Note: Descriptions are shown in the official language in which they were submitted.
CA 02309342 2000-06-14
PROTEIN PRODUCTION IN TRANSGENIC PLANT SEEDS
Background of the Invention
The use of transgenic plants for the production of pharmaceutical proteins
and industrial enzymes has been proposed. In general, expression of
recombinant
proteins relies on stable integration of a heterologous gene into a host plant
genome
using, e.g., Agrobacterium-mediated transformation or particle bombardment. In
terms of cost, production of commercially valuable proteins using crops in the
field
is more competitive than other biological production systems (such as yeast,
10 bacteria, or mammalian cell cultures) which require complex and high-
maintenance
bioreactors. Further, protein production in plant can be easily scaled-up to
produce
large quantities.
Summary of the Invention
15 The invention is based on the discovery that hydrolytic enzymes have a
specific activity higher than expected (as compared to, e.g., E. coli produced
enzyme) when expressed in transgenic seeds using a transgene having a promoter
active in a plant seed tissue and driving transcription of a sequence encoding
the
hydrolytic enzyme.
20 More specifically, the invention features a transgenic plant (e.g., a
transgenic
rice, barley, rye, or canola plant) whose genomic DNA includes a gene having a
promoter (e.g., an a-amylase promoter, such as that of the aAmy8 gene, or a
glutelin
promoter) and a nucleotide sequence encoding a hydrolytic enzyme and operably
linked to the promoter. The promoter drives expression of the hydrolytic
enzyme in
25 a plant seed tissue of a developing seed, germinating seed, germinated
seed, or
seedling of the transgenic plant. The enzyme optionally hydrolyzes a substrate
naturally occurring in a plant seed tissue. If the enzyme is a phytase, the
enzyme
can also be an acid phosphatase, such as the enzyme encoded by an appA gene,
or a
phytase from Selenomonas ruminantium (e.g., strain JY35, available as ATCC
30 55785), such as the enzyme encoded by the phyA gene. If the enzyme is a
CA 02309342 2000-06-14
amylopullulanase (e.g., of Therrnoanaerobacter ethanolicus), the enzyme can
also
be an a-amylase. The invention also includes a transgenic seed produced from a
transgenic plant as described above. As used herein, a "seed" can be a
developing
seed, a mature seed, a germinating seed, a germinated seed, or a seedling.
Thus, the
5 invention further features a method of producing a hydrolytic enzyme by
providing a
transgenic plant of the invention, harvesting a transgenic seed from the
transgenic
plant, germinating the seed, and optionally isolating the hydrolytic enzyme
from the
transgenic seed.
The invention also features a method of producing a polypeptide by
10 providing a transgenic plant whose genomic DNA contains a gene having an a-
amylase promoter and a nucleotide sequence encoding a polypeptide and operably
linked to the promoter; harvesting a transgenic seed from the transgenic
plant;
germinating the transgenic seed to produce a mature seed; and isolating the
polypeptide from the mature seed (e.g., from an embryo or endosperm tissue of
the
15 mature seed). Also included in the invention is a method of producing a
polypeptide
by providing a transgenic plant whose genomic DNA contains a gene having a
glutelin gene promoter and a nucleotide sequence encoding a polypeptide and
operably linked to the promoter; harvesting a transgenic seed from the
transgenic
plant; germinating the transgenic seed; and isolating the polypeptide from an
embryo
20 or endosperm tissue of the seed.
As used herein, a "phytase" is an enzyme that reacts with phytate or phytin to
release phosphorus or a moiety that contains phosphorus that is absorbable in
the GI
of a monogastric animal. Thus, a phosphatase that releases inorganic
phosphorus or
phosphate from phytate is also a phytase.
25 By one genetic element being "operably linked" to another is meant that one
genetic element (either in a plus strand, minus strand, or double stranded
form) is
structurally configured to operate or affect another genetic element. For
example, a
promoter operably linked to a sequence encoding a polypeptide means that the
promoter initiates transcription of a nucleic acid encoding the polypeptide.
CA 02309342 2000-06-14
Germination begins with water uptake by the seed (imbibition) and ends with
the start of elongation by the embryonic axis, usually the radicle. Therefore,
germination does not include seedling growth, which commences when germination
terminates. For germination to be completed, the radicle must expand and
penetrate
5 the surrounding structures. A developing seed is one that is germinating or
supporting growth of the seedling. As used herein, the term "seedling" means
the
juvenile plant grown from a germinating seed, as defined in de Vogel, "The
Seedling," In: Seedlings of Dicotyledons, Centre for Agricultural Publishing
and
Documentation, Wageningen, Netherlands, pp 9-25, 1983
An a-amylase gene promoter and signal peptide sequence can be fused
upstream of the enzyme open reading frame (ORF) or gene, which can be derived
from various sources. The chimeric gene is then introduced into a plant (e.g.,
cereal)
genome. For example, a malted transgenic cereal grain that produces high
levels of
phytase can be used for human consumption, animal feed, or food processing.
Malting is the process by which grains are germinated under controlled
condition
and in contained facilities to produce a consistent product.
The advantages of using transgenic malted seeds to produce a hydrolytic
enzyme as feed include: (1) the seeds contain a large quantity of nutrient and
minerals in an easily available form that is easily transportable and not
readily
20 perishable; (2) the nutrients in the seeds are not chelated by, e.g.,
phytate; (3) in the
case of malted seeds, little toxicity is expected for consumers of the feed,
because
the malted seeds have long been used in the brewing and food industries and
are
known to be harmless to humans and animals; and (4) the cost of manufacturing
feed for monogastric animals is reduced because an exogenous enzyme does not
need to be added to the raw material.
In addition, the transgenic plants of the irwention, or organs and seeds
thereof, can be used to produce hydrolytic enzymes for a variety of industrial
processes. Examples of such applications are in feed additives for non-
ruminants, in
soy processing, in food processing, or in the production of inositol or
inositol-
phosphates from phytate.
3
CA 02309342 2000-06-14
Other features or advantages of the present invention will be apparent from
the following detailed description, and also from the claims.
Brief Description of the Drawings
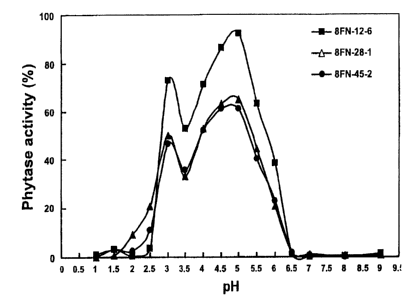
Fig. 1 is a line graph of pH versus phytase activity.
Fig. 2 is a line graph of pH versus acid phosphatase activity.
Fig. 3 is a bar graph of various transgenic plants versus amount of APU
produced therein. The filled bars represent plant samples grown in sucrose.
The
open bars represent plant samples grown without sucrose. "NT" is the non-
transformed control.
Figs. 4 and 5 are bar graphs of various transgenic lines versus amount of
APU. "NT" is the non-transformed control.
Fig. 6 is a bar graph of various transgenic lines versus specific activity for
APU.
Figs. 7 and 8 are bar graphs of various transgenic lines versus sugar
concentration. "NT" is the non-transformed control.
Detailed Description of the Invention
The invention relates to transgenic plants that produce seeds useful as the
20 raw material for a phosphorus or carbohydrate-rich feed. The superior
quality of the
seeds and the resulting feed is due to the insertion of a transgene containing
a
promoter active in a plant seed tissue and that highly expresses an enzyme in
a seed
tissue of a transgenic plant. Of course, the enzyme may be expressed elsewhere
in a
transgenic seed or plant without affecting the advantages that the invention
offers.
Seeds produced from such a plant can have reduced phytate and increased
dietary
phosphorus content, or reduced complex carbohydrates and increased simple
carbohydrates, compared to a control plant that does not contain the
transgene.
