Note: Descriptions are shown in the official language in which they were submitted.
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
l lie of Macrolides for the Treatment of Cancer and Macular Degeneration
Technical Field
The presem invention relates to novel utilities of semi-synthetic macrolides,
specifically
antitumor activity and activity against macular degeneration. More
particularly, the invention
relates to the use of 6-O-substituted erythromycin derivatives and
compositions containing
such compounds for the treatment of tumors and macular degeneration.
S
~ack~,round of the Invention
Erythromycins A through D, represented by formula (E), are well-known and
CHs
NMez
p
HO.."
OH
C ~ 1
H3
,,~
~ Ervthromvcin R~ R~
Ho.,",
CH,'...
o
o
cH3
Ra A -OH -CHI
HsC B -H -CH3
CHs
H
O
H
CHs
O ~
0........ C -OH -H
CHs
CHs D -H -H
H
~'
oH
s.
O
CHs
ORn
(E)
potent antibacterial agents. However, reports have appeared over the past few
years which
suggest that Erythromycin may possess antitumor as well as other beneficial
activities.
Hamada et al, have reported that tumor bearing mice treated with oral
erythromycin at
doses of 1-10 mg/kg had a two- to three-fold increase in survival times
compared to control
IS mice (Chemotherapy, 41, 59-69 (1995) ). Mikasa et al. reported that long-
term
clarithromycin treatment of lung cancer patients prolonged survival time
(Chemotherapy, ~,
288-296 (1997)). WO 95/28939 discloses that 14- or 15-membered macrolide
compounds,
including clarithromycin and erythromycin B, have a potent antitumor effect on
non-small cell
lung cancers and are therefore useful as practical therapeutic agents for this
type of cancer.
2o These tumors are considered the most difficult to treat with surgery or
chemotherapy.
- Much research and many resources have been devoted to the development of
antitumor
drugs, and some successful chemotherapeutic agents have been developed.
However, new
-1-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/IJS98/Z3043
antitumor agents and methods for inhibiting, remitting, or controlling growth
of tumors are still
needed.
Angiogenesis is the process by which new blood vessels invade a tissue. This
occurs
normally during wound healing, menstruation and development of an embryo.
However,
s angiogenesis is ven~ important in the development of some tumors. This
process of generating
new capillary blood vessels, also referred to as neovascularization. is an
essential feature of
fibroproiiferative processes representing solid tumor growth and inflammation.
Numerous
reviews of this subject exist, however, and that by Phillips et al. (Int. J.
Cancer, 17, 549-588
(19761 is a useful introduction to the subject.
tU Unlike angiogenesis in normal physiological situations, tumor-induced
angiogenesis
continues indefinitely until the cancer kills the host or unless the tumor is
eradicated.
Therefore, inhibition of the angiogenic process would be expected to slow the
growth of some
solid tumors. Marshall and Hawkins have reviewed the clinical experience with
anti-
angiogenic compounds in the treatment of both malignant and benign disease
(Breast Cancer
t 5 R~~sc~arch ami Treatment, ~, 253-261 ( 1995)).
The pathological growth of new blood vessels also underlies most eye diseases
that
cause catastrophic loss of vision. These new vessels -- which proliferate
under the sensory
retina -- leak serum and blood, which in turn leads to a fibrotic reaction or
disciform scar.
When left untreated, choroidal neovascularization generally results in severe
visual loss in 50%
20 to 90% of the affected eye. Additionally, patients with a neovascular
membrane in one eye are
at substantial risk of a neovascular membrane in the second eye within 5
years. Therefore,
patients with macular degeneration must be treated with antiangiogenic agents
before extensive
neovascularization and permanent scarring have occurred.
Age-related macular degeneration (AMD) is the most common cause of visual loss
in
''S elderly Americans. Choroidal neovascularization associated with severe
visual loss will
develop in 10-20% of patients with age-related macular degeneration. Current
treatments for
age-related macular degeneration fall into four categories: subretinal
surgery, laser surgery,
radiation, and pharmacological therapies. No satisfactory pharmacological
treatment exists for
choroidal neovascularization or age-related macular degeneration.
Antiangiogenic agents are
3o among the latest compounds to be used for the pharmacological treatment of
macular
degeneration. These antiangiogenic drugs are useful in treating the
neovascular phase of this
disorder. Antiangiogenic therapy primarily inhibits the growth of new vessels,
rather than
promoting regression of existing vessels. Therefore, there exists a need for
agents that could
prevent the development, growth or recurrence of these medical conditions.
35 The present invention provides new utilities of semi-synthetic 6-O-alkyl
erythromycin
derivatives, specifically for the treatment of tumors and macular
degeneration.
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Summary of the Invention
The present invention provides a method of treating tumors and macular
degeneration
in a human or veterinary patient comprising administering to a patient a
therapeutically effective
amount of a compound selected from the group consisting of:
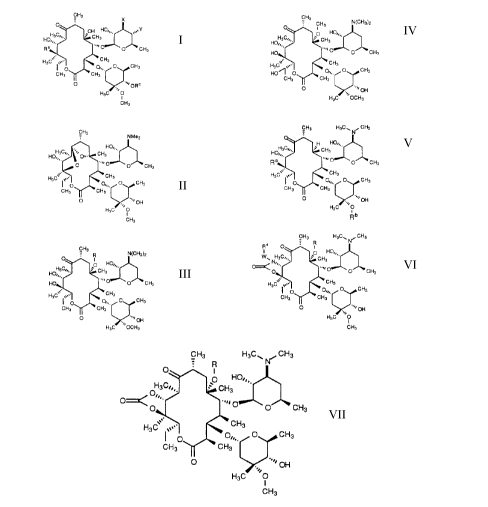
CH3
X
O
Of.~ HO,,, ~~,, Y
HO,,
H3C/'I ' CH3
Ra .~~~ O O CH3
H3C' '~,,~ ~ CH3
O ~ 0,,, O CH3
CH3 CH3 .
O ...i O R c
H3C ~~O
(I), 0~3 ,
CH3
NMe2
HO,,,.
