Note: Descriptions are shown in the official language in which they were submitted.
CA 02306205 2000-04-10
This invention concerns methods for reducing the chlorophyll content the seeds
of oil
plants, in particular rapeseed, based on the expression of chlorophyll
synthesis antisense genes.
In addition, the method concerns seeds of oil plants that have reduced
chlorophyll content
compared to the wild types, as well as the use of these seeds to produce
vegetable oils.
Next to soy and cottonseeds, the crucifers rape (Brassica napus) and turnip
(Brassica
rapa) are among the most important oil plants. The seeds of the rape plant
contain about 40%
fatty oil, the so called rapeseed oil or turnipseed oil, which as a rule is
obtained from the crushed
seeds by pressing or extraction in a yield of about 40% and then is refined.
The rapeseed oil that
is obtained can then be used as food oil, mineral lubricant oil additive, for
production of
margarine after hydrogenation, and as raw material in the production of
grafting wax, plasters,
leather dressings, and so forth. Rapeseed oil is also known as a good source
for, among other
things, C2o and C22 fatty acids, which are important as agents in plastics
processing and
detergents. In addition to their use as industrial raw materials, vegetable
oils, especially rapeseed
oil, are becoming increasingly important as biodiesel fuels. Generally
speaking, the ranges of use
of vegetable oils have become considerably broader in recent years. With
rising environmental
awareness, environmentally compatible industrial products, for example,
lubricants and
hydraulic fluids, have increasingly been developed.
The problem of high chlorophyll levels in rapeseeds is generally known,
especially in
rape cultivation with short growing seasons. With every harvest growers and
seed concerns must
weigh the risk of losing the harvest to frost against the additionally
necessary ripening of the
seeds, during which, moreover, decomposition of pigments occurs. For this
reason rape is
frequently allowed to lie for several days after harvesting for further
ripening before the harvest
is brought in.
A further complication is that low temperatures, frost temperatures that are
sublethal for
the seeds promote chlorophyll biosynthesis in rapeseeds and thus additionally
counteract the
decomposition of pigments that takes place during ripening, due to which
undesirably high
chlorophyll levels in rapeseeds can occur in particular in areas of
cultivation with relatively short
growing seasons.
In refining the rapeseed oil the pigments, especially the chlorophylls
contained in the
seeds and their photosensitive precursors, must be extracted at high cost.
Apart from the fact that
these extractions are costly from the standpoint of time and money, they also
always mean a loss
of the rapeseed oil yield. Although turnips make somewhat lesser demands than
rape with regard
to growing time and location, these Brassicaceae have similar problems with
regard to the
ripening of the seeds and excess chlorophyll content. The recovery and use of
turnipseed oil
largely correspond to those of rapeseed oil.
CA 02306205 2000-04-10
'L.
Chlorophyll synthesis in rape and turnip plants and their seeds could be
affected by
means of molecular biotechnology to the extent that the amount of excess
chlorophyll in the
rapeseeds could be reduced or completely chlorophyll-free seeds could be
produced.
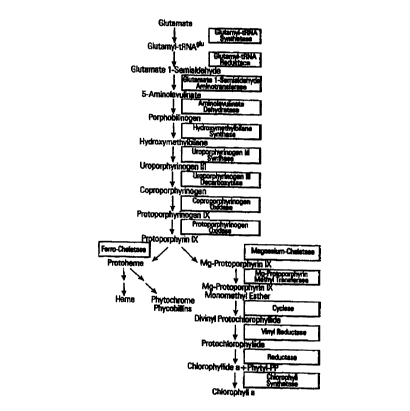
Tetrapyrrole biosynthesis, which in plants takes place chiefly in plastids,
progresses, as is
currently assumed, according to the following metabolic pathway (see also in
this regard Figure
1). 5-Aminolevulinic acid (ALA) is produced from glutamate via three enzyme
activities
(glutamyl tRNA synthetase, glutamyl tRNA reductase and glutamate 1-
semialdehyde
aminotransferase). Two ALA molecules condense to a cyclic compound,
porphobilinogen, which
is converted to the first tetrapyrrole, hydroxymethylbilane by concatenation
of 4 units.
Protoporphyrin IX results from hydroxymethylbilane through oxidations and side
chain
modifications via uroporphyrinogen III, coproporphyrinogen and
protoporphyrinogen IX.
Incorporation of a bivalent metal cation results in magnesium protoporphyrin
IX, which converts
to chlorophyll a through additional modifications, which involve, among other
things, the
incorporation of an additional isocyclic ring on ring B and the particularly
important
esterification of a propionate with a phytol chain.
The knowledge of plant genes that code for enzymes of the above indicated
chlorophyll
synthesis path and the possibility of transfernng such genes in a targeted way
to plants is an
important basis of this invention.