Phytate (myo-inositol hexakisphosphate) is the main storage form of
phosphorus in many seeds. Phytin is the insoluble mixed potassium, magnesium,
and calcium salt of myo-inositol hexaphosphoric acid (phytic acid). In
cereals, oil
4
CA 02309342 2000-06-14
seeds, and legumes, phytate accumulates in seeds during maturation and
accounts
for 50 to 80% of total phosphorus content of the seed. Soybean and corn meal
are
major components of animal feed. They contain adequate phosphorus levels to
meet
animal growth requirements, so long as phosphorus from the phytate is
digestible
5 (i.e., absorbable) by the animal. Ruminants (multigastric animals) readily
cleave
phytate to release digestible phosphorus. However, monogastric animals (e.g.,
pigs,
poultry, and fish) utilize phytate extremely poorly because they are deficient
in
gastrointestinal (GI) tract enzymes capable of hydrolyzing phytate. This
deficiency
necessitates supplementation of animal feed with phosphorus to meet dietary
10 requirements. Further, phytate chelates important cations (including iron,
magnesium, manganese, zinc, calcium, copper, and molybdenum) and forms
phytate-cation-protein complexes, thereby lowering the bioavailability of
minerals
and amino acids in the feed. Thus, the invention provides a means to release
phosphorus by degrading phytate in raw materials used for animal feed.
15 Phytase produced in a transgenic plant or seed of the invention can also be
used in a process for steeping corn or sorghum kernels. The plant tissue or
seed can
be ground before adding to steeping corn. Phytase liberated from the plant
tissue or
seed can act on phytin, which is present in many corn preparations. The
transgenic
cereal grains, if they are corn or sorghum grains, can produce phytase to
degrade
20 phytin in situ. Degradation of phytin in corn steeping increases the
nutrient content
and consequently the commercial value of corn steep liquor, which is used as
animal
feed or as a nutrient in microbial fermentation. Furthermore, the degradation
of
phytin can prevent problems relating to the accumulation of phytin deposits in
filters, pipes, reactor vessels, etc. during concentration, transport and
storage of corn
25 steep liquor. The action of phytase can also accelerate the steeping and
separation
processes involved in corn wet milling. _
In addition, the use of transgenic seeds containing starch-hydrolyzing
enzymes (e.g., amylopullulanase) is compatible and desirable with currently
used
processes in food and wine industries for production of sugars using cereal
grain
30 starch. Rice provides the main source of food for more than 50% of the
world
CA 02309342 2000-06-14
population and is the most important and widely grown crop on earth. Due to
the
large biomass, rice seeds are an ideal sources of soluble sugars for the food
and wine
industries. Amylopullulanase (APU) from Thermoanaerobacter ethanolicus 39E,
harboring both pullulanase and a-amylase activities, is capable of hydrolyzing
both
5 a-1,4 and a-1,6 bonds of polysaccharides and is heat stable with a catalytic
optimum of 90°C. Generally, rice contains 6-10% protein and 70-80%
starch by
weight. APU can hydrolyze the starch to produce soluble sugars and at the same
time increase the relative composition of insoluble rice flour. High protein
rice flour
has high nutritional value and is useful for the production of pudding, gruel,
instant
10 milk, baby food, etc. As shown below, a 2.9-kb DNA fragment of the APU gene
that encodes amino acids 75 to 1029 of the mature APU (total of 1481 amino
acids)
of T. ethanolica~s was isolated and characterized. This truncated APU
maintains
both a-amylase and pullulanase activities. The use of transgenic rice seeds
containing the dual active APU in the production of sugars from seed starch
15 simplifies the production process and significantly reduces production cost
for rice
flour.
The genetic elements necessary for constructing the transgene to be inserted
into the plant is readily available. For example, a-amylase promoters are
described
in U.S. Patent No. 5,460,952 and in the Example below. Other suitable
promoters
20 are described in McElroy et al., Plant Mol. Biol. 15:257-268, 1990;
Brusslan et al.,
Proc. Natl. Acad. Sci. USA 89:7791-7795, 1992; Wissenbach et al., Plant J.
4:411-
422, 1993; Reynolds et al., Plant Mol. Biol. 29:885-896, 1995; Kaukinen et
al., Plant
Mol. Biol. 30:1115-1128, 1996; Beaudoin et al., Plant Mol. Biol. 33:835-846,
1997;
Moore et al., Proc. Natl. Acad. Sci. USA 94:762-767, 1997; Odell et al.,
Nature
25 313:810-812, 1985; and U.S. Patent Application entitled "Plant Seedling and
Embryo Promoter," filed May 22, 2000, naming-inventors Su-May Yu and Yu-Chan
Chao. Other genetic elements, such as cis-regulatory sequences are also
available to
the skilled artisan. See, e.g., U.S. Patent No. 5,969,127.
The expression constructs containing the transgene can be inserted into a
30 vector, such as a plasmid, which is then introduced into plants using
Agrobacterium
6
CA 02309342 2000-06-14
tumefaciens-mediated DNA delivery or particle bombardment, which are standard
methods known in the art.
The DNA sequence encoding an enzyme may be obtained from a variety of
sources such as microbial, plant, or animal sources. For example, a DNA
sequence
encoding a phytase can be cloned from a ruminal bacterium by, e.g., screening
of
bacterial cDNA libraries or PCR amplification of nucleic acids using
heterologous
or degenerate probes.
The cloned genes described herein may be used as starting materials for the
construction of "second generation" enzymes whose amino acid sequence has been
altered by mutagenesis (e.g., site-directed mutagenesis), which have
beneficial
properties that differ from those of wild-type or recombinant enzymes. For
example, the temperature or pH optimum, specific activity, or substrate
affinity can
be altered so as to be better suited for application in a defined process.
Several techniques are available for the introduction of the expression
15 construct containing the enzyme-encoding DNA sequence into plants or cells
from
which transgenic plants can be produced. Such techniques include
electroporation,
microinjection, the ultrasonic method, polyethylene glycol-mediated protoplast
transformation, the poly-L ornithine method, the calcium phosphate method, and
particle bombardment.
In addition to these so-called direct DNA transformation methods,
transformation systems involving vectors are widely available, such as viral
vectors
(e.g., from the cauliflower mosaic virus, CaMV) and bacterial vectors (e.g.,
from the
genus Agrobacterium). After selection and/or screening, the protoplasts,
cells, or
plant parts (e.g., explants) that have been transformed can be regenerated
into whole
plants, using methods known in the art.
7
CA 02309342 2000-06-14
For transformation and regeneration of cereal crops, an embodiment of the
present invention uses the principle of the binary vector system (Hoekema et
al.,
Nature 303:179-180. 1983; and European Patent Application No. 0120516) in
which
Agrobacterium strains containing a vir plasmid with the virulence genes and a
5 compatible plasmid with the gene construct to be transferred. The binary
vectors as
used in this example contain, between the left- and right-border sequences of
the T-
DNA, an hph gene coding for hygromycin resistance and a multiple cloning site
for
insertion of gene constructs.
The promoter used for directing the expression of the phytase can be an
10 a-amylase gene promoter. The a-amylase gene promoters are active in
germinating
seeds (Huttly et al., EMBO J. 8:1907-1913, 1989; Skriver et al., Proc. Natl.
Acad.
Sci. USA 88:7266-7270, 1991; Lanahan et al. Plant Cell 4:203-21 l, 1992;
Tanida et
al., Mol. Gen. Genet. 244:127-134, 1994; Itoh et al., Plant Physiol. 107:25-
31,
1995), sucrose-starved suspension cells (Chan et al., J. Biol. Chem. 269:17635-
15 17641, 1994), and other tissues and organs (Chan et al., Plant Mol. Biol.
22:491-
506, 1993) of cereal crops.