H3C~~...~~~' CH
HO,,,. ~ 0~.. s
O ~~~~ O O CH3
H3C~~. '~,,~ ~ CH3
p ' 0,,, O CH3
CH3 CH3 .
O .~~~ OH
H3C ,~~0
(II ), ~H3 ,
CH3
R N(Chl3)2
I
O HO~.,
HOH3C~~'' ' CH3
HO ,I. ~~~~ O O CH3
H3C: . v.,,. w CHs
p ~ On. O CH3
CH3 CH3 .
O .~~~ OH
to (III), H3c .~'ocH3 ,
-3
SUBSTlTUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
CH3 N(CH3)2
O CH3
O HOi.,
HOH3C~~'' ' CH3
HO,~. ~~~~ O O CH3
H3C~~. ,~~.~ ~ CH3
HO~ O On. O CH3
CH3 CH3 .
O .~~~ OH
(IV) H3C .~~OCH3
CH3 H3C.,, N, CH3
H HO~..
HOH3Ci., ' CH3
O CH3
~ CH
H3C ~~.~' _ 3
( O 0~.,, O CH3
CH3 CH3
O .~~~ OH
H3C .~~0
(V), R5
CH3 H3C~ ~ CH3
Ra O R
I O HO,,~
Ww HsC~., . CH
3
O~ N~~,.
O .~~~ O O CH3
H3C~' ~ ,~,.. ~ CH3
O CH3
I O O
CH3 CH3
O .~~~ OH
H3C .~~0
(VI), ~H3 , and
-4
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
CH3 H3C~ ~ CH3
O R
'O HO,,,.
H3C~~. CH3
O~ 0~~~ ~''~ O
O~ O CH3
H3~' '~~ ' CH3
0~.. O CH3
CH3 CH3
O .~~~ OH
H3C .~~0
(VII), ~H3
wherein
R~ is hydrogen or hydroxy;
Rb is hydrogen or methyl;
R~ is hydrogen or hydroxy protecting group;
X is -NR1R~. wherein R~ and R~ are independently selected from
(1) hydrogen, and
(2) C1-C3-alkyl optionally substituted with a substituent selected from the
group
consisting of
(a) aryl,
(b) substituted-aryl,
(c) heteroaryl, and
(d) substituted-heteroaryl,
Y is hydrogen or hydroxyl;
or
X and Y taken together form a bond;
W is absent or is selected from the group consisting of -O-, -NH-, -NH-CO-,
and -N=CH;
RW is selected from the group consisting of
(1) hydrogen,
2o (2) CI-C6-alkyl optionally substituted with one or more substituents
selected from
the group consisting of
(a) aryl,
(b) substituted-aryl,
(c) heteroaryl,
' 25 (d) substituted-heteroaryl,
(e) hydroxy,
(f) Ct-C~,-alkoxy,
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
(g) NRIR2, wherein R1 and R2 are as defined previously.
and
{h) -CH2-M-R3
wherein M is selected from the group consisting
of:
(i) -C(O)-NH-,
(ii) -NH-C(O)-,
(iii) -NH-,
(iv) -N=,
(v) -N(CH3j-,
(vi) -NH-C(O)-O-
(vii) -NH-C(O)-NH-
(viiij -O-C(O)-NH-
(ix) -O-C(O)-O-
(x) -O-,
(xij -S(O)S-, wherein n is 0, 1 or 2,
(xii ) -C(O)-O-,
(xiii) -O-C(O)-, and
(xiv) -C(O)-,
and
R3 is selected from the group consisting of:
(i) C~-C6-alkyl, optionally substituted with a substituent
selected from the group consisting of
(aa) aryl,
(bb) substituted-aryl,
(cc ) heteroaryl, and
(dd) substituted-heteroaryl,
(ii) aryl,
(iii) substituted-aryl,
(iv) heteroaryl,
{v) substituted-heteroaryl, and
(vi) heterocycloaikyl,
(3) C3-C7-cycloalkyl,
(4) aryl,
(5) substituted-aryl,
- 35 (Ei) heteroaryl, and
(7) substituted-heteroaryl;
R is selectedfrom the group consisting of
-6
SUBST1TUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
( 1 ) methyl
substituted
with a moiety
selected
from the
group consisting
of .
(a~ CN,
(b) F,
(c) -C02R4 wherein R4 is selected from the group
consisting of C~-
C3-alk yl, aryl substituted C1-C3-alkyl, and heteroaryl
substituted
C 1-C3-alkyl,
(d) S(O)"R4 where n is 0, 1 or 2 and R4 is as previously
defined,
(e) NHC(O)R4 where R4 is as previously defined,
(f) NHC(O)NR1R2 wherein R1 and R2 are as previously
defined,
1c~ (g > aryl,
(h ) substituted aryl,
(i) heteroaryl, and
(j) substituted heteroaryl;
(2) C2-Clp-alkyl;
(3) C2-Cip-alkyl
substituted
with one
or more substituents
selected
from the
group consisting of
(a) halogen,
(b) hydroxy,
(c) C1-C3-alkoxy,
2u (d) C1-C3-alkoxy-C~-C3-alkoxy,
(e) oxo,
(17 -N3.
