl-2-furoic acid (74mg) and 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide
hydrochloride (156mg). The reaction mixture was stirred for 3 days. The
acetonitrile was removed in vacuo and the residue was partitioned between
dichloromethane and saturated sodium bicarbonate solution. The organic layer
was washed with brine, dried (MgS04) and concentrated in vacuo. The residue
was purified by flash column chromatography (Merck 9385 silica; eluent
dichloromethane:acetonitrile 9:1 ) to give the title compound (80mg) as a
white
solid. Mass spec. MH' (found) 309 MH+ (calculated) 369.
Intermediate 12
rel-(3R,3aR,6aS)-3-(6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-
pyrrolo[3,2-b]pyrrole-1-carbonyl)-furan-2-carbaldehyde
To a stirred solution of Intermediate 10 (540mg) in acetonitrile (40m1) was
added
2-formyl-3-furoic acid (400mg) and 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (841 mg). The reaction mixture was stirred for
16h. The acetonitrile was removed in vacuo and the residue was partitioned
between dichioromethane and saturated sodium bicarbonate solution. The
aqueous layer was extracted with dichloromethane. The combined organics
were washed with brine, dried (Na2S04) and concentrated in vacuo. The residue
was purified by flash column chromatography (Merck 9385 silica; eluent
CA 02303176 2000-03-07
WO 99/12933 pCT/EP98/05609
dichloromethane:acetonitrile 9:1 ) to give the title compound (626mg) as a
cream
solid. Mass spec. MH+ (found) 369 MH+ (calculated) 369.
Intermediate 13
rel-(3R,3aR,6aS)-5-(6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-
pyrrolo[3,2-b]pyrrole-1-carbonyl)-thiophene-2-carbaldehyde
To a stirred solution of Intermediate 10 (250mg) in acetonitrile (10m1) was
added
5-formylthiophene-2-carboxylic acid (206mg) and 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (389mg). The reaction mixture was stirs-ed for
3h. The acetonitrile was removed in vacuo and the residue was partitioned
between dichloromethane and saturated sodium bicarbonate solution. The
aqueous layer was extracted with dichloromethane. The combined organics
were washed with brine, dried (MgS04) and concentrated in vacuo to leave a
foam. The foam was purified by flash column chromatography (Merck 9385
silica; eluent dichloromethane:acetonitrile 9:1) to give the title compound
(280mg) as a cream solid. Mass spec. MH+ (found) 385 MH+ (calculated) 385.
Intermediate 14
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(1 H-pyrrole-2-carbonyl)-
hexahydro-p~~rrolo[3,2-b]pyrrol-2-one
A solution of pyrrole-2-carboxylic acid (60mg), 1-hydroxybenzotriazole (81
mg),
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (115mg) and
triethylamine (101 mg) in dimethylformamide (3ml) was stirred for 5 min. then
treated with Intermediate 10 (100mg). The reaction mixture was stirred for 6h.
then partitioned between 8% sodium bicarbonate solution (25m1) and ethyl
acetate (50m1). The organic phase was separated, washed with water (2x50m1)
and the solvent removed in vacuo to leave a solid. A suspension of the solid
in
diethyl ether (25m1) was stirred for 10 min. then filtered under suction. The
residue was dried to give the title compound (123mg) as a white powder.
41
CA 02303176 2000-03-07
WO 99/12933 pCT/EP98/05609
Melting point 200-203°C Mass spec. MH' (found) 340 MH+
(calculated} 340.
Intermediate 15
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(1-methyl-1 H-pyrrole-2-
carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
A solution of 1-methylpyrrole-2-carboxylic acid (150mg), 1-
hydroxybenzotriazole
(180mg), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (250mg)
and triethylamine (240mg) in dimethylformamide (5ml) was stirred for 15 min.
then treated with Intermediate 10 (246mg). The reaction mixture was stirred
for
18h. then partitioned between 2% sodium bicarbonate solution (135m1) and
ethyl acetate (150m1). The organic phase was separated, washed with water
(2x120m1), dried (Na2S04) and the solvent removed in vacuo to leave a semi-
solid. The semi-solid was suspended in diethyl eater (20m1) and the suspension
was stirred for 10 min. The ether was decanted and replaced by more ether
(10m1). The resultant suspension was stirred for 10 min. The ether was again
decanted and the residue dried to give the title compound (227mg} as a pale
brown solid. Melting point 176-178°C Mass spec. MH+ (found) 354 MH+
(calculated) 354.
Intermediate 16
~2;2-Dimethyl-propionyloxymethyl)-thiazole-4-carboxylic acid
A mixture of a-bromopyruvic acid (1.85g), 1-(tert-butylcarbonyfoxy)
thioacetamide (1.75g) and activated 4 Angstrom molecular sieves (10g) in
ethanol (100m1) was stirred for 24h. The solvent was removed in vacuo and
replaced by dichloromethane (100m1). The resultant suspension was stirred for
5
min. then filtered through Harborlite J2. The filtrate was evaporated to leave
a
solid, which was dissolved in ethyl acetate, dried (Na2SOA) and the solvent
removed in vacuo to give the title compound (1.83g) as a pale yellow, waxy
solid.
42
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Melting point 155-158°C Mass spec. MH+ (found) 244 MH+
(calculated) 244.
Intermediate 17
2-Hydroxymethyl-thiazole-4-carboxylic acid
A solution of Intermediate 16 (1.78g) and potassium carbonate (1.80g) in
methanol (90m1) and water (30m1) was stirred and heated at reflux for 4.5h.,
cooled, concentrated to 30m1, acidified with 2M hydrochloric acid and
extracted
with dichloromethane (5x60m1), then concentrated in vacuo to leave a solid
which was extracted with a hot (2:1 ) mixture of industrial methylated spirits
and
ethy) acetate (2x150m1). These extracts were combined with the
dichloromethane extracts and the solvents were removed in vacuo. The residual
gum was crystallised from diethyl ether to give the title compound (834mg) as
a
brown powder. Melting point 121-127°C Mass spec. MH+ (found) 160 MH+
(calculated) 160.
Intermediate 18
rel-(3S,3aS,6aR)-4-(2-Hydroxymethyl-thiazole-4~carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo(3,2-b]pyrrol-2-one
A solution of Intermediate 17 (787mg), Intermediate 10 (1.OOg), triethylamine
(655mg), 1-hydroxybenzotriazole (718mg) and 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (997mg) in dimethylformamide (5ml) was stirred
for 17h. then partitioned between 8% sodium bicarbonate solution (250m1) and
ethyl acetate (250m1). The aqueous phase was separated and extracted with
ethyl acetate (250m1). The combined organics were washed with 0.5M
hydrochloric acid (2x200m1) and water (2x200mi), dried (Na2S04) and the
solvent removed in vacuo to give a foam. The foam was suspended in diethyl
ether (100m1), with stirring, for 5 min. The resultant solid suspension was
filtered
under suction. The residue was dried to leave the title compound (1.23g) as a
43
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
brown powder. Melting point 197-201°C Mass spec. MH+ (found) 388 MH+
(calculated) 388.
Intermediate 19
2-Dimethylaminomethyl-thiazole-4-carboxylic acid
A mixture of a-bromopyruvic acid (370mg), 1-(dimethylamino)thioacetamide
hydrochloride (300mg) and sodium bicarbonate (200mg) in ethanol (25m1) was
stirred and heated at reflux for 1.5h. Potassium carbonate (213mg) was added
and refluxing was maintained for 0.75h. The reaction mixture was cooled and
the solvent was removed in vacuo. The residue was stirred in ethyl acetate
(30m1) for 0.5 h. The solvent was decanted. The residual solid was partitioned
between 0.5M hydrochloric acid (16m1) and ethyl acetate {20m1). The aqueous
phase was separated and concentrated in vacuo to leave a gum. The gum was
treated with methanol (20m1) and filtered. The filtrate was concentrated in
vacuo
and the residue dried to leave the title compound (430mg) as a dark brown
powder. Mass spec. MH+ (found) 187 MH+ (calculated) 187.
Intermediate 20
5-Fonnyl-isoxazole-3-carboxylic acid
A solution of ethyl-5-formylisoxazole-3-carboxylate (25mg) in 1,4-dioxan (3ml)
and 2M hydrochloric acid (1 ml) was stirred and heated at reflux for 5h.;
cooled
and the solvents removed in vacuo. The residue was triturated in diethyl
ether.
The solvent was removed and the residue dried to leave the title compound
(l8mg) as an orangelbrown solid. T.Lc. (dichloromethane:methanol 9:1)
Rf = 0.32.
Intermediate 21
rel-(3R,3aR,6aS)-3-(6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-
pyrrolo(3,2-b]pyrrole-1-carbonyl)-isoxazole-5-carbaldehyde
44
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
To a stirred solution of Intermediate 10 (670mg) in acetonitrile (50m1) was
added
Intermediate 20 (500mg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (1.04g). The reaction mixture was stirred for 4h. The
acetonitrile
was removed in vacuo and the residue was partitioned between
dichloromethane and saturated sodium bicarbonate solution. The organic phase
was separated and passed through a Varian SPE bond elution silica cartridge,
eluting sequentially with dichloromethane, chloroform, diethyl ether, ethyl
acetate, acetonitrile and methanol. Fractions containing the required product
were combined and evaporated to give the title compound (660mg) as a white
solid. T.Lc. (dichloromethane:acetonitrile 9:1) Rf = 0.33.
Intermediate 22
1-Methyl-5-styryl-1 H-pyrazole-3-carboxylic acid ethyl ester
A solution of (E)-ethyl-2,4-dioxo-6-phenylhex-5-enoate (40g) and
methylhydrazine (9g) in ethanol (250m1) was heated at reflux for 2h. The
solvent
was removed in vacuo and the residue was purified by flash column
chromatography on silica, using a (1:1) mixture of diethyl ether and
cyclohexane
as the eluent. Fractions containing the more polar of the two major new
components were combined and the solvent evaporated to leave the title
compound (25.4g) as yellow crystals. T.Lc. (diethyl ether:cyclohexane 1:1) Rf
=
0.14.
Also isolated from this reaction was Intermediate 23:
Intermediate 23
2-Methyl-5-styryl-2H-pyrazole-3-carboxylic acid ethyl ester
Fractions containing the less polar of the two major new components obtained
from the chromatographic purification of Intermediate 22 were combined and the
solvent evaporated to leave the title compound (11.4g) as a yellow oil.
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
T.Lc. (diethyl ether:cyclohexane 1:1 ) Rf = 0.62.
Intermediate 24
5-Formyl-1-methyl-1 H-pyrazole-3-carboxylic acid ethyl ester
Ozone was bubbbled through a stirred solution of Intermediate 22 (156mg) in
ethyl acetate (10m1) at -78°C for 2h. Nitrogen was then bubbled through
the
solution, triphenylphosphine (500mg) was added, the solution was warmed to
room temperature and the solvent was removed in vacuo. The product was
passed through a Varian SPE bond elution silica cartridge, eluting
sequentially
with dichloromethane, chloroform and diethyl ether. Fractions containing the
required product were combined and evaporated to give the title compound
(127mg) as white crystals. T.f.c. (diethyl ether:cyclohexane 1:1) Rf = 0.58.
