Note: Descriptions are shown in the official language in which they were submitted.
CA 02309891 2000-06-19
1
Description
PRODUCTION OF FIBRINOGEN IN TRANSGENIC ANIMALS
Backcround of the Invention
The final step in the blood coagulation cascade
is the thrombin-catalyzed conversion of the soluble plasma
protein fibrinogen to insoluble fibrin. Thrombin cleaves
a small peptide (fibrinopeptide A) from one of the three
component chains (the Aa-chain) of fibrinogen. Fibrin
monomers subsequently polymerize and are cross-linked by
activated factor XIII to form a stable clot.
Fibrinogen is a key component of biological
tissue glues (see, e.g., U.S. Patents Nos. 4,377,572 and
4,442,655), which mimic the formation of natural blood
clots to promote hemostasis and repair damaged tissue.
Tissue glues provide an adjuct or alternative to sutures,
staples and other mechanical means for wound closure.
However, the principal ingredients of these products
(fibrinogen, factor XIII and thrombin) are prepared from
pooled human plasma by cryoprscipitation (e. g. U.s.
Patents No. 4,377,572; 4,362,567; 4,909,251) or ethanol
precipitation (e. g. U.S. Patent No. 4,442,655) or from
single donor plasma (e. g. U.S. Patent No. 4,627,879;
Spotnitz et al., Am. Sub ~: 166-168, 1989). The
resultant fibrinogen/factor XIII preparation is mixed with
bovine thrombin immediately before use to convert the
fibrinogen to fibrin and activate the factor XIII, thus
initiating coagulation of the adhesive.
Commercially available adhesives are of pooled
plasma origin. Because blood-derived products have been
associated with the transmission of human immunodeficiency
virus (HIV), hepatitis virus and other etiologic agents,
the acceptance and availability of such adhesives is
CA 02309891 2000-06-19
2
limited. At present they are not approved for use in the
United States.
While the use of autologous plasma reduces the
risk of disease transmission, autologous adhesives can
only be used in elective surgery when the patient is able
to donate the necessary blood in advance.
As noted above, fibrinogen consists of three
polypeptids chains, each of which is present in two copies
in the assembled molecule. These chains, designated the
Aa, B~,and y-chains, are coordinately expressed, assembled
and secreted by the liver. While it might be expected
that recombinant DNA technology could provide an
alternative to the isolation of fibrinogen from plasma,
this goal has proven to be elusive. The three fibrinogen
chains have been individually expressed in E. coli (Lord,
~1 ~,: 33-38, 1985; Bolyard and Lord, Gene ~~: 183-192,
1988; Bolyard and Lord, ; lood ~: 1202-1206), but
functional fibrinogen has not been produced in a
prokaryotic system. Expression of biologically competent
fibrinogen in yeast has not been reported. Cultured
transfected mammalian cells have been used to express
biologically active fibrinogen (Farrell et al., Blood
55a, 1989; Hartwig and Danishefsky, J. Biol. Chem. 266:
6578-6585, 1991; Farrell et al., Biochemistry ~Q: 9414-
9420, 1991), but expression levels have been so low that
production of recombinant fibrinogen in commercial
quantities is not feasible. Experimental evidence
suggests that lower transcription rates in cultured cells
as compared to liver may be a factor in the low expression
rates achieved to date, but increasing the amount of
fibrinogen chain mRNA in transfected BHK cells did not
produce corresponding increases in fibrinogen protein
secretion (Drunkard and Foster, XIV Congress of the
International Society on Thrombosis and Haemostasis,
1993). These latter results suggest that proper assembly
and processing of fibrinogen involves tissue-specific
mechanisms not present in common laboratory cell lines.
CA 02309891 2000-06-19
3
There remains a need in the art for methods of
producing large quantities of high quality fibrinogen for
use in tissue adhesives and other applications. There is
a further need fot fibrinogen that is free of blood-borne
pathogens. The present invention fulfills these needs and
provides other, related advantages.
Summary of the Invention
It is an aspect of the present invention to
l0 provide commercially useful quantities of recombinant
fibrinogen, particularly recombinant human fibrinogen. It
is a further object of the invention to provide materials
and methods for expressing fibrinogen in the mammary
tissue of transgenic animals, particularly livestock
animals such as cattle, sheep, pigs and goats.
Within one aspect, the present invention
provides a method for producing fibrinogen comprising (a)
providing a first DNA segment encoding a secretion signal
operably linked to a fibrinogen Aa chain, a second DNA
segment encoding a secretion signal operably linked to a
fibrinogen H~ chain, and a third DNA segment encoding a
secretion signal operably linked to a (fibrinogen ~r chain,
wherein each of the first, second and third segments is
operably linked to additional DNA segments required for
its expression in the mammary gland of a host female
mammal; (b) introducing the DNA segments into a fert_lized
egg of a non-human mammalian species; (c) inserting the
egg into an oviduct or uterus of a female of the species
to obtain offspring carrying the DNA constructs; (d)
breeding the offspring to produce female progeny that
express the first, second and third DNA segments and
produce-milk containing biocompetent fibrinogen encoded by
the segments; (e) collecting milk from the female progeny;
and (f) recovering the fibrinogen from the milk. Within
one embodiment, the egg containing the introduced segments
is cultured for a period of time prior to insertion.
CA 02309891 2000-06-19
4
Within another aspect, the invention provides a
method of producing fibrinogen comprising the steps of (a)
incorporating a first DNA segment encoding a secretion
signal operably linked to an Aa chain of fibrinogen into a
S-lactoglobulin gene to produce a first gene fusion; (b)
incorporating a second DNA segment encoding a secretion
signal operably linked to a H~ chain of fibrinogen into a
~-lactoglobulin gene to produce a second gene fusion; (c)
incorporating a third DNA segment encoding a secretion
signal operably linked to a y chain of fibrinogen into a ~-
lactoglobulin gene to produce a third gene fusion; (d)
introducing the first, second and third gene fusions into
the germ line of a non-human mammal so that the DNA
segments are expressed in a mammary gland of the mammal or
i~s female progeny and biocompetent fibrinogen is secreted
into milk of the mammal or its female progeny; (e)
obtaining milk from the mammal or its female progeny; and
(f) recovering the fibrinogen from the milk. Within
preferred embodiments, the mammal is a sheep, pig, goat or
2o bovine.
Within another aspect, the invention provides a
method for producing ffibrinogen comprising the steps of
(a) providing a transgenic female non-human mammal
carrying in its germline heterologous DNA segments
encoding Aa, 8~ and ~ chains of fibrinogen, wherein the DNA
segments are expressed in a mammary gland of the mammal
and fibrinogen encoded by the DNA segments is secreted
into milk of the mammal; (b) collecting milk from the
mammal; and (c) recovering the fibrinogen from the milk.
Within another aspect, the invention provides a.
non-human mammalian embryo containing in its nucleus
heterologous DNA segments encoding Aa, B~ and ~ chains of
fibrinogen. Within a related aspect, the invention
provides a transgenic non-human female mammal that
produces recoverable amounts of human fibrinogen in its
milk.
CA 02309891 2000-06-19
Within another aspect, the invention provides a
method for producing a transgenic offspring of a mammal
comprising the steps of (a) providing a first DNA segment
encoding a fibrinogen Aa chain, a second DNA segment
5 encoding a fibrinogen B~B chain, and a third DNA segment
encoding a fibrinogen y chain, wherein each of said first,
second and third segments is operably linked to additional
DNA segments required for its expression in a mammary
gland of a host female mammal and secretion into milk of
the host female mammal; (b) introducing the DNA segments
into a fertilized egg of a mammal of a non-human species;
(c) inserting the egg into an oviduct or uterus of a
female of the non-human species to obtain an offspring
carrying the first, second and third DNA segments. In a
related aspect, the invention provides non-human mammals
produced according to this process.
Within an additional aspect, the invention
provides a non-human mammal carrying its germline DNA
segments encoding heterologous Aa, H~ and ~ chains of
2o fibrinogen, wherein female progeny of the mammal express
tho DNA segments in a mammary gland to produce
biocompetent fibrinogen.
These and other aspects of the invention will
become evident to the skilled practitioner upon reference
to the following detailed description and the attached
drawings.
CA 02309891 2000-06-19
6
Brief Descristi_on of the
--------- Drawings a human
Figure, 1 illustrates the subcloning of
fibrinogen Aa chain DNA sequence.
Figure 2 is a partial restriction map of the
vector Zem228. Symbols used era MT-1
p, mouse
matallothionsin promoter;
SV4ot, SV4o tenainator;
and
SV40p, SV40 promoter.
Figure 3 illustrates the subcloning of a human
fibrinogen B~ chain DNA sequence.
Figure 4 illustrates the subcloning of a human
fibrinogen ~ chain DNA
sequence.
Figure 5 is a partial restriction map of the
vector Zsm219b. Symbols used are MT-1
p, mouse
metallothionein pr omoter; hGHt, human growth hormone
terminator; SV40p, SV40 promoter; DHFR, dihydrofolate
reductass gene; and SV40t, SV40 terminator.
Detai_ied Descri&~ion of the Inventiow
Prior to setting forth the invention in detail,
2o it will be helpful to define certain terms used herein:
As used herein, the term "biocompetent
fibrinogen" is used to denote fibrinogen that polymerizes
when treated with thrombin to form insoluble fibrin.
The term "egg" is used to denote an unfertilized
ovum, a fertilized ovum prior to fusion of the pronuclei
or an early stage embryo (fertilized ovum with fused
pronuclei).
A "female mammal that produces milk containing
biocompatent fibrinogen" is one that, following pregnancy
and delivery, produces, during the lactation period, milk
containing recoverable amounts of biocompstent fibrinogen.
Those skilled in the art will recognized that such animals
will produce milk, and therefore the fibrinogen,
discontinuously.
The term "progeny" is used in its usual sense to
include children and descendants.
CA 02309891 2000-06-19
7
The term "heterologous" is used to denote
genetic material originating from a different species than
that into which it has been introduced, or a protein
produced from such genetic material.
Within the present invention, transgenic animal
technology is employed to produce fibrinogen within the
mammary glands of a host female mammal. Expression in the
mammary gland and subsequent secretion of the protein of
interest into the milk overcomes many difficulties
l0 encountered in isolating proteins from other sources.
Milk is readily collected, available in large quantities,
and well characterized biochemically. Furthermore, the
major milk proteins are present in milk at high
concentrations (from about 1 to 15 g/1).
From a commercial point of view, it is clearly
preferable to use as the host a species that has a large
milk yield. While smaller animals such as mice and rats
can be used (and are preferred at the proof-of-concept
stage), within the present invention it is preferred to
use livestock mammals including, but not limited to, pigs,
goats, sheep and cattle. Sheep are particularly preferred
due to such factors as the previous history of
transgenesis in this species, milk yield, cost and the
ready availability of equipment for collecting sheep milk.
See WO 88/00239 for a comparison of factors influencing
the choice of host species. It is generally desirable to
select a breed of host animal that has been bred for dairy
use, such as East Friesland sheep, or to introduce dairy
stock by breeding of the transgenic line at a later date.
In any event, animals of known, good health status should
be used.
Fibrinogen produced according to the present
invention may be human fibrinogen or f ibrinogen of a non-
human animal. For medical uses, it is preferred to employ
proteins native to the patient. The present invention
thus provides fibrinogen for use in both human and
veterinary medicine. Cloned DNA molecules encoding the
CA 02309891 2000-06-19
8
component chains of human fibrinogen are disclosed by
Rixon et al. (Biochem.
,~"~: 3237, 1983) , Chung et al.
(Biochem. ~: 3244, 1983), Chung et al. (Biochem.
3250, 1983), Chung et al. (Adv. Exn. Med. Biol
,~, : 3 9-
48, 1990) and Chung et al. (Ann. NY Acad. Sci
~Q$. 449-
456, 1983). Bovine fibrinogen clones are disclosed by
Brown et al. (Nuc. Acids Res. ,~: 6397, 1989) and Chung et
al. (Proc. Natl. Acad. Sci. USA ~: 1466-1470, 1981).
Other mammalian fibrinogen clones are disclosed by
Murakawa et al. (Thromb. Haemost. ~"~: 351-360, 1993).
Representative sequences of human Aa, B~ and ~ chain genes
are shown in SEQ ID NOS: 1, 3 and 5, respectively. Those
skilled in the art will recognize that allelic variants of
these sequences will exist;.that additional variants can
be generated by amino acid substitution, deletion, or
insertion; and that such variants are useful within the
present invention. In general, it is preferred that any
engineered variants comprise only a limited number of
amino acid substitutions, deletions, or insertions, and
2o that any substitutions are conservative. Thus, it is
preferred to produce fibrinogen chain polypeptides that
are at least 90~, preferably at least 95~, and more
preferably 99~ or more identical in sequence to the
corresponding native chains. The term "y chain" is meant
to include the alternatively spliced y' chain of
fibrinogen (Chung et al., Biochem. ~: 4232-4236, 1984).
A human fir' chain amino acid sequence is shown in SEQ ID
NO: 6. The shorter y chain is produced by alternative
splicing at nucleotides 9511 and 10054 of SEQ ID NO: 5,
resulting in translation terminating after nucleotide
10065 of SEQ ID NO: 5. .
To obtain expression in the mammary gland, a
transcription promoter from a milk protein gene is used.
Milk protein genes include those genes encoding caseins,
beta-lactoglobulin (BLG), a-lactalbumin, and whey acidic
protein. The beta-lactoglobulin promoter is preferred.
In the case of the ovine beta-lactoglobulin gene, a region
CA 02309891 2000-06-19
9
of at least the proximal 406 by of 5' flanking sequence of
the ovine HLG gene (contained within nucleotides 3844 to
4257 of SEQ ID' N0:7) will generally be used. Larger
portions of the 5' flanking sequence, up to about 5 kbp,
are preferred. A larger DNA segment encompassing the 5'
flanking promoter region and the region encoding the 5'
non-coding portion of the beta-lactoglobulin gene
(contained within nucleotides 1 to 4257 of SEQ ID N0:7) is
particularly preferred. Sae Whitelaw et al., Hiochem ,T.
l0 ~ø: 31-39, 1992. Similar fragments of promoter DNA from
other species are also suitable.
Other regions of the beta-lactoglobulin gene may
also be incorporated in constructs, as may genomic regions
of the gene to be expressed. It is generally accepted in
the art that constructs lacking introns, for example,
express poorly in comparison with those that contain such
DNA sequences (see Hrinster et al., Proc. Natl. Aced. Sci.
y,$~ ,$~: 836-840, 1988; Palmiter et al. , Proc. Natl. Aced.
Sci. USA ~: 478-482, 1991; Whitelaw et al., Transg!enic
Res. ~: 3-13, 1991; WO 89/01343; WO 91/02318). In this
regard, it is generally preferred, where possible, to use
genomic sequences containing all or some of the native
introns of a gene encoding the protein or polypeptide of
interest. within certain embodiments of the invention,
the further inclusion of at least some introns from the
beta-lactoglobulin gene is preferred. One such region is
a DNA segment which provides for intron splicing and RNA
polyadenylation from the 3' non-coding region of the ovine
beta-lactoglobulin gene. When substituted for the natural
3' non-coding sequences of a gene, this ovine beta-
lactoglobulin segment can both enhance and stabilize
expression levels of the protein or polypeptide of
interest. Within other embodiments, the region
surrounding the initiation ATG of one or more of the
fibrinogen sequences is replaced with corresponding
sequences from a milk specific protein gene. Such
replacement provides a putative tissue-specific initiation
CA 02309891 2000-06-19
environment to enhance expression. It is convenient to
replace the entire fibrinogen chain pre-pro and 5' non-
coding sequences with those of, for example, the BLG gene,
although smaller regions may be replaced.
5 For expression of fibrinogen, DNA segments
encoding each of the three component polypeptide chains of
fibrinogen are operably linked to additional DNA segments
required for their expression to produce expression units.
Such additional segments include the above-mentioned milk
to protein gene promoter, as well as sequences which provide
for termination of transcription and polyadenylation of
mRNA. The expression units will further include a DNA
segment encoding a secretion signal operably linked to the
segment encoding the fibrinogen polypeptide chain. The
secretion signal may be a native fibrinogen secretion
signal or may be that of another protein, such as a milk
protein. The term "secretion signal" is used herein to
denote that portion of a protein that directs it through
the secretory pathway of a call to the outside. Secretion
signals are most commonly found at the amino-termini of
proteins. See, for example, von Hainje, Nuc. Acids Res.
,: 4683-4690, 1986; and Meade et al., LT.S. Patent No.
4,873,316,
Construction of expression units is conveniently
carried out by inserting a fibrinogen chain sequence into
a plasmid or phage vector containing the additional DNA
segments, although the expression unit may be constructed
by essentially any sequence of ligations. It is
particularly convenient to provide a vector containing a
3o DNA segment encoding a milk protein and to replace the
coding sequence for the milk protein with that of a
fibrinogen chain (including a secretion signal), thereby
creating a gene fusion that includes the expression
control sequences of the milk protein gene. In any event,
cloning of the expression units in plasmids or other
vectors facilitates the amplification of the fibrinogen
sequences. Amplification is conveniently carried out in
CA 02309891 2000-06-19
11
bacterial (e. g. E. coli) host cells, thus the vectors will
typically include an origin of replication and a
selectable marker functional in bacterial host cells.
In view of the size of the fibrinogen chain
genes it is most practical to prepare three separate
expression units, mix them, and introduce the mixture into
the host. However, those skil7.ed in the art will
recognize that other protocols may be followed. For
example, expression units for the three chains can be
introduced individually into different embryos to be
combined later by breeding. In a third approach, the
three expression units can be linked in a single suitable
vector, such as a yeast artificial chromosome or phage P1
clone. Coding sequences for two or three chains can be
combined in polycistronic expression units (see, e.g.,
Levinson et al., U.S. Patent No. 4,713,339).
The expression units) is(are) then introduced
into fertilized eggs (including early-stage embryos) of
the chosen host species. Introduction of heterologous DNA
can be accomplished by ons of several routes, including
microinjection (e. g. U.S. Patent No. 4,873,191),
retroviral infection (Jasnisch, Scie~y~g ~: 1468-1474,
1988) or site-directed integration using embryonic stem
(ES) cells (reviewed by Bradley et al., Bio/Technolocv ~0:
534-539, 1992). The eggs are then implanted into the
oviducts or uteri of pseudopregnant females and allowed to
develop to term. Offspring carrying the introduced DNA in
their germ line can pass the DNA on to their progeny in
the normal, Mendelian fashion, allowing the development of
transgenic herds. General procedures for producing
transgenic animals are known in the art. see, for
example, Hogan et al., ManiDUlati;p_v the Mouse Embrvo~ A
Laboratory Manual, Cold Spring Harbor Laboratory, 1986;
Simons et al., Bio~Technolocv ø: 179-183, 1988; Wall et
al., Biol. ReDrod. ~,: 645-651, 1985; Buhler et al.,
Bio~ITechnoloav $: 140-143, 1990; Ebert et al. ,
Bio/Technolocrv ~: 835-838, 1991; Krimpenfort et al.,
CA 02309891 2000-06-19
12
Bio/Technolocrv ~: 844-847, 1991; Wall et al., J. Cell.
Biochem, g~: 1~3-120, 1992; and WIPO publications WO
88/00239, WO 90/05188, WO 92/11757; and GB 87/00458.
Techniques for
introducing foreign DNA sequences into mammals and their
germ cells were originally developed in the mouse. See,
e.g., Gordon et al., Proc. Natl. Acad. Sci. USA
7380-
7384, 1980; Gordon and Ruddle, Science ~: 1244-1246,
1981; Palmiter and Hrinster, Cell ~: 343-345, 1985;
Brinster et al., proc. Natl. Acad. Sci. USA
4438-4442,
1985; and Hogan et al. (ibid.). These techniques were
subsequently adapted for use with larger animals,
including livestock species (see e.g., WIPO publications
WO 88/00239, WO 90/05188, and WO 92/11757; and Simons et
al., Hio/Technoloav ~,: 179-183, 1988). To summarize, in
the most efficient route used to date in the generation of
transgenic mice or livestock, several hundred linear
molecules of the DNA of interest are injected into one of
the pro-nuclei of a fertilized egg. Injection of DNA into
the cytoplasm of a zygote can also be employed.
It is preferred to obtain a balanced expression
of each fibrinogen chain to allow for efficient formation
of the mature protein. Ideally, the three expression
units should b~ on the same DNA molecule for introduction
into eggs. This approach, however, may generate technical
problems at, for example, the injection and manipulation
stages. For example, the size of fibrinogen expression
units may necessitate the use of yeast artificial
chromosomes (YACs) or phage P1 to amplify and manipulate
the DNA prior to injection. If this approach is followed,
segments of DNA to be injected, containing all three
expression units, would be very large, thus requiring
modification of the injection procedure using, for
example, larger bore needles. In a more simple approach,
a mixture of each individual expression unit is used. It
is preferred to combine equimolar amounts of the three
expression units, although those skilled in the art will
CA 02309891 2000-06-19
13
recognize that this ratio may be varied to compensate for
the characteristics of a given expression unit. Some
expression, generally a reduced level, will be obtained
when lesser molar amounts of one or two chains are used,
and expression efficiencies can generally be expected to
decline in approximate proportion to the divergence from
the preferred equimolar ratio. In any event, it is
preferred to use a mixture having a ratio of Aa:B~:7
expression units~in the range of 0.5-1:0.5-1:0.5-1. When
l0 the ratio is varied from equimolar, it is preferred to
employ relatively more of the B~ expression unit.
Alternatively, one or a mixture of two of the expression
units is introduced into individual eggs. However,
animals derived by this approach will express only one or
two fibrinogen chains. To generate an intact fibrinogen
molecule by this approach requires a subsequent breeding
program designed to combine all three expression units in
individuals of a group of animals.
In general, female animals are superovulated by
treatment with follicle stimulating hormone, then mated.
Fertilized eggs are collected, and the heterologous DNA is
injected into the eggs using known methods. See, for
example, U.S. Patent No. 4,873,191; Gordon et al., Proc.
Natl. Aced. Sci. USA ,~: 7380-7384, 1980; Gordon and
Ruddle, ,~~,ence ~: 1244-1246, 1981; Palmiter and
Brinster, Cell g,~: 343-345, 1985; Brinster et al., Proc.
Natl. Aced. Sci. USA $~: 4438-4442, 1985; Hogan et al.,
Mani_DUiatina the Mouse Embryo: A Laboratory Manual, Cold
Spring Harbor Laboratory, 1986; Simons et al.
BioJTechnoloav g: 179-183, 1988; Wall et al., Biol.
Reprod. ~,: 645-651, 1985; Buhler et al., BiQ,~Technoloav
$: 140-143, 1990; Ebert et al. , Bio/Technolow $: 835-838,
1991; Krimpenfort et al., Bio/Te~hnoloav ~,: 844-847, 1991;
Wall et al., ~. Cell. Biochem. ,4$: 113-120, 1992; WIPO
publications WO 88/00239, WO 90/05118, and WO 92/11757;
and GB 87/00458.