The transgene can further includes a signal peptide sequence derived from
the a-amylase genes or another gene that is capable of directing the
recombinant
proteins toward the endoplasmic reticulum, vacuole, protein body, or
extracellular
20 space. Other regulatory sequences, such as terminator sequences and
polyadenylation signals that function in plants, can be included in the
transgene. An
example of one such sequence is the 3' untranslated region of the nopaline
synthase
(Nos) gene ofAgrobacterium tumefaciens or the 3' untranslated region of a rice
a-
amylase gene (U.S. Patent No. 5,969,127).
25 Once a transgenic plant of the invention is produced, expression in tissues
other than a seed tissue can be beneficial. For example; the transgenic plant
can be
used as a producer of a recombinant phytase, whether the phytase is isolated
from a
leaf, sheath, stem, or root of the transgenic plant.
Phytase activity can be measured using any method known in the art,
30 including ELISA, Western blotting, and direct enzyme assays using
colorimetric
8
CA 02309342 2000-06-14
techniques or native gel assays. See e.g., Shimizu, Biosci. Biotech. Biochem.
56:1266-1269, 1992; Yanke et al., Microbiology 144:1565-1573, 1998; and Kim et
al., Enzyme Microb. Technol. 22:2-7, 1998.
The transgenic plants, plant organs, or malted seeds can be used directly,
i.e.,
without further processing, or can first be processed via conventional means
such as
grinding to a consistency suitable for a particular use. Alternatively, the
phytase can
be extracted from the plant, plant organ, or malted seeds and, if desired,
purified
before use using conventional extraction methods and purification techniques.
See,
e.g.; Laboure et al., Biochem. J. 295:413-419, 1993; and Golovan et al., Can.
J.
Microbiol. 46:59-71, 2000.
Without further elaboration, it is believed that one skilled in the art can,
based on the above disclosure, and the production of transgenic plants and
seeds as
described below, utilize the present invention to its fullest extent. The
following
examples are to be construed as merely illustrative of how one skilled in the
art can
isolate and use the transgenic plants of the invention, and are not limitative
of the
remainder of the disclosure in any way. Any publications, patents, or patent
applications cited in this disclosure are hereby incorporated by reference.
ny a r~rnr ~ ~
The methods, materials, and procedures used in the present example are first
described.
Methods and Materials
Plant Material. The rice variety used in this example was Oryza sativa L.
cv. Tainung 67. Immature seeds were dehulled, sterilized with 2.4% NaOCI for 1
hour, washed extensively with sterile water, and placed on N6D agar medium for
callus induction as described in Toki, Plant Mol. biol. Rep. 15:16-21, 1997.
After
one month, calli derived from scutellae were subcultured in fresh N6D medium
for
transformation or in a liquid MS medium containing 3% sucrose and 10 pM 2,4-D
to establish a suspension cell culture as previously described in Yu et al.,
J. Biol.
Chem. 266:21131-21137, 1991.
9
CA 02309342 2000-06-14
Plasmids. Plasmid aAmyB-C carries a 1.4-kb rice a-amylase cDNA insert
in pBluescript KS+ (Stratagene) (Yu et al., Gene 122:247-253, 1992). Plasmid
pRYl8 carries a 3.8 kb DNA fragment that contains a rice genomic rDNA cluster,
including the 3' halfportion of 18S rRNA gene, the complete 5.85 rRNA gene,
and
5 the 5' half portion of the 25S rRNA gene in pUCl3 (Sano et al., Genome
33:209-
218, 1990). Plasmid RAMYG6a contains the 3' half portion and 3' flanking
region
of aAmy8 genomic DNA and was generated from screening a rice genomic DNA
library (Clontech) using aAmyB-C as a probe. The selected clone was
subsequently
subcloned from a positive EMBL-3 phage clone into pBluescript (Stratagene).
10 Plasmid RAMYG 17 contains the 5' flanking region, the entire coding region,
and
the 3' flanking region of aAmy7 genomic DNA and was generated from screening a
rice genomic DNA library (Clontech) using aAmyB-C as a probe. Further details
regarding RAMYG6a and RAMYG17 can be found in Ho et al., Master's Thesis,
Department of Biology, National Taiwan Normal University, 1991. The selected
15 clone was subsequently subcloned from a positive EMBL-3 phage clone into
pBluescript.
Plasmid Construction. The 5' end of the S. ruminantium phytase gene
(SrPf~ was modified by PCR using the plasmid pSrPf6 (U.S. Patent No.
5,939,303)
as a DNA template and the primers 5'-TTAAGCGATATCGCCAA
20 GGCCCCGGAACAGA-3' (SEQ ID NO:1; EcoRV site underlined) and
5'-ACGCAGGATCCACCTCATAAAACC-3' (SEQ ID N0:2; BamHI site
underlined) to yield a recombinant gene in which an EcoRV site was
incorporated
immediately 5' to the codon (bolded in SEQ ID NO:1 ) specifying the first
amino
acid of the mature, processed phytase enzyme and a BamHI site at the end of
the
25 phytase gene. The EcoRV site allows for the fusion of the phytase gene to
the signal
peptide sequence of the a-amylase gene aAmyB.~ The PCR product was subcloned
into the EcoRV and BamHI sites of pBluescript (Stratagene) to generated
pBS/SrPf6.
The E. coli phosphatase gene appA was similarly modified by PCR at the 5'
30 and 3' ends using plasmid pET-appA (Golovan et al., Can. J. Microbiol.
46:59-71,
CA 02309342 2000-06-14
2000) as a DNA template and the oligonucleotides 5'-
ACGGCGGATATCCAGAGTGAGCCGG AGCTGA-3' (SEQ ID N0:3; EcoRV site
underlined) and S'-AGGTTGGATCCTTACA AACTGCACGAAGG GT-3' (SEQ
ID N0:4; BamHI site underlined). The resulting PCR product was subcloned into
pBluescript to generate pBS/appA.
A 1.2 kb promoter and signal peptide region of aAmy8 was excised with SaII
and Hi~zdIII from pAG8 (Chan et al., Plant Mol. Biol. 22:491-506, 1993) and
subcloned into pBluescript to generated pBS/BSP. The aAnzy8 3'untranslated
region
(3'UTR) was PCR-amplified using RAi~IYG6a as the DNA template and the
10 oligonucleotides 5'-CG GGATCCTAGCTTTAGCTATAGCGAT-3' (SEQ ID NO:S;
BamHI site underlined) and 5'-TCCCCGCGGGTCCTCTAAGTGAACCGT-3'
(SEQ ID N0:6; SacII underlined). The nopaline synthase gene (Nos) 3'UTRs was
PCR-amplified using pBI221 (Clontech) as the DNA template and the
oligonucleotides 5'-TCCGGATCCCAGATCGTTCAAAC ATTT-3' (SEQ ID N0:7;
15 BamHI site underlined) and 5'-AGCCCGCGGGATCGATCTA GTAACAT-3' (SEQ
ID N0:8; SacII underlined).
The aAmy8 and Nos 3'UTRs were subcloned into the BamHI and SacII sites
in pBS/8SP to generate pBS/8SP8U and pBS/8SPNos, respectively. The SrPf6 was
excised with EcoRV and BamHI from pBS/SrPf6 and subcloned into the same sites
20 in pBS/8SP8U and pBS/8SPNos to generate pBS/8F8U and pBS/BFN, respectively.
The appA gene was excised with EcoRV and BamHI from pBS/appA and subcloned
into the same sites in pBS/8SP8U and pBS/BSPNos to generate pBS/8A8U, and
pBS/BAN. The correct in-frame fusion of the aAnry8 signal peptide sequence
with
the SrPf6 or appA coding region, and the junction regions which link the SrPf6
or
25 appA coding region with the aAmy8 3'UTR or Nos 3'UTR were all verified by
DNA sequencing.
