Note: Descriptions are shown in the official language in which they were submitted.
A
75181-43D
CA 02311059 2000-07-07
This is a Division of our co-pending Canadian Patent
Application No. 2,307,122 dated 27 October 1998 and entitled:
Device and Methods for Determination of Analyte in a Solution.
BACKGROUND OF THE INVENTION
This invention relates to the field of assay
technology, and in particular embodiments, to devices and
methods for quantification of analytes, e. g., biological
material, in a sample.
Many industries need to detect and qualify the
concentration and level of biological material or other analyte
in a sample. For example, the determination of bacterial
concentration in food and water is an essential part of food
and water quality testing. EPA regulations require that no
coliform such as Escherichia coli can be present in potable
water. The "presence/absence" format of a testing medium, such
as Colilert R chemical mixture (IDEXX Laboratories, ME) which
is used as a testing medium for Escherichia coli and all coli-
form bacteria, is very useful in making this determination.
Colilert R chemical mixture is based on the Defined Substrate
Technology described in Edberg, "Method and Medium for Use in
Detecting Target Microbes In Situ in a Specimen Sample of a
Possibly Contaminated Material", U. S. Patent Nos. 4,925,789
and 5,492,933.
However, there are areas where the quantification,
not just the detection, of bacterial concentration is important.
Examples of such areas include waste water, incoming water in
water purification systems, surface water, and food testing.
For example, numerous restaurant chains will only accept raw
ground beef or poultry that contains less than a certain
concentration of bacterial contamination. Therefore, food
processing plants must carry out the necessary microbiological
tests to determine the bacterial concentration of these food
items before they can be released to customers.
The classical methods of quantification of biological
material are the standard plate count method or the multiple
tube fermentation (MTF) method. A quantity of sample being
1
CA 02311059 2000-07-07
75181-43D
tested for microbial contamination is first dispensed in a
Petri dish. Then 15 ml of the appropriate media is poured
over the sample. The Petri dish is then swirled to mix the
sample in the medium and
la
CA 02311059 2000-07-07
WO 99/22017 PCT/iTS98I22628
the Petri-dish is left to solidify at room temperature for approximately 20
minutes. The medium
is then incubated at a specific temperature for a specific time, and any
resulting colonies are
counted.
The multiple tube fermentation method is described in Recles et al., "Most
Probable
Number Techniques" published in "Compendium of Methods for the Micmbiological
Examination of Foods", 3rd ed. 1992, at pages l OS-199, and in Greenberg et
al., "Standard
Methods For the Examination of Water and Wastewater" $th ed. 1992). In this
method, a
volume of sample is dispensed into several tubes representing this dilution
range. The tubes are
then incubated at the appropriate temperature so that the bacteria in each
tube are allowed to
grow. After incubation at a specific temperature for a specific time, the
number of positive tubes
is counted. The most probable number can be determined from the formula
described in Recles
et al., supra.
Water testing is mostly done by membrane filtration, where a certain volume of
water is
passed through the membrane and the membrane is incubated in a medium for a
certain period of
time. After appropriate incubation, the colonies are counted.
In many industries there is also a need to qualitatively and/or quantitatively
detect the
presence of an analyte in a liquid solution. For example, the detection of
inorganic ions may be
important in a manufacturing process using a test solution.
Heretofore, the methods and devices generally relied upon for measuring an
analyte in
2 0 solution have either required removing all of an aliquot from the test
solution or exposing a dip
stick to a test solution. Although these methods and devices can detect an
analyte in solution,
they suffer from a number of disadvantages.
The dip stick-related methods are not quantitative. For example, in the
general dip stick
embodiments there is no capability for determining or quantifying the presence
of an analyte in a
2 5 unit volume of that solution. Instead, the dip stick is simply brought
into contact with the test
solution.
Other methods require a user to manually remove an aliquot from a test
solution and
transfer it to a separate device for detection or quantification of the
analyte.
Therefore, despite the ability of these methods and devices to detect an
analyte in
3 0 solution, the methods and devices currently used have proven to have
limited accuracy; and/or to
2
CA 02311059 2000-07-07
WO 99/Z2017 PC'T/US98/22628
be costly, complicated, time consuming; and/or to have a limited range of uses
because of the
particular assay technology.
Thus, there exists a need for a simple, accurate, and inexpensive method for
the
determination of an analyte in solution without the disadvantages known in the
prior art. In
particular, there is a need for devices and methods capable of providing a
quantitative assay of
an analyte in a particular volume of a solution.
SUMMA_12Y OF INVENTION
The present invention provides devices and methods for detecting and
enumerating the
presence or absence of biological materials, analytes, and microorganisms in
sample solutions.