Intermediate 25
5-Formyl-1-meth-1 H-pyrazole-3-carboxylic acid
A solution of Intermediate 24 (1.02g) in 1,4-dioxan (10m1) and 2M hydrochloric
acid (10m1) was stirred and heated at reflux for 24h. The reaction mixture was
cooled and the solvent was removed in vacuo to leave the title compound as a
pale yellow solid (0.85g). T.Lc. (dichloromethane:methanol 9:1) Rf = 0.19
(streaking).
Intermediate 26
rel-(3R,3aR,6aS)-5-(6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-
p~~rrolo[3,2-b]pyrrole-1-carbonyl)-2-methyl-2H-pyrazole-3-carbaldehyde
To a stirred solution of Intermediate 10 (783mg) in acetonitrile (60m1) were
added Intermediate 25 (645mg) and 1-(3-dimethyiaminopropyl)-3-
ethylcarbodiimide hydrochloride (1.22g). The reaction mixture was stirred
overnight. The acetonitrile was removed in vacuo and the residue was
partitioned between dichloromethane and saturated sodium bicarbonate
46
CA 02303176 2000-03-07
WO 99/I2933 PCT/EP98/OS609
solution. The organic phase was separated and passed through a Varian SPE
bond elution silica cartridge, eluting sequentially with dichloromethane,
chloroform, diethyl ether and ethyl acetate. Fractions containing the required
product were combined and evaporated to give the title compound (315mg} as a
white solid. T.Lc. (dichloromethane:acetonitrile 9:1) Rf = 0.24.
Interrnediate 27
rel-(3R,3aR,6aS)-5-(6-isopropyl-4-methanesulfonyl-5-oxo-hexahydro-
pyrrolo[3,2-b]pyrrole-1-carbonyl)-2-methyl-2H-pyrazole-3-carbaldehyde
Ozone was bubbbled through a stirred solution of Intermediate 23 (124mg) in
ethyl acetate (15m1) at -78°C for 3h. Nitrogen was then bubbled through
the
solution, triphenylphosphine (500mg) was added, the solution was warmed to
room temperature and the solvent was removed in vacuo. The product was
passed through a Varian SPE bond elution silica cartridge, eluting
sequentially
with dichloromethane, chloroform and diethyl ether. Fractions containing the
required product were combined and evaporated to give the title compound
(62mg) as white crystals. T.Lc. (diethyl ether:cyclohexane 1:1) Rf = 0.70.
Intermediate 28
5-Formyl-2-methyl-2H-pyrazole-3-carboxylic acid
A solution of Intermediate 27 (1.Og) in 1,4-dioxan (10m1) and 2M hydrochloric
acid (10m1) was stirred and heated at reflux overnight. The reaction mixture
was
cooled and the solvent was removed in vacuo to leave the title compound as a
pale yellow solid (0.8g). T.Lc. (dichloromethane:methanol 9:1) Rf = 0.54
(streaking).
Intermediate 29
rel-(3R,3aR,6aS)-5-(6-Isoprop,~l-4-methanesulfonyl-5-oxo-hexahydro-
pyrrolo[3,2-b]pyrrole-1-carbonyl)-1-methyl-1 H-pyrazole-3-carbaldehyde
47
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98I05609
To a stirred solution of Intermediate 10 (812mg) in acetonitrile (45m1) was
added
Intermediate 28 (660mg) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (1.26g). The reaction mixture was stirred for 22h. The
acetonitrile
was removed in vacuo and the residue was partitioned between
dichloromethane and saturated sodium bicarbonate solution. The organic phase
was separated and passed through a Varian SPE bond elution silica cartridge,
eluting sequentially with dichloromethane, chloroform, diethyl ether and ethyl
acetate. Fractions containing the required product were combined and
evaporated to give the title compound (955mg) as a white solid.
T.Lc. (dichloromethane:acetonitrile 9:1) Rf = 0.22.
Intermediate 30
6-Bromomethyl-nicotinic acid
A mixture of methyl 2-(bromomethyl)pyridine-5-carboxylate (3.8g) and bis(tri-
"butyltin)oxide (16.5m1) in toluene (80m1) was stirred and heated at
80°C for 24h.
The reaction mixture was cooled and extracted with 2M hydrochloric acid
(2x50m1). The combined aqueous extracts were washed with toluene (40m1) and
concentrated in vacuo to leave the title compound (3.Og) as a yellow/brown
solid. Mass spec. MH+ (found) 216,218 MH'' (calculated) 216,218.
Intermediate 31
rel-(3R,3aR,6aS)-4-(6-Chloromethyl-pyridine-3-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
To a stirred suspension of Intermediate 3.0 (177mg) and 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (236mg) in acetonitrile
(5ml) was added Intermediate 10 (100mg). The reaction mixture was stirred for
2h. The acetonitrile was removed in vacuo and the residue was partitioned
between dichloromethane (40m1) and 2M sodium carbonate solution (40m1). The
organic phase was separated, washed with 2M sodium carbonate solution
48
CA 02303176 2000-03-07
WO 99/12933 PCTlEP98/05609
(20m1) and water (20m1), dried (MgS04) and concentrated to leave an oil. The
oil
was purified using flash column chromatography on silica, with
dichloromethane:acetonitrile (7:3) as the eluent, to give the title compound
(76mg) as a white foam. T.Lc. (dichloromethane:acetonitrile 7:3) Rf = 0.45.
Intermediate 32
5-Bromomethyl-pyrazine-2-carboxylic acid methyl ester
A mixture of Methyl(5-methyl)pyrazine-2-carboxylate (5.3g), N-
bromosuccinimide (6.3g) and dibenzoylperoxide (0.33g) in carbon tetrachloride
(125m1) was stirred and heated at reflux, with irradiation using a 200W
tungsten
lamp, for 5h. The reaction mixture was cooled, washed with 10% sodium
sulphite solution (2x20m1), water (20m1) and saturated brine (15m1), dried
(MgS04) and concentrated to leave an oil. The oil was purified using flash
column chromatography on silica, with cyclohexane:ethyl acetate (3:2) as the
eluent, to give the title compound (3.8 g) as a brown solid. T.Lc.
(cyclohexane:ethyl acetate 3:2) Rf = 0.28.
Intermediate 33
5-Bromomethyl-pyrazine-2-carboxylic acid hydrochloride
A mixture of Intermediate 32 (3.48g) and sodium hydroxide (6.OOg) in water
(40m1) was stirred for 2h.;acidified with 2M hydrochloric acid and extracted
with
ethyl acetate (4x30m1). The combined extracts were washed with saturated
brine (15m1), dried (MgS04) and the solvent removed in vacuo to leave the
title
compound (2.58g) as a pale yellow solid. Mass spec. MH'' (found) 217,219
MH+ (calculated) 217,219.
Intermediate 34
rel-(3R,3aR,6aS)-4-(5-Chloromethyl-pyrazine-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
49
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/OS609
To a stirred solution of Intermediate 10 (800mg) and 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (934mg) in acetonitrile
(10m1) was added Intermediate 33 (1.23g). The reaction mixture was stirred for
18h. The acetonitrile was removed in vacuo and the residue was partitioned
between dichloromethane (20m1) and 2M sodium carbonate solution (25m1). The
aqueous phase was separated and extracted with dichloromethane (2x20m1).
The combined organics were washed with water (15m1) and saturated brine
(15m1), dried (MgS04) and concentrated to leave an oil. The oil was purified
using flash column chromatography on silica, with dichloromethane:acetonitrile
(7:3) as the eluent, to give the title compound (562mg) as a white foam.
T.Lc. (dichloromethane:acetonitrile 7:3) Rf = 0.42.
Intermediate 35
2R-(2,2,2-Trifluoro-acetylamino)-succinamic acid
To a stirring suspension of D-Asparagine (37.98, powdered and dried at
110°C
for 48hrs) in methanol (144m1, dried over 3A sieves for 5 hours) under an
atmosphere of nitrogen was added triethylamine (40.2m1) followed by methyl
trifluoroacetate (36m1). The resumng mixture was left to stir for 48hrs. To
the
reaction mixture was added dry methanol (145m1) then Dowex 50 resin H+ form
(1158, dried at 56°C for 24 hours). The resultant mixture was stin~ed
for 10
minutes, filtered and the solvent removed in vacuo to give a crude white solid
containing the title compound. This crude product was combined with crude
product from a similar experiment and recrystallised from hot water to afford
the
title compound as a white crystalline solid (106g). Mass spec MNH4+
(found) 246 MNH4+ (calculated) 246
Intermediate 36
2R-(2,2,2-Trifluoro-acetylamino)-succinamic acid methyl ester
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
A stirring solution of Intermediate 35 (95.14g) in Methanol (1150m1, dried
over
3A molecular sieves) was cooled to -70°C. Acetyl chloride (162m1) was
slowly
added whilst maintaining the reaction temperature below -60°C. The
reaction
mixture was allowed to warm to -20°C and was left for 48 hours at this
temperature. The solvent was removed in vacuo to give a clear and colourless
oil containing the title compound. This was triturated with diethyl ether and
the
resultant white solid was recrystallised from boiling water to afford the
title
compound as a white crystalline solid (42g). Mass spec MH+ {found) 243
MH+ (calculated) 243
Intermediate 37
3-Cyano-2R-(2,2,2 trifluoro-acetylamino)-propionic acid methyl ester
To a stirring suspension of Intermediate 36 (3.Og) in dichloromethane (20m1)
was added pyridine {4.92m1) and p-toluene sutfonyl chloride (4.92g). More
dichloromethane (15m1) was added and the brown solution left to stir at room
temperature for 48 hours. The reaction mixture was diluted with
dichloromethane (25m1), washed with 1 M aqueous H3P04 (74m1), dried
(Na2S04) filtered and the solvent removed in vacuo to give a crude brown solid
(3.57g) containing the title compound. The crude mixture was purified by flash
chromatography (Si02, Merck, 9385) eluting with 1:3 then 1:2'/z ethyl
acetate:cyclohexane. The eluent was evaporated in vacuo to give the title
compound as a white crystalline solid (1.62g). T.L.C (1:1 Ethyl acetate:
cyclohexane) Rf 0.5 Mass spec MNH4~' (found) 242 MNH4'' (calculated)
242
Intermediate 38
2,2,2-Trifluoro-N-(2-oxo-pyn-olidin-3R-yl)-acetamide
A solution of Intermediate 37 (200mg) in ethanol (10m1) was stirred under an
atmosphere of hydrogen gas with 5% Rhodium on alumina (1.OOg) for 3 hours.
51
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98105609
The catalyst was removed by filtration and the filtrate concentrated in vacuo
to
afford a crude gum containing the title compound. The mixture was purified by
flash chromatography (Si02, Merck, 9385) eluting with acetonitrile. The eluent
was evaporated in vacuo to afford the title compound as a white solid (40mg).
