Note: Descriptions are shown in the official language in which they were submitted.
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
NICKEL CARBONYL VAPOUR DEPOSITION APPARATUS
AND PROCESS
FIELD OF THE INVENTION
This invention relates to nickel vapour deposition processes for use in the
manufacture of nickel or nickel coated objects, particularly, to effluent gas
recycling in
said processes; and to apparatus for use in said processes.
BACKGROUND TO THE INVENTION
1 S Chemical vapour deposition is a well-known method for depositing films or
coatings on substrates. One known chemical vapour used for depositing a nickel
film or
coating on a substrate is nickel carbonyl in the so-called Nickel Vapour
Deposition
process (NVD). Typically, the substrates to be nickel coated are heated within
a reaction
or deposition chamber to a predetermined suitable reaction temperature,
typically 110°C -
180°C in an atmosphere of nickel carbonyl, Ni(CO)4. The nickel carbonyl
reacts at the
surface of the heated substrate to deposit the Ni film or coating thereon.
Nickel carbonyl from a liquid supply tank flows through a vapourizer where it
is
converted into a gas stream to which gaseous stream may be added a small
amount of
carrier gas, such as carbon monoxide.
Typically, nickel carbonyl vapour is continuously introduced to the deposition
chamber, wherein it reacts to produce elemental nickel and carbon monoxide by-
product.
The spent gas is continuously purged from the chamber in order to maintain
proper
circulation of reactive nickel carbonyl across the surfaces of the substrates.
The
substrates may be heated according to well-known methods, such as heat
conduction,
radiation, inductance and the like.
The spent gases which contain nickel carbonyl in excess of 30% W/W generally
undergo a nickel carbonyl reclamation process to substantially remove the
nickel carbonyl
before the spent stream enters an incinerator. The incinerator is used to
ensure complete
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
2
thermal destruction of nickel carbonyl prior to letting the combustion
products into the
environment.
The recovered nickel carbonyl is, typically, passed to a liquid supply tank.
Nickel
carbonyl is produced in a nickel carbonyl generator containing nickel powder
of a suitable
morphology in a packed bed through which is passed fresh carbon monoxide gas
from a
storage cylinder to generate fresh nickel carbonyl. The gaseous mixture is
passed through
a condenser wherein the nickel carbonyl is condensed and fed to a storage
tank. A
compressor recirculates the resultant gas back to the nickel carbonyl
generator.
The above general process represents a typical operation involving, in effect
two
distinctive processes for generating, reacting and re-generating nickel
carbonyl with non
recycled carbon monoxide.
The above process, thus suffers from the disadvantages of wasting carbon
monoxide generated as a by-product by the burning thereof in an incinerator
and, also, the
need to have the incinerator continuously operating in a continuous NVD
process.
Moreover, nickel oxide is produced in the incinerator during nickel carbonyl
combustion.
United States Patent No. 5,766,683, issued June 16, 1998, to New American TEC
describes a reclaim system for cooling the gases received from the plating
system and
cooling them to a temperature just above the freezing point of nickel carbonyl
to
condense out and recover the liquid carbonyl. The reclaim system includes a
reclaim
condenser and a vapor recovery gas receiver for receiving vapors from the
reclaim
system. The vapor recovery system includes a first stage compressor
operatively
connected to a first stage receiver for pressurizing the vapor to about 25
PSIG, and a first
stage condenser operatively connected to the first stage compressor for
cooling the
vapors. A conduit communicates the vapor recovery system to the reactor system
for
forwarding the cooled vapors to a recycle pump receiver in the reactor system.
A further
compressor is provided in the reactor system for compressing the gases from
the recycle
pump receiver to about 65 PSIG. This system recovers and recycles substantial
amounts
of the nickel carbonyl and wherein the requirements for carbon monoxide are
substantially reduced. However, U.S.P. 5,766,683 does not satisfactorily
address the full
recovery of nickel carbonyl for recycle within the system.
There is, therefore, a need for an improved NVD process which is more
economic, safe and reliable.