European Patent Application No. 0 779 364 A2 describes an approach to reducing
the
chlorophyll content in transgenic plants, in which the transcript and protein
content of the
chlorophyll-binding proteins (chlorophyll alb binding (CAB) proteins) of the
aerial complex of
the photosystem II (light harvesting complex associated with photosystem II,
LHCII) is reduced.
This approach, which is based on the expression of antisense genes for LHCII
thus concerns
proteins that already bind synthesized chlorophyll in photosystem II, but not
enzymes that are
directly involved in chlorophyll synthesis.
In addition, experimental data from Flachmann and Kiihlbrandt (Plant Cell
(1995) 7, 149-
160) indicate that as a consequence of the expression of antisense genes for
LHCII the RNA
contents in the leaves of transgenic tobacco plants are reduced by up to 5% of
the control values
in wild types, but neither a reduction of the LHC protein content nor a
chlorophyll reduction is
observed. Flachmann and Kiihlbrandt were unable to establish a correlation
between the decrease
of the LHC-RNA level and the reduction of the protein and chlorophyll content.
In contrast to the strategy pursued in the prior art, it ought to be possible
to achieve a
successful and reliable reduction of the chlorophyll content in transgenic
plants by means of
direct intervention in the tetrapyrrole synthesis.
CA 02306205 2000-04-10
3
R
Therefore, a task of this invention is to point out ways by which chlorophyll
synthesis in
transgenic oil plant seeds can be reduced or blocked and in this way the
chlorophyll content
reduced by means of genetic engineering methods.
In addition, an important task of the invention is to make available
transgenic oil seeds
with chlorophyll content that is lower than that of seeds from wild species of
plants.
Other tasks of the invention arise from the following description. These tasks
are solved
through the objects of the independent claims, in particular on the basis of
making available of
the methods for reduction of the chlorine content in plant seeds in accordance
with the invention
as well as the plants and parts and products of plants with chlorophyll
content reduced compared
to wild species of plants in accordance with the invention.
This invention thus concerns the use of DNA sequences that code for
chlorophyll
synthesis enzymes and whose targeted transfer to and expression in transgenic
plant cells result
in a reduction of chlorophyll synthesis. More precisely, the invention
concerns the transfer of
suitable antisense gene constructs to plants and their expression in the plant
tissue.
The invention is based on experiments in which it was possible to reduce
significantly the
amount of chlorophyll in plant seeds by the seed-specific expression of
antisense genes to certain
enzyme steps of tetrapyrrole synthesis and the resulting seed-specific
inhibition of the activity of
specific enzymes of chlorophyll synthesis.
The antisense technique employs the complementarity of nucleic acid molecules
in an
elegant way. Antisense genes that code for tetrapyrrole metabolism enzymes
complementarily to
endogenous RNA reduce the endogenous RNA contents or the number of RNA
molecules
available for subsequent protein biosynthesis. A reduced content of endogenous
RNA necessarily
results in reduced translation, and thus a reduced amount of protein, which
again is expressed in
a reduced activity of the target enzyme.
The invention concerns all genes whose gene products catalyze steps of
tetrapyrrole
synthesis. Since the expression and activity of any enzyme involved in
tetrapyrrole synthesis can
be reduced or inhibited by targeted antisense RNA synthesis, the method for
reducing the
chlorophyll content of seed cells uses all of the genes of this metabolic
pathway, i.e., individually
or in combination. These genes are above all genes that code for glutamyl tRNA
synthetase,
glutamyl tRNA reductase, glutamate 1-semialdehyde aminotransferase, magnesium
chelatase or
its subunits CHL I, CHL D, CHL H, chlorophyll synthetase and Mg protoporphyrin
monomethyl
ester transferase.
The invention also concerns fragments of genes that are involved in
chlorophyll synthesis
and whose use within an antisense construct results in reduced activity of the
target enzyme.
Such fragments could in connection with this invention be called "antisense-
active" fragments,
CA 02306205 2000-04-10
g,
i.e., their transfer in the form of a suitable construct brings about a
reduction of the
corresponding endogenous enzyme activity.
In addition, the invention concerns the use of alleles and derivatives of the
genes in
accordance with the invention to reduce the chlorophyll content of plant
seeds, thus the use of
nucleic acid molecules whose sequences, due to degeneration of the genetic
code, differ from the
genes in accordance with the invention and whose transfer to plant cells
results in a reduction of
the content of the desired enzyme of chlorophyll metabolism caused by the
antisense gene.
In addition, the invention concerns the use in accordance with the invention
of nucleic
acid molecules that contain the antisense genes in accordance with the
invention or that result
from them by naturally occurring or by genetic engineering or chemical
processes and synthesis
methods or derived from them. These can be, for example, DNA or RNA molecules,
cDNA,
genomic DNA, mRNA, etc.
The following genes of chlorophyll synthesis are preferably used for reduction
of the
chlorophyll content of seed tissue within the scope of the invention.