In one aspect, the invention provides a sterile incubation plate for
determining the
presence or amount of a biological material in a test sample. The plate is
generally described by
a flat, horizontal surface containing recessed wells. Each well is adapted to
hold an aliquot of
liquid, and has a size and shape, and is made of a material, suitable for
holding the liquid aliquot
within the well by the forces of surface tension. At least one well contains
at least one reagent
for the detection of the biological material. No positive response is
generated in the absence of
the target biological material.
In a preferred embodiment, the reagent is deposited into the wells by corona
treatment
and drying. In another embodiment, the plate may also contain a lid. The well
or wells may
2 0 contain a plurality of reagents, and different wells may contain different
reagents or different
combinations of reagents, so that numerous assays can be conducted on a single
plate. The plate
is preferably constructed of plastic, however, it may be constructed of other
hydrophobic
materials) which are suitable for conducting the assay. In a preferred
embodiment, the plates of
the present invention will be rectangular in shape, however, they may also be
circular in shape,
2 5 or of any shape. in a preferred embodiment the wells are about 0.15 inches
in diameter. In
another preferred embodiment, the wells of the plate hold a total of about 1-
milliliter of solution.
In another preferred embodiment, each well of the plate holds between 0.1 and
100 ~.1 of liquid.
The well or wells of the plate may be chamfered to aid in the removal of
excess fluid. The plate
may further comprise a handle portion so that the operator may manipulate the
plate and conduct
3 0 the assay without risk of contacting the test sample.
3
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/ZZ628
In another aspect, the invention provides a sterile incubation plate similar
to the plate
described above, and may be adapted with each of its embodiments, with the
further feature that
it comprises a lid which contains at least one protrusions) which fits) into
the well(s). In this
aspect, the reagent or combination of reagents is contained on the end of the
protrusions) such
that the reagents) will dissolve in the test sample upon closing or affixing
the lid to the plate. In
one embodiment, the protrusions may feature a cavity. The reagents) or
combination of
reagents will be dried onto the end of each protrusion or onto the surface of
the cavity. The
surface of the cavity can also be corona treated before depositing the
reagent. In this aspect as
well, a reagents) or combinations) of reagents can be dried on, or the
protrusions) corona
treated, and a combination of reagents may be utilized among multiple
protrusions so that
multiple assays can be conducted on a single plate. The lower plate portion
can be adapted to all
of the embodiments with respect to the plate described above.
In another aspect, the invention describes a device for determining the
presence or
amount of an analyte(s) or microorganisms) in a test solution. The device
features a
substantially hydrophobic support structure with at least one reagent island
immobilized on the
support structure which is capable of absorbing a predetermined volume of test
solution. The
device also comprises a means for determining the presence or amount of the
analyte or
microorganism, the means being positioned on or within the reagent island(s).
This aspect of the
invention may take the form of a plastic or polymer dip stick with one or more
reagent islands)
2 0 immobilized on it. The reagent island may be made of any absorbent
material, for example
cellulose.
The support structure may have multiple reagent islands immobilized on it. The
means
for determining the presence or amount of microorganisms or analyte(s) may
comprise a powder
which is incorporated onto or within the material from which the reagent
island is prepared. The
2 5 means may be a reagent or a combination of reagents which leads to a
production of a detectable
signal when target anaiyte(s) or microorganisms) is present. The device may
contain multiple
reagent islands which can contain different reagents or combinations of
reagents so that
numerous assays can be conducted on a single device. In a preferred
embodiment, the reagent
islands are comprised of cellulose. The support structure can be constructed
of plastic, polymer,
3 0 or any suitable materials onto which a reagent island can be mounted and
which will not
4
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/22628
interfere with the assay. In another embodiment, the device also comprises a
container for
holding the support structure before, during, and after conducting the assay.
The container will
typically comprise a test tube and may further comprise a cap for the test
tube to further protect
the device.
In another aspect, the invention provides an assay device for determining the
presence or
amount of an analyte(s) or microorganisms) in a sample which comprises a
substantially
hydrophobic solid support structure with at least one discrete reagent island
immobilized thereon
which is capable of absorbing and holding a predetermined volume of test
solution. Positioned
on or within the reagent island is a means for determining the presence or
absence of a target
anasyte or microorganism. This aspect of the device further comprises a
container which is open
at opposite ends. The container may typically take the form of a laboratory
pipette. This aspect
of the invention may comprise any of the various embodiments described with
respect to the
device described above. The reagent islands can also be arranged in zones such
that each zone
provides a separate assay. The reagent islands can be adapted to retain
aliquots of liquid by
surface tension or by absorption. The device may typically comprise an
elongated strip. The
reagent comprised in the reagent islands may be a growth medium or an
indicator growth
medium. The reagent may also be an enzyme or an enzyme substrate.
