Note: Descriptions are shown in the official language in which they were submitted.
CA 02309486 2000-05-26
- 1 - I
Title: Chelating Silicone Polymers 5
FIELD OF THE INVENTION
This invention relates to silicone-based surfactants, and in
particular silicone-based surfactants useful as chelating agents.
BACKGROUND OF THE INVENTION
A wide variety of applications require control of the interfacial
properties between immiscible components, such as water-in-oil
emulsions or oil-in-water emulsions. Generally, to obtain good
performance it is necessary to stabilize the interface between the two
immiscible components. One simple example is the use of coupling
agents to modify silica surfaces so that silica may be used to reinforce
organic polymers, with which it is otherwise incompatible. Another
example is the use of surfactants to stabilize oils in water, such as in
cleaning and conditioning applications.
Silicones are among the most surface-active materials
("surfactants") known. They diffuse rapidly to interfaces and readily
spread. Spreading of the silicone may be facilitated by the incorporation
of polar groups on the silicone backbone. Some of the most effective
spreading compounds, particularly at solid/liquid/air surfaces, are the so-
called "superwetters" made by manufacturers including Crompton Corp.
and Dow Corning. The general structure of these superwetters is
(Me3SiO)ZSiMe(CH2)3(OCH2CHz)õOZ, where Z may be H, CH3, CH3COO,
etc.
Liquid-liquid interfaces are generally stabilized with silicones
bearing non-ionic hydrophilic groups. Common examples include
derivatives of so-called silicone polyols; that is silicones containing
polyether sidechains. US Patent No. 5,707,613 to Hill teaches that these
compounds are particularly useful at stabilizing water/silicone interfaces.
CA 02309486 2000-05-26
-2-
Ionic silicone copolymers can also be used to stabilize such interfaces. US
Patent No. 5,124,466 to Azechi et al. (Shin-Etsu) teaches that ammonium-
modified silicone surfactants are useful in the stabilization of silicone
emulsions in water.
The surface activity of silicones, whether cationic, zwitterionic or
non-ionic, cannot be readily changed, although pH modifications may
affect the behavior of some types of ammonium compounds. There are
advantages in being able to change the surface activity of a surface active
material so as to change the properties of systems in accordance with its
particular use, for example, to flocculate emulsions on demand. For
example, carboxylic acids and polymers derived from them (e.g.,
CARBOPOLTM (available from BF Goodrich)) change their ability to swell
water and to stabilize interfaces upon pH changes: bases convert neutral
carboxylic acids to carboxylates. In this respect, silicones having a pH
sensitivity, by virtue of amine or carboxylic acid groups, are known. US
Patent No. 5,447,997 to Releigh et al. teaches silicones containing
carboxylic acids whose surface properties change as a function of pH.
The properties of ionic surfactants may not only be changed by pH,
but by the nature of the counterions. For example, carboxylates with
monovalent counterions such as sodium swell well with water. In
contrast, multivalent counterions in the same system, lead to ionic
crosslinking and a reduction of swelling. At an interface, the surface
activity of such materials are similarly affected by the nature of the
counterion.
Multidentate ligands (or "chelating agents") bind metals very
tightly. The classic example is EDTA (ethylenediaminetetraacetic acid).
EDTA, normally in its calcium, disodium salt form, is frequently found
in food products. Heavy metal ions coming into contact with the EDTA
will complex with the amine and carboxylic acid groups, displacing the
sodium/calcium ions. The binding efficiency of EDTA and its
derivatives is known for many metals and their different oxidation
states. Chelating agents are added to many different formulations for
different purposes. They have also been bound to polymers. For
CA 02309486 2000-05-26
- 3 - I
example, chelating groups similar to those mentioned above are used as
supports in affinity chromatography.
However, there still exists a need for silicones that are effective at
chelating metal ions using complementary binding, whose properties
may be controlled through the relative amounts and morphology of the
hydrophilic and hydrophobic blocks, the chelating agent, the pH of the
solution, the presence or absence of multivalent counterions, and the
specific nature of the multivalent ions.
SUMMARY OF THE INVENTION
The present invention relates to silicone polymers useful as both
surfactants and chelating agents. The polymers contain a hydrophobic
component (the silicone polymer backbone) and a hydrophilic
component. The hydrophilic component may act as a chelating agent; i.e.
it will bind a variety of metals. The hydrophilic component may be
hydrophilic prior to binding to a metal, or after binding to a metal.
The hydrophobic nature of the silicone is provided by organic
radicals, such as methyl or other alkyl groups, modified alkyl groups such
as fluoroalkyl groups, aryl groups, and related hydrophobic moieties,
bound to the silicon atoms in the polymer. The hydrophilic component
includes multiple ligands to cooperatively bind one or more metal
centers. Examples of such ligands are well known in the art, and include
hydrophilic groups such as carboxylic acids and their derivatives, amines,
phosphines, alcohols, and unsaturated systems (multiple bonds) that are
or are rendered hydrophilic upon complexation with a metal ion.
In one aspect, the present invention relates to a silicone polymer
comprising a hydrophobic polysiloxane backbone and at least one metal
binding site which is covalently bound to the hydrophobic polysiloxane
backbone, the at least one metal binding site comprising at least two
ligands which are optionally bound to a metal.
In one embodiment, at least one of the ligands is hydrophilic either
before or after being bound to a metal. The ligand may include groups
selected from functional alkyl groups bearing heteroatom-based ligands,
functional aryl groups bearing heteroatom-based ligands, functional alkyl
CA 02309486 2009-04-29
-4-
groups bearing heteroatom-based ligands where the ligands have
exchangeable hydrogen atoms, functional aryl groups bearing
heteroatom-based ligands where the ligands have exchangeable hydrogen
atoms, functional alkyl groups having n-ligands, and functional aryl
groups having n-ligands. Preferably, the metal binding sites include two
or more carboxylic acids which may act as ligands.
The metal binding site may be covalently bonded to the silicone
polymer backbone by a linker, which is at least as stable to hydrolysis as
the siloxane linkage in the silicone polymer. The linker may be selected
from single atoms including C, N, 0, S, or P, or groups including amides,
esters, thioesters, urethanes, ureas, alkyl or aryl groups.
The polymers of the invention may have molecular weights from
about 500 to about 500,000 g/mol.
In one embodiment, the invention relates to a compound of the
formula I:
TI-Qq T2 (I)
where q is between 0 to 7,000; and
where Q is an internal siloxane group of the formula II:
1 4
i
~
R5
(II~
and, where R4 and R5, for each internal siloxane group, are the same or
different, and where R4 and R5 are independently, H with the proviso
that both R4 and R5 are not H on the same internal siloxane group,
alkoxy, alkyl, aryl, functional alkyl, functional aryl, a metal-binding site
comprising at least two ligands optionally bound to a metal, or a group
having an internal siloxane group of the formula III:
CA 02309486 2009-04-29
7
~iI is , Ds
R ! (III)
where r is between 0 to 7,000;
R', and R8, are for each internal siloxane group of the formula III the
5 same or different, and R6, R', and R8 are independently, H with the
proviso that not more than one of R6, R', and RB on each internal
siloxane group is H, alkoxy, siloxy, alkyl, aryl, functional alkyl, functional
aryl, or a metal binding site comprising at least two ligands optionally
bound to a metal;
T, is a group of the formula (IV):
R1
R2 IO
Si
R3 (IV)
T2 is a group of the formula (V):
R9
0`l Rto
R1i (V)
where, R', R2, R3, R9, Rlo, R11, arc independently, H with the proviso that
each silicon atom has no more than one H, alkoxy, siloxy, alkyl, aryl,
functional alkyl, functional aryl, or a metal binding site comprising at
least two ligands optionally bound to a metal;
with the proviso that at least one of R' to R" is a metal binding site
comprising at least two ligands optionally bound to a metal, and with the
proviso that the molecular weight of the compound is between 500 and
500,000 g/mol.
CA 02309486 2000-05-26
-6-
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention will be better understood when the
following description is read in connection with the accompanying
drawings, in which:
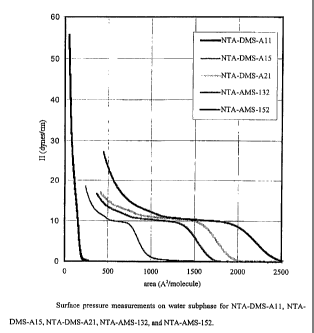
Figure 1 is a graph of surface pressure versus area on water
subphase for various compounds made in accordance with the present
invention;
Figure 2 is a graph of surface pressure versus area on water
subphase for various compounds made in accordance with the present
invention incorporating an NTA chelating ligand;
Figure 3 is a graph of surface pressure versus area on various
aqueous subphases for N-,N--Bis(carboxymethyl)-L-lysine terminated
polydimethylsiloxane DMS-All;
Figure 4 is a graph of surface pressure versus area on various
aqueous subphases for Succinyl-Terminated Polydimethylsiloxane DMS-
All;
Figure 5 is a graph of surface pressure versus area on various
aqueous subphases for N-,N--Bis(carboxymethyl)-L-lysine-terminated
polydimethylsiloxane DMS-A21; and
Figure 6 is a graph of surface pressure versus area on various
aqueous subphases for Succinyl-Terminated Polydimethylsiloxane DMS-
A21.
DESCRIPTION OF THE PREFERRED EMBODIMENT
In this document, the following terms have the meaning defined,
unless otherwise described:
Hydrophobic: groups or molecules that would not normally be soluble in
water;
Hydrophilic: groups or molecules that would normally be soluble in
water;
Ligand: a chemical group capable of binding to a metal;
CA 02309486 2000-05-26
-7
Alkyl: an aliphatic hydrocarbon, linear, branched and/or cyclic having up
to 20 carbon atoms;
Aryl: a hydrocarbon residue base, having up to 20 carbons and containing
at least one conjugated cyclic substructure, which cyclic structure may
contain an 0 or N, and which cyclic structure may be substituted at a
substitutable position with an alkyl group;
Functional alkyl: an alkyl group having one or more functional groups
selected from halogens (F, Cl, Br, I); hydroxy groups (OH); thiols (SH),
sulfides (SR, where R = alkyl, functional alkyl, aryl, or functional aryl),
disulfides (SSR, where R = alkyl, functional alkyl, aryl or functional aryl
groups), alkoxy groups (RO, where R = alkyl, functional alkyl, aryl or
functional aryl); primary amine (NH2)1 secondary amine (RNR', where R
and R' = alkyl, functional alkyl, aryl or functional aryl groups) or tertiary
amino groups (R2N, where R = independently, alkyl, functional alkyl,
aryl or functional aryl groups); primary phosphino (PHZ), secondary
phosphino (RPH, where R = alkyl, functional alkyl, aryl or functional
aryl) or tertiary phosphino groups (RR'P, where R and R' = alkyl,
functional alkyl, aryl or functional aryl groups); carboxylic acids (COOH)
and their derivatives including esters (COOR, where R = alkyl, functional
alkyl, aryl or functional aryl), thioesters (COSR, CSOR, where R = alkyl,
functional alkyl, aryl or functional aryl) and amides (CONH2, CONHR,
CONRR', where R and R' = alkyl, functional alkyl, aryl or functional aryl
groups), carbonates (ROCO2R') or derivatives (urethanes OCONH2,
OCONHR, OCONRR', NHCOOR, NR'COOR, ureas (NHCONH2,
NRCONH2, NHCONRH, NHCONRR', NRCONHR', NRCONR'R",
where R,R' and R" = alkyl, functional alkyl, aryl or functional aryl
groups), aldehydes (CHO), ketones (COR, where R = alkyl, functional
alkyl, aryl or functional aryl), alkenes (C=C) and alkynes (C=C);
Functional aryl: an aryl group having one or more functional groups
substituted at a substitutable position groups selected from halogens (F,
Cl, Br, I); hydroxy groups (OH); thiols (SH), sulfides (SR, where R = alkyl,
functional alkyl, aryl, or functional aryl), disulfides (SSR, where R =
alkyl, functional alkyl, aryl or functional aryl groups), alkoxy groups (RO,
where R = alkyl, functional alkyl, aryl or functional aryl) ; primary amine
CA 02309486 2000-05-26
-$-
(NH2), secondary amine (RNR', where R and R' = alkyl, functional alkyl,
aryl or functional aryl groups) or tertiary amino groups (R2N, where R =
independently, alkyl, functional alkyl, aryl or functional aryl groups);
primary phosphino (PH2)1 secondary phosphino (RPH, where R = alkyl,
functional alkyl, aryl or functional aryl) or tertiary phosphino groups
(RR'P, where R and R' = alkyl, functional alkyl, aryl or functional aryl
groups); carboxylic acids (COOH) and their derivatives including esters
(COOR, where R = alkyl, functional alkyl, aryl or functional aryl),
thioesters (COSR, CSOR, where R = alkyl, functional alkyl, aryl or
functional aryl) and amides (CONH2, CONHR, CONRR', where R and R'
= alkyl, functional alkyl, aryl or functional aryl groups), carbonates
(ROCO2R') or derivatives (urethanes OCONH2, OCONHR, OCONRR',
NHCOOR, NR'COOR, ureas (NHCONH2, NRCONH2, NHCONRH,
NHCONRR', NRCONHR', NRCONR'R", where R, R' and R" = alkyl,
functional alkyl, aryl or functional aryl groups), aldehydes (CHO),
ketones (COR, where R = alkyl, functional alkyl, aryl or functional aryl),
alkenes (C=C) and alkynes (C=C);
Functional alkyl groups bearing heteroatom-based ligands: the subset of
functional alkyl, having one or more 0, N, or S atoms including
hydroxy, thiols, sulfides, disulfides, alkoxy, primary, secondary and
tertiary amino groups, primary, secondary and tertiary phosphino
groups, carboxylic acids and their derivatives including esters, thioesters,
amides, carbonates or their derivatives including urethanes, ureas,
aldehydes, and ketones;
Functional aryl groups bearing heteroatom-based ligands: the subset of
functional aryl, having one or more 0, N, or S atoms including hydroxy,
thiols, sulfides, disulfides, alkoxy, primary, secondary and tertiary amino
groups, primary, secondary and tertiary phosphino groups, carboxylic
acids and their derivatives including esters, thioesters, amides,
carbonates or their derivatives including urethanes, ureas, aldehydes,
and ketones;
Functional alkyl groups bearing heteroatom-based ligands where the
ligands possess exchangeable hydrogens: the subset of functional alkyl,
having a group selected from OH, NH, SH, or PH;.
