Note: Descriptions are shown in the official language in which they were submitted.
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98/00432
1
i3enzimidazole compounds, pharmaceutical compositions containing the compounds
and
their use.
This invention relates to novel benzimidazole compounds, pharmaceutical
compositions
containing these compounds, methods of treating therewith, and to method of
preparing such
benzimidazole compounds. The novel compounds are useful in the treatment of
central nervous
system diseases and disorders, which are responsive to modulation of the GABAA
receptor
complex, such as for example anxiety, sleep disorders, memory disorders, and
epilepsia or other
conwlsive disorders.
Background of the invention
Receptors for ~aminobutyric acid (GABA), GABAA receptors are the most abundant
inhibitory
receptors in mammalian brain. The GABAA receptor are structurally constituted
as
1S macromolecular heteropentameric assemblies (combinations of a, p, and 'y/8
protein subunits).
Several subtypes of such GABAA receptors have been described by techniques of
modem
molecular biology.
Each GABAA receptor complex comprises a chloride ion channel that controls
chloride flux
across the neuronal membrane, and multiple recognition sites for small
modulatory molecules
such as benzodiazepines, barbiturates, picrotoxin, and certain steroids. When
GAGA interacts
with its receptor, the ion channel is opened, chloride influx is enhanced, the
membrane is
hyperpolarized and the cell becomes less responsive to excitatory stimuli.
This GAGA induced
ion current can be regulated by diverse agents, including agents that interact
with the
benzodiazepine receptor or recognition site.
Agents that bind or interact with the modulatory sites on the GABAA receptor
complex, such as
for example the benzodiazepine receptor, can have either enhancing effect on
the action of
GAGA, i.e. a positive modulatory effect of the receptor (agonists, partial
agonfsts), an
attenuating effect on the action of GAGA, i.e. negative modulation of the
receptor (inverse
agonists, partial inverse agonists), or they can block the effect of both
agonists and inverse
agonists by competitive block (antagonists or ligands without intrinsic
activity).
Agonists generally produce muscle relaxant, hypnotic, sedative, anxiolytic,
andlor
anticonvulsant effects, while inverse agonists produce proconvuisant, anti-
inebriant, and
anxiogenic effects. Partial agonists are characterized as compounds with
anxiolytic effects but
SUBSTfTUT'E SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98100432
2
without or with reduced muscle relaxant, hypnotic and sedative effects,
whereas partial inverse
agonists are considered to be useful as cognition enhancers.
Numerous compounds belonging to different series of compounds having affinity
for the
benzodiazepine receptors have been synthesized during the last three decades.
However,
although the benzodiazepine receptor sites are still considered as very
attractive biological
sites for interfering with the CNS to treat various disorders and diseases,
then nearly all
previously synthesized compounds acting at these receptor sites have failed
during clinical
development because of unacceptable side effects.
WO 96133191 describes oxime-substituted benzimidazoles having GAGA-receptor
activity.
The present invention provides novel benzimidazole compounds that interact
with the
benzodiazepine receptor of the GABAA receptor complex. The compounds of the
present
invention are valuable modulators of the GABAA receptor complex with a
favorable
pharmacodynamic.
Object of the invention
It is an object of the present invention to provide novel benzimidazole
compounds and
pharmaceutically-acceptable acid addition salts thereof, which are useful in
the treatment of
central nervous system disortters, aiseases or aliments, wWCn are res~urmvC
mnc
modulation of the GABAA receptor complex, and especially the positive
modulation of the
GABAA receptor.
30
Another object of the present invention is to provide pharmaceutical
compositions comprising
the novel benzimidazoie compounds being useful for the above purposes. Still
another object
of the present invention is to provide a novel method of treating with the
novel benzimidazole
compounds.
A further object of the present invention is to provide a method of preparing
the novel
pharmaceutical composit;ons.
Additional objects will be obvious from the following description, and others
will be obvious to
one skilled in the art.