T.L.C (Acetonitrile) Rf 0.63 Mass spec MNH4+ (found) 214 MNH,,
(calculated) 214
Intem~ediate 39
2-Oxo-3R-(2,2,2-trifluoro-acetytamino)-pyrrolidine-1-carboxylic acid benzyl
ester
To a stirring solution of Intermediate 38 (1.04g) in tetrahydrofuran cooled to
-
70°C, was added n-butyl lithium (1.6M in hexanes, 3.31 ml). After 5
minutes
benzylchloroformate (833.1) was added and the reaction mixture was allowed to
warm to room temperature. After 2'/z hours the reaction mixture was diluted
with
ethyl agitate (100m1) and washed with 1 M hydrochloric acid (2x150m1). The
combined organic extracts were dried (MgS04), filtered and concentrated in
vacuo to give a crude orange/white solid which was purified by trituration
with
diethyl ether to afford the title compound as a white solid (1.25g). Mass spec
MNH4+ (found) 348 MNH4+ (calculated) 348 Chiral HPLC (Chiracel AD,
eluent system ethanol:heptane 15:85, flow rate = 1 mUmin). Retention time of R
enantiomer =10.08 min (71.8%). Retention time of S enantiomer =12.50 min
(28.2%)
Intermediate 40
2-Ethoxy-3R-(2,2,2-trifluoro-acetylamino)-pyrrolidine-1-carboxytic acid benzyl
ester
Intermediate 39 (100mg) was dissolved in dry tetrahydrofuran (1ml), cooled to -
20°C and lithium borohydride (2.OM in THF, 0.15m1) added. After' hour
ethanol (1ml) was added followed by concentrated H2S04 (33w1) and the
resultant stirring solution was left at room temperature for 3%z hours. The
52
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98105609
reaction mixture was adjusted to pH8-9 by addition of saturated aqueous
sodium bicarbonate and the organic solvents were removed in vacuo. The
resultant residue was partitioned between ethyl acetate (20m1) and water
(l0ml)
and the acqueous phase extracted with further ethyl acetate (10m1). The
combined organic layers were dried (Na2S04), filtered and concentrated in
vacuo to afford the title compound as a clear oil (101mg) which was used
without further purfication. Mass Spec. MNH4+ (found) 378
MNH4+(calculated) 378
Intermediate 40 (Alternative Synthesis)
2-Ethoxy-3R-(2,2,2-t~~luoro-acetylamino)-pyrrolidine-1-carboxylic acid benzyl
ester
A solution of Intermediate 39 (214.8g) in dry THF (1200m1) was stirred and
cooled to -30°C. Lithium borohydride (2.OM in THF, 336m1) was added
(after an
initial temperature rise to -12°C, the temperature was maintained below
-17°C
throughout the addition). The mixture was stirred at -20°C for 90
minutes before
ethanol (760m1) was added to the mixture whilst maintaining the temperature
below -19°C. A cooled mixture of concentrated sulphuric acid (75m1) in
ethanol
(215m1) was slowly added to the mixture whilst maintaining the internal
temperature below -18°C. The cooling bath was removed and the reaction
left
to stir for 90 minutes, whereupon the internal temperature had risen to
+15°C. A
saturated solution of sodium bicarbonate (1600m1) was carefully added to the
mixture over 35 min before removal of the volatiles in vacuo. The residual
aqueous phase was extracted with ethyl acetate (1000m1 + 2x800m1) the
combined extracts washed with brine (800m1), dried (Na2S04) overnight and the
solvent removed in vacuo to give the title compound (211.6g) as an orange oil.
Tlc (4:1; CH2C 12: Et20) Rf = 0.64 and 0.43
Intermediate 41
53
CA 02303176 2000-03-07
WO 99/11933 PCT/EP98/05609
(2S, 3R)-2-(rel-1 S-Ethoxycarbonyl-2-methyl-propyl)-3-(2, 2, 2-trifluoro-
acetylamino)-pyrrolidine-1-carboxylic acid benzyl ester
Intermediate 40 (90mg), (1-Ethoxy-3-methyl-but-1-enyloxy)-triisopropyl-silane
(Intemlediate 95 ftom International Patent Application No W097/36903) (0.22g)
and dichloromethane (1.1 ml) were cooled to 5°C and boron trifluoride
dietherate
(0.15m1) added. After 55 min the reaction was quenched with 2M aqueous
sodium bicarbonate (15m1) and diluted with dichloromethane (10m1). The
aqueous layer was separated and the organic layer was washed with a
saturated aqueous solution of sodium chloride (10m1). The organic extract was
dried (MgS04), filtered and concentrated in vacuo to afford the title compound
as a colourless oil (106mg). Mass spec MH+ (found) 445 MH+
(calculated) 445
Intermediate 41 (Alternative Synthesis)
(2S,3R)-2-(rel-1 S-Ethoxycarbonyl-2-methyl-propyl)-3-(2,2,2-trifluoro-
acetylamino)-pyrrolidine-1-carboxylic acid benzyl ester
Intem~ediate 40 (97.9g), (Z)-(1-ethoxy-3-methyl-but-I-enyloxyl-triisopropyl-
silane)
(233g) and dichloromethane (600m1) were cooled to 5°C under nitrogen
and
boron trifluoride diethyl etherate (200m1) added over 15 minutes. After a
further
15 minutes, 2M sodium carbonate (750m1) was added, keeping the temperature
below 20°C. The reaction mixture was filtered through Hyflo and the
solid
material washed with dichloromethane (2x200m1). After adding the washes to
the 2-phase mixture the aqueous layer was separated and extracted with
dichloromethane (2x400m1). The combined extracts were washed with brine
(2x250m1), dried (MgS04) and concentrated in vacuo to give the title compound.
(154g). Tlc Si02 (1:3 ; ethyl acetate:cylcohexane) Rf = 0.49 (~i-anomer), 0.42
(a-anomer). Mass spec. (found) MH'' = 445 (talc) MH+ = 445
Intermediate 42
54
CA 02303176 2000-03-07
WO 99/I2933 ~ PCT/EP98/05609
(2S,3R)-3-Amino-2-( 1-ethoxycarbonyl-2-methyl-propyl)-pyrrolidine-1-carboxylic
acid benzyl ester
intermediate 41 (97mg), potassium carbonate (300mg), ethanol (2ml) and water
(2ml) were warmed at reflux for 2'/4 hours. The ethanol and water were
evaporated. in vacuo and the residue was partitioned between ethyl acetate
(l0ml) and water (10m1). The aqueous extract was taken to pH9-10 by addition
of 2M aqueous sodium hydroxide solution and extracted with diethyl ether
(3x20m1). The combined organic extracts were dried (MgS04), filtered and
concentrated in vacuo to afford the title compound as a clear oil (56mg).
Intermediate 42 (Alternative Synthesis)
(2S,3R)-3-Amino-2-(1-ethoxycarbonyl-2-methyl-propyf)-pyrrolidine-1-carboxylic
acid benzyl ester
Intermediate 41 (153g), potassium carbonate (183.3g), ethanol (1000m1) and
water (1000m1) were refluxed together for 5h. The organic layer was then
separated and concentrated in vacuo. The residue, the aqueous layer and brine
(200m1) were extracted with ether (2x500m1, + 250m1) and the combined
extracts extracted with 1 M hydrochloric acid (3x500m1). The combined acidic
extracts were then taken to pH8 with solid sodium hydrogen carbonate (150g)
and extracted with dichloromethane (600m1, + 3x300mi). The combined
dichloromethane extracts were dried (MgS04) and concentrated in vacuo to
afford the title compound (87.9g). Tlc SI02 (100:8:1
dichloromethane:ethanol:ammonia) Rf = 0.55 Mass spec (found) MH+ = 349
(talc) MH~ = 349
Intermediate 43
(3aR,6S,6aS)-6-Isopropyl-5-oxo-hexahydro-pyrrolo[3,2-b]pyrrole-1-carboxylic
acid benzyl ester
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98I056~9
Intermediate 42 (50mg) was dissolved in tetrahydrofuran (1 ml) and
tetramethylethylenediamine (1 ml) then 1 M t-butylmagnesium chloride in
tetrahydrofuran (0.4m1) added. After stirring for 3 hours the reaction was
quenched with saturated ammonium chloride solution (1 ml). The aqueous layer
was separated and extracted with ethylacetate (4ml). The combined organic
extracts were evaporated in vacuo. The residue was partitioned between
dichloromethane (10m1) and 2M hydrochloric acid (10m1). The aqueous phase
was separated and extracted with dichloromethane (3x5m1). The combined
organic extracts were dried (MgS04), filtered and concentrated in vacuo to
give
a crude white solid containing the title compound. Purfication by flash
chromatography (Si02, Merck, 9385) eluting with 1:1 ethyl acetate:cyclohexane
afforded the title compound as a white solid (16mg). T.L.C (2:1 ethyl
acetate:cyclohexane) Rf 0.38 Chiral HPLC (chiracel AD Column, eluent
system ethanol:heptane 10:90, flow rate 1mllmin). Retention time of RRS
lactam = 9.92min (73.6%). Retention time of SSR lactam = 13.12min (26.40)
Intermediate 44
~3aR,6S,6aS)-6-Isopropyl-4-methanesulfonyl-5-oxo-hexahydro-pyrrolo[3,2-
b]pyrrole-1-carboxylic acid benzyl ester
To Intermediate 43 (0.46g) in dry tetrahydrofuran (30m1) at -70°C under
nitrogen
was added 1 M lithium hexamethykiisilazide in tetrahydrofuran (2.Oml). The
solution was warmed to 0°C for 15 minutes and then retooled to -
70°C when
methane sulphonyl chloride (0.30m1) was added. After 1.5 hours, saturated
aqueous ammonium chloride was added (30m1) and the mixture extracted with
ethyl acetate (3x5m1). The combined extracts were washed with brine (2x25m1),
dried (MgS04) and the solvent removed in vacuo. Flash chromatography of the
residue on silica with 1:1 ethyl aoetate:cyclohexane gave the title compound
as
a white solid (0.34g). T.Lc Si02(1:1 ethyl acetate:cyclohexane) Rf 0.4 Mass
spec MNH4+ (found) = 398 MNH4+ (calculated) = 398
56
CA 02303176 2000-03-07
WO 99/12933 PC"T/EP98/05609
Intermediate 45
(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-
one
Intermediate 44 (0.31 g), 10% palladium hydroxide on carbon (0.24g) 1,4-dioxan
(25m1) and ethyl acetate (25m1) were mixed under hydrogen for 3 hours. The
catalyst was then removed by filtration through hyflo and the filtrate
concentrated in vacuo to afford the title compound as a pale yellow, solid
(0.20g). T.Lc Si02(9:1 chloroform: methanol) Rf = 0.36 Mass spec MH''
(found) = 247 MH+ (calculated) = 247
Intermediate 46
(3S,3aS,6aR~4-(5-Chloromethyl-pyrazine-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
To a stirred solution of Intermediate 45 (900mg) and 1-(3-dimethylaminopropyl)-
3-ethylcarbodiimide hydrochloride (1.25g) in acetonitrile (25m1) was added
Intermediate 1 (1.16g). The reaction mixture was stirred for 1 h and further
acetonitrile (25m1) was added. The mixture was stirred for 19 hours before the
acetonitrile was removed in vacuo and the residue was partitioned between
dichloromethane (30m1) and 2M sodium carbonate solution (30m1). The
aqueous phase was separated and extracted with dichloromethane (2x30m1).
