Note: Descriptions are shown in the official language in which they were submitted.
CA 02303714 2000-04-14
1
MULTIFUNCTIONAL APPARATUS FOR TREATMENT
OF RENAL INSUFFICIENCY
The present invention is a division of the
Canadian patent application 2,077,848 filed on September 9,
1992.
The present invention relates to an apparatus enabling to put on
to different types of treatment patients suffering from kidney failure,
in particular following an accident or a surgical operation.
In addition, the invention relates to an apparatus enabling the
accurate dosage in blood of substances such as medicines ( i n
particular antibiotics), glucose, or certain blood electrolytes
(potassium, magnesium; and bicarbonate, in particular). The invention
is described below in its application to dosing bicarbonate, but i t
will be understood that this particular example is given purely by
way of illustration and is not limiting in any way.
2o It is known that in addition to purifying plasma wastes (urea,
creatinine) and to excreting water, the kidneys play an important part
in maintaining the acid-base equilibrium of the internal medium, i n
particular by eliminating weak acids (phosphates, monosodium acids)
and by producing ammonium salts.
In people who have lost their kidney function either temporarily
or permanently, because this regulating mechanism is no longer
operating, an increase is observed in the acidity of the internal
medium (acidosis), i.e. a drop in the pH of the blood serum towards 7
30 (where blood pH normally lies within the very narrow limits of 7.35
to 7.45).
The conventional way of mitigating this deficiency of the
CA 02303714 2000-04-14
1a
regulating mechanism of the kidneys is to act on another mechanism
for regulating the acid-base equilibrium of the internal medium,
which mechanism is constituted by buffer systems of the blood, and
the main such system comprises carbonic acid as a weak acid i n
association with its alkaline salt, bicarbonate. Thus, to combat the
acidosis of a person suffering from kidney failure, bicarbonate i s
caused to pass into the blood, generally simultaneously with a
CA 02303714 2000-04-14
2
session during which the blood is purified by hemofiltration or by
hemodialysis.
During treatment by hemofiltration, where blood is purified by
ultrafiltration of plasma water through a semi permeable membrane
accompanied by convective transfer of plasma wastes, bicarbonate i s
added by infusing a solution of sodium bicarbonate.
During hemodialysis treatment where blood is purified by
plasma wastes being transferred by diffusion through a
semipermeable membrane with blood being circulated on one face o f
the membrane and a dialysis liquid being circulated on the other face,
bicarbonate may be added in two ways, depending on whether the
dialysis liquid contains bicarbonate or whether it has none.
When the dialysis liquid contains bicarbonate, then bicarbonate
is added to the blood by diffusion from the dialysis liquid through the
semipermeable membrane into the blood, and the bicarbonate
concentration in the dialysis liquid is adjusted accordingly.
When the dialysis liquid does not contain bicarbonate, then a
solution of sodium bicarbonate is infused into the patient as during
hemofiltration treatment, and in sufficient quantity to compensate
for the diffusive losses (or the convective losses in hemofiltration)
that occur in the membrane exchanger and to compensate for the
deficit from which the patient in an acidosis state is suffering.
The final concentration of bicarbonate in the blood of a patient
subjected to either of these treatments depends on the concentration
of bicarbonate in the infusion solution or in the dialysis liquid, on the
respective flow rates thereof, and on the flow rate of the patient's
blood through the membrane exchanger. With the exception of the
concentration of the sodium bicarbonate solution which is fixed by
the manufacturer, these parameters are, at present, determined
CA 02303714 2000-04-14
3
empirically by the doctor on the basis of blood pH measurements that
are performed regularly for such patients in a state of shock, whose
blood is being dialyzed or ultrafiltered on a permanent basis, or as
performed after one treatment session and before the following
session for patients who have lost kidney function permanently. I t
results therefrom that the concentration of bicarbonate in the blood
of the patient seldom corresponds to the desired concentration.
An object of the invention is to provide a blood treatment
apparatus which is particularly suited to continuously carry out a
plurality of treatments, and which enables an accurate control and
monitoring of the flow rates of the medical and corporeal liquids
which are circulated.