SUBSTIME SHEET (RUL~ 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98/00432
3
Summary of the Invention
The invention then, inter alia, comprises the following, alone or in
combination:
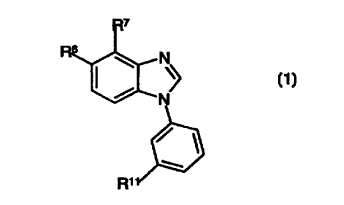
A compound represented by the general formula
_s N
N
R~
!0 wherein,
R" represents a 5- or 6-membered monocyclic heteroaryl group containing at
least one
nitrogen, or a 5- or 6-membered monocyclic heteroaryl group containing at
least one nitrogen
as the N-oxide;
one of R6 and R'represents hydrogen, and the other represents R'-C=O or
CR'=NOR",
wherein R' and R" each independently may be hydrogen, C,_e_alkyl which may be
substituted
one or more times with OH, CZ.s.alkenyl which may be substituted vne or more
times with OH,
C2.~.alkynyl which may be substituted one or more times with OH, or phenyl
which may be
substituted one or more times with OH;
provided however that when R" represents hydrogen, C,.~_alkyl, C2.s.alkenyl or
C2.s.alkynyl
then R" represents a 5- or 6-membered monocyclic heteroaryl containing at
least one nitrogen
as the N-oxide;
or a pharmaceutically acceptable salt thereof.
A compound as above, wherein R" represents C,.6-alkyl substituted with one
hydroxy-group.
A compound as above, wherein R' represents hydrogen.
A compound as above, wherein R" represents pyridyl-N-oxide.
SUBST1ME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCTIDK98I00432
4
A compound as above which is
5-Acetyl-1-(3-(3-pyridyl)phenyl)benzimidazoie O-(2-hydroxyethyl) oxime;
5-Acetyl-1-(3-(3-pyridyl-N-oxide)phenyl)benzimidazole oxime, hydrochloride;
5-Acetyl-1-(3-(3-pyridyl-N-oxide)phenyl)benzimidazole O-ethyl oxime, or
5-Acetyl-1-(3-(3-pyridyl-N-oxide)phenyl)benzimidazole;
yr a pharmaceutically acceptable addition salt thereof.
A method of preparing a compound as abave, by which method,
(a) a compound represented by the general formula
_s N
N
1
R~
I S wherein,
R" represents a 5- or 6-membered monocycfic heteroaryi group containing at
least one
nitrogen, or a 5- or 6-membered monocyclic heteroaryl group containing at
least one nitrogen
as the N-oxide;
one of R6 and R'represents hydrogen, and the other represents CR'=O, wherein
R' represents
hydrogen, C,.~-alkyl which may be substituted one or more times with OH, C2.s-
alkenyl which
may be substituted one or more times with OH, C2.s-alkynyf which may be
substituted one or
more times with OH, or phenyl which may be substituted one or more times with
OH;
is reacted with a compound of the formula R"-ONH2, or an acid-addition salt
thereof, wherein
R" represents hydrogen, C,.~-alkyl which may be substituted one or more times
with OH, Cz~-
alkenyl which may be substituted one or more times with OH, C2.s-alkynyl which
may be
substituted one or more times with OH, or phenyl which may be substituted one
or more times
with OH;
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98/00432
or
(b} a compound represented by the general formula
5
s N
I w y
N
R'
wherein,
R" represents a 5- or 6-membered monocyclic heteroaryl group containing at
least one
nitrogen;
one of R6 and R' is hydrogen, and the other represents R'-C=O or CR'=NOR",
wherein the
meaning of R' and R" is set forth above;
is subjected to oxidation with m-chloro-peroxobenzoic acid.
A method of preparing an intermediate compound of the formula
~OH
H2N0
characterized by reacting a compound of the formula
O
~Br
\N-OO
O
with HNR9Ra, wherein R9 and Ra independently may be hydrogen, C,-a-alkyl, C2_6-
alkenyl, C2.a-
alkvnvl C:~ -.-~:vclnalkvl_ C"-cycloalkyl-C,.B-alkyl, or R9 and Ra together
form a C~_, ring.