The combined organics were dried (MgS04), filtered and concentrated to leave
a brown foam. The foam was purified using flash column chromatography on
silica (Merck 9385), with 20% acetonitrileldichloromethane as the eluent. The
required fractions were evaporated to dryness in vacuo to give the title
compound (1.095g) as a white foam.
T.Lc. (20% acetonitrile/dichloromethane) Rf = 0.52.
Mass spec. MH+ (found) 401,403, MH+ (calc) 401,403
57
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Intermediate 47
5-((tert-Butoxycarbonyl-cyclopropyl-amino)-methyl]-pyrazine-2-carboxylic acid
Bromine was added to a stirred suspension of 2-methylpyrazin-5-carboxylic acid
(60g} in acetic acid (300m1). The reaction mixtire was then heated to
80°C for
one hour. The solvent was evaporated in vacuo and the residue partitioned
between ethyl acetate (250m1) and 2M aqueous HCI (250m1). The aqueous
phase was extracted with further ethyl acetate (5x250m1), the combined
organics were washed with 2M HCI (100m1) and saturated brine solution
(100m1), dried (MgS04), filtered and the solvent evaporated in vacuo to leave
a
brown solid. The solid was stirred in acetonitrile (900m1} and triethylamine
(60m1) and cyclopropylamine (30m1) were added. After stirring for 20 hours at
room temperature, further cyclopropylamine (30m1) was added and the mixture
stirred for a further 15 minutes. The volatiles were evaporated in vacuo, and
the
residue partitioned between ethyl acetate (200m1) and 2M aqueous HCI (300m1).
The organic phase was extracted with further 2M HCI (4x200m1), the combined
aqueous extracts were washed with ethyl acetate (50m1), cooled in an ice bath
and based with 10M aqueous sodium hydroxide (120m1). The solution was
washed with ethyl acetate (3x200m1) and diethyl ether (200m1), and remaining
organic volatiles were removed in vacuo to give a brown aqueous solution. To
the solution was added 1,4-dioxane (500m1) and di-tert-butyldicarbonate (71g)
and the mixture was stirred at room temperature for 20 hours. Further di-tert-
butyldicarbonate (10g) was added and stirring continued for a further 24
hours.
Citric acid (85g) was added to the stirred mixture before extracting it with
ethyl
acetate (2x200m1+3x150m1+2x100m1). The combined extracts were dried
(MgS04), filtered and the solvent removed in vacuo to give a brown oil which
was purified by flash column chromatography on silica (Merck 9385), with
100:8:1 dichloromethanelmethanol/acetic acid as the eluent. The required
fractions were evaporated to dryness in vacuo to give a tan coloured solid
which
58
CA 02303176 2000-03-07
W0 99/12933 PGT/EP98/05609
was stirred vigorously in 5:1 cyclohexaneldiethyl ether until finely divided.
The
solid was filtered off and dried in vacuo to give the title compound as a
orange/brown solid (16.65g).
T.Lc. (100:8:1 dichloromethane/methanoUacetic acid) Rf = 0.31.
Mass spec. MH'' (found) 294, MH'' (calc) 294
Intem~ediate 48
Cyclopropyl-[5-(6S-isopropyl-4-methanesutfonyl-5-oxo-hexahyd ro-(3aR,6aS)-
pyrrolo[3,2-b]pyrrole-1-carbonyl)-pyrazin-2-ylmethyl]-carbamic acid tert-butyl
ester
Intermediate 45 (11.36g), Intemnediate 47 (13.53g) and O-(7-azabenzotriazol-1-
yIrN,N,N',N' tetramethyluronium hexafluorophosphate (19.3g) were stirred in
acetonitrile (260m1) at room temperature and N,N-diisopropylethylamine (1fiml)
added. After stirring for two hours, the solvent was removed in vacuo, the
residue diluted with dichloromethane (250m1) and washed with 1 M sodium
carbonate solution (250m1). The aqueous phase was extracted with
dichloromethane (3x150m1). The combined organics were washed with 1 M
sodium carbonate solution (50m1), dried (MgS04), filtered and the solvent
evaporated in vacuo to leave a yellow-brown solid. The solid was purred by
flash column chromatography (Merck 9385 silica) and eluted with 50% ethyl
acetate/cyclohexane, and the required fractions evaporated to dryness in vacuo
to give the title compound as a white foam (21.55g)
Mass spec. MH+ (found) 522, MH+ (calc) 522.
[a]o2° +69.5 (c = 0.8, MeCN)
Intem~ediate 49:
2-Pyrrolidin-1-ylmethyl-oxazole-4-carboxylic acid ethyl ester
To a stirred solution of 2-(bromomethyl)oxazole-4-carboxylic acid ethyl ester
(43.9g) in acetonitrile (300m1) was added pyrrolidine (15.7m1). After stirring
for
59
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
minutes more pyrrolidine (7.8m1) was added. After a further 30 minutes the
solvent was removed in vacuo to leave an orange oil. The oil was partitioned
between 1 M sodium carbonate (400m1) and dichloromethane (500m1) and the
phases were separated. The organic phase was washed with water (100m1),
5 dried (MgS04), filtered and the solvent removed in vacuo to give the title
compound as an orange oil (24.Og).
Mass spec MH'' (found) = 225. MH+ (calculated) = 225.
Intermediate 50
10 2-Pyrrolidin-1-ylmethyl-oxazole-4-carboxylic acid methyl ester
Pyrrolidine (14mg) was added to a suspension of potassium carbonate (25mg)
and 2-(bromomethyl)oxazole-4-carboxylic acid methyl ester (27.5mg) in
acetonitrile (2.5m1). The reaction mixture was stirred for 6h. The solvent was
removed in vacuo. The residue was partitioned between ethyl acetate (15m1)
and water (5m1). The organic phase was dried (Na2S04) and the solvent
removed in vacuo to give the title compound (23mg) as a pale brown oil.
Mass spec MH'' (found) = 211. MH+ (calculated) = 211.
The following Intermediates 51-55 were prepared in a similar manner to
Intermediate 2 from 2-(bromomethyl)oxazole-4-carboxylic acid methyl ester:
Intermediate 51:
2-[(Cyclopropyl-methyl-amino)-methyl]-oxazole-4-carboxylic acid methyl ester
~~drochloride
isolated as the hydrochloride: a pale brown gum.
Mass spec MH+ (found) = 211. MH+ (calculated) = 211.
Intermediate 52:
2-[(Dicyclohexylamino)-methyl]-oxazote-4-carboxylic acid methyl ester
CA 02303176 2000-03-07
WO 99/12933 PC'T/EP98/05609
Pale yellow, waxy solid. Mass spec MH~ (found) = 321. MH+ (calculated) _
321.
Intermediate 53:
2-Piperidin-1-ylmethyl-oxazole-4-carboxylic acid methyl ester
Pale brown, waxy solid. Mass spec MH+ (found) = 225. MH+ (calculated) _
225.
Intermediate 54:
2-(4-Phenyl-piperazin-1-ylmethyl)-oxazole-4-carboxylic acid methyl ester
Pale yellow, waxy solid. Mass spec MH~ (found) = 302. MH+ (calculated) _
302.
Intermediate 55:
2-Dibutytaminomethyl-oxazole-4-carboxylic acid methyl ester
Viscous, pale yellow oil. Mass spec MH+ (found) = 269. MH+ (calculated) _
269.
Intermediate 56:
2-Pyrrolidin-1-ylmethyl-oxazole-4-carboxylic acidl2-Pyrrolidin-1-ylmethyl-
oxazole-4-carboxylic acid potassium salt
First Preparation:
Potassium carbonate (14.8g) was added to a solution of Intermediate 49 (24.Og)
in ethanol (150m1) and water (150m1). The reaction mixture was refluxed with
stirring for 4h. The solvent was removed in vacuo. The orangelbrown residue
was azeotroped with toluene (x3) and then dried in vacuo. The solid obtained
was stirred vigorously with ether (100m1) and filtered off before drying in
vacuo
to give a mixture of the title compound and potassium bicarbonate as a brown
solid (34.5g). This material was used without further purfication.
61
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Second Preparation:
A solution of Intermediate 50 (22mg) in dioxan (1.5m1) and 1.OM. sodium
hydroxide {0.3m1) was stirred for 5.Oh. The solution was neutralised (pH ca 7)
by
the dropwise addition of 2.OM. hydrochloric acid. The solvents were removed in
vacuo and the solid residue was dried further in vacuo to give a mixture of
the
title compound and sodium chloride as a pale yellow solid {40mg).
Mass spec MH+ (found) = 197. MH+ (calculated) = 197.
The following Intermediates 57-59 were prepared in a similar manner to
Intermediate 56 (second preparation) from Intermediates 53-55 respectively.
Intermediate 57:
2-Pii~eridin-1-yimethyl-oxazole-4-carboxylic acid
Cream solid. Mass spec MH'' (found) = 211. MH+ (calculated) = 211.
intermediate 58:
2 ~4-Phenyl-piperazin-1-ylmethyl)-oxazole-4-carboxylic acid
White solid. Mass spec MH+ (found} = 288. MH+ (calculated) = 288.
Intermediate 59:
2-Dibutylaminomethyl-oxazole-4-carboxylic acid
Pale yellow semi-solid. Mass spec MH+ (found) = 255. MH+ (calculated) _
255.
Examples
In the foregoing, dihydrochloride salts are indicated by the qualification
°(2:1)"
after the chemical name.
Example 1
62
CA 02303176 2000-03-07
WO 99/12933
PCT/EP98/05609
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-pyrrolidin-1-ylmethyl-
furan-
2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of intermediate 11 (80mg) and pyrrolidine (17mg) in dichloromethane
(6ml) was stirred for 2h. Sodium triacetoxyborohydride (69mg) was added and
stirring was continued overnight. The reaction mixture was washed with 8%
aqueous sodium bicarbonate solution and water. The organic phase was
passed through a Varian SPE bond elution silica cartridge (which had been pre-
conditioned by eluting a column volume of dichloromethane), eluting
sequentially with dichloromethane, chloroform, diethyl ether, a (1:1) mixture
of
diethyl ether and ethyl acetate, ethyl acetate, acetonitrile and methanol.
Fractions containing the required product were combined and evaporated to
give a gum which was treated with 1.OM. hydrogen chloride in diethyl ether to
give the title compound as a yellow solid (80mg). Mass Spec MH+ (found) _
424 MH+ (calculated) = 424 T.Lc. (dichloromethane: methanol 9:1 ): Rf =
0.27.
The following Examples 2-4 were prepared in a similar manner to Example 1
from Intermediate 11:
Example 2
rel-(3R, 3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-more holin-4-ylmethyl-
furan-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream solid: Mass Spec MH'' (found) = 440 MH+ (calculated) = 440
T.Lc. (dichloromethane:methanol 9:1 ): Rf = 0.60.
Example 3
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[5-(4-phenyl-piperazin-1-
yimethyl)-furan-2-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Red solid. Mass Spec MH+ (found) = 515 MH+ (calculated) = 515
63
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
T.Lc. (dichloromethane:methanol 9:1): Rf = 0.65.