(g) -CHO,
(h) O-S02-(substituted C1-C~-alkyl),
(i) -NRSR6 wherein RS and R6 are selected from
the group
consisting
of
(i) hydrogen,
(ii) C1_C12_a~Yl,
(iii) substituted C1-C12-alkyl,
(iv) C~-C12-alkenyl,
(v) substituted C~-C~2-alkenyl,
(vi) C~-C12-alkynyl,
(vii) substituted C1-C~2-alkynyl,
(viii) aryl,
- 35 (ix) C3-Cg-cycloalkyl,
(x) substituted C3-Cg-cycloalkyl, -
(xi) substituted aryl,
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
(xii) heterocycloalkyl,
(xiii) substituted heterocycloalkyl,
(xiv) C~-C~2-alkyl substituted with aryl,
(xv) C~-Ct2-alkyl substituted with substituted
aryl,
(xvi) CI-C12-alkyl substituted with heterocycloalkyl,
(xvii) C1-C12-alkyl substituted with substituted
heterocycloalkyl,
(xviii) C1-C12-alkyl substituted with C3-Cg-cycloalkyl,
(xix) C~-C12-alkyl substituted with substituted
C3-Cg-cycloalkyl,
(xx) heteroaryl,
(xxi ) substituted heteroaryl,
(xxii) C1-Ciz-alkyl substituted with heteroaryl,
and
(xxiii) C1-C12-alkyl substituted with substituted
heteroaryl,
or
R5 and R6 are taken together with the atom to
which they are attached
form a 3-10 membered heterocycloalkyl ring which
may be substituted
with one or more substituents
independently selected
from the group
consisting of
(aa> halogen,
(bb) hydroxy,
(cc) C1-C3-alkoxy,
(dd) C~-C3-alkoxy-CI-C3-allcoxy,
(ee) oxo.
(~ C 1-C3-~Yl.
(gg) halo-C1-C3-alkyl, and
(hh) C1-C3-alkoxy-C~-C3-alkyl,
(j) -C02R4 wherein R4 is as previously defined,
(k) -C(O)NR1R2 wherein R~ and R2 are as previously
defined,
(1) =N-O-R4 wherein R4 is as previously defined,
(m) -CN,
(n) O-S(O)"R4 wherein n is 0, 1 or 2 and R4 is
as previously defined,
(o) aryl,
(p) substituted aryl,
(q) heteroaryl,
(r) substituted heteroaryl,
- 35 (s) C3-Cg-cycloalkyl,
(t) substituted C3-Cg-cycloalkyl,
(u) C~-C12-alkyl substituted with heteroaryl,
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCTNS98/23043
(v) heterocycloallcyl,
(w) substituted heterocycloalkyl,
(x ) NHC(O)R4 where R4 is as previously defined,
(y) NHC(O)NRiR2 wherein R1 and R2 are as previously
defined,
(z) =N-NRSR6 wherein R5 and R6 are as previously
defined,
(aa) =N-R3 wherein R4 is as previously defined,
(bb) =N-NHC(O)R4 wherein R4 is as previously defined,
and
(cc) =N-NHC(O)NR1R2 wherein R~ and R2 are as previously
defined;
(4) C3-alkenyl
substituted
with a moiety
selected
from the
group consisting
of
(a) halogen,
(b) -CHO,
(c) -C02R4 where R4 is as previously defined,
t5 (d) -C(O)-R3 where R3 is as previously defined,
(e) -C(O)NR 1 R2 wherein R 1 and R2 are as previously
defined,
(f> -CN,
(g) aryl,
(h) substituted aryl,
(i) heteroaryl,
{j) substituted heteroaryl,
(k) C3-C7-cycloalkyl,
and
(1) C1-CI2-alkyl substituted with heteroaryl,
(5) C4-C1 0-alkenyl;
(6) C4-C1 0-aikenyl substituted with one or more substituents
selected from the
group consisting of
(a) halogen,
(b) Ci-C3-alkoxy,
(c) oxo,
(d) -CHO,
(e) -C02R4 where R4 is as previously defined,
(f) -C(O)NR 1 R2 wherein R 1 and R2 are as previously
defined,
(g) -NRSR6 wherein R5 and R6 are as previously
defined,
- 35 (h) =N-O-R4 where R4 is as previously defined,
(i) -CN,
(i) O-S(O)~R4 where n is 0, 1 or 2 and R4 is as
previously defined,
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
(k i aryl, _
(1 i substituted aryl,
(m) heteroaryl,
(n) substituted heteroaryl.
(o J C3-C7-cycloalkyl,
(p) C1-C~2-alkyl substituted with heteroaryl,
(q) NHC(O)R4 where R4 is as previously defined,
(r) NHC(O)NR~R2 wherein R1 and R2 are as previously
defined,
(s) =N-NR5R6 wherein R5 and R~ are as previously
defined,
(t) =N-R-~ wherein R3 is as previously defined,
(u) =N-NHC(O)R4 where Ra is as previously defined,
and
(v) =N-NHC(O)NR1R2 wherein RI and R2 are as previously
defined;
(7) C3-C~0-alkynyl;
and
(X) C3-C~0-alkynyl substituted with one or more substituents selected from the
group consisting of
(a) trialkylsiiyl,
(b) aryl,
(c) substituted aryl,
(d) heteroaryl,
and
(e) substituted heteroaryl.
30
The present invention also provides pharmaceutical compositions for treating
tumors
and macular degeneration in a human or veterinary patient comprising a
pharmaceutically
acceptable carrier and a compound selected from formulas (I) - (VII) above in
combination
with a pharmaceutically acceptable corner.
Detailed Des rintion of the Invention
Definitions
As used throughout this specification and the appended claims, the following
terms
have the meanings specified.
- 35 The terms "C1-C3-alkyl", "C~-C6-alkyl", and "C1-C12-alkyl" as used herein
refer to
saturated, straight- or branched-chain hydrocarbon radicals derived from a
hydrocarbon moiety
containing between one and three, one and six, and one and twelve carbon
atoms, respectively,
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
by removal of a single hydrogen atom. Examples of C)-C3-alkyl radicals include
methyl, _
ethyl, propyl and isopropyl, examples of C)-C~-alkyl radicals include, but are
not limited to,
methyl, ethyl, propyl, isopropyl, n-butyl. tent-butyl, neopentyl and n-hexyl.
Examples of C~-
C)2-alkyl radicals include, but are not limited to, all the foregoing examples
as well as n-
s heptyl, n-octyl, n-nonyl, n-decyl, n-undecyl and n-docecyl.
The term "C1-C~-allcoxy" as used herein refers to an C1-C6-alkyl group, as
previously
defined, attached to the parent molecular moiety through an oxygen atom.