SUBSTITUTE SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98/00432
6
to form a compound of the formula
ONR9R8
wherein R9 and RB has the meaning set forth above,
and subsequently heating the compound in acidic environment.
a pharmaceutical composition comprising an effective amount of a compound as
any above or
a pharmaceutically acceptable salt thereof or an oxide thereof together with
at least one
pharmaceutically acceptable carrier or diluent;
the use of a compound as any above for the preparation of a medicament for the
treatment of
a disorder or disease of a living animal body, including a human, which
disorder or disease is
responsive to the modulation of the GABAA receptor complex of the central
nervous system;
20
the use of a compound as any above for the preparation of a medicament for the
treatment of
a disorder or disease of a living animal body, including a human, which
disorder or disease is
responsive to positive modulation of the GABAA receptor complex of the central
nervous
system;
the use of a compound as any above for the preparation of a medicament for the
treatment of
a disorder or disease selected from anxiety, sleep disorders, memory
disorders, epilepsy or
any other convulsive disorder;
a method of treating a disorder or disease of a living animal body, including
a human, which
disorder or disease is responsive to modulation of the GABAA receptor complex
of the central
nervous system comprising administering to such a living animal body,
including a human, in
need thereof a therapeutically effective amount of a compound as any above;
the method as above, wherein a disorder or disease responsive to positive
modulation of the
GABAA receptor complex is treated;
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98100432
7
the method as above, wherein anxiety, sleep disorders, memory disorders,
epilepsy or any
other convulsive disorder is treated; and
the method as above, wherein the active ingredient is administered in form of
a
pharmaceutical composition thereof, in which it is present together with a
pharmaceutically
acceptable carrier or diluent.
Definition of substitutents
15
Alkyl means a straight chain or branched chain of from one to eight carbon
atoms or cyclic
alkyl of from three to seven carbon atoms, including but not limited to,
methyl, ethyl, propyl,
isopropyl, butyl, isobutyl, t-butyl, pentyl, hexyl, cyciopropyl, cyctobutyf,
cyclopentyl, cyclohexyi;
methyl, ethyl, propyl, isopropyl and t-butyl are preferred groups.
Alkenyl means a straight chain or branched chain of from two to six carbon
atoms containing
one double bond, including but not limited to ethenyl, 1-propenyl, 2-propenyl,
1-butenyi, 2-
butenyl, and 3-butenyl.
Alkynyl means a straight chain or branched chain of from two to six carbon
atoms containing
one triple bond, including but not limited to ethynyl, 1-propynyl, 2-propynyl,
1-butynyl, 2-
butynyl, and 3-butynyl.
A 5- or 6-membered monocyclic heteroaryl group includes, for example, oxazol-2-
yl, oxazol-4-
yl, oxazol-5-yl, isoxazol-3-yl, isoxazol-4-yl, isoxazol-5-yl, thiazol-2-yl,
thiazol-4-yl, thiazol-5-yl,
isothiazol-3-yl, isothiazol-4-yl, isothiazoi-5-yl, 1,2,4-oxadiazol-3-yl, 1,2,4-
oxadiazol-5-yl, 1,2,4-
thiadiazol-3-yi, 1,2,4-thiadiazol-5-yl, 1,2,5-oxadiazoi-3-yl, 1,2,5-oxadiazol-
4-yl, 1,2,5-thiadiazol-
3-yl, 1,2,5-thiadiazol-4-yl, 1-imidazoiyl, 2-imidazolyl, 4-imidazolyl, 1-
pyrrolyl, 2-pyrrolyl, 3-
pyrrolyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-
pyrimidinyl, 3-pyridazinyl,
4-pyridazinyl, 2-pyrazinyl, 1-pyrazolyl, 3-pyrazolyl, and 4-pyrazolyl.
Examples of pharmaceutically-acceptable addition salts include inorganic and
organic acid
addition salts such as the hydrochloride, hydrobromide, phosphate, nitrate,
perchlorate,
sulphate, citrate, lactate, tartrate, maleate, fumarate, mandeiate, benzoate,
ascorbate,
cinnamate, benzenesulfonate, methanesulfonate, stearate, succinate, glutamate,
glycollate,
SUBST1ME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98100432
8
toluene-p-sulphonate, formate, maionate, naphthalene-2-sulphonate, salicylate
and the
acetate for example.
Other acids such as oxalic acid, while not in themselves pharmaceutically
acceptable may be
useful in the preparation of salts useful as intermediates in obtaining
compounds of the
invention and their pharmaceutically acceptable acid addition salts. Such
salts are formed by
procedures well known in the art.