CA 02309891 2000-06-19
14
For injection into fertilized eggs, the
expression units ,are removed from their respective vectors
by digestion with appropriate restriction enzymes. For
convenience, it is preferred to desigr the vectors so that
the expression units are removed by cleavage with enzymes
that do not cut either within the expression units or
elsewhere in th~ vectors. The expression units are
recovered by conventional methods, such as electro-elution
followed by phenol extraction and ethanol precipitation,
sucrose density gradient centrifugation, or combinations
of these approaches.
DNA is injected into eggs essentially as
described in Hogan et al., ibid. In a typical injection,
eggs in a dish of an embryo culture medium are located
using a stereo zoom microscope (x50 or x63 magnification
preferred). Suitable media include Hepes (N-2-
hydroxyethylpiperazina-N~-2-ethanesulphonic acid) or
bicarbonate buffered media such as M2 or M16 (available
from Sigma Chemical Co., St. Louis, USA) or synthetic
oviduct medium (disclosed below). The eggs are secured
and transferred to the center of a glass slide on an
injection rig using, for example, a drummond pipette
complete with capillary tube. Viewing at lower (e.g. x4)
magnification is used at this stage. Using the holding
pipette of the injection rig, the eggs are positioned
centrally on the slide. Individual eggs are sequentially
secured to the holding pipette for injection. For each
injection process, th~ holding pipette/egg is positioned
in the center of the viewing field. The injection needle
3o is than positioned directly below the egg. Preferably
using x40 Nomarski objectives, both manipulator heights
are adjusted to focus both the egg and the needle. The
pronuclei are located by rotating the egg and adjusting
the holding pipette assembly as necessary. Once the
pronucleus has been located, the height of the manipulator
is altered to focus the pronuclear membrane. The
injection needle is positioned below the egg such that the
CA 02309891 2000-06-19
needle tip is in a position below the center of the
pronucleus. The position of the needle is then altered
using the injection manipulator assembly to bring the
needle and the pronucleus into the same focal plane. The
5 needle is moved, via the joy stick on the injection
manipulator assembly, to a position to the right of the
egg. With a short, continuous jabbing movement, the
pronuclear membrane is pierced to leave the needle tip
inside the pronucleus. Pressure is applied to the
10 injection needle via the glass syringe until the
pronucleus swells to approximately twice its volume. At
this point, the needle is slowly removed. Reverting to
lower (e. g. x4) magnification, the injected egg is moved
to a different area of the slide, and the process is
15 repeated with another egg.
After the DNA is injected, the eggs may be
cultured to allow the pronuclei to fuse, producing one-
cell or later stage embryos. In general, the eggs are
cultured at approximately the body temperature of the
species used in a buffered medium containing balanced
salts and serum. Surviving embryos are than transferred
to psaudopregnant recipient females, typically by
inserting them into the oviduct or uterus, and allowed to
develop to term. During embryogenesis, the injected DNA
integrates in a random fashion in the genomes of a small
number of the developing embryos.
Potential transgenic offspring are screened via
blood samples and/or tissue biopsies. DNA is prepared
from these samples and examined for the presence of the
3o injected construct by techniques such as polymerise chain
reaction (PCR; sea Mullis, U.S. Patent No. 4,683,202) and
Southern blotting (Southern, J. Mol. Biol. x:503, 1975;
Maniatis et al., Molecular Cloninq: A Laborator~r Manual,
Cold Spring Harbor Laboratory, 1982). Founder transgenic
animals, or GOs, may be wholly transgenic, having
transgenes in all of their cells, or mosaic, having
transgenes in only a subset of cells (see, for example,
CA 02309891 2000-06-19
16
Wilkie et al., DeveloB. Biol, ~; 9-18, 1986). In the
latter case, groups. of germ cells may be wholly or
partially transgenic. In the latter case, the number of
transgenic progeny from a founder animal will be less than
the expected 50~ predicted from Mendelian principles.
Founder GD animals era grown to sexual maturity and mated
to obtain offspring, or Gis. The Gis are also examined
for the presence of the transgene to demonstrate
transmission from~foundsr GO animals. In the case of male
GOs, these may be mated with several non-transgenic
females to generate many offspring. This increases the
chances of observing transgene transmission. Female GO
founders may be mated naturally, artificially inseminated
or superovulated to obtain many eggs which are transferred
to surrogate mothers. The latter course gives '-..he best
chance of observing transmission in animals having a
limited number of young. The above-described breeding
procedures are used to obtain animals that can pass the
DNA on to subsequent generations of offspring in the
normal, Mendelian fashion, allowing the development of,
for example, colonies (mice), flocks (sheep), or herds
(pigs, goats and cattle) of transgenic animals.
The milk from lactating GO and G1 females is
examined for the expression of the heterologous protein
using immunological techniques such as ELISA (see Harlow
and Lane, Antibodies. A Laboratory anu
]~~, Cold Spring
Harbor Laboratory, 1988) and Western blotting (Towbin et
al., Proc. Natl. Aced. Sci. USA ~: 4350-4354, 1979). For
a variety of reasons known in the art, expression levels
0! the heterologous protein will be expected to differ
between individuals.
A satisfactory family of animals should satisfy
three criteria: they should be derived from the same
founder GO animal; they should exhibit stable transmission
of the transgene; and they should exhibit stable
expression levels from generation to generation and from
lactation to lactation of individual animals. These
CA 02309891 2000-06-19
17
principles have been demonstrated and discussed (Carver et
al., Hio/Techno~ow ~: 1263-1270, 1993). Animals from
such a suitable family are referred to as a "line."
Initially, male animals, GO or G1, are used to derive a
flock or herd of producer animals by natural or artificial
insemination. In this way, many female animals containing
the same transgene integration event can be quickly
generated from which a supply of milk can be obtained.
The fibrinogen is recovered from milk using
standard practices such as skimming, precipitation,
filtration and protein chromatography techniques.
Fibrinogen produced according to the present
invention is useful within human and veterinary medicine,
such as in the formulation of surgical adhesives.
Adhesives of this type are known in the art. See, for
example, U.S. Patents No. 4,377,572; 4,442,655; 4,462,567;
and 4,627,879,
In general, fibrinogen and factor XIII are combined to
form a first component that is mixed just prior to use
with a second component containing thrombin. The thrombin
converts the fibrinogen to fibrin, causing the mixture to
gel, and activates the factor XIII. The activated factor
XIII cross links the fibrin to strengthen and stabilize
the adhesive matrix. Such adhesives typically contain
from about 30 mg/ml to about 100 mg/ml fibrinogen and from
about 50 ;tg/ml to about 500 ~tg/ml factor XIII. They may
also contain additional ingredients, such as aprotinin,
albumin, fibronectin, bulking agents, and solubilizers.
Methods far producing factor XIII are known in the art.
See, for example, U.S. Patent No. 5,204,447. The
fibrinogen is also useful for coating surfaces of
polymeric articles, e.g. synthetic vascular grafts, as
disclosed in U.S. Patent No. 5,272,074.
The invention is further illustrated by the
following non-limiting examples.
CA 02309891 2000-06-19
18
The multiple cloning site of the vector pUCl8
(Yanisch-Perron et al., Gene x:103-119, 1985) was removed
and replaced with a synthetic double stranded
oligonucleotide (the strands of which are shown in SEQ ID
NO: 8 and SEQ ID NO: 27) containing the restriction sites
Pvu I/Mlu I/Eco ~RV/Xba I/Pw I/Mlu I, and flanked by 5~
overhangs compatible with the restriction sites Eco RI and
Hind III. pUClB was cleaved with both Eco RI and Hind
III, the 5~ terminal phosphate groups were removed with
calf intestinal phophastase, and the oligonucleotide was
ligated into the vector backbone. The DNA sequence across
the junction was confirmed by sequencing, and the new
plasmid was called pUCPM.
The ~-lactoglobulin (BLG) gene sequences from
pSSitgXS (disclosed in WIPO publication WO 88/00239) were
excised as a Sal I-Xba I fragment and recloned into the
vector pUCPM that had been cut with Sal I and Xba I to
construct vector pUCXS. pUCXS is thus a pUClB derivative
containing the entire HLG gene from the sal I site to the
Xba I site of phage SS1 (Ali and Clark, J. Mol. Biol.
415-426, 1988).
The plasmid pSSitgSE (disclosed in WIPO
publication WO 88/00239) contains a 1290 by BLG fragment
flanked by Sph I and EcoR I restriction sites, a region
spanning a unique Not I site and a single Pw II site
which lies in the 5 ~ untranslated leader of the BLG mRNA.
Into this Pvu II site was ligated a double stranded, 8 by
JNA linker (5~-GGATATCC-3~) encoding the recognition site
for the enzyme Eco RV. This plasmid was called
pSSitgSE/RV. DNA sequences bounded by Sph I and Not I
restriction sites in pSSitgSE/RV were excised by enzymatic
digestion and used to replace the equivalent fragment in
pUCXS. The resulting plasmid was called pUCXSRV. The
sequence of the BLG insert in pUCSXRV is shown in SEQ ID
CA 02309891 2000-06-19
19
NO: 7, with the unique Eco RV site at nucleotide 4245 in
the 5~ untranslated leader region of the BLG gene. This
site allows insertion of any additional DNA sequences
under the control of the BLG promoter 3' to the
transcription initiation site.
Using the primers BLGAMP3 (5~-TGG ATC CCC TGC
CGG TGC CTC TGG-3' ; SEQ ID NO: 9 ) and BLGAMP4 ( 5 ~ -AAC GCG
TCA TCC TCT GTG AGC CAG-3'; SEQ ID NO: 10) a PCR fragment
of approximately 650 by was produced from sequences
immediately 3~ to the stop codon of the BLG gene in
pUCXSRV. Tha PCR fragment was engineered to have a BamH I
site at its 5~ and and an Mlu I site at its 3~ end and was
cloned as such into BamH I and Mlu I cut pGEM7zf(+)
(Promega) to give pDAM200(+).
pUCXSRV was digested with Kpn I, and the
largest, vector containing band was gel purified. This
band contained the entire pUC plasmid sequences and some
3~ non-coding sequences from the BLG gene. Into this
backbone was ligatad the small Kpn I fragment from
pDAM200(+) which, in the correct orientation, effectively
engineered a BamH I site at the extreme 5~ end of the 2.6
Kbp of the BLG 3~ flanking region. This plasmid was
called pBLAC200. A 2.6 Kbp Cla I-Xba I fragment from
p8LAC200 way ligatad into Cla I-Xba I cut pSP72 vector
(Promaga), thus placing an EcoR V site immediately
upstream of th~ BLG sequences. This plasmid was called
pBLAC210.
Tha 2.6 Kbp Eco RV-Xba I fragment from p8LAC210
was ligatad into Eco RV-Xba I cut pUCXSRV to form pMAD6.
This, in aff.ct, excised all coding and intros sequences
from pUCXSRV, forming a BLG minigane consisting of 4.3 Kbp
of 5~ promoter and 2.6 Kbp of 3~ downstream sequences
flanking a unique EcoR V site. An oligonucleotida linker
(ZC6839: ACTACGTAGT; SEQ ID NO: 11) was inserted into the
Eco RV site of pMAD6. This modification destroyed the Eco
RV site and created a Sna BI site to be used for cloning
purposes. The vector was designated pMAD6-Sna. Messenger
CA 02309891 2000-06-19
RNA initiates upstream of the Sna HI site and terminates
downstream of the Sna BI site. The precursor transcript
will encode a single BLG-derived intros, intros 6, which
is entirely within the 3' untranslated region of the gene.
5
Exam~rle II
Clones encoding the individual fibrinogen chains
wars obtained from the laboratory of Dr. Earl W. Davie,
10 University of Washington, Seattle. A genomic fibrinogen
Aa-chain clone (Chung et al., 1990, ibid.) was obtained
from the plasmid BS4. This plasmid contains the Aa clone
inserted into the Sal I and Ham HI sites of the vector
pUCl8, but lacks the coding sequence for the first four
15 amino acids of the Aa chain. A genomic H~-chain DNA (Chung
et al. , ibid. ) was isolated from a lambda Charon 4A phage
clone (designated ~a4) as two EcoRI fragments of ca. 5.6
Kbp each. The two fragments wars cloned separately into
pUCl9 that had been digested with Eco RI and treated with
20 calf intestinal phosphatass. The resulting clones were
screened by digestion with the restriction enzyme Pvu II
to distinguish plasmids with the 5' and 3' B~ inserts
(designated Beta5'RI/puc and Bsta3'RI/puc, respectively).
Genomic y-chain clones were isolated as described by Rixon
et al. (Hiochemistrv ~q: 2077-2086, 1985). Clone
py12A9
comprises 5' non-coding sequencss and approximately 4535
by of ~r-chain coding sequence. Clone p~r12F3 comprises the
remaining coding sequence and 3' non-coding nucleotides.
Both are pBR322-based plasmids with the fibrinogen
sequences inssrted at the EcoRI site. Thsss plasmids were
used as templates for the respective PCR reactions.
The fibrinogen chain coding sequences were
tailored for insertion into expression vectors using the
polymerase chain reaction (PCR) as generally described by
Mullis (U. S. Patent No. 4,683,202). This
procedure
removed native 5' and 3' untranslated sequences, added a 9
base sequence (CCT GCA GCC) upstream of the first ATG of
CA 02309891 2000-06-19
21
each coding sequence, supplied the first four codons for
the Aa-chain sequence, removed an internal Mlu I site in
the Aa sequence ,and added restriction sites to facilitate
subsequent cloning steps.
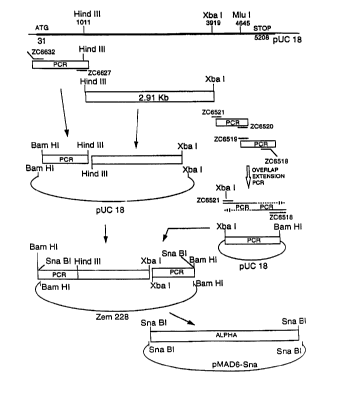
Referring to Figure 1, the 5~ end of the Aa
coding sequence was tailored in a PCR reaction containing
20 pmola for each of primers ZC6632 (SEQ ID NO: 12) and
ZC6627 (SEQ ID NO: 13), approximately 10 ng of plasmid BS4
template DNA, 10 ~ ~tl of a mix containing 2 . 5 mM each dNTP,
l0 7.5 ~tl lOx Pyrococcus furiosus (Pfu) nNA polymerise buffer
,~l (200 mM Tris-HC1, pH 8.2, 100 mM KCl, 60 mM (NH4) 2504,
20 mM MgCl2, 1~ Triton X-100; 100 ;tg/ml nuclease free
bovine serum albumin)(Stratagene, La Jolla, CA), and water
to 75 ;tl. The mixture was heated to 94°C in a DNA thermal
cyclar (Perkin-Elmer Corp., Norwalk, CT). To the heated
mixture was added 25 ul of a mixture containing 2.5 ~tl lOx
Pfu buffer ;1, 22 ;tl H20 and 1 ;tl 2.5 units/~tl Pfu DNA
polymerise (Stratagena). Ths reactions ware run in a DNA
thermal cycler (Perkin-Elmer) for five cycles of 94°, 45
seconds; 40°, 90 seconds; 72°, 120 seconds; 20 cycles of
94°, 45 seconds; 45°, 90 seconds; 72°, 120 seconds; then
incubated at 72° for 7 minutes. The 5~ PCR-generated
fragment was digested with Bam HI and Hind III, and the
Sam HI-Hind III fragment was then ligated to an internal
2.91 Kbp Hind III-Xba I fragment and Bam HI, Xba I-
digested pUCl8. PCR-generated axon sequences were
sequenced.
Referring again to Figure 1, the 3~ end of the
Aa coding sequence was tailored in a series of steps in
which the Mlu I site 563 bases upstream from the stop
codon of the Aa sequence was mutated using an overlap
extension PCR reaction (Ho et al. , Gene ,~: 51-59, 1989) .
In the first reaction 40 pmols of each of primers ZC6521
(SEQ ID NO: 14) and ZC6520 (SEQ ID NO: 15) were combined
with approximately 10 ng of plasmid BS4 template DNA in a
reaction mixture as described above. The reaction was run
for 5 cycles of 94°, 45 seconds; 40°, 60 seconds; 72°,
120
Trade-mark
CA 02309891 2000-06-19
22
seconds; 15 cycles of 94°, 45 seconds; 45°, 60 seconds;
72°, 120 seconds,~, then incubated at 72° for 7 minutes. A
second reaction was carried out in the same manner using
40 pmole of each of primers ZC6519 (SEQ ID NO: 16) and
ZC6518 (SEQ ID NO: 17) and BS4 as template. The PCR-
generated DNA fragments from the first and second
reactions were isolated by gel electrophoresis and elution
from the gel. Approximately 1/10 of each recovered
reaction product was combined with 40 pmole of each of
primers ZC6521 ( SEQ ID NO : 14 ) and ZC6518 ( SEQ ID NO: 17 )
in a PCR reaction in which the complementary 3' ends of
each fragment (containing the single base change) annealed
and served as a primer for the 3' extension of the
complementary strand. PCR was carried out using the same
reaction conditions as in the first and second 3' PCR
steps. The reaction product was then digested with Xba I
and Bam HI, and the Xba I-Ham HI fragmsnt was cloned into
Xba I, Bam HI-digested pUCl8. PCR-generated exons were
sequenced.
As shown in Figure l, the 5' Bam HI-Xba I
fragment (3.9 Kbp) and the 3' Xba I-Bam HI fragment (1.3
Kbp) were inserted into the Bam HI site of the vector
Zem228. Zem228 is a pUCl8 derivative comprising a Bam HI
cloning site between a mouse MT-1 promoter and SV40
terminator, and a neomycin resistance marker flanked by
Sv40 promoter and terminator sequences. See European
Patent Office Publication EP 319,944 and Fig. 2. The
entire Aa coding sequence was isolated from the Zem228
vec~~or as an Sna BI fragment, which was inserted into the
Sna BI sits of the plasmid pMAD6-Sna.
Referring to Fig. 3, the 5' end of the Bp-chain
was tailored by PCR using the oligonucleotides ZC6629 (SEQ
ID NO: 18) , ZC6630 (SEQ ID NO: 19) and ZC6625 (SEQ ID NO:
20). These primers were used in pairwise combinations
(ZC6629 + ZC6625 or ZC6630 + ZC6625) to generate B~ coding
sequences beginning at the first ATG codon (position 470
in SEQ ID NO: 3)(designated N1-Heta) or the third ATG
CA 02309891 2000-06-19
23
codon (position 512 in SEQ ID NO: 3)(designated N3-Beta).
Approximately 5, ng of Beta5~RI/puc template DNA was
combined with 20 pmole of each of the primers (N1-
Beta:ZC6629, SEQ ID NO: 18 + ZC6625, SEQ ID NO: 20; or N3-
Bsta:ZC6630, SEQ ID NO: 19 + ZC6625, SEQ ID NO: 20) in a
reaction mixture as described above. Ths mixtures ware
incubated for 5 cycles of 94°, 45 seconds; 40°, 120
seconds; (N1-Bsta) or 90 seconds (N3-Beta); 72°, 120
seconds; 20 cycles of 94°, 45 seconds; 45°, 120 seconds;
(N1-Beta) or 90 seconds (N3-Beta); 72°, 120 seconds; then
incubated at 72° for 7 minutes. The two reaction products
N1, 555 by or N3, 510 bp) wars each digested with Eco RI
and Bgl II, and the fragments were ligated to the internal
Bgl II-Xba I fragment and Eco RI + Xba I-digested pUCl9.
The 3~ end of the BQ sequence was tailored in a reaction
mixture as described above using the oligonucleotide
primers ZC6626 (SEQ ID NO: 21) and ZC6624 (SEQ ID NO: 22)
and approximately 5 ng of Bsta3'RI/puc template. The
mixtures ware incubated for 5 cycles of 94°, 45 seconds;
40°, 90 seconds; 72°, 120 seconds; 15 cycles of 94°, 45
seconds; 45°, 90 seconds; 72°, 120 seconds; than incubated
at 72° for 7 minutes. A 990 by Bgl II-Eco RI fragment was
isolated. This 3~ fragment was ligatsd to the adjacent
coding fragment (340 bp, SphI-Hgl II) and Sph I + Eco RI-
digested pUCl9. Tha 3~ and 5~ PCR-generated axons were
sequenced. A third interansdiats vector was constructed by
combining two internal fragments (4285 by Xba I-Eco RI and
383 kb Eco RI-Sph I) in Xba I + Sph I-digested pUCl9. The
entire B~3 coding sequence (two forms) was then assembled
by ligating one of the 5' Eco RI-Xba I fragments, the,
internal Xba I-Sph I fragment, the 3' Sph I-Eco RI
fragment and Eco RI-digested vector pUCl9. The B~
sequence was then isolated as a 7.6 Kbp Sna BI fragment
and inserted into the Sna BI site of pMAD6-Sna.
Referring to Fig. 4, the 5' end of the gamma
chain sequence was tailored by PCR using the
oligonucleotide primers ZC6514 (SEQ ID NO: 23) and ZC6517
CA 02309891 2000-06-19
24
(SEQ ID NO: 24) and approximately 50 ng of p~12A9 as
template. The PCR reaction was run as described above
using 40 pM of each primer. The reaction was run for 5
cycles of 94°, 45 seconds; 40°, 60 seconds, 72°, 120
seconds, followed by 15 cycles of 94°, 45 seconds; 45°, 60
seconds; 72°, 120 seconds. The resulting 213 by fragment
was digested with Bam HI and Spe I, and the resulting
restriction fragment was ligated with the adjacent
downstream 4.4 kb Spe I-Eco RI fragment and Bam HI + Eco
l0 RI digested pUCl9. The 3' end of the gamma chain sequence
was tailored using oligonucleotide primera ZC6516 (SEQ ID
NO: 25) and ZC6515 (SEQ ID NO: 26) using 40 pM of each
primer, approximately 50 ng of p~r12F3 template and the
same thermal cycling schedule as used for the 5' fragment.
The resulting 500 by fragment was digested with Spe I and
Bam HI, and the resulting restriction fragment was ligated
with the upstream 2.77 kb Eco RI-Spe I fragment and Eco RI
+ Bam HI-digested pUCl9. All PCR-generated exons were
sequenced. The entire y'-chain coding sequence was then
assembled by ligating a 4.5 Kbp Bam HI-Eco RI 5' fragment,
a 1.1 Kbp Eco RI-Pst I internal fragment and a 2.14 Kbp
Pst I-Xba I 3' fragment in Bam HI + Xba I-digested
Zem219b. Zem219b is a pUCl8-derived vector containing a
mouse metallothionein promoter and a DHFR selectable
marker operably linked to an SV40 promoter (Fig. 5).
Plasmid Zem219b has been deposited with American Type
Culture Collection as an E. coli XL1-blue transformant
under Accession No. 68979. The entire ~r~-chain coding
sequence was then isolated as a 7.8 Kbp Sna B1 fragment
and inserted into the Sna BI site of pMAD6-Sna.
Exam'! a III
Mice for initial breeding stocks (C57BL6J,
CBACA) were obtained from Harlan Olac Ltd. (Hicester, UK).