CA 02309486 2000-05-26
_g_
Functional aryl groups bearing heteroatom-based ligands where the
ligands possess exchangeable hydrogens: the subset of functional aryl,
having a group selected from OH, NH, SH or PH;
Functional alkyl groups bearing -ligands: the subset of functional alkyl
groups, having unsaturation in the form of bouble bonds between C and
Y and/or between N and Y, where is S, 0, NR, PR, CRR', and/or triple
bonds between C and Z, where Z is CR or N; and where R, and R' are
independently selected from alkyl, functional alkyl, aryl, functional aryl,
OH, NH, SH, or PH;
to Functional aryl groups bearing ligands: the subset of functional aryl
groups, having unsaturation in the form of bouble bonds between C and
Y and/or between N and Y, where is S, 0, NR, PR, CRR', and/or triple
bonds between C and Z, where Z is CR or N; and where R, and R' are
independently selected from alkyl, functional alkyl, aryl, functional aryl,
OH, NH, SH, or PH;
Alkoxy: OR, where R is alkyl, functional alkyl, aryl or functional aryl;
Siloxy: OSiRR'R", where R, R', and R" are independently alkyl,
functional alkyl, aryl or functional aryl groups, alkoxy, other siloxy
groups, or OH;
Metal: all metals of the periodic table, including without limitation,
alkali metals, alkaline earth metals, transition metals, lanthanides,
actinides, and Group 13 elements including Boron.
Those skilled in the art will appreciate that there are combinations
of functional groups that will react (e.g., amines + alkyl halides) which
are thus mutually incompatible. These combinations are not to be
inferred in the following discussion.
In accordance with the present invention, a silicone polymer
backbone has at least one covalently bonded metal binding site. The
metal binding site has at least two ligands such that any metallic binding
is at least bidentate, although it may also have three, four, five or more
binding sites (i.e. it may be tri, tetra, penta, hexa, etc. dentate).
Generally,
the silicone polymer may be any polysiloxane structure which is
hydrophobic in nature. The term "silicone polymer" and
"polysiloxane"are used interchangeably herein.
CA 02309486 2009-04-29
-10-
More preferably, the silicone polymer backbone may be a
compound of the formula I:
Tj-Qq T2 (I)
where q is between 0 to 7,000;
Q is an internal siloxane group of the formula II:
R4
~
i
RS
(II)
R4 and R5, for each internal siloxane group , are the same or different, and
where R4 and RS are independently, H with the proviso that both R4 and
RS are not both H on the same internal siloxane group, alkoxy, alkyl, aryl,
functional alkyl, functional aryl, a metal-binding site comprising at least
two ligands optionally bound to a metal, or a group having an internal
siloxane group of the formula III:
'
Ti+ R6
R8
(III)
where r is between 0 to 7,000;
R', and R8, for each internal siloxane group are the same or different, and
R6, R', and R$ are independently H with the proviso that not more than
one of R6, R', and R$ is H on any siloxane group, alkoxy, siloxy, alkyl,
functional alkyl, aryl, functional aryl, or a metal binding site comprising
at least two ligands optionally bound to a metal;
Tl is a group of the formula (IV):
._._.. .w_v.,Q..~. . ,,. , ,
CA 92309486 29UU=US-26
CA 02309486 2008-04-17
-11-
R1
R2 Ii-**,O
1 Rs (IV)
T2 is a group of the formula (V):
Rs
0 R1o
gi
Iõ (V)
where, R', R2, R3, R9, R10, R", are independently, H with the proviso that
each silicon atom has no more than one H, alkoxy, siloxy, alkyl,
functional alkyl, aryl, functional aryl, or a metal binding site comprising
at least two ligands optionally bound to a metal;
with the proviso that at least one of R' to R" is a metal binding site
comprising at least two ligands optionally bound to a metal and with the
proviso that the molecular weight of the compound is between about 500
and about 500,000 g/mol.
Some more specific examples of suitable substituents for R' to R"
include linear, branched and cyclic saturated alkyl groups having up 20
carbons such as methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, t-butyl,
pentyl, n-hexyl, cyclohexyl etc., linear, branched and cyclic alkoxy groups
having up to 20 carbon atoms such as methoxy, ethoxy, propoxy, butoxy,
cyclopentyloxy, etc., unsaturated non-cyclic hydrocarbon groups having
up to 20 carbon atoms such as vinyl, allyl, propargyl, etc., unsaturated
derivatives of fatty acids having up to 20 carbon atoms such as linolenyl
groups, unsaturated cyclic hydrocarbon groups such as cyclopentadienyl,
and aryl groups such as phenyl, tolyl, benzyl, naphthyl, etc. These
substituents may be substituted at a substitutable position with a halogen
such as fluorine, chlorine, bromine or iodine, or with a hydroxy, alkoxy,
amino group, etc. It will be appreciated, however, that the substituents
should not materially affect the hydrophobic properties of the silicone
polymer backbone.
v~a V./JV /~V V rVV V VJ rV
CA 02309486 2008-04-17
-12-
The silicone polymer may have a molecular weight (g/mol)
between about 500 and about 500,000, preferably between about 500 and
about 15,000 and more preferably between about 1,500 and about 15,000.
It will be appreciated that compounds of the formula I include
those polysiloxanes having a"linear" backbone, as well as those having a
"branched" backbone structure.
The compounds include at least one covalently bound site capable
of binding a metal. This site will possess multiple ligands (>1, i.e., bi-,
tri-
, tetra-, penta-, or hexadentate), which may be the same or different.
Preferably, the site will have three or more ligands capable of binding a
metal.
The metal-binding sites may be covalently bonded to the silicone
polymer backbone at one or both of the polymer's terminal ends.
Alternatively, or in addition, the ligands may be covalently bonded to the
polymer backbone in a periodic fashion. Preferably, the metal-binding
sites are periodically bonded to the silicone polymer backbone at intervals
between about every 5' to about every 200' internal Si-O- group. It will
be appreciated by those skilled in the art that the desired periodic interval
between the metal-binding sites will depend upon factors such as steric
hindrance, polarity of the resulting compound and the specific demands
of the application in which the compounds are used.
The metal-binding site includes ligands capable of binding a metal,
provided that, either before or after metal complexation, they are
hydrophilic. Many metal ligands are known to those skilled in the art,
and for example, are described in Cotton F. A. and Wilkinson G.,
Advanced Inorganic Chemistry, 3' ed (1972: Wiley & Sons, NY), and
King R. B., Encyclopedia of Inorganic Chemistry, (1994: Wiley and Sons,
Chichester, Vol. 2, pp. 777-821).
Preferably, the ligands are functional alkyl groups bearing
heteroatom-based ligands, functional aryl groups bearing heteroatom-
based ligands, functional alkyl groups bearing heteroatom-based ligands
where the ligands possess exchangeable hydrogens, functional aryl
groups bearing heteroatom-based ligands where the ligands possess
CA 02309486 2000-05-26
CA 02309486 2008-04-17
-13-
exchangeable hydrogens, functional alkyl groups bearing n-ligands, or
functional aryl groups bearing n-ligands. More preferably, the metal
binding sites have as ligands two or more carboxylic acid groups. Most
preferably, the metal binding sites are selected from the following groups:
0
0
HO O O OH
yy HO p
Y OH OH
OH 'J~
OH
O
O
O O
O OH
HO IO HO p
t IfOH X
W OH X W" v W`\/x` Wy
HO O
O OH
0
0
where W is N, P or C; X is C, N, or 0; Y is N, 0, S, P or C; t is between 1
and 10; and m is between 0 and 10.
The metal binding sites include a linker to covalently bond the
metal binding site to the silicone polymer backbone. Suitable linkers,
and methods of their preparation, are known to those skilled in the art,
and include those described in Brook M. A., Silicon in Organic,
Organometallic and Polymer Chemistry (2000: Wiley & Sons, N.Y),
Preferably, the linkers are selected from single C, N, 0, S, or P
atoms, or more complex functional groups including amides, esters,
thioesters, urethanes, ureas, alkyl, aryl, functional alkyl and functional
aryl groups.
Most preferably, the linkers are selected from the following groups
including -CO-(CHz),SiR3 . -XCO-(CHZ),,SiR3, -(CHZ)kSiR3 or -OCX-
(CH2)kSiR3 where X is C, 0, N, or S, k is between 0 and 10, and SiR3
represents the silicone polymer backbone.
CA 02309486 2008-04-17
-14-
Some examples of specific linkers, include:
o
R3Si R3Si`_{` _..,J' r ,'N R3Si` r 7/~ R3Si~
N k N k
k
O
Ria
R3Si 1~1 N R3Si R3Si N\ x
`O k SiR3
Riz Ria
where k is between 0 and 10; R12 is hydrogen, alkyl, aryl, functional alkyl or
functional aryl; and R13 and R14 are independently hydrogen, alkyl, aryl,
functional
alkyl, functional aryl, alkoxy or siloxy, and where R3Si is the silicone
polymer
backbone
Further linkers include compounds of the following general formula
I 1 I.~ O
jj Si Me3Si Si ~Si-SiMe3
~O- o~
and
Z
Z Z
where Z is a group of the formula:
0
O
HO O
X --I) II
N
N
LTOH
O
where X is 0 or N; and n and m are independently greater than or equal to 0,
with the
proviso that the total molecular weight of the compound is between about 500
and
500,000 g/mol.
CA 02309486 2008-04-17
- 14A -
Some specific examples of metal binding sites including linkers are:
O o
0 HO 0 0 HO O
R,si W` x R,Si N W` ~
k X v `OH ~ X v ~OH
OH O OH
O O OH O
0 O
a,si k OH
O HO O HO
t
R,Si`( ' ^ 'W 0 W m OH
'~ X W' \/ OH O
` jk OH
0
HO O
O O
R3SiW
k OH
HO O 0 1~
O OH
R3siw w~OH R13 HO 0
x
I,: OH R,si~ w w k
O OH
R19 HO O
0 OH
O
O
where W is N, P, or C; X is C, N, 0 or S; n is between 1 and 10; k is between
0 and 10; R12 is hydrogen, alkyl, aryl, functional alkyl or functional aryl;
and
R13 and R14 are independently hydrogen, alkyl, aryl,
CA 02309486 2000-05-26
- 15-functional alkyl, functional aryl, alkoxy or siloxy, and where R3Si is
the
silicone polymer backbone.
The metal to which the ligand may bind may be any metal.
Preferably, the metal is charged metal ion, which may be bound by a
s metal binding site. More preferably, the metal ion is selected from the
group of charged metal ions including Ca2+, Mg2+, Niz+, Fe3+, Cu2+, and
Co2+.
As will be appreciated, by incorporating the described metal
binding sites, the properties of the silicone polymers may be changed by
modification of solution pH, and by complexing metals of different
charge states to the polymer. For example, the following scheme
demonstrates how a neutral compound may be converted into an ionic
species by increasing pH or by the binding of a multivalent metal ion.
a,n vLJVY4a0 LVVV M~~
CA 02309486 2008-04-17
-16-
0 0
0 OH Ri R4 R9 HO 0
~' ~/ N Ii Si\ \E N\ LOH
R3 R5 Rtt
HO OH
O 0
I increase pH
O 0
0 0 Ri i4 is HO 0
/N SIIi Si Si\ N HO" / E I O+I An I E o
~/ -
R3 R5 Rtl
HO or
0 increase pH O
0 0
O 0 ii i4 i9 '0 0
N Si Si\
E 0 Anf '~1 I
R3 Rs Ril
O O
O multivalent metals O
% O
O 0 Ri R4 R9 O 0
N li o
\ + 0- I
R3 R5 Rii
M ~0 ro'---M.
O 0
The compounds of the present invention may be prepared by the
following general synthesis, which is exemplified in the later described
specific examples.
Preferably, in the first instance, a linker is grafted to the chelating
group. In a subsequent or concomitant step, the linker is covalently
grafted to a functionalized silicone. There are many convenient routes
CA 02309486 2000-05-26
- 17-that may be utilized, which will be readily apparent to those skilled in
the
art. Some, without limitation, are provided to demonstrate the flexibility
of the approach.
The linker (already bonded with the chelator) may be grafted to a
functionalized silicone polymer in any of the following methods:
Amine: To a haloalkyl-modified silicone (e.g., R3Si[CHz]3Cl), in a
suitable organic or aqueous solvent, is added a linker that is bound to a
chelating group containing an amine. Preferably the amine is a primary
or secondary amine (HZNR-cxEL) The reaction leads to the chelating
silicone R3Si[CH2]3NHR CHEL.
Alternatively, to an alcohol-modified silicone (e.g., R3Si[CH2]30H),
in a suitable organic or aqueous solvent, is added a linker that is bound to
a chelating group containing a haloalkane group, (X[CH2]õCHEL). The
reaction leads to the chelating silicone R3Si[CH2]3N[CH2]nCHEL.