Genes that code for glutamate 1-semialdehyde aminotransferase (GSA-AT). This
enzyme
catalyzes the conversion of glutamate 1-semialdehyde (GSA) to aminolevulinic
acid (ALA)
through the net transfer of an amino group from C2 to C 1. The expression of a
tobacco antisense
RNA for GSA-AT up to now has been investigated only in the leaves of tobacco
plants (Hofgen
et al. (1994) Proc. Natl. Acad. Sci. USA 91, 1726-1730). DNA sequences of two
tobacco full
length cDNA clones are available in the Gene Bank, Accession Nos. X65973 and
X65974.
Genes that code for the subunits CHL I and CHL H of magnesium chelatase. The
subunits of Mg chelatase are involved in the incorporation of magnesium into
protoporphyrin IX.
Suitable DNA sequences of full length cDNA clones that code for CHL I and CHL
H are
described in Kruse et al. (1997) Plant Mol. Biol. 35, 1053-1056 and under
accession Nos.
AF014053 (Chl I) or AF014051 and AF 14052 (Chl H) in the gene bank.
Genes that code for the plastid glutamyl tRNA synthetase. This enzyme
catalyzes the
formation of glutamyl tRNA from glutamic acid.
Genes that code for glutamyl tRNA reductase. This enzyme catalyzes the
reduction of
activated glutamate to glutamate 1-semialdehyde. Suitable DNA sequences of
full length cDNA
clones from a barley cDNA bank that code for the reductase are described in
Bougri and Grimm
(1996) Plant J. 9, 7867-878 and under Accession Nos. X86101, X86102 and X92403
in the gene
bank.
In an antisense gene construct in accordance with the invention a gene of
chlorophyll
synthesis or a fragment thereof, preferably the coding region of such a gene
or a fragment, is
bonded in antisense orientation, i.e., 3' -~ 5' orientation, with the 3' end
of a promoter, thus a
regulator element that guarantees the transcription of the coupled gene into
plant cells.
CA 02306205 2000-04-10
The genes in accordance with the invention can be expressed in plant cells,
for example,
under control of constitutive, but also inducible or tissue- or development-
specific promoters.
These are preferably seed-specific promoters whose use enables the targeted
inhibition of
chlorophyll synthesis in seed cells.
Examples of seed-specific promoters that can be used in connection with the
invention
that may be mentioned are the USP promoter (described, among other places, in:
Baumlein et al.
(1991) Mol. Gen. Genet. 459-467; Fiedler et al. (1993) Plant Mol. Biol. 22,
669-679; DE-C2-39
20 034), the napin promoter (Ericson et al. (1991) Eur. J. Biochem. 197, 741-
746; Accession No.
X 58142), the 2S albumin promoter (Krebbers et al. (1988) Plant Physiol. 87,
859-866;
Accession No. Z 24745), the legumin promoter (Baumlein et al. (1986) Nucl.
Acids Res. 14,
2707-2720; Accession No. X 03677) and the hordeine promoter (Entwistle et al.
(1991) Plant
Mol. Biol. 17, 1217-1231; Accession No. X60037).
Optionally, the antisense constructs used in accordance with the invention can
additionally include enhancer sequences or other regulator sequences.
It is likewise a task of the invention to make available new plants, plant
cells, plant parts
or plant products that are characterized by a reduced chlorophyll content
compared to the wild
species.
These tasks are solved through the transfer of the antisense nucleic acid
molecules in
accordance with the invention and their expression in plants. The making
available of the nucleic
acid molecules in accordance with the invention now presents the possibility
of altering plant
cells by means of genetic engineering methods to the extent that they have a
reduced chlorophyll
biosynthesis power compared to wild types. In accordance with the invention
these are seeds of
oil plants, especially rape and turnip, that have significantly reduced
chlorophyll content
compared to seeds of the wild species. The transgenic rape plants are
especially preferably spring
rape plants. Likewise 00 rape plants, i.e., rape plants that are erucic acid-
free and low-
glucosinolate, are also suitable for use of the methods in accordance with the
invention.
The plants that are transformed with the nucleic acid molecules in accordance
with the
invention and in which a lower amount of chlorophyll is synthesized because of
the integration
of such a molecule into their genome can in principle be any plants.
Preferably, these are oil
plants like rape and turnip from which a vegetable oil is obtained and in the
recovery of which
high chlorophyll contents are undesirable.
An obj ect of the invention is in particular propagation material of plants in
accordance
with the invention, for example, seeds, fruits, cuttings, bulbs, root stock,
etc., where this
propagation material optionally contains the above described transgenic plant
cells, as well as
parts of these plants like protoplasts, plant cells and calli; especially
preferably these are seeds.