These were mated in pairs to produce F1 hybrid cross
(B6CBAF1) for recipient female, superovulated females,
stud males and vasectomized males. All animals were kept
CA 02309891 2000-06-19
on a 14 hour light/10 hour dark cycle and fed water and
food (Special Diet Services RM3, Edinburgh, Scotland) ad
libitum.
Transgc.-is mice were generated essentially as
5 described in Hogs; et al. , ManiDUlatinqy the Mouse Embrv~
A Laborato~y~ Manual, Cold Spring Harbor Laboratory, 1986,
Female B6CBAF1 animals were supsrovulatad at 4-5 weeks of
age by an i.p. infection of pregnant mares' .serum
10 gonadotrophin (FOLLIGOM"' Vet-Drug, Falkirk, Scotland) (5
iu) followed by an i.p. injection of human chorionic
gonadotrophin (CHORULON; Vet-Drug, Falkirk, Scotland) (5
iu) 45 hours later. They were then mated with a stud male
overnight. Such females were next examined for copulation
15 plugs. Those that had mated were sacrificed, and their
eggs were collected for microinjection.
DNA was injected into the fertilized eggs as
described in Hogan at al. (ibid.) Briefly, each of the
vectors containing the Aa, Bp and y expression units was
20 digested with Mlu I, and the expression units were
isolated by sucrose gradient centrifugation. All
chemicals used ware reagent grads (Sigma Chemical Co., St.
Louis, MO, U.S.A.), and all solutions wars sterile and
nuclease-Eras. Solutions of 20% and 40% sucrose in 1 M
25 NaCl, 20 mM Tris pH 8.0, 5 mM EDTA were prepared using UHP
water and filter sterilized. A 30% sucrose solution was
prepared by mixing equal volumes of the 20% and 40%
solutions. A gradient was prepared by layering 0.5 ml
steps of the 40%, 30% and 20% sucrose solutions into a 2
ml polyallomer tube and allowed to stand for one hour.
100 ~tl of DNA solution (max. 8 ~,cg DNA) was loaded onto the
top of the gradient, and the gradient was centrifuged for
17-20 hours at 26,000 rpm, 15~C in a Beckman TL100
ultracentrifuge using a TLS-55 rotor (Beckman Instruments,
Fullerton, CA, USA). Gradients were fractionated by
puncturing the tube bottom with a 20 ga. needle and
collecting drops in a 96 well microtiter plate. 3 ~tl
# Trade-mark
CA 02309891 2000-06-19
26
aliquots were analyzed on a 1% agarose mini-gel.
Fractions containing~the desired DNA fragment were pooled
and ethanol precipitated overnight at -20°C in 0.3M sodium
acetate. DNA pellets were resuspended in 50-100 ~l UHP
water and quantitated by fluorimstry. The expression
units were diluted in~Dulbecco~s phosphate buffered saline
without calcium and magnesium (containing, per liter, 0.2
g KCI, 0.2 g KH2P04, 8.0 g NaCl, 1.15 g Na2HP04), mixed
(using either the~Ni-Beta or N3-Beta expression unit) in a
1:1:1 molar ratio, concentration adjuste3 to about 6
Etg/ml, and injected into the eggs (-2 pl total DNA
solution per egg).
Recipient females of b-8 weeks of age are
prepared by mating 86CBAF1 females in natural estrus with
vasectomized males. Females possessing copulation plugs
are then kept for transfer of microinjected eggs.
Following birth of potential transgenic animals,
tail biopsies ars taken, under anesthesia, at four weeks
of age. Tissue samples are placed in 2 ml of tail buffer
(0.3 M Na acetate, 50 mM HCl, 1.5 mM MgCl2, 10 mM Tris-
HC1, pH 8.5, 0.5% NP40, 0.5% Tween ZO) containing 200
;tg/ml proteinase K (Boehringer Mannheim, Mannheim,
Germany) and vortexed. The samples are shaken (250 rpm)
at 55°-60° for 3 hours to overnight. DNA prepared from
biopsy samples is examined for the presence of the
injected constructs by PCR and Southern blotting. The
digested tissue is vigorously vortexed, and 5 ;tl aliquots
are placed in 0.5 ml microcentrifuge tubes. Positive and
negative tail samples are included as controls. Forty ;tl
of silicone oil (HDH, Pools, UK) is added to each tube,
and the tubes are briefly centrifuged. The tubes are
incubated in the heating block of a thermal cycler (e. g.
Omni-gene, Hybaid, Teddington, UK) to 95°C for 10 minutes.
Following this, each tube has a 45 ~tl aliquot of PCR. mix
added such that the final composition of each reaction mix
is: 50 mM KCl; 2 mM MgCl2; 10 mM Tris-HC1 (pH 8.3) ; 0.01%
gelatin; 0.1% NP40, 10% DMSO; 500 nM each primer, 200 ~M
CA 02309891 2000-06-19
27
dN''Ps; 0.02 U/~l Taq polymerise (Boehringer Mannheim,
Mannheim, Germany). The tubas are then cycled through 30
repeated temperature changes as required by the particular
primers used. The primers may bs varied but in all cases
must target the BLG promoter region. This is specific for
the injected DNA fragments because the mouse doss not have
a BLG gene. Twelve ~tl of 5x loading buffer containing
Orange G marker dye (0.25 Orange G [Sigma] 15~ Ficoll
type 400 [Pharmacia Biosystsms Ltd., Milton Keynes, UK])
is then added to each tubs, and the reaction mixtures are
electrophoresad on a 1.6~ agarose gel containing ethidium
bromide (Sigma) until the marker dye has migrated 2/3 of
the length of the gel. Ths gel is visualized with a UV
light source emitting a wavelength of 254 nm. Transgenic
mice having one or more of the injected DNA fragments are
identified by this approach.
Positive tail samples are processed to obtain
puts DNA. The DNA samples are screened by Southern
blotting using a BLG promoter probe (nucleotides 2523-4253
of SEQ ID NO: 7). Specific cleavages with appropriate
restriction enzymes (a.g. Eco RI) allow the distinction of
the three constructs containing the Aa, 8S and y sequences.
Southern blot analysis of transgsnic mice
prepared essentially as described above demonstrated that
more than 50~ of progeny contained all three fibrinogen
sequences. Examination of milk from positive animals by
reducing SDS polyacrylamide gel electrophoresis
demonstrated the presence of all three protein chains at
concentrations up to 1 mg/ml. The amount of fully
assembled fibrinogen was related to the ratios of
individual subunits present in the milk. No apparent
phenotype was associated with high concentrations of human
fibrinogen in mouse milk.
Example IV
Donor ewes are treated with an intravaginal
progesterone-impregnated sponge (CIiRONOGEST# Goat Sponge,
Trade-mark
CA 02309891 2000-06-19
28
Intervet, Cambridge, UK) on day 0. Sponges are left in
situ for ten or twelve days.
Superowlation is induced by treatment of donor
ewes with a total of one unit of ovine follicle
stimulating hormone (OFSH) (OVAGEN; Horizon Animal
Reproduction Technology Pty. Ltd., New Zealand)
administered in eight intramuscular injections of 0.125
units per injection starting at 5:00 pm on day -4 and
ending at 8:00 am on day 0. Donors are injected
intramuscularly with 0.5 ml of a luteolytic agent
(ESTRUMATE; Vet-Drug) on day -4 to cause regression of the
corpus luteum, to allow return to estrus and owlation.
To synchronize owlation, the. donor animals are injected
intramuscularly with 2 ml of a synthetic releasing hormone
analog (RECEPTAL; Vet-Drug) at 5:00 pm on day 0.
Donors are starved of food and water for at
least 12 hours before artificial insemination (A.I.). The
animals are artificially inseminated by intrauterine
laparoscopy under sedation and local anesthesia on day 1.
Either xylazine (ROMPUN; Vet-Drug) at a dose rate of 0.05-
0.1 ml per 10 kg bodywaight or ACP injection 10 mg/ml
(Vet-Drug) at a doss rate of 0.1 ml per 10 kg bodyweight
is injected intramuscularly approximately fifteen minutes
before A.I. to provide sedation. A.I. is carried out
using freshly collected semen from a Poll Dorset ram.
Semen is diluted with equal parts of filtered phosphate
buffered saline, and 0.2 ml of the diluted semen is
injected per uterine horn. Immediately pre- or post-A. I.,
donors are given an intramuscular injection of AMOXYPEN #
(Vet-Drug).
Fertilized eggs are recovered on day 2 following
starvation of donors of food and water from 5:00 pm on day
1. Recovery is carried out under general anesthesia
induced by an intravenous injection of 5% thiopentone
sodium (INTRAVAL SODIUM; Vet-Drug) at a dose rate of 3 ml
per 10 kg bodyweight. Anesthesia is maintained by
inhalation of 1-2% Halothane/02/N20 after intubation. To
Trade-mark
CA 02309891 2000-06-19
29
recover the fertilized eggs, a laparotomy incision is
made, and the ,uterus is exteriorized. The eggs are
recovered by retrograde flushing of the oviducts with Ovum
Culture Medium (Advanced Protein Products, Brierly Hill,
Wast Midlands, UR) supplemented with bovine serum albumin
of Naw Zealand origin. After flushing, the uterus is
returned to the abdomen, and the incision is closed.
Donors are allowed to recover post-operatively or are
euthanized. Donors that are allowed to recover are given
an intramuscular injection of Amoxypen L.A. a, the
manufacturer's recommended dose rate immediately pre- or
post-operatively.
Plasmids containing the three fibrinogen chain
expression units are digested with Mlu I, and the
expression unit fragments are recovered and purified on
sucrose density gradients. The fragment concentrations
are determined by lluorimetry and diluted in Dulbecco's
phosphate bulfarad saline without calcium and magnesium as
described above. Tha concentration is adjusted to 6 ~tg/ml
and approximately 2 pl of the mixture is microinjected
into one pronucleus o! each fertilized eggs with visible
pronuclei.
All fertilized eggs surviving pronuclear
microinjaction era cultured in vitro at 38.5°C in an
atmosphere o! 5~ C02:5~ 02:90 N2 and about -100 humidity
in a bicarbonate bu!lered synthetic oviduct medium (see
Table) supplemented with 20~ v/v vasectomized ram serum.
Tha serum may be heat inactivated at 56°C !or 30 minutes
and stored lrozan at -20°C prior to use. The fertilized
eggs era cultured for a suitable period of time to allow
early embryo mortality (caused by the manipulation
techniques) to occur. These dead or arrested embryos are
discarded. Embryos having developed to 5 or 6 cell
divisions are transferred to synchronized recipient ewes.
CA 02309891 2000-06-19
Table
Synthetic oviduct Medium
5 Stock A (Lasts '~ Mo nths)
NaCl 6.29 g
KCl 0.534 g
~2P04 0.162 g
MgS04.7H O 0.182 g
to Penicillin 0.06 g
Sodium Lactate 60$ syrup 0.6 mls
Super H20 99.4 mls
Htock B (Lasts 2 we eks)
15 NaHC03 0.21 g
Phenol red 0.001 g
Super H20 10 mls
Mock C (Lasts 2 we eks)
20 Sodium Pyruvate 0.051 g
Super H20 10 mls
Stock D (Lasts 3 mo nths)
CaC12.2H20 0.262 g
25 Super H20 10 mls
Stock E (Lasts 3 mo nths(
Hepss 0.651 g
Phenol red 0.001 g
30 Super H20 10 mls
TQ make i
lO
l
f
u~ B
m carbonate Buffered
s o
STOCK A 1 ml
STOCK B 1 ml
STOCK C 0.07 ml
STOCK D 0.1 ml
Super H20 7.83 ml
Osmolarity should be 265-285
mOsm.
Add 2.5 ml of heat inactivated sheep serum
and filter sterilize.
To make uo 10 mls o f HEPES Buffered Med
STOCK A 1 ml
STOCK 8 0.2 ml
STOCK C 0.07 ml
STOCK D 0.1 ml
STOCK E 0.8 ml
Super H20 7.83 ml
CA 02309891 2000-06-19
31
Osmolarity should be 265-285 mOsm.
Add 2.5 ml of heat inactivated sheep serum
and filter sterilize.
Recipient ewes are treated with an intravaginal
progesterone-impregnated sponge (Chronogest Ewe Sponge or
Chronogest Ewe-Lamb Sponge, Interest) left in situ for 10
or 12 days. The ewes are injected intramuscularly with
1.5 ml (300 iu) of a follicle stimulating hormone
substitute (P.M.S.G., Interest) and with 0.5 ml of a
luteolytic agent (Estrumate, Coopers Pitman-Moors) at
sponge removal on day -1. The~ewes era tested for estrus
with a vasectomized ram between 8:00 am and 5:00 pm on
days 0 and 1.
Embryos surviving in vitro culture are returned
to recipients ( starved from 5 : 00 pm on day 5 or 6 ) on day
6 or 7. Embryo transfer is carried out under general
anesthesia as described above. The uterus is exteriorized
via a laparotomy incision with or without laparoscopy.
Embryos are returned to one or both uterine horns only in
ewes with at least ons suitable corpora lutea. After
replacement of the uterus, the abdomen is closed, and the
recipients are allowed to recover. The animals are given
an intramuscular injection of Amoxypen L.A. at the
manufacturer's recommended dose rate immediately pry- or
post-operatively.
Lambs are identified by ear tags and left with
their dams for rearing. Ewes and lambs are either housed
and fed complete diet concentrates and other supplements
and or ad lib. hay, or are let out to grass.
Within the (first week of life (or as soon
thereafter as possible without prejudicing health), each
lamb is tested for the presence of the heterologous DNA by
two sampling procedures. A 10 ml blood sample is taken
from the jugular vein into an EDTA vacutainer. If fit
enough, the lambs also have a second 10 ml blood sample
CA 02309891 2000-06-19
32
taken within one week of the first. Tissue samples are
taken by tail biopsy as soon as possible after the tail
has become desensitized after the application of a rubber
elastrator ring to its proximal third (usually within 200
minutes after "tailing"). The tissue is placed
immediately in a solution of tail buffer. Tail samples
are kept at room temperature and analyzed on the day of
collection. All lambs are given an intramuscular
infection of Amoxypen L.A. at' the manufacturer's
recommended dose rate immediately post-biopsy, and the cut
end of the tail is sprayed with an antibiotic spray.
DNA is extracted from sheep blood by first
separating white blood cells. A 10 ml sample of blood is
diluted in 20 ml of Hank's buffered saline (HHS; obtained
from Sigma Chemical Co. ) . Ten ml of the diluted blood is
layered over 5 ml of Histopaque (Sigma) in each of two 15
ml screw-capped tubes. The tubes are centrifuged at 3000
rpm (2000 x g max.), low brake for 15 minutes at room
temperature. White call interfaces are removed to a clean
15 ml tube and diluted to 15 ml in HBS. The diluted cells
are spun at 3000 rpm for 10 minutes at room temperature,
and the call pellet is recovered and resuspended in 2-5 ml
of tail buffer.
To extract DNA from the white cells, 10% SDS is
added to the resuspendsd cells to a final concentration of
1%, and the tube is inverted to mix the solution. One mg
of fresh proteinase K solution is added, and the mixture
is incubated overnight at 45°C. DNA is extracted using an
equal volume of phenol/chloroform (x3) and
chloroform/isoamyl alcohol (xi). Ths DNA is then
precipitated by adding 0.1 volume of 3 M NaOAc and 2
volumes of ethanol, and the tubs is inverted to mix. The
precipitated DNA is spooled out using a clean glass rod
with a sealed end. The spool is washed in 70% ethanol,
and the DNA is allowed to partially dry, then is
redissolved in TE (10 mM Tris-HC1, 1 mM EDTA, pH 7.4).
CA 02309891 2000-06-19
33
DNA samples from blood and tail are analyzed by
Southern blotting using probes for the BLG promoter region
and the fibrinogen chain coding regions.
From the foregoing, it will be appreciated that,
although specific embodiments of the invention have been
described herein for purposes of illustration, various
modifications may be made without deviating from the
spirit and scope of the invention. Accordingly, the
invention is not limited except as by the appended claims.
CA 02309891 2000-06-19
34
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: ZymoGenetics, Inc.
1201 Eastlake Avenue East
Seattle, Washington 98102
United States of America
Pharmaceutical Proteins Ltd.
Roslin
Edinburgh
Midlothian, Scotland EH25 9PP
(ii) TITLE OF INVENTION: Production of Fibrinogen in Transgenic
Animals
(iii) NUMBER OF SEQUENCES: 27
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: ZymoGenetics, Inc.