Ether: To a haloalkyl-modified silicone (e.g., R3Si[CH2]3Cl), in a
suitable organic or aqueous solvent, is added a linker that is bound to a
chelating group containing an alcohol (HORIEL). The reaction leads to
the chelating silicone R3Si[CHZ]3OR CHEL
Ester: To an alcohol-modified silicone (e.g., R3Si[CHz]3OH), in a
suitable organic or aqueous solvent, is added a linker that is bound to a
chelating group having an activated carboxylic acid group (acid
anhydride, acid halide, activated ester, e.g., N-hydroxysuccimide ester)
ZCOCHEL. The reaction leads to the chelating silicone,
R3Si[CHZ]3OCOCHEL.
Amide: To an amine-modified silicone (e.g., R3Si[CHz]3NHZ)1 in a
suitable organic or aqueous solvent, is added a linker that is bound to a
chelating group having an activated carboxylic acid group (acid
anhydride, acid halide, activated ester, e.g., N-hydroxysuccimide ester)
ZCOCHEL. The reaction leads to the chelating silicone,
R3Si[CH2]3NCOCHEL.
The linker may be grafted to the chelating group in an analogous
manner, using standard organic functional group chemistry, e.g., via
amines, esters, ethers and amides.
CA 02309486 2000-05-26
-18-
The compounds of the present invention may have a variety of
applications. While the materials possess interesting surface activity in
the absence of metal, they have the additional possibility of undergoing
changes in surface activity as a function of stimuli including pH and the
addition of different metals of different valency and charge. They may be
used in applications requiring the interaction of silicone polymers with
proteins, for example personal care products such as hair conditioners or
hand creams, in the isolation and purification of proteins by affinity
chromatography, in the stabilization of proteins for delivery in washing
powders or in drug delivery systems. They may also be used as
surfactants and emulsifiers in a wide variety of applications requiring
control of the surface activity, since their properties may be modified on
demand. They may also be used in the stabilization of interfaces of
minerals, for example, calcium carbonate, in hydrophobic media or to
hydrophobize such compounds for use in aqueous systems. These
examples of uses are intended only to be illustrative, and not limiting.
Skilled artisans will understand and appreciate a wide range of useful
applications for the compounds of the present invention.
The following examples, which are non-limiting, are illustrative
of the present invention. The scope of the invention is limited only by
the claims.
EXAMPLES
General
As more completely described below, poly(dimethylsiloxane)
oligomers functionalized with terminal and pendant NTA chelating
groups were prepared by a linear multistep synthesis utilizing, until the
last step, ester-protected carboxylic acids. The starting materials were
commercially available aminopropylsilicones. This approach avoided
separation steps that were difficult because of the high surface activity of
both the intermediate and ultimate hydrophilically-modified silicones (the
final compounds and their intermediates were capable of efficiently
stabilizing emulsions, as evidenced during attempted washing of reaction
mixtures containing the compounds, water and either ether or chlorinated
hydrocarbons).
CA 1123119486 2UUU-U5-Z6
CA 02309486 2008-04-17
-19-
Chart 1: NTA- and succinyl-silicone chelators: terminal functionalization.
\~~ =SI~
v.Si
~ tJn
H HN NH
O O
O
X X HOOC
~
X = 0H X = COZH
O 2 H
Compound MW Yieido Compound MW Yielda
SUCC-DMS-A11 1075 95 NTA-DMS-A11 1550 95
SUCC-DMS-A15 3200 95 NTA-DMS-A15 3690 52
SUCC-DMS-A21 5200 95 NTA-DMS-A21 5690 65
a starting from the amino siiioone
Chart 2: NTA- and succinyl-silicone chelators: pendant functionalization.
Me Si ~O+ S i O'O X= OH moPh
s +M+Ji õ SiMe3 Compound MW cheiator Yield
SUCC-AMS-132 4670-5670 2-3 86
SUCC-AMS-152 7440-8440 4-5 66
SUCC-AMS-162 4370-5370 6-7 70
H
X = ~OzH
HOOC \-.COZH
moP/o
Compound MW chefator Yield
NTA,AMS-132 5060-6060 2-3 25
NTA-AMS-152 8500-9500 4-5 .12
NTA-AMS-162 5270-6270 6-7 74'
q,A uc4OY450 Lovv-uo-co
CA 02309486 2008-04-17
-20-
The chelating agent used in these reactions, N-,N--bis(carboxymethyl)-L-lysine
(NTA), was derived from lysine according to the procedure presented by Hochuli
et al. (J. Chromatogr. 1987, 411, 177) that utilizes carboxymethylation of Z-
protected (Z = C6HSCH2OCO-) lysine and subsequent deprotection/reprotection
steps (Scheme 1 below). The final product (NTA) was purified by trituration
with
hot methqnol and then recrystallization, or by desalting on a column filled
with
Sephadex -G10 size exclusion gel (MW cutoff < 700), followed by
lyophilization.
These purification steps were not trivial.
Scheme 1
OOH 900H
H2N OOH ~ OpH OOH
1. BrCHZCOZH HOOC ~ HOOC ~
NaOH 1. H./Pd/C in 1M NaOH
2. ~I 2. HCI
ZHN
to MZ-Lysine zHN N~ Z-NTA Hz NTA
The synthesis of the chelating silicones began with commercially available
aminopropylsilicones, both terminal and pendant. The general synthesis is
shown in Schemes 2 and 3. Standard reactions for peptide synthesis were
utilized. N-tert-Butyloxycarbonyl-NTA tricesium salt was synthesized from
NTA and di-tert-butyldicarbonate. and used for the next step without further
purification (BOC = IV -tert-Butyloxycarbonyl). The introduction of the benzyl
groups, using benzyl bromide met with some difficulties and the low yield (45%
after silica gel chromatography) was due to the fact that significant amounts
of
mono and di-benzyl esters were obtained along with the desired tribenzyl
ester.
The BOC group was removed by treatment with trifluoroacetic acid and
the product (as a TFA salt) was used for the next step without further
purification. The primary amino group of the NTA-benzyl ester was reacted
with succinic anhydride (96% yield), and the free carboxylic acid group of the
succinyl-NTA-benzyl ester was esterified with N-hydroxysuccinimide, to give
the
activated ester that, after silica gel chromatography, was stable at 8 C for
at least 2
weeks (Scheme 2).
CA 02309486 2000-05-26
-21 -
In the penultimate step (Scheme 3), the aminopropyl-functional silicone
was reacted with a 10-15% molar excess of N--SSU-NTA-Bn esterto generate a
stable amide bond. Excess NTA reactant was consumed by small amounts of
AMS-162, an aminopropyl-methylsiloxane-dimethylsiloxane having 7-8% mol
amino groups and M,,, 4000-5000. These compounds, and the excess NTA with
which they reacted, were completely retained by silica gel during the column
chromatography purification step. The final step of the synthesis involved the
removal of the benzyl protective groups by hydrogenation in the presence of
palladium on charcoal. The end functional-silicone chelators were obtained in
to higher yields (93-100%) after catalyst and solvent removal, than the
pendant
silicone chelators: NTA-AMS-132 and NTA-AMS-152 were obtained in 47% and
33% yield, respectively. The removal of the catalyst was quite difficult for
these
two cases, due to the formation of very fine water/oil dispersions. This route
provided a generic two step synthesis for a variety of silicone chelators,
with the
chelating moiety attached either to the ends or pendant from the polysiloxane
backbone. Although these compounds were somewhat difficult to characterize as
the free carboxylic acids, except by electrospray-mass spectroscopy, the
structures
of the penultimate tribenzyl esters were easily determined by 1H-NMR, 13C-NMR,
FT-IR, GPC.
CA 02309486 2000-05-26
-22-
H2N OOH t-Boc2O O OOH
HOOC vCOOH 1:1 Dioxane/ 0 HOOC TPO0H
1 M NaOH
H
BnBr, Cs2CO3, 0 OOBn TFA
DMF
BnOOC OOBn
45% over 2 steps
0
H2N 0OBn 0
Bn
OO
BnOOC COOBn NE CH CI HO
~~ 2 2 BnOOC pOBn
96% (2 steps)
0
-OH 0
0
-0 OOBn no- EDCI, CHZCI2 BnOOC OOBn
CH3OCH2CH2OCH3
87%
Scheme 2
CA 02309486 2000-05-26
-23-
O O
N COOBn
N -O
O 0 BnOOC-,,~N-,,,COOBn
35-70% CH2CI2, SILICONE"~~NH2
0
H
SILICONE"~N N,, COOBn
H
O BnOOC.,-,.N.1.,,OICOOBn
NTA-Silicone-Bn ester
H 2/Pd/C
CH3OCH2CH2OCH3
O
H
SILICONE'*~~N N COOH
H
O HOOC~NN-~ COOH
NTA-DMS-A11, -DMS-A15, -DMS-A21: 93 - 100%
NTA-AMS-132, -AMS-152: 37 - 47%
Scheme 3
Alternatively, the chelating silicone may be prepared starting from the
aminosilicones. In this case, ester protection was not used. Activation of the
carboxyl groups on the silicones (2.5 parts) was performed by reaction with N-
hydroxysuccinimide (5 parts) in the presence of 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (5 parts) in 1,2-
dimethoxyethane (220 parts). After removal of the solvents in vacuo, the
residue
was dissolved in methylene chloride (900 parts), washed with water, 1N HC1 and
then dried over sodium sulfate (Scheme 4).
The succinimidyl-terminated polydimethylsiloxane (65 parts) dissolved in
1,2-dimethoxyethane (150 parts) was vigorously stirred with N",N'-
bis(carboxymethyl)-L-lysine sodium salt (1.5 parts) dissolved in water (270
parts)
overnight. The two layers were separated and the solvents were removed in
vacuo from the bottom aqueous layer. The solid obtained was purified by
dialysis
CA 02309486 2000-05-26
-24-
against deionized water. Lyophilization led to the desired product as a white
solid.
OH O
SILICONE"`~~NHZ
0 O O 0
O
RT, N2, 16 h EDCI/NHS/CH2CI~DMF O
+ O
NEt3, CH2CI2 1NH RT, N2, 12 h 0
NH
LICONE
85-90% > 95% (1 H-NMR)
ILICONE
NTA 0
NEtJCH2CI2/MeOH N COOH
S I LICONE -'~-~N
RT, N2, 14-16 h H HOOC\/N\/COOH
NTA-DMS-A11, NTA-DMS-A15
36-49%
Scheme 4
Properties of the Chelating Silicones
In order to test the ability of NTA-DMS-A11 to form complexes with metal
cations, the chelating silicone was dissolved/dispersed in deionized water (5
x 10'
M) and treated with 10-3 M solutions of CuC12. FeC13, and CoC12.
The solutions obtained were analyzed by electrospray-mass-spectroscopy
lo (ES-MS). Ammonium hydroxide (1 drop of 0.1% NH4OH solution) was added
and the data were recorded in negative ion mode. It is known that Fe3+, Co2+,
and
Cu2+ form stable complexes with iminodiacetic acid type chelators (tridentate
ligands) and EDTA (a pentadentate ligand for CuZ+ and Co2+, and a hexadentate
ligand for Fe3+) at pH values larger than 5. Under these conditions, it is
also
known that silicones undergo depolymerization. Thus, it was not surprising to
observe the molecular ions of complexed fragments containing only one silicon-
based residue: the highest molecular weight ions [(Si-(CHz)3NCO(CH2)2CON-
NTA-M"+) I- were 529, 533, and 536, for M + = Fe3+, Co2+, and Cuz+,
respectively.
q-A uL3uY46o duau-ua-La
CA 02309486 2008-04-17
-25-
Svecific ExaW e9
Chemical Reagents
N`-Benzyloxycarbonyl-L-lysine (99%, Bachem), bromoacetic acid (97%, Aldrich),
palladium on activated charcoal (Degussa type E101NE/W, wet/Pd 10% dry
5-weight basis, water 50%, Aldrich), Celite (Aldrich), benzyl bromide (98%,
Aldrich), cesium carbonate (99%, Aldrich), di-t-butyl pyrocarbonate (99%,
Aldrich), anhydrous N,N-dimethylformamide (99.8%, Aldrich), trifluoroacetic
acid (99+%, Aldrich), succinic anhydride (99%, Aldrich), N-hydroxysuccinimide
(97%, Aldrich), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
to (98%, Aldrich), 4-nitrobenzyl chloride (99%, Aldrich), hydrogen bromide
(30%
solution in acetic acid, Aldrich), allyl alcohol (99%, Aldrich), n-
butyllithium (1.6
M solution in hexanes, Aldrich), chlorotrimethylsilane (redistilled, 99+%,
Aldrich), dicumyl peroxide (98%, Aldrich), 1,1,1,3,3,3-hexamethyldisilazane
(99.9%, Aldrich), platinum-divinyl-tetramethyldisiloxane complex (Karstedt's
15 catalyst) in xylene (Gelest), and vinylmagnesium bromide (1.0 M solution in
tetrahydrofuran, Aldrich), were used as received. Sodium bicarbonate,
potassium
hydroxide, and sodium hydroxide were obtained from BDH Chemicals, Acetic
acid and hydrochloric acid were obtained from Fischer Scientific. The NMR
solvents DZO, CDC13, CDZC12, CD3OD, and CD3CN were obtaised from Cambridge
20 Isotope Laboratories. Bio-Rad Laboratories supplied Bio-beads S-X1
(divinylbenzene crosslinked styrene). Toyopearl HW-40C gel filtration was
supplied by Supelco (Sigma-Aldrich Canada Ltd.). Amino-functionalized
silicones were purchased from Gelest Inc. (Table 1).
aa~Tyuawyyao tuuu-v~-~o
CA 02309486 2008-04-17
-26-
Table 1: Commercial Aminopropyl-Terminated Polydimethylsiloxanes
Aminopropylmethylsiloxane - Dimethylsiloxane Copolymers
H2N-0S-O-- ti-O~-- ji~~NH2
!