Further, the compounds of this invention may exist in unsolvated as well as in
solvated forms
with pharmaceutically acceptable soivents such as water, ethanol and the like.
In general, the
solvated forms are considered equivalent to the unsolvated forms for the
purposes of this
invention.
Some of the compounds of the present invention exist in (+) and (-) forms as
well as in
racemic forms. Racemic forms can be resolved into the optical antipodes by
known methods,
for example, by separation of diastereomeric salts thereof, with an optically
active acid, and
liberating the optically active amine compound by treatment with a base.
Another method for
resolving racemates into the optical antipodes is based upon chromatography on
an optical
active matrix. Racemic compounds of the present invention can thus be resolved
into their
optical antipodes, e.g., by fractional crystallization of d- or I- (tartrates,
mandelates, or
camphorsulphonate) salts for example. The compounds of the instant invention
may also be
resolved by the formation of diastereomeric amides by reaction of the
compounds of the
present invention with an optically active activated carboxylic acid such as
that derived from
{+) or (-) phenyiaianine, (+) or (-) phenylglycine, (+) or (-) camphanic acid
or by the formation
of diastereomeric carbamates by reaction of the compounds of the present
invention with an
optically active chloroformate or the like.
Additional methods for the resoivation of optical isomers, known to those
skilled in the art may
be used, and will be apparent to the average skilled in the art. Such methods
include those
discussed by J. Jaques, A. Collet, and S. Wilen in °Enantiomers,
Racemates, and
Resolutions", John Wiley and Sons, New York (1981).
Furthermore, as the compounds of the invention are oximes they can exist in
two forms, Z-
and E-form, depending on the arrangement of the substituents around the
SUBSTITUTE SHEET (RULE 26}
CA 02304379 2000-03-22
WO 99/19323 PCTIDK98/00432
9
-C=N- double bond. The present invention includes both the Z- and E-form of
the compounds
of the invention as well as mixtures thereof.
The compounds of the invention may be prepared in numerous ways.
The compounds of the invention and their pharmaceutically acceptable
derivatives may thus
be prepared by any method known in the art for the preparation of compounds of
analogous
structure and as shown in the representative examples which follows.
Starting materials for the processes described in the present patent
application are known or
can be prepared by known processes from commercially available chemicals.
The products of the reactions described herein are isolated by conventional
means such as
extraction, crystallization, distillation, chromatography, and the like.
Biology:
4-aminobytyric acid (GAGA) is the major inhibitory neurotransmitter and has
been shown to act
throughout both the central and peripheral nervous system. At present two
types of GAGA
receptors are known, the GABAA and the GABAB receptors. Recent molecular
biology has
demonstrated that the GABAn receptors can be subdivided into numerous
subreceptors
consistant with the selective and or partial pharmacological effects observed
with certain
benzodiazepine receptor ligands as opposed to the unselective effects observed
for the
classical benzodiazepine receptor ligands such as for example diazepam.
Activation of GAGA
receptors leads to alternations in membrane potential (hyperpotarization). The
GABAa
receptors are associated with chloride influx through its associated and
integrated chloride
channel, whereas GABAB receptor activation indirectly alters potassium and
calcium channels
as well as modifies second messenger production. The GABAA recognition sites
can be
activated by GAGA, muscimol; and isoguvacine for example, but not by GABAB
agonists such
as for example bactofen. The modulatory GABAA recognition site at the
benzodiazepine
receptor sites can be selectively radiolabelled with 3H-flunitrazepam. The
affinity of various
potential ligands for the benzodiazepine receptor sites can thus be evaluated
by estimating the
ability of test compounds to displace 3H-flunitrazepam.
Method
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98100432
Tissue Preparation: Preparations are performed at 0-4°C unless
otherwise indicated. Cerebral
cortex from male Wistar rats (150-200 g) is homogenized for 5-10 sec in 20 ml
Tris-HCI (30
mM; pH 7.4) using an Ultra-Turrax homogenizes. The suspension is centrifuged
at 27,000 x g
5 for 15 min and the pellet is washed three times with buffer (centrifuged at
27,000 x g for 10
min). The washed pellet is homogenized in 20 ml of buffer and incubated on a
water bath
(37°C) for 30 min to remove endogenous GAGA and then centrifuged for 10
min at 27,000 x g.