(B) STREET: 1201 Eastlake Avenue East
(C) CITY: Seattle
(D) STATE: WA
(E) COUNTRY: USA
(F) ZIP: 98102
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release X1.0, Version X1.25
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION;
(A) NAME: Parker, Gary E
(B) REGISTRATION NUMBER: 31-648
(C) REFERENCE/OOCKET NUMBER: 93-15PC
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 206-442-6673
(B) TELEFAX: 206-442-6678
CA 02309891 2000-06-19
(2j INFORMATION FOR SEQ ID N0:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 5943 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomicj
(viij IMMEDIATE SOURCE:
(B) CLONE: Human Fibrinogen A-alpha chain
(ixj FEATURE:
(A) NAME/KEY: COS
(B) LOCATION: ~oin(31..84, 1154..1279, 1739..1922, 3055..3200,
3786..5210)
(xij SEQUENCE DESCRIPTION: SEQ ID N0:1:
GTCTAGGAGC CAGCCCCACC CTTAGAAAAG AT6 TTT TCC ATG AGG ATC GTC TGC 54
Met Phe Ser Met Arg Ile Val Cys
1 5
CTA GTT CTA AGT GTG GTG GGC ACA GCA TGG GTATGGCCCT TTTCATTTTT 104
Leu Val Leu Ser Val Val Gly Thr Ala Trp
10 15
TCTTCTTGCT TTCTCTCTGG TGTTTATTCC ACAAAGAGCC TGGAGGTCAG AGTCTACCTG 164
CTCTATGTCC TGACACACTC TTAGCTTTAT GACCCCAGGC CTGGGAGGAA ATTTCCTGGG 224
TGGGCTTGAC ACCTCAAGAA TACAGGGTAA TATGACACCA AGAGGAAGAT CTTAGATGGA 284
TGAGAGTGTA CAACTACAAG GGAAACTTTA GCATCTGTCA TTCAGTCTTA CCACATTTTG 344
TTTTGTTTTG TTTTAAAAAG GGCAAGAATT ATTTGCCATC CTTGTACCTA TAAAGCCTTG 404
GTGCATTATA ATGCTAGTTA ATGGAATAAA ACATTTTATG GTAAGATTTG TTTTCTTTAG 464
TTATTAATTT CTTGCTACTT GTCCATAATA AGCAGAACTT TTAGTGTTAG TACAGTTTTG 524
CTGAAAGGTT ATTGTTGTGT TTGTCAAGAC AGAAGAAAAA GCAAACGAAT TATCTTTGGA 584
AATATCTTTG CAGTATCAGA AGAGATTAGT TAGTAAGGCA ATACGCTTTT CCGCAGTAAT 644
CA 02309891 2000-06-19
36
GGTATTCTTT TAAATTATGA ATCCATCTCT AAAGGTTACA TAGAAACTTG AAGGAGAGAG 704
GAACATTCAG TTAAGATAGT CTAGGTTTTT CTACTGAAGC AGCAATTACA GGAGAAAGAG 764
CTCTACAGTA GTTTTCAACT TTCTGTCTGC AGTCATTAGT AAAAATGAAA AGGTAAAATT 824
TAACTGATTT TATAGATTCA AATAATTTTC CTTTTAGGAT GGATTCTTTA AAACTCCTAA 884
TATTTATCAA ATGCTTATTT AAGTGTCACA CACAGTTAAG AAATTTGTAC ACCTTGTCTC 944
CTTTAATTCT CATAACAACT CCATAAAATG GGTCCTAGGA TTTCCATTTG AAGATAAGAA 1004
ACCTGAAGCT TGCCGAAGCC CTGTGTCTGC TCTCCTTAAT CTCTGTGAGA GTGCCATCTC 1064
TTCCTGGGGA CTTGTAGGCA TGCCACTGTC TCCTCTTCTG GCTAACATTG CTGTTGCTCT 1124
CTTTTGTGTA TGTGAATGAA TCTTTAAAG ACT GCA GAT AGT GGT GAA GGT GAC 1177
Thr Ala Asp Ser Gly Glu Gly Asp
20 25
TTT CTA GCT GAA GGA GGA GGC GTG CGT GGC CCA AGG GTT GTG GAA AGA 1225
Phe Leu Ala Glu Gly Gly Gly Yal Arg Gly Pro Arg Val Val Glu Arg
30 35 40
CAT CAA TCT GCC TGC AAA GAT TCA GAC TGG CCC TTC TGC TCT GAT GAA 1273
His Gln Ser Ala Cys Lys Asp Ser Asp Trp Pro Phe Cys Ser Asp Glu
45 50 55
GAC TGG GTAAGCAGTC AGCGGGGGAA GCAGGAGATT CCTTCCCTCT GATGCTAGAG 1329
Asp Trp
GGGCTCACAG GCTGACCTGA TTGGTCCCAG AAACTTTTTT AAATAGAAAA TAATTGAATA 1389
GTTACCTACA TAGCAAATAA AGAAAAGGAA CCTACTCCCA AGAGCACTGT TTATTTACCT 1449
CCCCAACTCT GGATCATTAG TGGGTGAACA GACAGGATTT CAGTTGCATG CTCAGGCAAA 1509
ACCAGGCTCC TGAGTATTGT GGCCTCAATT TCCTGGCACC TATTTATGGC TAAGTGGACC 1569
CTCATTCCAG AGTTTCTCTG CGACCTCTAA CTAGTCCTCT TACCTACTTT TAAGCCAACT 1629
TATCTGGAAG AGAAAGGGTA GGAAGAAATG GGGGCTGCAT GGAAACATGC AAAATTATTC 1689
TGAATCTGAG AGATAGATCC TTACTGTAAT TTTCTCCCTT CACTTTCAG AAC TAC 1744
Asn Tyr
CA 02309891 2000-06-19
37
AAATGC CCTTCT GGCTGCAGG AT6AAA GGGTTGATT GATGAA GTCAAT 1792
LysCys ProSer GlyCysArg MetLys GlyLeuIle AspGlu YalAsn
65 . 70 75
CAAGAT TTTACA AACAGAATA AATAAG CTCAAAAAT TCACTA TTTGAA 1840
GlnAsp PheThr AsnArgIle AsnLys LeuLysAsn SerLeu PheGlu
80 85 90
TATCAG AAGAAC AATAAGGAT TCTCAT TCGTTGACC ACTAAT ATAATG 1888
TyrGln LysAsn AsnLysA_spSerHis SerLeuThr ThrAsn IleMet
95 100 105 110
GAAATT TTGAGA GGCGATTTT TCCTCA GCCAATA 1932
GTAAGTATTA
GluI1~ LeuArg GlyAspPhe SerSer AlaAsn
115 120
CATATTTACTTCTTTGACTTTATAACAGAAACAACAAAAATCCTAAATAAATATGATATC 1992
CGCTTATATCTATGACAATTTCATCCCAAAGTACTTAGTGTAGAAACACATACCTTCATA 2052
ATATCCCTGAAAATTTTAAGAGGGAGCTTTTGTTTTCGTTATTTTTTCAAAGTAAAAGAT 2112
GTTAACTGAGATTGTTTAAGGTCACAAAATAAGTCAGAATTTTGGATTAAAACAAGAATT 2172
TAAATGTGTTCTTTTCAACAGTATATACTGAAAGTAGGATGGGTCAGACTCTTTGAGTTG 2232
ATATTTTTGTTTCTGCTTTGTAAAGGTGAAAACTGAGAGGTCAAGGAACTTGTTCAAAGA 2292
CACAGAGCTGGGAATTCAACTCCCAGACTCCACTGAGCTGATTAGGTAGATTTTTAAATT 2352
TAAAATATAGGGTCAAGCTACGTCATTCTCACAGTCTACTCATTAGGGTTAGGAAACATT 2412
GCATTCACTCTGGGCATGGACAGCGAGTCTAGGGAGTCCTCAGTTTCTCAAGTTTTGCTT 2472
TGCCTTTTTACACCTTCACAAACACTTGACATTTAAAATCAGTGATGCCAACACTAGCTG 2532
GCAAGTGAGTGATCCTGTTGACCCAAAACAGCTTAGGAACCATTTCAAATCTATAGAGTT 2592
AAAAAGAAAAGCTCATCAGTAAGAAAATCCAATATGTTCAAGTCCCTTGATTAAGGATGT 2652
TATAAAATAATTGAAATGCAATCAAACCAACTATTTTAACTCCAAATTACACCTTTAAAA 2712
TTCCAAAGAAAGTTCTTCTTCTATATTTCTTTGGGATTACTAATTGCTATTAGGACATCT 2772
TAACTGGCATTCATGGAAGGCTGCAGGGCATAACATTATCCAAAAGTCAAATGCCCCATA 2832
CA 02309891 2000-06-19
38
GGTTTTGAACTCACAGATTAAACTGTAACCAAAATAAAATTAGGCATATTTACAAGCTAG2892
TTTCTTTCTTTCTTTTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTT2952
CTTTCTTTCT TTCTCCTTCC TTCCTTTCTT CCTTTCTTTT TTGCTGGCAA TTACAGACAA 3012
ATCACTCAGC AG AC 3065
AGCTACTTCA CGT
ATAACCATAT GAT
TTTCGATTTC AAT
Asn
Arg
Asp
Asn
125
ACCTACAACCGA GTGTCA GAGGATCTG AGAAGCAGA ATTGAA GTC CTG 3113
ThrTyrAsnArg YalSer GluAspLeu ArgSerArg IleGlu Yal Leu
130 135 140
AAGCGCAAAGTC ATAGAA AAAGTACAG CATATCCAG CTTCTG CAG AAA 3161
LysArgLysVal IleGlu LysValGln HisIleGln LeuLeu Gln Lys
145 150 155
AATGTTAGAGCT CAGTTG GTTGATATG AAACGACTG GAGGTAAGTATGT 3210
AsnYalArgAla GlnLeu YalAspMet LysArgLeu Glu
160 165 170
GGCTGTGGTCCCGAGTGTCCTTGTTTTTGAGTAGAGGGAA ATAGTTATGC3270
AAGGAAGGCG
ACTGAGTGTCTACTATATGCAGAGAAAAGTGTTATATCCaTCATCTACCTAAAAGTAGGT3330
ATTATTTTCCTCACTCCACAGTTGAAGAAAAAAAAATTCAGAGATATTAAGTAAATTTTC3390
CAACGTACATAGATAGTAATTCAAAGCAATGTTCAGTCCCTGTCTATTCCAAGCCATTAC3450
ATCACCACACCTCTGAGCCCTCAGCCTGAGTTCACCAAGGATCATTTAATTAGCGTTTCC3510
TTTGAGAGGGAATAGCACCTTACTCTTGATCCATTCTGAGGCTAAGATGAATTAAACAGC3570
ATCCATTGCTTATCCTGGCTAGCCCTGCAATACCCAACATCTCTTCCACTGAGGGTGCTC3630
GATAGGCAGAAAACAGAGAATATTAAGTGGTAGGTCTCCGAGTCAAAAAAAATGAAACCA3690
GTTTCCAGAAGGAAAATTAACTACCAGGAACTCAATAGACGTAGTTTATGTATTTGTATC3750
TACATTTTCTCTTTATTTTTCTCCCCTCTCTCTAG ATT AAG 3803
GTG GAC
ATT GAT
Val Asp Ile Lys
Ile Asp
175
CA 02309891 2000-06-19
39
ATC CGA TCT TGT CGA GGG TCA TGC AGT AGG GCT TTA GCT CGT GAA GTA 3851
Ile Arg Ser Cys Arg Gly Ser Cys Ser Arg Ala Leu Ala Arg Glu Val
180 185 190
GAT CTG AAG GAC TAT GAA~GAT CAG CAG AAG CAA CTT GAA CAG GTC ATT 3899
Asp Leu Lys Asp Tyr Glu Asp Gln Gln Lys Gln Leu Glu Gln Val Ile
195 200 205
GCC AAA GAC TTA CTT CCC TCT AGA GAT AGG CAA CAC TTA CCA CTG ATA 3947
Ala Lys Asp Leu Leu Pro Ser Arg Asp Arg Gln His Leu Pro Leu Ile
210 215 22tr
AAA ATG AAA CCA GTT CCA GAC TTG GTT CCC GGA AAT TTT AAG AGC CAG 3995
Lys Met Lys,Pro Yal Pro Asp Leu Yal Pro Gly Asn Phe Lys Ser Gln
225 230 235 240
CTT CAG AAG GTA CCC CCA GAG TGG AAG GCA TTA ACA GAC ATG CCG CAG 4043
Leu Gln Lys Yal Pro Pro Glu Trp Lys Ala Leu Thr Asp Met Pro Gln
245 250 255
ATG AGA ATG GAG TTA GAG AGA CCT GGT GGA AAT GAG ATT ACT CGA GGA 4091
Met Arg Met Glu Leu Glu Arg Pro Gly Gly Asn Glu Ile Thr Arg Gly
260 265 270
GGC TCC ACC TCT TAT GGA ACC GGA TCA GAG ACG GAA AGC CCC AGG AAC 4139
Gly Ser Thr Ser Tyr G1y Thr Gly Ser Glu Thr Glu Ser Pro Arg Asn
275 280 285
CCT AGC AGT GCT GGA AGC TGG AAC TCT GGG AGC TCT GGA CCT GGA AGT 4187
Pro Ser Ser Ala Gly Ser Trp Asn Ser Gly Ser Ser Gly Pro Gly Ser
290 295 300
ACT GGA AAC CGA AAC CCT GGG AGC TCT GGG ACT GGA GGG ACT GCA ACC 4235
Thr Gly Asn Arg Asn Pro Gly Ser Ser Gly Thr Gly Gly Thr Ala Thr
305 310 315 320
TGG AAA CCT GGG AGC TCT GGA CCT GGA AGT GCT GGA AGC TG~ AAC TCT 4283
Trp Lys Pro Gly Ser Ser Gly Pro Gly Ser Ala Gly Ser Trp Asn Ser
325 330 335
GGG AGC TCT GGA ACT GGA AGT ACT GGA AAC CAA AAC CCT GGG AGC CCT 4331
Gly Ser Ser Gly Thr Gly Ser Thr Gly Asn Gln Asn Pro Gly Ser Pro
340 345 350
AGA CCT GGT AGT ACC GGA ACC TGG AAT CCT GGC AGC TCT GAA CGC GGA 4379
Arg Pro Gly Ser Thr Gly Thr Trp Asn Pro Gly Ser Ser Glu Arg Gly
355 360 365
CA 02309891 2000-06-19
AGT GCT GGG CAC TGG ACC TCT GAG AGC TCT GTA TCT GGT AGT ACT GGA 4427
Ser Ala Gly His Trp Thr Ser Glu Ser Ser Val Ser Gly Ser Thr Gly
370 375 ?80
CAATGGCAC TCTGAATCT GGAAGTTTT AGGCCA GATAGCCCA GGCTCT 4475
GlnTrpHis SerGluSer GlySerPhe ArgPro AspSerPro GlySer
385 390 395 400
GGGAACGvG AGGCCTAAC AAGCCAGAC TGGGGC ACATTTGAA GAGGTG 4523
GlyAsnAla ArgProAsn AsnProAsp TrpGly ThrPheGlu GluVal
405 410 415
TCAGGAAAT,GTAAGTCCA GGGACAAGG AGAGAG TACCACACA GAAAAA 4571
SerGlyAsn YalSerPro GlyThrArg ArgGlu TyrHisThr GluLys
420 425 430
CTGGTCACT TCTAAAGGA GATAAAGAG CTCAGG ACTGGTAAA GAGAAG 4619
LeuValThr SerLysGly AspLysGlu LeuArg ThrGlyLys GluLys
435 440 445
GTC ACC TCT GGT AGC ACA ACC ACC ACG CGT CGT TCA TGC TCT AAA ACC 4667
Val Thr Ser Gly Ser Thr Thr Thr Thr Arg Arg Ser Cys Ser Lys Thr
450 455 460
GTT ACT AAG ACT GTT ATT GGT CCT GAT GGT CAC AAA GAA GTT ACC AAA 4715
Yal Thr Lys Thr Yal Ile G1y Pro Asp Gly His Lys Glu Yal Thr Lys
465 470 475 480
GAA GTG GTG ACC TCC GAA GAT GGT TCT GAC TGT CCC GAG GCA ATG GAT 4763
Glu Yal Val Thr Ser Glu Asp Gly Ser Asp Cys Pro Glu Ala Met Asp
485 490 495
TTA GGC ACA TTG TCT GGC ATA GGT ACT CTG GAT GGG TTC CGC CAT AGG 4811
Leu Gly Thr Leu Ser Gly Ile Gly Thr Leu Asp Gly Phe Arg His Arg
500 505 510
CAC CCT GAT GAA GCT GCC TTC TTC GAC ACT GCC TCA ACT GGA AAA ACA 4859
His Pro Asp Glu Ala Ala Phe Phe Asp Thr Ala Ser Thr Gly Lys Thr
515 520 525
TTC CCA GGT TTC TTC TCA CCT ATG TTA GGA GAG TTT GTC AGT GAG ACT 4907
Phe Pro Gly Phe Phe Ser Pro Met Leu Gly Glu Phe Val Ser Glu Thr
530 535 540
CA 02309891 2000-06-19
41
GAG TCT AGG GGC TCA GAA TCT GGC ATC TTC ACA AAT ACA AAG GAA TCC 4955
Glu Ser Arg Gly Ser Glu Ser Gly Ile Phe Thr Asn Thr Lys Glu Ser
545 550 555 560
AGT TCT CAT CAC CCT GGG,ATA GCT GAA TTC CCT TCC CGT GGT AAA TCT 5003
Ser Ser His His Pro Gly Ile Ala Glu Phe Pro Ser Arg Gly Lys Ser
565 570 575
TCA AGT TAC AGC AAA CAA TTT ACT AGT AGC ACG AGT TAC AAC AGA GGA 5051
Ser Ser Tyr Ser Lys Gln Phe Thr Ser Ser Thr Ser Tyr Asn Arg G1y
580 585 590
GAC TCC ACA TTT GAA AGC AAG AGC TAT AAA ATG GCA GAT GAG GCC GGA 5099
Asp Ser Thr Phe Glu Ser Lys Ser Tyr Lys Met Ala Asp Glu Ala Gly
595 600 605
AGT GAA GCC GAT CAT GAA GGA ACA CAT AGC ACC AAG AGA GGC CAT GCT 5147
Ser Glu Ala Asp His Glu Gly Thr His Ser Thr Lys Arg Gly His Ala
610 615 620
AAA TCT CGC CCT GTC AGA GGT ATC CAC ACT TCT CCT TTG GGG AAG CCT 5195
lys Ser Arg Pro Val Arg Gly Ile His Thr Ser Pro leu Gly Lys Pro
625 630 635 640
TCC CTG TCC CCC TAGACTAAGT TAAATATTTC TGCACAGTGT TCCCATGGCC 5247
Ser Leu Ser Pro
645
CCTTGCATTT CCTTCTTAAC TCTCTGTTAC ACGTCATT6A AACTACACTT TTTTGGTCTG 5307
TTTTTGTGCT AGACTGTAAG TTCCTTGGGG GCAGGGCCTT TGTCTGTCTC ATCTCTGTAT 5367
TCCCAAATGC CTAACAGTAC AGAGCCATGA CTCAATAAAT ACATGTTAAA TGGATGAATG 5427
AATTCCTCTG AAACTCTATT TGAGCTTATT TAGTCAAATT CTTTCACTAT TCAAAGTGTG 5487
TGCTATTAGA ATTGTCACCC AACTGATTAA TCACATTTTT AGTATGTGTC TCAGTTGACA 5547
TTTAGGTCAG GCTAAATACA AGTTGTGTTA GTATTAAGTG AGCTTAGCTA CCTGTACTGG 5607
TTACTTGCTA TTAGTTTGTG CAAGTAAAAT TCCAAATACA TTTGAGGAAA ATCCCCTTTG 5667
CAATTTGTAG GTATAAATAA CCGCTTATTT GCATAAGTTC TATCCCACTG TAAGTGCATC X727
CTTTCCCTAT GGAGGGAAGG AAAGGAGGAA GAAAGAAAGG AAGGGAAAGA AACAGTATTT 5787
GCCTTATTTA ATCTGAGCCG TGCCTATCTT TGTAAAGTTA AATGAGAATA ACTTCTTCCA 5847
CA 02309891 2000-06-19
42
ACCAGCTTAA TTTTTTTTTT AGACTGTGAT GATGTCCTCC AAACACATCC TTCAGGTACC 5907
CAAAGTGGCA TTTTCAATAT CAAGCTATCC GGATCC 5943
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 644 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Met Phe Ser Met Arg Ile Val Cys Leu Val Leu Ser Val Yal Gly Thr
1 5 10 15
Ala Trp Thr Ala Asp Ser Gly Glu Gly Asp Phe Leu Ala Glu Gly Gly
20 25 30
Gly Val Arg Gly Pro Arg Val Yal Glu Arg His Gln Ser Ala Cys Lys
35 40 45
Asp Ser Asp Trp Pro Phe Cys Ser Asp Glu Asp Trp Asn Tyr Lys Cys
50 55 60
Pro Ser Gly Cys Arg Met Lys Gly Leu Ile Asp Glu Val Asn Gln Asp
65 70 75 80
Phe Thr Asn Arg Ile Asn Lys Leu Lys Asn Ser Leu Phe Glu Tyr Gln
85 90 95
Lys Asn Asn Lys Asp Ser His Ser Leu Thr Thr Asn Ile Met Glu Ile
100 105 110
Leu Arg Gly Asp Phe Ser Ser Ala Asn Asn Arg Asp Asn Thr Tyr Asn
115 120 125
Arg Yal Ser Glu Asp Leu Arg Ser Arg Ile Glu Val Leu Lys Arg Lys
130 135 140
Yal Ile Glu Lys Val Gln His Ile Gln Leu Leu Gln Lys Asn Yal Arg
145 150 155 160
CA 02309891 2000-06-19
43
Ald Gln Leu Yal Asp Met Lys Arg Leu Glu Val Asp lle Asp Ile Lys
165 170 175
Ile Arg Ser Cys Arg Gly Ser Cys Ser Arg Ala Leu Ala Arg Glu Val
180 185 190
Asp Leu Lys Asp Tyr Glu Asp Gln Gln Lys Gln Leu Glu Gln Yal Ile
195 200 205
Ala Lys Asp Leu Leu Pro Ser Arg Asp Arg Gln His Leu Pro Leu Ile
210 215 220
Lys Met Lys Pro Yal Pro Asp Leu Val Pro Gly Asn Phe Lys Ser Gln
225 230 235 240
Leu Gln Lys Val Pro Pro Glu Trp Lys Ala Leu Thr Asp Met Pro Gtn
245 250 255
Met Arg Met Glu Leu Glu Arg Pro Gly Gly Asn Glu Ile Thr Arg Gly
260 265 270
Gly Ser Thr Ser Tyr Gly 'fhr Gly Ser Glu Thr Glu Ser Pro Arg Asn
275 280 285
Pro Ser Ser Ala Gly Ser Trp Asn Ser Gly Ser Ser Gly Pro Gly Ser
290 295 300
Thr Gly Asn Arg Asn Pro Gly Ser Ser Gly Thr Gly Gly Thr Ala Thr
305 310 315 320
Trp Lys Pro Gly Ser Ser Gly Pro Gly Ser Ala Gly Ser Trp Asn Ser
325 330 335
Gly Ser Ser Gly Thr Gly Ser Thr Gly Asn Gln Asn Pro Gly Ser Pro
340 345 350
Arg Pro Gly Ser Thr Gly Thr Trp Asn Pro Gly Ser Ser Glu Arg Gly
355 360 365
Ser Ala Gly His Trp Thr Ser Glu Ser Ser Val Ser Gly Ser Thr Gly
370 375 380
Gln Trp His Ser Glu Ser Gly Ser Phe Arg Pro Asp Ser Pro Gly Ser
385 390 395 400
Gly Asn Ala Arg Pro Asn Asn Pro Asp Trp Gly Thr Phe Glu Glu Val
405 410 415
CA 02309891 2000-06-19
44
Ser
Gly
Asn
Val
Ser
Pro
Giy
Thr
Arg
Arg
Glu
Tyr
His
Thr
Glu
Lys
420
425
430
Leu
Val
Thr
Ser
Lys
Gly
Asp
Lys
Glu
Leu
Arg
Thr
Gly
Lys
Glu
Lys
435
440
445
YalThr Ser Ser Thr Thr Thr Thr Arg Arg Ser
Gly Cys Ser Lys Thr
450 455 460
YalThr Lys Val Ile Gly Pro Asp Gly His Lys Thr