Homopolymers
Code Viscosity Molecular % Amine
(cSt) weight* (NH2)
DMS-A11 10 -15 850 - 900 3.2 - 3.8
DMS-A15 50 - 60 3000 1.0 -1.2
DMS-A21 100 -120 5000 0.6 - 0.7
Copolymers
Code Viscosity Molecular o Amine
(cSt) weight* (NH2)
AMS-132 80 -100 4500 - 5500 2-3
AMS-152 150 - 260 7000 - 8000 4-5
AMS-162 80-120 4000 - 5000 6-7
* Value provided by supplier; based on polystyrene standards.
Purification
Triethylamine (99%, Aldrich) was dried over KOH pellets, distilled and
stored over molecular sieves (4 A). Methylene chloride (98%, Aldrich) was
distilled from calcium hydride. 1,1,1-Trichloroethane (reagent grade, Caledon)
was filtered using a type HA 0.45 _m Millipore filter. Chlorodimethylsilane
(97%,
Aldrich), chlorodimethylvinylsilane (97%, Gelest), and chlorotrimethylsilane
l s (98%, Aldrich) were distilled under an inert (nitrogen) atmosphere and
stored
over molecular sieves. Diethyl ether (99.9%, Aldrich) was distilled over
lithium
aluminum hydride. Tetrahydrofuran (99%, Aldrich) was first distilled from
lithium aluminum hydride and then from potassium/benzophenone. A Milli-Q
,, ,
CA 02309486 2000-05-26
-27-
purification
system (Waters Associates, Millford, Massachusetts) was used to
deionize and further purify distilled water.
General Procedures
All syntheses were carried out in dry apparatus under a dry nitrogen
atmosphere utilizing conventional bench-top techniques.
Compound Characterization
'H NMR Fourier spectra were recorded on a Bruker DRX-500 (500 MHz)
spectrometer, Bruker AC-300 (300 MHz) spectrometer or Bruker AC-200 (200
MHz) spectrometer. 13C and 29Si NMR were performed on a Bruker DRX-500
spectrometer (at 125.7 MHz and 99.3 MHz for carbon and silicon, respectively),
a
Bruker AC-200 spectrometer (at 50.3 MHz for carbon), and a Bruker AC-300
spectrometer (at 75.4 MHz and 59.60 MHz for carbon and silicon, respectively).
Two dimensional 'H-, 13C-, and 1H-1H chemical shift correlation experiments
were recorded on a Bruker DRX-500 spectrometer. Chemical shifts for 'H NMR
spectra are reported with respect to the following standards: residual
chloroform
set at 7.24 ppm, CDHC12 set at 5.32 ppm, CD2HOD set at 3.30 ppm, the HDO peak
at
4.67 ppm, and tetramethylsilane set at 0 ppm. 13C NMR spectra are reported
with
respect to the following standards: chloroform set at 77 ppm, methylene
chloride
set at 53.8 ppm, and tetramethylsilane set at 0 ppm. Chemical shifts for 29Si
NMR
spectra are reported with respect to tetramethylsilane set at 0 ppm. Coupling
constants (J) are recorded in hertz (Hz). The abbreviations s = singlet, d =
doublet,
t = triplet, dd = doublet of doublets, m = multiplet, are used in reporting
the
spectra.
Mass spectrometry by chemical ionization (CI), with ammonia as the
reagent gas (NH3 CI), and electron impact (EI) mass spectra were recorded on a
VG Analytical ZAB-E double focusing mass spectrometer. Low-resolution spectra
were recorded for routine sample analysis of non-polar samples where
appropriate. Typical experimental conditions were: mass resolution 1000,
electron energy 70 eV, source temperature 200 C, source pressure of 2 x 10"6
mbar
for EI and 4 x 10-5 mbar for CI. Mass spectra were reported as percent
intensity (%)
versus mass/charge (m/z) ratio.
1.t1 VLJOyqaocVVV-U,-LO mm
CA 02309486 2008-04-17
-28-
Pneumatically-assisted electrospray.4Qnization mass spectrometry ESMS
was performed on a Micromass Quattro-LCO Triple quadrupole mass spectrometer
with dichloromethane, dichloromethane:methanol (50/50) or methanol as the
mobile phase at a flow rate of 15 L/min, with use of a Brownlee Microgradient
syringe pump. Samples were dissolved in dichloromethane:methanol (50/50) or
pure methanol. Ammonia or NH4OAc was added for analysis in the negative
mode ; for analysis in the positive mode, formic acid was added .
Infrared spectra in the 4000 - 400 cm 1 region were recorded on a BioRad
FTS-40 Fourier transform spectrometer. Solid samples were prepared as KBr
1o pellets (1-5 % w/w). Ultraviolet spectra were recorded on a Hewlett-Packard
8451
diode array spectrometer.
The molecular weight distributions of oligomers, grafted products, and
functional silicones were analyzed using a Waters Gel Permeation
Chromatograph equipped with a Waters 410 Differential Refractive Index
detector. Two Waters Styragel HR-4E (7.8x300 mm) columns in series were
utilized with 1,1,1-trichloroethane as solvent flowing at I mL/min for
functional
silicone analysis. Narrow molecular weight polydimethylsiloxane standards
(Polymer Laboratories) were used for calibration of the chromatographic
system.
Synthetic Procedures
Preparation of NTA
N",N `-Bis(carboxymethyl)-NE-(benzyloxycarbonyl)-L-lysine [N -Z NTA]
Id`-(Benzy1oxycarbonyl)-L-lysine (14.0 g, 0.05 mol) was dissolved in 2M
NaOH (125 mL, 0.25 mol) with stirring, and cooled to 0 C. Bromoacetic acid
(27.8
g, 0.2 mol) was added gradually with stirring and the pH of the solution was
adjusted to 12.5 - 13.0 by the addition of NaOH. After 2 h the reaction
mixture
was warmed to room temperature and the reaction was allowed to continue
overnight. Several pH adjustments were necessary in order to maintain a pH
value above 12. The reaction mixture was heated to 50 C for 4 h with stirring
3o and pH adjustments to maintain a pH above 12. After cooling to room
temperature, the product was precipitated from the solution by adding 1N HCI
to
CA 02309486 2000-05-26
-29-
1.8, filtered, and dried overnight under high vacuum at 50 C. A white solid
pH
(18.85 g, 0.048 mol, 95%) was obtained (m.p. 171 - 174 C).
1H NMR (DMSO-d6, 200. MHz) 8 9.19 (s, br, 3H, COZH), 7.33 (m, 5H, Ph), 7.24
(t, J= 4.8 Hz, 1H, NH), 4.99 (s, 2H, PhCH2), 3.46 (s, 4H, HOZCCHZ), 3.32 (m,
1H,
HO2CCHCH2), 2.95 (m, 2H, ZNHCHz), 1.57 (m, 2H, HO2CCHCH2), 1.36 (m, 4H,
ZNHCH2CH2CH2); 13 C NMR (D20: CD3CN (1:1), 50.3 MHz) S 175.4 (COZH), 175.1 (2
x COzH), 158.4 (HNC=O), 138.0 (Ph), 129.6 (Ph), 129.1 (Ph), 128.8 (Ph), 67.2
(HO2CCHCH2), 66.6 (2 x HO2CCH2), 55.5 (PhCHZ), 41.2 (ZNHCH2), 30.0
(HOZCCHCH2), 29.7 (ZNHCH2CH2), 24.2 (ZNHCH2CH2CH2); ESMS (-ve ion mode
+ 1 drop 0.2 % NH4OH): m/z (% intensity), 395 (100) [M"]; FT-IR (KBr): v(cm"1)
3377 (COO-H), 3024 (CH), 2942 (CH), 1728 (C=O), 1698 (N-C=O), 1536 (N-C=O).
N `,N `-Bis(carboxymethyl)-L-lysine [NTA]
NE-Z NTA (9.35 g, 0.027 mol) was dissolved in 1N NaOH (60 mL, 0.06 mol),
a spatula tip of 10% Pd/C was added, and hydrogenation was conducted at
normal pressure and room temperature overnight. The catalyst was removed by
vacuum filtration through a Celite pad. The clear colorless filtrate was
acidified
to pH 2 by dropwise addition of concentrated HCI, followed by the removal of
solvent in vacuo. The crude solid obtained was triturated with hot methanol (3
x
250 mL). The solvent was removed in vacuo and the product was dried under
vacuum at 50 C overnight: yield 5.24 g (0.02 mol, 83.3 %) white solid.
'H NMR (CD3OD: D20 (1:1), 200.13 MHz) S 3.84 (s, 5H, 2 x CH2COZH,
CHCO2H), 2.90 (t, 2H, J= 6.9 Hz, H2NCHZ), 1.82 (m, 2H, HOZCCHCH2), 1.56 (m,
4H,
H2NCH2CH2CH2); 13C NMR (CD3OD: D20 (1:1), 50.32 MHz) fi 173.2 (COZH), 171.4
(2x COZH), 68.8 (CHCOzH), 56.3 (2 x CH2CO2H ), 40.2 (H2NCH2), 27.7 (CH2), 27.5
(CHZ), 24.2 (CH2); ESMS (-ve mode + 1 drop 0.1 % NH4OH): m/z (% intensity),
261
(100) [M"]; FT-IR (KBr): - (cm"1) 3561 (COO-H), 3005 (CH), 2963 (CH), 1734
(C=O),
1627 (C=O).
CA 02309486 2000-05-26
-30-
N",N"-Bis(carboxymethyl)-N-(tert-butyloxycarbonyl)-L-lysine cesium-salt [N--
BOC NTA-Cs-salt
N",N"-Bis(carboxymethyl)-L-lysine (4.15 g, 15.8 mmol) was added to a
mixture of cesium carbonate (7.746 g, 23.8 mmol), water (25 mL), and dioxane
(25
mL). The solution was cooled to 0 C and di-tert-butyl dicarbonate (3.457 g,
15.8
mmol) was added with stirring. The reaction was continued at room temperature
for 45 min and the pH of the solution was maintained at a value of 8.5 by
addition of small amounts of cesium carbonate. Complete disappearance of
to N",N-bis(carboxymethyl)-L-lysine was shown by TLC on silica-gel (95% EtOH:
H20 (7:3), ninhydrin). The organic solvent was removed in vacuo and the
residual water was removed by lyophilization yielding the product as a white
powder. The product (as a Cs-salt) was used in the next step without further
purification. TLC in 95% EtOH: H20 (7:3), ninhydrin: Rf (pr d.) = 0.40, Rf
(Start.,r,at.) _
0.25.
N `,N"-Bis(carboxymethyl)-NE-(tert-butyloxycarbonyl)-L-lysine tribenzyl ester
[N--
BOC NTA-Bn-ester]
Benzyl bromide (1.436 g, 1 mL, 8.4 mmol) was added to a stirred
suspension of N-BOC NTA-Cs-salt (1.88 g, 2 mmol) in anhydrous DMF (50 mL).
The reaction was continued at room temperature overnight under nitrogen
atmosphere and vigorous stirring. The DMF was removed in vacuo (<1 mm Hg)
at 45 - 50 C. The product was purified by silica gel chromatography with
hexanes
/ ethyl acetate (80:20) as eluent. The solvents were removed in vacuo,
yielding a
pale yellow oil (0.563 g, 0.89 mmol, 44.5%). TLC in hexanes / ethyl acetate
(80:20),
ninhydrin: Rf = 0.09.
1H NMR (CDC13, 200.13 MHz) S 7.25 (m, 16H, 3 x Ph + NH), 5.01 (m, 6H, 3x
CH2Ph), 3.63 (s, 4H, 2 x CHzCOZBn), 3.39 (t, 1H, J= 5.1 Hz, CHCO2Bn), 2.94 (m,
2H,
NHCHz), 1.60 (m, 2H, NCH2CH2), 1,37 (s, 9H, BOC), 1.33 (m, 4H, CHZCHZCH); 13C
3o NMR (CDCl3, 50.32 MHz) 6 172.3 (CHCOzBn), 171.0 (2 x CH2CO2Bn), 155.9
(NHC=O), 135.7 (Ph), 135.6 (2 x Ph), 128.4 (Ph), 128.2 (Ph), 126.8 (Ph), 78.9
(CMe3),
66.3 (2 x CH2Ph), 65.1 (NCH), 64.7 (CH2Ph), 52.7 (2 x NCH2), 40.2 (NHCH2),
30.0
CA 02309486 2000-05-26
-31 -
(CH2), 29.4 (CH2), 28.4 (3 x CH3), 23.0 (CH2); ESMS (+ve mode + MeOH): m/z (%
intensity), 633 (100 %) [M+].
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester [NTA-Bn-ester]
N",N"-Bis(carboxymethyl)-Ne-(tert-butyloxycarbonyl)-L-lysine tribenzyl
ester (1.625 g, 2.6 mmol) in trifluoroacetic acid (20 mL) was stirred under a
nitrogen atmosphere at room temperature. After 45 min the reaction was
complete as shown by TLC on silica gel in hexanes / ethyl acetate (70:30)
(ninhydrin and UV detection). After solvent removal in vacuo (< 1 mm Hg) a
clear oil was obtained (1.68 g, 100% yield, product is the TFA salt), which
was used
in the next step without further purification. TLC in hexanes/ethyl acetate
(70:30): Rf cstart. mat.) = 0.25, Rf (Proauct) = 0.00.
1H NMR (CDC13, 200.13 MHz) S 7.32 (m, 15H, Ph), 5.10 (m, 6H, 3 x CH2Ph),
3.59 (m, 4H, 2 x NCH2), 3.53 (m, 1H, NCH), 3.05 (m, 2H, H2NCHZ), 1.75-1.43 (m,
6H, H2NCH2CHZCHZCH2).