Examples of C~-C6-
alkoxy, but are not limited to, methoxy, ethoxy, propoxy, isopropoxy, n-
butoxy, tert-butoxy,
neopentoxy and n-hexoxy.
1e) The term "Cl-C12-alkenyl" denotes a monovalent group derived from a
hydrocarbon
moiety containing from two to twelve carbon atoms and having at least one
carbon-carbon
double bond by the removal of a single hydrogen atom. Alkenyl groups include,
for example,
ethenyl, propenyl, butenyl, 1-methyl-2-buten-1-yl, and the like.
The term "C~-C12-alkynyl" as used herein refers to a monovalent group derived
from a
15 hydrocarbon containing from two to twelve carbon atoms and having at least
one carbon-
carbon triple bond by the removal of a single hydrogen atom. Representative
alkynyl groups
include ethynyl, 2-propynyl (propargyl), 1-propynyl and the like.
The term "alkylene" denotes a divalent group derived from a straight or
branched chain
saturated hydrocarbon by the removal of two hydrogen atoms, for example
methylene, 1,2-
2e ethylene, l,l-ethylene, 1,3-propylene, 2,2-dimethylpropylene, and the like.
The term "C1-C3-alkylamino" as used herein refers to one or two C~-C3-alkyl
groups,
as previously defined, attached to the parent molecular moiety through a
nitrogen atom.
Examples of C~-C3-alkylamino include. but are not limited to methylamino,
dimethylamino,
ethylamino, diethylamino, and propylamino.
25 The term "oxo" denotes a group wherein two hydrogen atoms on a single
carbon atom
in an alkyl group as defined above are replaced with a single oxygen atom
(i.e. a carbonyl
group ).
The term "aryl" as used herein refers to a mono- or bicyclic carbocyclic ring
system
having one or two aromatic rings including, but not limited to, phenyl,
naphthyl,
3o tetrahydronaphthyl, indanyl, indenyl and the like. Aryl groups (including
bicyclic aryl groups)
can be unsubstituted or substituted with one, two or three substituents
independently selected
from loweralkyl, substituted loweralkyl, haloalkyl, allcoxy, thioalkoxy,
amino, alkylamino,
dialkylamino, acylamino, cyano, hydroxy, halo, mercapto, vitro,
carboxaldehyde, carboxy,
alkoxycarbonyl and carboxamide. In addition, substituted aryl groups include
35 tetrafluorophenyl and pentafluorophenyl.
The term "C3-C12-cycloalkyl" denotes a monovalent group derived from a
monocyclic
or bicyclic saturated carbocyclic ring compound by the removal of a single
hydrogen atom.
SUBSTITUTE SHEET (RULE 2fi)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Examples include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
bicyclo[2.2.1]heptyl, and
bicyclo[2.2.2]octyl.
The terms "halo" and "halogen" as used herein refer to an atom selected from
fluorine.
chlorine, bromine and iodine.
The term "alkylamino" refers to a group having the structure -NHR' wherein R'
is
alkyl, as previously defined. Examples of alkylamino include methylamino,
ethylamino, iso-
propylamino and the like.
The term "diallcylamino" refers to a group having the structure -NR'R" wherein
R' and
R" are independently selected from alkyl, as previously defined. Additionally,
R' and R"
1o taken together may optionally be -(CH2)t;- where k is an integer of from 2
to 6. Examples of
diallcylamino include, dimethylamino, diethylaminocarbonyl, methylethylamino,
piperidino,
and the like.
The term "haloalkyl" denotes an alkyl group, as defined above, having one,
two, or
three halogen atoms attached thereto and is exemplified by such groups as
chloromethyl,
bromoethyl, trifluoromethyl, and the like.
The term "alkoxycarbonyl" represents an ester group; i.e. an alkoxy group,
attached to
the parent molecular moiety through a carbonyl group such as methoxycarbonyl,
ethoxycarbonyl, and the like.
The term "thioallcoxy" refers to an alkyl group as previously defined attached
to the
z« parent molecular moiety through a sulfur atom.
The term "carboxaldehyde" as used herein refers to a group of formula -CHO.
The term "carboxy" as used herein refers to a group of formula -C02H.
The term "carboxamide" as used herein refers to a group of formula -CONHR'R"
wherein R' and R" are independently selected from hydrogen or alkyl, or R' and
R" taken
together may optionally be -(CH2)k- where k is an integer of from 2 to 6.
The term "heteroaryl", as used herein, refers to a cyclic aromatic radical
having from
five to ten ring atoms of which one ring atom is selected from S. O and N;
zero, one or two
ring atoms are additional heteroatoms independently selected from S, O and N;
and the
remaining ring atoms are carbon, the radical being joined to the rest of the
molecule via any of
3o the ring atoms, such as, for example, pyridyl, pyrazinyl, pyrimidinyl,
pyrrolyl, pyrazolyl,
imidazolyl, thiazolyl, oxazolyl, isoxazolyl, thiadiazolyl, oxadiazolyl,
thiophenyl, furanyl,
quinolinyl, isoquinolinyl, and the like.
The term "heterocycloalkyl" as used herein, refers to a non-aromatic partially
unsaturated or fully saturated 3- to 10-membered ring system, which includes
single rings of 3
to $ atoms in size and bi- or tri-cyclic ring systems which may include
aromatic six-membered
aryl or heteroaryl rings fused to a non-aromatic ring. These heterocycloalkyl
rings include
those having from one to three heteroatoms independently selected from oxygen,
sulfur and
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
nitrogen, in which the nitrogen and sulfur heteroatoms may optionally be
oxidized and the _
nitrogen heteroatom may optionally be quaternized.
Representative heterocycles include, but are not limited to, pyrrolidinyl,
pyrazolinyl,
pyrazolidinyl, imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl,
oxazoiidinyl,
isoxazolidinyl, morpholinyl, thiazolidinyl, isothiazolidinyl, and
tetrahydrofuryl.
The term "heteroarylallcyl" as used herein, refers to a heteroaryl group as
defined above
attached to the parent molecular moiety through an alkylene group wherein the
alkylene group
is of one to four carbon atoms.
