Note: Descriptions are shown in the official language in which they were submitted.
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
SEROLOGICAL DIAGNOSIS OF CHAGAS' DISEASE
Background of the Invention
Chagas'disease is characterized by a short-term acute phase, with very few
s clinical symptoms, and a long-term chronic phase, usually accompanied by
severe
gastrointestinal and/or cardic complications which result in permanent
physical
disability or death.
Chagas'disease is an endemic disease caused by the flagellate Trypanosoma
cruzi. In Latin America, approximately 16 to 18 million individuals are
already
~o infected and as many as 90 million individuals are at risk of infection
(W.H.O.,
1991 ). The disease is transmitted in Nature by Triatominae vectors. As a
result of
effective public health measures for the control of the vector in most
countries.
blood transfusion is quantitatively the most important form of transmission of
the
disease today. In Latin America, blood samples with antibodies associated with
is Chagas'disease represent 1-4% of the total blood samples in major
Hemocenters.
More recently, Chagas'disease has also become a major public health concern in
North America, owing to the increasing number of immigrants from Latin
American countries, in the last decade. Recent studies estimate that there may
be
in the United States approximately 100,000 Trypanosoma cruzi-infected
?o individuals with potential risk of transmitting Chagas'disease by blood
transfusion {Hagar and Rahimtoola, 1991 ).
The diagnosis of acute Chagas'disease is not a problem because of the large
number of parasites in the blood. In contrast, the chronic phase is diagnosed
by
serological methods because of the very small number or absence of circulating
is parasites. This has also restricted so far the use of polymerase chain
reaction
(PCR) with specific primers, as the final diagnostic test of Chagas' disease,
before
a major epidemiologic survey of sera from chronic patients is carried out. The
three serological methods that are currently being used in blood banks -
hemmagglutination (HA}, indirect immunofluorescence (IIF) and enzyme-linked
1
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
immunosorbent assay (ELISA)- utilize mixtures of antigens prepared from the
epimastigote form of the parasite. According to the World Health Organization
(WHO), at least two positive tests of the three cited above are necessary for
the
diagnosis of the disease. Blood samples that are positive to only one of the
three
s tests are classified as "indeterminate or inconclusive" and, in consequence,
discarded. The indeterminate diagnosis is associated with 20 to 90% of all
blood
sampies that gave one or more positive tests for Chagas'disease, depending on
the
methods employed and how they are applied. This high percentage of
indeterminate results represents a serious problem in blood banks, both in
terms of
io volume of discarded blood and doubtful diagnosis of Chagas'disease. In
fact, a
blood sample with a false positive test is no longer used for transfusion or
isolation of cells and other blood components. Such loss of donated blood also
affects the production of blood derivatives such as albumin, immunoglobulins
and
clotting factors which are of commercial value. Conversely, a blood sample
with a
~ s false negative test is a dangerous source of contamination by the
parasite.
The disadvantages of the current serological methods can be summarized as
follows:
1. Low sensitivity: current methods use human sera at low dilutions, with a
consequent increase in the background due to the cross-reactivity with natural
ao antibodies and low-titer antibodies resulting from nonspecific polyclonal
activation. Specific recombinant or synthetic epimastigote antigens, singly or
in
mixtures, are not sufficiently sensitive because they react only with a
limited
number of specific antibodies present in the sera of chronic Chagasic patient.
2. Low specificity: serological tests using epimastigote extracts cross-react
with
Zs antigens from microbial sources other than Trypanosoma cruzi, notably
Leishmania and some fungal and bacterial antigens.
Brief Summary of invention
The invention describes the purification of the A&T and EpEx antigens,
and their use in a chemiluminescent a 2 yme-linked immunosorbent assay (CL-
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
ELISA), for the accurate diagnosis of Chagas'disease. When carried out in
parallel, the results of the tests taken together provide high sensitivity and
high
specificity not obtainable with conventional methods described in the
literature
and/or which are commercially available.
s Detailed description of the invention
Purification of the A&T antigen
A&T antigen is purified from trypomastigote forms of Trypanosome cruzi
according to Almeida et al., 1993 and Almeida et al., 1994a . Trypomastigote
forms are obtained from infected green monkey kidney fibroblasts (LLC-MK2
~o cells) cultured in Dulbecco's modified Eagle medium (D-MEM) containing 10%
fetal bovine serum. The cell-derived trypomastigotes are collected 6-7 days
later,
following their release from infected cells, from the top fluid after
sedimentation
of the cell debris and incubation for 1.5 h at 37°C. Parasites are
washed 3 times in
0.15 M phosphate-buffered saline (PBS), pH 7.4, centrifuged at 12,OOOg, and
kept
i s at -70°C until lyophilization. Lyophilized trypomastigotes are
sequentially
extracted 5 times with 10 volumes of chloroform/methanol (2:1 ),
chloroform/methanol ( 1:2), chloroform/methanol/water ( 10:20:8), for 30 min
each
time, at room temperature. After centrifugation at 12,OOOg, the organic
extracts
are discarded and the final delipidated pellet is dried under a stream of
nitrogen.