The pellet is then homogenized in buffer and centrifuged for 10 min at 27,000
x g. The final
pellet is resuspended in 30 ml buffer and the preparation is frozen and stored
at -20°C.
Assay: The membrane preparation is thawed and centrifuged at 2°C for 10
min at 27,000 x g.
The pellet is washed twice with 20 ml 50 mM Tris-citrate, pH 7.1 using an
Ultra-Turrax homo-
genizes and centrifuged for 10 min at 27,000 x g. The final pellet is
resuspended in 50 mM
Tris-citrate, pH 7.1 (500 ml buffer per g of original tissue), and then used
for binding assays.
Aliquots of 0.5 ml tissue are added to 25 pl of test solution and 25 pl of 3H-
FNM (1 nM, final
concentration), mixed and incubated for 40 min at 2°C. Non-specific
binding is determined
using clonazepam (1 pM, final concentration). After incubation the samples are
added 5 ml of
ice-cold buffer and poured directly onto Whatman GF/C glass fibre filters
under suction and
immediately washed with 5 ml ice-cold buffer. The amount of radioactivity on
the filters is
determined by conventional liquid scintillation counting. Specific binding is
total binding minus
non-specific binding.
The test value is calculated as the ICSa (the concentration (nM) of the test
substance which
inhibits the specific binding of all-FNM by 50%).
Test results obtained by testing selected compounds of the present invention
appear from the
following table:
Table
Test compound: ICS (nM)
5-acetyl-1-(3-(3-pyridyl)-phenyl)-benzimidazole O-(2-hydroxyethyl)oxime 4.2
5-acetyl-1-(3-(3-pyridyl-N-oxide)-phenyl)-benzimidazole oxime 14.0
5-acetyl-1-(3-(3-pyridyl-N-oxide)-phenyl)-benzimidazole O-ethyl oxime 10.0
5-acetyl-1-(3-(3-pyridyl-N-oxide)-phenyl)-benzimidazole 12.0
SUBSTITUTE SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCTIDK98/00432
11
Pharmaceutical Compositions
While it is possible that, for use in therapy, a compound of the invention may
be administered
as the raw chemical, then it is preferable to present the active ingredient as
a pharmaceutical
formulation.
The invention thus further provides a pharmaceutical formulation comprising a
compound of
LO the invention or a pharmaceutically acceptable salt or derivative thereof
together with one or
more pharmaceutically acceptable carriers therefor and, optionally, other
therapeutic andlor
prophylactic ingredients. The carrierls) must be "acceptable" in the sense of
being compatible
with the other ingredients of the formulation and not deleterious to the
recipient thereof.
I S Pharmaceutical formulations include those suitable for oral, rectal,
nasal, topical (including
buccal and sub-lingual), vaginal or parenteral (including intramuscular, sub-
cutaneous and
intravenous) administration or in a form suitable for administration by
inhalation or insufflation.
The compounds of the invention, together with a conventional adjuvant,
carrier, or diluent, may
20 thus be placed into the form of pharmaceutical compositions and unit
dosages thereof, and in
such form may be employed as solids, such as tablets or filled capsules, or
liquids such as
solutions, suspensions, emulsions, elixirs, or capsules filled with the same,
all for oral use, in
the form of suppositories for rectal administration; or in the form of sterile
injectable solutions
for parenteral (including subcutaneous) use. Such pharmaceutical compositions
and unit
25 dosage forms thereof may comprise conventional ingredients in conventional
proportions, with
or without additional active compounds or principles, and such unit dosage
forms may contain
any suitable effective amount of the active ingredient commensurate with the
intended daily
dosage range to be employed. Formulations containing one (i ) milligram of
active ingredient
or, more broadly, 0.01 to one hundred (100) milligrams, per tablet, are
accordingly suitable
30 representative unit dosage forms.
The compounds of the present invention can be administrated in a wide variety
of oral and
parenteral dosage forms. It will be obvious to those skilled in the art that
the following dosage
forms may comprise as the active component, either a compound of the invention
or a
35 pharmaceutically acceptable salt of a compound of the invention.