In another aspect, the invention provides an assay device for determining the
presence or
amount of an analyte(s) or microorganisms) in a sample comprising a solid
support structure
2 0 with at least one reagent island immobilized thereon. Each reagent island
is adapted to hold an
aliquot of liquid and is of a size and shape and made of a material suitable
to hold the aliquot
within the reagent island. At least one of the reagent islands contains at
least one reagent for the
detection of the analyte(s) and/or microorganisms) of interest. The device
does not provide a
positive response for the analyte(s) or microorganisms) when the analyte(s) or
2 5 microorganisms) is not present in the test sample.
In a preferred embodiment, the reagent islands of the device may take up a
preselected
total volume. In one embodiment the device may comprise a combination of
reagents located in
separate reagent islands. In another embodiment, the reagent islands are
arranged in zones,
wherein each zone provides a separate assay so that multiple assays may be
conducted on a
3 0 single device. The reagent islands may be adapted to retain aliquots of
liquid sample by surface
5
CA 02311059 2000-07-07
WO 99/22017 PCT/US98~22628
tension or by absorption. The reagent islands may be adapted to each take up
equal volumes of
liquid. Or the reagent islands may exist in subsets of islands, wherein each
subset consists of at
least one island, and each subset takes up different volumes from the other
subsets. The device
may be constructed so that the reagent islands are immobilized on more than
one surface. In
different embodiments, the device may be a plate or an elongated strip. In a
preferred
embodiment, the support structure may be constructed of a hydrophobic
material. In one
embodiment, the at least one reagent may be a growth medium. In a specific
embodiment, the
growth medium may be a indicator growth medium. In various embodiments, the at
least one
reagent may be cells of at least one bacterial strain, an enzyme, or an enzyme
substrate.
The invention also provides methods for detecting the presence or amount of
biological
material in a sample. In one aspect the method comprises the steps of i)
providing an incubation
plate with at least one well which contains at least one reagent for detecting
the biological
material, the plate comprising a generally flat horizontal surface defining
multiple recessed
wells, each well being adapted to hold an aliquot of liquid, and being of a
size and shape and
constructed of a material suitable to hold the liquid aliquot within the well
by surface tension
and wherein at least one well contains at least one reagent for the detection
of the biological
material; 2) liquefying the test sample if necessary, and distributing the
sample over the surface
of the incubation plate; 3) draining off any excess liquid; and 4) incubating
the plate until the
presence or absence of the biological material in one or more wells is
determined so that the
2 0 amount of biological materials can be determined. The distributing step
may comprise dipping
the plate into the sample, or pouring, and may optionally include swirling or
tipping.
This aspect of the invention can be adapted for all of the separate
embodiments with
respect to the plate devices discussed above.
In another aspect, the invention provides a method of detecting the presence
or amount of
2 5 a biological material in a sample, comprising the steps of 1 ) providing a
sterile incubation plate
with a lower portion which is a generally flat horizontal surface and contains
multiple recessed
wells adapted to hold an aliquot of liquid and being sized and shaped and
formed of a material
suitable to hold the liquid within the well by surface tension, and a lid with
at least one
protrusion containing at least one reagent for the detection of biological
materials, wherein the
3 0 protrusion fits into individual wells when the lid is closed on the lower
plate portion; 2)
6
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/Z2628
liquefying the test sample if necessary, and distributing the sample over the
surface of the
incubation plate; 3) draining off any excess liquid from the plate; 4) closing
the lid on the Iower
plate portion such that the reagents) on the at least one protrusion contacts
aliquots of liquid in
the wells, thereby allowing dissolution of the reagent(s); and 4) incubating
the plate until the
presence or absence of the biological material in one or more wells is
determined so that the
amount of biological materials can be determined.
This aspect of the invention can be adapted with all of the embodiments with
respect to
the methods discussed previously, and all of the embodiments with respect to
the plate and lid
device discussed previously.
1 o In another aspect, the invention provides a method for making an
incubation plate with at
Least one reagent, the method comprising the steps of 1 ) providing a plate
with a generally flat
horizontal surface with recessed wells which are sized and shaped and formed
of a material
suitable to hold aliquots of test sample within each well by surface tension;
and 2) drying at least
one reagent into the well(s).
I5 In preferred embodiments at least one well may be corona treated prior to
the drying step.
In another embodiment, the corona treated portion may consist essentially of
an inner surface of
at least one well. In a preferred embodiment the reagent may be a cellular
growth medium, or a
bacterial growth medium. The plate may be made to suit all of the embodiments
of the plates
discussed previously.