CA 02306205 2000-04-10
6
~e
This invention additionally has the task of making available methods for
producing plant
cells and plants and parts thereof, especially seeds, that are characterized
by a reduced
chlorophyll content.
This task is solved by methods with which the generation of new plant cells
and plants
that are characterized by a reduced chlorophyll content compared to the wild
types due to the
transfer and expression of antisense genes that are directed toward endogenous
genes that code
for the enzymes of chlorophyll synthesis is possible.
There are various genetic engineering transformation methods for generation of
such new
plant cells and plants. In accordance with the invention, plant cells that are
characterized by a
reduced chlorophyll content due to the expression of an antisense gene
construct in accordance
with the invention are produced by a method that includes the following steps:
a) Preparation of an expression cassette, which includes the following DNA
sequences:
- a promoter that ensures transcription into plant cells;
- at least one nucleic acid sequence that codes for an enzyme or a fragment
thereof that
participates in chlorophyll synthesis, where the nucleic acid sequence is
coupled in antisense
orientation to the 3' end of the promoter, and
- optionally a termination signal for termination of transcription and
addition of a poly
(A) tail to the corresponding transcript that is coupled to the S' end of the
nucleic acid sequence.
b) Transformation of plant cells with the expression cassette prepared in step
(a).
c) Regeneration of transgenic plants and optionally propagation of the plants.
.
Furthermore, the invention concerns the use of the antisense constructions in
accordance
with the invention to generate plants, especially plant seeds, that have a
reduced chlorophyll
content. Preferably the invention concerns the use of the antisense
constructions in accordance
with the invention to generate seeds of oil plants, especially rape and
turnip, that have a reduced
chlorophyll content.
Another task of the invention is to point out the possibilities of using the
plants in
accordance with the invention or their cells, parts and products, especially
their seeds.
An object of the invention is in particular the use of the plants in
accordance with the
invention, especially their seeds, to obtain vegetable oils as raw materials
for the chemical,
cosmetics, pharmaceuticals and food industry and as energy Garners.
The plants in accordance with the invention thus represent an important source
for
obtaining vegetable oils, especially rapeseed and turnipseed oil, for a broad
spectrum of
commercial purposes.
Possibilities for said promoter are in principle any of the functional
promoters in the
plants chosen for transformation that satisfy the requirement that the
expression that they
regulate leads to a reduced chlorophyll synthesis power in plant cells. In
view of the use of the
CA 02306205 2000-04-10
1
transgenic plants as providers of vegetable oils promoters that ensure seed-
specific expression
appear to be particularly meaningful here. Examples of such promoters are the
USP, napin,
2S albumin, legumin and hordeine promoters mentioned above.
If such promoters are not known or are not available, in any case the concept
of isolating
such promoters is well known to the specialist. Here poly(A)+ RNA is isolated
from seed tissue
and a cDNA bank is set up in a first step. In a second step cDNA clones that
are based on
poly(A)+ RNA molecules from tissue not deriving from the seeds from the first
bank are used to
identify by means of hybridization those clones whose corresponding poly(A)+
RNA molecules
are expressed only in the plant tissue. Then the cDNAs identified in this way
are used to isolate
promoters that then can be used for antisense expression.
A large number of cloning vectors that contain a replication signal for E.
coli and a
marker gene for selection of transformed bacterial cells is available for
preparation for the
insertion of foreign genes into higher plants. Examples of such vectors are
pBR322, the pUC
series, MI3mp series, pACYC 184, and so forth. The desired sequence can be
introduced at a
convenient restriction cut site in the vector. The resulting plasmid is used
for transformation of
E. coli cells. The transformed E. coli cells are grown in a suitable medium
and then harvested
and lysed. The plasmid is recovered. In general restriction analyses, gel
electrophoreses and
other biochemical/molecular biological methods are used as analysis methods to
characterize the
resulting plasmid DNA. After each manipulation the plasmid DNA can be split
and the
recovered DNA fragments can be bonded to other DNA sequences. Each plasmid DNA
sequence
can be cloned in the same or other plasmids.
A larger number of known techniques are available for introduction of DNA into
a plant
host cell, and the specialist can easily determine the method that is suitable
in each case. These
techniques include the transformation of plant cells with T-DNA using
Agrobacterium
tumefaciens or Agrobacterium rhizogenes as transformation agents, fusion of
protoplasts, direct
gene transfer of isolated DNA in protoplasts, electroporation of DNA, the
insertion of DNA by
means of biolistic methods as well as other possibilities.
In the injection and electroporation of DNA in plant cells no special
requirements per se
are made on the plasmids that are used. This is also true for direct gene
transfer. Simple plasmids
like pUC derivatives can be used. However, if whole plants are to be
regenerated from the cells
that are transformed in this way, the presence of a selectable marker gene is
necessary. The
common selection markers are known to the specialist and selecting a suitable
marker will not be
problem.