Example 4
rel-(3R,3aR,6aS)-3-isopropyl-1-methanesulfonyl-4-[5-(4=methyl-piperazin-1
ylmethyl)-furan-2-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochlorid_e_
White solid. Mass Spec MH+ (found) = 453 MH+ (calculated) = 453
T.Lc. (dichloromethane:methanol 9:1): Rf = 0.16.
Example 5
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesutfonyl-4-(2-morpholin-4-ylmethyl-
furan-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 12 (50mg) and morpholine (l3mg} in dichloromethane
(3.5m1) was stirred for 1.5h. Sodium triacetoxyborohydride (43mg) was added
and stirring was continued overnight. The reaction mixture was diluted with
dichloromethane and extracted with 2M hydrochloric acid. The aqueous extracts
were made basic with 8% aqueous sodium bicarbonate solution and extracted
with dichloromethane. The dichloromethane extracts were washed with brine,
dried (Na2S04) and the solvent removed in vacuo to leave a solid, which was
treated with 1.OM. hydrogen chloride in diethyl ether to give the title
compound
as a cream solid (32mg). Mass Spec MH'" (found) = 440 MH+ (calculated)
= 440 T.Lc. (dichloromethane:ethanol:ammonia 100:8:1}: Rf = 0.68.
The following Examples 6-10 were prepared in a similar manner to Example 5
from Intermediate 12:
Example 6
rel-(3R,3aR,6aS)-4-(2-Dimethylaminomethyl-furan-3-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream solid. Mass Spec MH+ (found} = 398 MH+ (calculated) = 398
64
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.70.
Example 7
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(2-piperidin-1-ylmethyl-furan-
3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Beige solid. Mass Spec MH+ (found) = 438 MH+ (calculated) = 438
T.l.c. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.68.
Example 8
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-ylmethyl-
furan-
3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Yellow solid. Mass Spec MH' (found) = 424 MH+ (calculated) = 424
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.67.
Example 9
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfon I-4-[2-(4-phenyl-piperazin-1-
ylmethyl)-furan-3-carbonyl]-hexahydro-pyn-olo[3,2-b]pyrrol-2-one hydrochloride
Yellow solid. Mass Spec MH+ (found) = 515 MH'' (calculated) = 515
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.86.
Example 10
rel-(3R, 3aR, 6aS)-3-Isopropyl-1-methanesulfonyl-4-[2-(4-methyl-piperazin-1-
ylmethyl) furan-3-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream solid. Mass Spec MH+ (found) = 453 MH' (calculated) = 453
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.50.
Example 11
rel-(3R,3aR,6aS)-4-(5-Dimethylaminomethyl-thiophene-2-carbonyl)-3-isopropyl-
1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
A mixture of Intermediate 13 (60mg), dimethylammonium chloride (36mg) and
sodium triacetoxyborohydride (66mg) in dichloromethane (6ml) was stirred
overnight. The reaction mixture was diluted with dichloromethane and extracted
with 2M hydrochloric acid. The aqueous extracts were washed with
dichloromethane then made basic with 2M sodium carbonate solution and
extracted with dichloromethane. These dichloromethane extracts were washed
with brine, dried (MgS04) and the solvent removed in vacuo to leave a gum,
which was treated with 1.OM. hydrogen chloride in diethyl ether to give the
title
compound as a yellow solid (15mg). Mass Spec MH+ (found) = 414 MH'
(calculated) = 414 T.Lc. (dichloromethane:ethanol:ammonia 100:8:1 ): Rf =
0.44.
The following Example 12 was prepared in a similar manner to Example 11 from
Intermediate 13:
Example 12
rel-(3R, 3aR, 6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-piperid in-1-ylmethyl-
thiophene-2-carbonyl)-hexahydro-pyrroloL,2-b]pyrrol-2-one hydrochloride
Yellow solid. Mass Spec MH'' (found) = 454 MH+ (calculated) = 454
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.52.
Example 13
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(5-morpholin-4-ylmethyl-1 H-
pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 14 (80mg), paraformaldehyde (28mg) and morpholine
(40mg) in ethanol (4ml) and glacial acetic acid (1.5m1) was stirred and heated
at
reflux for 18h. The mixture was cooled to room temperature and partitioned
between 8% aqueous sodium bicarbonate solution (25m1) and ethyl acetate
(25m1). The aqueous phase was further extracted with ethyl acetate (30m1). The
66
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
combined organic phases were dried (Na2S04) and the solvent evaporated in
vacuo to leave a gum. The gum was purfied by flash column chromatography
(Merck 9385 silica; eluent dichloromethane:ethanol:ammonia 200:8:1 ) to give a
white powder (21 mg) which was treated with 1.OM. hydrogen chloride in diethyl
ether to give the title compound as a white powder (22mg) Melting Point 184-
188° Mass Spec MH+ (found) = 439 MH~ (calculated) = 439
The following Examples 14-20 were prepared in a similar manner to Example 13
from Intermediate 14:
Example 14
rel-(3S,3aS,6aR)-4-(5-Dimethylaminomethyl-1 H-pyrrole-2-carbonyl)-3-isopropyl-
1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White powder, Melting Point 233-236°C
Mass Spec MH+ (found) = 397 MH+ (calculated) = 397
T.Lc. (Dichloromethane:ethanol:ammonia 100:8:1) Rf = 0.45
Example 15
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4.-(5-piperidin-1-ylmethyl-1 H-
pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White powder, Melting Point 160-164°C
Mass Spec MH+ (found) = 437 MH+ (calculated) = 437
Example 16
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(5-pyrrolidin-1-ylmethyl-1 H-
p~~rrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White powder, Melting Point 175-178°C
Mass Spec MH' (found) = 423 MH'' (calculated) = 423
67
CA 02303176 2000-03-07
WO 99/11933 PCT/EP98/05609
Example 17
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-[5-(4-phenyl-piperazin-1-
ylmethyl)-1 H-pyrrole-2-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride (1:2)
Cream powder, Melting Point 156-160°C
Mass Spec MH+ (found) = 514 MH+ (calculated) = 514
Example 18
rel-(3S, 3aS,6aR)-3-1 sopropyl-1-methanesu Ifonyl-4-[5-(4-methyl-piperazin-1-
ylmethyl)-1 H-pyrrole-2-carbonyl]-hexahydro-pyrrolo[3,2-b]pyn-ol-2-one
hydrochloride (1:2)
Cream powder, Melting Point 177-181°C
Mass Spec MH+ (found) = 452 MH'' (calculated) = 452
Example 19
rel-(3S,3aS,6aR)-4-(5-Dibutylaminomethyl-1 H-pyrrole-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Pale yellow powder, Melting Point 116-120°C
Mass Spec MH+ (found) = 481 MH+ (calculated) = 481
Example 20
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(5-methylaminomethyl-1 H-
pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream powder, Melting Point 210-215°C
Mass Spec MH+ (found) = 383 MH'' (calculated) = 383
Example 21
68
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
rel-(3S, 3aS,6aR)-3-Isopropyl-1-methanesu Ifonyl-4-( 1-methyl-5-piperid i n-1-
ylmethyl-1 H-pyrrole-2-carbonyl)-hexahydro-pyrrol~3,2-b]pyrrol-2-one
hydrochloride
A mixture of Intermediate 15 (40mg), paraformaldehyde (l5mg) and piperidine
(23mg) in ethanol (3ml) and glacial acetic acid (1.5m1) was stirred and heated
at
reflux for 22h. The mixture was cooled to room temperature and partitioned
between 8% aqueous sodium bicarbonate solution (30m1) and ethyl acetate
(20m1). The organic phase was dried (Na2S04) and the solvent evaporated in
vacuo to leave a gum. The gum was purled by flash column chromatography
(Merck 9385 silica; eluent dichloromethane:ethanol:ammonia 100:8:1 ) to give a
white solid (26mg) which was treated with 1.OM. hydrogen chloride in diethyl
ether to give the title compound as a white powder (28mg). Melting Point 149-
153°C Mass Spec MH' (found) = 451 MH+ (calculated) = 451
The following Examples 22-25 were prepared in a similar manner to Example 21
from Intermediate 15:
Example 22
rel- 3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(1-methyl-5-morpholin-4-
ylmethyl-1 H-pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Pate yellow powder, Melting Point 150-154°c
Mass Spec MH~ (found) = 453 ~ MH+ (calculated) = 453
Example 23
rel-(3S,3aS,6aR)-4-(5-Dimethylaminomethyl-1-methyl-1 H-pyrrole-2-carbonyl)-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White powder, Melting Point 137-141°C
Mass Spec MH+ (found) = 411 MH+ (calculated) = 411
69
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/OS609
Example 24
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(1-methyl-5-pyrrolidin-1-
ylmethyl-1 H-pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Yellow powder, Melting Point 135-139°C
Mass Spec MH+ (found) = 437 MH+ (calculated) = 437
Exami~le 25
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(1-methyl-5-
methylaminomethyl-1 H-pyrrole-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
White powder, Melting Point 216-218°c
Mass Spec MH+ (found) = 397 MH+ (calculated) = 397
Example 26
rel-(3S,3aS,6aR)-4-~4-Dimethylaminomethyl-1 H-pyrrole-2-carbonyl)-3-isopropyl-
1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 14 (80mg), paraformaldehyde (25mg),
dimethylammonium chloride (38mg) and activated 4 Angstrom molecular sieves
(200mg) in ethanol (10m1) was stirred and heated at reflux for 24h. The
mixture
was cooled to room temperature and tha solvent was evaporated in vacuo to
leave a gum. The gum was purified by flash column chromatography, using two
columns (Merck 9385 silica; eluent dichloromethane:ethanol:ammonia; 80:8:1
for the first column, 100:8:1 for the second column) to isolate a white powder
(16mg) which was treated with 1.OM. hydrogen chloride in diethyl ether to give
the title compound as a white powder (16mg). Melting Point 160-165°c
Mass Spec MH+ (found) = 397 MH+ (calculated) = 397
T.l.c (dichloromethane:ethanol:ammonia 100:8:1 ) Rf = 0.20
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/OS609
Example 27
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl~-(2-pyrrolidin-1-ylmethyl-
thiazole-4-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A solution of Intermediate 18 (387mg) and triethylamine (202mg) in
dichloromethane (42m1) was stirred and treated with methanesulphonyl chloride
(172mg). The reaction mixture was stirred for 1.5h. An aliquot (7ml) was
removed and added to a stirred solution of pyrrolidine (30mg) in
dichloromethane (2ml). The solution was stirred for 2 days. Aqueous 8% sodium
bicarbonate solution (12m1) was added, with vigorous stirring. The aqueous
phase was separated and extracted with dichloromethane (15m1). The organic
phases were combined and dried (Na2S04). The solvent was removed in vacuo
to leave a semi-solid, which was triturated in diethyl ether (10m1) to give a
solid
suspension. Cyclohexane (l0ml) was added to the suspension and the solvent
was decanted. The residual solid was dried in vacuo to leave a white solid
which
was treated with 1.OM. hydrogen chloride in diethyl ether to give the title
compound as a cream powder {51 mg). Melting Point 130-134°c Mass Spec
MH'' (found) = 441 MH+ (calculated) = 441
The following Examples 28-35 were prepared in a similar manner to Example 27
from Intermediate 18:
Example 28
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(2-morpholin-4-ylmethyl-
thiazole-4-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Pale buff powder, Melting Point 138-143°C
Mass Spec MH+ (found) = 457 MH+ (calculated) = 457
Example 29
71
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4.-(2-piperidin-1-ylmethyl-
thiazole-4-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream powder, Melting Point 153-158°C
Mass Spec MH+ (found) = 455 MH+ (calculated) = 455
Example 30
ref-(3S, 3aS,6a R)-3-Isopropyl-1-methanesulfonyl-4-[2-(4-methyl-piperazin-1-
ylmethyl)-thiazole-4-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride (1:2)
White powder, Melting Point 153-158°C
Mass Spec MH+ (found} = 470 MH+ (calculated) = 470
Example 31
rel-(3S,3aS,6aR)-4-1;-2-Cyclopropylaminomethyl-thiazole-4-carbonyl)-3-
isopropyl-
1-methanesulfonyl-hexahydro-pyn-olo[3,2-b]pyrrol-2-one hydrochloride
Pale grey powder, Melting Point 160-163°c
Mass Spec MH'' (found) = 427 MH'' (calculated) = 427
Example 32
rel-(3S;3aS,6aR)-4-{2-[(4-Fluoro-benzylamino)-methyllthiazole-4-carbonyl}-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Pale yellow powder, Melting Point 141-145°c
Mass Spec MH+ (found) = 495 MH+ (calculated) = 495
Example 33
rel~3S, 3aS, 6aR)-3-Isopropyl-1-methanesu Ifonyl-4-[2-(4-phenyl-piperazin-1-
ylmethyl)-thiazole-4-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride (1:2)
Cream powder, Melting Point 156-161°c
72
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
Mass Spec MH' (found) = 532 MH'' (calculated) = 532
Example 34
rel-(3S, 3aS,6aR)-4-(2-Dibutylaminomethyl-thiazole-4-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Pale yellow powder, Melting Point 81-86°C
Mass Spec MH+ (found) = 499 MH+ (calculated) = 499
Example 35
rel-(3R,3aR,6aS)-3-Isopropyl-4-{2-[(1-isopropyl-2-methyl-propylamino)-methyl]
thiazole~-carbonyl}-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Cream powder, Melting Point 192-195°C
Mass Spec MH+ (found) = 485 MH+ (calculated) = 485
Example 36
rel-(3S,3aS,6aR)-4-(2-Dimethylaminomethyl-thiazole-4-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b~pyrrol-2-one hydrochloride
Oxalyl chloride (127mg) was added to a stirred suspension of Intermediate 19
(125mg) in dichloromethane (10m1), followed by dimethylformamide (1 drop).