2 o In another aspect, the invention provides a method for making an
incubation plate
comprising the steps of 1 ) providing a sterile incubation plate with a lower
portion which is a
generally flat horizontal surface and contains recessed wells, each well
adapted to hold an
aliquot of liquid and being sized and shaped and formed of a material suitable
to hold the aliquot
by surface tension, and a lid with at least one protrusion which fits into the
individual wells
2 5 when the lid is closed on the lower plate portion; and 2) drying the
reagents) onto at least one
said protrusion. This aspect may contain all of the embodiments of the
invention described
previously with respect to the other methods and plate devices.
In another aspect, the invention provides a method of manufacturing a device
useful for
detecting an analyte(s) or microorganisms) in a test solution, the method
comprising the steps of
30 1) providing a material capable of absorbing a predetermined volume of
liquid per amount of
7
CA 02311059 2000-07-07
WO 99/Z2017 PCTNS98~226Z8
material; 2) preparing at least one reagent island from the material; 3)
combining the material
with a means for detecting the presence or amount of the analyte or
microorganism; and 4)
securing the reagent island to a substantially hydrophobic support structure.
In various embodiments, the preparing step may comprise preparing multiple
reagent
islands. In another embodiment, the reagent islands may be capable of
absorbing a
predetermined volume of solution.
In another aspect, the invention provides a method of detecting an analyte or
microorganism in a test sample comprising the steps of 1 ) contacting the test
sample with at least
one reagent island capable of absorbing a predetermined volume of test
solution and
immobilized on a support structure, and which comprises a means for
determining the presence
or absence of an analyte or microorganism positioned on or within the reagent
island(s); 2)
separating the test sample from the reagent islands) after the reagent
islands) has absorbed a
predetermined amount of sample; 3) subjecting the reagent islands) to reaction
parameters
which allow development of the reagent and generation of a sensible signal;
and 4) determining
the presence or amount of an analyte or microorganism.
In another aspect, the invention provides a method for detecting an analyte or
microorganism in
a test sample which comprises the steps of 1 ) selecting a test solution for
the detection of the
analyte or microorganism; 2) providing a device which comprises a
substantially hydrophobic
support structure and at least one reagent islands) immobilized on the support
structure, and
2 0 which is capable of absorbing a predetermined volume of the test solution
and comprises a
means for determining the presence or amount of the analyte(s) or
microorganism(s); 3)
contacting the device with the test solution for a time sufficient to allow
the reagent islands) to
absorb the predetermined volume; and 4) allowing the means for determining the
presence or
amount of an analyte(s) or microorganisms) to determine the presence or amount
of the
2 5 analyte(s) or microorganism. In one embodiment, the contacting step may
involve introducing
the device into the test solution and removing it from the test solution. In
another embodiment,
the providing step may comprise providing a determining means which comprises
a reagent
which produces a sensible signal that signifies the presence or amount of an
analyte(s) or
microorganism(s). In another embodiment, the allowing step may comprise
subjecting the dcvice
3 0 to reaction parameters sufficient to allow development of the reagent.
Another step may be
8
CA 02311059 2000-07-07
WO 99IZ2017 PCT/US98/22628
added to the method comprising observing the determining means, or a step of
determining the
presence or amount of an anaIyte(s) or microorganism(s), or a step of
determining the quantity of
the analyte or microorganism.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. lA is a plan view of a first embodiment of the device for the
determination of
analyte in solution of the present invention;
Fig. 1B is an enlarged cross section of Fig. lA taken at lines 1B-1B;
Fig.IC is a side view of the first embodiment of the present invention of Fig.
lA;
Fig. 2A is an enlarged view of Fig. 1 B showing a well without a chamfer; Fig.
2B is an
enlarged view of Fig. 1B showing a well with a chamfer;
Fig. 3A is a plan view of a lid for the device of the first embodiment of Fig.
1 A;
Fig. 3B is an enlarged, partial side view of the lid of Fig. 3A;
Fig. 3C is a side view of the lid of Fig. 3A;
Fig. 3D is a perspective view, partially exposed of the lid of Fig. 3A placed
over the
device of Fig. lA;
Fig. 4A is a plan view of a second embodiment of a lid having a plurality of
protrusions
on the inner surface thereof;
2 0 Fig. 4B is an enlarged section of the lid of Fig. 4A taken at lines 4B-4B;
Fig. 4C is a side view of the lid of Fig. 4A;
Fig. 4D is a partially exposed, enlarged perspective view of a portion of the
lid of Fig.