Additional DNA sequences may be necessary, in each case according to the
method of
insertion of desired genes into the plant cell. If, for example, Ti or Ri
plasmid is used for
transformation of the plant cell, then at least the right boundary, and
frequently the right and left
CA 02306205 2000-04-10
boundary, of the T-DNA contained in the Ti and Ri plasmid must be bonded to
the genes to be
introduced as a flanking region.
If agrobacteria are used for the transformation, the DNA to be introduced must
be cloned
in special plasmids, namely either in an intermediary or a binary vector. The
intermediary
vectors can be integrated into the Ti or Ri plasmid of the agrobacteria by
homologous
recombination by means of sequences that are homologous to sequences in the T-
DNA. The
plasma additionally contains the vir region necessary for transfer of the T-
DNA. Intermediate
vectors cannot replicate in agrobacteria. Using a helper plasmid, the
intermediary vector can be
transferred to Agrobacterium tumefaciens (conjugation). Binary vectors can
replicate both in E.
coli and in agrobacteria. They contain a selection marker gene and a linker or
polylinker which
are framed by the right and left T-DNA boundary region. They can be
transformed directly to the
agrobacteria (Holsters et al. (1978) Molecular and General Genetics 163, 181-
187). The
agrobacterium that serves as host cell should contain a plasmid that carries a
vir region. The vir
region is necessary for transfer of the T-DNA to the plant cells. Additional T-
DNA can be
present. The agrobacterium transformed in this way is used for transformation
of plant cells.
The use of T-DNA for transformation of plant cells has been intensively
investigated and
described extensively in EP 120 515; Hoekema in: the Binary Plant Vector
System,
Offsetdrokkerij Kanters B.V., Alblasserdam (1985) Chapter V; Fraley et al.
(1993) Crit. Rev.
Plant. Sci., 4, 1-46 and An et al. (1985) EMBO J. 4, 277-387.
For the transfer of the DNA into the plant cells plant, explants can be
cultured
expediently with Agrobacterium tumefaciens or Agrobacterium rhizogenes. Then
from the
infected plant material (for example, leaf pieces, stem segments, roots, as
well as protoplasts or
suspension-cultured plant cells) whole plants can be regenerated in a suitable
medium, which can
contain antibiotics or biocides for selection of transformed cells. The
regeneration of the plants
takes place by conventional regeneration methods using known nutrient media.
The plants
obtained in this way can then be tested for presence of the introduced DNA.
Other possibilities
for the introduction of foreign DNA using the biolistic method or by
protoplast transformation
are well known (see, for example, Willmitzer L. (1993) Transgenic Plants, in:
Biotechnology, A
Multi-volume Comprehensive Treatise (H.J. Rehm. G. Reed, A. Piihler, P.
Stadler, eds.) Vol. 2,
627-659, V.C.H. Weinheim - New York - Basel - Cambridge).
Once the introduced DNA is integrated into the genome of the plant cell, as a
rule it is
stable there and continues to remain in the descendants of the original
transformed cell. It
normally contains a selection marker that confers resistance to a biocide or
antibiotic like
kanamycin, 6418, bleomycin, hygromycin, methotrexate, glyphosate,
streptomycin,
sulfonylurea, gentamycin or phosphinotricin, etc., to the transformed plant
cells. The individually
CA 02306205 2000-04-10
selected marker should then allow the selection of the transformed cells
compared to cells that
lack the introduced DNA.
The transformed cells grow within the plant in the usual way (see also
McCormick et al.
(1986) Plant Cell Reports 5, 81-84). The resulting plants can be grown in the
normal way and
crossed with plants that have the same transformed genetic makeup or another
genetic makeup.
The resulting hybrid individuals have the corresponding phenotypic properties.
Seeds can be
obtained from the plants.
Two or more generations should be grown in order to guarantee that the
phenotypic trait
will continue to remain stable and be inherited. In addition, seeds should be
harvested in order to
guarantee that the corresponding phenotype or other characteristics have
remained preserved.
Likewise, conventional methods can be used to determine transgenic lines that
are
homozygous for the new nucleic acid molecules and their phenotypic behavior
with respect to
altered chlorophyll content tested and compared with that of hemizygous lines.
The transfer and expression of the antisense gene constructs in accordance
with the
invention can take place with the aid of traditional molecular biological and
biochemical
methods. These techniques are known to the specialist and he is capable of
easily selecting a
suitable detection method, for example, a Northern blot analysis, for
qualitative and quantitative
detection of RNA that is specific for the coding region of the relevant
antisense gene, or a
Southern blot analysis for identification of the transferred DNA sequences.
The resulting transgenic plant cells or plants as well as parts and products
thereof can
then be tested far their chlorophyll content. The following analysis methods,
for example,
suggest themselves on this regard:
- Determination of the chlorophyll and carotinoid contents after Porra et al.