Zo The dry pellet is then extracted 5 times with 10 volumes of 9% 1-butanol
for 2 h
each time, at room temperature. The soluble extract corresponds to the
fraction
containing at A&T antigen together with some hydrophilic and hydrophobic
contaminants. The A&T-containing fraction is then lyophilized for 24 h and
chromatographed on a column of octyl-Sepharose (Pharmacia-LKB, Upsala,
is Sweden), pre-equilibrated with 5% 1-propanol in 0.1 M ammonium acetate
buffer,
pH 7.2. The A&T-containing fraction dissolved in 5 % 1-propanol in 0.1 M
ammonium acetate buffer, pH 7.2 is applied to the column at a low flow rate.
The
colum is washed with 5% 1-propanol in 0.1 M ammoniun acetate buffer, pH 7.2
and eluted with a 1-propanol gradient (S-60%). The column fractions containing
3
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
the A&T antigen are tested for immunoreactivity with a specific polyclonal
antibody generated against the A&T antigen (anti-A&T antibody). The A&T-
positive fractions from the octyl-Sepharose column are pooled, dried and
partitioned between water and 1-butanol. The aqueous phase is lyophilized for
24
s h, resuspended in 5% 1-propanol in ammonium acetate 0.1 M, pH 7.2 and
applied
to the phenyl-Superose column (Pharmacia-LKB, Sweden) (pre-equilibrated with
% 1-propanol in ammonium acetate 0.1 M, pH 7.2). The column is eluted with a
1-propanol gradient (5-60%). Material eluting in earlier fractions (column
void)
and containing the A&T antigen is pooled and lyophilized for 24 h. The
material
i o included in the column is basically constituted of hydrophobic
contaminants,
mainly phospholipids. Finally, as a final purification step to eliminate
hydrophilic
contaminants, A&T antigenic preparation is re-applied to a column of octyl-
Sepharose (Pharmacia-LKB, Sweden), pre-equilibrated with 5% 1-propanol in
O.1M ammonium acetate buffer, pH 7.2. The A&T-containing fraction dissolved
~s in 5% 1-propanol in 0.1 M ammonium acetate buffer, pH 7.2 is applied to the
column at a low flow rate. The column is washed with 5% 1-propanol in 0.1 M
ammonium acetate buffer, pH 7.2 and eluted with a 1-propanol gradient (5-70%)
and eluted with a shallow 1-propanol gradient (20-40%). The fractions are
assayed for irnmunoreactivity with the anti-A&T antibody by dot-blotting and
ao Western-blotting. Antibody binding fractions are pooled, exhaustively
dialyzed
against deionized water, lyophilized for 48 h, redissolved in deionized water
and
stored at -70°C.
Purification of the EpEx anti eon
EpEx antigen is prepared from epimastigote forms of Trypanosoma cruzi,
as Tulahuen strain. Parasites are cultured at 28°C, in Schneider's
insect medium
containing 20% fetal calf serum. After 7-10 days, the parasites are collected
from
the culture supernatant, washed three times with 100 mM phosphate-buffered
saline, pH 7.4 and centrifuged at 12,OOOg for 30 min, at 4°C. Pelleted
parasites
are immediately resuspended in IO mM Tris-HCI buffer, pH 7.5, 0.2 mM
4
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
leupeptin, 2 mM EDTA, 1 % nonanoyl-N-methylglucamide (lysis buffer), and
submitted to four cycles of freezing and thawing, in liquid nitrogen and water
bath (37°C), respectively. The resulting lysate is centrifuged at
10,0008 for 5 min,
at 4°C. The supernatant, containing the EpEx antigenic preparation, is
removed
s and stored at -70°C.
Chemiluminescent enzyme-linked immunosorbent assay (CL-ELISA) usin~EpEx
and A&T antigens
Chemiluminescent ELISA is carried out according to protocols previously
described (Almeida et al., 1993, 1994b). A&T (at 0.15~,g dry weight/pl
deionized
~o water) and EpEx (at O.lSp.g protein/~,1 of lysis buffer) antigens are
diluted in 50
mM sodium carbonate buffer, pH 9.6, for a final concentration of 0.2 ng/p,l
and
0.8 ng/p,l, respectively. Fifty microliters of each antigen are separately
added to
each well of milky-white 96-well Maxisorp FluoroNunc plates (Nunc, Denmark).