The 1.7-kb promoter and signal peptide region of aAmy7 was PCR-amplified
using RAMYGI7a as a DNA template and the oligonucleotides 5'-
ACCGGGTCGAC GTATACATGTCACCTACA-3' (SEQ ID N0:9; SaII site
30 underlined) and 5'-GGTGATATCCAGGACTT GCCCGGCTGT-3' (SEQ ID
11
CA 02309342 2000-06-14
NO:10; EcoRV site underlined) to yield a PCR product having a SaII site at the
5'
end of the aAnZy7 promoter and an EcoRV site immediately 3' to the signal
peptide
sequence. The PCR product was subcloned into the SaII and EcoRV sites in
pBluescript to generate pBS/7SP. The aAmy7 3'UTR was PCR-amplified using
RAMYG17 as the DNA template and the oligonucleotides 5'-
GCTCTAGAAATCTGAGCGCACGATG-3' (SEQ ID NO:11; XbaI site underlined)
5'-TCCCCGCGGTAAGCATTAAGCAGTGCA-3'(SEQ ID N0:12; SacII site
underlined. The PCR product was subcloned into the XbaI and SacII sites in
pBS/7SP to generate pBS/7SP7U. The Nos 3'UTR was excised withXbaI and SacII
10 from pBS/BSPN and subcloned into the same sites in pBS/7SP to generate
pBS/7SPNos. The SrPfG gene was excised with EcoRV and BamHI from pBS/SrPf6
and subcloned into the same sites in pBS/7SP7U and pBS/7SPNos to generate
pBS/7F7U and , pBS/7FN, respectively. The appA gene was excised with EcoRV
and BamHI from pBS/appA and subcloned into the same sites in pBS/7SP7U and
15 pBS/7SPNos to generate pBS/7A7U and pBS/7AN, respectively. The correct in-
frame fusion of the aAmy7 signal peptide sequence with the SrPf6 or appA
coding
region, and the junction regions which link the SrPf6 or appA coding region
with the
aAmy7 3'UTR or Nos 3'UTR are all verified by DNA sequencing.
The cauliflower mosaic virus 35S RNA (CaMV35S) promoter-hygromycin
20 B phosphotransferase (hph) coding sequence-tumor morphology large gene
3'UTR
(tml) fusion gene was excised with EcoRI from pTRA 1 S 1 (Zheng et al., Plant
Physiol. 97:832-835, 1991) and subcloned into the EcoRI site of the binary
vector
pPZP200 (Hajdukiewicz et al., Plant Mol. Biol. 25:989-994, 1994) to generate
pPZP/HPH. pPZP/HPH was linearized with SaII and blunted-ended to served as a
25 vector for cloning. pBS/8F8U, pBS/BFN, pBS/8A8U, pBS/8AN, pBS/7F7U,
pBS/7FN, pBS/7A7U, and pBS/7AN were lineanzed with PvuII to serve as inserts
for cloning. The inserts were ligated with the vector to generate pPZP/8F8U,
pPZP/8FN, pPZP/8A8U, pPZP/BAN, pPZP/7F7U, pPZP/7FN, pPZP/7A7U, and
pPZP/7AN, respectively.
12
CA 02309342 2000-06-14
Rice Transformation. Plasmids were introduced into Agrobacteriz~m
tumefaciens strain EHA101 (Hood et al., J. Bacteriol. 168:1291-1301, 1986)
with an
electroporator (BTX) following the manufacturer's instruction. Calli induced
from
immature rice seeds were co-cultured with Agrobacterium according to the
methods
described by Hiei et al., Plant J. 6:271-282, 1994 and Toki et al., supra.
Northern Blot Analysis. Total RNA was isolated from endosperms of
germinating seeds as described in Yu et al., Plant Mol. Biol. 30:1277-1289,
1996 and
isolated from cultured suspension cells using a TRIZOL reagent (GIBCO BRL).
RNA gel blot analysis was performed as described in Thomas, Methods Enzymol.
100:255-266, 1983. Briefly, 10 ~g of total RNA was electrophoresed on a 1%
agarose gel containing 10 mM sodium phosphate buffer (pH 6.5), transferred to
a
nylon filter, and hybridized with 32P random primer labeled SrPf6 DNA, appA
DNA,
czAmyB gene-specific DNA, or rDNA probe. The aAmy8 gene-specific DNA was
prepared as described in Sheu et al., J. Biol: Chem. 271:26998-27004, 1996.
The
blot was visualized using autoradiography and quantified using software
accompanying the of a PhosphoImager (Molecular Dynamics).
Western Blot Analysis. Total proteins were extracted from endosperms of
germinating seeds using an extraction buffer (SO mM Tris-HCl [pH 8.8], 1 mM
EDTA, 10% glycerol, 1% Triton X-100, 10 mM (3-mercaptoethanol, and 0.1%
sarkosyl). Western blot analysis was performed as described by Yu et al., J.
Biol.
Chem. 266:21131-21137, 1991.
Phytase Activity Assay. Protein extract was prepared from the germinating
seeds as described by Li et al., Plant Physiol. 114:1103-1111, 1997. The
phytase
activity was determined as described in Shimizu, Biosci. Biotech. Biochem.
56:1266-1269, 1992.
Results
The aAmy8 promoter confers sucrose starvation-enhanced accumulation of
appA and SrPf6 mRNAs in transformed rice suspension cells. To determine
whether
the crAmy8 promoter controls sugar-dependent expression of appA in rice, a 1.2-
kb
DNA fragment containing the 5' regulatory and signal peptide sequences of
aAmyB
13
CA 02309342 2000-06-14
was fused in-frame at the S' end of the appA or SrPf6 gene. The chimeric gene
was
inserted into a binary vector and introduced into Agrobacterium for rice
transformation. Many transformed cell lines were obtained and eight lines were
selected for further study. The transformed calli were cultured as suspension
cells,
and the suspension cells were then cultured in medium with or without sucrose.
Total RNA was purified and subjected to gel blot analysis using appA DNA,
SrPf6
DNA, crAmy8 gene-specific DNA, or rDNA as a probe. Accumulation of appA,
SrPf6, and aAmy8 mRNAs were detectable in cells starved of sucrose but not in
cells provided with sucrose. No appA or SrPf6 mRNA was detected in the non-
transformed (NT) cells.
The aAmy8 promoter confers sucrose starvation-enhanced activity of
phytase in transformed rice suspension cells. To determine whether the
transformed
rice suspension cells carrying an aAmyB-appA or aAmyB-SrPf6 chimeric gene
synthesize and secrete phytase into the culture medium, the phytase activity
in
15 suspension cells and in culture medium were determined. The phytase
activity was
detectable in the transformed cells and in the culture medium. In both cases,
phytase
activity was significantly enhanced by sucrose starvation. The phytase
activity was
also detectable in the non-transformed cells under sucrose starvation, which
was
probably caused by the endogenous phytase gene.
The aAmy7 and aAmy8 promoters control expression of SrPf6 in
germinating transgenic rice seed. Transgenic rice plants were regenerated from
the
transformed rice calli carrying aAmy7-SrPf6 or crAmyB-SrPf6. The T1 seeds of
some transgenic lines were randomly selected for further study. To determine
the
role of the aAmy7 and aAmyB promoters in the expression of appA and SrPf6
genes
during seed germination, the transgenic rice seeds were germinated for 5 days.
Total
RNA was purified from the entire germinated seed (including the shoots, roots,
and
endosperms) and subjected to gel-blot analysis using SrPf6 DNA, crAmyB gene-
specific DNA, or rDNA as a probe. Under the control of czAmy7 promoter,
expression of SrPf6 could be detected in some of the germinated seeds. Under
the
14
CA 02309342 2000-06-14
control of aAmyB promoter, expression of SrPf6 could be detected in all the
germinated seeds. No SrPfG mRNA was detected in non-transformants.