(1989)
Biochem. Biophys. Acta 975, 384-394;
- determination of ALA synthesis power (as described, for example, in
Zavgorodnyaya et
al. (1997) The Plant J. 12, 169-178);
- determination of the glutamate 1-semialdehyde aminotransferase activity (see
also
Zavgorodnyaya et al. (1997) supra).
Often a reduced chlorophyll content in transgenic plants or their parts and
products
compared to the wild types can be detected with the naked eye or with optical
aids.
Common methods such as are described in the relative laboratory manuals like
Sambrook
et al. (1989) Molecular Cloning: A Laboratory Manual, 2"d edition, Cold Spring
Harbor
Laboratory Press, Cold Spring Harbor, New York, can be used for DNA and RNA
isolation,
sequence analysis, restriction, cloning, gel electrophoresis, radioactive
labeling, Southern,
Northern and Western blot analyses, hybridization and the like.
The following examples serve to illustrate the invention.
CA 02306205 2000-04-10
~b
Examples
Example 1 .
Preparation of an antisense construct based on DNA sequences that code for a
glutamate 1-
semialdehyde aminotransferase from tobacco
For construction of a GSA-AT antisense mRNA expression vector the complete
1714 by
tobacco GSa-AT-cDNA fragment (Gene Bank Accession No. X65974; see Hofgen et
al., supra)
as EcoRV/XbaI restriction fragment was cloned into the binary vector BinHyg-
Tx, a plasmid
derivative of the vector BinAR (Hofgen and Willmitzer (1990) Plant Sci. 66,
221-230), which
was cut beforehand with the restriction enzymes SmaI and XbaI.
For preparation of a binary vector with a fusion of an USP promoter and the
GSA-AT-
cDNA sequence in antisense orientation the vector pP30T containing the USP
promoter from
Vicea faba, a pUC 18 derivative (see also Baumlein et al. ( 1991 ), supra) was
cut with PstI and
BgIII. The isolated USP promoter fragment was then cloned in a BamHI/PstI-cut
pBluescript
vector (-> pUSPblue). This vector construct pUSPblue was then cut with EcoRI
and XbaI in
order to obtain an EcoRI/XbaI promoter fragment. The 355 CaMV promoter was
removed from
the binary vector BinAR by restriction digestion with EcoRI and XbaI and, by
ligation of the
EcoRI/XbaI vector fragment with the USP promoter fragment replaced by this
seed-specific
promoter (~ pUSPbin). The complete cDNA sequence (XbaI-3'-GSA-AT-cDNA-S'-SaII)
cut
from the pBluescript with XbaI and SaII was then inserted in the XbaI-SaII-cut
pUSPbin.of
tobacco GSA aminotransferase. The resulting binary vector was characterized as
pUSPASGSAT
(see also Figure 2).
In place of the said binary vectors BinAR or BinHyg-Tx, which contain a
tetracycline-
inducible CaMV 355 promoter, any suitable vector for plant transformation can
be used to
prepare an antisense gene consisting of a fusion of a promoter, preferably a
seed-specific
promoter that guarantees transcription and translation in plant cells, and DNA
sequences that
code for enzymes that participate in chlorophyll synthesis.
Example 2:
Transformation of rape plants and regeneration of intact plants
The transformation in summer rape was carried out by the method of De Block et
al.
(1989, Plant Physiol. 91, 694-701) and Damgaard et al. (1997, Transgenic
Research 6, 279-288).
For this a recombinant culture of Agrobacterium tumefaciens (strain GV 3101)
was
washed and resuspended in medium 1 (MS medium (Murashige and Skoog (1962)
Physiol. Plant
15, 473), 2.SmM MES, pH 5.5, 1 mg/L benzylaminopurine (BAP), 0.1 mg/L
napthylacetic acid
(NAA), 0.01 mg/L gibberellic acid (GA3), and 200 pM acetosyringone).
CA 02306205 2000-04-10
The hypocotyl of 14 day old Brassica napus seedlings were cut into segments of
0.5 to
1 cm long, incubated for 30 min with the bacterial suspension and then kept
for 5 days in the
dark at 21 °C in 0.7% agar containing medium 1. For callus induction
the hypocotyl was
incubated at 25°C on medium 2 (MS medium, 2.SmM MES, pH 5.7, 30 g/L
sucrose, 1 mg/L
kinetin, 1 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.01 mg/L GA3, S00
mg/L
polyvinylpyrrolidone (PVP), 5 mg/L AgN03, 5 g/L agarose, 250 mg/L
carbenicillin, 100 mg/L
kanamycin; at 150 pM photon/m2/sec).
Formation of the callus was followed by induction of shoots on medium 3 (MS
medium,
2.SmM MES, pH 5.5, 20 g/L sucrose, 40 mg/L adenine, 1 mg/L BAP, 0.1 mg/L NAA,
0.01 mg/L
GA3, 500 mg/L PVP, 5 mg/L AgN03, 100 mglL kanamycin, 5 gIL agarose, 250 mg/L
carbenicillin).