"Hydroxy-protecting group", as used herein. refers to an easily removable
group
1 which is known in the an to protect a hydroxyl group against undesirable
reaction during
synthetic procedures and to be selecaively removable. The use of hydroxy-
protecting groups is
well known in the art for protecting groups against undesirable reactions
during a synthetic
procedure and many such protecting groups are known, cf., for example, T.H.
Greene and
P.G.M. Wuts, Protective Groups in Organic Synthesis. 2nd edition, John Wiley &
Sons,
15 New York ( 1991 ). Examples of hydroxy-protecting groups include, but are
not limited to,
methylthiomethyh tort-dimethylsilyl, tort-butyldiphenylsilyl, ethers such as
methoxymethyl,
and esters including acetyl benzoyl, and the like.
A the term "protected-hydraxy" refers to a hydroxy group protected with a
hydroxy
protecting group, as defined above, including benzoyl, acetyl, trimethylsilyl,
triethylsilyl,
20 methaxymethyl groups, for example.
The term "substituted aryl" as used herein refers to an aryl group as defined
herein
substituted by independent replacement of one, two or three of the hydrogen
atoms thereon
with Cl, Br, F, I, OH, CN. C1-C3-alkyl, C1-C6-alkoxy, C1-C6-alkoxy substituted
with aryl,
haloalkyl, thioallcoxy, amino, allcylamino, dialkylamino, mercapto, vitro,
carboxaldehyde,
25 carboxy, alkoxycarbonyl and carboxamide. In addition, any one substitutent
may be an aryl,
heteroaryl, or heterocycloallcyl group. Also, substituted aryl groups include
tetrafluorophenyl
and pentafluorophenyl.
The term "substituted heteroaryi" as used herein refers to a heteroaryl group
as defined
herein substituted by independent replacement of one, two or three of the
hydrogen atoms
3o thereon with Cl, Br, F, I, OH, CN, C1-C3-alkyl, C1-C6-alkoxy, C~-C6-alkoxy
substituted
with aryl, haloallcyl, thioalkoxy, amino. alkylamino, diallcylamino, mercapto,
vitro,
carboxaldehyde, carboxy, alkoxycarbonyl and carboxamide. In addition, any one
substitutent
may be an aryl, heteroaryl, or heterocycloallcyl group.
The term "substituted heterocycloalkyl" as used herein, refers to a
heterocycloalkyl
35 group, as defined above, substituted by independent replacement of one, two
or three of the
hydrogen atoms thereon with Cl, Br, F. I, OH, CN, C~-C3-alkyl, C1-C6-alkoxy,
C1-C6-
alkoxy substituted with aryl, haloalkyl, thioalkoxy, amino, alkylamino,
diallcylamino,
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
mercapto, nitro, carboxaldehyde, carboxy, allcoxycarbonyl and carboxamide. In
addition, any
one substitutent may be an aryl, heteroaryl. or heterocycloalkyl group.
Numerous asymmetric centers may exist in the compounds of the present
invention.
Except where otherwise noted, the present invention contemplates the various
stereoisomers
S and mixtures thereof. Accordingly, whenever a bond is represented by a wavy
line, it is
intended that a mixture of stereo-orientations or an individual isomer of
assigned or unassigned
orientation may be present.
As used herein, the term "pharmaceutically acceptable salt" refers to those
salts which
are, within the scope of sound medical judgment, suitable for use in contact
with the tissues of
W humans and lower animals without undue toxicity, irritation, allergic
response and the like, and
are commensurate with a reasonable benefit/risk ratio. Pharmaceutically
acceptable salts are
well known in the art. For example, S. M. Berge, et al. describe
pharmaceutically acceptable
salts in detail in J. Pharm. Sci., ~: 1-19 (1977), incorporated herein by
reference. The salts
can be prepared in situ during the final isolation and purification of the
compounds of the
15 invention, or separately by reacting the free base function with a suitable
organic acid.
Examples of pharmaceutically acceptable, nontoxic acid addition salts are
salts of an amino
group formed with inorganic acids such as hydrochloric acid, hydrobromic acid,
phosphoric
acid, sulfuric acid and perchloric acid or with organic acids such as acetic
acid, oxalic acid,
malefic acid, tartaric acid, citric acid, succinic acid or malonic acid or by
using other methods
20 used in the art such as ion exchange. Other pharmaceutically acceptable
salts include adipate,
alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate,
butyrate,
camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate>
dodecylsulfate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate,
gluconate, hemisulfate>
heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate,
lactate, laurate,
2S lauryl sulfate, malate, maleate, malonate, methanesulfonate, 2-
naphthalenesulfonate,
nicotinate, nitrate, oleate, oxalate, palmitate, pamoate, pectinate,
persulfate, 3-
phenylpropionate, phosphate, picrate, pivalate> propionate, stearate,
succinate, sulfate,
tartrate, thiocyanate, p-toluenesulfonate, undecanoate, valerate salts, and
the like.
Representative alkali or alkaline earth metal salts include sodium, lithium,
potassium, calcium,
3o magnesium, and the like. Further pharmaceutically acceptable salts include,
when appropriate,
nontoxic ammonium, quaternary ammonium, and amine cations formed using
counterions
such as halide, hydroxide, carboxylate, sulfate, phosphate, nitrate,
loweralkyl sulfonate and
aryl sulfonate.
As used herein, the term "pharmaceutically acceptable ester" refers to esters
which
35 hydrolyze in. vivo and include those that break down readily in the human
body to leave the
parent compound or a salt thereof. Suitable ester groups include, for example,
those derived
from pharmaceutically acceptable aliphatic carboxylic acids, particularly
alkanoic, alkenoic,
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
cycloalkanoic and alkanedioic acids, in which each alkyl or aikenyl moiety
advantageously has
not more than fi carbon atoms. Examples of particular esters includes
formates. acetates,
propionates, butyrates, acrylates and ethylsuccinates.