The aAmy8 promoters control expression of appA ira germinating transgenic
rice seed. Transgenic rice plants were regenerated from the rice calli
carrying
5 aAmyB-appA. The T1 seeds of some transgenic lines were randomly selected for
further study. Seeds were germinated for 5 days and total RNA was purified
from
the entire germinated seeds (including the shoots, roots, and endosperms) and
subjected to gel-blot analysis using appA DNA, aAmy8 gene-specific DNA, or
rDNA as a probe. Under the control of the aAmy8 promoter, expression of appA
could be detected in all germinated seeds. No appA mRNA was detected in the
non-
transformants.
The aAnZy8 promoter confers high phytase activity in germinating transgenic
rice seeds. To determine the expression level of phytase in germinated
transgenic
seeds, phytase activity in the T1 transgenic seeds were analyzed. The
transgenic rice
15 seeds were germinated for 5 days. Cell extract was prepared from the entire
germinated seeds (including the shoots, roots, and endosperms) and subjected
to
phytase activity analysis. Phytase activity was higher in all the germinated
transgenic seeds carrying the aAmyB-SrPf6-Nos chimeric gene than in the non-
transformants. One of the transgenic lines, 8FN-8, had significantly higher
phytase
activity as compared with other transgenic lines. Phytase activity was higher
in all
the germinated transgenic seeds carrying the aAmyB-appA chimeric gene than in
the
non-transformant. One transgenic line, 8AN-14 had significantly higher phytase
activity as compared with other transgenic lines including 8FN-8.
The introduced phytase and acid phosphatase genes are inherited to the
progenies of transgenic rice. To determine whether the introduced appA and
SrPf6
DNAs were inherited to the progenies of Tl transgenic rice, the T2 seeds of
transgenic lines 8AN-14 and 8FN-8 were germinated for 5 days. Total RNA was
purified from the entire germinated seeds and subjected to gel blot analysis
using
appA DNA, SrPf6 DNA, or rDNA as a probe. Expression of appA and SrPf6 was
30 detected in most of the germinated T2 seeds.
CA 02309342 2001-11-15
The aAmy8 promoter confers temporal expression of phytase in germinating
transgenic rice seed. To determine the expression pattern of phytase in
germinated
transgenic rice seed carrying aAmyB-appA or aAmyB-SrPf6, the T2 seeds of
transgenic lines 8FN-8-8 and 8AN-14-10 were germinated for various lengths of
time, and phytase activity was determined. The phytase activity in the
transgenic
seeds increased as germination proceeded and reached a peak at days 5-6 while
declining afterwards. Phytase activity was also detectable in the non-
transformed
seeds, but remained at low level throughout the entire germination period.
The molecular weight of phytase produced in germinating transgenic rice
seeds is similar to that produced in Pichia pastoris. SrPf6 was cloned into a
plasmid
under the control of the glyceraldehyde-3-phosphate dehydrogenase (GAP)
promoter and overexpressed in Pichia pastoris. The SrPf6 cDNA was PCR-
amplified using pSrPf6 as the DNA template and oligonucleotides 5' -
CGGAATTCGCCAAGGCGCCGGAGC AGAC-3' (SEQ ID N0:25) (EcoRI site
underlined) as S' primer and 5' -GCTCTAGATACGCCTTC GCCGGATGGCT-3'
(SEQ )D N0:26) (Xbal site underlined) as 3' primer. The DNA fragment
containing
SrPf6 cDNA was digested with EcoRI and Xbal and ligated into the same sites in
pGAPZaA (Invitrogen) to generate pGAP-SrPf6. SrPf6 was led by a signal peptide
a-factor and was under the control of GAP promoter. pGAP-SrPf6 was linearized
by
restriction enzyme AvrII and transferred into P. pastoris host strain SMD
1168H by
electroporation. The transformed cells were plated on YPDS (1% yeast extract,
2%
peptone, 2% dextrose, 1 M sorbitol, pH 7.5) plus zeocin (100 mg/ml) at
30°C for 3
days. Production and purification of phytase were performed according to the
manufacturer's instruction provided with pGAPZaA.
To compare the molecular weight of phytase produced in germinating
transgenic rice seeds with that of the phytase overexpressed in Pichia, seeds
of
transgenic rice line 8FN-8 were germinated for 5 days. Total proteins were
extracted from the entire germinated seeds and subjected to Western blot
analysis
using phytase polyclonal antibodies. The full-length cDNA of SrPf6 was PCR-
amplified using plasmid pSrPf6 as DNA template and oligonucleotides 5'-
16
CA 02309342 2001-11-15
CCCGAATTCATGAAATACTGGCAG-3' (SEQ ID N0:27) (EcoRI site underlined)
as 5' -primer and 5' -CCCGAGCTCTTACGCCTTCGCCGG-3' (SEQ ID N0:28)
(SacI site underlined) as 3' primer. The amplified DNA fragment was digested
with
EcoRI and SacI and ligated into the same sites in pET20b(+) (Novagen) to
generate
pET-SrPf6. pET-SrPf6 was transferred to E. coli strain BL21 (DE3) and
expressed.
Purification of phytase was performed according to the instruction provided by
Novagen. One hundred micrograms of purified phytase was injected into a New
Zealand White rabbit successively at 4-6 week interval according to the
methods
generally described in Williams et al., "Expression of foreign proteins in E.
coli using
plasmid vectors and purification of specific polyclonal antibodies," In: DNA
Cloning
2. Expression Systems: A Practical Approach, Glover et al., eds., IRL Press,
New
York, 1995.
The molecular weight of the full-length phytase produced by germinating
transgenic seeds was similar to that produced by Pichia. It was known that the
phytase encoded by SrPf6 had a molecular weight of 36.5 kD when expressed in
E.
coli (U.S. Patent No. 5,939,303). The molecular weight of phytase expressed in
Pichia was about 36.5 kD and 38 kD. Similarly, when the phytase was expressed
in
transgenic rice seeds the molecular weight was about 36.5 kD and 40 kD. It was
known that there is one potential glycosylation site in the primary structure
of SrPf6
(U.S. Patent No. 5,939,303). The 38 kD and 40 kD phytases expressed in Pichia
and transgenic rice seeds, respectively, were probably caused by differential
glycosylation. One protein with molecular weight of 34 kD was also present in
the
germinating rice seeds, which could have been a degradation product of the
phytase.
These results indicate that the mature phytase produced in the transgenic
plants and
seeds described herein were properly post-translationally modified.
Transgenic germinated rice seedlseedling contained phytase with high
specific activity and broad pHprofiles for high activity. Unexpectedly, it was
also
discovered that the phytase specific activity expressed in the germinated
transgenic
seeds is two fold higher than that expressed in E. coli. The reason for this
surprising
result could be that there are many endogenous hydrolytic enzymes
simultaneously
expressed in the germinated seeds. These hydrolytic enzymes may have a
17
CA 02309342 2000-06-14
synergistic effect on the phytase activity present in germinating seeds.
Alternatively,
or in conjunction, the post-translationally modified phytase in the
germinating seeds
may have increased the enzyme's specific activity, which resulted in the
observed
increase in phytase activity. Thus, besides the advantages of expressing
phytase in
transgenic malted seeds, the specific activity of the recombinantly produced
enzyme
was also increased.
The ruminal bacterial phytase has a pH optimum of 4.0-5.5 (U.S. Patent
No. 5,939,303). To determine the optimal pH of phytase expressed in the rice
seeds
described above, T2 seeds of three transgenic rice lines were germinated for 5
days,
10 and the optimal pH for phytase expressed in rice germinated seed/seedling
was
determined. The results showed that the activity of phytase expressed in all
three
transgenic rice lines was optimal at pH 3 and pH 4.5-5.0 (Fig. 1). In the
digestive
tract of monogastric animals, the pH ranges from 2-3 in the stomach and 4-7 in
the
small intestine. The broader and more acidic optimal pH profile of phytase
expressed in rice allows the enzyme to function well in the stomach and small
intestine of animals and is therefore an advantage for its use as a feed
additive. The
reason for this surprising result could be that the phytase expressed in rice
was post-
translationally modified, which resulted in the increase in activity and/or
stability
over a broad range of pH.