4A showing the protrusions thereof;
Fig. SA is a plan view showing the lid of Fig. 4A placed on the device of Fig.
lA;
2 5 Fig. SB is an enlarged, exposed view of Fig. SA;
Fig. SC is a side view of Fig. SA;
Fig. 6A is a plan view of a second embodiment of the device of the present
invention
having a handle;
Fig. 6B is a cross sectional view of Fig. 6A taken at lines 6B-6B;
3 0 Fig. 6C is a side view of the second embodiment of the device of Fig. 6A;
9
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/22628
Fig. 7A is a plan view of the embodiment of Fig. 6A having a lid placed
thereon;
Fig. 7B is a cross sectional view taken at lines 7B-7B of Fig. 7A;
Fig. 7C is a side view of Fig. 7A;
Fig. 8 is a plan view of a third embodiment of the present invention
employable as a
single assay dip stick device;
Fig. 9 is a side view of Fig. 8;
Fig. 10 is a fourth embodiment of the device of the present invention
employable as dip
stick assay device having a multiple reagents islands;
Fig. I 1 shows the device of Fig. 10 in which multiple assays have been
performed;
Fig. 12 is a perspective view of the fifth embodiment of the device of the
present
invention in which a dip stick assay device having two planes with multiple
reagent islands
thereon is removably insertable in a tube with a cap thereon;
Fig. 13 is a perspective view of a sixth embodiment of the device of the
present invention
in which a plurality of reagent islands are located in more than one plane on
a stick and are
removably locatable in a tube having a removable cap thereon; and
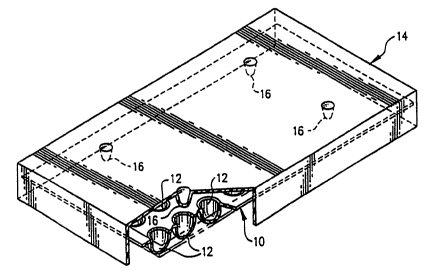
Fig. 14 is a perspective view of a seventh embodiment of the device of the
present
invention in which a laboratory pipette contains an elongate block having a
plurality of reagent
islands on one or more sides thereof.
2 0 DESCRIPTION OF THE PREFERRED EMBODIMENTS
Definitions
"Microorganism" - microorganisms means any microbe, including bacteria, fungi,
or protists.
"Bacteria" - all organisms belonging to the Kingdom Monera.
"Biological Material" - any material derived from a biological organism.
2 5 "Target Organisms" - any "Microorganim" preferably but not limited to E.
coli, yeast and/or
mold.
"Cell" - any cell found in a plant, animal, bacteria, or any other living
organism.
"Analyze" - any atom or molecule or ion which is involved in cellular
metabolism in a living
organism.
3 0 "Target Analyte" - any "Analyte", preferably but not limited to
metabolites and/or enzyme
CA 02311059 2000-07-07
WO 99/22017 PCTNS98/2262$
activity.
"Proteinaceous Material" - proteins, peptides, enzymes, or amino acids.
"Hydrophobic" - having a sufficient degree of hydrophobicity to prevent
"crosstalk" or bridging
of liquids, such as samples or reagents, or adjacent incubation cells, wells
or islands.
Structure
The present invention provides a simpler method for accurate quantification of
the
number of microorganisms in a sample, or for quantification of any other type
of analyze, such as
discrete particulate biological material within a sample. Such biological
materials include fungi
or other living organisms, as well as aggregates of proteins, such as enzymes,
or even co-factors,
using reaction mixtures well known to those in the art. The invention
generally makes use of a
novel article which is designed to take-up and hold a pre-selected volume of a
liquid sample and
to provide a reagent or reagents to such sample volume. In preferred
embodiments, the pre-
selected volume is divided into a plurality of sample aliquots. The plurality
of sample aliquots
may be all of the same volume or may be in sets of aliquots, where each set
contains aliquots of
different volumes.
The device used is generally in the form of a sterile incubation plate having
a multitude
of wells able to hold separate aliquots of liquid. The device is constructed
to contain at least one
reagent provided to the at least one well and preferably to a plurality or all
of the individual
wells. Because the plate, which can include a lid, contains the reagent or
reagents, the reagents)
2 0 is present in the plate prior to introduction of a sample. For example,
such a reagent may be a
specific growth medium for bacteria. The reagents) is provided prior to sample
addition in a
manner such that the reagent is not significantly washed or dissolved away
during sample
addition. The provision of the reagent or reagents within the plate eliminates
the need for a
tester to separately prepare the reagent and then add it to a sample or to the
test plate. In
2 5 addition, in many applications, the construction of the incubation plate
is preferably arranged so
that no pipetting is required, the plate is simply dipped into the sample, or
an approximate
quantity of the liquefied sample is poured onto the surface of the plate, and
a designed volume of
sample is retained within the wells. In this mode, the plate provides an auto-
dispensing function.