According to the invention, this object is achieved by a
multifunction apparatus for circulating corporeal and medical liquids
in an exchanger having two compartments separated by a
semipermeable membrane, a first compartment being connected to an
extracorporeal blood circuit connectable to a patient, and a second
compartment having an inlet and an outlet, the apparatus comprising:
~ first pumping means for circulating a first sterile liquid;
~ second pumping means for circulating a second sterile liquid;
~ ~ third pumping means for circulating blood in the
extracorporeal blood circuit;
~ extraction means for causing ultrafiltration of a corporeal
liquid through the membrane of the exchanger;
~ first weighing means for weighing a first reservoir for the
first sterile liquid, and a second reservoir for a waste liquid, the
first reservoir being connectable to the inlet of the second
compartment of the exchanger, and the second reservoir being
CA 02303714 2000-04-14
4
connectable to the outlet of the second compartment of the
exchanger;
~ second weighing means for weighing a third reservoir for the
second sterile liquid, this reservoir being connectable to the
S extracorporeal blood circuit; and
~ control means for receiving at least weight information fro m
the first weighing means and the second weighing means and for
controlling at least one of the first, the second and the third pumping
means and the extraction means, from at least this weight
information and from at least one information corresponding to a
desired flow rate of at least one of the first sterile liquid, the
second sterile liquid, the blood and the corporeal liquid.
The first weighing means includes either two independent
weighing means for weighing the first reservoir and the second
reservoir respectively, or a single weighing means for weighing both
the first reservoir and the second reservoir.
According to a characteristic of the invention, the apparatus
further comprises connecting means for permitting the f i r s t
reservoir to be selectively connectable to the inlet of the second
compartment of the exchanger or to the extracorporeal blood circuit.
According to another characteristic of the invention, the
apparatus further comprises dosage means for adjusting to a desired
concentration [A]DES, in the blood of the patient, the concentration o f
a substance (A) present in the second sterile liquid, whereby the
diffusive and/or convective transfer of the substance (A) through the
membrane of the exchanger is taken into account
According to a variant of the invention, the dosage means
comprises means for regulating the flow rate (QA) of the second
sterile liquid as a function of the flow rate (Qour) of the waste
CA 02303714 2000-04-14
liquid, whereby the flow rate (QA) of the second sterile liquid and
the flow rate (QouT) of the waste liquid are related by the equation:
QA - [A]DES x QoUT
[A]soy
5
where [A]SOS is the of the substance (A) in
concentration the f i r s t
sterile liquid.
According to another variantof the invention, the means
dosage
comprises means for regulatingthe flow rate (QA) of second
the
sterile liquid function of clearance CI of the artificialkidney
as a the
for the substance (A), whereby the flow rate (QA) of second
the
sterile liquid and the clearance are related by the equation:
CI
[A]DES x CI
OA [A]sou
where [A]soy is the concentration of the substance (A) in the f i r s t
sterile liquid.
Other characteristics and advantages of the invention appear on
reading the following description. Reference is made to the
accompanying drawings, in which:
Figure 1 is a simplified diagram of a first embodiment of the
invention; and
Figure 2 is a simplified diagram of a second embodiment of the
invention.
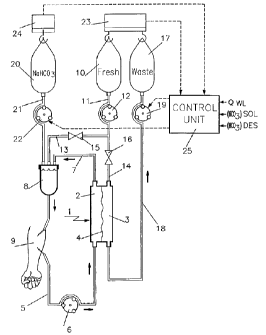
The artificial kidney shown in Figure 1 comprises an exchanger
1 having two compartments 2 and 3 separated by a semipermeable
membrane 4. The compartment 2 is connected to a circuit for
CA 02303714 2000-04-14
6
extracorporeal blood circulation and comprising an upstream duct 5
having a circulation pump 6 disposed thereon, and a downstream duct
7 fitted with a bubble trap 8. The ducts 5 and 7 have their free ends
provided with respective needles or catheter connectors f o r
connecting the circuit for extracorporeal blood circulation to the
vascular system of a patient 9.
A container 10 containing sterile substitution/dialysis liquid
that does not contain any bicarbonate is connected via common length
of duct 11 which has a circulation pump 12 disposed thereon to two
ducts 13 and 14 that are connected respectively to the bubble trap 8
and to an inlet of the second compartment 3 of the exchanger 1.