20 The E. coli acid phosphatase has a pH optimum of 2.5 (Dassa et al., J.
Biol.
Chem. 257:6669-6676, 1982; and Dassa et al., J. Bacteriol. 172:5497-5500,
1990).
To determine the optimal pH of acid phosphatase expressed in rice, T2 seeds of
transgenic rice line 8AN-14-6 were germinated for 5 days, and the optimal pH
for
phytase expressed in germinated rice seed/seedling was determined. The results
25 show that activity of acid phosphatase expressed in rice was optimal over a
pH range
of 3-5.5 (Fig, 2) . As with the ruminal bacterial phytase expressed in rice,
the
broader and more acid optimal pH profile of E. coli acid phosphatase would
allow
the enzyme to function well in the stomach and small intestine of animals and
is
therefore an advantage for its use as a feed additive. The reason for this
surprising
30 result also could be that the acid phosphatase expressed in rice was post-
18
CA 02309342 2000-06-14
translationally modified, which resulted in increase in activity and/or
stability over a
broad range of pH.
~~r a r~rpr ~ ~
The methods, materials, and procedures used in the present example are first
described.
Materials and Methods
Plant Material. All pant materials were prepared as described in Example 1.
Preparation of genomic DNA. Rice seeds were germinated and grown in the
dark for 1 week. Bacteria T. ethanolicus 39E was obtained from the American
Type
Culture Collection (ATCC 53033). The bacterial and rice genomic DNA was
purified according to the method described in Sheu et al., J. Biol. Chem
271:26998-
27004, 1996.
PCR. The 1351-by glutelin gene promoter region (Fragment I; Zheng et al.,
Plant J. 4:357-366, 1993; and Wu et al., Plant Cell Physiol. 39:885-889, 1998)
was
PCR-amplified using rice genomic DNA as template and primers B1-5 (5'-
GGGGAATTCGA TCTCGATTTTTGAGGAAT-3' [SEQ ID N0:13], EcoRI site
underlined) and B 1-3 (5'-GGGGGATCCCATAGCTATTTGTACTTGCT-3' [SEQ ID
N0:14], BamHI site underlined). The glutelin gene promoter plus 75-by putative
signal peptide sequence (Fragment II) was PCR-amplified using rice genomic DNA
as template and primers B1-5 and B1-sp
(5'GGGGGATCCGGGATTAAATAGCTGGGCCA-3' [SEQ ID N0:15], BamHI
site underlined). The glutelin gene promoter plus putative signal peptide and
propeptide sequences (Fragment III) was PCR-amplified using rice genomic DNA
as template and primers B1-5 and B1-pro
(5'GGGGGATCCCCTCACTTTCCGAAG TGGTT-3' [SEQ ID N0:16], BamHI
site underlined).
The truncated apu ORF encoding only amino acid 75 to 1029 was PCR-
amplified using the genomic DNA of T. ethanolicus 39E as template and
oligonucleotides 5'-CGG GATCCTTAAGCTTGCATCTTGA-3' (SEQ ID N0:17;
19
CA 02309342 2000-06-14
BamHI site underlined) as forward primer and 5'-
CCGGCGGCCGCCTACATATTTTCCCCTTGGCCA-3' (SEQ ID N0:18; NotI site
underlined) as reverse primer.
Plasmid construction. The PCR-amplified Fragments I, II, and III were
5 digested with EcoRI and BamHI and subcloned into the same sites in
pBluescript
(Stratagene) to generate pBS-G, pBS-Gp, and pBS-Gpp, respectively. The
truncated
apu was digested with BamHI and NotI and fused downstream of the GIuB-1
promoter, GIuB-1 promoter-signal peptide sequence, and GIuB-1 promoter-signal
peptide-propeptide sequence in pBS-G, pBS-Gp, and pBS-Gpp, respectively, to
10 make translational fusions and to generate plasmids pBS-G-apu, pBS-Gp-apu,
and
pBS-Gpp-apu, respectively. The nopaline synthase gene (Nos) 3' untranslated
(UTR) was PCR-amplified using pBI221 (Clontech) as DNA template and
oligonucleotide 5'-TCCGAGCTCCAGATCGTTC AAACATTT-3' (SEQ ID
N0:19; SacI site underlined) as forward primer and oligonucleotide 5'-
15 AGCGAGCTCGATCGATCTAGTAACAT-3' (SEQ ID N0:20; SacI underlined) site
as reverse primer. The Nos 3'UTR was digested with SacI and fused downstream
of
apai in pBS-G-apu, pBS-Gp-apu, and pBS-Gpp-apu to generate pBS-G-apu-Nos,
pBS-Gp-apu-Nos, and pBS-Gpp-apu-Nos, respectively.
The 1.2 kb promoter and signal peptide sequence of crAmy8 was excised with
20 SalI and HindIII from pAG8 (Chan et al., Plant Mol. Biol. 22:491-506, 1993)
and
subcloned into pBluescript to generate pBS/8sp. The aAmy8 3'UTRs was PCR-
amplified using RAMYG6a as a DNA template and oligonucleotide 5'-
CGCCGCGGTAGCTTTA GCTATAGCGAT-3' (SEQ ID N0:21; SacII site
underlined) as forward primer and oligonucleotide 5'-
25 TCCCCGCGGGTCCTCTAAGTGAACCGT-3' (SEQ ID N0:22; SacII site
underlined) as reverse primer. Plasmid RAMYG6a contains the 3' half portion of
the coding sequence and 3' flanking region of aAmy8 genomic DNA and was
generated by screening of a rice genomic DNA library (Clontech) using crAmyB-C
(Yu et al., Gene 122:247-253, 1992) as a probe. The aAmyB 3'UTRs was subcloned
30 into the SacII sites in pBS/8sp to generate pBS/8sp8U. The truncated apu
was cut
CA 02309342 2000-06-14
with BamHI and NotI and subcloned into the same sites in pBS-8sp8U to generate
pBS-aAmyB-sp-apu-8U.
The 1.1-kb promoter and signal peptide sequence of aAmy3 was excised
with SaII and HindIII from p3G-132II (Lu et al., J. Biol. Chem. 273:10120-
10131,
1998) and subcloned into pBluescript to generate pBS-asp. The aAmy3 3'UTR was
excised with HindIII and SacI from pMTC37 (Chan et al., Plant J. 15:685-696,
1998) and subcloned into the same sites in pBS-asp to generate pBS-3sp3U. The
truncated apu was digested with BamHI and NotI and subcloned into the same
sites
in pBS-3sp3U to generate pBS-aAmy3-sp-apu-3U.
10 The correct in-frame fusion of the GIuB, aAnry3, and aAmy8 signal peptide
or propeptide sequence with the apu coding region, the junction regions which
link
the apu coding region with the aAmy3, aAnzyB, or Nos 3'UTR were all verified
by
DNA sequencing. The GIuB-apu-Nos, GIuB-sp-apu-Nos, GIuB-spp-apu-Nos,
aAmy3-sp-apu-aAmy3 3'UTR, and aAmyB-sp-apu-aAmy8 3'UTR chimeric genes
were excised from pBS-G-apu-Nos, pBS-Gp-apu-Nos, pBS-Gpp-apu-Nos, pBS-
aAmy3-sp-apu-3U, and pBS-aAmyB-sp-apu-8U with SaII, blunt-ended, and
inserted into the HindIII-digested and blunt-ended binary vector pSMYIH (Ho et
al.,
Plant Physiol. 122:57-66, 2000) to generate pSMYI/Gapu, pSMYl/Gpapu,
pSMYI/Gppapu, and pSMYI/3apu and pSMYI/8apu, respectively.