465Thr Glu Yal Lys
470 475
480
GluVal Val Ser Glu Asp Gly Ser Asp Cys Pro Met
Thr Glu Ala Asp
485 490 495
LeuGly Thr Ser Gly Ile Gly Thr Leu Asp Gly His
Leu Phe Arg Arg
500 505 510
HisPro Asp Ala Ala Phe Phe Asp Thr Ala Ser Lys
Glu Thr Gly Thr
515 520 525
PhePro Gly Phe Ser Pro Met Leu Gly Glu Phe Glu
Phe Val Ser Thr
530 535 540
GluSer Arg Ser Glu Ser Gly Ire Phe Thr Asn Glu
545G1y Thr Lys Ser
550 . 555
560
SerSer His Pro Gly Ile Ala Glu Phe Pro Ser Lys
His Arg Gly Ser
565 570 575
SerSer Tyr Lys Gln Phe Thr Ser Ser Thr Ser Arg
Ser Tyr Asn Gly
580 585 590
AspSer Thr Glu Ser Lys Ser Tyr Lys Met Ala Ala
Phe Asp Glu Gly
595 600 605
SerGlu Ala His
Asp His Ala
Glu Gly
Thr His
Ser Thr
Lys Arg
Gly
610 615
620
Lys Lys
Ser Pro
Arg
Pro 640
Val
Arg
Gly
Ile
His
Thr
Ser
Pro
Leu
Gly
625
630
635
Ser
Leu
Ser
Pro
CA 02309891 2000-06-19
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8878 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(iij MOLECULE TYPE: DNA (genomic)
(vii) IMMEDIATE SOURCE:
(Bj CLONE: human fibrinogen B-beta chain
('ix) FEATURE:
(A) NAME/KEY: misc_RNA
(B) LOCATION: 1..469
(ix) FEATURE:
(A) NAME/KEY: exon
(B) LOCATION: 470..583
(ixj FEATURE:
(Aj NAME/KEY: intron
(B) LOCATION: 584..3257
(ixj FEATURE:
(A) NAME/KEY: exon
(B) LOCATION: 3258..3449
(ix) FEATURE:
(Aj NAME/KEY: intron
(Bj LOCATION: 3450..3938
(ixj FEATURE:
(Aj NAME/KEY: exon
(B) LOCATION: 3939..4122
(ixj FEATURE:
(Aj NAME/KEY: intron
(B) LOCATION: 4123..5042
(ix) FEATURE:
(Aj NAME/KEY: exon
(B) LOCATION: 5043..5270
CA 02309891 2000-06-19
(ix) FEATURE:
(A) NAME/KEY: intros
(B) LOCATION: 5271..5830
(ix) FEATURE: '
(A) NAME/KEY: exon
(B) LOCATION: 5831..5944
(ix) FEATURE:
(A) NAME/KEY: intros
(B) LOCATION: 5945..6632
(ix) FEATURE:
(A) NAME/KEY: exon
(B) LOCATION: 6633..6758
(ix) FEATURE:
(A) NAME/KEY: intros
(B) LOCATION: 6759..6966
(ix) FEATURE:
(A) NAME/KEY: exon
(B) LOCATION: 6967..7252
(ix) FEATURE:
(A) NAME/KEY: intros
(B) LOCATION: 7253..7870
(ix) FEATURE:
(A) NAME/KEY: axon
(B) LOCATION: 7871..8102
(ix) FEATURE:
(A) NAME/KEY: 3'UTR
(8) LOCATION: 8103..8537
(ix) FEATURE:
(A) NAME/KEY: misc_RNA
(B) LOCATION: 8538..8878
46
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LOCATION: ~oin(470..583, 3258..3449, 3939..4122, 5043..5270,
5831..5944, 6633..6758, 6967..7252, 7871..8102)
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
CA 02309891 2000-06-19
47
GAATTCATGC CCCTTTTGAA ATAGACTTAT GTCATTGTCA GAAAACATAA GCATTTATGG 60
TATATCATTA ATGAGTCACG ATTTTAGTGG TTGCCTTGTG AGTAGGTCAA ATTTACTAAG 120
CTTAGATTTG TTTTCTCACA TATTCTTTCG GAGCTTGTGT AGTTTCCACA TTAATTTACC 180
AGAAACAAGA TACACACTCT CTTTGAGGAG TGCCCTAACT TCCCATCATT TTGTCCAATT 240
AAATGAATTG AAGAAATTTA ATGTTTCTAA ACTAGACCAA CAAAGAATAA TAGTTGTATG 300
ACAAGTAAAT AAGCTTTGCT GGGAAGATGT TGCTTAAATG ATAAAATGGT TCAGCCAACA 360
AGTGAACCAA AAATTAAATA TTAACTAAGG AAAGGTAACC ATTTCTGAAG TCATTCCTAG 420
CAGAGGACTC AGATATATAT AGGATTGAAG ATCTCTCAGT TAAGTCTAC ATG AAA 475
Met Lys
1
AGG ATG GTT TCT TGG AGC TTC CAC AAA CTT AAA ACC ATG AAA CAT CTA 523
Arg Met Yal Ser Trp Ser Phe His Lys Leu Lys Thr Met Lys His Leu
10 15
TTA TTG CTA CTA TTG TGT GTT TTT CTA GTT AAG TCC CAA GGT GTC AAC 571
Leu Leu Leu Leu Leu Cys Yal Phe Leu Val Lys Ser Gln Gly Val Asn
20 25 30
GAC AAT GAG GAG GTGAATTTTT TAAAGCATTA TTATATTATT AGTAGTATTA 623
Asp Asn Glu Glu
TTAATATAAG ATGTAACATA ATCATATTAT GTGCTTATTT TAATGAAATT AGCATTGCTT 683
ATAGTTATGA AATGGAATTG TTAACCTCTG ACTTATTGTA TTTAAAGAAT GTTTCATAGT 743
ATTTCTTATA TAAAAHCAAA GTAATTTCTT GTTTTCTAGT TTATCACCTT TGTTTTCTTA 803
AGATGAGGAT GGCTTAGCTA ATGTAAGATG TGTTTTTCTC ACTTGCTATT CTGAGTACTG 863
TGATTTTCAT TTACTTCTAG CAATACAGGA TTACAATTAA GAGGACAAGA TCTGAAAATC 923
TCACAAACTA TAAAATAATA AAAGAGCAGA ATTTTAAGAT AAAAGAAACT GGTGGTAGGT 983
AGATTGTTCT TTGGTGAAGG AAGGTAATAT ATATTGTTAC TGAGATTACT ATTTATAAAA 1043
ATTATAACTA AGCCTAAAAG CAAAATACAT CAAGTGTAAT GATAGAAAAT GAAATATTGC 1103
CA 02309891 2000-06-19
48
TTTTTTCAGA TGAAAAGTTC AAATTAGAGT TAGTGTGTAT TGTTATTATT AATAGTTATG 1163
AAACACGGTT CAGTCTAATT TATTTATTTG TAGAACAGTT TGTCCTCAAC TATTATTTTT 1223
GCTGACTTAT TGCTGTTAAT TTGCAGTTAC TAAAAATACA GAAATGCATT TAGGACAATG 1283
GATATTTAAG AAATTTAAAT TTTATCATCA AACGTATCAT GGCCAAATTT CTTACATATA 1343
GCATAGTATC ATTAAACTAG AAATAAGAAT ACACAATAAT ATTTAAATGA AGTGATTCAT 1403
TTCGGATCAT TAT~GAGTTT CAAGGGAACT TGAGTGTTGT ACTTATCAGA CTCTACATGT 1463
AAGAACATAT AGTTAATCTG GTTGTGTGTG TAAAAACATA TGGTTAATCT GGTTAAGTCT 1523
GGTTAATCAT ATTAGGTAAG AAAAATGTAA AGAATGTGTA AGACGAAATT TTTGTAAAGT 1583
ACTCTGCAAA GCACTTTCAC ATTTCTGCTT ATCAACTAAA CCTCACAGAG ATAGTTTAAT 1643
AGTTTAGGCT TTAAAATGGA TTTTGATTAT TCAACAAGTG GCCTTCATAA TTTCTTTAAG 1703
TGTTTTTCTT TAAGTATATA CTTTCTTTAA ATATTTTTTA AAATTTCCTT TTCTCTAGTA 1763
AAGCCAGACC ATCCATGCTA CCTCTCTAGT GGCACTCTGA AATAAAAAGA AAATAGTTTT 1823
CTCTGTTATA ATTGTATTTG TAATAAGCAG ATGAATCACA TTTCTTAAAA TTTGTTTTAG 1883
AGAGGGTAAG CTCTGACTAG GACCATGACT TCAATGTGAA ATATGTATAT ATCCTCCGAA 1943
TCTTTACATA TTAAGAATGT ATATAGTCAA CTGGTTAAAC AGGAAAATCT GGAACAGCCT 2003
GGCTGGGTTT TAATCTTAGC ACCATCCTAC TAAATGTTAA ATAATATTAT AATCTAATGA 2063
ATAAATGACA ATGCAATTCC AAATAGAGTT CATCTGATGA CTTCTAGACT CACAAAATTG 2123
CAAGAGAGCT CAGTTGTTGC TCAGTTGTTC CAAATCATGT CGTTTGTTAA TTTGTAATTA 2183
AGCTCCAAAG GATGTATAGC TACTGACAAA AAAAAAAATG AGAATGTAGT TAATCCAAAT 2243
CAAAACTTTC CTATTGCAAT GCGTATTTTC TGCTTCATTA TCCTTTAATA TAATATTTTA 2303
AGTTAGCAAG TAATTTTAAT TACAATGCAC AAGCCTTGAG AATTATTTTA AATATAAGAA 2363
AATCATAATG TTTGATAAAG AAATCATGTA AGAAATTTCA AGATAATGGT TTAACAAATA 2423
ATTTTGTTGA TAGAAGATAA GACTAAAAGT GAAATTCGAA GTGGAGAGGA CACTTAAACT 2483
GTAGTACTTG TTATGTGTGA TTCCAGTAAA AATAGTAATG AGCACTTATT ATTGCCAAGT 2543
CA 02309891 2000-06-19
49
ACTGTTCTGA GGGTACCATA TGCAATAAGT TATTTAATCC TTACAATAAT CTTGTAAGGC 2603
AGATTCAAAC TATCATTACA CTTATTTTAC AGATGAGAAA ACTGGGGCAC AGATAAAGCA 2663
AC?TGCCCAA GGTCTCATAG CTGTAAGTCA ACCCTACGGT CAAGACCTAC AAGTAGCCGA c723
GCTCCAGAGT ACATTATGAG GGTCAAAGAT TGTCTTATTA CAAATAAATT CCAAGTAGAA 2783
TCAACCTTTA ATAAGTCTTT AATGTCTCTT AAATATGTTT ATATAGGAGT CTAATCACCA 2843
ATTCACAAAA ATGAAAGTAG GGAAATGATT AACAATAATC ATAGGAATCT AACAATCCAA 2903
GTGGCTTGAG AATATTCATT CTTCTTGACA GTATAGATTC TTTACAATTT CGTAAGTTCC 2963
AATGTATGTT TTAGGAATAT GAGGTCATTA CTATTCATAA TCTGATACAG CTTTATCCTA 3023
AGGCCTCTCT TTAAAAACTA CACTGCATCA TAGCTTTTTT GTGCAGTTGG TCTTTCTACT 3083
GTTACTGAAC AGTAAGCAAC CTACAGATTC ACTATCACCA ACCAGCCAGT T~aATGGATCT 3143
TAAGCAAATT ATC4AGCTTG TGATAACCTA AATTATAAAA TGAGGGTGTT GGAATAGTTA 3203
CATTCCAAAT CTTCTATAAC ACTCTGTATT ATATTTCTGC CTCATTCCTT GTAG GGT 3260
Gly
TTC TTC AGT GCC CGT GGT CAT CGA CCC CTT GAC AAG AAG AGA GAA GAG 3308
Phe Phe Ser Ala Arg Gly His Arg Pro Leu Asp Lys Lys Arg Glu G1u
40 45 50 55
GCT CCC AGC CTG AGG CCT GCC CCA CCG CCC ATC AGT GGA GGT GGC TAT 3356
Ala Pro Ser Leu Arg Pro Ala Pro Pro Pro Ile Ser Gly Gly Gly Tyr
60 65 70
CGG GCT CGT CCA GCC AAA GCA GCT GCC ACT CAA AAG AAA GTA GAA AGA 3404
Arg Ala Arg Pro Ala Lys Ala Ala Ala Thr Gln Lys Lys Ilal Glu Arg
75 80 85
AAA GCC CCT GAT GCT GGA GGC TGT CTT CAC GCT GAC CCA GAC CTG 3449
Lys Ala Pro Asp Ala Gly Gly Cys Leu His Ala Asp Pra Asp Leu
90 95 100
GTGGGTGCAC TGATGTTTCT TGCAGTGGTG GCTCTCTCAT GCAGAGAAAG CCTGTAGTCA 3509
TGGCAGTCTG CTAATGTTTC ACTGACCCAC ATTACCATCA CTGTTATTTT GTTTGTTTAT 3569
CA 02309891 2000-06-19
50
TTTGGAAATA AAATTCAAAACATAAACATA TTGGGCCTTTGGTTTAGGCT TTCTTTCTTG3629
TTTTCTTTGG TCTGGGCCCAAAATTTCAAA TTAGGATATGTGGGTGCCAC CTTTCCATTT3689
GTATTTTGCC ACTGCCTTTGTTTAGTTGGT AAAATTTTCATAGCCCAATT ATATTTTTTC3749
TGGGGTAAGT AATATTTTAAATCTCTATGA GAGTATGATGATGACTTTCG AATTTCTGGT3809
CTTACAGAAA ACCAAATAATAAATTTTTAT GTTGGCTAATCGTATCGCTG AATTTTCCTA3869
TGTGCTATTT TAACAAATGTCCATGACCCA AATCCTTCATCTAATGCCTG CTATTTTCTT3929
TGTTTTTAG GGG GTG TGT CCT ACA GGA 3977
TTG TGT CAG TTG CAA
GAG GCT
Gly Val Leu Cys Pro Thr Gly
Cys Gln Leu Gln
Glu Ala
105 110 115
TTG CTA CAA CAG GTT GAT GAG TTA AAT 4025
GAA AGG CCA ATC
AGA AAT AGT
Leu Leu Gln Gln Val Asp Glu Leu Asn
Glu Arg Pro Ile
Arg Asn Ser
120 125 130
AAC AAT GTG GAA TCT TCC TTT CAG TAC 4073
GCT GTT TCC CAG
ACC TCC TCT
Asn Asn Val Glu Ser Ser Phe Gln Tyr
Ala Val Ser Gln
Thr Ser Ser
135 140 145
ATG TAT TTG CTG CAG AAG CAA GTA AAA 4122
AAA GAC CTG TGG G
CAA AAG AGG
Met Tyr Leu Leu Gln Lys Gln Val Lys
Lys Asp Leu Trp
Gln Lys Arg
150 155 160
GTAGATATCC TTGTGCTTTCCATTCGATTT TCAGCTATAAAATTGGAACC GTTAGACTGC4182
CACGAGAATG CATGGTTGTGAGAAGATTAA CATTTCTGGGTTAGTGAATA GCATTCATAC4242
GCTTTTGGGC ACCTTCCCCTGCAACTTGCC AGATAAGCACTATTCAGCTC TTATTCCCAG4302
TCTGACATCA GCAAGTGTGATTTTCTATGA AAAATTCTACTATGACTCCT TATTTTAAGT4362
ATACAAGAAA CTTGTGACTCAGAAGATAAT ATTTACAGAGTGGAAAAAAA CCCCTAGCAT4422
TTATAGTTTT AACATTTGAGGTTTTGAATG AGAGAGTTATCCATAATATA TTCAATTGTG4482
TTGTGGATAA TGACACCTAACCTGTGAATC TTGAGGTCAGAATGTTGAGT GCTGTTGACT4542
TGGTGGTCAG GAAACAGCTAGTGCGTGAGC CTGGCACAGGCATCTCAGTG AGTAGCATAC4602
CCACAGTTGG AAATTTTTCAAAGAAATCAA AGGAATCATGACATCTTATA AATTTCAAGG4662
TTCTGCTATA CTTATGTGAAATGGATAAAT AAATCAAGCATATCCACTCT GTAAGATTGA4722
CA 02309891 2000-06-19
51
ACTTCTCAGA TGGAAGACCC CAATACTGCT TTCTCCTCTT TTCCCTCACC AAAGAAATAA 4782
ACAACCTATT TCATTTATTA CTGGACACAA TCTTTAGCGT ATACCTATGG TAAATTACTA 4842
GTATGGTGGT TAGGATT' T GTTAATTTGT ATATGTCATG CGCCAAATCA TTTCCACTAA 4902
ATATGACTAT ATATCATAAC TGCTTGGTGA TAGCTCAGTG TTTAATAGTT TATTCTCAGA 4962
AAATCAAAAT TGTATAGTTA AATACATTAG TTTTATGAGG CAAAAATGCT AACTATTTCT 5022
ACAT4ATTTC ATTTTTCCAG AT AAT GAA AAT GTA GTC AAT GAG TAC TCC 5071
Asp Asn Glu Asn Val Val Asn Glu Tyr Ser
165 170
TCA GAA CTG GAA AAG CAC CAA TTA TAT ATA GAT GAG ACT GTG AAT AGC 5119
Ser Glu Leu Glu Lys His Gln Leu Tyr Ile Asp Glu Thr Val Asn Ser
175 180 185
AAT ATC CCA ACT AAC CTT CGT GTG CTT CGT TCA AiC CTG GAA AAC CTG 5167
Asn Ile Pro Thr Asn Leu Arg Val Leu Arg Ser Ile Leu Glu Asn Leu
190 195 20C 205
AGA AGC AAA ATA CAA AAG TTA GAA TCT GAT GTC TCA GCT CAA ATG GAA 5215
Arg Ser Lys Ile Gln Lys Leu Glu Ser Asp Yal Ser Ala Gln Met Glu
210 215 220
TAT TGT CGC ACC CCA TGC ACT GTC AGT TGC AAT ATT CCT GTG GTG TCT 5263
Tyr Cys Arg Thr Pro Cys Thr Val Ser Cys Asn Ile Pro Val Yal Ser
225 230 235
GGC AAA G GTAACTGATT CATAAACATA TTTTTAGAGA GTTCCAGAAG AACTCACACA 5320
Gly Lys
CCAAAAATAA GAGAACAACA ACAACAACAA AAATGCTAAG TGGATTTTCC CAACAGATCA 5380
TAATGACATT ACAGTACATC ATAAAAATAT CCTTAGCCAG TTGTGTTTTG GACTGGCCTG 5440
GTGCATTTGC TGGTTTTGAT GAGCAGGATG GGGCACAGGT AGTCCCAGGG GTGGCTGATG 5500
TGTGCATCTG CGTACTGGCT TGAACAGATG GCAGAACCAC AGATAGATGT AGAAGTTTCT 5560
CCATTTTGTG TGTTCTGGGA GCTCATGGAT ATTCCAGGAC ACAAAAGGTG GAGAAGAGCT 5620
TTGTTCATCC TCTTAGCAGA TAAACGTCCT CAAAACTGGG TTGGACTTAC TAAAGTAAAA 5680
CA 02309891 2000-06-19
52
TGAAAATCTA ATATTTGTTA TATTATTTTC AAAGGTCTAT AATAA~ACAC TCCTTAGTAA 5740
CTTATGTAAT GTTATTTTAA AGAATTGGTG ACTAAATACA AAGTAATTAT GTCATAAACC 5800
CCTGAACATA ATGTTGTCTT~ACATTTGCAG AA TGT GAG GAA ATT ATC AGG AAA 5853
Glu Cys Glu Glu Ile Ile Arg Lys
240 245
GGA GGT GAA ACA TCT GAA ATG TAT CTC ATT CAA CCT GAC AGT TCT GTC 5901
Gly Gly Glu Thr Ser Glu Met Tyr Leu Ile Gln Pro Asp Ser Ser Val
250 255 260
AAA CCG TAT AGA GTA TAC TGT GAC ATG AAT ACA GAA AAT GGA G 5944
Lys Pro Tyr Arg Val Tyr Cys Asp Met Asn Thr Glu Asn Gly
265 270 275
GTAAGCTTTC GACAGTTGTT GACCTGTTGA TCTGTAATTA TTTGGATACC GTAAAATGCC 6004
AGGAAACAAG GCCAGGTGTG GTGGCTCATA CCTGTAATTC CAGCACCTTG GGAGGCCAAA 6064
GTGGGCTGAT AGCTTGAGCC TAGGAGTTTG AAACTAGCCT GGGCAACATA ATGAGACCCT 6124
AACTCTACAA AAAAAAAAAA AATACCAAAA AAAAAAAAAA AATCAGCTGT GTTGGTAGTA 6184
TGTGCCTGTA GTCCCAGCTA TCCAGGAGGC TGAGATGGGA GATCACCTGA GCCCACAACC 6244
TGGAGTCTTG ATCATGCTAC TGAACTGTAG CCTGGGCAAC AGAGGATAGT GAGATCCTGT 6304
CTCAAAAAM AAAATTAATT AAAAAGCCAG GAAACAAGAC TTAGCTCTAA CATCTAACAT 6364
AGCTGACAAA GGAGTAATTT GATGTGGAAT TCAACCTGAT ATTTAAAAGT TATAAAATAT 6424
CTATAATTCA CAATTTGGGG TAAGATAAAG CACTTGCAGT TTCCAAAGAT TTTACAAGTT 6484
TACCTCTCAT ATTTATTTCC TTATTGTGTC TATTTTAGAG CACCAAATAT ATACTAAATG 6544
GAATGGACAG GGGATTCAGA TATTATTTTC AAAGTGACAT TATTTGCTGT TGGTTAATAT 6604
ATGCTCTTTT TGTTTCTGTC AACCAAAG GA TGG ACA GTG ATT CAG AAC CGT 6655
Gly Trp Thr Val Ile Gln Asn Arg
280 285
CAA GAC GGT AGT GTT GAC TTT GGC AGG AAA TGG GAT CCA TAT AAA CAG 6703
Gln Asp Gly Ser Val Asp Phe Gly Arg Lys Trp Asp Pro Tyr lys Gln
290 295 300
CA 02309891 2000-06-19
53
GGA TTT GGA AAT GTT GCA ACC AAC ACA GAT GGG AAG AAT TAC TGT GGC 6751
Gly Phe Gly Asn Val Ala Thr Asn Thr Asp Gly Lys Asn Tyr Cys Gly
305 310 315
CTA CCA G GTAACGAACA GGCATGCAAA ATAAAATCAT TCTATTTGAA ATGGGATTTT 6808
Leu Pro
TTTTAATTAA AAAACATTCA TTGTTGGAAG CCTGTTTTAG GCAGTTAAGA GGAGTTTCCT 6868
GACAAAAATG TG~AAGCTAA AGATAAGGGA AGAAAGGCAG TTTTTAGTTT CCCA.4AATTT 6928
TATTTTTGGT GAGAGATTTT ATTTTGTTTT TCTTTTAG GT GAA TAT TGG CTT 6980
Gly Glu Tyr Trp Leu
320
GGA AAT GAT AA4 ATT AGC CAG CTT ACC AGG ATG GGA CCC ACA GAA CTT 7028
Gly Asn Asp Lys Ile Ser Gln Leu Thr Arg Met Gly Pro Thr Glu Leu
325 330 335 340
TTG ATA GAA ATG GAG GAC TGG AAA GGA GAC AAA GTA AAG GCT CAC TAT 7076
Leu Ile Glu Met Glu Asp Trp Lys Gly Asp Lys Val Lys Ala His Tyr
345 350 355
GGA GGA TTC ACT GTA CAG AAT GAA GCC AAC AAA TAC CAG ATC TCA GTG 7124
Gly Gly Phe Thr Val Gln Asn Glu Ala Asn Lys Tyr Gln Ile Ser Val
360 365 370
AAC AAA TAC AGA GGA ACA GCC GGT AAT GCC CTC ATG GAT GG~i GCA TCT 7172
Asn Lys Tyr Arg Gly Thr Ala Gly Asn Ala Leu Met Asp Gly Ala Ser
375 380 385
CAG CTG ATG GGA GAA AAC AGG ACC ATG ACC ATT CAC AAC GGC ATG TTC 7220
Gln Leu Met Gly Glu Asn Arg Thr Met Thr Ile His Asn Gly Met Phe
390 . 395 400
TTC AGC ACG TAT GAC AGA GAC AAT GAC GGC TG GTATGTGTGG 7262
Phe Ser Thr Tyr Asp Arg Asp Asn Asp Gly Trp
405 410 415
CACTCTTTGC TCCTGCTTTA AAAATCACAC TAATATCATT ACTCAGAATC ATTAACAATA 7322
TTTTTAATAG CTACCACTTC CTGGGCACTT ACTGTCAGCC ACTGTCCTAA GCTCTTTATG 7382
CATCACTCGA AAGCATTTCA ACTATAAGGT AGACATTCTT ATTCTCATTT TACAGATGAG 7442
ATTTAGAGAG ATTACGTGAT TTGTCCAATG TCACACAACT ACCCAGAGAT AAAACTAGAA 7502
CA 02309891 2000-06-19
54