The term "pharmaceutically acceptable prodrugs" as used herein refers to those
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgment. suitable for use in contact with the tissues of humans and
lower animals
with undue toxicity, irritation, allergic response, and the like, commensurate
with a reasonable
benefit/risk ratio, and effective for their intended use, as well as the
zwitterionic forms. where
possible, of the compounds of the invention. The term "prodrug" refers to
compounds that are
1n rapidly transformed in vivn to yield the parent compound of the above
formula, for example by
hydrolysis in blood. A thorough discussion is provided in T. Higuchi and V.
Stella, Pro-
drugs as Novel Delivery S sums, Vol. 14 of the A.C.S. Symposium Series, and in
Edward
B. Roche, ed., Bioreversible Carriers in Drug Desien, American Pharmaceutical
Association
and Pergamon Press> 1987, both of which are incorporated herein by reference.
Pharmaceutical Compositions
The pharmaceutical compositions of the present invention comprise a
therapeutically
effective amount of a compound of the present invention formulated together
with one or more
pharmaceutically acceptable carriers. As used herein, the term
"pharmaceutically acceptable
2O carrier" means a non-toxic, inert solid, semi-solid or liquid filler,
diluent, encapsulating
material or formulation auxiliary of any type. Some examples of materials
which can serve as
pharmaceutically acceptable carriers are sugars such as lactose, glucose and
sucrose; starches
such as corn starch and potato starch; cellulose and its derivatives such as
sodium
carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered
tragacanth: malt;
'_'S gelatin; talc: excipients such as cocoa butter and suppository waxes;
oils such as peanut oil,
cottonseed oil; safflower oil: sesame oil: olive oil; corn oil and soybean
oil; glycols: such a
propylene glycol: esters such as ethyl oleate and ethyl laurate; agar;
buffering agents such as
magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water;
isotonic
saline; Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as
well as other non-
30 toxic compatible lubricants such as sodium lauryl sulfate and magnesium
stearate, as well as
coloring agents, releasing agents, coating agents, sweetening, flavoring and
perfuming agents,
preservatives and antioxidants can also be present in the composition,
according to the
judgment of the formulator. The pharmaceutical compositions of this invention
can be
administered to humans and other animals orally, rectally, parenterally,
intracisternally,
35 intravaginally, intraperitoneally, topically (as by powders, ointments, or
drops), bucally, or as
an oral or nasal spray.
-15-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCTNS98/23043
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions. solutions. suspensions, syrups and elixirs. In
addition to the
active compounds. the liquid dosage forms may contain inert diluents commonly
used in the art
such as, for example, water or other solvents, solubilizing agents and
emulsifiers such as ethyl
alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol.
benzyl benzoate.
propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular,
cottonseed,
groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl alcohol,
polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
Besides inert
diluents, the oral compositions can also include adjuvants such as wetting
agents, emulsifying
to and suspending agents, sweetening, flavoring, and perfuming agents.
lnjectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or wetting
agents and suspending agents. The sterile injectable preparation may also be a
sterile injectable
solution, suspension or emulsion in a nontoxic parenterally acceptable diluent
or solvent, for
15 example, as a solution in 1,3-butanediol. Among the acceptable vehicles and
solvents that may
be employed are water, Ringer's solution, U.S.P. and isotonic sodium chloride
solution. In
addition. sterile, fixed oils are conventionally employed as a solvent or
suspending medium.
For this purpose any bland fixed oil can be employed including synthetic mono-
or
diglycerides. In addition, fatty acids such as oleic acid are used in the
preparation of
2~~ injectables.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter, or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium prior to use.
25 In order to prolong the effect of a drug, it is often desirable to slow the
absorption of
the drug from subcutaneous or intramuscular injection. This may be
accomplished by the use
of a liquid suspension of crystalline or amorphous material with poor water
solubility. The
rate of absorption of the drug then depends upon its rate of dissolution
which, in turn, may
depend upon crystal size and crystalline form. Alternatively, delayed
absorption of a
3o parenterally administered drug form is accomplished by dissolving or
suspending the drug in
an oil vehicle. Injectable depot forms are made by forming microencapsule
matrices of the
drug in biodegradable polymers such as polylactide-polyglycolide. Depending
upon the ratio
of drug to polymer and the nature of the particular polymer employed, the rate
of drug release
can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
35 poly(anhydrides) Depot injectable formulations are also prepared by
entrapping the drug in
liposomes or microemulsions which are compatible with body tissues.
-16-
SUBSTITUTE SHEET (RULE 26~
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Compositions for rectal or vaginal administration are preferably suppositories
which
can be prepared by mixing the compounds of this invention with suitable non-
irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which are
solid at ambient temperature but liquid at body temperature and therefore melt
in the rectum or
vaginal cavity and release the active compound.
Solid dosage forms for oral administration include capsules. tablets, pills,
powders,
and granules. Ln such solid dosage forms, the active compound is mixed with at
least one
inert, pharmaceutically acceptable excipient or carrier such as sodium citrate
or dicalcium
phosphate and/or a) fillers or extenders such as starches, lactose, sucrose,
glucose, mannitol,
tU and silicic acid, bl binders such as, for example, carboxymethylcellulose,
alginates, gelatin,
polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as glycerol,
d) disintegrating
agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain
silicates, and sodium carbonate, e) solution retarding agents such as
paraffin, f) absorption
accelerators such as quaternary ammonium compounds, g) wetting agents such as,
for
example, cetyl alcohol and glycerol monostearate, h) absorbents such as kaolin
and bentonite
clay, and i) lubricants such as talc, calcium stearate, magnesium stearate,
solid polyethylene
glycols, sodium lauryl sulfate. and mixtures thereof. In the case of capsules,
tablets and pills,
the dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
2U filled gelatin capsules using such excipients as lactose or milk sugar as
well as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well known in the
pharmaceutical formulating art. They may optionally contain opacifying agents
and can also be
of a composition that they release the active ingredients) only, or
preferentially, in a certain
part of the intestinal tract, optionally, in a delayed manner. Examples of
embedding
compositions which can be used include polymeric substances and waxes.