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
3
SLTIvIMARY OF Tf~ INVENTION
It is an object of the present invention to provide an improved NVD process
which is more economic to operate, safe and more operably reliable than
present prior art
NVD processes.
It is a further object of the invention to provide a more environmentally
acceptable
NVD process having a reduced need for use of a carbon monoxide incinerator
during
operations.
It is a yet fi~rther object of the invention to provide apparatus for use in
aforesaid
NVD processes.
Accordingly, in one broad aspect the invention provides a closed loop, carbon
monoxide self contained process for the production of nickel or a nickel-
coated object by
the nickel vapour deposition process, comprising
(a) placing an object to be treated with nickel carbonyl by said nickel vapour
deposition process in a deposition chamber;
(b) feeding a gaseous mixture comprising nickel carbonyl and carbon monoxide
to
said deposition chamber;
(c) depositing nickel on said object to produce said nickel or nickel-coated
object
in said chamber and a nickel carbonyl-depleted gaseous mixture;
(d) removing said nickel carbonyl-depleted gaseous mixture from said chamber;
(e) removing nickel carbonyl from said nickel carbonyl-depleted gaseous
mixture
in a primary nickel carbonyl condensation unit to produce a first reduced-
concentration nickel carbonyl-containing gas; the improvement comprising
(f) condensing said first reduced-concentration nickel carbonyl-containing gas
in a
secondary condensation unit operative in an alternate freeze-thaw mode by,
stepwise,
(l) freezing said first reduced-concentration nickel carbonyl-containing gas
to
produce a first solid nickel carbonyl;
(ii) melting said first solid nickel carbonyl to produce a first liquid nickel
carbonyl;
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
4
(iii) removing said first liquid nickel carbonyl from said secondary
condensation
unit to produce a second reduced concentration nickel carbonyl-containing
gas having a nickel carbonyl concentration of less than 5 V/V %; and
(g) feeding said second reduced-concentration nickel carbonyl-containing gas
to a
nickel carbonyl reactor containing nickel powder to produce a fresh
gaseous mixture comprising fresh nickel carbonyl and carbon monoxide.
Two process loops, one nickel carbonyl generation and the other decomposition
are connected via recycled carbon monoxide and liquid carbonyl transfer links.
The two
loops can operate independently from each other or in tandem as is preferred
for reasons
outlined hereinbefore.
The preferred nickel carbonyl reclaim system of use in the invention provides
for
successful operation and integration of the two loops. After the deposition
chamber there
is a preferred 3-stage carbonyl reclamation process. In the first or primary
condensation
unit nickel carbonyl is condensed. The secondary reclamation unit comprises a
pair of
condensation units which essentially reduces the nickel carbonyl concentration
to below
1 %. The double units, alternatively and simultaneously, freeze or thaw out
nickel
carbonyl. The switching from on unit to the other is accomplished by a
suitable nickel
carbonyl concentration detection system in the process stream.
Accordingly, the invention provides in a preferred embodiment the process as
hereinbefore defined, for continuous operation having a plurality of said
secondary
condensation units linked in parallel arrangement and operative in
alternating, alternate
freeze-thaw modes.
In the next stage, carbon monoxide-nickel carbonyl gas mixture is compressed
and traces of nickel carbonyl are removed by a 3'd reclaim system comprising a
pair of
condensation units, which reclaims traces of nickel carbonyl to yield pure
carbon
monoxide wherein carbon monoxide was the sole carrier gas. Reclaim 3 system
operates
on the same switching principles as reclaim 2.
Accordingly, in a more preferred embodiment, the invention, as hereinabove
defined, further comprising condensing said second reduced-concentration
nickel
carbonyl-containing gas in a tertiary condensation unit operative in an
alternate freeze-
thaw mode, by stepwise,
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
(iv) freezing said second reduced-concentration nickel carbonyl-containing gas
to
produce a second solid nickel carbonyl;
(v) melting said second solid nickel carbonyl to produce a second liquid
nickel
carbonyl;
S (vi) removing said second liquid nickel carbonyl from said tertiary
condensation unit; to produce a third reduced-concentration nickel
carbonyl-containing gas having a nickel carbonyl concentration of less
than 500 p.p.m.; and
(h) feeding said third reduced-concentration nickel carbonyl-containing gas to
a
nickel carbonyl reactor containing nickel powder to produce a further fresh
gaseous mixture comprising nickel carbonyl and carbon monoxide.
