Note: Descriptions are shown in the official language in which they were submitted.
CA 02306592 2000-05-30
- 1 -
Fuel Cell
The present invention is concerned with a fuel cell
and in particular with a fuel cell which provides
increased operating efficiency by maximising the
available interfacial area between high and low
density fluid phases or between gas-liquid-solid
phases, across which phases heat and mass transfer and
chemical reactions may take place. The present
invention also provides for a novel method of
improving the efficiency and performance of fuel cells
by subjecting them to forced rotation.
Fuel cells are devices for utilizing the
electrochemical conversion of the free-energy changes
of a chemical reaction directly into electrical
energy. By making use of gaseous or solid reactants
(e.g. hydrogen, oxygen, or metallic powders) the
anodic and cathodic reactants can be fed into their
respective chambers where the electrochemical energy
conversion proceeds. An electrolyte layer (often a
liquid) is provided between the two electrodes of an
electrochemical cell. At the anode, the half-cell
reaction involving the anodic reagent yields electrons
which are transported through an external circuit to
the cathode where they are taken up in the half-cell
reaction involving the cathodic reactant, usually
oxygen. The circuit is completed by the transport of
ions from one electrode to the other through the
electrolyte, Current passing through the external
electrical circuit provides electrical power and
allows mecha~ical work to be done via, for example, an
electric motor.
Unlike batteries which store elec~rical energy, =uel
cells are energy producers which convert the e:.ergy of
CA 02306592 2000-05-30
- 2 -
chemical reactions directly into electricity. They do
so in an environmentally clear. way, with no harmful
pollutants such as those whic~ arise from the normal
burning of fuels in conventio~:al combustion processes.
Because fuel cells are not limited to the
thermodynamic (Cannot) efficiencies of internal
combustion engines (typically i0-50o), they offer much
greater prospects for achieving high efficiencies (70-
1000) and energy conversion rages. In order to attain
these high efficiencies, however, new designs of
compact fuel cells are required which can extract the
electrochemical energy in a more effective manner.
Achievable power output and performance is limited by
the slow diffusion of ions and electrons at the
reactant-electrolyte-electrode interface, especially
in the case where the reactant is a gas.
Fuel cells are often classified according to their
basic system configuration. Tie most common
classifications include: phosc~oric acid fuel cells
(PAFCs); molten carbonate fue,~ cells (MCFCs); solid
oxide fuel cells (SOFCs); pro~on exchange membrane
fuel cells (PEMFCs); alkaline =uel cells (AFCs); and
direct methanol fuel cells (D~'.FCs). In another
classification, fuel cell types are grouped according
to the fuel and oxidant consuTed, e.g. hydrogen-oxygen
(or air) fuel cells; organic compounds-oxygen (or air)
fuel cells; carbon or carbon ...onoxide-oxygen (or air)
fuel cells; nitrogenous compo~~nds-oxygen (or air) fuel
cells; and metal-oxygen (or a_=) fuel cells.
Conventional fuel cells are t~-cically composite quasi-
static structures incorporatir_; numerous individual
electrochemical cells stacked _n series and parallel
to generate the required outpv~ voltage and current
density. T~e present invention is particularly suited
to the metal-oxygen type of fuel cell, and also the
hydrogen-oxygen type of fuel cell, but is not
CA 02306592 2000-05-30
- 3 -
exclusively limited to them.
One objectW:e of the present invention is therefore to
provide a fuel cell with increased working efficiency
which alle-~=ates to some extent problems associated
with previously used fuel cells.
Accordingly, the present invention provides for a fuel
cell compr=ping:
a cha~-.ber suitable for holding an electrolyte
there; z;
a mecranism which enables rotation of the
electrolyte about an axis of rotation of the
chamber;
one or more structures which define one or more
inlets for introducing an oxidant and/or a fuel
into t~:e chamber, which one or more inlets are
spaces from the axis of rotation of the chamber;
at leGyt one electrode contactable with the
electrolyte and the oxidant; and
at least one electrode contactable with the
electrolyte and the fuel;
Fuel cells currently known in the art frequently
employ a 1_~uid electrolyte and a gaseous oxidant
(e.g. air c. oxygen) and/or fuel (e.g. hydrogen). The
circulatio:: of the gaseous oxidant and/or fuel throuch
the electrc_yte relies upon buoyancy-driven natural
convection processes. In all buoyancy-driven natural
convection processes on earth, the driving forces
arise thro~:~h the interaction of matter with the
earth's grw,-itational field. The 'rise velocity', i.e.
the natural velocity attained by the lighter phase,
such as a ~~bble of gas, with respect to the heavier
phase, usu~_ly a liquid, is therefore governed by tre
value of t=-~ local gravitational acceleration, whic:,:
on earth i~ roughly constant at 9.81m/s2. This rise
CA 02306592 2000-05-30
- 4 -
velocity can be increased substantially by
intensifying the local acceleration field using
rotating frames such as centrifuges or other spinning
devices.