Solid compositions of a similar type may also be employed as fillers in soft
and hard
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high molecular
3o weight polethylene glycols and the like.
The active compounds can also be in micro-encapsulated form with one or more
excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills, and
granules can be prepared with coatings and shells such as enteric coatings,
release controlling
coatings and other coatings well known in the pharmaceutical formulating art.
In such solid
dosage forms the active compound may be admixed with at least one inert
diluent such as
sucrose, lactose or starch. Such dosage forms may also comprise, as is normal
practice,
additional substances other than inert diluents, e.g., tableting lubricants
and other tableting aids
-17-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 - PCT/US98/23043
such a magnesium stearate and microcrystalline cellulose. In the case of
capsules, tablets and
pills, the dosage forms may also comprise buffering agents. They may
optionally contain
opacifying agents and can also be of a composition that they release the
active ingredients)
only, or preferentially, in a certain part of the intestinal tract,
optionally, in a delayed manner.
Examples of embedding compositions which can be used include polymeric
substances and
waxes.
Dosage forms for topical or transdermal administration of a compound of this
invention
include ointments, pastes, creams, lotions, gels, powders, solutions, sprays,
inhalants or
patches. The active component is admixed under sterile conditions with a
pharmaceutically
acceptable earner and any needed preservatives or buffers as may be required.
Ophthalmic
formulations, ear drops, eye ointments, powders and solutions are also
contemplated as being
within the scope of this invention.
The ointments, pastes, creams and gels may contain, in addition to an active
compound
of this invention, excipients such as animal and vegetable fats, oils, waxes,
paraffins, starch,
tragacanth, cellulose derivatives, polyethylene glycols, silicones,
bentonites, silicic acid, talc
and zinc oxide, or mixtures thereof.
Powders and sprays can contain, in addition to the compounds of this
invention,
excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium
silicates and
polyamide powder, or mixtures of these substances. Sprays can additionally
contain
customary propellants such as chlorofluorohydrocarbons.
Transdermal patches have the added advantage of providing controlled delivery
of a
compound to the body. Such dosage forms can be made by dissolving or
dispensing the
compound in the proper medium. Absorption enhancers can also be used to
increase the flux
of the compound across the skin. The rate can be controlled by either
providing a rate
controlling membrane or by dispersing the compound in a polymer matrix or gel.
According to the methods of treatment of the present invention, tumors are
treated and
macular degeneration is treated or prevented in a patient such as a human or
lower mammal by
administering to the patient a therapeutically effective amount of a compound
of the invention,
in such amounts and for such time as is necessary to achieve the desired
result. By a
"therapeutically effective amount" of a compound of the invention is meant a
sufficient amount
of the compound to treat bacterial infections, at a reasonable benefit/risk
ratio applicable to any
medical treatment. It will be understood, however, that the total daily usage
of the compounds
and compositions of the present invention will be decided by the attending
physician within the
scope of sound medical judgment. The specific therapeutically effective dose
level for any
particular patient will depend upon a variety of factors including the
disorder being treated and
the severity of the disorder; the activity of the specific compound employed;
the specific
composition employed; the age, body weight, general health, sex and diet of
the patient; the
-18
'SUBSTIT#JTE MEET (RUl:E 26)~
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
time of administration, route of administration, and rate of excretion of the
specific compound
employed: the duration of the treatment: drugs used in combination or
coincidental with the
specific compound employed; and like factors well known in the medical arts.
The total daily dose of the compounds of this invention administered to a
human or
S other mammal in single or in divided doses can be in amounts, for example,
from 0.01 to 200
mg/kg body weight or more usually from 10 to 100 mg/kg body weight. Single
dose
compositions may contain such amounts or submultiples thereof to make up the
daily dose. In
general, treatment regimens according to the present invention comprise
administration to a
patient in need of such treatment from about 10 mg to about 2000 mg of the
compounds) of
m this invention per day in single or multiple doses.
In a preferred method of the invention of treating tumors and macular
degeneration the
compound is selected from the group of compounds having the formulas (I) -
(V). In a more
preferred method the compound is selected from the group of compounds having
the formulas:
Compound of Formula (I): R~ = OH, X = NHMe, Y = H, Rc = H;
1 i Compound of Formula (I): R~~ = OH, X = NMe2, Y = H, Rc = Formyl;
Compound of Formula (I): R~~ = OH, X and Y taken together form a bond, Rc = H;
Compound of Formula (I): R<< = OH. X = NMe2, Y = OH, Rc = H;
Compound of Formula (I): R<< = OH, X = NMe(Benzyl ), Y = H, Rc = H;
Compound of Formula (II);
2n Compound of Formula (III): R = -CH2CH=CH2
Compound of Formula (III): R = -CH2CH=N-O-(Benzyl);
Compound of Formula (III): R = -CH3; (clarithromycin);
Compound of Formula (IV);
Compound of Formula (V): R~~ = OH, Rh = H; and
''S Compound of Formula (V): R~~ = OH, Rh = CH3.
In a preferred pharmaceutical composition of the invention the compound is
selected
from the group of compounds having the formulas (I) - (V). In a more preferred
pharmaceutical composition the compound is selected from the group of
compounds listed in
the paragraph above.
The foregoing may be better understood by reference to the following examples
which
are presented for the purpose of illustrating the 6-O-substituted erythromycin
derivatives that
may be utilized for the treatment of tumors and macular degeneration.
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Exam 1
In Vitro Anti-Angiogenic Active of selected Macrolides
Representative compounds of the present invention were assayed in vitro for
their
ability to induce giant cells in culture of mouse peritoneal macrophages.
Since the anti-
angiogenic activity of macrolides correlates to their ability to induce giant
cells in culture of
mouse peritoneal macrophages, the in vines activity of macrolides were
determined according to
the method described by E. Kita et al., in Nat. Immun., 12, 326-338 (1993).