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98/00432
12
For preparing pharmaceutical compositions from the compounds of the present
invention,
pharmaceutically acceptable carriers can be either solid or liquid. Solid form
preparations
include powders, tablets, pills, capsules, cachets, suppositories, and
dispersible granules. A
S solid carrier can be one or more substances which may also act as diluents,
flavouring agents,
solubilizers,
lubricants, suspending agents, binders, preservatives, tablet disintegrating
agents, or an
encapsulating material.
In powders, the carrier is a finely divided solid which is in a mixture with
the finely divided
active component.
In tablets, the active component is mixed with the carrier having the
necessary binding
capacity in suitable proportions and compacted in the shape and size desired.
The powders and tablets preferably contain from one to about seventy percent
of the active
compound. Suitable carriers are magnesium carbonate, magnesium stearate, talc,
sugar,
lactose, pectin, dextrin, starch, gelatin, tragacanth, methylcellulose, sodium
carboxymethylcellulose, a low melting vax, cocoa butter, and the like. The
term "preparation" is
intended to include the formulation of the active compound with encapsulating
material as
. carrier providing a capsule in which the active component, with or without
carriers, is
surrounded by a carrier, which is thus in association with it. Similarly,
cachets and lozenges
are included. Tablets, powders, capsules, pills, cachets, and lozenges can be
used as solid
forms suitable for oral administration.
30
For preparing suppositories, a low melting vax, such as a mixture of fatty
acid glycerides or
cocoa butter, is first melted and the active component is dispersed
homogeneously therein, as
by stirring. The molten homogenous mixture is then poured into convenient
sized molds,
allowed to cool, and thereby to solidify.
Formulations suitable for vaginal administration may be presented as
pessaries, tampons,
creams, gels, pastes, foams or sprays containing in addition to the active
ingredient such
carriers as are known in the art to be appropriate.
SUBSTITUTE SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98I00432
13
Liquid form preparations include solutions, suspensions, and emulsions, for
example, water or
water propylene glycol solutions. For example, parenteral
injection liquid preparations can be formulated in solutions in aqueous
polyethylene glycol
solution.
The compounds according to the present invention may thus be formulated for
parenterai
administration (e.g. by injection, for example bolus injection or continuous
infusion) and may
be presented in unit dose form in ampoules, pre-filled syringes, small volume
infusion or in
multi-dose containers with an added preservative. The compositions may take
such forms as
LO suspensions, solutions, or emulsions in oily or aqueous vehicles, and may
contain formufatory
agents such as suspending, stabilising and/or dispersing agents.
Alternatively, the active
ingredient may be in powder form, obtained by aseptic isolation of sterile
solid or by
lyophilisation from solution, for constitution with a suitable vehicle, e.g.
sterile, pyrogen-free
water, before use.
Aqueous solutions suitable for oral use can be prepared by dissolving the
active component in
water and adding suitable colorants, flavours, stabilizing and thickening
agents, as desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided active
component in water with viscous material, such as natural or synthetic gums,
resins,
methylcellulose, sodium carboxymethylceilulose, and other well known
suspending agents.
Also included are solid form preparations which are intended to be converted,
shortly before
use, to liquid form preparations for oral administration. Such liquid forms
include solutions,
suspensions, and emulsions. These preparations may contain, in addition to the
active
component, colorants, flavours, stabilizers, buffers, artificial and natural
sweeteners,
dispersants, thickeners, solubilizing agents, and the like.
For topical administration to the epidermis the compounds according to the
invention may be
formulated as ointments, creams or lotions, or as a transdermal patch.
Ointments and creams
may, for example, be formulated with an aqueous or oily base with the addition
of suitable
thickening andlor gelling agents. Lotions may be formulated with an aqueous or
oily base and
will in general also contain one or more emulsifying agents, stabilising
agents, dispersing
agents, suspending agents, thickening agents, or colouring agents.
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98/00432
14
Formulations suitable for topical administration in the mouth include lozenges
comprising
active agent in a flavoured base, usually sucrose and acacia or tragacanth;
pastilles
comprising the active ingredient in an inert base such as gelatin and glycerin
or sucrose and
acacia; and mouthwashes comprising the active ingredient in a suitable liquid
carrier.
