Note: Descriptions are shown in the official language in which they were submitted.
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
1
SEWING RING HAVING INCREASED ANNULAR COAPTATION
Field of the Invention
~ The present invention relates generally to medical devices and
particularly to heart valve prostheses having an improved sewing ring which
facilitates valve coaptation with surrounding tissue, improves the valve
attachment methodology and increases valve stability when the prostheses is
implanted iri the heart.
Background of the Invention
Prosthetic heart valves are used to replace damaged or diseased heart
valves. In vertebrate animals, the heart is a hollow muscular organ having
four
pumping chambers: the left and right atria and the left and right ventricles,
each
provided with its own one-way valve. The natural heart valves are identified
as
1 S the aprtic, mitral (or bicuspid), tricuspid and pulmonary valves.
Prosthetic heart
valves can be used to replace any of these naturally occunring valves,
although
repair or replacement of the aortic or mitral valves are most common because
they
. reside in the left side of the heart where presswes are the greatest.
Two primary types of heart valve replacements or prostheses are known.
One is a mechanical-type heart valve which uses a ball and cage arrangement or
a
pivoting mechanical closwe to provide unidirectional blood flow. The other is
a
tissue-type or "bioprosthetic" valve which is constructed with natural-tissue
valve
leaflets which function much like a natural human heart valve's, imitating the
natwal action of the flexible heart valve leaflets which seal against each
other to
enswe the one-way blood flow. In both types of prosthetic valves, a
~ biocompatible cloth covered suture ring on the valve body (mechanical) or
stent
(tissue-type) provides a platform for attaching the valve to the annulus of
the
' particular valve being replaced.
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
2
The valves of the heart separate chambers therein, and are each mounted
in an annulus therebetween. The annuluses comprise dense fibrous rings
attached ,
either directly or indirectly to the atrial and ventricular muscle fibers. In
a valve
replacement operation, the damaged leaflets are excised and the annulus
sculpted
to receive a replacement valve. Ideally the annulus presents relatively
healthy
tissue which can be formed by the surgeon into a uniform ledge projecting into
the
orifice left by the removed valve. The time and spacial constraints imposed by
surgery, however, often dictate that the shape of the resulting annulus is
less than
perfect for attachment of a sewing ring. Moreover, the annulus may be
calcified
as well as the leaflets and complete annular debridement, or removal of the
hardened tissue, results in a larger orifice and less defined annulus ledge to
which
to attach the sewing ring. In short, the contours of the resulting annulus
vary
widely after the natural valve has been excised.
Conventional placement of the valve is infra-annular, with the valve body
deep within the narrowest portion of the annulus to enhance any seal effected
by
the sewing ring/suture combination and reduce the chance of perivalvular
leakage.
Surgeons report using at least 30 simple sutures or 20 mattress-type sutures
to
prevent leakage. Mattress sutures are more time consuming and essentially
comprise double passes of the needle through the tissue with one knot.
The four valves separate each ventricle from its associated atrium, or
from the ascending aorta (left ventricle) or pulmonary artery (right
ventricle).
After the valve excision, the annulus generally comprises a ledge extending
into
and defining the orifice between the respective chambers. Prosthetic valves
may attach on the upstream or downstream sides of the annulus ledge, but
outside of the ventricles to avoid interfering with the large contractions
therein.
Thus, for example, in the left ventricle a prosthetic valve is positioned on
the
inflow side of the mitral annulus (in the left atrium), or on the outflow side
of
the aortic annulus (in the ascending aorta). Besides the differing anatomies
of
the mitral and aortic annuluses, the pressures exerted on the attachment
sutures
CA 02303289 2000-03-13
,~ ' W099/13802 PCT/US98/14696
3
differ as well. The highest pressures to which the sutures are subjected in
use is
in the backflow half of the flow cycle when the valve closes. In systole, the
left
ventricle contracts to push blood through the body's circulatory system and
the
' mitral valve is forced closed by pressures of up to 140 mm Hg. Because the
prosthetic mitral valve is attached on the inflow side of the annulus opposite
the
ventricle chamber, the sutures are placed in direct tension. In contrast, the
backflow pressure of the ascending aorta on the aortic valve is much less, and
in
any event the back pressure pushes the prosthetic valve against the aortic
annulus so that the attaching sutures are not in tension. The end result is
that
care must be taken so that the mitral valve is more securely attached, and
pledgets are conventionally used in conjunction with sutures in both aortic
and
mitral implantations to avoid a "cheesewire" effect on the tissue. Pledgets
are
small pieces of biocompatible fabric attached to each individual suture that
are
positioned within the loop of the suture between the suture and the tissue to
prevent the suture when placed in tension from cutting into the tissue.
Naturally, the implantation of a prosthetic heart valve, either a
mechanical valve or a bioprosthetic valve (i.e., "tissue" valve), requires a
great
deal of skill and concentration given the delicate nature of the native heart
tissue, the spatial constraints of the surgical field and the criticality of
achieving
a secure and reliable implantation. It is of equal importance that the valve
itself
have characteristics that promote a long valve life and that have minimal
impact
on the physiological makeup of the heart environment.
Given the uneven nature of the annuluses, the design of the sewing ring
and the method with which the sewing ring is fixed into place are perhaps the
most crucial aspects of prosthetic heart valve implantation. Accordingly, an
' optimum sewing ring design contemplates a blend between structure highly
complimentary to the valve annulus tissue and a valve attachment platform that
simplifies the implantation procedure for the surgeon. Although prior art
sewing ring designs are widely varied and numerous, until the design of the
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
4
present invention, attempts to effectively blend improved structure/tissue
compatibility with a convenient "surgeon friendly" sewing platform have been
largely unsuccessful.
Many prior art sewing rings are designed to take up little space so as to .
increase the potential orifice opening for the valve within. One example of a
prior art sewing ring may be found in U.S. Patent No. 5,397,348 to Campbell et
al. which discloses a sewing ring made of a solid PTFE felt ring having a
cross-
sectional shape of a right triangle. The sewing ring is mounted to a
mechanical
valve, and one side of the ring extends perpendicular to the flow direction
through the valve, thus the right triangle designation. The PTFE felt ring is
enveloped by cloth that conforms to the right-triangular shape. When implanted
in the mitral position as shown in Figure 1 of the Campbell patent, the
hypotenuse of the right triangle mates with the tissue in the valve annulus.