In the fuel cell of the present invention, centrifugal
forces acting on the electrolyte phase, together with
inverse-centrifugal (centripetal) forces acting on the
oxidant and/or fuel phases, act jcintly to increase
the overall circulation flow rate. When the induced
inertial acceleration exceeds that of the normal local
gravitational field, this externally-forced flow
process may promote an increase in volumetric
throughput of chemical substances in a reactor and
hence improve the overall rate of chemical reaction.
In ordinary 'static' fuel cells, the overall rate of
chemical reaction and therefore tr:e overall power
density achievable from the device may be limited by
the local gravitational field. In the fuel cell of the
present invention the improved overall rate of
chemical reaction will increase the power density
achievable from the device.
Advantageously, the acceleration Meld induced in the
fuel cell of the present invention as a result of
rotation of the electrolyte about an axis of rotation
of the chamber and the improved ccnvection which
results can also promote and enha~:ce the internal
circulation of the electrolyte fluid without the use
of pumps. Under strong rotation, ,::yen the angular
velocity is sufficiently high to _nduce a local linear
acceleration substantially greater than the normal
local gravitational field (approx_M:ately 9.81 m/s2),
this flow process is referred tO GJ 'enhanced natural
circulation'. The present inventic:~ thus
advantageously utilises enhanced ~~tural circulation
to provide an increase in the power output of fuel
CA 02306592 2000-05-30
- 5 -
cells, such as for example metal-oxygen (or air) fuel
cells.
The increase in the bubble rise velocity also brings
with it concomitant increases in the throughput of
chemical substances which can substantially improve
the yield from an electrochemical reaction process.
This is particularly advantageous because, generally,
the performance of conventional electrochemical
reactors is limited by the maximum achievable flow
rate of oxidant and/or fuel, which are usually gases,
passing through a porous electrode in an electrolyte
(usually a liquid), and the available interfacial area
between the different phases across which heat and
mass transfer and electrochemical reactions take
place.
It will be appreciated by those skilled in the art
that the rotation of the electrolyte about an axis of
symmetry of said chamber may be achieved in a number
of ways. However, preferably, the mechanism which
enables rotation of said electrolyte comprises a
mechanism c~hich enables rotation of said chamber.
Obviously, rotating the chamber containing the
electrolyte automatically rotates the electrolyte
itself. This may, for example, be achieved by means of
an electric motor. Thus, the reactor may, in one
embodiment, comprise a centrifuge or the like.
The chamber may, in another embodiment, be rotated by
providing a plurality of guide vanes and/or impellers
thereon, w~ich cause said chamber to rotate upon
introducticn of the oxidant and/or fuel into the fuel
cell. In t~is embodiment of the invention, the
impellers/cuide vanes may be provided outside said
chamber, w:~ich _~self may be provided inside an
external m using. Thus, advantageously, introduction
CA 02306592 2000-05-30
- 6 -
of the oxidant and/or fuel into the external housing
imparts movement to the chamber by impinging on said
guide vanes prior to its introduction into the chamber
for reaction. Thus, an external rotation mechanism,
such as an electric motor or the like, may not be
required, the rotation being induced primarily by the
pressure and momentum change of the introduced oxidant
and/or fuel impinging on said guide vanes to impart
movement to the chamber.
Alternatively, the mechanism which enables rotation of
the electrolyte may comprise rotating impellers or
baffles or the like provided within said chamber.
When the electrolyte is rotated about an axis of
rotation of the chamber, centrifugal and Coriolis
forces serve to increase the buoyancy forces on the
oxidant and/or fuel entering the fuel cell through the
inlet for introducing oxidant and/or fuel into said
chamber. The fuel cell thus works on an 'inverse
centrifuge' type principle. The 'inverse centrifuge'
principle provides the action by which a continuous
flow of a lower density fluid through another higher
density fluid can be maintained, the induced buoyancy
forces being caused by centripetal forces acting on
the lighter phase.
Fuel cells which rely on a continuous recirculation of
liquid electrolyte could benefit from the 'inverse
centrifuge' effect. For example a gas, which may
constitute either the fuel and/or the oxidant,
introduced into the electrolyte will red~~ce the local
fluid density and this density reduction can be used
to drive a continuous 'enhanced natural circulation'
around the fuel cell. This circulating flow is
increased in direct proportion to the increase in
acceleration field resulting from an increase in
CA 02306592 2000-05-30
angular velocity or spin speed of the electrolyte
about the axis of rotation. Further, the 'bubbling
action' of the gas can substantially increase the
electrochemical reaction rate by increasing the gas-
liquid-solid interfacial area at the reaction sites.