Selection of suitable carbon monoxide diaphragm type compressors is most
preferred for safe running of the process.
The reactor in the carbonyl generation loop can have a continuous feed of
nickel
powder. Suitable controlled reactor bed temperature is maintained via a
separate heat
management system. Highly toxic reactor residue is handled by an auxiliary
suction
system.
Transfer of liquid nickel carbonyl during the process between generation to
decomposition loop is accompanied without pumps by pressurizing storage
cylinders.
In a further feature, the invention provides a method of recovering nickel
carbonyl
in a nickel deposition system which includes a reactor system, a plating
system, a reclaim
system and a vapor recovery system comprising the steps of producing liquid
nickel
carbonyl, vaporizing the liquid nickel carbonyl and applying the vaporized
nickel
carbonyl to a substrate to deposit nickel thereon and to release carbon
monoxide, cooling
the gases after the deposition of nickel on said substrate to a temperature
below the
freezing point of nickel carbonyl to condense out nickel carbonyl to a solid;
subsequently
allowing said solid nickel carbonyl to melt and recovering liquid nickel
carbonyl from
said solid nickel carbonyl.
In a further aspect the invention provides an improved nickel deposition
apparatus
having a nickel carbonyl reactor unit, a nickel deposition unit, a nickel
carbonyl vapour
recovery system having a primary condensation unit and a secondary
condensation unit
the improvement comprising means for freezing nickel carbonyl to a solid
within said
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
6
secondary condensation unit, means for melting said solid nickel carbonyl to a
liquid;
means for recovering said liquid nickel carbonyl; and means for recovering
nickel
carbonyl depleted gaseous mixture.
Preferably, the apparatus has a plurality, preferably, a pair of said
secondary
condensation units linked in parallel and means for operatively controlling
said units in
alternating, alternate nickel carbonyl freeze-thaw modes.
More preferably, the apparatus further comprises a tertiary condensation unit
for
freezing nickel carbonyl out of said recovered nickel carbonyl-depleted
gaseous mixture
to a solid state, means for melting said frozen nickel carbonyl to a liquid
and means for
recovering said liquid nickel carbonyl.
Yet more, preferably, the apparatus comprises a pair of said tertiary
condensation
units linked in parallel and means for operatively controlling said units in
alternating,
alternate nickel carbonyl freeze-thaw modes.
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the invention may be better understood, a preferred embodiment
will
now be described by way of example only wherein:
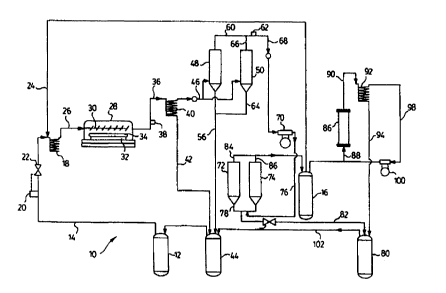
Fig. 1 represents a schematic flow sheet of a process and apparatus according
to the
invention.
DETAILED DESCRIPTION OF A PREFERRED EMBODIIUViENT
Fig. 1 shows generally as 10, an apparatus and process flow sheet according to
the
invention.
Liquid nickel carbonyl from storage tank 12 is passed at ambient temperature
through conduit line 14 at 40 psi with carbon monoxide Garner gas prior to
entering
vaporizer 18. The liquid nickel carbonyl flow rate in conduit 14 is measured
by a mass
flow meter 20, which sends the information to a flow control valve 22 whereby
the flow
is controlled and adjusted to the desired value. Carbon monoxide carrier gas
from carbon
monoxide storage tank 16 (84) is mixed from line 24 with the nickel carbonyl
to form a
10% CO W/W 90% Ni(CO)4.