The design typified by the Campbell patent has a number of significant
drawbacks. For example, the solid nature of the PTFE felt ring does not easily
conform to an irregularly shaped annulus and introduces an inherent stiffness
that limits the ability of the sewing ring to flex with the annulus tissue as
that
tissue is stressed during normal heartbeat activity. The lack of flexibility
or low
compliance, in turn, increases the loads exerted on the sutures used to attach
the
sewing ring potentially leading to leakage problems or damage to the annulus
tissue. For example, unduly stiff sewing rings must be sutured in place fairly
tightly to prevent perivalwlar leakage between sutures. This added tension may
strangle the annulus tissue and result in a decubitous ulceration.
The inherent stiffness (low compliance) also severely narrows the
margin for error when selecting the appropriate size sewing ring/valve for a
given patient. If the selected size is slightly too large, the inability of
the PTFE
felt ring to easily compress requires undue deformation of the annulus tissue
in
order to adequately attach the valve. Similarly, if the selected size is
slightly
too small, the inability of the PTFE felt ring to easily stretch results in
undue
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
tension on the tissue and sutures in order to achieve attachment. As a result,
a
great deal of care and accuracy by the surgeon are needed in the selection of
a
valve size that precisely matches the valve annulus of the patient.
~ Unfortunately, standard sizing tools are provided in increments based on an
5 overall orifice size, and may not be able to accurately measure a less than
optimally formed annulus. The surgeon thus must use informed judgment in
selecting an approximate valve size.
The combination of the stiffness in the PTFE felt ring with the right
triangle shape also has drawbacks. For example, the valve annulus tissue
typically does not have a cross-section which matches the linear hypotenuse,
and given the inherently stiff and bulky nature of the PTFE felt ring, there
is
insufficient flexibility for the hypotenuse edge of the ring to bend in a
manner
that adequately conforms to the irregular, nonlinear shape of the sculpted
annulus cross-section. This again potentially results in perivalvular leakage
and
tissue damage.
The stiffness/right triangle shape combination also is a limiting factor in
providing adequate sewing ring cross-sectional area for suturing (or other
attachment methods, e.g., stapling) the valve to the annulus tissue. The
annular
band of material around the periphery of the sewing ring which serves as the
suturing platform is relatively narrow in a radial dimension which
necessitates
the use of pledgets in conjunction with the sutures. Obviously, the use of
pledgets increases the complexity and time required for valve implantation.
The
annular band of a right triangular sewing ring is so radially narrow that the
suture loop passes through a relatively thin portion of the annulus tissue
near the
annulus tip, and so pledgets must be used.
The implantation problem caused by narrow sewing rings is aggravated
in many prior prosthetic valves by rigid structure extending outward from the
valve body into the interior of the sewing ring. See, for example, the
compressible stiffening ring of Campbell (U.S. Patent No. 5,397,348). This
CA 02303289 2000-03-13
, WO 99/13802 PCT/US98/14696
6
structure further limits the placement of sutures in the sewing ring to the
radially
outer regions thereof. Moreover, if attempts were made to increase the annular
band of the sewing ring, or to at least increase the cross-sectional angle of
the
hypotenuse in order to provide a larger suture platform, the solid nature of
the
S PTFE felt ring would only cause an undesirable increase in stiffness and
bulkiness. Such a result would then simply amplify the problems already
discussed with regard to sewing ring stiffness and low compliance.
In view of the foregoing, it is evident that an improved sewing ring that
addresses the apparent deficiencies in existing sewing rings is necessary and
desired. That is, there is a need for an advanced design that improves
compatibility of the ring to the annulus tissue and simultaneously simplifies
for
the surgeon the technique used to attach the valve.
Summary of the Invention
The present sewing ring is designed with a larger radial profile to enable
deep passes into the surrounding annulus tissue, and has increased material
close to the valve body to enable deep passes into the ring material, both
factors
enabling a reduction in the number of sutures used. The sewing ring is highly
compliant and resilient to better cooperate with movements of the surrounding
tissue and accordingly reduce the tension needed for each suture. Moreover,
the
increased size and novel shapes enable great flexibility in valve placement
within the annulus. In short, the present invention provides a sewing ring
which
is more surgeon-friendly, more secure in preventing leaks, and more flexible.
The present invention addresses deficiencies apparent in the prior art,
including improving the coaptation characteristics of the sewing ring and
simplifying the surgical methodology for attaching prosthetic heart valves to
the .
valve annulus. In that regard, the present invention provides a novel and non-
obvious sewing ring shape and structural makeup that complements the
CA 02303289 2000-03-13
7
physiological and anatomical characteristics of the annulus and provides an
attachment platform that that reduces the need for tedious suturing
techniques.
In accordance with the present invention, there is provided a sewing ring that
includes a suture-penetrable ring member made of a resilient material that has
a
plurality of ribs defining adjacent cells or voids that enhance the resiliency
of the ring
member. The ring member has a radial width that results in the sewing ring
providing
a coaptation area with the annulus tissue that is sufficiently large so as to
enable the
attachment of the sewing ring to the annulus tissue without a load
distributing device
such as a pledget.
In addition, the sewing ring of the present invention combines a resilient
ring
member with a novel ring member geometry so as to ensure the sufficient
coaptation
area between the sewing ring and the tissue without unduly stressing the
annulus
tissue. In the case of a mitral valve implantation, the present invention may
include a
smoothly contoured blend or coaptation surface to conrform to the mitral valve
annulus. In the case of an aortic valve implantation, the present invention
may include
a outwardly extending coaptation side which extends a particular radial
distance to
ensure the adequate coaptation distance.
In accordance with an object of an aspect of the invention there is provided a
sewing ring for use in implanting a prosthetic heart valve to a support
annulus,
comprising:
a suture-penetrable annular ring member formed of a plurality of deformable
walls, some of which are radially aligned to define open cells therebetween,
the
annular ring member being oriented about an axis and having a top end and a
bottom
end spaced along the axis, the annular ring member having a triangular cross
section
defined by an axially extending inner ring side, a top side projecting
radially outward
from the top end of the inner ring side, and a coaptation side extending
between an
outermost projection of the top side and the inner ring side, wherein a radial
distance
between the inner ring side and the outermost projection of the top side is at
least
about 3.18 mm; and
a fabric covering surrounding at least an outer portion of the annular ring
member.