The fuel and/or oxidant gases may be supplied under
pressure through the one or more inlets located away
from the axis of rotation. Preferably, the one or more
inlets are provided in the peripheral walls of said
chamber which are furthest from the axis of rotation.
The rates of heat and mass transfer are also
increased. Rotation therefore serves to promote and
increase the continuous natural circulation of the
electrolyte and to promote and increase the throughput
of oxidant and/or fuel in a fuel cell in a manner
analogous to the flow in a centrifugal bubble column
or disk, without the use of pumps. The exploitation of
'enhanced natural circulation' by the fuel cell of the
present invention reduces the need for external pumps,
piping or other components which are ordinarily
required for the circulation of the electrolyte within
the fuel cell. This results in a more compact and
cost-effective design with substantially fewer moving
parts, thus increasing overall reliability. The
enhanced throughput of oxidant and/or fuel also
enables the use of higher flow resistance (and hence
denser) electrodes, in turn contributing to a higher
energy yield. As well as enhancing ionic transport
between the electrodes the enhanced natural
circulation of the electrolyte also reduces the
dendritic growth of species such as metal oxide
crystals which can be responsible for short circuiting
fuel cell devices.
Another advantage of the fuel cell of the present
invention is the controllability of its power output.
This may be achieved by variation of the spin speed of
CA 02306592 2000-05-30
_ g _
the electrolyte. Increasing the spin speed increases
the throughput of reactants thus increasing the rate
of reaction. This improves the uniformity of power
generation.
The incorporation of a spin-axis is also a useful
engineering feature. Having a single common axis of
rotation, a fully integrated 'stand alone' power unit
can be designed having the fuel cell reaction chamber,
oxidant and/or fuel delivery system, exhaust discharge
systems, electric drive motor and spin-speed regulator
all on one shaft. Direct coupling to an inertial
flywheel or drive/transmission system is also
possible. For applications in space where earth-
derived gravitational forces are practically absent,
the creation of artificial gravity by spinning
centrifuges may be the only means of achieving induced
natural convection flow processes.
Optionally, the fuel cell may also provide at least
one structure defining at least one outlet for removal
of said oxidant and/or said fuel adjacent said axis of
rotation.
Preferably, when one or more outlets are present, the
inlets) and outlets) are located at opposite ends of
the chamber. This advantageously provides the maximum
interfacial area between the electrodes and the
oxidant and/or fuel for electrochemical reactions to
occur.
Preferably, introduction of the oxidant and/or fuel
into the chamber and subsequently into contact with
said electrolyte and said electrodes may be by way of
sparging mans. This, advantageously, provides a
plurality of inlets which deliver the oxidant and/or
fuel and o~~=mises the reactive surface area in the
CA 02306592 2000-05-30
g -
chamber. Advantageously, when a gas is used as the
oxidant and/or fuel, the sparging means delivers
bubbles of a uniform size.
Preferably, the fuel cell also comprises one or more
compressors which pressurise the oxidant and/or fuel
prior to introduction into the fuel cell via the one
or more inlets for the oxidant and/or fuel. Preferably
the compressor is driven using energy supplied by the
fuel cell.
Preferably, the fuel cell also comprises one or more
low-pressure turbines which de-pressurise any oxidant
and/or fuel leaving the fuel cell via the one or more
outlets for the oxidant and/or fuel. The recovered
energy can be redirected back to other components of
the fuel cell such as the compressor or the mechanism
which enables rotation of the electrolyte. This
improves the energy efficiency of the device.
Preferably the fuel cell also comprises a feedback
mechanism which regulates the flow of oxidant and/or
fuel through the fuel cell so as to minimise any
undesirable vibrations which may occur due to non-
uniform distribution of oxidant and/or fuel.
Preferably, the electrodes comprise porous
electrically conductive material. Preferably, the
electrodes comprise three-dimensional blocks rather
than two-dimensional plates. In a metal-oxygen (cr
air) fuel cell, the metal fuel is itself the elec~rode
which contacts the oxidant. That is to say, the metal
acts both as oxidant and electrode. The metal fuel may
be fabricated in the form of a solid porous block or
supplied as a loosely compacted metal powder retained
within a porous metal cage ~frhich cage acts as an
electrical contact as well as providing structural
CA 02306592 2000-05-30
- 10 -
support. The electrode which contacts the oxidant
preferably comprises a porous electrically-conducting
solid material such as porous sodium-tungsten-bronze
or a porous carbon material such as pyrolized
porphyrins.