Blocking means 15 and 16 such as electromagnetically-operated
clamps are provided on the ducts 13 and 14 respectively to enable the
container 10 to be isolated or connected selectively to the exchanger
1 or to the circuit for extracorporeal blood circulation.
A second container 17 for waste liquid (ultrafiltrate and/or
waste dialysis liquid) is connected to an outlet of the second
compartment 3 of the exchanger 1 by a duct 18 which has an
extraction pump 19 for the waste liquid disposed thereon. The pump
19 serves to establish a variable pressure drop in the compartment 3
of the exchanger 1, i.e. it serves to vary the transmembrane pressure
and consequently the ultrafiltration flow rate.
A third container 20 containing a sterile solution of sodium
bicarbonate is connected to the bubble trap 8 by means of a duct 21
which has a circulation pump 22 disposed thereon.
In accordance with the invention, the artificial kidney shown i n
Figure 1 includes means for measuring the difference between the
liquids) infused into the patient 9 and the waste liquid, optionally
for determining a desired weight loss to be achieved by extracting a
CA 02303714 2000-04-14
7
quantity of plasma water that is greater than the quantity of infused
liquid(s), and to establish a determined value of bicarbonate
concentration in the plasma of the patient. These means comprise
first scales 23 for weighing the container 10 o f
substitution/dialysis liquid and the container 17 of waste liquid,
second scales 24 for weighing the container 20 of sodium bicarbonate
solution, and a control unit 25 suitable for receiving the data
delivered by the scales 23 and 24 as input signals, a reference value
QW~ for the desired weight loss flow rate, the value [HC03]SOS of the
concentration of bicarbonate in the solution contained in the
container 20, and a reference value [HC4s]pES for the desired
concentration of bicarbonate in the blood. The control unit 25 i s
designed to control the waste liquid extraction pump i 9 taking into
account the desired weight loss QW~ and the flow rate Q~N imposed on
the pump 12 for circulating the substitution/dialysis liquid, and t o
control the pump 22 for infusing the bicarbonate solution taking into
account the flow rate QpuT of the waste liquid extraction pump 19.
In accordance with the invention, the flow rate C~pa of the
infusion pump 22 can be controlled as a function of the flow rate Qpu'r
of the extraction pump 19 regardless of the type of treatment being
delivered to the patient (hemofiltration with or without infusion o f
substitution liquid, hemodialysis, or hemodiafiltration) by the
equation:
~co3 = QouT x~C~IoES (1 )
[HC03Jso~
The above-described artificial kidney operates as follows:
In hemofiltration mode without any substitution liquid being
infused, the clamps 15 and 16 are closed, the pump 12 for circulating
CA 02303714 2000-04-14
g
the substitution/dialysis liquid is off, and the pumps 19 and 22 for
extracting the blood filtrate and the infusion of bicarbonate solution
are on. The control unit 25 continuously adjusts the flow rate QpuT of
the extraction pump 19 as measured by means of the scales 23 so
that the flow rate is permanently equal to the sum of the desired
weight loss flow rate QWL and the flow rate Q~pa of the infusion o f
bicarbonate solution as measured by means of the scales 24. The
control unit 25 also continuously adjusts the flow rate C~pa of the
pump 22 for infusing the bicarbonate solution as a function of the
desired concentration of bicarbonate [HC03]pES in the blood of the
patient, of the concentration [HC03]soL of the solution contained i n
the container 20, and of the connective losses that occur in the
exchanger 1, which losses are equal to C~n- x [QHCOa]BLD~ where
[QHCO3]BLD is the concentration of bicarbonate in the blood of the
patient, and where the transmittance of the high permeability
membranes used for hemofiltration is equal to 1 for blood
electrolytes (recall that the general formula giving the mass f I ow
rate Js of a substance passing through a membrane as a function o f
the volume flow rate Jv of plasma water is the following:
Js = Jv x Tr x Cs
where Cs is the concentration of the substance in the blood and where
Tr is the transmittance of the membrane relative to said substance).
The pump 22 for infusing the bicarbonate solution being servo-
controlled in compliance with equation (1) given above enables thus
the blood of the patient 9 to be brought progressively to an
equilibrium state where its concentration of bicarbonate is equal to
[HC~3] DES
In hemofiltration mode with infusion of substitution liquid, the
clamp 16 is closed, the clamp 15 is open and all three pumps 12, 19,
CA 02303714 2000-04-14
9
and 22 are on, with the flow rate of the pump 12 being fixed by the
operator to a constant value at the beginning of a treatment session.