CA 02303289 2002-08-08
7a
In accordance with another object of an aspect of the invention there is
provided a sewing ring for use in implanting a prosthetic heart valve to a
support
annulus, comprising:
a suture-penetrable annular ring member formed of a plurality of deformable
walls, some of which are radially aligned to define open cells therebetween,
the
annular ring member being oriented about an axis and having a top end and a
bottom
end spaced along the axis, the annular ring member having a cross section
defined by
an axially extending inner ring side, a top side projecting radially outward
from the
top end of the inner ring side, and a coaptation side extending between an
outermost
projection of the top side and the inner ring side, wherein the coaptation
side is at least
partly concavely curved; and
a fabric covering surrounding at least an outer portion of the annular ring
member.
According to a further aspect of the invention, there is provided a sewing
ring
for use in implanting a prosthetic heart valve to a support annulus,
comprising:
a suture-penetable annular ring member formed of a plurality of deformable
walls, some of which are radially aligned to define open cells therebetween,
the
annular ring member being oriented about an axis and having a top end and a
bottom
end spaced along the axis, the annular ring member having a cross section
defined by
an axially extending inner ring side, a top side projecting radially outward
from the
top end of the inner ring side, and a coaptation side extending between an
outermost
projection of the top side and the inner ring side, wherein the coaptation
side is at least
partly concavely curved and is defined by one of the deformable walls to
thereby
provide a concavely curved tissue coaptation surface around the periphery of
the ring
that compliantly conforms to the support annulus and resists perivalvular
leaking
therebetween; and
a fabric covering surrounding at least an outer portion of the annular ring
member.
i i
CA 02303289 2002-08-08
7b
Further objects and advantages of the present invention shall become apparent
to those skilled in the art upon reading and understanding the following
detailed
description of a presently preferred embodiment of the invention.
Brief Description of the DraWInQS.
Figure la is an exploded perspective of a mitral annulus sewing ring in
accordance with the present invention showing a mechanical valve in phantom;
Figure lb is a perspective assembly view of the mitral annulus sewing of
Figure 1 a;
CA 02303289 2000-03-13
V1'O 99/13802 PCT/US98/14696
8
Figures 2a and 2b are top and bottom perspective views of a sponge used
in a mitral annulus sewing ring in accordance with the present invention;
Figure 2c is a cross-sectional view of the sponge of Figure 2b as taken
along the lines 2c-2c;
S Figures 3a-c are schematic cross-sectional views of one side of a
mechanical valve and three embodiments of the mitral sewing ring of Figure lb;
Figure 4a is a schematic sectional view of a mitral annulus;
Figure 4b is a schematic sectional view of a valve sizer in the mitral
annulus in preparation for implanting the sewing ring of Figure lb;
Figure 4c is a schematic sectional view of a mechanical valve having the
mitral sewing ring of Figure 1 b in the mitral annulus;
Figure Sa is a schematic cross-sectional view of one side of a mechanical
valve and sewing ring of Figure 1 b placed in a mitral valve annulus;
Figure Sb is a schematic cross-sectional view of one side of a
mechanical valve and prior art sewing ring placed in a mitral valve annulus;
Figure 6a is an exploded perspective view of an aortic annulus sewing
ring in accordance with the present invention showing a mechanical valve in
phantom;
Figure 6b is a perspective assembly view of the aortic annulus sewing of
Figure 6a;
Figure 7a is a perspective view of a sponge used in an aortic annulus
sewing ring in accordance with the present invention;
Figure 7b is a cross-sectional view of the sponge of Figure 7a as taken
along the lines 7b-7b;
Figure 8a is a perspective view of a further embodiment of a sponge for
use in smaller annulus sewing rings in accordance with the present invention; -
Figure 8b is a cross-sectional view of the sponge in Figure 8a taken
along the line 8b-8b;
CA 02303289 2000-03-13
WO 99/13802 PCTNS98/14696
9
Figures 9a-c are schematic cross-sectional views of one side of a
mechanical valve and three embodiments of the aortic sewing ring of Figure 6b;
Figure l0a is a schematic sectional view of an aortic annulus;
- Figure 1Ob is a schematic sectional view of a valve sizer in the aortic
annulus in preparation for implanting an aortic sewing ring similar to that
shown in Figure 6b;
Figure lOc is a schematic sectional view of a mechanical valve having
an aortic sewing ring similar to that shown in Figure 6b in an aortic annulus;
Figure l la is a schematic sectional view of a further valve sizer in an
aortic annulus in preparation for implanting an aortic sewing ring of Figure
6b
in an supra-annular position;
Figure llb. is a schematic sectional view of an aortic sewing ring of
Figure 6b in an supra-annular position of an aortic annulus;
Figure 11 c is a schematic sectional view of an aortic sewing ring of
1 S Figure 6b in an infra -annular position of an aortic annulus;
Figure l ld is a schematic sectional view of a downsized aortic sewing
ring of Figure 6b in an infra-annular position of an aortic annulus;
Figure 12a is a plan view of a mechanical valve as placed in an intra-
annular position in an aortic valve annulus with an fabric covering of an
sewing
' 20 ring of an present invention removed to illustrate an annular sponge
similar to
that shown in Figures 7a or 8a in compression;
Figure 12b is a top cross-sectional view similar to Figure 12a with an
mechanical valve placed in an supra-annular position in an aortic valve
annulus
and showing an annular sponge in a relatively uncompressed state;
25 Figure 13a is a schematic cross-sectional view of one side of a
- mechanical valve and sewing ring of Figure 9b or 9c as placed in a supra-
annular position in an aortic valve annulus;
CA 02303289 2000-03-13
Figure 13b is a schematic cross-sectional view of one side of a
mechanical valve and prior art sewing ring as placed in an supra-annular
position in an aortic valve annulus;
Figure 14a is a schematic cross-sectional view of one side of a
5 mechanical valve and sewing ring of Figure 9b or 9c as placed in an intra-
annular position in an aortic valve annulus;
Figure 14b is a schematic cross-sectional view of one side of a
mechanical valve and prior art sewing ring as placed in an infra-annular
position
in an aortic valve annulus.