Each well to which a reagent or reagents is provided may receive the same or
different
3 0 reagents. Thus, where a plurality of reagents is used, separate wells may
receive different single
11
CA 02311059 2000-07-07
WO 99122017 PCTNS98IZZ6Z8
reagents, different combinations of reagents, or combinations of these
possibilities.
The phrase "at least one well is supplied with at least one reagent" means
that one or
more reagents are contained in the plate prior to sample addition in a manner
so that the reagent
or reagents will be present in a significant amount in at least one of the
individual wells during
incubation. Thus, the phrase distinguishes from situations where the user adds
the reagent along
with the sample or in such a manner that the reagent is substantially diluted
or dissolved in the
sample prior to draining off any excess sample liquid from the plate. In some
embodiments, not
every well will contain each reagent or may even contain no reagent. Thus,
preferably the
reagent or reagents are present in a manner such that a discrete quantity of
reagent or reagents is
supplied in or to each intended recipient well individually. While it is
possible for a user to
prepare a plate by depositing a reagent in the wells of a plate prior to
sample addition, preferably
the plate is provided to the user with the reagents) already deposited and
preferably dried in
place.
Generally, the wells are designed to form separate incubation chambers for
each sample
aliquot. The wells can be of the same size or of different sizes and shapes to
increase counting
range and/or simulate dilution effects.
Thus, in a first aspect, the invention features a sterile incubation plate
having a generally
flat horizontal surface. The surface defines a plurality of recessed wells (in
preferred
embodiments, at least 40, 60, 90 or even 200 recessed wells are provided) and
each well is
2 0 adapted to hold aliquots of liquid by surface tension. Any excess liquid
from the liquid sample
drains from the surface of the plate outside the wells due to the
hydrophobicity of the material
used to form the plate. The plate may be constructed of plastic or other
hydrophobic material.
In other embodiments, the plate may be generally circular in shape, or have
any shape.
The plate is constructed to contain at least one reagent, e.g., a growth
medium, and to
2 5 provide that at least one reagent individually to at least one well. Thus,
the reagents) is
provided within the plate rather than being added separately. In general, the
reagent or reagents
are provided directly to individual wells. In other embodiments, different
wells can contain
different reagents or different combinations of reagents to provide a variety
of applications. For
example, the provision of different reagents or different combinations of
reagents to different
3 0 wells can provide a plurality of different assays on a single plate. The
reagents) is provided in
I2
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/22628
such a manner that it is not appreciably washed away during the aliquoting or
auto-aliquoting
process (as described below).
In other preferred embodiments, a lid is also provided to prevent
contamination of liquid
within the wells; and the plate is provided in a sterile form so that no
positive aliquots are noted
unless at least one biological material particle is present in the sample. In
embodiments in which
a lid is present, the lid is regarded as part of the incubation plate, whether
attached or separate
from the plate portion containing the wells. In this context, the lid is the
"lid portion", and the
plate portion containing the wells is the "lower plate portion". The lid can
be a top cover, but
can also be constructed as a container, such as a clamshell arrangement, so
that the plate portion
containing the wells can be fully contained within the lid/container. In
another preferred
embodiment, the lid is designed as a rectangular tube, such that the plate
portion is inserted into
the open end of the tube; generally an enlarged portion of the plate will
close the open end of the
tube-shaped lid.
In a preferred embodiment, the reagent is coated into individual wells in a
manner so that
the reagent will not be appreciably washed or dissolved out of the well during
the process of
distributing the sample into the wells. As an example, the inner surface of
the wells can be
corona treated so that the reagent will tightly adhere to the plate when dried
and will therefore
dissolve only slowly when contacted with the liquid sample. Additional agents
can be added to
the medium or coated on the medium in the wells to further control the
dissolution rate.
2 o In other preferred embodiments, the plate includes a lid which has a
structure designed
and adapted to deliver the reagents) to the individual well(s). For example,
the lid can be
constructed with a projection or protuberance which holds the reagent, and
preferably a plurality
of such projections or protuberances, such as a projection for each well. The
lid may be attached
to the lower portion of the plate or may be separate. When the lid is closed,
or placed onto the
2 5 lower portion of the plate after aliquoting of the sample, the reagent
will contact the liquid
sample in the well and will then dissolve into the liquid sample. The reagent
may be coated onto
the projection, such as by using corona treatment of the surface of the
projection to adhere the
reagent to the projection. The reagent may also be dried onto the protrusion
or protrusions of the
lid. The projection may also have a cavity to hold the reagent, the surface of
which may be
3 o treated to improve adhesion of the reagent. Different reagents, or
different combinations of
13
CA 02311059 2000-07-07
wo ~naom rcr~s9sn2~s
reagents may be coated onto each of the projections.