The reaction mixture was stirred for 1.Oh. then concentrated in vacuo. The
residue was suspended in dichloromethane (15m1) and treated, with stirring;
with
Intermediate 10 (43mg) and sodium bicarbonate (175mg). After stirring the
reaction mixture for 16h aqueous 8% sodium bicarbonate solution (12m1) was
added. The aqueous phase was separated and extracted with dichloromethane
(15m1). The combined organic extracts were dried (Na2S04) and evaporated to
give a brown gum. The gum was chromatographed on silica (Merck 9385), using
a mixture of dichloromethane, ethanol and ammonia (160:8:1 ) as the eluent, to
?3
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
give a brown gum, which was treated with 1.OM. hydrogen chloride in ether to
give the title compound (23mg) as a cream solid. Melting Point 122-
127°C
Mass Spec MH+ (found) = 415 MH+ (calculated) = 415
Example 37
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-morpholin-4-ylmethyl-
isoxazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 21 (50mg) and morpholine (13mg) in dichloromethane
(5ml) was stirred for 1.5h. Sodium triacetoxyborohydride (43mg) was added
and stirring was continued for 2.5h. The reaction mixture was washed with 8%
aqueous sodium bicarbonate solution. The organic phase was passed through a
Varian SPE bond elution silica cartridge (which had been pre-conditioned by
eluting a column volume of dichloromethane), eluting sequentially with
dichloromethane, chloroform, diethyl ether, ethyl acetate, acetonitrile and
methanol. Fractions containing the required product were combined and
evaporated to give a foam which was treated with 1.OM. hydrogen chloride in
diethyl ether to give the title compound as a white solid (39mg).
Mass Spec MH+ (found) = 441 MH+ (calculated) = 441
T.Lc. (dichloromethane:methanol 9:1 ): Rf = 0.65.
The following Examples 38-44 were prepared in a similar manner to Example 37
from Intermediate 21:
Example 38
rel-(3R,3aR,6aS1-4-(5-Dimethylaminomethyl-isoxazole-3-carbonyl)-3-isopropyl-
1-methanesulfon~-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White solid. Mass Spec MH+ (found) = 399 MH+ (calculated) = 399
T.Lc. (dichloromethane:methanol 9:1): Rf = 0.58.
74
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98I05609
Example 39
rel-(3 R, 3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-pyrrolid in-1-ylmethyl-
isoxazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream solid. Mass Spec MH'' (found) = 425 MH+ (calculated) = 425
T.Lc. (dichloromethane:methanol 9:1): Rf = 0.55.
Example 40
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-piperidin-1-ylmethyl-
isoxazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White solid. Mass Spec MH+ (found) = 439 MH+ (calculated) = 439
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1 ): Rf = 0.52.
Example 41
rel-(3R,3aR,6aS)-3-isopropyl-1-methanesulfonyl-4-[5-(4-methyl-piperazin-1-
~~Imethyl)-isoxazole-3-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
White solid. Mass Spec MH+ (found) = 454 MH+ (calculated) = 454
T.Lc. (dichloromethane:methanol 9:1): Rf = 0.13.
Example 42
rel-(3R,3aR,6aS)-4-{5-[(4-Fluoro-benzylamino)-methyl]-isoxazole-3-carbonyl}-3-
isopropyl-1-methanesulfonyi-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Cream solid. Mass Spec MH+ (found) = 479 MH+ (calculated) = 479
T.Lc. (dichforomethane:ethanol:ammonia 100:8:1): Rf= 0.59.
Example 43
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[5-(4-phenyl-piperazin-1-
ylmethyl)-isoxazole-3-carbonyl]-hexahydro-pyn-olo[3,2-b]pyrrol-2-one
hydrochloride
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
Cream solid. Mass Spec MH+ (found) = 516 MH+ (calculated) = 516
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.62.
Example 44
rel-(3R,3aR,6aS)-4-(5-Dibutylaminomethyl-isoxazole-3-carbonyl)-3-isoproplrl-1-
methanesulfonyl-hexahydro-pyrrolo(3,2-b]pyrrol-2-one hydrochloride
Cream solid. Mass Spec MH+ (found) = 483 MH+ (calculated) = 483
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.75.
Example 45
rel 3R,3aR,6aS)-4-(5-Dimethylaminomethyl-1-methyl-1 H-pyrazole-3-carbonyl)-
3-isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
A mixture of Intermediate 26 (50mg) and dimethylammonium chloride (32mg) in
dichloromethane (5ml) was stirred for 2h. Sodium triacetoxyborohydride
(55mg) was added and stirring was continued for 3h. The reaction mixture was
washed with 8% aqueous sodium bicarbonate solution. The organic phase was
passed through a Varian SPE cartridge (which had been pre-conditioned by
eluting through a column volume of dichloromethane), eluting with
dichloromethane, chloroform, diethyl ether, ethyl acetate, acetonitrile and
methanol. Fractions containing the required product were combined and
evaporated to give a pale brown oil which was treated with 1.OM. hydrogen
chloride in diethyl ether to give the title compound as a cream solid (28mg).
Mass Spec MH'' (found) = 412 MH+ (calculated) = 412 T.Lc.
(dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.68.
The following Examples 46-49 were prepared in a similar manner to Example 45
from Intermediate 26:
76
CA 02303176 2000-03-07
WO 99/12933
PCT/EP98/05609
Example 46
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(1-methyl-5-pyrrolidin-1-
ylmethyl-1 H-pyrazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Cream solid. Mass Spec MH+ (found) = 438 MH+ (calculated) = 438
T.Lc: (dichloromethane:ethanol:ammonia 100:8:1 ): Rf = 0.65.
Example 47
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-( 1-methyl-5-morpholin-4-
ylmethyl-1 H-pyrazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Cream solid. Mass Spec MH+ (found) = 454 MH+ (calculated) = 454
T.t.c. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.69.
Example 48
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfony!-4-(1-methyl-5-piperidin-1-
ylmethyl-1 H-pyrazole-3-carbonyl)-hexahydro-~~yrrolo[3,2-b]pyrrol-2-one
hydrochloride
Cream solid. Mass Spec MH'' (found) = 452 MH+ (calculated) = 452
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1 ): Rf = 0.67.
Example 49
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[1-methyl-5-(4-methyl-
piperidin-1-ylmethyl)-1 H-pyrazole-3-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-
2-
one hydrochloride
Cream solid. Mass Spec MH+ (found) = 466 MH'' (calculated) = 466
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.78.
77
CA 02303176 2000-03-07
WO 99/12933
PCT/EP98/05609
Example 50
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[2-methyl-5-(4-phenyl-
eiperazin-1-ylmethyl)-2H-pyrazole-3-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-
one hydrochloride
A mixture of Intermediate 29 (50mg) and phenylpiperazine (28mg) in
dichloromethane (5ml) was stirred for 2h. Sodium triacetoxyborohydride
(50mg) was added and stirring was continued overnight. The reaction mixture
was washed with 8% aqueous sodium bicarbonate solution. The organic phase
was passed through a Varian SPE cartridge (which had been pre-conditioned by
eluting through a column volume of dichloromethane), eluting sequentially with
dichloromethane, chloroform, diethyl ether, ethyl acetate, acetonitrile and
methanol. Fractions containing the required product were combined and
evaporated to give a gum which was treated with 1.OM. hydrogen chloride in
diethyl ether to give the title compound as a dark yellow solid (16mg). Mass
Spec MH+ (found) = 529 MH+ (calculated) = 529 T.Lc. (dichloromethane:
ethanol:ammonia 100:8:1): Rf = 0.77.
The following Examples 51-55 were prepared in a similar manner to Example 50
from Intermediate 29:
Example 51
rel-(3R,3aR,6aS)-4-(5-Dimethylaminomethyl-2-methyl-2H-pyrazole-3-carbonyl)-
3-isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Yellow solid. Mass Spec MH+ (found) = 412 MH+ (calculated) = 412
T:Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.66.
Example 52
CA 02303176 2000-03-07
WO 99/12933 pCT/EP98/05609
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(2-methyl-5-pyrrolidin-1-
ylmethyl-2H-pyrazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Yellow solid. Mass Spec MH+ (found) = 438 MH+ (calculated} = 438
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.63.
Example 53
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(2-methyl-5-morpholin-4-
ylmethyl-2H-pyrazole-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
Yellow solid. Mass Spec MH+ (found) = 454 MH+ (calculated) = 454
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.65.
Example 54
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(2-methyl-5-piperidin-1-
ylmethyl-2H-pyrazole-3-carbonyl)-hexahydro-p~~rrolo[3,2-b]pyrrol-2-one
hydrochloride
Yellow solid. Mass Spec MH'' (found) = 452 MH+ (calculated) = 452
T.l.c. (dichloromethane:ethanol:ammonia 100:8:1): Rf = 0.65.