Solutions or suspensions are applied directly to the nasel cavity by
conventional means, for
example with a dropper, pipette or spray. The formulations may be provided in
single or
multidose form. In the latter case of a dropper or pipette this may be
achieved by the patient
administering an appropriate, predetermined volume of the solution or
suspension. In the case
of a spray this may be achieved for example by means of a metering atomising
spray pump.
Administration to the respiratory tract may also be achieved by means of an
aerosol
formulation in which the active ingredient is provided in a pressurised pack
with a suitable
propellant such as a chlorofluorocarbon (CFC) for example
dichforodifluoromethane,
l5 trichlorofluoromethane, or dichlorotetrafluoroethane, carbon dioxide or
other suitable gas. The
aerosol may conveniently also contain a surfactant such as lecithin. The dose
of drug may be
controlled by provision of a metered valve.
Alternatively the active ingredients may be provided in the form of a dry
powder, for example a
powder mix of the compound in a suitable powder base such as lactose, starch,
starch
derivatives such as hydroxypropylmethyl cellulose and polyvinylpyrrolidine
(PVP).
Conveniently the powder carrier will form a gel in the nasal cavity. The
powder composition
may be presented in unit dose form for example in capsules or cartridges of
e.g. gelatin or
blister packs from which the powder may be administered by means of an
inhaler.
30
In formulations intended for administration to the respiratory tract,
including intranasal
formulations, the compound will generally have a small particle size for
example of the order of
5 microns or less. Such a particle size may be obtained by means known in the
art, for
example by micronization.
When desired, formulations adapted to give sustained release of the active
ingredient may be
employed.
The pharmaceutical preparations are preferably in unit dosage forms. In such
form, the
preparation is subdivided into unit doses containing appropriate quantities of
the active
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98/00432
component. The unit dosage form can be a packaged preparation, the package
containing
discrete quantities of preparation, such as packeted tablets, capsules, and
powders in vials or
ampoules. Also, the unit dosage form can be a capsule, tablet, cachet, or
lozenge itself, or it
can be the appropriate number of any of these in packaged form.
5
Tablets or capsules for oral administration and liquids for intravenous
administration are
preferred compositions.
Method of Treating
The compounds of this invention are extremely useful in the treatment of
disorders or diseases
of a living animal body due to their affinity for the benzodiazepine binding
site of the GABAA
receptor. These properties make the compounds of this invention extremely
useful in the
treatment of convulsions, anxiety, sleep disorders, memory disorders as well
as other
disorders sensitive to modulation of the GABAA receptor. The compounds of this
invention
may accordingly be administered to a subject, including a human, in need of
treatment,
alleviation, or elimination of a disorder or disease associated with the GABAn
receptors. This
includes especially convulsions, anxiety, sleep disorders and memory
disorders.
Suitable dosage range are 0.01-100 milligrams daily, 0.1-50 milligrams daily,
and especially
0.1-30 milligrams daily, dependent as usual upon the exact mode of
administration, form in
which administered, the indication towards which the administration is
directed, the subject
involved and the body weight of the subject involved, and further the
preference and
experience of the physician or veterinarian in charge.
Example 1
The following example will illustrate the invention further; however they are
not to be construed
as limiting.
The starting material of the following examples can be obtained as described
in WO 96/33191
5-Acetyl-1-(3-(3-pyridyl-N-oxide}phenyl}benzimidazole O-ethyl oxime (7)
SUBST(ME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCT/DK98/00432
16
mCPBA
CH2C12
(s~
n)
To a stirred solution of 6 (1.7 g, 4.77 mmol) in dichloromethane (25 ml) was
added m-
chloroperbenzoic acid (mCPBA) (1.23 g, 7.13 mmol) in portions at ambient
temperature and
stirring was continued overnight. The reaction mixture was washed with aqueous
sodium
hydroxide (1 M), dried over sodium sulfate and concentrated under reduced
pressure. The
residue was chromatographied on a silica gel column using a mixture of ethyl
acetate and
methanol (9:1 vlv) as the eluent. The crude product obtained from the eluate
was
recrystailized from ethanol to yield 7(1.15 g, 65%). M.p. 189-190°C.