TTTGAGCACA GTTACTTTCT GAATAATGAG CATTTAGATA AATACCTATA TCTCTATATT 7562
CTAAAGTGTG TGTGAAAACT TTCATTTTCA TTTCCAGGGT TCTCTGATAC TAAGGGTTGT 7622
AAAAGCTATT ATTCCAGTAT AAAGTAACAA ACACAGTCCC TAGATGGATT GCCACAAAGG 7682
CCCAGTTATC TCTCTTTCTT GCTATAGGGC ACAGGAGGTC TTTGGTGTAT TAGTGTGACT 7742
CTATGTATAG CACCCAAAGG AAAGACTACT GTGCACACGA GTGTAGCAGT CTTTTATGGG 7802
TAATCTGCAA AACGTAACTT,GACCACCGTA GTTCTGTTTC TAATAACGCC AAACACATTT 7862
TCTTTCAG G TTA ACA TCA GAT CCC AGA AAA CAG TGT TCT AAA GAA GAC 7910
Leu Thr Ser Asp Pro Arg Lys Gln Cys Ser Lys Glu Asp
420 425
GGT GGT GGA TGG TGG TAT AAT AGA TGT CAT GCA GCC AAT CCA AAC GGC 7958
Gly Gly Gly Trp Trp Tyr Asn Arg Cys His Ala Ala Asn Pro Asn Gly
430 435 440
AGA TAC TAC TGG GGT GGA CAG TAC ACC TGG GP.C ATG GCA AAG CAT GGC 8006
Arg Tyr Tyr Trp Gly Gly Gln Tyr Thr Trp Asp Met Ala Lys His Gly
445 450 455 460
ACA GAT GAT GGT GTA GTA TGG ATG AAT TGG AAG GGG TCA TGG TAC TCA 8054
Thr Asp Asp Gly Yal Val Trp Met Asn Trp Lys Gly Ser Trp Tyr Ser
465 470 475
ATG AGG AAG ATG AGT ATG AAG ATC AGG CCC TTC TTC CCA CAG CAA TAGTCCCCAA
8109
Met Arg Lys Met Ser Met Lys Ile Arg Pro Phe Phe Pro Gln Gln
480 485 490
TACGTAGATT TTTGCTCTTC TGTATGTGAC AACATTTTTG TACATTATGT TATTGGAATT 8169
TTCTTTCATA CATTATATTC CTCTAAAACT CTCAAGCAGA CGTGAGTGTo ACTTTTTGAA 8229
AAAAGTATAG GATAAATTAC ATTAAAATAG CACATGATTT TCTTTTGTTT TCTTCATTTC 8289
TCTTGCTCAC CCAAGAAGTA ACAAAAGTAT AGTTTTGACA GAGTTGGTGT TCATAATTTC 8349
AGTTCTAGTT GATTGCGAGA ATTTTCAAAT AAGGAAGAGG GGTCTTTTAT CCTTGTCGTA 8409
GGAAAACCAT GACGGAAAGG AAAAACTGAT GTTTAAAAGT CCACTTTTAA AACTATATTT 8469
ATTTATGTAG GATCTGTCAA AGAAAACTTC CAAAAAGATT TATTAATTAA ACCAGACTCT 8529
CA 02309891 2000-06-19
GTTGCAATAA GTTAATGTTT TCTTGTTTTG TAATCCACAC ATTCAATGAG TTAGGCTTTG 8589
CACTTGTAAG.GAAGGAGAAG CGTTCACAAC CTCAAATAG: TAATAAACCG GTCTTGAATA 8649
TTTGAAGATT TAAAATCTGA CTCTAGGACG GGCACGGTGG CTCACGACTA TAATCCCAAC 8709
ACTTTGGGAG GCTGAGGCGG GCGGTCACAA GGTCAGGAGT TCAAGACCAG CCTGACCAAT 8769
ATGGTGhAAC CCCATCTCTA CTAAAAATAC AAAAATTAGC CAGGCGTGGT GGCAGGTGCC 8829
TGTAGGTCCC AGCTAGCCTG TGAGGTGGAG ATTGCATTGA GCCAAGATC 8878
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 491 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Met Lys Arg Met Val Ser Trp Ser Phe His Lys Leu Lys Thr Met Lys
1 5 10 15
His Leu Leu Leu Leu Leu Leu Cys Val Phe Leu Val Lys Ser Gln Gly
20 25 30
Val Asn Asp Asn Glu Glu Gly Phe Phe Ser Ala Arg Gly His Arg Pro
35 40 45
Leu Asp Lys Lys Arg Glu Glu Ala Pro Ser Leu Arg Pro Ala Pro Pro
50 55 60
Pro Ile Ser Gly Gly Gly Tyr Arg Ala Arg Pro Ala Lys Ala Ala Ala
70 75 80
Thr Gln Lys Lys Val Glu Arg Lys Ala Pro Asp Ala Gly Gly Cys Leu
85 90 95
His Ala Asp Pro Asp Leu Gly Val Leu Cys Pro Thr Gly Cys Gln Leu
100 105 110
CA 02309891 2000-06-19
56
Gln Glu Ala Leu Leu Gln Gln Glu Arg Pro Ile Arg Asn Ser Val Asp
115 120 125
Glu Leu Asn Asn Asn Val Glu Ala Val Ser Gln Thr Ser Ser Ser Ser
130 ' 135 140
Phe Gln Tyr Met Tyr Leu Leu Lys Asp Leu Trp Gln Lys Arg Gln Lys
145 150 155 160
Gln Val Lys Asp Asn Glu Asn Val Val Asn Glu Tyr Ser Ser Glu Leu
165 170 175
Glu Lys His Gln Leu Tyr Ile Asp Glu Thr Val Asn Ser Asn Ile Pro
180 185 190
Thr Asn Leu Arg Val Leu Arg Ser Ile Leu Glu Asn Leu Arg Ser Lys
195 200 205
Ile Gln Lys Leu Glu Ser Asp Val Ser Ala Gln Met Glu Tyr Cys Arg
210 215 220
Thr Pro Cys Thr Val Ser Cys Asn Ile Pro Val Val Ser Gly Lys Glu
225 230 235 240
Cys Glu Glu Ile Ile Arg Lys Gly Gly Glu Thr Ser Glu Met Tyr Leu
245 250 255
Ile Gln Pro Asp Ser Ser Val Lys Pro Tyr Arg Val Tyr Cys Asp Met
260 265 270
Asn Thr Glu Asn Gly Gly Trp Thr Val Ile Gln Asn Arg Gln Asp Gly
275 280 285
Ser Val Asp Phe Gly Arg Lys Trp Asp Pro Tyr Lys Gln Gly Phe Gly
290 295 300
Asn Val Ala Thr Asn Thr Asp Gly Lys Asn Tyr Cys Gly Leu Pro Gly
305 310 315 320
Glu Tyr Trp Leu Gly Asn Asp Lys Ile Ser Gln Leu Thr Arg Met Gly
325 330 335
Pro Thr Glu Leu Leu Ile Glu Met Glu Asp Trp Lys Gly Asp Lys Val
340 345 350
Lys Ala His Tyr Gly Gly Phe Thr Val Gln Asn Glu Ala Asn Lys Tyr
355 360 365
CA 02309891 2000-06-19
57
Gln Ile Ser Val Asn Lys Tyr Arg Gly Thr Ala Gly Asn Ala Leu Met
370 375 380
Asp Gly Ala Ser Gln Leu Met Gly Glu Asn Arg Thr Met Thr Ile His
385 390 395 400
Asn Gly Met Phe Phe Ser Thr Tyr Asp Arg Asp Asn Asp Gly Trp Leu
405 410 415
Thr Ser Asp Pro Arg Lys Gln Cys Ser Lys Glu Asp Gly Gly Gly Trp
420 425 430
Trp Tyr Asn Arg Cys His Ala Ala Asn Pro Asn Gly Arg Tyr Tyr Trp
435 440 445
Gly Gly Gln Tyr Thr Trp Asp Met Ala Lys His Gly Thr Asp Asp Gly
450 455 460
Val Val Trp Met Asn Trp Lys Gly Ser Trp Tyr Ser Met Arg Lys Met
465 470 475 480
Ser Met Lys Ile Arg Pro Phe Phe Pro Gln Gln
485 490
(2) INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10564 base pairs
(B) TYPE; nucleic acid
(C) STRANDEONESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(vii) IMMEDIATE SOURCE:
(B) CLONE: human fibrinogen gamma chain
(ix) FEATURE:
(A) NAME/KEY: COS
(B) LOCATION: join(1799..1876, 1973..2017, 2207..2390, 2510
..2603, 4211..4341, 4645..4778, 5758..5942, 7426
..7703, 9342..9571)
CA 02309891 2000-06-19
58
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
CTACACACTT CTTGAAGGCA AAGGCAATGC TGAAGTCACC TTTCATGTTC AAATCATATT 60
AAAAAGTTAG CAAGATGTAA TTATCAGTGT ACTATGTAAA TCTTTGTGAA TGATCAATAA 120
TTACATATTT TCATTATATA TATTTTAGTA GATAATATTT ATATACATTC AACATTCTAA 180
ATATAGAAAG TTTACAGAGA AAAATAAAGC CTTTTTTTCC AATCCTGTCC TCCACCTCTG 240
CATCCCATTC TTCTTCACAG AGGCAACTGA TTCAAGTCAT TACATAGTTA TTGAGTGTTA 300
ACTACAACTA TGTTAAGTAC AGCTATATAT GTTAGATGCC GTAGCCACAG AAATCAGTTT 360
ACAATCTAAT GCAGTGGATA CAGCATGTAT ACATATAATA TAAGGTTGCT ACAAATGCTA 420
TCTGAGGTAG AGCTGTTTGA AAGAATACTA ATACTTAAAT GTTTAATTCA ACTGACTTGA 480
TTGACAACTG ATTAGCTGAG TGGAAAAGAT GGATGAGAAA GATTGTGAGA CTTAATTGGC 540
TGGTGGTATG GTGATATGAT TGACAATAAC TGCTAAGTCA GAGAGGGATA TATTAAGGAG 600
GAGAAGAAAA GCAACAAATC TGGTTTTGAT GTGTTCACTT TGTTATAATT ATTGATTATT 660
TACTGAATAT GAATATTTAT CTTTGTTTTT GAGTCAATAA ATATACCTTT GTAAAGACAG 720
AATTAAAGTA TTAGTATTTC TTTCAAACTG GAGGCATTTC TCCCACTAAC ATATTTCATC . 780
AAAACTTATA ATAAGCTTGG TTCCAGAGGA AGAAATGAGG GATAACCAAA AATAGAGACA 840
TTAATAATAG TGTAACGCCC AGTGATAAAT CTCAATAGGC AGTGATGACA GACATGTTTT 900
CCCAAACACA AGGATGCTGT AAGGGCCAAA CAGAAATGAT GGCCCCTCCC CAGCACCTCA 960
TTTTGCCCCT TCCTTCAGCT ATGCCTCTAC TCTCCTTTAG ATACAAGGGA GGTGGATTTT 1020
TCTCTTCTCT GAGATAGCTT GATGGAACCA CAGGAACAAT GAAGTGGGCT CCTGGCTCTT 1080
TTCTCTGTGG CAGATGGGGT GCCATGCCCA CCTTCAGACA AAGGGAAGAT TGAGCTCAAA 1140
AGCTCCCTGA GAAGTGAGAG CCTATGAACA TGGTTGACAC AGAGGGACAG GAATGTATTT 1200
CCAGGGTCAT TCATTCCTGG GAATAGTGAA CTGGGACATG GGGGAAGTCA GTCTCCTCCT 1260
GCCACAGCCA CAGATTAAAA ATAATAATGT TAACTGATCC CTAGGCTAAA ATAATAGTGT 1320
TAACTGATCC CTAAGCTAAG AAAGTTCTTT TGGTAATTCA GGTGATGGCA GCAGGACCCA 1380
CA 02309891 2000-06-19
59
TCTTAAGGAT AGACTAGGTT TGCTTAGTTC GAGGTCATAT CTGTTTGCTC TCAGCCATGT 1440
ACTGGAAGAA GTTGCATCAC ACAGCCTCCA GGACTGCCCT CCTCCTCACA GCAATGGATA 1500
ATGCTTCACT AGCCTTTGCAGATAATTTTGGATCAGAGAA GCTGGGCCAA1560
AAAACCTTGA
AAAGGAGGAG CTTCAACCTGTGTGCAAAATCTGGGAACCTGACAGTATAGGTTGGGGGCC1620
AGGATGAGGA AAAAGGAACGGGAAAGACCTGCCCACCCTTCTGGTAAGGAGGCCCCGT6A1680
TCAGCTCCAG CCATTTGCAGTCCTGGCTATCCCAGGAGCTTACATAAAGGGACAATTGGA1740
GCCTGAGAGG TGACAGTGCTGACACTACAAGGCTCGGAGCTCC6GGCACTCAGACATC 1798
ATG AGT TGG TCC CTC TAC TAT GCT 1846
TTG CAC CCC CGG TTC
AAT TTA ATT
Met Ser Trp Ser Leu Tyr Tyr Ala
Leu His Pro Arg Phe
Asn Leu Ile
1 5 10 15
CTT TTA TTT CTC TCT TCA ACA TGT GTA GCA GTAAGTGTGC TCTTCACAAA 1896
Leu Leu Phe Leu Ser Ser Thr Cys Val Ala
20 25
ACGTTGTTTA AAATGGAAAG CTGGAAAATA AAACAGATAA TAAACTAGTG AAATTTTCGT 1956
ATTT~TTCTC TTTTAG TAT GTT GCT ACC AGA GAC AAC TGC TGC ATC TTA 2005
Tyr Val Ala Thr Arg Asp Asn Cys Cys Ile Leu
30 35
GAT GAA AGA TTC GTAAGTAGTT TTTATGTTTC TCCCTTTGTG TGTGAACTGG 2057
Asp Glu Arg Phe
AGAGGGr,CAG AGGAATAGAA ATAATTCCCT CATAAATATC ATCTGGCACT TGTAACTTTT 2117
TAAAAACATA GTCTAGGTTT TACCTATTTT TCTTAATAGA TTTTAAGAGT AGCATCTGTC 2177
TACATTTTTA ATCACTGTTA TATTTTCAG GGT AGT TAT TGT CCA ACT ACC TGT 2230
Gly Ser Tyr Cys Pro Thr Thr Cys
GGC ATT GCA GAT TTC CTG TCT ACT TAT CAA ACC AAA GTA GAC AAG GAT 2278
Gly Ile Ala Asp Phe Leu Ser Thr Tyr Gln Thr Lys Val Asp Lys Asp
55 60 65
CA 02309891 2000-06-19
CTA CAG TCT TTG GAA GAC ATC TTA CAT CAA GTT GAA AAC AAA ACA TCA 2326
Leu Gln Ser Leu Glu Asp Ile Leu His Gln Yal Glu Asn Lys Thr Ser
75 80
GAA GTC AAA CAG CTG ATA AAA GCA ATC CAA CTC ACT TAT AAT CCT GAT 2374
Glu Yal Lys Gln Leu Ile Lys Ala Ile Gln Leu Thr Tyr Asn Pro Asp
85 90 95
GAA TCA TCA AAA CCA A GTGAGAAAAT AAA6ACTACT GACCAAAAAA 2420
61u Ser Ser Lys Pro
100
TAATAATAAT AATCTGTGAA GTTCTTTTGC TGTTGTTTTA GTTGTTCTAT TTGCTTAAGG 2480
ATTTTTATGT CTCTGATCCT ATATTACAG AT ATG ATA GAC GCT GCT ACT TTG 2532
Asn Met Ile Asp Ala Ala Thr Leu
105 110
AAG TCC AGG ATA ATG TTA GAA GAA ATT ATG AAA TAT GAA GCA TCG ATT 2580
Lys Ser Arg Ile Met Leu Glu Glu Ile Met Lys Tyr Glu Ala Ser Ile
115 120 125
TTA ACA CAT GAC TCA AGT ATT CG GTAAGGATTT TTGTTTTAAT TTGCTCTGCA 2633
Leu Thr His Asp Ser Ser Ile Arg
130
AGACTGATTT AGTTTTTATT TAATATTCTA TACTTGAGTG AAAGTAATTT TTAATGTGTT 2693
TTCCCCATTT ATAATATCCC AGTGACATTA TGCCTGATTA TGTTGAGCAT AGTAGAGATA 2753
GAAGTTTTTA GTGCAATATA AATTATACTG GGTTATAATT GCTTATTAAT AATCACATTG 2813
AAGAAAGAT~ TTCTAGATGT CTTCAAATGC TAGTTTGACC ATATTTATCA AAAATTTTTT 2873
CCCCATCCCC CATTTATCTT ACAACATAAA ATCAATCTCA TAGGAATTTG GGTGTTGAAA 2933
ATAAAATCCT CTTTATAAAA ATGCTGACAA ATTGGTGGTT AAAAAAATTA GCAAGCAGAG 2993
GCATAGTAAG GATTTTGGCT CCTAAAGTAA ATTATATTGA ATGTGGAGCA GGAAGAAACA 3053
TGTCTTGAGA GACTAAGTGT GGCAAATATT GCAAAGCTCA TATTGATCAT TGCAGAATGA 3113
ACCTGCATAG TCTCTTCCCT TCATTTGGAA GTGAATGTCT CTGTTAAAGC TTCTCAGGGA 3173
CTCATAAACT TTCTGAACAT AAGGTCTCAG ATACAGTTTT AATATTTTTC CCCAATTTTT 3233
TTTTCTGAAT TTTTCTCAAA GCAGCTTGAG AAATTGAGAT AAATAGTAGC TAGGGAGAAG 3293
CA 02309891 2000-06-19
61
TGGCCCAGGA AAGATTTCTC CTCTTTTTGC TATCAGAGGG CCCTTGTTAT TATTGTTATT 3353
ATTATTACTT GCATTATTAT TGTCCATCAT TGAAGTTGAA GGAGGTTATT GTACAGAAAT 3413
TGCCTAAGAC AAGGTAGAGG GAAAACGTGG ACAAATAGTT TGTCTACCCT TTTTTACTTC 3473
AAAGAAAGAA CGGTTTATGC ATTGTAGACA GTTTTCTATC ATTTTTGGAT ATTTGCAAGC 3533
CACCCTGTAA GTAACTACAA AAGGAGGGTT TTTACTTCCC CCAGTCCATT CCCAAAGCTA 3593
TGTAACCAGA AGCATTAAAG_AAGAAAGGGG AAGTATCTGT TGTTTTATTT TACATACAAT 3653
AACGTTCCAG ATCATGTCCC TGTGTAAGTT ATATTTTAGA TTGAAGCTTA TATGTATAGC 3713
CTCAGTAGAT CCACAAGTGA AAGGTATACT CCTTCAGCAC ATGT6AATTA CTGAACTGAG 3773
CTTTTCCTGC TTCTAAAGCA TCAGGGGGTG TTCCTATTAA CCAGTCTCGC CACTCTTGCA 3833
GGTTGCTATC TGCTGTCCCT TATGCATAAA GTAAAAAGCA AAATGTCAAT GACATTTGCT 3893
TATTGACAAG GACTTTGTTA TTTGTGTTGG GAGTTGAGAC AATATGCCCC ATTCTAAGTA 3953
AAAAGATTCA GGTCCACATT GTATTCCTGT TTTAATTGAT TTTTTGATTT GTTTTTCTTT 4013
TTCAAAAAGT TTATAATTTT AATTCATGTT AATTTAGTAA TATAATTTTA CATTTTCCTC 4073
AAGAATGGAA TAATTTATCA GAAAGCACTT CTTAAGAAAA TACTTAGCAG TTTCCAAAGA 4133
AAATATAAAA TTACTCTTCT GAAaGGAATA CTTATTTTTG TCTTCTTATT TTTGTTATCT 4193
TATGTTTCTG TTTGTAG A TAT TTG CAG GAA ATA TAT AAT TCA AAT AAT CAA 4244
Tyr Leu Gln Glu Ile Tyr Asn Ser Asn Asn Gln
135 140 145
AAG ATT GTT AAC CTG AAA GAG AAG GTA GCC CAG CTT GAA GCA CAG TGC 4292
Lys Ile 11a1 Asn Leu Lys Glu Lys Ilal Ala Gln Leu Glu Ala Gln Cys
150 155 160
CAG GAA CCT TGC AAA GAC ACG GTG CAA ATC CAT GAT ATC ACT GGG AAA G 4341
Gln Glu Pro Cys lys Asp Thr Val Gln Ile His Asp Ile Thr Gly Lys
165 170 175
GTAACTGATG AAGGTTATAT TGGGATTAGG TTCATCAAAG TAAGTAATGT AAAGGAGAAA 4401
GTATGTACTG GAAAGTATAG GAATAGTTTA GAAAGTGGCT ACCCATTAAG TCTAAGAATT 4461
CA 02309891 2000-06-19
62
TCAGTTGTCT_AGACCTTTCTTGAATAGCTA TTTAAAAGGAATGCTGATGT 4521
AAAAAAACAG
GAAAAGTAAGAAAATTATTCTTGGAAAATG TACATGTTAAAAGCTATTTT 4581
AATAGTTTAC
TCAAGGCTGGCACAGTCTTACCTGCATTTCAAACCACAGTAAAAGTCGATTCTCCTTCTC 4641
TAG AT AAG GGA AAA CAG 4688
TGT CAA GCT AGC GGG
GAC ATT CTT
GCC AAT
Asp Cys Lys Gly Lys Gln
Gln Asp Ala Ser Gly
Ile Ala Leu
Asn
180 185 190
TAC TTT AAC CAG TTC TTA 4736
ATT AAA CAA GTC TAC
CCT CTG TGT
AAA GCT
Tyr Phe Asn Gln Phe leu
Ile Lys Gln Val Tyr
Pro Leu Cys
Lys Ala
~
195 200 205
GAA ATC TGG ACT TTT CAG 4778
GAT GGG GTG AAG
TCT GGA
AAT GGA
Glu Ile Trp Thr Phe Gln
Asp Gly Val Lys
Ser Gly
Asn Gly
210 215 220
GTAATTTTTTCCCCACCATGTGTATTTAATAAATTCCTACATTGTTTCTGCCATATGGCA 4838
GATACTTTTCTAAGCACCTTGTGAACCGTAGCTCATTTAATCCTTGCAATAGCCCTAAGA 4898
GGAAGGTACTTCTGTTACTCCTATTTACAGAAAAGGAAACTGAGGCACACAAGGTTAAAT 4958
AACTTGCCCAAGACCACATAACTAATAAGCAACAGAGTCAGCATTTGAACCTAGGCAGTA 5018
TAGTTTCAGAGTTTGTGACTTGACTCTATATTGTACTGGCACTGACTTTGTAGATTCATG 5078
GTGGCACATAATCATAGTACCACAGTGACAAATAAAAAGAAGGAAACTCTTTTGTCAGGT 5138
AGGTCAAGACCTGAGGTTTCCCATCACAAGATGAGGAAGCCCAACACCACCCCCCACCAC 5198
CCCACCACCATCACCACCCTTTCACACACCAGAGGATACACTTGGGCTGCTCCAAGACAA 5258
GGAACCTGTGTTGCATCTGCCACTTGCTGATACCCACTAG~GAATCTTGGCTCCTTTACTT 5318
TCTGTTTACCTCCCACCACTGTTATAACTGTTTCTACAGGGGGCGCTCAGAGGGAATGAA 5378
TGGTGGAAGCATTAGTTGCCAGACACCGATTGAGCAATGGGTTCCATCATAAGTGTAAGA 5438
ATCAGTAATATCCAGCTAGAGTTCTGAAGTCGTCTAGGTGTCTTTTTAATATTAGCACTC 5498
ATTTAGAATTTATGATGTGCCAGAAACCCTCTTAAGTATTTCTCTTATATTCTCTCTCAT 5558
GATCCTTGCAGCAACCCTAAGAAGTAACCATCATTTTTCCTATTTGATACATGAGGAAAC 5618
TGAGGTAGCTTGGCCAAGATCACTTAGTTGGGAGTTGATAGAACCAGTGCTCTGTATTTT 5678
CA 02309891 2000-06-19
63
TGACAAAATG TTGACAGCAT TCTCTTTACA TGCATTGATA GTCTATTTTC TCCTTTTGCT 5738
CTTGCAAATG TGTAATTAG AGA CTT GAT GGC AGT GTA GAT TTC AAG AAA AAC 5790
A~g Asp
Leu Phe
Asp Lys
Gly Lys
Ser Asn
Val
225 230
TGGATTCAA TATAAAGAA GGATTT CAT CTGTCTCCT GGC ACA 5838
GGA ACT
TrpIleGln TyrLysGlu GlyPhe His LeuSerPro Gly Thr
Gly Thr
235 240 245
ACAGAATTT TGGCTGGGA AATGAG ATT CATTTGATA ACA CAG 5886
AAG AGC
ThrGluPhe TrpLeuGly AsnGlu Ile HisLeuIle Thr Gln
Lys Ser
250 255 260 265
TCTGCCATC CCATATGCA TTAAGA GAA CTGGAAGAC AAT GGC 5934
GTG TGG
SerAlaIle ProTyrAla LeuArg Glu LeuGluAsp Asn Gly
Val Trp
270 275 280
AGAACCAG GTACTGTTTT GAAATGACTT 5982
CCAACTTTTT
ATTGTAAAGA
ArgThrSer
TTGCCTGGAATGTGCACTTTCCAACTATCAATAGACAATGGCAAATGCAGCCTGACAAAT 6042