CA 02307036 2003-02-03
WO QOn6432 ~GT/CA98J01OZ3
7
Vaporizer 18 vaporizes the liquid nickel carbonyl to a temperature of
approximately 87°C. The vaporized mid is passed through heated conduit
26 to
deposition chamber 28, wherein ttxe gas is dispersed tlxrough a manifold 30 to
contact a
. heated maadrel 32, having a substrate 34 at a temperature of approximately
180°C. At
this temperatwre, the nickel carbonyl decomposes at the surFa~ce of substrate
34 to produce
a nickel z~aetal coating on substrate 34. The temperat~ue and residence tune
of the
gaseous mixture in the chamber is such as to effect about a 60% nickel
carbonyl
decomposition rate. The remaining non-decomposed nickel carbonyl, CO carrier
gas and
C~? produced from the decomposition exits chamber 28 through heated conduit
3fi at
about 60°C to a nefrigetation unit 40, wherein most of the nickel
carbonyl is liquefied and
fed through, line 42 to storage tanlt 44. The gas concentration in Iine 36 is
measured by a
UV gas concentration cell meter (act shownj, and, typically, has a nickel
carbonyl
concentration ranging, from, 30 - 80% V/V, temperature of between 82 -
85°C and a
pressure of about 0.05 bar..
Tlxe gaseous mixture exiting unit 40, typically, has a nickel carbonyl
concentration
o~ between 6.5 -~ 8.0 V/V %, temperature of -15° to --17°C,
pressure of about 0.05 bat,
and is passed through conduit 46 and then to one of two refrigerated
reclamation units 48
or54. " ' . y , _ _
LFnits 48, 50 are identical in construction and operaiioa and Kepresent a pair
of
refrigeration units which operate in parallel in distinct fi~ng cycles, from,
either a
"freezing of nickel carbonyl" mode or a "thawing of carbonyl" mode as may be
selected.
The units in their freeze mode, typically, provide solid nickel carbonyl at a
temperatlme
selected from -55° to -~8°C. Ju operation, when. unit 48 is in
its fixing mode, ~t.e.
operating at its coldest temperature, exhaust gas fram conduit 46 is passed
through
conduit 52 to unit 48, wherein the vast majority of the nickel carbonyl in the
gas is fi~oren.
Small amounts of nickel carbonyl gas pass through nail. 48 until either the
back pressure
is the system exceeds a pre-determined value, or if the nickel carbonyl gas
level passing
through lie 60 exiting uilit 48 exceeds a pre-dat~myned level as measured by
UV
ar~alyur 62. Typically, the gaseous mixture in line d0 has s nickel carbonyl
concaatration
less than 5 VIV %, and generally of betw~n 0.35 - 0.55 'VN % and a temperature
of
between 15° to 20°C at a pressure of about 30 bat afiar
com~nessi~on as hereixabelow
descn~bed.
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
8
When either of the above two conditions is met, other unit 50 is switched to
the
freezing mode and the exhaust gas flow is switched to unit 50. First unit 48
is then
switched over to the "thaw" mode which means that the unit freezing system is
switched
off and the unit heaters {not shown) are turned on. The thaw mode melts the
frozen
nickel carbonyl into a liquid form, which is gravity drained to storage tank
44 via line 56.
The above procedure in respect of unit 50 occurs when unit SO reaches its
critical
condition whereby the thaw/freeze process switches back to the original thaw
setting with
liquid nickel carbonyl dropping through conduits 64 and 56 into storage tank
44.
Thus, further nickel carbonyl-depleted exhaust gas leaves units 48 and 50
through
lines 60, 66, respectively, to pass through common line 68 to a diaphragm
compressor 70
to provide a compressed gas mixture. The compressed gas passes subsequently
through
an additional or third reclaiming system consisting of dual refrigeration
units 72, 74
analogous to units 48, 50 and related analogous conduits so designed as to be
identical in
operation to reclaim system 2 with the exception that reclaim system 3
operates at a
higher pressure. Thus, units 72,74 are identical in constructions and
operation and
represent a pair of refrigeration units which also operate in parallel in
distinct freezing
cycles, from either a "freezing of nickel carbonyl" mode or a "thawing of
carbonyl" mode
as may be selected. In operation, when unit 72 is in its freezing mode, i.e.
operating at its
coldest temperature, compressor 70 exhaust gas from conduit 76 is passed
through
conduit 78 to unit 72, wherein the vast majority of the nickel carbonyl in the
gas is frozen
out. Essentially, nickel carbonyl-free gas passes out of unit 72 until the
back-pressure in
the system exceeds a pre-determined value. Typically, the nickel carbonyl is
at a
concentration of less than 500 p.p.m. and, generally, between 100 - 200
p.p.m., at a
gaseous mixture temperature of about -50°C and pressure of 30 bar.