Example 55
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[2-methyl-5-(4-methyl-
piperazin-1-ylmethyl)-2H-pyrazole-3-carbonyl]-hexahydro-pyrrolo[3,2-bjpyrrol-2-
one hydrochloride
Yellow solid. Mass Spec MH+ (found) = 467 MH+ (calculated) = 467
T.Lc. (dichloromethane:ethanol:ammonia 100:8:1): Rf=0.29.
Example 56
79
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
rel-(3R,3aR,6aS)-4-(6-[(Dicyclohexylamino)-methy!]-pyridine-3-.carbonyl}-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 31 (90mg), dicyclohexylamine (104.6p,1), sodium
iodide
(79mg) and potassium carbonate (169mg) in acetonitrile (2ml) was stirred for 5
days. The solvent was evaporated and the residue was partitioned between
2M sodium carbonate solution (2ml) and dichloromethane (3ml). The phases
were separated. The organic phase was passed through a Varian SPE cartridge
(which had been pre-conditioned by eluting through a column volume of
dichloromethane), eluting sequentially with dichloromethane, chloroform,
diethyl
ether, ethyl acetate, aceton~rile and methanol. Fractions containing the
required product were combined and evaporated to give a colourless oil which
was treated with 1.OM. hydrogen chloride in diethyl ether to give the title
compound as a cream solid (60mg). Mass Spec MH+ (found) = 545 MH+
(calculated) = 545 T.Lc. (ethyl acetate): Rf = 0.47.
The following Examples 57-64 were prepared in a similar manner to Example 56
from Intermediate 31:
Example 57
rel-(3R,3aR,6aS)-4-(6-Dibutylaminometh~~l-pyridine-3-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo(3,2-b]pyrrol-2-one hydrochloride (1:2)
Brown glass. Mass Spec MH+ (found) = 493 MH+ (calculated) = 493
T.Lc. (ethyl acetate) Rf = 0.49
Example 58
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4.-(6-morpholin-4-ylmethyl-
pyridine-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Orange glass. Mass Spec MHO (found) = 451 MH+ (calculated) = 451
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
T.Lc. (ethyl acetate) Rf = 0.38
Example 59
rel-(3R,3aR,6aS)-4-(6-Cyclopropylaminomethyl-pyridine-3-carbonyl)-3-isopropyl-
1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Pale brown solid. Mass Spec MH'' (found) = 421 MH+ (calculated) = 421
T.Lc. (ethyl acetate) Rf = 0.36
Example 60
rel- 3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(6-piperidin-1-ylmethyl-
pyridine-3-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Green solid. Mass Spec MH' (found) = 449 MH+ (calculated) = 449
T.Lc. (ethyl acetate) Rf = 0.36
Example 61
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(6-(4-methyl-piperazin-1-
ylmethyl)-pyridine-3-carbonyl]-hexahyd ro-pyrrolo(3,2-b]pyrrol-2-one
hydrochloride (1:2)
Orange glass. Mass Spec MH+ (found) = 464 MH+ (calculated) = 464
T.Lc. (ethyl acetate) Rf = 0.05
Example 62
rel-(3R,3aR,6aS)-3-Isopropyl-4-(6~(1-isopropyl-2-methyl-propylamino)-methyl]-
pyridine-3-carbonyl}-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride (1:2)
Yellow solid. Mass Spec MH+ (found) = 479 MH+ (calculated) = 479
T.Lc. (ethyl acetate) Rf = 0.42
Example 63
81
CA 02303176 2000-03-07
WO 99/12933 PC'F/EP98/05609
rel-(3R,3aR,6aS)-4-(6-Dimethylaminomethyl-pyridine-3-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Pale yellow glass. Mass Spec MH'. (found) = 409 MH+ (calculated) = 409
T.Lc. (ethyl acetate) Rf = 0.21
Example 64
rel-(3R,3aR,6aS)-4-{6-[(4-Fluoro-benzylamino)-methyl]-pyridine-3-carbonyl}-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
Yellow/brown solid. Mass Spec MH+ (found) = 489 MH+ (calculated) = 489
T.Lc. (ethyl acetate) Rf = 0.41
Example 65
rel-(3R,3aR,6aS)-4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A mixture of Intermediate 34 (45mg), cyclopropyiamine (23.3p1) and sodium
iodide (25mg) in dichloromethane (1 ml) was stirred for 18h. More
cyclopropylamine (23.3w1) was added and stirring was continued for a further
24h. The reaction mixture was partitioned between 2M sodium carbonate
solution (3ml) and dichloromethane {3ml). The phases were separated. The
organic phase was passed through a Varian SPE cartridge {which had been
pre-conditioned by eluting through a column volume of dichloromethane),
eluting
sequentially with dichloromethane, chloroform, diethyl ether, ethyl acetate,
acetonitrile and methanol. Fractions containing the required product were
combined and evaporated to give a pale brown oil (17mg) which was treated
with 1.OM. hydrogen chloride in diethyl ether to give the title compound as a
brown solid (20mg). Mass Spec MH+ (found) = 422 MH; (calculated) = 422
T.Lc. (ethyl acetate): Rf = 0.12.
82
CA 02303176 2000-03-07
WO 99/12933 ~ PCT/EP98/05609
The following Examples 66-75 were prepared in a similar manner to Example 65
from Intermediate 34:
Example 66
rel-(3R,3aR,6aS)-4-(5-Dibutylaminomethyl-pyrazine-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Pale brown solid. Mass Spec MH' (found) = 494 MH+ (calculated) = 494
T.Lc. (ethyl acetate) Rf = 0.63
Example 67
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-morpholin-4-ylmethyl-
p~razine-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Cream solid. Mass Spec MH+ (found) = 452 MH+ (calculated) = 452
T.Lc. (ethyl acetate) Rf = 0.12
Example 68
rel-(3R,3aR,6aS)-4-(5-[(Dicyclohexylamino)-methyl]-pyrazine-2-carbon rLJI h3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]p~~rroi-2-one
hydrochloride
1~:2~
Cream solid. Mass Spec MH+ (found) = 546 MH+ (calculated) = 546
T.Lc. (ethyl acetate) Rf = 0.65
Example 69
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-~iperidin-1-ylmethyl-
p~razine-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Cream solid. Mass Spec MH+ (found) = 450 MH+ (calculated) = 450
T.Lc. (ethyl acetate) Rf = 0.12
83
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
Example 70
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[5-(4-methyl-piperazin-1-
~methyl)-pyrazine-2-carbonyl]-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride (1:2)
Brown glass. Mass Spec MH' (found) = 465 MH+ (calculated) = 465
T.Lc. (ethyl acetate) Rf = 0.02
Example 71
rel-(3R, 3aR, 6aS)-3-Isopropyl-4-(5-[( 1-isopropyl-2-methyl-propylamino)-
methyl]-
p~razine-2-carbonyl}-1-methanesulfonyl-hexahydro-pyrrolo[3,2-]pyrrol-2-one
hydrochloride (1:21
Cream solid. Mass Spec MH+ (found) = 480 MH+ (calculated) = 480
T.Lc. (ethyl acetate) Rf = 0.45
Example 72
rel-(3R,3aR,6aS)-4-(5-Dimethylaminomethyl-pyrazine-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Pale brown solid. Mass Spec MH+ (found) = 410 MH+ (calculated) = 410
T.Lc. (ethyl acetate) Rf = 0.06
Example 73
rel-(3 R, 3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-[5-~4phenyl-piperazin-1-
ylmethyl)-pyrazine-2-carbonyl]-hexahyd ro-pyrrolo[3, 2-b]pyrro I-2-one
hydrochloride (1:2)
Pale yellow glass. Mass Spec MH+ (found) = 527 MH+ (calculated) = 527
T.Lc. (ethyl acetate) Rf = 0.27
Example 74
84
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
rel-(3 R, 3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-pyrrolid i n-1-ylmethyl-
pyrazine-2-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Orangelbrown solid. Mass Spec MH+ (found) = 436 MH+ (calculated} = 436
T.Lc. (ethyl acetate) Rf = 0.05
Example 75
rel-(3R,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-4-(5-methylaminomethyl-
pyrazine-2-carbonyl)-hexahydro-pyn-olo[3,2-b]pyrrol-2-one hydrochloride (1:2)
Pale brown solid. Mass Spec MH+ (found) = 396 MH'' (calculated) = 396
T.l.c. (ethyl acetate) Rf = 0.02
Example 76
(3S,3aS,6aR)-4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3-isopropyl-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
A solution of Intermediate 48 (21.53g) and 4.OM HCi in 1,4-dioxan (200m1) was
stirred at room temperature for 2 hours. The solvent was removed in vacuo to
give an off white solid. The solid was recrystallised ftom hot 5% water/2-
propanol (2.31) to give the title (single enantiomer) compound (15.54g) as a
white solid.
T.Lc. (Silica, eluent 200:8:1 dichloromethane:ethanol:0.880 ammonia) Rf =
0.21.
Mass spec. MH+ (found) 422.19, MH'' (calc) 422.19
[a]oz° +51.3 (c = 0.9, 1:1 HZOfMeCN)
M.pt. 183-185°C
Circular Dichroism: 7~250.2nm (AE -1.34M'~cni ~)
7~",eX285.4nm (OE +0.99M-~crri ~), (MeCN/H20)
Elemental analysis: Found C, 47.4; H, 6.4; N, 14.3; S, 6.5; CI, 7.8; water,
4.9%
(C~9H2~CI N5O4S .HCL1.3H20 requires C, 47.4; H, 6.4; N, 14.6; S, 6.7; CI, 7.4;
water, 4.9%).
Example 76 (alternative preparation)
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
A mixture of Intermediate 46 (1.0568}; cyclopropylamine (0.73m1) and potassium
iodide (481 mg) in acetonitrile (25m1) was stirred for 3 hours. The solvent
was
evaporated in vacuo and the mixture partitioned between sat. sodium
bicarbonate solution (20m1) and dichloromethane (20m1). The phases were
separated. The aqueous phase was further extracted with dichloromethane
(2x20m1) The combined organics were dried (MgS04), filtered and the solvent
removed in vacuo to leave an oil. The oil was purified by flash column
chromatography (Merck 9385 silica} and eluted with 200:8:1
dichloromethane:ethano1:0.880 ammonia. Fractions containing the required
product were combined and evaporated to give a white solid (924mg) which was
dissolved in dichloromethane (10m1) and treated with 1.OM. hydrogen chloride
in
diethyl ether (10m1) to give the title (single enantiomer) compound as a white
solid (1.008).