Description of the Preferred Embodiments
The detailed description set forth below in connection with the appended
drawings is intended as a description of the presently preferred embodiments
of
the invention, and is not intended to represent the only forms in which the
present invention may be constructed or utilized. The description sets forth
the
structures and functions for the present invention in connection with the
illustrated embodiments. Preferred embodiments of sewing rings for
prosthetic heart valves in accordance with the present invention are disclosed
in
this description and the Figures. The description and figures include
information for using the invention both in mitral valve replacement and
aortic
valve replacement. However, such description and figures are by way of
example only and not by away of limitation. Those skilled in the art will
appreciate that the sewing ring of the present invention may be utilized in
other
various applications.
CA 02303289 2000-03-13
V1'O 99/13802 PCT/US98/14696
11
Mitral Valve Sewing,_$j~g
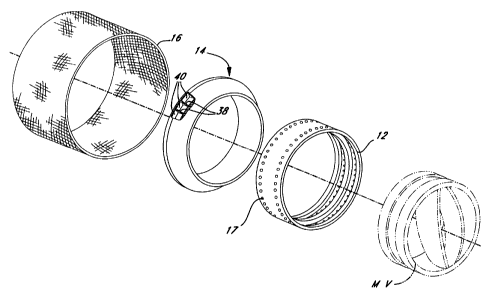
Referring to Figures la and lb, a first embodiment of the present
- invention generally comprises a sewing ring 10 configured for use with a
prosthetic mitral valve MV. The sewing ring 10 generally includes a ring
member or stent 12 to which an annular sponge 14 is attachable. A fabric
material 16 generally covers the stent 12 and the annular sponge 14. It should
be noted that the sewing ring 10 is particularly suited for implantation in
the
mitral annulus because it conforms to the particular anatomy of that annulus,
and valves other than the mechanical valve shown may be used in conjunction
therewith. Thus, MV designates mitral valve, whether mechanical or
bioprosthetic.
The flow direction of blood though the sewing ring (with the valve MV
removed) is seen in Figwe lb. As used herein, the term proximal refers to that
1 S end or edge of the device which is on the upstream or inflow side thereof
and
the term distal refers to that end or edge of the device which is on the
downstream or outflow side of the device. The proximal end of the device is
indicated by the letters PE and the distal end of the device is indicated by
the
letters DE. Notice that the mitral sewing ring 10 expands radially from the
outflow or distal end DE to the inflow or proximal end PE. This is because the
mitral valve MV is implanted on the inflow side of the mitral annulus from the
side of the left atrium.
According to a preferred embodiment of the present invention, the stent
12 is comprised of a polyacetal material, one example of which is DELRIN (a
registered trademark of E.LDuPont DeNemours & Co., Inc. Wilmington,
- Delaware). As those skilled in the art will appreciate, the stmt 12 may be
comprised of various other polymer materials such as polyacetals, polyesters,
ultra high molecular weight polyethylene, polysuIfones, polyimides, polyether
keytones (e.g., PEEK), liquid crystalline polymers (e.g., LCP's), and/or
carbon
CA 02303289 2005-10-04
12
filter composites. The ring member may alternatively be formed of
biocompatible metal or metal alloy, such as titanium Eigiloy or zirconium.
The needle-penetrable fabric ,material 16 preferably comprises a
biocompatible woven or knitted material, such as polyester or other suitable
material. The fabric may be treated or coated with various chemical
materials/coatings to improve biocompatability (e:g., heparin, chemically
bound
heparin, carbon coatings, etc.).
The annular sponge 14 is comprised of a biocompatible resilient
material, preferably silicone rubber. The needle-penetrable annular sponge 14
preferably comprises a plurality of cells or voids 40 (best shown in Figure
2b)
as described below and is assembled to the stmt L2 and fabric material 16 with
sutures. More particularly, the stent 12 includes a plurality of apertures 17,
preferably two circumferential rows; through whioh a needle ~,d suture may be
breaded. The stmt 12 is first attached to the sponge 14, and xhen the fabric
material 16 is wrapped around and covers both entirely, except for an utwardly
PrW~fi~ ~ular rib 19 (see Figure la) used to secure the .assembled ring to
the valve body. Such an assembly procedure is described in U:S. Patent
5,755,783, filed July 29, 1996, entitled "SUTURE RINGS FOR
ROTATABLE ARTIFICIAL HEART VALVES". Other sewing ring
assemblies may be suitable, of course.
Referring more particularly to Figures 2a-2e, the annular sponge 14 has
a projected cross-sectional conf guration characterized by a circumferential
inner surface 30 having a dimension ~I, a radial top .surface 32 having a
dimension W, a circumferential outer surface 34 having a dimension b, and a
smoothly contoured blending surface 36 extending between the bottom ends of .
the inner surface and he outer surface. The outer surface 34 is substantially
smaller than the inner surface 30 and thus defines the periphery of an
outwardly
extending flange of the sponge 14. The .inner surface 30 and outer surface 34
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
13
are desirably parallel and axially disposed, although other configurations are
. possible. A plurality of radially oriented ribs 38 extend between the
aforementioned surfaces to divide the interior of the sponge into a plurality
of
circumferentially arrayed and desirably evenly spaced cells 40. In the
illustrated
embodiment, discrete walls having faces define the inner surface 30, outer
surface 34 and blending surface 36, while the elongated top surface 32 is
defined by the top edges of the ribs 38 and is substantially open to the cells
40.
In a preferred embodiment, the dimension H of the inner surface 30 is
approximately 4.57mm, the dimension W of the elongated top surface 32 is
. 10 approximately 4.32mm, the dimension h of the outer surface 34 is
approximately 1.57mm and the smooth contoured blending surface 36 has a
substantially constant radius R of approximately 4.45mm. The overall diameter
D of the annular sponge 14 is seen in Figure 2a and is generally determined by
the size of the annulus into which the sewing ring 10 is received. These
dimensions are given as exemplary only, and other dimensions or ranges may be
used.