N",N"-Bis(carboxymethyl)-Ne-succinyl-L-lysine tribenzyl ester [SUCC NTA-Bn-
ester]
Succinic anhydride (0.257 g, 2.6 mmol) was added to a stirred solution of
N",N"-bis(carboxymethyl)-L-lysine tribenzyl ester TFA salt (1.68 g, 2.6 mmol)
and
triethylamine (2.5 mL) in dry methylene chloride (30 mL), under nitrogen
atmosphere at room temperature. More triethylamine was added in order to
maintain basic conditions (pH 8.5 on wet litmus paper). After 3.5 h the
reaction
was complete as shown by TLC on silica gel in methylene chloride / acetic acid
(99.8:0.02), detected by UV-light and molybdenum reagent. The organic phase
was
washed with 1N HCl (80 mL) and brine (3 x 80 mL) and dried over anhydrous
sodium sulfate. Solvent removal in vacuo yielded the product as a clear
colorless
oil (1.422 g, 2.25 mmol, 87.5%). TLC in CH2C12 / AcOH (99.8: 0.02): Rf 1slut.
0.38,
Rf (proawt) = 0.13, Rf (succi,;c wd,.) = 0.90.
1H NMR (CDC13, 200.13 MHz) S 7.31 (m, 15H, 3x Ph), 6.39 (s, 1H, NH), 5.05
(m, 6H, CH2Ph), 3.65 (s, 4H, 2 x NCH2), 3.47 (t, 1H, J= 4.6 Hz, NCH), 3.17 (m,
2H,
CA 02309486 2000-05-26
-32-
NHCH2), 2.62 (m, 2H, HO2CCHZ), 2.45 (m, 2H, HO2CCH2CH2), 1.67 (m, 2H,
NCHCH2) 1.43 (m, 4H, NHCH2CH2CH2);13C NMR (CDC13, 50.32 MHz) 8 175.3
(C=O), 172.9 (C=O), 172.6 (C=O), 171.4 (2 x BnOC=O), 135.6 (Ph), 135.5 (Ph),
128.5
(Ph), 128.3 (Ph), 128.2 (Ph), 128.1 (Ph), 66.5 (2 x CH2Ph), 66.4 (CH2Ph), 64.1
(NCH),
52.9 (2 x NCH2), 39.4 (NHCH2), 30.6 (CH2), 30.3 (CHZ), 29.4 (CH2), 27.7 (CH2),
22.4
(CH2); ES-MS (+ve mode in MeOH): m/z (% intensity), 633 (100) [M+]; FT-IR
(neat): - (cm 1) 3369 (COO-H), 3036 (CH), 2950 (CH), 1737 (C=O), 1635 (N-C=O),
1554
(N-C=0).
1o N `,N `-Bis(carboxymethyl)-1V-succinimidylsuccinyl-L-lysine tribenzyl
ester [111~-
SSU NTA-Bn-ester]
N-Hydroxysuccinimide (0.78 g, 6.7 mmol) in dry 1,2-dimethoxyethane (80
mL) was added to N",N-bis(carboxymethyl)-N-succinyl-L-lysine tribenzyl ester
(4.234 g, 6.7 mmol) in dry methylene chloride (80 mL) under a nitrogen
atmosphere. After cooling to 0 C, 1-ethyl-3-(3-dimethylaminopropyl)-
carbodiimide hydrochloride (1.311 g, 6.7 mmol) was added with stirring. After
4 h
the solution was gradually warmed to room temperature and the reaction
continued overnight. The solvents were removed in vacuo. Silica gel
chromatography in hexanes/ ethyl acetate (15:85) (UV and molybdenum reagent
visualization) yielded the product as colorless oil (3.676 g, 5.8 mmol, 87%).
TLC in
hexanes: ethyl acetate (15:85): Rf (pr auct) = 0.13.
1H NMR (CDC13, 200.13 MHz) S 7.31 (m, 15H, 3 x Ph), 6.09 (s, 1H, NH), 5.07
(m, 6H, CH2Ph), 3.68 (s, 4H, 2 x NCH2), 3.47 (t, 1H, J= 7.5 Hz, NCH), 3.17 (m,
2H,
NHCH2), 2.95 (t, 2H, J= 7.3 Hz, NHS-OZCCH2), 2.74 (s, 4H, NHS), 2.55 (t, 2H,
J= 7.3
Hz, NHS-O2CCH2CH2), 1.67 (m, 2H, NCHCH2)11.43 (m, 4H, NHCH2CH2CH2);13C
NMR (CDC13, 50.32 MHz) 8172.5 (C=0), 171.2 (2x C=0),169.9 (C=O), 169.0 (2 x
BnOC=O), 168.2 (C=0), 135.6 (Ph), 135.5 (Ph), 128.5 (Ph), 128.3 (Ph), 128.1
(Ph), 66.4
(3 x CH2Ph), 64.2 (NCH), 52.8 (2 x NCH2), 39.1 (NHCH2), 30.6 (CHZ), 29.4
(CH2), 28.0
(CH2), 26.9 (CH2), 25.5 (2 x CH2), 22.5 (CH2); FT-IR (neat): v(cm 1) 3387 (COO-
H),
3036 (CH), 2946 (CH), 1816 (C=0),1786 (C=O), 1740 (C=O), 1674 (C=0),1544 (N-
C=O); ES-MS (+ve mode in MeOH): m/z (% intensity), 730 (100) [M+].
CA 02309486 2000-05-26
-33-
of Chelating Silicones (Condensation and Deprotection)
Preparation
Terminal Chelators
Monocarboxylic acids
Succinyl-Terminated Polydimethylsiloxane DMS-A11 [SUCC-DMS-A11].
Succinic anydride (1.6 g, 16 mmol) and aminopropyl terminated
polydimethylsiloxane DMS-All (7.2 g, 8 mmol) were dissolved in 200 mL dry
methylene chloride and triethylamine (10 mL) was added. The solution was
1o stirred under nitrogen at room temperature overnight. The organic phase was
washed with 1M HCl (2x 150 mL) and water (4 x 150 mL). After drying over
anhydrous sodium sulfate the solvent was removed in vacuo, yielding a pale
yellow oil (8.36 g, 95 %).
1H-NMR (CDC13, 200.13 MHz) S 3.17 (q, 4H, J= 6.4 Hz, 2 x
SiOCH2CH2CH2NH), 2.60 (m, 4H, 2 x O=CCH2), 2.50 (m, 4H, 2 x O=CCH2), 1.48 (m,
4H, 2 x SiOCH2CH2), 0.48 (m, 4H, 2 x SiOCH2), 0.03 (m, -65H, SiCH3); 13C-NMR
(CDC13, 50.32 MHz) S 176.4 (2 x O-C=O), 172.4 (2 x NH-C=O), 42.7 (2 x
SiOCH2CH2CH2NH), 30.7 (2 x OC(O)CHZ), 29.9 (2 x NC(O)CH2), 23.3 (2 x
SiOCHZCH2), 15.3 (2 x SiOCH2), 1.0 (SiCH3)1 0.1 (SiCH3); 29Si-NMR (CH2ClZ,
2o 59.63MHz, TMS ext. std): S 7.23, -19.33, -21.31, -22.04; FT-IR (neat): v(cm-
1) 3305
(COO-H), 2964 (CH), 1709 (C=O), 1651 (N-C=O), 1557 (N-C=O), 1261 (Si-CH3)1
1020
(Si-O), 802 (Si-CH3); GPC: neg. signal (RI det.): M,, = 1687, MW = 1908, PD =
1.13;
pos. signal (RI det.): Mn = 543, M,, = 562, PD = 1.03.
Succinyl-Terminated Polydimethylsiloxane DMS-A15 [SUCC-DMS-A15].
Succinic anydride (2.11 g, 21 mmol) and aminopropyl-terminated
polydimethylsiloxane DMS-A11 (30.0 g, 10 mmol) were dissolved in 700 mL dry
methylene chloride under a nitrogen atmosphere and dry triethylamine (10 mL)
was added. The solution was stirred under nitrogen at room temperature
overnight. The organic phase was washed with 1M HCl (2 x 150 mL) and water (4
CA 02309486 2000-05-26
-34-
x 150 mL). After drying over anhydrous sodium sulfate the solvent was removed
in vacuo, yielding a pale yellow oil (30.78 g, 95%).
1H-NMR (CDC13, 200.13 MHz) S 3.22 (q, 4H, J= 6.4 Hz, 2 x
SiCH2CH2CH2NH), 2.67 (m, 4H, 2 x O=CCHz), 2.49 (m, 4H, 2 x O=CCH2), 1.52 (m,
4H, 2 x SiCH2CH2), 0.50 (m, 4H, 2 x SiCH2)1 0.05 (m, -256H, SiCH3); 13C-NMR
(CDC13, 50.32 MHz) 8 172.4 (4 x C=O), 42.7 (2 x SiCH2CH2CH2NH), 30.7 (2 x
OC(O)CH2), 30.0 (2 x NC(O)CH2), 23.3 (2 x SiCHzCH2)1 15.3 (2 x SiCH2)1 1.8
(SiCH3)1
1.0 (SiCH3), 0.1 (SiCH3); FT-IR (neat): v(cm 1) 3300 (COO-H), 2965 (CH), 1713
(C=O),
1652 (N-C=O), 1557 (N-C=O), 1266 (Si-CH3), 1005 (Si-O); ES-MS (-ve mode in
to CHZCIz / MeOH): doubly charged ion series with peaks from 297 [M2-, n = 2]
to
1261 [Mz-, n = 28]; GPC: Mn = 1185, MW = 3552, PD = 3.0 (from 1H-NMR, MW =
3,408, n = 40-41, based on end-group analysis).
Succinyl-Terminated Polydimethylsiloxane DMS-A21 [SUCC-DMS-A21].
Succinic anydride (1.05 g, 10.5 mmol) and aminopropyl-terminated
polydimethyl-siloxane DMS-A21 (25.0 g, 5 mmol) were dissolved in 700 mL dry
methylene chloride under nitrogen atmosphere and dry triethylamine (7 mL)
was added. The solution was stirred under nitrogen at room temperature
overnight. The organic phase was washed with 1M HCl (2x 110 mL) and
deionized water (4x 110 mL). After drying over anhydrous sodium sulfate the
solvent was removed in vacuo, yielding a pale yellow oil (25.86 g, 4.97 mmol,
95
1H-NMR (CDC13, 200.13 MHz) S 3.23 (q, 4H, J= 6.7 Hz, 2 x
SiCH2CH2CH2NH), 2.68 (m, 4H, 2 x O=CCH2), 2.49 (m, 4H, 2 x O=CCH2), 1.52 (m,
4H, 2 x SiCH2CH2), 0.51 (m, 4H, 2 x SiCH2), 0.05 (m, ~422H, SiCH);13C-NMR
(CDC13, 50.32 MHz) S 175.3 (2 x C=O), 172.4 (2 x C=O), 42.8 (2 x
SiCH2CH2CH2NH),
30.8 (2 x OC(O)CH2), 30.0 (2 x NC(O)CH2), 23.4 (2 x SiCH2CHz)115.4 (2 x
SiCH2)01.8
(SiCH3), 1.0 (SiCH3), 0.1 (SiCH3); 29Si-NMR (CHZCIZ, 59.63MHz, TMS ext. std):
S
3o 7.16, -21.33, -21.47, -22.14; FT-IR (neat): v(cm') 3300 (COO-H), 2965 (CH),
1715
(C=O), 1651 (N-C=O), 1558 (N-C=O), 1261 (Si-CH3), 1096 (Si-O), 1024 (Si-O),
801 (Si-
CA 02309486 2000-05-26
-35-
CH3); GPC: Mn = 1369, Mti = 5746, PD = 4.19 (from 1H-NMR, MW = 5,500, n = 68-
69,
based on end-group analysis).
Tricarboxylic Acids
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated
polydimethylsiloxane DMS-A11 [NTA-DMS-Al1-Bn-Ester]
Aminopropyl-terminated poly-dimethylsiloxane DMS-A11 (0.394 g, 0.45
lo mmol) was added to a stirred solution of N",N"-bis(carboxymethyl)-Ne-
succinimidylsuccinyl-L-lysine tribenzyl ester (0.72 g, 1 mmol) in dry
methylene
chloride (40 mL) under a nitrogen atmosphere. After stirring for 5 h at room
temperature the solvent was removed in vacuo. Silica gel chromatography using
methylene chloride/methanol (97:3) yielded the product as a clear pale yellow
solid (0.900 g, 0.43 mmol, 95%). TLC in CH2C12 / MeOH (97:3): Rf (product) -
0.14 -
0.40, Rf (NHS-OH) = 0.04, Rf (DMS-A11) -Of Rf (NHS-ester start. mat.) = 0.58.
1H NMR (CDC13, 200.13 MHz) S 7.31 (m, 30H, 6 x Ph), 6.20 (s, br, 4H, NH), 5.06
(m,
12H, CH2Ph), 3.67 (s, 8H, 4 x NCH2), 3.47 (t, 2H, J= 7.5 Hz, 2 x NCH), 3.15
(m, 8H, 4
x NHCHz), 2.46 (s, 8H, 2 x O=CCH2CH2C=O), 1.65 (m, 4H, 2 x NCHCHZ), 1.43 (m,
12H, 2 x SiCH2CH2, 2 x NHCH2CH2CH2), 0.49 (m, 4H, 2 x SiCHZ), 0.04 (m, -55H,
SiCH3);13C NMR (CDC13, 50.32 MHz) 5 172.5 (C=O), 172.3 (C=O), 172.1 (C=O),
171.2
(C=O), 135.7 (Ph), 135.6 (Ph), 128.5 (Ph), 128.3 (Ph), 128.1 (Ph), 66.4 (6 x
CH2Ph), 64.4
(2 x NCH), 52.8 (4 x NCH2), 42.5 (2 x NHCH2), 39.2 (2 x NHCH2), 32.0 (2 x
CH2), 31.8
(2 x CH2), 29.7 (2 x CH2), 28.4 (2 x CH2), 23.4 (2 x CH2), 22.8 (2 x CH2),
15.4 (2 x CH2),
1.2 (CH3), 1.0 (CH3)10.3 (CH), 0.1 (CH3); 29Si NMR (CH2ClZ, 59.63MHz, TMS ext.
std): S 7.22, -20.9, -21.50, -22.10; ES-MS (+ve mode in MeOH + 1 drop 0.1 %
HCOOH): singly charged ion series with peaks from 1478 [M+, n = 0] to 2364
[M+, n
= 11]; doubly charged ion series with peaks from 739 [M2+, n = 0] to 1407
[Mz+, n =
181; FT-IR (neat): v(cm 1) 3301 (COO-H), 2962 (CH), 1743 (C=O), 1642 (N-C=O),
1549
(N-C=O), 1261 (Si-CH3)11091 (Si-O), 1028 (Si-O), 801 (Si-CH3).