When the above condition is met, refrigeration unit 74 is switched to its
freezing
mode and conduit 76 exhaust gas flow is switched to unit 74. First unit 72 is
then
switched over to its "thaw" mode, which means that its unit freezing system is
switched
off and its unit heaters (not shown) are turned on. In a thaw mode, the frozen
nickel
carbonyl melts into a liquid, which is then transferred to storage tank (80)
via line 82.
The above procedure in respect of unit 74 is conducted until unit 74 reaches
its critical
condition whereby the thaw/freeze process is switched back to its original
thaw setting,
CA 02307036 2003-02-03
we aor~~a ' ' Peiricw9sro~oa.~. ~. ,
9
with the resultant liquid nickel carbonyl being sub~quently trans~ened through
conduit
82 into storage tank 80.
Thus, further essentially nickel carbonyl-free compressed exhaust gas leaves
units
?2 and ?4 through liurnes 84, 85, respectively, to pass to carbon monoxide
storage teak 16.
Tank 16 is also a reservoir for a carbon monoxide supply to nickel carbaayl
producer
reactor 86 containing a bed of nickel powder raw material. Reactor 86 receives
carbon
mmnoxide from tank 16 through line 88 a~ad operates at a t~enuperature
selected from 70° _
115°C. The addition o~ CO through line 88 provides for the production
of nickel
carbonyl, wherein the flowthrough of CO depends on the reactivity of ff~e
nickel powder,
i.e. the greater the production rate, the more CO that is consumed.
Regenerated nickel carbanyllC4 mixture exits reactor 86 thraugb line 90 to
nickel
carbonyl liquid condenser 92 operating at a temperature of between -18°
and 0°C,
wfiereia lied nickel carbonyl is condensed and gravity fed t'kurough conduit
9~ to storage
taal~~t . The resultant gas mixture exits condenser 92 through line 98 to
generator
recirculatmg compressor 100, which recycles the gas back to reactor 86 for
nickel
carbonyl production until the zuickel powder is fully conk to nickel carbonyl.
Liquid nickel carbonyl is subsequently transferred From storage tank 96
through
conduit 102 to storage teaks 44 and 12, as required_
Thus, it can be seen that the embodiment hereinabove described provides for a
fully closed carbon monoxide recycle.sysrem wherein.iniriai carbon monoxide is
used as
a cagier gas, comrerted to nickel carbonyl and subseduently sa-generated from
the nicks!
carbonyl. The only material added and subsequently removed from the system is
riietalIic
nickel, which is added as powder and removed as an object or caaoting as
nickel plate, foil
gad the lace. Judicious selection of reactor temperatures, decomposition
chamber
temperattues and conduit temperatures enables proper utilization of the feat
reactants. Suitable gax pressures a~ad flow rates are rowdily attained and
controllable.
Use of a second stage double, in parallel, refrigeration u:rit ' systeux
enables
continuous operation of the process, Further, use of a third stage double, is
parallel,
refrigeration unit system further provides en;6aaced nickel ca~rba~xyl
recovery values for
recycle withi,ia the carbon monoxide fully closed system. '
Although this disclosure has described gad illustrated preferred embodimeat$
of
the invention, it is to be v~erstood that the invexd:ion is not restricted to
those paraicular
CA 02307036 2000-O1-21
WO 00/26432 PCT/CA98/01023
embodiments. Rather, the invention includes all embodiments which are
functional or
mechanical equivalence of the specific embodiments and features that have been
described and illustrated.