The contoured blending swface 36 may be a curve of constant radius or
a complex curve with several different radii of curvature or even an aspheric
curve with a constantly changing radius of curvature. The surface 36 desirably
mimics as near as possible the ideal shape of a mitral annulus after the
natural
mitral valve has been excised.
The soft material of the sponge 14 in conjunction with the cells 40
provides a highly compliant sewing ring to facilitate deformation thereof,
particularly at the flange or outer surface 34. Such compliance allows the
sponge 14 to conform to the sculpted mitral annulus and maximize the valve
- orifice to annulus ratio. The cells 40 also make the suture ring 10 more
easily
penetrable by a needle and mitigate dulling of the needle as sometimes occurs
with solid PTFE r7ngs.
CA 02303289 2000-03-13
WO 99/13802 PC?/US98/14696
14
Figures 3a-c illustrate various configurations of the present mitral valve
sewing ring on a mechanical valve V for various sized annuluses. Features
previously identified such as the stent 12 and sponge 14 will be given like
numbers. Figure 3a shows a sewing ring 10' for use in smaller mitral annuluses
having diameters between 23 and 29 mm. The fabric covering typically
comprises a long piece 16a on the inflow side and a short piece 16b on the
outflow side, the two pieces overlapping on the exterior of the stent 12. Two
friction controlling protuberances 42 are provided on the interior of the
stent 12
and serve to compress the fabric against the valve body V. Figure 3b shows a
sewing ring 10" for use in mitral annuluses having diameters of approximately
31 mm. The construction is the same as for the sewing ring 10' of Figure 3a
except for a spacer sleeve 44 interposed and sutured between the stent 12 and
sponge 14. This sleeve 44 in combination with a larger diameter sponge 14
1 S enables the assembly to fit in larger annuluses. Furthermore, the fabric
covering
comprises an inflow piece 16a, an outflow piece 16b, and a sponge retainer
piece 16c which encompasses the sponge 14 and is secured on the interior
thereof. Finally, Figure 3c shows a valve V and sewing ring 10"' for use in 33
mm annuluses. The construction is identical to the sewing ring 10" of Figure
3b except for a larger spacer sleeve 46.
Mitral Ann_ulu~, gizin~ and Imp]antation
Figure 4a schematically illustrates in section a mitral annulus 48 having
a diameter X. The well-defined ledge of the mitral annulus 48 may vary
depending on the extent of tissue resection required, but is typically more
pronounced than the aortic annulus shown in Figure 10a. Figure 4b shows a
valve sizer 50 shaped like the mitral sewing ring 10 and positioned within the
annulus for measurement. When the appropriate sizer is found, the
con espondingly sized valve is chosen for implantation. Figure 4c shows a
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
mechanical valve MV and mitral sewing ring 10 of the present invention as
placed into the annulus 48 for implantation.
Advantages ~f M;rrat R;"o
The combination of enhanced resiliency due to the plurality of cells 40
5 with the unique cross-sectional configuration, yields a sewing ring 10 that
provides enhanced and increased coaptation with the mitral annulus 48, as best
shown in Figure Sa. It should be noted that the cross-sections such as Figure
Sa
illustrating sewing rings attached to annuluses are only schematic, and the
precise dimensions may not be to scale. Indeed, the cross-section of the
sponge
10 14 seen in Figure 2c is accurate, but the cross-section seen in Figure Sa
is not..
Due to the smooth contoured blending surface 36 and the compliance of
the mufti-celled sponge 14, the sewing ring 10 is able to contact the annulus
tissue 48 over substantially all of the blending surface 36 to achieve a
substantial coaptation area 24. Moreover the increased coaptation is achieved
15 without unduly deforming or compressing the tissue. The sizable coaptation
area along with the enhanced resiliency improves the stability of the valve
during pumping of the heart without damaging the annulus tissue. It also
better
seals the valve within the annulus to negate the possibility of perivalvular ,
leaking.
It is understood to those skilled in the art that in order to attach a
prosthetic mitrat valve without pledgets, the surgeon must have a minimum
"bite" of about 4mm of mitral annulus tissue (as measured radially) upon which
to introduce and secure the sutures. Such a distance can be gauged from where
the annulus tissue touches the outer surface 34 of the sewing ring to where
the
tissue ends near or at the base of the sewing ring 10. Even if such a bite was
available using prior art rings, none had the flexibility and resiliency to
deform
in cooperation with the tissue and so reduce the stress on each suture. The
- present mitral rings 10 provide such compliance and resilience in
conjunction
with the larger shape, and thus enable pledget-free attachment.
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
16
Moreover, the unique configuration also yields a suturing platform of
sufficient area to allow the introduction of both a ventricular tissue suture
20 in
close proximity to the inner periphery of the sewing ring 10 and/or an atrial
tissue suture 22 more toward the outer periphery of the sewing ring all
without
the use of pledgets to distribute the load. 1n effect, the increased "bite" of
the
sutures into the annulus afforded by the larger coaptation area 24 enables the
tough annulus tissue itself to combat the "cheesewire" effect, thus obviating
pledgets. In the alternative, the suturing platform still allows for more
traditional horizontal mattress or running sutures, with or without pledgets.
In the preferred embodiment, surgeons are able to reduce the number of
sutures used while still ensuring proper sealing around the valve. Desirably,
about 20 simple sutures are sufficient to secure the sewing ring 10 to the
mitral
annulus. This is a decrease of 33% over prior designs which required 30 simple
sutures, and represents a decrease of 50% over designs requiring 20 mattress
I 5 sutures (in effect, doubling the number of passes of simple sutures). Even
with
the reduced number of sutures, the shape and characteristics of the sewing
ring
10 provide adequate protection against subsequent perivalvular leakage. This
attractive combination of features is facilitated by the resilient nature of
the
inner sponge 14 and shape and size of the ring 10. The sutures are passed both
deeper into the annulus and deep within the sewing ring to better distibute
and
stresses in tension of the sutures. Between each suture, the sewing ring 10
molds to the annulus tissue whether smooth or irregular. This "hugging" of the
annulus is not defeated by beating of the heart and movement of the valve
because of the flexible and resilient inner sponge which absorbs such
stresses.
In short, the soft ring 10 starts out better conforming to the annulus and
maintains that conformance at least until tissue ingrowth into the fabric 16
supplants the good seal provided.