After shoot formation has occurred, the calli, which show shoots are
transferred to a
shoot elongation medium (medium 4) in glass vessels (MS medium, 2.SmM MES, pH
5.7,
g/L sucrose, 0.0025 mg/L BAP, 100 mg/L kanamycin, 7 g/L agarose, 250 mg/L
carbenicillin).
After 2-3 weeks the shoots were transferred to medium 5 (MS medium, 2.SmM MES,
pH 5.5,
7 g/L agarose) in order to form roots.
Alternatively, rape plants were generated by the following protocol: a
recombinant
culture ofAgrobacterium tumefaciens (strain GV 3101) was washed and
resuspended in MS
medium with 2.SmM MES, pH 5.5. The hypocotyl of 5-7 day old rape seedlings
were cut into
explants about 7 mm long and precultured in liquid CIM medium for 24 h. Then
the exphnts
were cocultured for 2-3 days in the dark in 50 pL of an overnight culture of
the recombinant
agrobacterium strain in 10 mL of the CIM medium. The CIM medium consists (per
liter) of MS
medium, 30 g sucrose, 500 mg MES, pH 5.8, 1 mg 2,4-D, 1 mg kinetin. Then the
hypocotyl
pieces were washed and cultured for 7-10 days to callus induction on CIM
medium containing S
g/L agarose, 20 mg kanamycin, 250 mg betabactyl and 250 mg carbenicillin. Then
the slightly
swollen explantates were placed on SIM medium for shoot induction. The medium
was replaced
every 10-14 days. SIM medium consists (per liter) of MS medium, vitamins, 20 g
sucrose, 500
mg MES, pH 5.6-5.8, 2 mg zeatin, 2 mg BAP, 100 mg myoinositol, 5 g agarose,
mg kanamycin, S00 mg betabactyl or carbenicillin. After the end of shoot
formation, the kalli
that showed shoots were transferred to glass vessels containing MS medium, 500
mg/L MES,
pH 5.7, 20 g/L sucrose, 20 mg/L kanamycin, S/L agarose and S00 mg/L
carbenicillin.
In addition to the described transformation methods, rape plants can also be
transformed
by means of other techniques. For this purpose, the protocol of Moloney et al.
(1989, Plant Cell
Rep. 8, 238-242) suggests itself; in this protocol an agrobacteria-mediated
DNA transfer to
cotyledons of 7-day-old seedlings takes place via the cut site on the leaf
stem. In addition, the
transformation of protoplasts using cells of various tissues is suitable
(described, for example, in
CA 02306205 2000-04-10
12
1~.
Thomzik (1993) In: Biotechnology in agriculture and forestry, Vol. 23, Plant
protoplasts and
genetic engineering IV (Bajaj, ed.) Springer-Verlag, Berlin, 170-182).
Example 3:
Analysis of transgenic plants that express the antisense RNA to glutamate 1-
semialdehyde
aminotransferase under control of the USP promoter
Plants that were transformed with the vector construct pUSPASGSAT (see Example
1
and Figure 2) were then tested for expression of the antisense RNA to GSA-AT.
For this purpose the chlorophyll contents and synthesis rates in the
transgenic plants were
determined. For this chlorophylls were extracted from 100 mg plant material
ground in liquid
nitrogen with buffered, ice-cold 80% acetone until the pellet had become
colorless. The samples
were appropriately diluted and the extinction measured at 663, 646 and 750 nm
on a
spectrophotometer. The formulas given by Porra et al. (1989, supra) were
employed for the
calculation of the chlorophyll contents.
To determine chlorophyll synthesis rates, seed tissue was incubated in 20mM
K2HP04/KH2P04 (pH 7.1) with 34 kBq D-[4-'4C]-labeled ALA (5-aminolevulinic
acid) for 8 h
in light, then a chlorophyll extraction was carried out and separation was
done via HPLC. The
extraction and separation followed the technique of Gilmore and Yamamoto
(1991, J.
Chromatography 543, 137-145), modified by Kurse et al. (1995, EMBO J. 14, 3712-
3720), as
follows: 100 mg seed material ground in liquid nitrogen was weighed and
extracted with 100%
acetone and 10 , as follows: 100 mg seed material ground in liquid nitrogen
was weighed and
extracted with 100% acetone and 10 uM KOH until the pellet had become
colorless (1 time
400 pL, 3 times 200 pL). The extracts were diluted 4:1 with HZ for the HPLC
run in order to
achieve sharper separations. The chlorophylls were eluted by means of a
LiChrospher 100 HPLC
RP 18 column (5 um, Merck) at a flow rate of 1 rnL/min, using the following
gradients: 100%
eluent A (780 mL acetonitrile; 80 mL MeOH; 30 mL tris/HCI, O.1M, pH 8.0) for 7
min, in a
linear increase over 6 min to 100% eluent B (800 mL MeOH; 200 mL hexane), and
14 min
100% eluent B. The eluate was analyzed with a photodiode array (PDA) detector
and connected
radioactivity monitor. Radioactively labeled chlorophyll could be categorized
according to the
reaction times, which are known for the HPLC system.