GCAAACAGCACATCCAGCCACCATTTTCTCCAGGAGTGTGTTTGGTTCTTGGGCAATCCA 6102
AAAAGGTAAATTCTATTCAGGATGAATCTAAGTGTATTGGTACAATCTAATTACCCTGGA 6162
ACCATTCAGAGTAATAGCTAATTACTGAACTTTTAATCAGTCCCAGGAATTGAGCATAAA 6222
ATTATAATTTTATCTAGTCTAAATTACTATTTCATGAAGCAGGTATTATTATTAATCCCA 6282
TTTTATAGATTAACTTGCTCAAAGTCACATTGCTGATAAGTGGTAGAGGTAGAATTCAGA 6342
CTCAAGTAGTTTAACTTTAGAGCCTGTCCTCTTAACAACTATCCTGGTTGAAAAGCAAAT 6402
ACAGCCTCTTCAGACTTCTCAGTGCCTTGATGGCCATTTATTCTGTCAAATCATGAGCTA 6462
CCCTAAAAGTAAACCAGCTAGCTCTTTTGATGATCTAGAGGCTTCTTTTTGCTTGAGATA 6522
TTTGAAGGTTTTAAGCATTGTTACCTAATTAAAATGCAGAAAAATATCCAACCCTCTTGT 6582
TATGTTTAAGGAATAGTGAAATATATTGTCTTCAAACACATGGACTTTTTTTTATTGCTT 6642
GGTTGGTTTTTAATCCAGAAAGTGCTATAGTCAGTAGACCTTCTTCTAGGAAAGGACCTT 6702
CA 02309891 2000-06-19
64
CCATTTCCCA.GCCACTGGAGATTAGAAAATAAGCTAAATATTTTCTGGAAATTTCTGTTC6762
ATTCATTAAGGCCCATCCTTTCCCCCACTCTATAGAAGTGTTGTCCACTTGCACAATTTT6822
TTCCAGGAAAGAATCTCTCTAACTCCTTCAGCTCACATGCTTTGGACCACACAGGGAAGA6882
CTTTGATTGTGTAATGCCCTCAGAAGCTCTCCTTCTTGCCACTACCACACTGATTTGAGG6942
AAGAAAATCCCTTTAGCACCTAACCCTTCAGGTGCTATGAGTGGCTAATGGAACTGTACC7002
TCCTTCAAGTTTTGTGCAATAATTAAGGGTCACTCACTGTCAGATACTTTCTGTGATCTA7062
TGATAATGTGTGTGCAACACATAACATTTCAATAAAAGTAGAAAATATGAAATTAGAGTC7122
ATCTACACATCTGGATTTGATCTTAGAATGAAACAAGCAAAAAAGCATCCAAGTGAGTGC7182
AATTATTAGTTTTCAGAGATGCTTCAAAGGCTTCTAGGCCCATCCCGGGAAGTGTTAATG7242
AGCTGTGGACTGGTTCACATATCTATTGCCTCTTGCCAGATTTGCAAAAAACTTCACTCA7302
ATGAGCAAATTTCAGCCTTAAGAAACAAAGTCAAAAATTCCAAGGAAGCATCCTACGAAA7362
GAGGGAACTTCTGAGATCCCTGAGGAGGGTCAGCATGTGATGGTTGTATTTCCTTCTTCT7422
CAG T CA GAC GCC ATG CT GAC 7468
ACT G TAT TTC AAG
GTG GGA
CCT GAA
G
Thr Ala Ala Met la Asp
Asp Tyr Phe Lys
Val Gly
Pro Glu
A
285 290 295
AAG TAC CTA ACA GGG GAT GGA GAT 7516
CGC TAT GCC GCT
TAC TTC
GCT GGT
lys Tyr Leu Thr Gly Asp Gly Asp
Arg Tyr Ala Ala
Tyr Phe
Ala Gly
300 305 310
GCCTTTGAT GGCTTT GATTTTGGC GATGAT CCT GAC AAGTTT TTC 7564
AGT
AlaPheAsp GlyPhe AspPheGly AspAsp ProSerAsp LysPhe Phe
315 320 325 330
ACATCCCAT AATGGC ATGCAGTTC AGTACC TGGGACAAT GaCAAT GAT 7612
ThrSerHis AsnGly MetGlnPhe SerThr TrpAspAsn AspAsn Asp
335 340 345
AAGTTTGAA GGCAAC TGTGCTGAA CAGGAT GGATCTGGT TGGTGG ATG 7660
LysPheGlu GlyAsn CysAlaGlu GlnAsp GlySerGly TrpTrp Met
350 355 360
AACAAGTGT CACGCT GGCCATCTC AATGGA GTTTATTAC CAAG 7703
AsnLysCys HisAla GlyHisLeu AsnGly ValTyrTyr Gln
365 370 375
CA 02309891 2000-06-19
ss
GTATGTTTTC CTTTCTTAGA TTCCAAGTTA ATGTATAGTG TATACTATTT TCATAAAAAA 7763
TAATAAATAG ATATGAAGAA ATGAAGAATA ATTTATAAAG ATAGTAGGGA TTTTATCATG 7823
TTCTTTATTT CAACTAAGTT CTTTGAAACT GGAAGTGGAT AATACCAAGT TCATGCCTAA 7883
AATTAGCCCT TCTAAAGAAA TCCACCTGCT GCAAAATATC CAGTAGTTTG GCATTATATG 7943
TGAAACTATC ACCATCATAG CTGGCACTGT GGGTTGTGGG ATCTCCTTTA GACATACAAC 8003
ATAAATGATC TGGATGGATT AACATTACTA CATGGATGCT TGTTGACACA TTAACCTGGC 8063
TTCCCATGAG CTTTGTGTCA GATACACGCA GTGAACAGGT GTTTGGAGGA ACAGAATAAA 8123
GAGAAGGCAA GCACTGGTAA GGGCAGGGGT TTGTGAAAGC TTGAGAGAAG AGACCAGTCT 8183
GAGGACAGTA GACACTTATT TTAGGATGGG GGTTGGATGA GGAGGCTATA GTTTGCTATA 8243
AGCTTGGAAT GGTTTGGAAC ACTGGTTTCA CTCACCTACC CAGCAGTTAT GTGTGGGGAA 8303
GCCTTACCGA TGCTAAAGGA TCCATGTTAC AATAATGGCA TTATTTGGAA ATCCCAGTGG 8363
TATTCCATGA ATAAAACCAC TATGAAGATA ATCCCACTCA ACAGACTCTC CGTTGGAGAA 8423
GGACAGCAAC ACCACCCTGG GAAAGCCAAA CAGTCAGACC AGACCTGTTT AGCATCAGTA 8483
GGACTTCCCT ACCATATCTG CTGGGTAGAT GAGTGAAACC AGTGTTCCAA ACCACTCCGG 8543
GCTTGTAGCA AACCATAGTC TCCTCATCTA CCAAGATGAG CAACCTTACC TCCTGATGTC 8603
CTAGCCAATC ACCAACTAGG AAACTTTGCA CAGTTTATTT AAAGTAACAG TTTGATTTTC 8663
ACAATATTTT TAAATTGGAG AAACATAACT TATCTTTGCA CTCACAAACC ACATAATGAG 8723
AAGAAACTCT AAGG~AAAAT GCTTGATCTG TGTGACCCGG GGCGCCATGC CAGAGCTGTA 8783
GTTCATGCCA GTGTTGTGCT CTGACAAGCC TTTTACAGAA TTACATGAGA TCTGCTTCCC 8843
TAGGACAAGG AGAAGGCAAA TCAACAGAGG CTGCACTTTA AAATGGAGAC ATAAAATAAC 8903
ATGCCAGAAC CATTTCCTAA AGCTCCTCAA TCAACCAACA AAATTGTGCT TTCAAATAAC 8963
CTGAGTTGAC CTCATCAGGA ATTTTGTGGC TCCTTCTCTT CTAACCTGCC TGAAGAAAGA 9023
TGGTCCACAG CAGCTGAGTC CGGGATGGAT AAGCTTAGGG ACAGAGGCCA ATTAGGGAAC 9083
CA 02309891 2000-06-19
66
TTTGGGTTTGTAGCCCTACT AGTAGTGAATAAATTTAAAGTGTGGATGTGACTATGAGTC9143
ACAGCACAGATGTTGTTTAA TAATATGTTTATTTTATAAATTGATATTTTAGGAATCTTT9203
GGAGATATTTTCAGTTAGCA ~GATAATACTATAAATTTTATGTAACTGGCAATGCACTTCG9263
TAATAGACAGCTCTTCATAG ACTTGCAGAGGTAAAAAGATTCCAGAATAATGATATGTAC9323
ATCTACGACT GT GGCACTTAC TCA AAAGCATCTACT CCTAAT 9373
TGTTTTAG
Gly GlyThrTyr Ser LysAlaSerThr ProAsn
380 385
GGTTATGAT AATGGCATT ATTTGGGCC ACT TGGAAAACCCGG TGGTAT 9421
GlyTyrAsp AsnGlyIle IleTrpAla Thr TrpLysThrArg TrpTyr
390 395 400
TCCATGAAG AAAACCACT ATGAAGATA ATC CCATTCAACAGA CTCACA 9469
SerMetLys LysThrThr MetLysIle Ile ProPheAsnArg LeuThr
405 410 415
ATTGGAGAA GGACAGCAA CACCACCTG GGG GGAGCCAAACAG GTCAGA 9517
IleGlyGlu GlyGlnGln HisHisLeu Gly GlyAlaLysGln ValArg
420 425 430 435
CCAGAGCAC CCTGCGGAA ACAGAATAT GAC TCACTTTACCCT GAGGAT 9565
ProGluHis ProAlaGlu ThrGluTyr Asp SerLeuTyrPro GluAsp
440 445 450
GATTTGTAGAAAATTA CACAAAGTTT CHGAAATTCT
9621
ACTGCTAACT
TCTATTGACC
AspLeu
CTGAAAGTTTCTTCCTTTTTTCTCTTACTATATTTATTGATTTCAAGTCTTCTATTAAGG9681
ACATTTAGCCTTCAATGGAAATTAAAACTCATTTAGGACTGTATTTCCAAATTACTGATA9741
TCAGAGTTATTTAAAAATTGTTTATTTGAGGAGATAACATTTCAACTTTGTTCCTAAATA9801
TATAATAATAAAATGATTGACTTTATTTGCATTTTTATGACCACTTGTCATTTATTTTGT9861
CTTCGTAAATTATTTTCATTATATCAAATATTTTAGTATGTACTTAATAAAATAGGAGAA9921
CATTTTAGAGTTTCAAATTCCCAGGTATTTTCCTTGTTTATTACCCCTAAATCATTCCTA9981
TTTAATTCTTCTTTTTAAATGGAGAAAATTATGTCTTTTTAATATGGTTTTTGTTTTGTT10041
ATATATTCACAGGCTGGAGACGTTTAAAAGACCGTTTCAAAAGAGATTTACTTTTTTAAA10101
CA 02309891 2000-06-19
67
GGACTTTATC TGAACAGAGA GATATAATAT TTTTCCTATT GGACAATGGA CTTGCAAAGC 10161
TTCACTTCAT TTTAAGAGCA AAAGACCCCA TGTTGAAAAC TCCATAACAG TTTTATGCTG 10221
ATGATAATTTATCTACATGCATTTCAATAAACCTTTTGTTTCCTAAGACTAGATACATGG10281
TACCTTTATTGACCATTAAAAAACCACCACTTTTTGCCAATTTACCAATTACAATTGGGC10341
AACCATCAGTAGTAATTGAGTCCTCATTTTATGCTAAATGTTATGCCTAACTCTTTGGGA10401
GTTACAAAGGAAATAGCAATTATGGCTTTTGCCCTCTAGGAGATA~AGGACAAATACAGG10461
AAAATACAGCAACCCAAACTGACAATACTCTATACAAGAACATAATCACTAAGCAGGAGT10521
CACAGCCAf,ACAACCAAGATGCATAGTATCCAAAGTGCAGCTG 10564
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 453 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
Met Ser Trp Ser Leu His Pro Arg Asn Leu Ile Leu Tyr Phe Tyr Ala
I 5 10 15
Leu Leu Phe Leu Ser Ser Thr Cys Yal Ala Tyr Val Ala Thr Arg Asp
20 25 30
Asn Cys Cys Ile Leu Asp Glu Arg Phe Gly Ser Tyr Cys Pro Thr Thr
35 40 45
Cys Gly Ile Ala Asp Phe Leu Ser Thr Tyr Gln Thr Lys Val Asp Lys
50 55 60
Asp Leu Gln Ser Leu Glu Asp Ile Leu His Gln Val Glu Asn Lys Thr
65 70 75 80
Ser Glu Val Lys Gln Leu Ile Lys Ala Ile Gln Leu Thr Tyr Asn Pro
85 90 95
CA 02309891 2000-06-19
68
Asp Glu Ser.Ser Lys Pro Asn Met Ile Asp Ala Ala~ Thr Leu Lys Ser
100 105 110
Arg Ile Met Leu Glu Glu Ile Met Lys Tyr Glu Ala Ser Ile Leu Thr
115 v 120 125
His Asp Ser Ser Ile Arg Tyr Leu Gln Glu Ile Tyr Asn Ser Asn Asn
130 135 140
Gln Lys I1e Val Asn Leu Lys Glu Lys Val Ala Gln Leu Glu Ala Gln
145 150 155 160
Cys Gln Glu Pro Cys Lys Asp Thr Val Gln Ile His Asp Ile Thr Gly
165 170 175
Lys Asp Cys Gln Asp Ile Ala Asn Lys Gly Ala Lys Gln Ser Gly Leu
180 185 190
Tyr Phe Ile Lys Pro Leu Lys Ala Asn Gln Gln Phe Leu Yal Tyr Cys
195 200 205
Glu Ile Asp Gly Ser Gly Asn Gly Trp Thr Val Phe Gln Lys Arg Leu
210 215 220
Asp Gly Ser Val Asp Phe Lys Lys Asn Trp Ile Gln Tyr Lys Glu Gly
225 230 235 240
Phe Gly His Leu Ser Pro Thr Gly Thr Thr Glu Phe Trp Leu Gly Asn
245 250 255
Glu Lys Ile His Leu Ile Ser Thr Gln Ser Ala Ile Pro Tyr Ala Leu
260 265 270
Arg Yal Glu Leu Glu Asp Trp Asn Gly Arg Thr Ser Thr Ala Asp Tyr
275 280 285
Ala Met Phe Lys Yal Gly Pro Glu Ala Asp Lys Tyr Arg Leu Thr Tyr
290 295 300
Ala Tyr Phe Ala Gly Gly Asp Ala Gly Asp Ala Phe Asp Gly Phe Asp
305 310 315 320
Phe Gly Asp Asp Pro Ser Asp Lys Phe Phe Thr Ser His Asn Gly Met
325 330 335
Gln Phe Ser Thr Trp Asp Asn Asp Asn Asp Lys Phe Glu Gly Asn Cys
340 345 350
CA 02309891 2000-06-19
69
Ala Glu Gln Asp Gly Ser Gly Trp Trp Met Asn Lys Cys His Ala Gly
355 360 365
His Leu Asn Gly 11a1 Tyr Tyr Gln Gly Gly Thr Tyr Ser Lys Ala Ser
370 375 380
Thr Pro Asn Gly Tyr Asp Asn Gly Ile Ile Trp Ala Thr Trp Lys Thr
385 390 395 400
Arg Trp Tyr Ser Met Lys Lys Thr Thr Met Lys Ile Ile Pro Phe Asn
405 . 410 415
Arg Leu Thr Ile Gly Glu Gly Gln Gln His His Leu Gly G?y Ala Lys
420 425 430
Gln Ilal Arg Pro Glu His Pro Ala Glu Thr Glu Tyr Asp Ser Leu Tyr
435 440 445
Pro Glu Asp Asp Leu
450
(2) INFORMATION FOR SEQ ID N0:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10807 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ovine beta-lactoglobulin
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:7:
ACGCGTGTCG ACCTGCAGGT CAACGGATCT CTGTGTCTGT TTTCATGTTA GTACCACACT 60
GTTTTGGTGG CTGTAGCTTT CAGCTACAGT CTGAAGTCAT AAAGCCTGGT ACCTCCAGCT 120
CTGTTCTCTC TCAAGATTGT GTTCTGCTGT TTGGGTCTTT AGTGTCTCCA CACAATTTTT 180
AGAATTGTTT GTTCTAGTTC TGTGAAAAAT GATGCTGGTA TTTTGATAAG GATTGCATTG 240
AATCTGTAAA GCTACAGATA TAGTCATTGG GTAGTACAGT CACTTTAACA ATATTAACTC 300
CA 02309891 2000-06-19
TTCACATCTG TGAGCATGAT ATATTTTCCC CCTCTATATC ATCTTCfIATT CCTCCTATCA 360
GTTTCTTTCA TTGCAGTTTT CTGAGTACAG GTCTTACACC TCCTTGGTTA GAGTCATTCC 420
TCAGTATTTT ATTCCTTTGA TACAATTGTG AATGAGGTAA TTTTCTTAGT TTCTCTTTCT 480
GATAGCTCAT TGTTAGTGTA TATATAGAAA AGCAACAGAT TTC'.'ATGTAT TAATTTTGTA 540
TCCTGCAACA GATTTCTATG TATTAATTTT GTATCCTGCT ACTTTACGGA ATTCACTTAT 600
TAGCTTTTTG GTGACATCTT GAGGATTTTC TGAAGAAAAT GGCATGGTAT GGTAGGACAA 660
GGTGTCATGT CATCTGCAAA CAGTGGCAGT TTTCCTTCTT CCCTTCCAAC CTGGATTTCT 720
TTGATTTCTT TCTGTCTGAG TACGACTAGG ATTCCCAATA CTATACCGAA TAAAAGTGGC 780
AAGAGTGGAC ATCCTTGTCT TATTTTTCTG ACCTTAGAGG AAATGCTTTC AGTTTTTCAC 840
CATTAATTAT AATGTTTACT GTGGGCTTGT CATATGTGGC CTTCATTATA TGGAGGTCTA 900
TTCCCTCTAT ACCCACCTTG TTGAGAGTTT TTATCATAAA AGTATGTTGA ATTTTGTCAA 960
AAGTTTTTCC TGCATCTATT GAGATGATTT TTACTCTTCA ATTCATTAAT GATTTTTATT 1020
CTTCATTTTG TTAATGATTT CCATTCTTCA ATTTGTTAAC GTGGTATATC ACATTGATTG 1080
ATTTGTGGAT ACCTTTGTAT CCCTGGGATA AACCTCACTT GATCATGAGC TTTCAATGTA 1140
TTTTTGAATT CACTTTGCTA ATATTCTGTT GGGTATTTTT GCATCTCTAT TCATCAATGA 1200
TATTGGCCTA AGAAAGGTTT TGTCTGGTTT TAGTATCAGG GTGATGCTGG CCTCATAGAG 1260
AGAGTTTAGA AGCATTTCCT CCTCTTTGAT TTTTCGGAAT AGTTTGAGTA GGATAGGTAT 1320
TAACTCTTCT TTAAATGTTT GGGGACTTCC CTGGTGAGCC GGTGGTTGAG AATCCGCCTC 1380
AGGGATGTGG GTTTGATCCC TGGTCAGGGA ACCATTAATA AGATCCCACA TGCTGCAGGC 1440
AACAAGCCCC CAAGCTGCAA CCACTGAGCT GCAACCGCTG CAGTGCCCAC AGGCCACGAC 1500
CAGAGAAAGC CCACATACAG CAGGGAAGAC CCAGCACAAC CGGAAAAAGG AGTTTGGTGG 1560
AATACAGCTG TGAAGCCGTC TGGTCCTGGA CTCCTGCTTG AGGGAATTTT TTAAAAATTA 1620
TTGATTCAAT TTCATTACTG GTAACTGGTC TGTTCATATT TTCTATTTCT TCCGGGTTCA 1680
GTCTTGGGAG ATTGTACATG CCTAGGAATG TGTCCGTTTC TTCTAGGTTG TCCATTTTAT 1740
CA 02309891 2000-06-19
71
TGGACATGCA TGGGAGCACA CAGCACCGAC CAGCGAGACT CATGCTGGCT TCCTGGGGCC 1800
AGGCTGGGGC CCCAAGCAGC ATGGCATCCT AGAGTGTGTG AAAGCCCACT GACCCTGCCC 1860
AGCCCCACAA TTTCATTCTG AGAAGTGATT CCTTGCTTCT GCACTTACAG GCCCAGGATC 1920
TGACCTGCTT CTGAGGAGCA GGGGTTTTGG CAGGACGGGG AGATGCTGAG AGCCGACGGG 1980
GGTCCAGGTC CCCTCCCAGG CCCCCCTGTC TGGGGCAGCC CTTGGGAAAG ATTGCCCCAG 2040
TCTCCCTCCT ACAGTGGTCA GTCCCAGCTG CCCCAGGCCA GAGCTGCTTT ATTTCCGTCT 2100
CTCTCTCTGG ATGGTATTCT CTGGAAGCTG AAGGTTCCTG AAGTTP.TGAA TAGCTTTGCC 2160
CTGAAGGGCA TGGTTTGTGG TCACGGTTCA CAGGAACTTG GGAGACCCTG CAGCTCAGAC 2220
GTCCCGAGAT TGGTGGCACC CAGATTTCCT AAGCTCGCTG GGGAACAGGG CGCTTGTTTC 2280
TCCCTGGCTG ACCTCCCTCC TCCCTGCATC ACCCAGTTCT GAAAGCAGAG CGGTGCTGGG 2340
GTCACAGCCT CTCGCATCTA ACGCCGGTGT CCAAACCACC CGTGCTGGTG TTCGGGGGGC 2400
TACCTATGGG GAAGGGCTTC TCACTGCAGT GGTGCCCCCC GTCCCCTCTG AGATCAGAAG 2460
TCCCAGTCCG GACGTCAAAC AGGCCGAGCT CCCTCCAGAG GCTCCAGGGA GGGATCCTTG 2520
CCCCCCCGCT GCTGCCTCCA GCTCCTGGTG CCGCACCCTT GAGCCTGATC TTGTAGACGC 2580
CTCAGTCTAG TCTCTGCCTC CGTGiTCACA CGCCTTCTCC CCATGTCCCC TCCGTGTCCC 2640
CGTTTTCTCT CACAAGGACA CCGGACATTA GATTAGCCCC TGTTCCAGCC TCACCTGAAC 2700
AGCTCACATC TGTAAAGACC TAGATTCCAA ACAAGATTCC AACCTGAAGT TCCCGGTGGA 2760
TGTGAGTTCT GGGGCGACAT CCTTCAACCC CATCACAGCT TGCAGTTCAT CGCAAAACAT 2820
GGAACCTGGG GTTTATCGTA AAACCCAGGT TCTTCATGAA ACACTGAGCT TCGAGGCTTG 2880
TTGCAAGAAT TAAAGGTGCT AATACAGATC AGGGCAAGGA CTGAAGCTGG CTAAGCCTCC 2940
TCTTTCCATC ACAGGAAAGG GGGGCCTGGG GGCGGCTGGA GGTCTGCTCC CGTGAGTGAG 3000
CTCTTTCCTG CTACAGTCAC CAACAGTCTC TCTGGGAAGG AAACCAGAGG CCAGAGAGCA 3060
AGCCGGAGCT AGTTTAGGAG ACCCCTGAAC CTCCACCCAA GATGCTGACC AGCCAGCGGG 3120
CA 02309891 2000-06-19
72
CCCCCTGGAAAGACCCTACAGTTCAGGGGGAAGAGGGGCTGACCCGCCAGGTCCCTGCT 3180
G
ATCAGGAGACATCCCCGCTATCAGGAGATTCCCCCACCTTGCTCCCGTTCCCCTATCCCA 3240
ATACGCCCACCCCACCCCTGTGATGAGCAGTTTAGTCACTTAGAATGTCAACTGAAGGCT 3300
TTTGCATCCCCTTTGCCAGAGGCACAAGGCACCCACAGCCTGCTGGGTACCGACGCCCAT 3360
GTGGATTCAGCCAGGAGGCCTGTCCTGCACCCTCCCTGCTCGGGCCCCCTCTGTGCTCAG 3420
CAACACACCCAGCACCAGCATTCCCGCTGCTCCTGAGGTCTGCAGGCAGCTCGCTGTAGC 3480
CTGAGCGGTGTGGAGGGAAGTGTCCTGG6AGATTTAAAATGTGAGAGGCGGGAGGTGGGA 3540
GGTTGGGCCCTGTGGGCCTGCCCATCCCACGTGCCTGCATTAGCCCCAGTGCTGCTCAGC 3600
CGTGCCCCCGCCGCAGGGGTCAGGTCACTTTCCCGTCCTGGGGTTATTATGACTCTTGTC 3660
ATTGCCATTGCCATTTTTGCTACCCTAACTGGGCAGCAGGTGCTTGCAGAGCCCTCGATA 3720
CCGACCAGGTCCTCCCTCGGAGCTCGACCTGAACCCCATGTCACCCTTGCCCCAGCCTGC 3780
AGAGGGTGGGTGACTGCAGAGATCCCTTCACCCAAGGCCACGGTCACATGGTTTGGAGGA 3840
GCTGGTGCCCAAGGCAGAGGCCACCCTCCAGGACACACCTGTCCCCAGTGCTGGCTCTGA 3900
CCTGTCCTTGTCTAAGAGGCTGACCCCGGAAGTGTTCCTGGCACTGGCAGCCAGCCTGGA 3960
CCCAGAGTCCAGACACCCACCTGTGCCCCCGCTTCTGGGGTCTACCAGGAACCGTCTAGG 4020
CCCAGAGGGGACTTCCTGCTTGGCCTTGGATGGAAGAAGGCCTCCTATTGTCCTCGTAGA 4080
GGAAGCCACCCCGGGGCCTGAGGATGAGCCAAGTGGGATTCCGGGAACCGCGTGGCTGGG 4140
GGCCCAGCCCGGGCTGGCTGGCCTGCATGCCTCCTGTATAAGGCCCCAAGCCTGCTGTCT 4200
CAGCCCTCCACTCCCTGCAGAGCTCAGAAGCACGACCCCAGGGATATCCCTGCAGCCATG 4260
AAGTGCCTCCTGCTTGCCCTGGGCCTGGCCCTCGCCTGTGGCGTCCAGGCCATCATCGTC 4320.