Example 77:
(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(2-p~~rrolidin-1-ylmethyl-oxazole-
4-carbonyl)-hexahydro-pyrrolo[3,2-b)pyrrol-2-one hydrochloride
Intermediate 56 (32.28) was added rapidly to a stirred solution of 1
hydroxybenzotriazole (13.08) in acetonitrile (350m1}. A solution of
(3S,3aS,6aR)-3-isopropyl-1-methanesulfonyl-hexahydropyrrolo[3,2-b)pyrrol-2
one (Intermediate 122 from International Patent Application W097/36903)
(21.78) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
(37.08) in acetonitrile (70m1) was then added and the reaction mixture was
stirred for 20h. The solvent was removed in vacuo and the residue was
partitioned between dichloromethane (900m1} and 1.OM. sodium carbonate
solution (600m1). The aqueous phase was separated and extracted with
dichloromethane (150m1). The combined organics were washed with brine
(250m1}, dried (MgS04) and concentrated in vacuo to leave a brown solid. The
solid was purified by flash column chromatography (Merck 9385 silica; eluent
86
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
dichloromethane:ethanol:ammonia 150:8:1 to 135:8:1 ) to give a cream solid
(29.3g). The solid was dissolved in dichioromethane (150m1} and treated with
1.OM. hydrogen chloride in ether (75m1). The solvent was removed in vacuo to
leave a solid which was again dissolved in dichloromethane (150m1) and treated
with 1.OM. hydrogen chloride in ether (75m1). The solvent was removed in vacuo
to leave a solid which was recrystallised from acetone to give the title
compound
(26.3g) as a white solid.
Meting point 156-158°C.
T.f.c. (Silica; dichloromethane:ethanol:ammonia 100:8:1; double elution) Rf =
0.66.
'H NMR (400 MHz; D-6 DMSO): d 8.78 (s, 1H}, 4.68 (s, 2H), 4.13 (ddd, J=
11,11,7 Hz, 1 H), 4.08 (dd, J=11,10 Hz, 1 H), 3.80 (ddd, J=12,10.5,5.5 Hz, 1
H},
3.60 (m, 2H}, 3.55 (dd, J=12,10.5 Hz, 1H), 3.31 (s, 3H}, 3.20 (m, 2H), 3.03
(dd,
J=12,2.5 Hz, 1 H}, 2.88 (md, J=2.5 Hz, 1 H), 2.34 (m, 1 H), 2.12 (m, 1 H),
1.96 (m,
4H), 1.19 (d, J=7 Hz, 3H), 0.98 (d, J=7 Hz, 3H). Contains 0.16 Mol. % acetone.
Infra-red (KBr diffuse reflectance) 3633, 3474, 3149, 3102, 2956, 2882, 2668,
2576, 2475, 1747, 1709, 1639, 1634, 1567, 1442, 1380, 1347, 1161, 1146, 967,
810, 547 crri ~ .
Mass spec MH+ (found) = 425.186372. MH+ (calculated} = 425.185867 (error
1.2ppm).
Combustion analysis.
Found: C, 48.65; H, 6.39; N, 11.41; S, 6.19; CI, 7.13%.
Ct9H28N405S.HCL0.75H20Ø2Me2C0 requires: C, 48.43; H, 6.57; N, 11.53; S,
6.60; CI, 7.29%.
Example 78:
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-ylmethyl-
oxazole-4-carbonyl)-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
8~
CA 02303176 2000-03-07
WO 99/12933 PCT/EP98/05609
A stirred suspension of Intermediate 56 (second preparation: 40mg) in
dichloromethane (4ml) was treated with oxalyl chloride (63mg) followed by
dimethylfom~amide (1 drop). The reaction mixture was stirred for 1.5h. The
solvent was removed in vacuo and replaced by toluene (10 ml). The resultant
suspension was triturated vigorously for 10 min. The toluene was removed in
vacuo to leave a gum which was suspended -in dichloromethane (5 ml) and
treated with Intermediate 10 (20mg) and sodium bicarbonate (35mg). The
reaction mixture was stirred for 3.75h. then partitioned between
dichloromethane (2x10m1) and water (5ml). The combined organics were dried
(NaaS04) and concentrated in vacuo to leave a solid. The solid was triturated
in
ether (4ml) for 10 min. The ether was decanted. The residue was dried in vacuo
to leave a white powder. The powder was treated with 1.OM. hydrogen chloride
in diethyl ether to give the title compound as a cream powder (17mg).
Melting point 116-120°C.
Mass spec MH' (found) = 425. MH+ (calculated) = 425.
The following Examples 79-81 were prepared in a similar manner to Example 78
from Intermediate 10 and Intermediates 57-59 respectively:
Example 79:
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4-(2-piperid in-1-ylmethyl-
oxazole-4-carbonyl)-hexahydro-pyrroloL,2-b]pyrrol-2-one hydrochloride
White powder, melting point 140-143°C.
Mass spec MH+ (found) = 439. MH+ (calculated) = 439
Example 80:
rel-(3S,3aS,6aR)-3-Isopropyl-1-methanesulfonyl-4- 2-(4-phenyl-piperazin-1-
ylmethyl)-oxazole-4-carbonyl]-hexahydro-pyrrolo 3,2-b]pyrrol-2-one
hydrochloride
88
CA 02303176 2000-03-07
WO 99/12933
PCT/EP98/05609
Cream solid, melting point 156-160°C.
Mass spec MH+ (found) = 516. MH+ (calculated) = 516.
Example 81:
rel-(3S,3aS,6aR~-4-(2-Dibutylaminomethyl-oxazole-4-carbonyl)-3-isoprop~~l-1-
methanesulfonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one hydrochloride
White powder, melting point 122-126°C.
Mass spec MH+ (found) = 483. MH'' (calculated) = 483.
Example 82:
rel-(3S,3aS,6aR)-4-~2-((Cyclopropyl-methyl-amino)-methyl]-oxazole-4-carbonyl}-
3-isopropyl-1-methanesuffonyl-hexahydro-pyrrolo[3,2-b]pyrrol-2-one
hydrochloride
A solution of Intermediate 51 (21mg) in dioxan (1.5m1) and 1.OM. sodium
hydroxide (0.4m1) was stirred for 3.5h. Hydrochloric acid (0.35m1) was added
with stirring. The solvents were removed in vacuo. The residue was triturated
in
dioxan (3ml) for 5 min. The solvent was removed in vacuo. The trituration was
repeated using more dioxan (3m1). The solvent was removed in vacuo and the
residue dried under vacuum to give a solid. A stirred suspension of this solid
in
dichloromethane (3ml) was treated with oxalyl chloride (50mg) followed by
dimethylformamide (1 drop). The reaction mixture was stirred for 1.Oh. The
solvent was removed in vacuo and the residue was triturated vigorously in a
(1:1) mixture of dichloromethane and toluene (10m1) for 5 min. The solvents
were removed in vacuo to leave a gum which was suspended in
dichloromethane (5 ml) and treated with Intermediate 10 (18mg) and sodium
bicarbonate (35mg). The reaction mixture was stirred for 0.75h., left at room
temperature for 3 days, diluted with dichloromethane (10m1) and washed with
water (10m1). The organic phase was dried (Na2S04) and concentrated in vacuo
to leave a gum. The gum was purified by flash column chromatography (Merck
89
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
9385 silica; eluent dichloromethane:ethanol:ammonia 150:8:1 ) to give a white
powder. The powder was treated with 1.OM. hydrogen chloride in diethyl ether
to
give the title compound as a white powder (7mg).
Melting point 116-119°C.
Mass spec MH+ (found) = 425. MH+ (calculated) = 425.
The following Example 83 was prepared in a similar manner to Example 82
from Intermediate 10 and Intermediate 52:
Example 83:
rel-(3S,3aS,6aR)-4-(2-[(Dicyclohexylamino)-methyl]-oxazole-4-carbonyl}-3-
isopropyl-1-methanesulfonyl-hexahydro-pyrrolo[3,2-b]p~~rrol-2-one
hydrochloride
White powder, melting point 130-133°C.
Mass spec MH+ (found) = 535. MH+ (calculated) = 535.
Biological Data
1. The compounds of Examples 1-83 were tested in the in vitro elastase test
described earlier in the description. The IC5a values are given in the
following
table:
CA 02303176 2000-03-07
WO 99/I2933 PCT/EP98/05609
Example ICSp~ Example ICS ,~M
1 0.123 39 0.021
2 0.081 40 0.030
3 0.164 41 0.014
4 0.065 42 0.057
5 0.039 43 0.051
6 0.071 44 0.099
7 0.082 45 0.019
8 0.086 46 0.014
9 0.129 47 0.024
10 0.114 48 0.030
11 0.139 49 0.051
12 0.104 50 0.054
13 0.032 ~ 51 0.021
14 0.021 52 0.012
15 0.021 53 0.015
16 0.019 54 0.011
17 0.068 55 0.020
18 0.022 56 0.038
19 0.057 57 0.041
20 0.013 58 0.045
21 0.029 59 0.028
22 0.043 60 0.037
23 0.026 61 0.030
24 0.024 62 0.041
25 0.009 63 0.025
26 0.017 64 0.048
27 0.008 65 0.013
28 0.011 66 0.076
29 0.014 67 0.019
30 0.017 68 0.087
31 0.016 69 0.022
32 0.009 70 0.013
33 0.005 71 0.050
34 0.013 72 0.012
35 0.056 73 0.074
36 0.013 74 0.012
37 0.017 75 0.093
38 0.014 76 0.011
91
CA 02303176 2000-03-07
WO 99/12933 PC'f/EP98/05609
Example ICS ~
-
77 0.010
78 0.014
79 0.016
80 0.100
81 0.076
82 0.084
83 0.137
2. Compounds of Examples 1, 2, 11, 12, 13, 15, 16, 17, 27, 28, 29, 31, 33,
34, 35, 37-50, 56, 57, 59, 60, 62-69, 72, 73, 76, 78, 80 and 81 were tested in
an
in vivo hamster test described above at an effective dose of less than 1
Omglkg,
and gave a duration of effect lasting at least 6 hours.
3. The compounds of Examples 1 to 83 were tested in the human whole
blood elastase inhibition assay described earlier in the description. The ICS
values are given in the table below.
92
CA 02303176 2000-03-07
WO 99/12933 PGT/EP98/05609
EXample ~CSO .~ EXample ICSp
1 0.355 41 1.76
2 1.882 42 0.43
3 2.195 43 0.429
4 4.185 44 0.528
3 45 0.518
6 4.015 46 0.524
7 3.04 47 1.043
8 3.46 48 0.414
9 3.615 49 0.539
5.565 50 1.92
11 1.16 51 >10
12 2.4 52 . 7.637
13 1.103 53 8.23
14 1.885 54 4.205
0.452 55 > 10
16 0.774 56 0.293
17 0.316 57 0.193
18 2.925 58 2.44
19 0.408 59 0.394
6.405 60 0.605
21 0.317 61 5.898
22 0.453 62 0.252
23 0.364 63 1.62
24 0.306 64 0.521
1.64 65 0.333
26 > 10 66 0.217
26 0.704 67 0.878
28 1.125 68 0.221
29 0.311 69 0.264
1.683 70 3.215
31 0.896 71 0.205
32 0.427 72 0.322
33 0.257 73 0.282
34 0.546 74 . 0.205
0.81 75 5.025
36 0.296 76 0.139
37 1.187 77 0.245
38 0.224 78 0.411
39 0.358 79 0.502
0.222 80 0.4
93
CA 02303176 2000-03-07
WO 99/12933 PC"T/EP98/05609
Exam to IC50 M
81 0.296
~82 0.717
83 0.55
94