A fuel cell wherein the fuel is a metal and the
oxidant is oxygen or air represents a preferred
embodiment of the present invention. In this case, the
metal fuel may be provided in the form of a powder for
continuous feeding or as a single 'charge' of
compacted porous metal. The electropositive metals
most suitable for metal-oxygen fuel cells include, in
order of decreasing electrochemical energy equivalent:
lithium, aluminium, magnesium, calcium, iron and zinc.
Aluminium is second only to lithium in terms of
gravimetric energy density but is superior on
volumetric grounds and is also cheaper and less toxic.
Thus aluminium is preferred as the metal fuel.
When the fuel used is a metal the chamber
advantageously additionally comprises a porous cage
for containing said metal. In this type of fuel cell,
the electrolyte preferably comprises hydroxide ions,
more preferably, said electrolyte comprises an aqueous
solution of potassium or sodium hydroxide.
In a particularly preferred embodiment of the present
invention the chamber additionally comprises interr_al
baffles which separate the oxidant electrode and ti-:e
fuel electrode and which define passageways for the
internal recirculation of the electrolyte.
Even more preferably the chamber may additionally
comprise a rap for the collection and/or removal c=
reaction products from the cell. For a fuel cell
wherein the fuel is a metal and the oxidant is oxycen
CA 02306592 2000-05-30
- 11 -
or air, the trap may comprise a chemical precipitate
trap (such as calcium oxide) which retains the
reaction by-product (such as metal hydroxide).
In a preferred embodiment of the present invention the
chamber may be subdivided into smaller chambers to
create a plurality of electrocher..ical cells spaced
around the circumference of the camber which may be
electrically connected to each ot~er in series or in
parallel.
Preferably, the speed of rotation of the electrolyte
may be variable. That is to say, the mechanism which
enables rotation of the electrolyte may provide for
rotation at variable speed. More preferably, the
rotation may driven by an external mechanism such as
an electric motor which may be integral with the fuel
cell. The electric motor which drives rotation may be
powered by electricity generated by the fuel cell
itself. A particularly preferred embodiment is a
'self-starting' cell wherein the fuel cell remains
operative even when it is not spinning so that it may
provide sufficient electrical power to start-up the
electric motor.
Preferably, the electrolyte may be rotated with its
spin axis oriented in the vertical direction.
In a further aspect of the invent_on there may also be
provided a fuel cell system comprising a plurality of
fuel cells according to the prese:~t invention which
fuel cells are physically connected in series with one
another and electrically connected in series or in
parallel wit: one another. The el=ctrica~ connections
allow the electrical characterise-cs of the system to
be varied. In a preferred embodir.~nt of the fuel cell
system the fuel cells are all mou~:ted on a common spin
CA 02306592 2000-05-30
- 12 -
axis.
The present invention also encompasses within its
scope a vehicle comprising a fuel cell or a fuel
system as herein described.
According to a further aspect of the present invention
there is provided a method for improving the
efficiency and performance of fuel cells which method
comprises introducing an oxidant and/or fuel into
contact with an electrolyte present in a fuel cell as
defined herein by means of the one or more inlets
spaced from the axis of rotation of said chamber and
rotating the electrolyte about the axis of rotation of
said chamber. Any unreacted oxidant and/or unreacted
fuel may be optionally removed by means of one or more
outlets adjacent said axis of rotation.
Preferably, the electrolyte in said chamber is rotated
by rotating said chamber. The chamber may be rotated
by external means (e.g. an electric motor) or
alternatively by providing guide vanes or baffles on
said chamber which cause said chamber to rotate upon
introduction of said oxidant and/or fuel into said
fuel cell.
The method of the present invention may further
comprise allowing solvent (usually water) to boil off
from the electrolyte. This is advan~ageous because it
helps to maintain the correct elect=olyte
concentrations and pH within the fuel cell. It also
helps to dissipate internal heat generation caused by
ohmic resistance and I=R losses.
The method of the present invention may further
comprise the step of regenerating tie parent chemical
fuel and oxidant from the reaction by-products.
CA 02306592 2000-05-30
- 13 -
Apart from the usual benefits claimed for all fuel
cells, such as clean electrochemical 'combustion' with
no harmful emissions to the atmosphere, the additional
advantages claimed of this invention are basically
threefold. (i) The centrifuge action serves to
increase the operating pressure within the fuel cell
without increasing the temperature. The reasons why
the electrical output is thereby increased may be
attributable to the increase in interfacial contact
area between the oxidant and/or fuel and electrolyte
at the reaction site within the pores of the
electrode, directly influencing the ion exchange
process. (ii) Intensification of the acceleration
field induced by rotation can be used to increase the
throughput of fuel and/or oxidant by the inward
centripetal force acting on the low density phase
(i.e. the oxidant and/or fuel), and the outward
centrifugal force acting on the high density phase
(i.e. the electrolyte), thereby increasing the oxidant
and/or fuel diffusion rate and hence the overall
reaction rate and power output. (iii) The rotation can
be readily sustained by a small electric motor
deriving energy from the fuel cell itself and mounted
on the common spin axis. Excess electrical power or
rotational kinetic energy could then be utilized for
mechanical traction in automotive applications by
direct drive electric motors, possibly incorporating
energy storage techniques (e.g. flywheels or
batteries).