ACCCAGACCATGAAAGGCCTGGACATCCAGAAGGTTCGAGGGTTGGCCGGGTGGG~GAGT 4380
TGCAGGGCGGGCAGGGGAGCTGGGCCTCAGAGAGCCAAGAGAGGCTGTGACGTTGGGTTC 4440
CCATCAGTCAGCTAGGGCCACCTGACAAATCCCCGCTGGGGCAGCTTCAACCAGGCGTTC 4500
ACTGTCTTGCATTCTGGAGGCTGGAAGCCCAAGATCCAGGTGTTGGCAGGGCTGGCTTCT 4560
CA 02309891 2000-06-19
73
CCTGCGGCCG CTCTCTGGGG AGCAGACGGC CGTCTTCTCC AGTCCTCTGC GCGCCCTGAT 4620
TTCCTCTTCC TGTGAGGCCA CCAGGCCTGC TGGAAACACG CCTGCCTGCG CAGCTTCACA 4680
CGACCTTTGT CATCTCTTTA AAGGCCATGT CTCCAGAGTC ATGTGTTGAA GTTCTGGGGG 4740
TTAGTGGGAC ACAGTTCAGC CCCTAAAAGA GTCTCTCTGC CCCTCAAATT TTCCCCACCT 4800
CCAGCCATGT CTCCCCAAGA TCCAAATGTT GCTACATGTG GGGGGGCTCA TCTGGGTCCC 4860
TCTTTGGGTT CAGTGTGAGT CTGGGGAGAG CATTCCCCAG GGTGCAGAGT TGGGGGGAGT 4920
ATCTCAGGGC TGCCCAGGCC GGGGTGGGAC AGAGAGCCCA CTGTGGGGCT GGGGGCCCCT 4980
TCCCACCCCC AGAGTGCAAC TCAAGGTCCC TCTCCAGGTG GCGGGGACTT GGCACTCCTT 5040
GGCTATGGCG GCCAGCGACA TCTCCCTGCT GGATGCCCAG AGTGCCCCCC TGAGAGTGTA 5100
CGTGGAGGAG CTGAAGCCCA CCCCCGAGGG CAACCTGGAG ATCCTGCTGC AGAAATGGTG 5160
GGCGTCTCTC CCCAACATGG AACCCCCACT CCCCAGGGCT GTGGACCCCC CGGGGGGTGG 5220
GGTGCAGGAG GGACCAGGGC CCCAGGGCTG GGGAAGAGGG CTCAGAGTTT ACTGGTACCC 5280
GGCGCTCCAC CCAAGGCTGC CCACCCAGGG CTTTTTTTTT TTTTAAACTT TTATTAATTT 5340
GATGCTTCAG AACATCATCA AACAAATGAA CATAAAACAT TCATTTTTGT TTACTTGGAA 5400
GGGGAGATAA AATCCTCTGA AGTGGAAATG CATAGCAAAG ATACATACAA TGAGGCAGGT 5460
ATTCTGAATT CCCTGTTAGT CTGAGGATTA CAAGTGTATT TGAGCAACAG AGAGACATTT 552
TCATCATTTC TAGTCTGAAC ACCTCAGTAT CTAAAATGAA CAAGAAGTCC TGGAAACGAA 5580
GCAGTGTGGG GATAGGCCCG TGTGAAGGCT GCTGGGAGGC AGCAGACCTG GGTCTTCGGG 5640
CTCAAGCAGT TCCCGCTACC AGCCCTGTCC ACCTCAGACG GGGGTCAGGG TGCAGGAGAG 5700
AGCTGGATGG GTGTGGGGGC AGAGATGGGG ACCTGAACCC CAGGGCTGCC TTTTGGGGGT 5760
GCCTGTGGTC AAGGCTCTCC CTGACCTTTT CTCTCTGGCT TCATCTGACT TCTCCTGGCC 5820
CATCCACCCG GTCCCCTGTG GCCTGAGGTG ACAGTGAGTG CGCCGAGGCT AGTTGGCCAG 5880
CTGGCTCCTA TGCCCATGCC ACCCCCCTCC AGCCCTCCTG GGCCAGCTTC TGCCCCTGGC 5940
CA 02309891 2000-06-19
74
GCTCAGTTCATCCTGATGAAAATGGTCCATGCCAATGGCTCAGAAAGCAGCTGTCTTTCA 6000
GGGAGAACGGCGAGTGTGCTCAGAAGAAGATTATTGCAGAAAAAACCAAGATCCCTGCGG 6060
TGTTCAAGATCGATGGTGAGTCCGGGTCCCTGGGGGACACCCACCACCCCCGCCCCCGGG 6120
GACTGTGGACAGGTTCAGGGGGCTGGCGTCGGGCCCTGGGATGCTAAGGGACTGGTGGTG 6180
ATGAAGACACTGCCTTGACACCTGCTTCACTTGCCTCCCCTGCCACCTGCCCGGGGCCTT 6240
GGGGCGGTGGCCATGGGCAGGTCCCGGCTGGCGGGCTAACCCACCAGGGTGACACCCGAG 6300
CTCTCTTTGCTGGGGGGCGGGCGGTGCTCTGGGCCCTCAGGCTGAGCTCAGGAGGTACCT 6360
GTGCCCTCCCAGGGGTAACCGAGAGCCGTTGCCCACTCCAGGGGCCCAGGTGCCCCACGA 6420
CCCCAGCCCGCTCCACAGCTCCTTCATCTCCTGGAGACAAACTCTGTCCGCCCTCGCTCA 6480
TTCACTTGTTCGTCCTAAATCCGAGATGATAAAGCTTCGAGGGGGGGTTGGGGTTCCATC 6540
AGGGCTGCCCTTCCGCCGGGCAGCCTGGGCCACATCTGCCCTTGGCCCCCTCAGGACTCA 6600
CTCTGACTGGAGGCCCTGCACTGACTGACGCCAGGGTGCCCAGCCCAGGGTCTCTGGCGC 6660
CATCCAGCTGCACTGGGTTTGGGTGCTGGTCCTGCCCCCAAGCTGCCCGGACACCACAGG 6720
CAGCCGGGGCTGCCCACTGGCCTCGGTCAGGGTGAGCCCCAGCTGCCCCCGCTCAGGGCT 6780
TGCCCCGACAATGACCCCATCCTCAGGACGCACCCCCCTTCCCTTGCTGGGCAGTGTCCA 6840
GCCCCACCCGAGATCGGGGGAAGCCCTATTTCTTGACAACTCCAGTCCCTGGGGGAGGGG 6900
GCCTCAGACTGAGTGGTGAGTGTTCCCAAGTCCAGGAGGTGGTGGAGGGTCCTGGCGGAT 6960
CCAGAGTTGACAGTGAGGGCTTCCTGGGCCCCATGCGCCTGGCAGTGGCAGCAGGGAAGA 7020
GGAAGCACCATTTCAGGGGTGGGGGATGCCAGAGGCGCTCCCCACCCCGTCTTCGCCGGG 7080
TGGTGACCCCGGGGGAGCCCCGCTGGTCGTGGAGGGTGCTGGGGGCTGACTAGCAACCCC 7140
TCCCCCCCCGTTGGAACTCACTTTTCTCCCGTCTTGACCGCGTCCAGCCTTGAATGAGAA 7200
CAAAGTCCTTGTGCTGGACACCGACTACAAAAAGTACCTGCTCTTCTGCATGGAAAACAG 7260
TGCTGAGCCCGAGCAAAGCCTGGCCTGCCAGTGCCTGGGTGGGTGCCAACCCTGGCTGCC 7320
CAGGGAGACCAGCTGCGTGGTCCTTGCTGCAACAGGGGGTGGGGGGTGGGAGCTTGATCC 7380
CA 02309891 2000-06-19
CCAGGAGGAG GAGGGGTGGG GGGTCCCTGA GTCCCGCCAG GAGAGAGTGG TCGCATACCG 7440
GGAGCCAGTC TGCTGTGGGC CTGTGGGTGG CTGGGGACGG GGGCCAGACA CACAGGCCGG 7500
GAGACGGGTG GGCTGCAGAA CTGTGACTGG TGTGACCGTC GCGATGGGGC CGGTGGTCAC 7560
TGAATCTAAC AGCCTTTGTT ACCGGGGAGT TTCAATTATT TCCCAAAATA AGAACTCAGG 7620
TACAAAGCCA TCTTTCAACT ATCACATCCT GAAAACAAAT GGCAGGTGAC ATTTTCTGTG 7680
CCGTAGCAGT CCCACTGGGC ATTTTCAGGG CCCCTGTGCC AGGGGGGCGC GGGCATCGGC 7740
GAGTGGAGGC TCCTGGCTGT GTCAGCCGGC CCAGGGGGAG GAAGGGACCC GGACAGCCAG 7800
AGGTGGGGGG CAGGCTTTCC CCCTGTGACC TGCAGACCCA CTGCACTGCC CTGGGAGGAA 7860
GGGAGGGGAA CTAGGCCAAG GGGGAAGGGC AGGTGCTCTG GAGGGCAAGG GCAGACCTGC 7920
AGACCACCCT GGGGAGCAGG GACTGACCCC CGTCCCTGCC CCATAGTCAG GACCCCGGAG 7980
GTGGACAACG AGGCCCTGGA GAAATTCGAC AAAGCCCTCA AGGCCCTGCC CATGCACATC 8040
CGGCTTGCCT TCAACCCGAC CCAGCTGGAG GGTGAGCACC CAGGCCCCGC CCTTCCCCAG 8100
GGCAGGAGCC ACCCGGCCCC GGGACGACCT CCTCCCATGG TGACCCCCAG CTCCCCAGGC 8160
CTCCCAGGAG GAAGGGGTGG GGTGCAGCAC CCCGTGGGGG CCCCCTCCCC ACCCCCTGCC 8220
AGGCCTCTCT TCCCGAGGTG TCCAGTCCCA TCCTGACCCC CCCATGACTC TCCCTCCCCC 8280
ACAGGGCAGT GCCACGTCTA GGTGAGCCCC TGCCGGTGCC TCTGGGGTAA GCTGCCTGCC 8340
CTGCCCCACG TCCTGGGCAC ACACATGGGG TAGGGGGTCT TGGTGGGGCC TGGGACCCCA 8400
CATCAGGCCC TGGGG~CCCC CCTGTGAGAA TGGCTGGAAG CTGGGGTCCC TCCTGGCGAC 8460
TGCAGAGCTG GCTGGCCGCG TGCCACTCTT GTGGGTGACC TGTGTCCTGG CCTCACACAC 8520
TGACCTCCTC CAGCTCCTTC CAGCAGAGCT AAGGCTAAGT GAGCCAGAAT GGTACCTAAG 8580
GGGAGGCTAG CGGTCCTTCT CCCGAGGAGG GGCTGTCCTG GAACCACCAG CCATGGAGAG 8640
GCTGGCAAGG GTCTGGCAGG TGCCCCAGGA ATCACAGGGG GGCCCCATGT CCATTTCAGG 8700
GCCCGGGAGC CTTGGACTCC TCTGGGGACA GACGACGTCA CCACCGCCCC CCCCCCATCA 8760
CA 02309891 2000-06-19
76
GGGGGACTAG_~AGGGACCAG GACTGCAGTCACCCTTCCTGGGACCCAGGCCCCTCCAGGC8820
CCCTCCTGGGGCTCCTGCTCTGGGCAGCTTCTCCTTCACCAATAAAGGCATAAACCTGTG8880
CTCTCCCTTCTGAGTCTTTGCTGGACGACGGGCAGGGGGTGGAGAAGTGGTGGGGAGGGA8940
GTCTGGCTCAGAGGATGACAGCGGGGCTGGGATCCAGGGCGTCTGCATCACAGTCTTGTG9000
ACAACTGGGGGCCCACACACATCACTGCGGCTCTTTGAAACTTTCAGGAACCAGGGAGGG9060
ACTCGGCAGAGA~ATCTGCCAGTTCACTTGGAGTGTTCAGTCAACACCCAAACT~GACAA9120
AGGACAGAAAGTGGAAAATGGCTGTCTCTTAGTCTAATAAATATTGATATGAAACTCAAG9180
TTGCTCATGGATCAATATGCCTTTATGATCCAGCCAGCCACTACTGTCGTATCAACTCAT9240
GTACCCAAACGCA~TGATCTGTCTGGCTAATGATGAGAGATTCCCAGTAGAGAGCTGGCA9300
AGAGGTCACAGTGAGAACTGTCTGCACACACAGCAGAGTCCACCAGTCATCCTAAGGAGA9360
TCAGTCCTGGTGTTCATTGGAGGACTGATGTTGAAGCTGAAACTCCAATGCTTTGGCCAC9420
CTGATGTGAAGAGCTGACTCATTTGAAAA~ACCCTGATGCTGGGAAAGATTGAGGGCAGG9480
AGGAGAAGGGGACGACAGAGGATGAGATGGTTGGATGGCATCACCAACACAATGGACATG9540
GGTTTGGGTGGACTCCAGGAGTTGGTGATGGACAGGGAGGCCTGGCGTGCTACGGAAGCG9600
GTTTATGGGGTCACAAAGACTGAGTGACTGAACTGAGCTGAACTGAATGGAAATGAGGTA9660
TACAGCAAAGTGGGGATTTTTTAGATAATAAGAATATACACATAACATAGTGTATACTCA9720
TATTTTTATGCATACCTGAATGCTCAGTCACTCAGTCGTATCTGACTCTGTGACCTATGG9780
ACCGTAGCCTTCCAGGTTTCTTCTGTCCACAGAATTCTCCAAGGCAAGAATACTGGAGTG9840
GGTAGCCATTTCCTCCTCCAGGGGATCCTCCCGACCCAGGGATTGAACCGGCATCTCCTG9900
TATTGGCAGGTGGATTCTTTACCACTGTGCCACCAGGGAAGCCCGTGTTACTCTCTATGT9960.
CCCACTTAATTACCAAAGCTGCTCCAAGAAAAAGCCCCTGTGCCCTCTGAGCTTCCCGGC10020
CTGCAGAGGGTGGTGGGGGTAGACTGTGACCTCGGAACACCCTCCCGCTTCAGGACTCCC10080
GGGCCACGTGACCCACAGTCCTGCAGACAGCCGGGTAGCTCTGCTCTTCAAGGCTCATTA10140
TCTTTAAAAAAAACTGAGGTCTATTTTGTGACTTCGCTGCCGTAACTTCTGAACATCCAG10200
CA 02309891 2000-06-19
77
TGCGATGGAC AGGACCTCCT CCCCAGGCCT CAGGGGCTTC AGGGAGCCAG CCTTCACCTA 10260
TGAGTCACCA GACACTCGGG GGTGGCCCCG CCTTCAGGGT GCTCACAGTC TTCCCATCGT 10320
CCTGATCAAA GAGCAAGACC AATGACTTCT TAGGAGCAAG CAGACACCCA CAGGACACTG 10380
AGGTTCACCA GAGCTGAGCT GTCCTTTTGA ACCTAAAGAC ACACAGCTCT CGAAGGTTTT 10440
CTCTTTAATC TGGATTTAAG GCCTACTTGC CCCTCAAGAG GGAAGACAGT CCTGCATGTC 10500
CCCAGGACAG CCACTCGGTG GCATCCGAGG CCACTTAGTA TTATCTGACC GCACCCTGGA 10560
ATTAATCGGT CCAAACTGGA CAAAAACCTT GGTGGGAAGT TTCATCCCAG AGGCCTCAAC 10620
CATCCTGCTT TGACCACCCT GCATCTTTTT TTCTTTTATG TGTATGCATG TATATATATA 10680
TATATATTTT TTTTTTTTTC ATTTTTTGGC TGTGCTGGCT GTTCGTTGCA GTTCGGTGCG 10740
CAGGCTTCTC TCTAGTTTCT CTCTAGTCTT CTCTTATCAC AGAGCAGTCT CTAGACGATC 10800
GACGCGT 10807
(2) INFORMATION FOR SEQ ID N0:8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 47 base pairs
(B) TYPE: nucleic acid
(C) STRANDEONESS: single
(D) TOPOLOGY: linear
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:8:
AATTCCGATC GACGCGTCGA CGATATACTC TAGACGATCG ACGCGTA 47
(2) INFORMATION FOR SEQ ID N0:9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
CA 02309891 2000-06-19
(vii) IMMEDIATE SOURCE:
(B) CLONE: BLGAMP3
78
(xi)~SEQUENCE DESCRIPTION: SEQ ID N0:9:
TGGATCCCCT GCCGGTGCCT CTGG 24
(2) INFORMATION FOR SEQ ID N0:10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: BLGAMP4
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:10:
AACGCGTCAT CCTCTGTGAG CCAG 24
(2) INFORMATION FOR SEQ IO N0:11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 base pairs
(B) TYPE: nucleic acid
(C) STRANOEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLUNE: ZC6B39
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:11:
ACTACGTAGT 10
(2) INFORMATION FOR SEQ IO N0:12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 42 base pairs
(B) TYPE: nucleic acid
CA 02309891 2000-06-19
(C) STRANOEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6632
79
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:12:
CGACGCGGAT CCTACGTAi,C TGCAGCCATG TTTTCCATGA GG 42
(2) INFORMATION FOR SEQ ID N0:13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6627
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:13:
AGGGCTTCGG CAAGCTTCAG G 21
(2) INFORMATION FOR SEQ ID N0:14:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6521
(xi) SEQUENCE DESCRIPTION: SEQ IO N0:14:
GCCAAAGACT TACTTCCCTC TAGA 24
CA 02309891 2000-06-19
(2) INFORMATION FOR SEQ ID N0:15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 30 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6520
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:15:
GCATGAACf;T CGCGTGGTGG TTGTGCTACC 30
(2) INFORMATION FOR SEQ ID N0:16:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 30 ba:,e pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(8) CLONE: ZC6519
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:16:
ACCACGCGAC GTTCATGCTC TAAAACCGTT 30
(2) INFORMATION FOR SEQ ID N0:17:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 36 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6518
CA 02309891 2000-06-19
81
(xi) SEQUENCE DESCQIPTION: SEQ ID N0:17:
GCTGCGGGAT CCTACGTACT AGGGGGACAG GGAAGG 36
(2) INFORMATION FOR SEQ ID N0:18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 45 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6629
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:18:
CGACGCGAAT TCTACGTACC TGCAGCCATG AAAAGGATGG TTTCT 45
(2) INFORMATION FOR SEQ ID N0:19:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 45 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(fl) TOPOLOGY: linear
(vii) IMMEDIATE aOURCE:
(8) CLONE: ZC6630
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:19:
CGACGCGAAT TCTACGTACC TGCAGCCATG AAACATCTAT TATTG 45
(2) INFORMATION FOR SEQ ID N0:20:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
CA 02309891 2000-06-19
(vi i ) IdhIEDIATE SOURCE:
(B) CLONE: ZC6625
82
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:20:
GTGAGATTTT CAGATCTTGT C 21
(2) INFORMATION FOR SEQ ID N0:21:
(i) SEQUENCE i,HARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANOEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6626
xi) SEQUENCE DESCpIPTION: SEQ ID N0:21:
:~.:...: iTACT GTGGCCTACC A 21
(2) INFORMATION FOR SEQ ID N0:22:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 33 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(6) CLONE: ZC6624
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:22:
GCTGCGGAAT TCTACGTACT ATTGCTGTGG GAA 33
(2) INFORMATION FOR SEQ ID N0:23:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 45 base pairs
(B) TYPE: nucleic acid
CA 02309891 2000-06-19
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6514
83
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:23:
CGACGCGGAT CCTACGTACC TGCAGCCATG AGTTGGTCCT TGCAC 45
(2) INFORMATION FOR SEQ ID N0:24:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6517
(xi) SEQUENCE DESCRIPTION: SEQ IO N0:24:
GTCTCTGGTA GCAACATACT A 2I
(2) INFORMATION FOR SEQ ID N0:25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6516
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:25:
GGGTTTCTAG CCCTACTAGT AG 22
(2) INFORMATION FOR SEQ ID N0:26:
CA 02309891 2000-06-19
84
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANOEDNESS: single
(D) TOPOLOGY: linear
(vii) IMMEDIATE SOURCE:
(B) CLONE: ZC6515
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:26:
GGGTTTCTA6 CCCTACTAGT AG 22
(2) INFORMATION FOR SEQ ID N0:27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 47 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:27:
AAGCTACGCG TCGATCGTCT AGAGTATATC GTCGACGCGT CGATCGG