CA 02309486 2000-05-26
-36-
N",N"-Bis(carboxymethyl)-L-lysine terminated polydimethylsiloxane DMS-A11
[NTA-DMS-A11]
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated
polydimethylsiloxane DMS-A11 (0.506 g, 0.24 mmol) was dissolved in dry 1,2-
dimethoxyethane (40 mL). 10% palladium on charcoal (a spatula tip) was added,
and hydrogenation at room temperature and normal pressure was allowed to
proceed for 8 h. The completion of the reaction was shown by the disappearance
of the starting material as checked by TLC using methylene chloride / methanol
(93:7). The catalyst was removed by filtration through a Celite pad and the
solvent was removed in vacuo yielding the product as a pale yellow clear solid
(0.370 g, 0.24 mmol, 100%).
1H NMR (CD3OD, 200.13 MHz) S 3.64 (s, 8H, 4 x NCH2), 3.47 (t, 2H, J= 6.8 Hz, 2
x NCH), 3.15 (m, 8H, 4 x NHCH2), 2.45 (s, 8H, 2 x O=CCH2CH2C=O),1.70 (m, 4H, 2
x
NCHCH2), 1.52 (m, 12H, 2 x SiCH2CHZ, 2 x NHCHZCHZCH2), 0.56 (m, 4H, 2 x
SiCH2), 0.08 (m, -60H, SiCH3); 13C NMR (CD3OD, 50.32 MHz) S 175.8 (C=O), 174.6
(C=O), 66.6 (2 x NCH), 55.3 (4 x NCHZ), 43.6 (2 x NHCH2), 40.1 (2 x
NHCH2)032.4 (4
x CH2), 30.7 (2 x CH2), 29.9 (2 x CHZ), 24.7 (2 x CHz), 24.4 (2 x CH2),16.4 (2
x CH2), 1.4
(CH3), 0.3 (CH3); ES-MS (-ve mode in CH2C12 / MeOH + 1 drop 0.25mM NH4OAc):
singly charged ion series with peaks from 936 [M-, n = 0] to 2120 [M", n =
16];
doubly charged ion series with peaks from 467 [M2- , n = 0] to 1209 [M2-, n =
20]; FT-
IR (neat): v(cm') 3297 (COO-H), 2963 (CH), 1730 (C=O), 1646 (N-C=O), 1553 (N-
C=O), 1261 (Si-CH3)11090 (Si-O), 1029 (Si-O), 801 (Si-CH3).
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated polydimethyl-
siloxane DMS-A15 [NTA-DMS-A15-Bn-Esterj
Aminopropyl-terminated poly-dimethylsiloxane DMS-A15 (1.340 g, 0.45
mmol) was added to a stirred solution of N",N"-bis(carboxymethyl)-NE-
succinimidylsuccinyl-L-lysine tribenzyl ester (0.72 g, 1 mmol) in dry
methylene
chloride (40 mL) under a nitrogen atmosphere. After stirring for 5 h at room
CA 02309486 2000-05-26
-37-
temperature a small amount of aminopropylmethylsiloxane-dimethylsiloxane
copolymer AMS-162 (0.5 g) was added in order to react with excess NHS-
activated
starting material and the stirring was continued for 15 min. The solvent was
removed in vacuo. Silica gel chromatography using methylene
chloride/methanol (95:5) yielded the product as a clear pale yellow solid
(1.136 g,
55 %). TLC in CH2C12 / MeOH (95:5): Rf (product) = 0.08 - 0.24, Rf (NHS-0H) =
0.02, Rf (DMs-
A15) = 0.00, Rf (AMS-162 NTA-Bn-ester) = 0.00.
'H NMR (CDC13, 200.13 MHz) S 7.31 (m, 30H, 6 x Ph), 6.21 (s, br, 4H, NH), 5.06
(m, 12H, CH2Ph), 3.67 (s, 8H, 4 x NCH2), 3.47 (t, 2H, j= 7.3 Hz, 2 x NCH),
3.16 (m,
lo 8H, 4 x NHCH2), 2.47 (s, 8H, 2 x O=CCH2CH2C=O), 1.66 (m, 4H, 2 x NCHCH2)
1.46
(m, 12H, 2 x SiCH2CH2, 2 x NHCH2CH2CH2), 0.50 (m, 4H, 2 x SiCH2), 0.06 (m,
-330H, SiCH);13C NMR (CDC13, 50.32 MHz) S 172.5 (C=O), 172.3 (C=O), 172.1
(C=0), 171.2 (C=0),135.7 (Ph), 135.6 (Ph), 128.6 (Ph), 128.3 (Ph), 128.2 (Ph),
66.4 (6 x
CH2Ph), 64.4 (2 x NCH), 52.8 (4 x NCH2), 42.5 (2 x NHCH2), 39.2 (2 x NHCH2),
32.0
(2 x CH2), 31.8 (2 x CH2), 29.7 (2 x CH2), 28.4 (2 x CH2), 23.5 (2 x CH2),
22.8 (2 x CH2),
15.4 (2 x CH2), 1.7 (CH3), 1.0 (CH3)10.3 (CH3), 0.1 (CH3); ES-MS (+ve mode in
MeOH
+ 1 drop 0.2 % HCOOH): doubly charged ion series with peaks from 1407 [M2+, n
=
18] to 2291 [M+, n = 41]; triply charged ion series with peaks from 1260 [M3+,
n = 31]
to 1851 [M3+, n = 55]; GPC: Mn = 2137, M,, = 2398, PD = 1.12; FT-IR (neat):
v(cm 1)
2o 3299 (COO-H), 2964 (CH), 1746 (C=O), 1641 (N-C=O), 1550 (N-C=O), 1261 (Si-
CH3),
1092 (Si-O), 1021 (Si-O), 800 (Si-CH3).
1V",N"-Bis(carboxymethyl)-L-lysine-terminated polydimethylsiloxane DMS-A15
[NTA-DMS-A15]
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated
polydimethylsiloxane DMS-A15 (0.547 g, 0.12 mmol) was dissolved in dry 1,2-
dimethoxyethane (25 mL), 10% palladium on charcoal (a spatula tip) was added,
and hydrogenation at room temperature and normal pressure was allowed to
proceed for 8 h. The completion of the reaction was shown by the disappearance
of the starting material as checked by thin layer chromatography using
methylene chloride / methanol (93 : 7). The catalyst was removed by filtration
CA 02309486 2000-05-26
-38-
through a Celite pad and the solvent was removed in vacuo yielding the product
as a pale yellow clear solid (0.397 g, 0.11 mmol, 95 %).
1H NMR (CD3OD, 200.13 MHz) S 3.62 (s, 8H, 4 x NCH2), 3.49 (t, 2H, J= 7.0 Hz, 2
x NCH), 3.13 (m, 8H, 4 x NHCH2), 2.69 (s, 8H, 2 x O=CCH2CH2C=O), 1.76 (m, 4H,
2 x
NCHCH2), 1.52 (m, 12H, 2 x SiCH2CH2, 2 x NHCH2CH2CH2), 0.59 (m, 4H, 2 x
SiCH2), 0.09 (m, -360H, SiCH3);13C NMR (CD3OD, 50.32 MHz) S 175.8 (C=O), 174.6
(C=O), 66.6 (2 x NCH), 55.3 (4 x NCH2), 43.6 (2 x NHCH2), 40.1 (2 x NHCH2),
32.5 (4
x CH2), 30.7 (2 x CH), 29.9 (2 x CH2), 24.7 (2 x CH2), 24.4 (2 x CH2), 16.4 (2
x CH2)1 1.5
(CH3)10.4 (CH3); ES-MS (-ve mode in CH2C12 / MeOH): doubly charged ion series
1o with peaks from 1096 [M2-, n 17] to 2392 [M2-, n 52]; triply charged ion
series
with peaks from 756.5 [M', n 18] to 1545 [M3-, n 51]; FT-IR (neat): v(cm-1)
3290
(COO-H), 2964 (CH), 1729 (C=O), 1642 (N-C=O), 1261 (Si-CH3)11091 (Si-O), 1020
(Si-
0), 800 (Si-CH3).
N",N-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated polydimethyl-
siloxane DMS-A21 [NTA-DMS-A21-Bn-Ester]
Aminopropyl-terminated polydimethylsiloxane DMS-A21 (1.125 g, 0.23
mmol) was added to a stirred solution of N",N"-bis(carboxymethyl)-Ne-
2o succinimidylsuccinyl-L-lysine tribenzyl ester (0.365 g, 0.5 mmol) in dry
methylene
chloride (60 mL) under a nitrogen atmosphere. After stirring for 5 h at room
temperature a small amount of aminopropylmethylsiloxane-dimethylsiloxane
copolymer AMS-162 (0.5 g) was added in order to react with excess NHS-
activated
starting material and the stirring was continued for 15 min. The solvent is
removed in vacuo. Silica gel chromatography using methylene
chloride/methanol (95:5) yielded the product as a clear pale yellow solid
(1.01 g,
0.16 mmol, 70 %). TLC in CH2C12 / MeOH (95:5): Rf (Proauct) = 0.07 - 0.29, Rf
(NHS-oH) -
0.02, Rf (DMS-A15) = 0.00, Rf (AMS-162 NTA-Bn-ester) - 0.00.
1H NMR (CDC13, 200.13 MHz) S 7.30 (m, 30H, 6 x Ph), 6.15 (s, br, 4H, NH), 5.07
(m, 12H, CH2Ph), 3.67 (s, 8H, 4 x NCH2), 3.45 (t, 2H, J= 7.1 Hz, 2 x NCH),
3.16 (m,
8H, 4 x NHCH2), 2.47 (s, 8H, 2 x O=CCH2CH2C=O), 1.66 (m, 4H, 2 x NCHCH2),1.44
(m, 12H, 2 x SiCH2CH2, 2 x NHCH2CH2CH2), 0.50 (m, 4H, 2 x SiCH2), 0.06 (m,
~490H, SiCH3);13C NMR (CDC13, 50.32 MHz) 8 172.5 (C=O), 172.3 (C=O), 171.2
CA 02309486 2000-05-26
-39-
(C=O), 135.7 (Ph), 135.6 (Ph), 128.6 (Ph), 128.3 (Ph), 128.1 (Ph), 66.4 (6 x
CH2Ph), 64.4
(2 x NCH), 52.8 (4 x NCH2), 42.6 (2 x NHCH2), 39.2 (2 x NHCH2), 31.7 (4 x
CH2), 29.7
(2 x CH2)028.3 (2 x CH2)023.4 (2 x CH2), 22.8 (2 x CH2)015.4 (2 x CHz), 1.7
(CH), 1.0
(CH3), 0.3 (CH3)1 0.1 (CH3); 29Si NMR (CH2C12, 59.63MHz, TMS ext. std): 6
7.10,
-19.19, -22.03, -22.76; ES-MS (+ve mode in MeOH + 1 drop 0.2 % HCOOH): doubly
charged ion series with peaks from 1407 [M2+, n = 18] to 2291 [M+, n 41];
triply
charged ion series with peaks from 1260 [M3+, n = 31] to 1851 [M3+, n 55];
GPC: Mn
= 1922, Mti, = 2813, PD = 1.46; FT-IR (neat): v(cm 1) 3299 (COO-H), 2964 (CH),
1746
(C=O), 1642 (N-C=O), 1550 (N-C=O), 1261 (Si-CH3)11092 (Si-O), 1021 (Si-O), 801
(Si-
to CH3).
N `,N"-Bis(carboxymethyl)-L-lysine-terminated polydimethylsiloxane DMS-A21
[NTA-DMS-A21]
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester-terminated
polydimethylsiloxane DMS-A15 (1.01 g, 0.16 mmol) was dissolved in dry 1,2-
dimethoxyethane (25 mL), 10% palladium on charcoal (a spatula tip) was added,
and hydrogenation at room temperature and normal pressure was allowed to
proceed for 8 h. The completion of the reaction was shown by the disappearance
of the starting material as checked by thin layer chromatography using
methylene chloride / methanol (93:7). The catalyst was removed by filtration
through a Celite pad and the solvent was removed in vacuo yielding the product
as a pale yellow clear solid (0.832 g, 0.15 mmol, 93%).