5-Acetyl-1-(3-(3-pyridyl-N-oxide)phenyi)benzimidazole oxime, hydrochloride (9)
NOH
N N N
mCPBA > H2NpH. HCI
N ~ N N
CHZCIp . HCI
b, b.
LS (5) (2.0 g, 6.4 mmol) was treated with mCPBA as described above to yield
(8)(1.0 g, 48%). This
N-oxide (1 g, 3.0 mmol) was suspended in methanol (50 ml) and hydroxylamine
hydrochloride
(0.32 g, 4.6 mmol) was added. The mixture was refluxed overnight, and the
cooled mixture was
poured into water (100 ml). The precipitate was filtered off, washed with
water and dried to yield
(9), hydrochloride (0.86 g, 82%). M.p. 298-300°C.
5-Acetyl-1-(3-(3-pyridyl)phenyl)benzimidazole O-(2-hydroxyethyl) oxime(1fl)
SUBSTfME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99119323 PCTIDK98100432
17
+ H2N0(CH2)20H, HCI -
10
To a suspension of (5)(5.0 g, 16 mmol) in ethanol (50 ml) was added O-(2-
hydroethyl)hydroxylamine, hydrochloride (2.7 g, 24 mmol) and triethylamine
(3.3 ml, 24 mmoi)
5 and the mixture was heated to reflux overnight. The cooled mixture was
poured into water (200
ml) and the resulting suspension was extracted with ethyl acetate. The organic
extract was dried
over sodium sulfate and concentrated under reduced pressure. The concentrate
was
chromatographied on a silica gel column using a mixture of ethyl acetate and
methanol (9:1 v/v)
as the eluent. The crude product obtained from the eluate was recrystallized
from ethanol to yield
(10) (1.95 g, 33%). M.p. 168-170°C.
O-(2-hydroxyethyl)hydroxvlamine. hydrochforide:
O ~Br O
Br gr Me2NH
N-OH _.-~",. ~ N-p~ --.r
p O
CONS CO~ ~H
HCI. D i ~ + HZNO--' , HCI
COOH
0.,, J
N-(2-bromoethoxv)phtalimide:
To a solution of N-hydroxyphtalimide (5.0 g, 30.7 mmol) in DMF (50 ml) was
added triethylamine
(4.27 ml, 30.7 mmol) and 1,2-dibromoethane (10.6 ml, 0.12 mmoi) and the
mixture was stirred in
a nitrogen atmosphere at ambient temperature overnight. The reaction mixture
was filtered, and
the filtrate was evaporated to dryness under reduced pressure. The residue was
trituated with
water to leave the crystalline product (6.56 g, 89%).
SUBSTIME SHEET (RULE 26)
CA 02304379 2000-03-22
WO 99/19323 PCT/DK98I00432
18
N N-nimethvl-2-(5 6-dihvdro-1.4,2-dioxazin-3-vl)benzamide:
N-(2-Bromoethoxy)phtalimide (6.56 g, 27.3 mmol) was suspended in THF (30 ml).
Gaseous
dimethylamine was gently bubbled through this suspension until a clear orange-
red solution had
formed. This resulting solution was stirred at ambient temperature overnight.
The reaction
mixture was filtered through celite and the filtrate was concentrated under
reduced pressure. The
residue was purified by column-chromatography on silica gel using a mixture of
ethyl acetate and
methanol (9:1 v/v) as the eluent. Yield: 4.53 g (71 %).
O-(2-Hydroxyethvl)hvdroxvlamine:
LO A solution of N,N-dimethyl-2-(5,6-dihydro-1,4,2-dioxazin-3-yl)benzamide
(4.53 g, 19.4 mmol) in
hydrochloric acid (50 ml, 6M) was heated to reflux overnight. The mixture was
filtered and the
filtrate was concentrated under reduced pressure. Toluene was added to the
residue and the
resulting mixture was evaporated to dryness to yield the desired product (3.3
g, contaminated
with dimethylammonium chloride) which was used without further purification.
SUBSTIME SHEET (RULE 26)