In yet other embodiments, the plate has a reagent delivery portion. The
reagent delivery
portion is constructed to hold a selected quantity or quantities of one or
more reagents and to
deliver that reagents) individually to one or more wells. For example, the
reagent delivery
portion can be a frame attached to the underside of a lid portion with rings
or cylinders which
hold the reagent or reagents. When the lid is closed following sample
distribution, the reagent or
reagents will contact the individual sample aliquots and disperse in the
liquid. Other
configurations can also be designed.
The generally flat horizontal surface is designed to allow the liquid to be
aliquoted
readily between the wells and then excess liquid to be drained from the plate
while retaining a
liquid sample aliquot within each of the wells. Those in the art will
recognize that the depth and
shape of the wells, as well as the material used to make the wells and the
plate, are chosen such
that surface tension can be used to hold the aliquots against gravity within
each well, dependent
on the type of the liquid used in the liquefied sample; those skilled in the
art will understand the
factors for selecting plate materials and well sizes appropriate for the
various liquids. Preferably
the sample is an aqueous solution, more preferably a dilute aqueous solution
or suspension. In a
preferred embodiment, the well has a diameter of about 0.15 or 0.16 inches.
Preferably a well
holds between 5 and 100 ~.1. Also in preferred embodiments, the well may have
a shape
designed to enhance retention of a sample aliquot, andlor retention of reagent
within the well.
2 0 An example of such an alternate well shape is a generally circular well
with ribs projecting
toward the center of the well, but other designs can also be used. In a
preferred embodiment the
wells are chamfered to allow liquid that is above the horizontal plane to be
poured off easily (see
Fig. 2B). The dimensions of the wells and the number of wells on a plate can
be selected so that
a particular total volume is retained on a plate. Preferably a plate retains a
total of between
about 0.1 and 10 ml., more preferably about 0.5, 1, or 2 ml., within the
wells.
The incubation plate may be formed of any desired material, but in particular
it is
desirable that a plastic be used which allows separate aliquots of the
liquefied sample to be held
by surface tension within each well without cross contamination of the wells.
Preferably, the
material is hydrophobic. The surface can be untreated or treated chemically or
physically to
3 0 enhance retention of liquid within the wells, even when the plate is
inverted or tipped at any
14
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/22628
angle from the horizontal. The plate may also be treated to enhance adhesion
of a reagent or
reagents within a well or on another desired surface of the plate, for example
comma treated.
Generally, the reagent will be dried onto the plate surface.
In yet other preferred embodiments, the incubation plate is clear or colored,
for example,
white or yellow (to enhance the appearance of color (e.g., blue)) within the
incubation plate), and
the plate has rectangular dimensions of about 1.5 by 2.5 inches or I by 4
inches, or for circular
plates, a diameter of about 3 or 5 inches .
In related aspects, the invention features methods for detection of a
biological material in
a sample using an incubation plate having at least one reagent as described
above. The methods
include the steps of liquefying the sample (if necessary) and distributing the
liquefied sample
over the surface of an incubation plate as described for the above aspect,
preferably by dipping
the plate into the sample. In an alternative embodiment, the sample is poured
into the plate and
the plate is tipped or swirled as needed to distribute the sample; in this
embodiment, the well-
containing area of the plate is surrounded by a wall which retains the sample
on the plate during
sample distribution. As described above, each well is adapted to hold an
aliquot of liquid and is
sized and shaped, and formed of a suitable material, to hold the aliquot
within the well by
surface tension. The aliquots of liquid enter the individual wells without
being applied
individually, and therefore the method incorporates automatic aliquoting (or
auto-aliquoting).
Any excess liquid from the liquefied sample drains from the surface of the
plate outside the
2 o wells due to the hydrophobicity of the material used to form the plate.
In one method the plate has at least one reagent in at least one well prior to
sample
addition, so that following autoaliqouting of the liquid sample into the
wells, the reagent or
reagents dissolve into the liquid. In a related method, the reagent or
reagents are contained in
the lid, such as on or in protrusions in the lid which project into wells when
the Iid is closed on
2 5 the lower plate portion. Thus, when the lid is closed, the reagents)
contacts the liquid aliquot in
the individual well or wells, and then disperses or dissolves in the liquid.
In the context of this invention, the term "dipping" refers to a brief
immersion of at least
a portion of an incubation plate in a liquid. Preferably the period of
immersion is less than about
3 seconds, more preferably less than about 2 seconds, and still more
preferably less than about 1
3 0 second.