In a typical hydrogen-oxygen fuel cell, gaseous fuel
and oxidant are pumped separately under pressure
through porcus electrodes in contact with an
electrolyte. Pressurisation permits them to operate in
zero-gravity conditions as well as on earth. However,
a rotating ~vel cell offers advantages not achievable
in a solely static pressurised system. These benefits
CA 02306592 2000-05-30
- 14 -
derive not only from the accompanying increase in
pressure. Through the 'inverse-centrifuge' effect, the
increased buoyancy forces dynamically stimulate the
process of oxidant/electrolyte and/or fuel/electrolyte
mixing and diffusion, thereby enhancing the chemical
reaction process. Increasing the local acceleration
field (by rotation) has a very strong influence on
buoyancy, on the dynamics of bubble motion and the
bubble 'rise velocity'. A rotating fuel cell can
therefore take advantage of dynamic effects not
present in conventional static pressurised fuel cells.
As previously mentioned, a particularly preferred
embodiment of the invention comprises a fuel cell for
'burning' metallic fuel (e.g. powdered aluminium or
zinc) comprising porous matrix 'oxygen' electrodes
within a liquid electrolyte. Such a fuel cell can also
benefit substantially from centrifugal effects when
constructed as a rotating fuel cell. The additional
advantages over conventional static metal-oxygen fuel
cell designs are as follows. (i) The oxidizing gas
(oxygen or air) injected into the porous matrix oxygen
electrode, by virtue of the intense 'bubbling action',
can significantly increase the reaction rate by
creating a pulsating gas-liquid film at the 'three-
phase boundary' within the oxygen electrode. Rapid
random movements of this localized wetted film region
promotes a significant increase in charge transfer at
the gas-liquid-solid interface. (ii) The rising gas
stream reduces the average fluid density in the
electrolyte surrounding the oxygen electrode, which,
together with the increased centrifugal force due to
rotation of the liquid in the fuel chamber, promotes
enhanced natural circulation of electrolyte around the
cell. (iii) Oxygen or air not consumed in the
reduction process is readily discharged from the fuel
cell by means of improved gas-liquid separation at the
CA 02306592 2000-05-30
- 15 -
electrolyte free surface assisted by the rotational
centrifuge effects. (iv) Reactant by-products can be
effectively 'trapped' away from the axis of rotation
by centrifugal forces without impeding the flow of
electrolyte around the cell. These chemical by-
products can either be continuously (or
intermittently) removed, or, in a closed loop system,
regenerated to yield the parent metal fuel plus oxygen
for subsequent re-use. (v) Centrifugal forces can be
utilized to an advantage as a means of feeding the
cell with fresh metallic fuel. These 'outward'
centrifugal forces can be used to distribute and
compact the powdered fuel, simplifying the design of
the fuel supply system. (vi) A 'fluidized bed' of fuel
particles can be maintained to yield a large increase
in interfacial area between the fuel and electrolyte.
This improves overall fuel cell performance and
efficiency by augmenting the electrochemical reaction
rate. (vii) Powdered aluminium is particularly
preferred because it is readily obtainable in a very
pure condition at low cost. Being strongly
electropositive, it therefore provides a suitably
cheap fuel which can be electrochemically 'burned'
without the release of noxious gases. The system is
also chemically regenerative with a low cost of
regeneration. (viii) The compactness of the metal fuel
together with the low temperature of operation result
in fewer material problems, higher reliability and
longer life for the cell. A spinning configuration
also provides for a smaller overall design, hence
reduced mass and increased power-to-weight ratio.
Depending cn the specific type, a rotating fuel cell
could be engineered in many different ways especially
with regard to the geometry of the gas and liquid flow
paths, electrode configuration, design of electrolyte
chambers and the mechanical/electrical connections
CA 02306592 2000-05-30
- 16 -
between individual cells. One feature which will
generally be common to rotating fuel cells however is
that the injected oxidant and/or fuel are introduced
at those regions corresponding to substantially the
outer-most radius of the particular electrochemical
cell. Thus, the typical back-to-back arrangement of
electrodes in conventional fuel cells should instead
be redesigned to facilitate the centrifuge-enhanced
diffusion of gases through the electrolyte-wetted
regions of the electrodes. For any given cell, the two
electrodes could for example be opened out edge-to-
edge fashion, preferably, but not necessarily, on a
plane of constant radius.