Transformation. Transformations were carried out as described in Example
1.
Expression of APU in E. coli and preparation of antibodies. The truncated
apu encoding amino acids 75 to 1029 was PCR-amplified using genomic DNA of T.
ethanolicz~s 39E as template and oligonucleotides 5'-CGCATATGTTAAGCTTGC
25 ATCTTGATTC-3' (SEQ ID N0:23; NdeI site underlined) as forward primer and 5'-
CCGCTCGAGCTACATATTTTCCCCTTGGCCA-3' (SEQ ID N0:24; XhoI site
underlined) as reverse primer. The amplified DNA fragment was digested with
NdeI
and XhoI and ligated into the same sites in pET20b(+) (Novagen) to generate
pET-
APU. pET-APU was transferred to E. coli strain BL21 (DE3), and APU was
30 expressed. Purification of APU was performed according to the instruction
provided
21
CA 02309342 2000-06-14
by Novagen. One hundred micrograms of purified APU was injected into a New
Zealand White rabbit successively at 4-6 week interval according to the
methods
described in Williams et al., supra.
APU activity assay and enzyme-linked imnzzmosorbent assay (ELISA). Rice
seeds or tissues were ground in liquid N2, lysed with a buffer (90.8 mM
KZHP04, 9.2
mM KH~P04, 10 mM EDTA, 10% glycerol, 1% Triton X-100, and 7 mM (3-
mercaptoethanol) and centrifuged at 15,000 X g for 10 minutes. The supernatant
was then collected. APU activity was assayed as described in Mathupala et al.,
J.
Biol. Chem. 268:16332-16344, 1993. ELISA was perfornled as described in
Ausubel et al., Short Protocols in Molecular Biology, 2nd ed., In: A
Compendium of
Methods from Current Protocols in Molecular Biology, John Wiley & Sons, New
York, 1992. The total protein concentration was determined using a Bio-Rad
protein
assay kit based on the dye binding assay of Bradford (Bradford, Anal. Biochem.
72:248-254, 1976).
Determination of sugar concentration. Mature rice seeds were ground to
powder with a mortar and pestle. Rice seedlings were ground to powder in
liquid NZ
with mortar and pestle. Water was added to the powder to make a 10% (w/v)
slurry.
The slurry derived from mature rice seeds was heated at 90°C for 30
minutes, total
soluble sugars were extracted, and the concentration of sugars was determined
as
described in Chen et al., Plant J. 6:625-636, 1994. The slurry derived from
rice
seedlings was heated at 85°C for 2 hours, total soluble sugars were
extracted, and
the concentrations of glucose, fructose, sucrose, maltose, and maltotriose
were
determined by high-performance liquid chromatography (HPLC) as described in
Shaw et al., Biosci. Biotech. Biochem. 56:1071-1073, 1992.
Results
Isolation of the rice glutelin gene promoter, signal peptide, and propeptide
sequences. Primers based on the nucleotide sequence of a glutelin gene (GIuB-1
)
(Takaiwa et al., Plant Mol. Biol. 17:875-885, 1991) were designed, and three
DNA
fragments containing the GIuB-I promoter, putative signal peptide sequence,
and
putative propeptide sequence were synthesized using PCR. The putative 25-amino
22
CA 02309342 2000-06-14
acid signal peptide cleavage site was predicted based on a statistical method
(von
Heijne, J. Mol. Biol. 184:99-105, 1985). The putative 36-amino acid propeptide
cleavage site was predicted based on the sweet potato sporamin propeptide
sequence
(Matsuoka et al., Proc. Natl. Acad. Sci. USA 88:834-838, 1991). Fragment I
contained the 1351 by GluB-I promoter region ending right at the translation
initiation codon. Fragment II contained Fragment I plus a 75 by signal peptide
sequence. Fragment III contained Fragment II plus a 108 by putative propeptide
sequence. The purpose for including the signal peptide or propeptide region
was to
target APU to different cellular compartments (e.g., cytoplasm, protein body,
or
vacuole). Effect of cellular localization on yield, stability, and activity of
APU in
transgenic rice seeds could then be compared. Transformed rice suspension
cells
expressed and secreted APU into the culture medium.
Transformed rice suspension cells expressed and secreted APU into the
culture medium. All plasmids were delivered into rice calli by particle
15 bombardment or Agrobacterium-mediated transformation systems. The
transfected
rice calli were grown under hygromycin selection. Genomic Southern blot
analyses
of the transformed cell lines revealed that, in general, the transgenic lines
obtained
via the Agrobacterium-mediated transformation contained 1 to 2 copies and
that, via
the particle bombardment, the transgenic plants contained multiple copies
(more
than 2) of the apu gene in the genomes.
The transformed rice calli were cultured in liquid MS medium to generate
suspension cell culture. Because APU was expressed with signal peptide or
propeptide sequence in most of the transgenic lines, the culture media were
collected
for analysis of APU accumulation. The amount of APU in the media of
transformed
rice suspension cell cultures was determined using ELISA and the purified E.
coli-
expressed APU as a standard. As shown in Fig. 3, the amount of APU in media of
_
transformed suspension cells was significantly higher than that in media of
non-
transformed cells. In media of cells carrying aAmy3 or aAmy8 promoters, the
amount of APU was higher in the absence of sucrose than in the presence of
sucrose.
Surprisingly, although the GIuB-I promoter had been shown to confer
23
CA 02309342 2000-06-14
endosperm-specific expression in developing rice seeds, it was found that APU
was
also expressed in cultured rice suspension cells. In media of cells carrying
the GIuB-
1 promoter, the amount of APU was higher in the presence of sucrose than in
the
absence of sucrose. Additionally, APU expressed without a signal peptide
sequence
was also present in the culture media. Computer analysis indicated that the N-
terminal of APU had amino acid sequence features resembling a signal peptide
sequence, which might explain the secretion of APU into the culture medium
without a leader peptide added. Thus, it was discovered that APU without a
signal
peptide sequence could be secreted into the culture medium.
Transgenic germinated rice seedlseedling contains high level ofAPU.
Transgenic calli carrying apu under the control of an a-amylase or glutelin
gene
promoter and with or without signal peptide sequence were regenerated. These
transgenic plants were self fertilized for two generations, and the T2
homozygous
seeds were obtained.
15 Homozygosity of transgenic seeds was determined by germination of 25
transgenic seeds in water containing 50 pg/ml hygromycin for 7 days, and then
calculating the ratio between numbers of growing and non-growing seeds.
Homozygous seeds was expected to germinate and grow under hygromycin
treatment. Five T2 homozygous seeds from each transgenic rice line carrying
20 various constructs were germinated and grown for S days. The entire
germinated
seeds/seedlings were collected, and the APU levels in cell extracts were
determined
using ELISA and purified E. coli-expressed APU as a standard. The average APU
level expressed under the control of either an a-amylase or glutelin gene
promoter
was higher than that of the non-transformants (Fig. 4). The expression of APU
in
25 germinated rice seed/seedling was unexpected, because it was known that the
GIuB-
I promoter was expressed specifically in endosperm during the development of
the
seed.
Transgenic matLrre rice seeds contain high levels ofAPU. Five T2
homozygous seeds of each transgenic rice line carrying a transgene were
selected for
30 APU analysis. The embryo and endosperm of mature seeds were separately
24
CA 02309342 2000-06-14
collected and homogenized in buffer to form an extract. APU levels in cell
extracts
were determined using ELISA. The average APU level expressed under the control
of either an a,-amylase or glutelin gene promoter in transgenic seeds was
higher than
that in the non-transformed seed (Fig. 5). Unexpectedly, the GIuB-1 promoter
5 conferred APU expression in embryo. Additionally, the fact that crAmy3 and
crAnry8
promoters conferred APU expression in embryo and endosperm of mature seed was
also unexpected because it was known that a-amylase genes were expressed
specifically in endosperm or transiently in embryo of germinating seed and not
in
the mature seed.