After 12 h at 4°C, plates are washed 5 times with 0.15 M phosphate-
buffered
i s saline, pH 7.4, 0.05 % Tween 20 (PB S-T) and blocked with 0.1 % bovine
serum
albumin (BSA) in 50 mM sodium carbonate buffer, pH 9.6, for 12 h at 4°C
or,
alternatively, for 2 h at 37°C. Plates are then washed 5 times with
0.15 M
phosphate-buffered saline, pH 7.4, 0.05% Tween 20 (PBS-T). The human sera,
diluted 1:2,000 in PBS-T containing 0.5% BSA (PBS-TB), are added to the plates
Zo and incubated for 30 min at 37°C. Plates are washed 5 times with PBS-
T, the
excess liquid removed by inversion or filter paper, and then incubated with
biotinylated goat anti-human IgG (Amersham, UK), diluted 1:2,000 with PBS-TB,
for 30 min at 37°C. After washing 5 times with PBS-T, a streptavidin-
horseradish
peroxidase conjugate (Amersham, UK) diluted 1:1,000 with PBS-TB is added,
2s following incubation for 30 min at 37°C. Plates are washed 5 times
with PBS-T,
the excess liquid removed by inversion on filter paper, and then incubated
with
luminol (ECL reagents, Amersham, UK), diluted 1:20 in 50 mM carbonate buffer,
pH 9.6, for 1-5 min at room ternperature. Thereafter, the reaction is
quantified
CA 02309705 2000-OS-10
WO 99/41610 PCT/BR98/00006
using a luminometer for 96-well polysterene plate readings. The results are
expressed as relative luminescent units (RLU). Cutoff values for A&T and EpEx
CL-ELISAs were first calculated by determining the reactivities of 200 normal
human sera (NHS). The mean and SD of these 200 reactivities were determined. A
s value of 10 times the SD was added to the mean for the cutoff value,.The
dispersion of the RLU readings for 200 NHS showed SD very close to the means
using both A&T and EpEx CL-ELISAs (143 ~ 123 and 177 ~ 151, respectively).
Therefore, for each plate in which a single negative control (pool of 100 NHS)
in
quadruplicate was included, the cutoff values for A&T and EpEx CL-ELISA were
i v established as 10 times the negative control mean minus the background
control
mean (cutoff value = 10 x negative control mean - background control mean ).
Result interpretation:
To interpret the results obtained, the luminometer reading of a serum
sample is divided by the predeterminated cutoff value. A positive result is
defined
~s when the relative serum reading (RLU) is greater than 1, which represents
the
cutoff value. Conversely, a negative sample has an RLU equal or lower than 1.
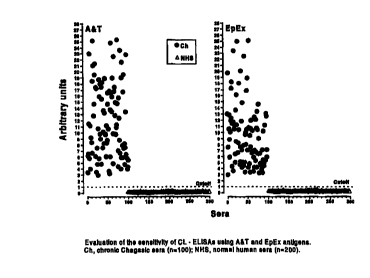
1. The high sensitivity of the chemiluminescent (CL)-ELISA method permits the
use of high dilutions of sera ( 1:2,000) (Fig. 1 ), and thus eliminates most
of the
nonspecific or false-positive reactions of current methods, which use serum
2o dilutions in the 1:30 to 1:400 range (Tables 1 and 2).
6
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
Table 1 - Comparison between CL-ELISA (with A&T and EpEx) and conventional
diagnostic tests with a panel of sera with inconclusive serology for Chagas'
disease.
Diagnostic dilution Negative InconclusivePositiveFalse-NegativeFalse-
Positive
test
Chemiluminescence '
A&T-CL-ELISA2 1:2,000 74 0 26 0 0
EpEx-CL-ELISA 1:2,000 72 0 28 0 2
western blotting-EpEx1:400 72 11 17 0 2
ELISA-EpEx 1:100 77 7 16 3 0
Commercial
kits
ELISA-A1 1:41 10 70 20 0 64
ELISA-A2 1:41 41 21 38 0 33
ELISA-B t:41 67 8 25 0 7
ELISA-C 1:41 69 17 14 0 5
HA 1:40 79 12 9 5 0
IF 1:30 79 6 15 5 0
No. Of false-positive
sera = [no.
Of positive
+ inconclusive
sera with
each test)
- no. Of positive
sera with
the reference
method
(A&T-CL-ELISA).
Z CL-ELISA
with A&T antigen
(CLE-A&T)
is considered
the gold method.
to Table 2 - CL-ELISA reactivity of A&T and EpEx antigens with
inconclusive and heterologous sera.