One possible back-to-back configuration for a fuel
cell utilising spin-enhanced natural circulation would
be to spatially separate the 'fuel electrode' from the
'oxidant electrode' by a common radial channel or
conduit in such a way as to permit a double-loop
recirculation of the electrolyte. Gaseous fuel such as
hydrogen may be injected by means of a sparger into
the outer periphery of the fuel electrode. Likewise,
gaseous oxidant such as air may be injected separately
into the outer periphery of the oxidant electrode.
Liquid electrolyte could then circulate freely by
flowing radially outwards in the common central
channel from where it would divide to flow into the
two sparger regions of the fuel and oxidant inlets.
The radial inward flow of gases, and the resu'-ting
reduction in two-phase mixture densities in the
separated fuel and oxidant secticns will promcte
enhanced circulation of liquid electrolyte around the
fuel cell. Further requirements to balance gas and
electrolyte pressures in order to control the position
of the wetted interface within ti-:e electrodes may be
achieved by pressure regulation or through co::trol of
rotational spin speed.
CA 02306592 2000-05-30
- 17 -
Electrode design in metal-oxygen fuel cells is
somewhat less complex and, therefore, allows better
use of the inverse-centrifuge principle to control the
gas and liquid flows through the porous structures of
the two electrodes.
In some fuel cell designs, the electrolyte, which may
be an acid or alkaline aqueous solution, continuously
circulates between the elec;.rodes. As stated already,
in a rotating fuel cell, centrifugal forces causes a
large increase in hydrostatic pressure in the
electrolyte which, up to a limit, is beneficial to
fuel cell operation and performance. By injecting gas
(fuel or oxidant) under pressure through small nozzles
at the outer periphery of the centrifuge chamber, a
drastic reduction in density of the gas-liquid mixture
is produced which promotes enhanced natural
circulation of the liquid electrolyte around the cell.
The pressure and circulation flow rate can both be
directly augmented by simply increasing the rotation
speed.
The advantages of rotation outweigh the additional
complexity of driving the rotation by, for example, an
electric motor. Useful advantages arise from the
coupling of the hydrodynamic and electrochemical
processes which together increase the overall
efficiency and specific power output from such a
device. Rotation can be used to set up and control an
enhanced convection-type flow of the gas-liquid two-
phase mixture forming the recirculating electrolyte.
This avoids the need for pumps to force the liquid
around the fuel cell circuit, thus eliminating
ancillary control circuits and reducing material
corrosion problems. Further, the pressure within the
fuel cell can be increased to optimize the
electrochemical reaction rates at the electrode
CA 02306592 2000-05-30
- 18 -
surfaces b~~ controlling spin speed. By injecting gas
(e.g. hydrogen, oxygen or air) directly into the
electrode, the resultant 'bubbling' action brings
about loca'_ized random movements of the electrolyte
film at the gas-liquid-solid interface which increases
the electrochemical reaction rate. This is known to
greatly irn~rove the exchange of electronic and ionic
charge at the 'three-phase boundary' and can produce a
100-fold increase in current density at the electrode.
A very lance increase in interfacial area between a
solid fuel (e. g. powdered aluminium) and the liquid
electrolyte can advantageously be sustained by
'fluidizin~' the solid particles. Producing a
'fluidized bed' makes the metallic fuel particles
dynamically buoyant, increasing the interfacial
contact area and thus the electrical power density
obtainable from a given quantity of fuel.
Further ad-~antages of a rotating fuel cell stem from
the typica_1y intermittent nature of power sources
needed for domestic transportation purposes. For these
applications, the means of rotation may be derived via
the main transmission system whilst consuming only a
small fraction of the total power produced. The
additional power required for maintaining this
rotation is low compared to the overall power needed
for vehicle acceleration. This additional power will
be used ma_:~ly to overcome frictional resistance in
bearings a:~d to compress the oxidant gas (e. g. air)
for pressu-ised operation. Such a drive mechanism
could be geared into the main transmission system for
road vehic-es and coupled to total power demand. In
this way, =uel cell spin rate could be geared to road
speed, posy=bly utilizing a f?ywheel effect to manage
and contro= spin velocity and thereby generate higher
specific pc~aer on demand for an increase in
acceleratic:~.
CA 02306592 2000-05-30
- 19 -
The invention may be more clearly understood from the
following description, which is given by way of
example only, with reference to the accompanying
drawings wherein:
Figure 1 is an embodiment of the basic concept as
applied to rotating metal-oxygen fuel cells. A
vertical cross section through such a rotating fuel
cell is indicated in section X-X and a horizontal
section near the mid-plane in section Y-Y.