The operation of the artificial kidney in this second treatment mode
differs from that described above only in that to control the
S extraction pump 19 the control unit 25 takes account of the emptying
of the container 10, with the flow rate ~ imposed on the pump 1 9
then being selected so that the difference between the flow rate o f
substitution liquid and the flow rate of waste liquid as measured by
the scale 23 is equal to the sum of the desired weight loss flow rate
QW~ and the infusion rate C~o3 of bicarbonate solution as measured
by the scales 24. The infusion pump 22 for the bicarbonate solution
is adjusted as before in compliance with the servo-control specified
by equation (1 ).
In hemodialysis mode, the clamp 15 is closed, the clamp 16 i s
open, and all three pumps 12, 19 and 22 are on. The control unit 25
continuously adjusts the flow rate QouT of the extraction pump 19 so
that the difference between the flow rate of dialysis liquid and the
flow rate of waste liquid as measured by the scales 23 i s
continuously equal to the infusion flow rate C~-~~ of bicarbonate
solution as measured by the scales 24, with the weight loss f I o w
rate reference value being zero.
The control unit 25 also controls the infusion flow rate QHCO3 of
the bicarbonate solution as a function of the desired bicarbonate
concentration [HC03]pES for the blood of the patient, of the
concentration [HC03Jso~ of the solution contained in the container 20,
and of the diffusive loss through the exchanger 1 which is given by CI
x [HC03]gLD, where [HC03]gLD is the concentration of bicarbonate i n
the blood of the patient and where CI is the clearance of th a
artificial kidney for bicarbonate (the clearance is defined in general
CA 02303714 2000-04-14
1 ~
terms as the ratio between the quantity of substance eliminated per
unit time and the concentration of the substance in the blood at the
inlet of the exchanger). To ensure that the concentration o f
bicarbonate in the blood reaches a given value [HC03]pES a t
equilibrium, it is therefore necessary to control the infusion f I o w
rate QHCO3 of the pump 22 for the bicarbonate solution in compliance
with the equation:
(HC~3~DES
QHC03 = CI X ~C~~SOL
which assumes that the clearance of the artificial kidney has
previously been determined, which clearance depends on the type o f
exchanger used (nature of the membrane, area) and, in general, on the
flow rates of blood and of dialysis liquid through the exchanger.
However, for certain values of blood flow rate and of dialysis
liquid flow rate, the clearance of the kidney for a given substance and
a given type of exchanger is substantially constant. This applies i n
particular when firstly the area of the membrane in the exchanger i s
sufficiently large relative to the blood flow rate and secondly the
blood flow rate is relatively large compared with the dialysis liquid
flow rate (being about three or more times said rate). Under such
circumstances, the blood and the dialysis liquid leaving the exchanger
have the same concentration of the substance under consideration and
the clearance CI is equal to the outlet flow rate of the waste liquid
QOUT. In other words, under these particular operating conditions, the
control of the pump 22 for infusing the bicarbonate solution i s
defined by equation (1). These conditions are applicable to continuous
dialysis treatment of patients in a state of shock for whom
purification must be performed at a moderate rate so that their
weakened organism can tolerate it.
CA 02303714 2000-04-14
11
The artificial kidney of the invention thus has a particular
advantage for treating patients who have temporarily lost kidney
function since, whatever the type of treatment to which they are
subjected, this artificial kidney makes it possible to act on th a i r
S acid-base equilibrium in a manner that is simple by controlling one
pump only using a single servo-control equation.
The kidney can also operate in hemodiafiltration mode in which
the positions of the clamps and the operation of the pumps are the
same as in hemodialysis mode, except that the pump 19 is controlled
so as to give rise to ultrafiltration in the kidney in compliance with a
given reference value for weight loss rate.
The artificial kidney shown in Figure 2 differs from that
described above in that its circuit for extracorporeal blood
circulation includes a second pump 26 disposed downstream from the
exchanger 1, thereby enabling the transmembrane pressure in th a
exchanger 1 to be varied and consequently enabling the flow rate o f
ultrafiltered plasma water to be varied (i.e. Caps in hemofiltration).