1H NMR (CDC13 / CD3OD (1:1), 200.13 MHz) S 3.64 (s, 8H, 4 x NCH2), 3.48
(m, 2H, 2 x NCH), 3.14 (m, 8H, 4 x NHCH2), 2.44 (s, 8H, 2 x O=CCHZCH2C=O),
1.68
(m, 4H, 2 x NCHCH2), 1.49 (m, 12H, 2 x SiCH2CH2, 2 x NHCH2CHZCHZ), 0.52 (m,
4H, 2 x SiCH2), 0.06 (m, -490H, SiCH);13C NMR (CDC13 / CD3OD (1:1), 50.32 MHz)
8174.6 (C=O), 173.2 (C=O), 66.6 (2 x NCH), 52.5 (4 x NCH2), 41.6 (2 x NHCH2),
38.1
(2 x NHCH2), 30.7 (2 x CHZ), 28.8 (2 x CH2), 28.5 (2 x CHZ), 27.8 (CH2), 22.4
(2x CH2),
3o 22.2 (2x CH2)114.5 (2x CHZ), -0.1 (CH3)1-1.2 (CH3), -3.0 (CH3); ES-MS (-ve
mode in
CH2ClZ / MeOH): doubly charged ion series with peaks from 1096 [Mz", n = 17]
to
CA 02309486 2000-05-26
-40-
2249 [MZ-, n = 48]; FT-IR (neat): v(cm-1) 3300 (COO-H), 2963 (CH), 1730 (C=O),
1643
(N-C=O), 1549 (N-C=O), 1261 (Si-CH3)11091 (Si-O), 1020 (Si-O), 798 (Si-CH3).
Pendant Chelating Silicones
Monocarboxylic Acids
Succinyl-Pendant Polydimethylsiloxane AMS-132 [SUCC-AMS-132].
Succinic anydride (1.34 g, 13.4 mmol) and aminopropylmethylsiloxane-
dimethylsiloxane copolymer AMS-132 (30.0 g, 6.7 mmol, 13.4 meq NH2) were
dissolved in 700 mL dry methylene chloride under a nitrogen atmosphere and
dry triethylamine (9.6 mL) was added. The solution was stirred under nitrogen
at
room temperature overnight. The organic phase was washed with 1M HCl (2 x 50
mL) and deionized water (4 x 90 mL). After drying over anhydrous sodium
sulfate, the solvent was removed in vacuo, yielding a pale yellow oil (26.85
g, 5.8
mmol, 86%).
1H-NMR (CDC13, 200.13 MHz) S 3.23 (q, 4H, J= 6.7 Hz, 2 x
SiCH2CH2CH2NH), 2.64 (m, 4H, 2 x O=CCHz), 2.50 (m, 4H, 2 x O=CCHZ), 1.52 (m,
4H, 2 x SiCH2CHz)1 0.51 (m, 4H, 2 x SiCH2)1 0.05 (m, -364H, SiCH3); 29Si-NMR
(CH2Clz, 59.63MHz, TMS ext. std): 8 6.99, -19.41, -21.65, -21.82, -22.13, -
22.45; FT-IR
(neat): v(cm 1) 3300 (COO-H), 2965 (CH), 1716 (C=O), 1648 (N-C=O), 1261 (Si-
CH3),
1090 (Si-O), 1021 (Si-O), 801 (Si-CH3); GPC: Mõ = 1348, M,, = 5101, PD = 1.62
(from
1H-NMR, MW = 7,700, n = 95, m = 1.63, calculated for 2.5% NHZ).
Succinyl-Pendant Polydimethylsiloxane AMS-152 [SUCC-AMS-152].
Succinic anydride (2.25 g, 22.5 mmol) and aminopropylmethylsiloxane-
dimethylsiloxane copolymer AMS-152 (35.0 g, 5 mmol, 22.5 meq NH2) were
dissolved in 700 mL dry methylene chloride under a nitrogen atmosphere and
dry triethylamine (16.7 mL) was added. The solution was stirred under nitrogen
at room temperature overnight. The organic phase was washed with 1M HCl (2 x
90 mL) and deionized water (4 x 150 mL). After drying over anhydrous sodium
CA 02309486 2000-05-26
-41 -
sulfate, the solvent was removed in vacuo, to yield a pale yellow oil (24.69
g, 3.32
mmol, 66%).
1H-NMR (CDC13, 200.13 MHz) S 3.20 (m, 4H, 2 x SiCH2CH2CH2NH), 2.63 (m,
4H, 2 x O=CCH2), 2.48 (m, 4H, 2 x O=CCHZ), 1.52 (m, 4H, 2 x SiCH2CHZ), 0.48
(m,
4H, 2 x SiCH2), 0.05 (m, - 163H, SiCH3);13C-NMR (CDC13, 50.32 MHz) S 176.4 (2
x
C=O), 172.4 (2 x C=O), 42.5 (2 x SiCH2CHzCH2NH), 30.7 (2 x OC(O)CHZ), 30.0 (2
x
NC(O)CHZ), 23.0 (2 x SiCHzCH2), 14.5 (2 x SiCHz), 1.8 (SiCH3)1 1.0 (SiCH3)10.3
(SiCH), -0.5 (SiCH3); 29Si-NMR (CH2C12, 59.63MHz, TMS ext. std): S 6.97, -
19.44, -
21.68, -22.16, -22.86; FT-IR (neat): v(cm') 3304 (COO-H), 2965 (CH), 1715
(C=O),
1o 1654 (N-C=O), 1558 (N-C=0), 1262 (Si-CH3)01094 (Si-O), 1022 (Si-O), 801 (Si-
CH);
GPC: Mõ = 1200, MW = 3162, PD = 2.63 (from'H-NMR, MW = 10,600, n = 116, m
4.5, calculated for 4.5% NHz).
Succinyl-Pendant Polydimethylsiloxane AMS-162 (SUCC-AMS-162].
Succinic anydride (2.71 g, 27 mmol) and aminopropylmethylsiloxane-
dimethylsiloxane copolymer AMS-162 (30.0 g, 7.5 mmol, 27 meq NH2) were
dissolved in 700 mL dry methylene chloride under a nitrogen atmosphere and
dry triethylamine (18.1 mL) was added. The solution was stirred under nitrogen
at room temperature overnight. The organic phase was washed with 1M HC1 (2 x
100 mL) and deionized water (4 x 160 mL). After drying over anhydrous sodium
sulfate, the solvent was removed in vacuo, to yield a pale yellow oil (22.73
g, 5.21
mmol, 70%).
1H-NMR (CDCl3, 200.13 MHz) 8 3.20 (m, 4H, 2 x SiCH2CH2CH2NH), 2.63 (m,
4H, 2 x O=CCH2), 2.49 (m, 4H, 2 x O=CCHZ), 1.53 (m, 4H, 2 x SiCH2CH2), 0.48
(m,
4H, 2 x SiCH2), 0.05 (m, -82H, SiCH3);13C-NMR (CDC13, 50.32 MHz) 8 176.2 (2 x
C=O), 172.5 (2 x C=O), 42.5 (2 x SiCH2CH2CH2NH), 30.7 (2 x OC(O)CH2), 30.0 (2
x
NC(O)CH2), 23.0 (2 x SiCHzCH2)014.5 (2 x SiCHZ), 1.7 (SiCH3), 1.0 (SiCH3)10.3
(SiCH3), -0.6 (SiCH3); 29Si-NMR (CHzCl21 59.63MHz, TMS ext. std): S 6.98, -
19.42, -
3o 21.66, -22.15, -22.52; FT-IR (neat): v(cm 1) 3310 (COO-H), 2959 (CH), 1711
(C=O),
1654 (N-C=O), 1553 (N-C=O), 1261 (Si-CH3)11091 (Si-O), 1021 (Si-O), 801 (Si-
CH);
CA 02309486 2000-05-26
-42-
GPC: Mõ = 785, M,, = 1709, PD = 2.18 (from 1H-NMR, MW = 5,100, n= 44-45, m
3.7, calculated for 6.5% NHZ).
Tricarboxylic Acids
N",N"-Bis(carboxymethyl)-L-lysine tribenzyl ester pendant polydimethyl-
siloxane
AMS-132 [NTA-AMS-132-Bn-Ester]
Aminopropylmethylsiloxane-dimethylsiloxane copolymer AMS-132 (1.377
1o g, 0.28 mmol, 0.46 meq NH2) was added to a stirred solution of N",N"-
bis(carboxymethyl)-NE-succinimidylsuccinyl-L-lysine tribenzyl ester (0.403 g,
0.55
mmol) in dry methylene chloride (50 mL) under a nitrogen atmosphere. After
stirring for 5 h at room temperature a small amount of
aminopropylmethylsiloxane-dimethylsiloxane copolymer AMS-162 (0.2 g) was
added in order to react with excess NHS-activated starting material and the
stirring was continued for 15 min. The solvent was removed in vacuo. Silica
gel
chromatography using methylene chloride/methanol (95:5) yielded the product
as a clear pale yellow solid (0.783 g, 0.15 mmol, 54%). TLC in CH2CI2 / MeOH
(95:5): Rf (prodõct) = 0.10 - 0.22, Rf (AMs-132) = 0.
1H NMR (CDC13, 200.13 MHz) 8 7.30 (m, 15H, 3 x Ph), 6.10 (s, br, 2H, NH), 5.06
(m, 6H, CH2Ph), 3.67 (s, 4H, 2 x NCH2), 3.44 (t, 1H, J= 6.9 Hz, NCH), 3.19 (m,
4H, 2
x NHCH2), 2.46 (s, 4H, O=CCH2CH2C=O), 1.65 (m, 2H, NCHCH2) 1.40 (m, 6H,
SiCH2CH2, NHCHZCH2CHZ), 0.47 (m, 2 H, SiCH2), 0.05 (m, -205H, SiCH);13C
NMR (CDC13, 50.32 MHz) S 172.5 (C=O), 171.2 (C=O), 135.7 (Ph), 128.6 (Ph),
128.3
(Ph), 128.2 (Ph), 66.4 (3 x CH2Ph), 64.5 (NCH), 52.8 (2 x NCH2), 42.4 (NHCH2),
39.2
(NHCH2), 31.8 (2 x CH2), 29.7 (CH2), 28.4 (CH2), 23.5 (CH2), 22.8 (CHZ), 15.4
(CH2)11.7
(CH3), 1.0 (CH3)10.3 (CH3); GPC: Mn = 3311, MN, = 5566, PD = 1.68 (from 1H
NMR,
MW = 5251, n = 53, m = 1.63, calculated for 2.5% NH2 ); FT-IR (neat): v(cm"1)
3300
(COO-H), 2965 (CH), 1747 (C=O), 1642 (N-C=O), 1550 (N-C=O), 1262 (Si-CH3)11091
(Si-O), 1021 (Si-O), 801 (Si-CH3).
CA 02309486 2000-05-26
-43-
N",N"-Bis(carboxymethyl)-L-lysine pendant polydimethylsiloxane AMS-132
[NTA-AMS-132]
N",N `-Bis(carboxymethyl)-L-lysine tribenzyl ester pendant
polydimethylsiloxane AMS-132 (0.783 g, 0.15 mmol) was dissolved in dry 1,2-
dimethoxyethane (25 mL), 10% palladium on charcoal (a spatula tip) was added,
and hydrogenation at room temperature and normal pressure was allowed to
proceed for 8 h. The completion of the reaction was shown by the disappearance
of the starting material as checked by TLC using methylene chloride/methanol
to (93:7). The catalyst was removed by filtration through a Celite pad. A
thick gel-
like material separated upon solvent removal in vacuo. This gel was dissolved
in methylene chloride (20 mL) and water (20 mL) was added to this solution
with
stirring. The product precipitated upon methylene chloride removal in vacuo.
The precipitation was repeated with 20 mL CH2C12 and 20 mL water. After drying
in vacuo (< 0.1 mm Hg) the final product was obtained as a white solid (0.332
g,
0.07 mmol, 47%).
'H NMR (CDC13 / CD3OD (1:1), 200.13 MHz) S 3.59 (m, 4H, 2 x NCH2), 3.48 (m,
1H, NCH), 3.13 (m, 4H, 2 x NHCH2), 2.45 (s, 4H, O=CCH2CH2C=O), 1.70 - 1.40 (m,
8H, NCHCH2 + SiCHzCH2 + NHCH2CHZCH2), 0.53 (m, 2H, SiCH2), 0.04 (m, -367H,
SiCH);13C NMR (CDC13 / CD3OD (1:1), 50.32 MHz) 6173.8 (C=O), 172.3 (C=O), 64.6
(NCH), 53.4 (2 x NCH2), 41.3 (NHCH2), 38.0 (NHCH2), 30.3 (2 x CHZ), 28.5 (2 x
CH2),
27.7 (CH2), 22.1 (CH2),13.6 (CHz), -0.2 (CH3), -0.5 (CH3); FT-IR (neat): v (cm
1) 3337
(COO-H), 2964 (CH), 1728 (C=0),1644 (N-C=O), 1261 (Si-CH3)11092 (Si-O),1021
(Si-
0), 800 (Si-CH3) (from 'H NMR, MW = 4838, n = 53, m = 1.63, calculated for
2.5%
NHZ).