The advantages are more clearly seen upon comparison of the sewing
ring 10 with a prior art sewing ring 52 depicted in Figure 3b. The prior art
CA 02303289 2000-03-13
. . WO 99/13802 PCTNS98/14696
17
sewing ring 52 is characterized by a solid Teflon felt or cloth filler and has
a
configuration essentially of a right triangle. The right triangle comprises a
top
surface 54, an inner surface 56 and a straight edge hypotenuse 58 that
connects
the top surface 54 and the inner surface 56.
Due to the limited resilience of the solid filler, the edge 58 contacting
the annulus 48 being a straight edge and the dimensions of the sewing ring 52,
the tissue 48 and the sewing ring 52 do not coapt to the same advantageous
degree offered by the sewing ring 10 of the present invention. Indeed, the
coaptation area 60 for the prior art sewing ring 52 is substantially smaller
than
the coaptation area 24 resulting from the sewing ring 10 of the present
invention. And if attempts were made to increase the coaptation area 60, the
tissue 48 would become unduly compressed and deformed so as to potentially
harm the tissue.
In addition, the prior art sewing ring does not provide a sufficient
suturing platform to allow the introduction of sutures 28 without the use of
pledgets. The small coaptation area 60 does not provide sufficient tissue
interface with the sewing ring to ensure safe attachment of the valve.
Pledgets
are needed in order to distribute the loads and thereby prevent concentrated
loads on the small tissue/sewing ring interface.
Aortic Valve Sewing Ring
Referring to Figures 6a and 6b, a second embodiment of the present
invention generally comprises a suture ring 110 configured for use with an
artificial aortic valve AV. Again, the valve AV may be of a number of types,
and is shown as a mechanical valve as an example only. As with the mitral
valve configuration discussed above, the sewing ring 110 generally includes a
ring member 112 to which an annular sponge 114 is attached. A fabric material
116 generally covers the ring member 112 and the annular sponge 114. The
CA 02303289 2000-03-13
WO 99/I3802 . PCT/US98/14696
18
material used for each of the components are the same as those described for
the
mitral version discussed above.
The sewing ring 110 for use with an aortic valve is generally similar to
the sewing ring used for the mitral valve. One exception is that the
configuration of the sponge member 114 is generally frusto-conical in shape,
thus defining a substantially constant outward taper from the proximal end PE
to the distal end DE thereof. Notice that in contrast with the mitraI valve of
Figures 1 a and 1 b, the aortic sewing ring 110 expands from the inflow or
proximal end PE to the outflow or distal end DE. This is because the aortic
valve AV is implanted on the outflow side of the aortic annulus. Additionally,
the valve body of the aortic valve AV, as well as the sewing ring 110
therefor,
are provided in a range of diameters which is less than in the mitral valve
because of the smaller aortic annulus.
Referring to Figures 6a, 7a and 7b, the sponge member 114 includes a
plurality of cells or voids 139 defined by walls 141 and ribs 143 which
provide
enhanced flexibility to the sponge member in much the same manner as
described with respect to the sewing 14 for the mitral valve. The sponge
member 114 has a projected triangular cross-sectional shape defined by three
surfaces, namely a coaptation side 140, an inner ring side 132 and a top edge
142 wherein each of the surfaces are separated by an angle A, B and C,
respectively. The walls 141 define the coaptation side 140 and inner ring side
132, while the top edge 142 remains open to the cells 139. For larger valves,
the lengths of each side and the associated angles are such that the sponge
114
provides a projected triangular area 138 that extends beyond a triangular area
144 that otherwise defines a right triangle within the cross-section of the
sponge
member 114. As seen in Figures 9b and 9c; the triangular area 138 projects
past .
the outflow side or distal end DE of the attached valve body. The coaptation
side 140 is desirably shaped to mimic the ideal shape of the aortic annulus
after
CA 02303289 2000-03-13
WO 99/13802 PCTNS98/14696
19
valve excision. The aortic annulus is less pronounced than the mitral annulus,
. and tends to be lass planar and somewhat scallop shaped.
In a preferred embodiments of the ring 110 for larger patients, the inner
- ring side 132 has a length of approximately 6.17mm and the angle A between
the inner ring side 132 and the coaptation side 140 is approximately 32.8
degrees. The coaptation side slopes such that it extends a distance 133 beyond
the inner ring side 132 of approximately 1.04mm and then connects with the top
edge 142 at an angle C of about 47.2 degrees. The top edge 142 then slopes
back to the inner ring side 132 for a horizontal, or radial, distance 136 of
approximately 3.18mm at an angle B of approximately 110 degrees. These
dimensions thus lead to the coaptation side 140 having a length of
approximately 7.88mm. Again, these dimensions are exemplary only and
should not be construed to limit the invention further than the appended
claims.
Once the cloth 116 has been sewed onto the sponge 114, the dimensions
will jncrease accordingly, due to the thickness of the cloth which can range
between .008 inch (0.20 mm) and 0.014 inch (0.36 mm). The overall projected
cross-sectional area of the sewing ring . of this preferred embodiment is
approximately 10.968 sq. mm.. For example, in the preferred embodiment just
described, the inner ring side 132 has a length of approximately 6.Smm and the
top edge 136 will have a length of approximately 4mm.
Rings for Small Aortic nn 1 ~ c
In certain patients, particularly children, the aortic annulus is quite small.
As a result, it is sometimes advantageous to utilize a smaller diameter valve
and
sewing ring (on the order of about l9mm or 2lmm) that are especially adapted
for placement into such a small location. Even with such small annulus
- diameters, intra-annular placement of a conventionally sized valve would
unduly restrict the flow of blood. Consequently, and referring to Figures 8a
and
8b, it is advantageous to use a sponge member 214 configured to have a cross-
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
section in the shape of a right triangle but dimensioned so as to maintain
increased coaptation with the aortic annulus in the supra-annular position.
As with the sponge described with previous embodiments, the sponge
214 is comprised of cells or voids 239 defined by walls 241 and ribs 243 to
5 enhance the resiliency of the sewing ring 210. In its projected cross-
sectional
shape, the sponge 214 is configured to have a coaptation side 238, an inner
ring
side 236 and a top edge 232. The inner ring side 236 and the coaptation side
238 are placed at an angle D from each other.