Figure 2 is another embodiment of the basic concept as
applied to rotating metal-oxygen fuel cells. This
embodiment demonstrates in more detail how the fuel
and oxidant may be introduced into the fuel cell.
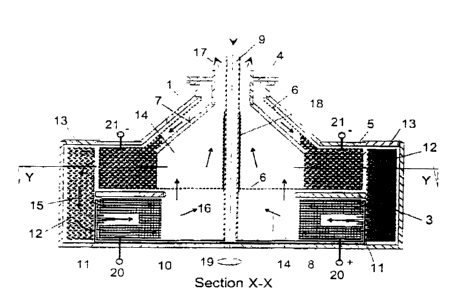
With reference to Figure 1, the main components for a
metal-oxyge:~: rotating fuel cell are as follows. A
chamber comprising a cylindrical non-conducting
chamber (1) capable of sustaining large
circumferential hoop stresses from centrifugal forces
caused by rotation about an axis of symmetry (19). The
main chamber (1) is separated into one or more cells
by non-conducting radial dividing plates or walls (2)
to electrically isolate individual electrochemical
cells, thus permitting 'stacking' of multiple series-
connected cells to increase overall output voltage.
Internal plate-type baffles (3) separate the upper and
lower halves of each cell and provide passageways for
internal recirculation of the electrolyte (14) as well
as structural support for the electrodes (5 and 8).
The radial dates (2) and baffles (3) also give
structural strength to the chamber under high spin-
induced stresses as well as providing support for the
two electrees (5 and 8). Liquid electrolyte (14)
however can :low freely and continuously between all
cells in the chamber without additional pumping.
CA 02306592 2000-05-30
- 20 -
Fuel, such as powdered aluminium, magnesium or zinc,
is introduced via a fuel supply inlet (4) into the
cells, and contained within a porous gauze-like
material in the form of a cage (6). The main purpose
of this cage is to retain the powdered fuel as well as
to provide an electrical contact (21) for power take-
off. This powdered fuel is fed directly into the fuel
cage by means of an internal conical channel (7) which
is configured to allow a continuous supply of fuel to
the cell as necessary. Once introduced into the cell
the metal fuel also becomes a constituent of the
electrode (5). The oxygen electrode (8) is fabricated
from a solid porous conducting matrix such as porous
sodium-tungsten bronze or porous carbon to complete
the electrochemical circuit of the fuel cell. Air or
oxygen, introduced under pressure via a central gas
supply tube (9), then flows into contact with the
various oxygen electrodes in each cell via a series of
radial channels (10) and fine nozzles or 'spargers'
(11). Pressurized air is supplied either via a
compressed air cylinder or by means of an on-board air
compressor (see figure 2). This gas, introduced at the
'bottom' of the electrode (8), serves two functions.
Firstly, it supplies air or oxygen directly to the
oxygen electrode (8) and promotes 'bubbling' at the
three-phase boundary of the electrode surface.
Secondly, gas bubbles injected into the electrolyte
(14) rise 'up' towards the spin ax_s (19), reducing
the fluid density in the 'riser' part of the cell and
creating a natural circulation 'up-::ards' through the
oxygen electrode (8) and 'downward' through the fuel
electrode (5).
After electrochemical reaction wit~:in the fuel cell,
the reaction products are retained in a trap (12) such
as a matrix of lime or similar substance, allowing
removal of reaction products from one cell via
CA 02306592 2000-05-30
- 21 -
removable access plates (13) or via an external
reaction product removal system (not shown). A closed-
loop regeneration system could also preferentially be
used to decompose the reaction products
electrolytically back to the parent metal and oxygen.
Such a regenerative system would permit autonomous
operation in oxygen-deficient environments such as in
space or under water.
As a result of rotation, the electrolyte (14) will
form a free surface near the axis of rotation.
Centrifugal and centripetal forces will maintain the
continuous natural circulation flow path (15) of
electrolyte around the cell, whilst permitting
separation (16) of unreacted gas from the liquid free
surface. The separated gaseous waste products may in
this way be removed via an outlet (17) for disposal or
re-use. Electrical insulation (18) is provided between
the oxidising gas supply pipe (9) and the metal powder
retaining cage (6) of the fuel compartment. Rotation
about the spin axis (19) of the fuel cell chamber can
be controlled to vary the rate of electrical power
generation. Electrical connections (20) to the oxygen
electrode and (21) to the fuel electrode allow
connection to an external electrical circuit.
Individual cell segments (22) within the centrifuge
chamber can be coupled in series internally or
externally to increase overall cell output voltage.