Reactive sera in
Number Conventional CL-ELISA
Conventional serologyof sera tests' A&T EpEx
Incondusive for Chagas100 100 26 28~
disease
Leishmaniasis
visceral 1 t 5 0 2
cutaneous 16 10 0 1
Autolmmune diseases 30 0 0 0
Infectious diseases
AIDS 24 0 0 0
Hepatitis 24 0 0 0
Syphilis 24 0 0 0
Paracaccidioidomycosis5 1 0 0
Poli A/C vaccinated
(Nelsseria meningltidls)
pre-immune 5 0 0 0
Immunized 5 5 0 0
Chagas' disease 100 100 100 100
Normal human sera 200 0 0 0
' Number of sera giving at least 1 positive reaction In the conventional
Chagas' disease serology
(indirect immunofluorescence, indirect hemagglutination and ELISA).
z Two reactions with EpEx are false-positive.
2. The A&T antigen is a purified preparation of closely related molecules thar
are
specific of the trypomastigote stage obtained in tissue culture of mammalian
7
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
cells, thus being very similar to the infective forms of the parasite that
cause the
disease in man.
3. Since the serological reactions with A&T antigen are highly specific, there
is
no cross-reactivity with antigens from a variety of other infectious agents
s including Leishmania, and with natural antibodies and low-titer antibodies
resulting from nonspecific polyclonal activation (Table 2).
4. The A&T antigen is easily obtainable in amounts sufficient for a great
number
of tests in appropriate ELISA plates for chemiluminescent reading. Moreover,
the
purified A&T antigen is highly stable when fixed on plates for prolonged
periods.
i o 5. Since A&T antigen reacts with lytic (protective) antibodies,
characteristic of
active infection and present in high titers in chronic patient sera, it can be
used to
monitor the response of patients to chemotherapy (Fig. 2).
6. The EpEx complex antigen is prepared from the epimastigote form and
contains
many components that are also expressed in the infective stage. It reacts with
Is antibodies that are recognized by conventional serology for Chagas'disease,
but
not with those antibodies whose reactions are due to artifacts such as
blocking
reagents, culture medium supplements, etc.
7. The EpEx antigen is readily prepared from fast growing epimastigote
culture,
and although it is not as specific as A&T purified antigen, it is highly
sensitive
2o and provide complementary and confirmatory data for the positive reactions
obtained with A&T antigen (Fig. 1 ).
8. The advantages of using both A&T and EpEx antigens in parallel tests are
the
following:
a) the antigens present in both tests are highly sensitive and therefore,
2s a positive result with both antigens provides a diagnosis with a
high level of confidence (Fig. 1, Table 1);
b) positive reactivity with EpEx, and negative with A&T, while
eliminating active Chagas' infection, suggests leishmaniasis or
another infectious disease (Table 2);
8
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
c) a decrease in the reactivity with A&T followed by a decrease with
EpEx has prognostic value, and is a criterion of cure in patients
submitted to chemotherapy (Fig. 2);
d) when applied to sera classified as "indeterminate" (i.e. sera which are
negative
s to one or two of the following tests: hemagglutination, immunofluorescence
and
ELISA), the A&T and EpEx tests provide unambiguous results, thereby
eliminating inconclusive serological diagnosis of Chagas'disease (Tables 1 and
2)
9
CA 02309705 2000-OS-10
WO 99/41610 PCTBR98/00006
REFERENCES
World Health Organization ( 1991 ) Control of Chagas' disease. WHO Tech. Rep.
Ser.
s 811:1-91
Hagar, J.M and Rahimtoola, S.H. ( 1991 ) Chagas'heart disease in the United
States. N. Engl. J. Med. 325:763-8
Almeida, LC., Krautz, G.M., Krettli, A.U. and Travassos, L.R. ( 1993)
Glyconjugates of Trypanosoma cruzi : a 74 kD antigen of trypomastigotes
i o specifically reacts with lytic anti a galactosyl antibodies from patients
with
chronic Chagas disease. J. Clin. Lab. Anal. 7: 307-316.
Almeida, LC., Ferguson, M.A.J., Schenkman, S. and Travassos, L.R.
( 1994a).Lytic anti-a-galactosyl antibodies from patients with chronic Chagas
disease recognise novel O-linked oligosaccharides on mucin-like GPI-anchored
i s glycoproteins of Trypanosoma cruzi. Biochem. J. 304: 793-802.
Almeida, LC., Rodrigues, E.G. and Travassos, L.R. (1994b) Chemiluminescent
immunoassays: discrimination between the reactivities of natural and human
patient antibodies with antigens from eukaryotic pathogens, Trypanosoma cruzi
and Paracocidioides brasiliensis. J. Clin. Lab. Anal. 8: 424-431.
1~