N",Na-Bis(carboxymethyl)-L-lysine tribenzyl ester pendant polydimethylsiloxane
AMS-152 [NTA-AMS-152-Bn-Ester]
Aminopropylmethyl-siloxane-dimethylsiloxane copolymer AMS-152
(1.233 g, 0.16 mmol, 0.72 meq NH2) was added to a stirred solution of N `,N `-
bis(carboxymethyl)-N-succinimidylsuccinyl-L-lysine tribenzyl ester (0.584 g,
0.8
mmol) in dry methylene chloride (50 mL) under a nitrogen atmosphere. After
CA 02309486 2000-05-26
-44-
stirring for 5 h at room temperature a small amount of
aminopropylmethylsiloxane-dimethylsiloxane copolymer AMS-162 (0.2 g) was
added in order to react with excess NHS-activated starting material and the
stirring was continued for 15 min. The solvent was removed in vacuo. Silica
gel
chromatography using methylene chloride / methanol (95:5) yielded the product
as a clear pale yellow solid (0.604 g, 0.06 mmol, 37%). TLC in CH2C12 / MeOH
(95:5): Rf (product) - 0.08 - 0.25, Rf (AMS132) = 0=
'H NMR (CDC13, 200.13 MHz) S 7.30 (m, 15H, 3 x Ph), 6.12 (s, br, 2H, NH), 5.06
(m, 6H, CH2Ph), 3.67 (s, 4H, 2 x NCH2), 3.44 (t, 1H, J= 7.4 Hz, NCH), 3.15 (m,
4H, 2
Io x NHCH2), 2.46 (s, 4H, O=CCH2CH2C=O), 1.64 (m, 2H, NCHCH2), 1.40 (m, 6H,
SiCH2CH2, NHCH2CH2CH2), 0.47 (m, 2 H, SiCH2), 0.05 (m, -175H, SiCH3);13C
NMR (CDC13, 50.32 MHz) 8172.5 (C=O), 171.2 (C=O), 135.7 (Ph), 128.6 (Ph),
128.3
(Ph), 128.2 (Ph), 66.4 (3 x CH2Ph), 64.5 (NCH), 52.8 (2 x NCH2), 42.4 (NHCH2),
39.2
(NHCH2), 31.9 (2 x CH2), 29.7 (CH2), 28.4 (CH2), 23.2 (CH2), 22.8 (CH2), 14.7
(CH2), 1.8
(CH3), 1.0 (CH3), 0.3 (CH3); FT-IR (neat): v(cm 1) 3301 (COO-H), 2965 (CH),
1747
(C=O), 1642 (N-C=O), 1550 (N-C=O), 1261 (Si-CH3),1091 (Si-O), 1019 (Si-O), 801
(Si-
CH3); GPC: Mõ = 3487, M,,, = 5082, PD = 1.45 (from 'H NMR, MW = 12,300, n =
121,
m= 4.35, calculated for 4.5% NH2).
2o N,N-Bis(carboxymethyl)-L-lysine pendant polydimethylsiloxane AMS-152
(NTA-AMS-152)
AMS-152 (0.6 g, 0.06 mmol) was dissolved in dry 1,2-dimethoxyethane (25
mL), 10% palladium on charcoal (a spatula tip) was added, and hydrogenation at
room temperature and normal pressure was allowed to proceed for 8 h. The
completion of the reaction was shown by the disappearance of the starting
material as checked by TLC using methylene chloride / methanol (93:7). The
catalyst was removed by filtration through a Celite pad. A thick gel-like
material
separated upon solvent removal in vacuo. This gel was dissolved in methylene
chloride (20 mL) and water (20 mL) was added to this solution with stirring.
The
product precipitated upon methylene chloride removal in vacuo. The
precipitation was repeated with 20 mL CH2Cl2 and 20 mL water. After drying in
CA 02309486 2000-05-26
-45-
vacuo (< 0.1 mm Hg) the final product was obtained as a white solid (0.16 g,
0.02
mmol, 33%).
'H NMR (CD3OD, 500.13 MHz) S 3.61 (m, 4H, 2 x NCH2), 3.46 (m, 1H, NCH),
3.14 (m, 4H, 2 x NHCH2), 2.45 (s, 4H, O=CCH2CH2C=O), 1.70 - 1.50 (m, 8H,
NCHCH2 + SiCH2CH2 + NHCH2CH2CH2), 0.54 (m, 2H, SiCHz), 0.09 (m, -120H,
SiCH);13C NMR (CD3OD, 125.76 MHz) S 175.9 (C=O), 174.6 (C=O), 66.8 (NCH), 55.6
(NCHz), 54.8 (NCH), 43.5 (NHCH2), 40.1 (NHCH2), 32.5 (CH2), 30.7 (CH2), 29.9
(CHZ), 28.0 (2 x CH2), 24.3 (CH2), 15.8 (CHz), 2.0 (CH3)11.5 (CH); FT-IR
(neat): v
(cm 1) 3303 (COO-H), 2963 (CH), 1737 (C=O), 1644 (N-C=O), 1568 (N-C=O), 1262
(Si-
i o CH3)11098 (Si-O), 1023 (Si-O), 808 (Si-CH3) (from 'H NMR, MW = 8400, n =
85, m
4.35, calculated for 4.5% NH2 ).
N `,N `-Bis(carboxymethyl)-L-lysine tribenzyl ester pendant-
polydimethylsiloxane
AMS-162 [NTA-AMS-162-Bn-Ester]
Aminopropylmethyl-siloxane-dimethyl-siloxane copolymer AMS-162 (1.1
g, 0.24 mmol, 0.9 meq NH2) was added to a stirred solution of N",N"-
bis(carboxymethyl)-NE-succinimidylsuccinyl-L-lysine tribenzyl ester (0.66 g,
0.9
mmol) in dry methylene chloride (50 mL) under a nitrogen atmosphere. After
stirring for 5 h at room temperature the solvent was removed in vacuo. Silica
gel
chromatography using methylene chloride/methanol (95:5) yielded the product
as a clear pale yellow solid (1.2 g, 0.18 mmol, 74%).
1H NMR (CDC13, 200.13 MHz) S 7.30 (m, 15H, 3 x Ph), 6.11 (s, br, 2H, NH), 5.06
(m, 6H, CH2Ph), 3.67 (s, 4H, 2 x NCH2), 3.44 (t, 1H, J= 7.5 Hz, NCH), 3.18 (m,
4H, 2
x NHCH2), 2.46 (s, 4H, O=CCH2CH2C=O), 1.66 (m, 2H, NCHCH2) 1.44 (m, 6H,
SiCH2CH2, NHCHZCH2CH2), 0.48 (m, 2 H, SiCH2), 0.05 (m, -86H, SiCH3);13C NMR
(CDC13, 50.32 MHz) S 172.5 (C=O), 172.2 (C=O), 172.1 (C=O), 171.2 (C=O), 135.7
(Ph),
135.6 (Ph), 128.5 (Ph), 128.3 (Ph), 128.2 (Ph), 66.4 (3 x CH2Ph), 64.5 (NCH),
52.8 (2 x
NCH2), 42.4 (NHCH2), 39.2 (NHCH2), 31.9 (CH2), 31.7 (CH2), 29.7 (CH2), 28.5
(CHZ),
3o 23.2 (CHz), 22.8 (CH2),14.7 (CHz), 1.8 (CH3), 1.0 (CH3)10.5 (CH3), -0.6
(CH3); FT-IR
(neat): v (cm 1) 3299 (COO-H), 2963 (CH), 1746 (C=O), 1642 (N-C=O), 1550 (N-
C=O),
CA 02309486 2000-05-26
-46-
1261 (Si-CH3)11091 (Si-O), 1021 (Si-O), 801 (Si-CH3); GPC: Mõ = 2143, MN, =
4178, PD
= 1.95 (from 1H NMR, MW = 5,900, n = 47, m = 3.7, calculated for 6.5% NH2).
Surface Pressure Analysis
Films of the prepared chelating silicones were examined for their surface
behavior using a Langmuir trough. The films were formed by spreading a very
small amount of the compound from a chloroform/methanol solution (9 : 1)
onto (subphase) liquids that included deionized water, NaHCO3 @ pH 8, 10' M
NiC12 in deionized water and 10"4 M CaC12 in NaHCO3 @ pH 8.
The surface pressure is equal to the difference between the surface tension
of the pure liquid surface and the one covered with a film, and may be
represented by as:
n=Yo - Y'
where Yo is the surface tension of the pure liquid and Y is the tension of the
film-
covered surface.
Three pressures characterize the stability of a monolayer with respect to its
own bulk phase: the collapse pressure (the highest pressure to which a
monolayer can be compressed without detectable expulsion of molecules to form
a new phase), the equilibrium spreading pressure (where the monolayer is in
equilibrium with the stable liquid bulk phase), and the monolayer stability
limit
(maximum pressure attainable in the film without the possibility of collapse).
Molecular weight exerts an important influence on pressure and
area/molecule at collapse, as may be seen in Table 2, and Figures 1 and 2. It
is
noted that the pressure at collapse (PF) decreases as the molecular weight
increases for both succinyl- ("SUCC") and NTA-end-functional polysiloxane
oligomers. For example, for the succinyl-end functional series the pressure at
collapse decreases from 34.6 mN/m (SUCC-DMS-A11) to 15.7 mN/m (SUCC-
DMS-A21). This difference is even more dramatic for NTA-end-functional
compounds. Collapse pressures for NTA-DMS-A11 and NTA-DMS-A21 is 55.8
and 17.2 mN/m, respectively.
The presence of multiple tethering functional groups pendant from the
backbone of SUCC-AMS-152 and NTA-AMS-152 also leads to better anchoring of
the film to the subphase and results in a significant increase of collapse
pressure,
in spite of the fact that these polymers have the highest molecular weights.
High
CA 02309486 2000-05-26
-47-
collapse pressure (27.1 mN/m) combined with a large area at collapse (444
AZ/molecule) for NTA-AMS-152 are consistent with molecules having multiple
anchoring to the subphase.
Table 2: Pressure-Area Isotherms for Succinyl- and NTA-Functional
Silicones on a Water Subphase.
Collapse Pressure Area at Collapse
Silicone MW n n/m PF A2/
b) `) mN/m Molecule
SUCC-DMS-A11
1100 8 4 34.6 110
SUCC-DMS-A15
3400 40-41 20 18.3 247
SUCC-DMS-A21
5500 68-69 34 15.7 320
SUCC-AMS-132
5160 a) 63-64 a) 39 13.8 289
SUCC-AMS-152
7935 a) 92-93 a) 21 32.4 305
NTA-DMS-A11
1530 8-9 4 55.8 42
NTA-DMS-A15
5230 58 29 18.6 237
NTA-DMS-A21
6860 80 40 17.2 411
NTA-AMS-132
5560 a) 63-64 a) 39 16.8 370
NTA-AMS-152
9000a) 92-93 a) 21 27.1 444
a) calculated from manufacturer specifications
b) n number of dimethylsiloxy units in the backbone
c) m number of functional groups per molecule
n-A isotherms were measured on aqueous subphases containing 5x10' M
NiC12 (pH 6.8 - 7.0), 5x10-4 M CaC12 (pH 7.9, NaHCO3 solution), and NaHCO3 (pH
CA 02309486 2000-05-26
-48-
7.9) for the various compounds prepared. Tables 3-5 summarize the experimental
results.
Table 3. Pressure-Area Isotherms for Succinyl- and NTA-Functional
Silicones on Water/NiC12 Subphase.
Collapse Pressure
Silicone PF
mN/m
SUCC-DMS-A11
39.2
SUCC-DMS-A15
16.1
SUCC-DMS-A21
17.4
SUCC-AMS-132
14.6
SUCC-AMS-152
34.3
NTA-DMS-A11
57.8
NTA-DMS-A15
17.4
NTA-DMS-A21
18.0
NTA-AMS-132
15.9
NTA-AMS-152
30.4
CA 02309486 2000-05-26
-49-
Table
4. n-A Isotherms on a NaHCO3 (pH 7.9) Subphase.
Collapse Pressure
Silicone MW PF
mN/m
SUCC-DMS-A11 1100 26.9
SUCC-DMS-A15 3400 19.7
SUCC-DMS-A21 5500 22.1
SUCC-AMS-132 5160a) 18.3
SUCC-AMS-152 7935a) 37.3
NTA-DMS-A11 1530 53.3
NTA-DMS-A15 5230 13.1
NTA-DMS-A21 6860 16.4
NTA-AMS-132 5560a) 17.0
NTA-AMS-152 9000a) 26.9
a) calculated from manufacturer specifications
CA 02309486 2000-05-26
-50-
Table 5. n-A Isotherms on a NaHCO3/CaC12 (pH 7.9) Subphase.
Collapse Pressures
Silicone MW PF
mN/m
SUCC-DMS-A11 1100 50.0
SUCC-DMS-A15 3400 23.2
SUCC-DMS-A21 5500 18.2
SUCC-AMS-132 5160a) 16.5
SUCC-AMS-152 7935a) 39.6
NTA-DMS-A11 1530 53.5
NTA-DMS-A15 5230 13.4
NTA-DMS-A21 6860 16.0
NTA-AMS-132 5560a) 15.7
NTA-AMS-152 9000a) 31.2
a) calculated from manufacturer specifications
Tables 3 - 5 demonstrate that the change in the solution pH from 6.8 - 7.0
(water) to 7.9 - 8.0 (NaHCO3 solution) has a small but noticeable influence on
the
isotherms of both NTA- and succinyl-functionalized silicones. The collapse
pressure PF for NTA-derivatives decreases at higher pH values. It is quite
likely
lo that the pH at the interface is actually lower than in the subphase, and
that a
significant number of the carboxylic acid groups in the tricarboxylic acid
CA 02309486 2000-05-26
-51-
anchoring component of the polymer are ionized. As more carboxylic acid groups
are ionized the electrostatic repulsive forces between these negatively
charged
groups might destabilize the cohesion of the monolayer. Collapse pressures for
succinyl-compounds increase at higher pH in the subphase.
As illustrated in Tables 3 - 5, and Figures 3 - 6, the presence of Niz+ or
Ca2+
in the subphase has a marked effect on the surface behavior of both NTA- and
succinyl-functional silicones and on the collapse pressure of functional
silicones
of all molecular weights and degrees of functionalization. Nickel and calcium
increase the collapse pressure of succinyl-functional silicones by 0.8 - 4.6
mN / m
lo and 2.5 - 15.4 mN/m respectively (with the exception of SUCC-DMS-A15). The
behavior of NTA-functional oligomers on NiZ+ and Ca2+ subphases was quite
different from the one exhibited by succinyl-functional silicones. These
differences may be attributed to the presence in the of the tricarboxylic
acids and
their ability to sequester ions, which simultaneously leads to a change in
surface
activity.
It will be appreciated that the metal binding site may be any site
which binds metals. Some common examples include EDTA, NTA, IDA
(iminodiacetic acid), NTAA (nitrylotriacetic acid), and DTPA (diethylene
triamine pentaacetic acid).