,The top edge 232 has approximately the same length as the
10 corresponding horizontal distance 136 in the sewing ring 114 described
previously and in a preferred embodiment, that dimension is approximately
3.I8mm. In the same preferred embodiment, the inner ring side 236 has a
length of approximately 3.68mm and the angle D is equal to approximately 40.4
degrees, thus resulting in a length of 4.865mm for the coaptation side 238.
15 Aortic Ring .onfig oration
Figures 9a-c illustrate various configurations of the present aortic valve
sewing ring 110 on a mechanical valve V for various sized annuluses. Features
previously identified such as the stent 112 and sponge 114 will be given like
numbers. Figure 9a shows a sewing ring 110' for use in smaller aortic
20 annuluses having diameters between 19 and 21 mm. The fabric covering
typically comprises a short piece 116a on the outflow side and a long piece
116b on the inflow side. Two friction controlling protuberances 150 are
provided on the interior of the stent 112 and serve to compress one end of the
short piece 116a and one end of the long piece 116b against the valve body V.
Figure 9b shows a sewing ring 110" for use in aortic annuluses having
diameters of between 21 and 29 mm. The construction is similar to the sewing -
ring 110' of Figure 9a except the sponge I 14 is that shown in Figure 7b which
extends above the valve V body. A coaptation face 151 makes a rake angle 152
with a plane 154 normal to the ring axis of between 10° and 20°.
More
CA 02303289 2000-03-13
, WO 99/13802 PCTNS98/14696
21
specifically, the rake angle 152 is preferably 10° for 21 mm annuluses
and 20°
for 23-29 mm annuluses. The coaptation face 151 meets the axial outer surface
of the stent 112 at a distance 156 of about lmm from an inflow end of the
valve
V. Finally, Figure 9c shows a valve V and sewing ring 110"' for use in 31 mm
aortic annuluses. The construction is identical to the sewing ring 110" of
Figure 9b except for a consistent rake angle of about 20°. Also, the
sponge 114
includes a thickened region 158 at an inflow end which serves to increase the
profile of the ring to fit the larger annulus. The region 158 is desirably
integrally formed in the silicone sponge 114 and has an axial dimension
approximately equal to the axial distance 156 of the smaller ring of Figure
9b.
Mitral Annul~cSi .ing and Implantation
Figure l0a schematically illustrates in section an aortic annulus 160
having a diameter X. The aortic annulus 160 is typically less pronounced than
the mitral annulus shown in Figure 4a but nevertheless exhibits a datum line
162 at its narrowest orifice. Figure lOb shows a valve sizer 164 shaped like
the
small diameter aortic sewing ring 110' of Figure 9a and positioned within the
annulus for measurement. When the appropriate sizer is found, the
correspondingly sized valve is chosen for implantation. Figure lOc shows a
aortic valve AV and sewing ring 110' of the present invention as placed supra-
annularly with respect to the annulus 160 for implantation.
Aortic Ring Placement Flexibility
One of the advantages in the use of the sponge 114 in the sewing rings
110" or 110"' is that it accommodates aortic valve placement either intra-
annularly or supra-annularly without damaging the annulus tissue or adding
difficult steps to the surgical methodology. ' Supra-annularly refers to
placement
of the valve body generally outside of the annulus itself, while infra-annular
position the valve body extends substantially within the annulus. And in
either
application, the sewing ring 110 offers an increased coaptation with the
annulus
CA 02303289 2000-03-13
. WO 99/13802 PCT/US98/14696
22
tissue much as described with regard to the mitral valve placement. These
advantages are best discussed with reference to Figures l la-l ld.
Figure l la shows a valve sizer 166 in position in the aortic annulus 160
prior to placement of a valve. The present invention enables the surgeon to
take
the dimension measured conventionally with the sizer 166 and choose various
sized valves depending on the need. That is, conventional wisdom teaches the
placement of prosthetic valves infra-annularly to help prevent perivalvular
leakage. In some situations, however, a supra-annular placement might be more
expedient if not for this leakage potential. Figure l lb shows a mechanical
valve
and aortic ring 110" or 110"' placed in the supra-annular position. Because of
the advantageous shape, compliance, compressibility and resiliency of the
present rings, they will conform to the annulus and provide leak free
coaptation
even in this unconventional position. Figure llc shows the same valve after
being gently manipulated into an infra-annular position. Finally, Figure l ld
shows a downsized valve with aortic ring 110" or 110"' of the present
invention positioned infra-annularly. The valve may be downsized by 1 or 2
mm based on the surgeon's examination of the needs of the patient. Previous
sewing rings were either too stiff and/or not large enough to accommodate all
of
the various implantation positions that the present rings 110" or 110"'
enable.
S~on_~ Compression
Referring next to Figures 12a and 12b, the configuration of the sponge
114 in the context of either supra-annular or infra-annular implantation is
better
understood. In Figure 12b, the sponge 114 is shown in the supra-annular
position prior to suturing and thus the sponge appears substantially in its
undeflected state, at least on the upper end. In Figure 12a, the sponge 114 is
shown in the infra-annular position prior to suturing and thus the sponge is .
deflected in order to account for the smaller annulus size. The ribs 143
dividing
the cells 139 are compressed radially inwardly and bend as a result. Also, the
outer peripheral wall 141 of each cell takes on a concave shape. This manner
of
CA 02303289 2000-03-13
WO 99/13802 PCT/US98/14696
23
deflection enables the sewing ring to provide the advantageous resiliency for
the
sewing ring as previously described. That is, some prior rings provided
compliance but no resiliency or spring back. The present ring not only
' conforms better to the annulus prior to placement, but when placed intra-
annularly, for example, springs out to coapt to the tissue.
Advan ages of Aortic Ring
The sewing ring 110 ( 110" or 110"' of Figure 9b or 9c) placed in the
supra-annular position is depicted in Figure 13a. Prior to being sutured into
place, the sewing ring has the undeflected shape as shown by the dotted lines
(having element numbers with prime designations), indicating that a
significant
portion of the sewing ring, including the projected area 138', is not in
intimate
contact with the tissue 160. In order to secure the sewing ring into place,
this
portion of the sewing ring 110 must be pulled into engagement with the
adjacent
tissue 160. Due to the enhanced compliance of the sponge 114, this is easily
done as shown by the solid line configuration of the sewing ring without
unduly
deforming the annulus. The sewing ring 114 is easily pulled into engagement
with the tissue without any significant reduction in the length of the
coaptation
face 151 of the sewing ring 110 and without any undue stretching being induced
in the tissue 160. Furthermore, because of the advantageous cross-sectional
area of the sewing ring 110, the coaptation area 124 extends along
substantially
the entire length of the coaptation face 151 of the sewing ring 1 I 0.