The numbers
to indicate the minimum concentration of analyte necessary to induce giant
cells. The data given
in Table I below indicate that the compounds were effective in inducing giant
cells and
therefore that the compounds possess anti-angiogenic activity.
Table 1
t5 In Vitr-n Activity of Selected Compounds*
in Inducine Giant Cells
Compound Minimum Conc. tn
Induce
No. f
1 2.0
2 1.0
3 2.5
4 1.0
5 3.5
6 2.0
7 5.0
8 2.0
11 I.0
12 2.0
* compounds
are identified
in Table
2 below
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Table 2
Identi~~~r and Source of Selected Macrolides
Tested by lrr Vitrn or In. Vivn Protocols
Cmpd Compound - Source or method of preparation
No.
_
1 Compound of Formula (I): Freiberg, U.S. Patent 3,725,385
( R<< = OH, (1973)
J X = NHMe. Y = H, Rc = H
2 Compound of Formula (I): Tanadier et al., J. Orb.
~ R~~ = OH. Chem. 39, 2495
X = NMe2. Y = H, Rc = Formyl( 1974)
3 Compound of Formula (I): Jones and Rowley, J. Org.
R~~ = OH, Chem., 33,
X and Y taken together 665 ( I 968)
form a bond,
Rc=H
4 Compound of Formula (I): Jones et al., Arttimicrob.
R~~ = OH, Agents
X = NMe2, Y = OH, Rc = Chemother., 123 ( 1970)
H
_ Compound of Formula (I): Freiberg, U. S. Patent 3,681,325,
R~ = OH,
Benz 1), Y = H,Rc=H issued August I, 1972
X = NMe(
6 _ Kurath et al. (Experientia,
Compound of Formula (II) 27, 362
(1971)
7 Compound of Formula (III):Or et al., disclosed in U.S.
R = - Patent
CH2CH=CHI Application Serial No. 08/841.038
filed
4/29/97.
$ Compound of Formula (III):Or et al., disclosed in U.S.
R = Patent
CH2CH=N-O-(Benzyl) Application Serial No. 08/841,038
filed
4/29/97.
9 Compound of Formula (III):Abbott Laboratories
I R =
CH3; (clarithromycin)
Compound of Formula (IV) Hardy et al., Antimicrob.
Agents
Chemother.. 32, 1710-1719
( 1988)
11 _ McAlpine et al., 30th Intl.
Compound of Formula (V): Conf.
R<< = OH,
Rh = H Antibiot. Agents. Chcmother.,
Atlanta,
USA, 1990, Abst. 810
12 Compound of Formula (V): McAlpine et al., 30th Intl.
Rs = OH, Conf.
Rn = CH3 Antibiot. Agents. Chemother..
Atlanta,
USA, 1990. Abst. 810
Examo~2
In Vivo Anti-AneioQenic Activity of Selected Macrolides
1U The anti-angiogenic properties of macrolides were tested in vivo in the
mouse cornea
model. In this model, the neovascularization is induced by bFGF-containing
pellet implanted
in the cornea. Macrolide was given orally, QD, from day 0 (day of pellet
implantation)
through day 5. At day 6, mice were perfused with saline and Indian ink. Eyes
were removed,
fixed and the cornea was isolated, mounted in a glass slide. Microvessel
density was
determined by image analysis system, and the anti-angiogenic effect the test
compound
estimated.
-21-
SUBSTITUTE SHEET (RULE 2fi)
CA 02307850 2000-04-27
WO 99/22722 PCT/US98/23043
Protocols
Pellet ~r~aration. Pellets were prepared which contained a mixture of three
substances: 1 ) HydronTM (polyhydroxyethylmethacrylate), a slow-release
polymer; 2)
SucralfateT"', to stabilize and to slow the release of basic fibroblast growth
factor (bFGF); and
S 3) bFGF, a potent angiogenic growth factor. The mixture, after being speed
vacuumed and re-
suspended into a small volume, was spread onto a sterile nylon mesh. After it
is dried, pellets
of approximately equal size of 0.4 x 0.4 x 0.2 mm were obtained by pulling
apart the fibers of
the mesh. Each pellet contains approximately 100 ng of bFGF, an optimal dose
established
previously.
lU Pellet implantation. Approximately 8 week old CF-1 albino mice were used in
this
study. Under anesthesia, and using a dissecting microscope, an intrastromal
micropocket was
made, at 0.7 mm of the temporal limbus. A single pellet was deposited on the
corneal surface
and inserted in the pocket. The control animals received no drug treatment.
Antibiotic
ointment was applied to pellet-implanted eyes immediately after surgery. The
eyes were
15 routinely examined postoperatively.
Theraw. Therapy was initiated on day 0, after the mice recovered from
anesthesia. In
three experiments. compounds were administered orally four times per day to
groups of three
mice per dose at doses from 25 to 200 mg/kg/day for a period of 6 days.
Compound 8 was
tested at 100 mg/kg/day, compound 9 was tested at 25 to 200 mg/kg/day, and
compound 9
2n was tested at 100 mg/kg/day. Control groups consisted of three mice per
experiment. In two
experiments taxol was administered intraperitineally four times per day to
groups of three mice
per dose at 10 mg/kg/day for b days as a toxicity control group.
Examination of Neovascuiarization. At day 6 postoperative. under deep
anesthesia,
mice were perfused with an intracardiac injection of about 25 mL of saline,
then perfused with
~S an Indian ink I :30 solution. Eyes were enucleated and fixed with
formaldehyde 10%
overnight. After fixation, corneas were dissected and mounted onto a glass
slide. Microvessei
density was measured by image analysis using a Image-Pro PlusT~~' software
program. The
microvessel density was measured with arbitrary units and are expressed as
mean ~ standard
error (n=6).
30 Results. Animals that received taxol had positive signs of toxicity. No
signs of gross
toxicity were observed in the macrolide treated mice. Compounds 8, 9 and 10 at
100
mg/kg/day decreased angiogenesis by SO% in the mouse cornea model as estimated
from the
reduced microvessel density in the treated animals.
-22-
SUBSTITUTE SHEET (RULE 26)