In addition, the S-aminolevulinic acid synthesis capacity in the transgenic
plants was
determined. Since enzyme activities in the CS pathway cannot be determined
without purifying
the enzymes, indirect methods were selected to measure the capacity for ALA
formation from
glutamate. For one, the accumulation of ALA after incubation of LA was
determined by the
following protocol: 100-300 mg seed tissue per batch was incubated in light
for 2-4 h with
40n~1VI levulinic acid, a potent inhibitor (substrate similarity) of ALA
dehydratase (ALAD), in
CA 02306205 2000-04-10
~3
1~
20mM K2HP04/KH2P04 (pH 7.1). The plant material was frozen in liquid nitrogen,
homogenized and, after adding 1 mL 20mM K2HP04/KH2P04 (pH 7.1), thoroughly
mixed. The
ALA determination followed Mauzerall and Granick (1956, J. Biol. Chem. 219,
435-446). After
centrifuging for 20 min at 15,000 g at 4°C, 250 ~L 20mM K2HP04/KH2P04
(pH 7.1 ) and
100 ~L ethyl acetoacetate was pipetted into 250 uL of the supernatant. Samples
that had been
extracted without levulenic acidincubation at time to served as control. All
samples were heated
for exactly 10 min at 100°C, then cooled on ice for 5 min, thoroughly
mixed with 500 mL
modified Ehrlich's reagent (373 mL glacial acetic acid; 90 mL 70% (w/v)
perchloric acid; 1.55 g
HgCl2; volume filled to 500 mL with H20; 2 g p-dimethylaminobenzaldehyde
dissolved in
110 mL of this solution) and centrifuged for 5 min at 15,000 g. Extinctions at
525, 553 and 600
nm were measured. The nonspecific turbidity, measured at 600 nm, was
subtracted from all of
the values; the ratio Ess3~szs should lie between 1.3 and 1.5. A reference
series was run in order
to be able to quantify the ALA contents.
In addition, the accumulation of ALA was determined after incubation in
glutamate and
levulinic acid. For this purpose 100-300 mg of seeds are incubated in 92.5 kBq
L-[U-'4C]
glutamate, 2mM glutamate, 20mM LA, 880 ~L 20mM K2HP04/KH2P04 (pH 7.2) for 8 h
at
room temperature in light. The plant material was homogenized in 0.5 mL 1N TCA
and 1%
SDS, centrifuged and the supernatant was diluted with 1 volume of eluent (7.8
g/L NaH2P04;
1.74 g/L SDS, 5 mL/L tert-amyl alcohol). The ['4C]-labeled ALA that formed was
detected in
accordance with Pontoppidan and Kannangara (1994, Eur. J. Biochem. 225, 529-
537) via HPLC
(flow rate 1 mL/min, isocratic mode; RP 8 column, Novapak C8, 4 um particle
size, 3.9 mm x
150 mm). The amount of radioactively labeled ALA was determined via an affixed
radioactivity
monomer. Peak identification was done by Co chromatography with D-[4-'4C]-
labeled ALA, and
quantification was done by means of reference series of the same substance.
In addition, Southern and Northern blot experiments were carned out to detect
the
integration of the trans genes and the effect on the RNA content for GSA-
aminotransferase
following standard methods (for example, (Sambrook et al. (1989) supra). The
amount of GSA
aminotransferase was also determined immunologically in the classic Western
Blot analysis,
with antibodies being obtained by immunization of rabbits or mice with
Freund's adjuvant.
The analysis of transgenic rapeseeds that express the antisense construct
contained in the
vector pUSPASGSAT showed that the method in accordance with the invention
results in a
significant reduction of the chlorophyll content.
If the molecular biological operations have not in any way been described
sufficiently,
they were carried out by standard techniques as described in Sambrook et al.
(1989) supra. With
regard to the transformation of plants, reference is made to generally known
survey articles as
well as to the publications mentioned above.
CA 02306205 2000-04-10
r ' , : . . . , 1$
Description of figures:
Figure 1 shows the metabolic pathway of tetrapyrrole biosynthesis. .
Figure 2 shows a restriction map of the binary vector pUSP-ASGSAT described in
Example 1, which contains a fusion of the USP promoter and the region coding
for GSA
aminotransferase in antisense orientation. The vector pUSP-ASGSAT bears a
kanamycin
resistance gene as the phase selection marker.
;.