Multiple units can be stacked in parallel and/or
series configura~ior_s to match the desired overall
power characteristics of the fuel cell stack to any
particular application.
Operation ef the metal-air fuel cell takes place as
follows when alu~:inium is used as the fuel.
Using an al'.~aline electrolyte, the aluminium-air fuel
CA 02306592 2000-05-30
- 22 -
cell operates according to two possible reaction
schemes.
1) For alkali concentrations below 3M,
4A1 + 302 + 6H20 = 4A1 (OH) 3
with the anode reaction
A1 + 30H- - Al (OH) 3 + 3eo-
2) For alkali concentrations greater than 3M,
4A1 + 302 + 40H- - 4A102- + 2H20
with the anode reaction
A1 + 40H- - AlOz- + 2Hz0 + 3eo-
Start-up from stationary conditions may take place as
follows. In the non-rotating state, electrolyte (14)
is introduced into the centrifuge chamber (1) oriented
with its spin axis (19) vertical so as to cover the
oxygen electrode (8) and partially wet the cage (6)
for holding the metallic fuel. Loose powdered fuel
(e. g. aluminum or zinc) is introduced into the fuel
cage (6), or alternatively a number of pre-formed
porous fuel 'blocks' are placed in each fuel cell
segment. In this embodiment there would be sufficient
'coverage' of the fuel with liquid electrolyte to
generate enough power to initiate self-rotation and
self-compression of the air supply.
When rotation is applied, the electrolyte level will
re-establish itself roughly parallel to the spin axis
(19), completely covering the fuel and oxygen
electrodes (5) and (8) and with adequate liquid
present to permit enhanced natural circulation flow
around the fuel cell. Until the gas pressure (air,
oxygen) is applied via the gas inlet pipe (9), some
liquid electrolyte will flow back through the gas
sparger nozzles (11) and channels (10) to an
CA 02306592 2000-05-30
- 23 -
intermediate equilibrium position due to centrifugal
effects. This liquid will be purged however once the
applied gas over-pressure is sufficient to push the
air (or oxygen) into the oxygen electrode (8) and into
the 'riser' part of the circuit. The initial 'start
up' air supply may be from a pressurized oxygen
cylinder until enough power is generated to drive the
air compressor.
The lower density two-phase mixture passing within and
around the oxygen electrode (8) creates a density
difference between the oxidising compartment and the
fuel compartment which is the main mechanism for
promoting spin-enhanced natural circulatio~:. Air or
oxygen not consumed in the oxygen electrode (8) will
separate (16) from the electrolyte free surface to be
discharged at the exit of the fuel cell via the outlet
(17). Natural circulation flow rate is strongly
governed by the local acceleration field which
obviously depends on spin speed. Likewise, gas-liquid
separation is governed by rotation. Control of spin
angular velocity therefore allows full control of the
major operating parameters: pressure, flow rates,
interfacial area between gas-liquid-solid (and hence
chemical reaction rate and power generation.), and
separation efficiency.
Inter-cell electrical connections (20) and (21) are
provided which may be internal or external. By
providing cross-linking of an oxygen electrode (8)
with a neighbouring fuel electrode (5), it is possible
to connect all the individual cell segments (22) in
series to increase the net output potentia~- of the
composite fuel cell configuration. Combinations of
series and/or parallel interconnections be~ween cells
and with additional in-line centrifuge chaT.bers on the
same axis provides a wide range of design
CA 02306592 2000-05-30
- 24 -
possibilities for different power output
specificaticns and geometrical size envelopes.
With refere:-:ce to figure 2, the metal-oxygen fuel cell
already described with reference to figure 1 is shown
together with extra detail of the means by which the
fuel and ox;,dant are supplied to the fuel cell and the
means by which exhaust gases are depressurised. The
metal-oxygen fuel cell already described with
reference to figure 1 is shown located at the bottom.
The detail cf the means by which the fuel and oxidant
are supplied to the fuel cell and the means by which
exhaust gases are depressurised are shown located at
the top. Air enters the inlet (1), is compressed from
slightly sub-atmostpheric pressure (2) by means of a
two-stage centrifugal compressor (3) to a central air
supply line (4). From this high pressure point, air
enters the soarger (5) at a pressure high enough to
overcome the hydrostatic pressure of the spinning
electrolyte. Air bubbles then pass through the cathode
region, expanding to leave the free surface (6) at an
intermediate pressure of about 4 bar. The free surface
of the electrolyte is thereby kept at a pressure high
enough to ccntrol boiling. Unused air then flows to
the exterior of the fuel cell via an exhaust duct,
first passing through a low-pressure turbine (7) where
part of the compression work can be recovered, thus
improving the overall efficiency of the process.