In addition, the containers 10 and 17 for the substitution/dialysis
and for the waste liquid are now weighed by independent scales 27,
28, and the duct 18 connecting the compartment 3 of the exchanger 1
to the waste liquid container 17 is not provided with a pump.
Moreover, a three-port valve 29 having the ducts 11, 13, and 14
connected thereto serves to connect the container 10 for the
substitution/dialysis liquid either to the bubble trap 8 or to the
compartment 3 of the exchanger 1, or else to isolate the container
10.
The operation of this second embodiment of the art i f i c i a l
kidney of the invention is not significantly different from that of the
preceding embodiment. In hemofiltration mode without infusion o f
CA 02303714 2000-04-14
12
substitution liquid, the pump 12 is off and the flow rate of the pump
26 is controlled by the control unit 25 so that the filtration flow
rate ~ measured by the scales 28 is equal to the sum of the
reference weight loss flow rate QW~ and the infusion flow rate o f
bicarbonate solution QHCO3 as measured by the scales 24.
In hemofiltration mode with infusion of substitution liquid, the
pump 12 is on at a rate that is initially adjusted by the operator, and
the rate of the pump 26 is controlled by the control unit 25 so that
the filtration rate C~~ is equal to the sum of the reference weight
loss rate Qw~, the infusion rate of bicarbonate solution QHC03~ and the
infusion rate Q~N of substitution liquid as measured by the scales 27.
In dialysis mode, the pumps 6 and 26 on the blood c i rcu it
respectively upstream and downstream from the exchanger 1 operate
at the same rate, and the pump 12 which then serves as a pump f o r
circulating the dialysis liquid operates at a rate that is set i n i t i a I I
y
by the operator.
In hemodiafiltration mode, the control unit 25 controls the f I o w
rate of the ~~pump 26 as in hemofiltration mode with infusion o f
substitution liquid.
Except for hemofiltration mode in which the pump is off, the
flow rate of the pump 12 for circulating the substitution/dialysis
liquid is controlled by the control unit 25 which compares the desired
flow rate stored initially in the memory of said unit with the flow
rate as measured by the scales 27. The flow rate (spa of the pump
22 for infusing bicarbonate is controlled, as before, as a function o f
the waste liquid flow rate r~ as measured by the scales 28, i n
compliance with equation (1 ), or equation (2), as the case may be.
The invention is not limited to the embodiments described
above and variants may be provided.
CA 02303714 2000-04-14
13
In particular, in contrast to the artificial kidney embodiments
described above, in modes where the substitution/dialysis liquid i s
circulated by the pump 12, it is possible to have the flow rate of the
pump 19 (26) that controls the ultrafiltration flow rate as the rate
that is fixed initially by the operator, with the flow rate of the pump
12 being controlled as a function of the difference between the fresh
liquids and the waste liquids as infused and ultrafiltered, and the
desired weight loss rate.
Moreover, the value of the liquid flow rates needed for
controlling the pump 19 (26) controlling the ultrafiltration flow rate
and for controlling the pump 22 for infusing the bicarbonate solution
could be determined by measurement means other than scales, f o r
example using flow rate meters or volume-measuring means.
Moreover, the pumps 12 and 22 used for controlling the f I ow
rate of substitution/dialysis liquid and the flow rate of bicarbonate
solution could be replaced by electromagnetically-operated clamps,
with the liquids then flowing under gravity.
Also the source 10 of infusion liquid could be directly
connected to the vascular system of the patient and not, as described
before, to the circuit 5, 7 for extracorporeal blood circulation.
Finally, as mentioned above, the dosage means fitted to an
artificial kidney of the invention may be used for dispensing all sorts
of substances into the blood of a patient undergoing a treatment
session by hemofiltration, hemodialysis, or hemodiafiltration. For a
medicine A, for example, the container 20 would contain a sterile
solution of the medicine with the container 10 containing a dialysis
liquid in which the main electrolytes of blood are present, including
bicarbonate. The operation of the kidney is not different from that
described above with reference to the embodiments of Figures 1 and
CA 02303714 2000-04-14
14
2, and in particular, the infusion pump 22 is controlled as a function
of the flow rate of waste liquid in application of equation (1 ) o r
equation (2) as the case may be.