Moreover,
the coaptation area 124 is substantially equal to the area achieved as if the
sewing ring 110 was in its undeflected state.
In addition, due to the geometry of the sewing ring as dictated in large
part by the sponge 114, the suturing platfon;n of the sewing ring 1 I O
results in
' an increased distance 168 between the sewing ring and the location in the
tissue
160 in which the surgeon may introduce a suture 169. This increased distance,
sometimes termed the "bite", enables suturing the sewing ring to the annulus
without using pledgets.
CA 02303289 2000-03-13
WO 99/13802 PCTNS98/14696
24
It is understood to those skilled in the art that in order to attach a
prosthetic aortic valve without pledgets, the surgeon must have a minimum
"bite" of about 3 mm of aortic annulus tissue (measured radially) upon which
to
introduce and secure the sutures. Such a distance can be gauged from where the
aortic annulus tissue touches the periphery of the sewing ring to where the
tissue
ends near or at the base of the sewing ring 110. Even if such a bite was
available using prior art rings, none had the flexibility and resiliency to
deform
in cooperation with the tissue and so reduce the stress on each suture. The
present aortic rings 110 provide such compliance and resilience in conjunction
with the larger shape, and thus enable pledget-free attachment.
In addition, the same advantages mentioned above for the mitral ring
embodiments are equally applicable to the aortic ring 110. More specifically,
less sutures with a better seal are provided along with the elimination of
pledgets and the ability to place infra- or supra-annularly. Finally, less
tension
1 S need be applied to each suture when implanting the valve because of the
compliant and resilient nature of the ring 110, thus reducing the potential
for
decubitous ulceration of the tissue within the suture loop.
These advantages are better understood with reference to Figure 13b
which shows the use of a prior art aortic annulus sewing ring 170 made of
either
solid Teflon felt or cloth filler as positioned in the supra-annular position.
The
prior art sewing ring does not have the necessary resiliency to allow
attachment
of the sewing ring to the tissue below without significantly deforming the
sewing ring and inducing undue stretching forces on the tissue 160. Moreover,
the geometry of the sewing ring 170 is such that the available coaptation area
is
already limited and becomes even more so when the sewing ring is deformed in
order to achieve attachment with the tissue. As is seen the coaptation area
172
is much less than the coaptation area 124 achieved with the sewing ring 110 of
the present invention. As a result, the surgeon is left with smaller amount of
CA 02303289 2000-03-13
WO 99/13802 ~ PCT/US98/14696
tissue upon which to attach the sewing ring 170, thus necessitating the use of
pledgets.
The sewing ring 110 (110" or 110"' of Figure 9b or 9c) placed in the
infra-annular position is depicted in Figure 14a_ Prior to suturing, the
sewing
5 ring 110 must be delicately manipulated and eased into the annulus by the
surgeon (hence, "infra-annular" placement) since the annulus for such
placement is smaller in diameter than the outer diameter of the sewing ring.
Due to the enhanced resiliency of the sewing ring 110, such placement is
achieved without adversely compressing the surrounding annulus tissue 160 and
10 without unduly compressing the sewing ring so as to lose coaptation area.
In
fact, the enhanced resiliency enables the sewing ring 110 to better match the
contour of the annulus tissue and thus further enhance the coaptation. The
enhanced resiliency combined with the sewing ring geometry thus results in a
coaptation area 125 that is substantially the same as what would be obtained
if
15 the coaptation face 151 was in the undeflected state. As discussed above,
this
combination also results in an increased bite 174 for the surgeon to introduce
a
suture 176 without using a pledget.
The advantages in the infra-annular placement context are better
understood with reference to Figure 14b which depicts the same prior art
sewing
20 ring 170 as discussed with respect to Figure 13b. Due to the limited
resiliency
of the sewing ring 170, the tissue 160 is unduly compressed when the ring is
positioned into infra-annular position. This makes the step of placing the
valve
more difficult for the surgeon. In addition, the geometry of the sewing ring
170
as compared to the sewing ring 110 of Figure 14a yields a coaptation area 178
25 that is significantly less than the coaptation area 125 offered by the
sewing ring
110 of the present invention.
The following Table I is a comparison of sizes of various sewing rings
available on the market and one example of the sewing ring of the present
invention. The sewing rings have the following sources:
~
CA 02303289 2000-03-13
26
A - Carbomedics Inc. of Houston, Texas
B - St. Jude Medical of Minneapolis, Minnesota
C - Baxter Healthcare Corp. of Irvine, California (Starry
D - Baxter Healthcare Corp. of Irvine, California (TEKNA)
S E - Present invention
TABLE I - COMPARISON OF SUTURABLE AREAS
SEWING RING A B C D E
USABLE RADIAL1.5 2.0 3.3 mm 2.5 4.06 mm
mm mm mm
WIDTII*
USABLE CROSS-3.9 4.0 10.3 mm' 5.2 11.0 mm'
mm' mm' mm'
SECTIONAL
AREA
COMPOSITION SOLID SOLID SOLID SILICONECELLEDCELLED
CLOTH CLOTH SPONGE SILICONESILICONE
SPONGESPONGE
* The term "usable radial width" is that width extending radially
outward from the valve body or stmt structure through which sutures can be
passed. This term takes into account any obstruction to the passage of sutures
on the valve body or stmt structure which decreases the absolute width of each
ring.
None of the aforementioned prior art sewing rings offers the
combination of enhanced resiliency with the unique geometry of an aortic
sewing ring in accordance with the present invention, nor the increased
coaptation between the sewing ring and the annulus tissue.
It is understood that the examples and embodiments described herein
and shown in the drawings represent only the presently preferred embodiments
of the invention, and are not intended to exhaustively describe in detail all
possible embodiments in which the invention may take physical form.