10 Trarasgenic germinated rice seedlseedling contains APU with high specific
activity. The data in Fig. 4 indicated that the APU level was significantly
higher in
transgenic germinated seed/seedling than in the non-transformed control. To
compare the specific activity of recombinant APU expressed in transgenic
germinated seed/seedling, five T2 homozygous seeds of each transgenic rice
lines
15 carrying various constructs were germinated and grown for 5 days. Cell
extracts of
the entire germinated seed/seedlings were prepared, and the APU level in the
cell
extract was determined using ELISA. APU activity per amount of APU in the cell
extracts was determined after incubation of the cell extract at 90°C
for 30 minutes.
As shown in Fig. 6, the specific activity of APU present in transgenic
germinated
20 seed/seedling varied from line to line regardless of the construct used.
Interestingly,
the specific activity of APU expressed in transgenic germinated seed/seedling
was
two to seven fold higher than that of the E. coli-expressed APU. The reason
for this
surprising result could be that there are many endogenous hydrolytic enzymes
present in the rice endosperm. These hydrolytic enzymes may have a synergistic
25 effect on the APU activity present in germinated seeds. Alternatively, or
in
conjunction, the post-translationally modified APU in the germinated seeds may
have increased the enzyme's specific activity, which resulted in the observed
increase in APU activity. Note that there are three potential glycosylation
sites in
the APU polypeptide, which may be differentially glycosylated depending on the
30 production method used. Thus, besides the above-mentioned advantages of
CA 02309342 2001-09-14
expressing APU in transgenic germinated seeds, the specific activity of the
recombinant enzyme was also increased.
Transgenic rice seed and germinated seedlseedling produce high level of
sugars. The results summarized in Figs. 4 and 5 revealed that APU level is
5 significantly higher in transgenic mature seed and germinated seed/seedling,
than in
the non-transformed control. To determine whether transgenic seeds produce
higher
levels of soluble sugars than the non-transformants, five T2 homozygous seeds
of
each transgenic rice line carrying apu under the control of the GIuB-1
promoter were
homogenized in water and incubated at 85°C for 2 hours. As shown in
Fig. 7, the
10 concentration of soluble sugar in transgenic seed was higher than that in
the non-
transformed seed. Five T2 homozygous seeds of each transgenic rice line
carrying
apu under the control of crAmy3 and cz.Anry8 promoter were also homogenized in
water and incubated at 90°C for 30 minutes. As shown in Fig. 8, the
concentration
of sugar in germinated seed/seedling was higher than that in the non-
transformed
15 control. These results demonstrated that the increased level of APU in
transgenic
seed or germinated seed/seedling facilitated production of sugars from seed
starch
without addition of exogenous APU.
26
CA 02309342 2001-11-15
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: ACADMIA SINICA
(ii) TITLE OF INVENTION: PROTEIN PRODUCTION IN TRANSGENIC PLANT
SEEDS
(iii) NUMBER OF SEQUENCES: 28
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Swabey Ogilvy Renault
(B) STREET: 1981 McGill College - Suite 1600
(C) CITY: Montreal
(D) STATE: Quebec
(E) COUNTRY: Canada
(F) ZIP: H3A 2Y3
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.25
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: CA 2,309,342
(B) FILING DATE: 2000-06-14
(C) CLASSIFICATION: AO1H-5/00
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: CAWTHORN, Christian
(B) REGISTRATION NUMBER: 11005
(C) REFERENCE/DOCKET NUMBER: 6070-222 CC
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (514) 847-4256
(B) TELEFAX: (514) 288-8389
(C) TELEX:
(2) INFORMATION FOR SEQ ID N0: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 31
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
26a
CA 02309342 2001-11-15
ttaagcgata tcgccaaggc cccggaacag a 31
(2) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 2:
acgcaggatc cacctcataa aacc 24
(2) INFORMATION FOR SEQ ID NO: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 31
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 3:
acggcggata tccagagtga gccggagctg a 31
(2) INFORMATION FOR SEQ ID NO: 4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 31
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 4:
aggttggatc cttacaaact gcacgaaggg t 31
(2) INFORMATION FOR SEQ ID NO: 5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
26b
CA 02309342 2001-11-15
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
cgggatccta gctttagcta tagcgat 27
(2) INFORMATION FOR SEQ ID N0: 6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 6:
tccccgcggg tcctctaagt gaaccgt 27
(2) INFORMATION FOR SEQ ID N0: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 7:
tccggatccc agatcgttca aacattt 27
(2) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 26
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
26c
CA 02309342 2001-11-15
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 8:
agcccgcggg atcgatctag taacat 26
(2) INFORMATION FOR SEQ ID N0: 9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
accgggtcga cgtatacatg tcacctaca 29
(2) INFORMATION FOR SEQ ID N0: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STR.ANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 10:
ggtgatatcc aggacttgcc cggctgt 27
(2) INFORMATION FOR SEQ ID NO: 11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 25
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 11:
gctctagaaa tctgagcgca cgatg 25
26d
CA 02309342 2001-11-15
(2) INFORMATION FOR SEQ ID N0: 12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 12:
tccccgcggt aagcattaag cagtgca 27
(2) INFORMATION FOR SEQ ID NO: 13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 13:
ggggaattcg atctcgattt ttgaggaat 29
(2) INFORMATION FOR SEQ ID N0: 14:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 14:
gggggatccc atagctattt gtacttgct 29
(2) INFORMATION FOR SEQ ID NO: 15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
26e
CA 02309342 2001-11-15
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotidefor PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID
NO: 15:
gggggatccgggattaaata gctgggcca 29
(2) INFORMATION
FOR SEQ
ID N0:
16:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotidefor PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID
NO: 16:
gggggatcccctcactttcc gaagtggtt 29
(2) INFORMATION
FOR SEQ
ID N0:
17:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 25
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotidefor PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID
N0: 17:
cgggatccttaagcttgcat cttga 25
(2) INFORMATION
FOR SEQ
ID N0:
18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 33
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotidefor PCR
26f
CA 02309342 2001-11-15
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 18:
ccggcggccg cctacatatt ttccccttgg cca 33
(2) INFORMATION FOR SEQ ID NO: 19:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 19:
tccgagctcc agatcgttca aacattt 27
(2) INFORMATION FOR SEQ ID N0: 20:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 26
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 20:
agcgagctcg atcgatctag taacat 26
(2) INFORMATION FOR SEQ ID N0: 21:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 21:
cgccgcggta gctttagcta tagcgat 27
(2) INFORMATION FOR SEQ ID NO: 22:
(i) SEQUENCE CHARACTERISTICS:
26g
CA 02309342 2001-11-15
(A) LENGTH: 27
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 22:
tccccgcggg tcctctaagt gaaccgt 27
(2) INFORMATION FOR SEQ ID NO: 23:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 23:
cgcatatgtt aagcttgcat cttgattc 28
(2) INFORMATION FOR SEQ ID NO: 24:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 31
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 24:
ccgctcgagc tacatatttt ccccttggcc a 31
(2) INFORMATION FOR SEQ ID N0: 25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
26h
CA 02309342 2001-11-15
a
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 25:
cggaattcgc caaggcgccg gagcagac 28
(2) INFORMATION FOR SEQ ID N0: 26:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 26:
gctctagata cgccttcgcc ggatggct 28
(2) INFORMATION FOR SEQ ID NO: 27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 27:
cccgaattca tgaaatactg gcag 24
(2) INFORMATION FOR SEQ ID NO: 28:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial Sequence
(ix) FEATURE:
(D) OTHER INFORMATION: oligonucleotide for PCR
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 28:
cccgagctct tacgccttcg ccgg 24
26i