CA 02311059 2000-07-07
WO 99/22017 PCT/US98l22628
As described, the plate is constructed to contain at least one reagent, such
as a growth
medium, and preferably all the reagents needed for the particular assay where
the reagent is in
the plate prior to distribution of the sample. In other embodiments, different
wells can contain
different reagents to provide a variety of applications, for example, to
provide a plurality of
different assays on a single plate. The reagents) is provided in such a manner
that it is not
appreciably washed away during the auto-aliquoting process. Any needed
reagents not provided
directly in the device can be provided in the sample or directly into the
plate at the time of use.
The method then involves incubating that incubation plate until the presence
or absence of the
biological material is deteralined.
Also in preferred embodiments, the plate is provided with a handle portion by
which the
user can grasp the plate while avoiding contact with the sample application
portion of the plate.
For applications where the sample is distributed over the surface of the plate
by dipping the plate
into the sample, at least a portion of the handle is not dipped into the
sample, thus aiding in
preventing contamination of the plate and/or the sample by contact with the
user's hand.
The shape of the incubation plate is not critical, but in preferred
embodiments is a
generally rectangular shape. Another example is a generally circular shape
(such as that of a
Petri dish). Indeed, the incubation plate can be used to take the place of a
Petri dish.
Specifically, the method of this invention can be used to replace those
existing tests that are
generally run on Petri dishes to score the number of microbial colonies. Since
discrete aliquots
2 0 of the sample are provided in the plate, one of ordinary skill in the art
need only score the
number of positive wells in the plate to define the Quantity of biological
material within the
original sample, as with the MPN test discussed above. In preferred
embodiments, the
incubation plates of these methods are constructed of plastic, or other
hydrophobic materials.
As noted above, the biological material that can be detected is any material
that fortes a
2 5 discrete particle, such as a microorganism, which may be quantified by
determining the presence
or absence of such a biological material within each well of the incubation
plate. The sample
may be any biological sample or environmental sample such as waste water,
food, a surface
swab, or swabs from other surfaces, such as a throat, or other samples well
known to those in the
art. This sample may be a liquid sample, or may be dissolved in a liquid to
form the liquefied
3 0 sample. Thus, the term "liquefying" refers to providing the sample in a
liquid that can be rapidly
16
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/22628
aliquoted within the incubation plate. The liquefied sample may remain as a
liquid or may be
solidified, e.g., gelled, in the wells after excess liquid is removed.
This invention provides an extremely useful device and method which allows
unskilled
personnel to rapidly determine the quantity of biological material within a
sample. Since the
S sample is readily liquefied by people without significant training in
microbiology, and the
materials for any specific tests can be provided by the manufacturer, such
people can readily
perform the tests with accuracy. The incubation plate is generally provided in
the sterile form so
that no inappropriate detection of biological material can occur.
While it is known to provide plastic containers which can hold liquid within a
plurality
of recesses, this device and method provides an automatic aliquoting (or auto-
aliquoting) method
in which the steps to be performed by the tester are reduced and simplified by
removing the
requirement for manual addition of a reagent and preferably also eliminating
the need for
pipetting a sample or other liquid onto the plate. This is an improvement over
the existing
products used to detect and quantify microorganisms because the potential for
contamination of
the reagent or errors in dilution are reduced.
The present device can be used particularly in food analysis and in testing of
clinical
samples. The separation of the wells of the present device prevents crosstalk
or contamination
between each aliquot. Because of this, many of the tests can be performed by
observing
fluorescence (which is not readily performed in an agar-containing Petri
dish). The device is
2 0 particularly useful when there is a large quantity of microorganisms
present in a sample, such as
more than one organism per one ml or per ten ml.
Other features and advantages of the invention will be apparent from the
following
description of the preferred embodiments thereof, and from the claims.
Referring to Figs. lA-1D, there is shown an incubation plate 10 having a
plurality of
2 5 wells 12 each having a diameter of about 0.16 inches. The incubation plate
10 has rectangular
dimensions of about 2.7 x 1.4 inches. The incubation plate is made of formed
plastic. Wells 12
are spaced apart sufficiently to prevent crosstalk between the wells. These
wells may have a
chamfer (Fig. 2B) if desired to prevent liquid remaining at the upper edge of
the well. The inner
surfaces of the wells can be corona treated and a reagent, such as a growth
medium, can be dried
3 0 onto those surfaces. The reagent can be either a single reagent or a
combination of reagents.
17
CA 02311059 2000-07-07
WO 99/22017 PGT/US98/22628
Those in the art will recognize that incubation plate 10 can be readily formed
by standard
procedures and manufactured with or without perimeter wall, and with or
without a lid 14 (Figs.
3A-3C). This lid is provided with dimples 16 to prevent contact of the lid
with plate 10.
Referring to Figs. 4A-4D and SA-SC, a rectangular incubation plate 10 is shown
with a
corresponding lid 20. The lid is constructed with a set of projections or
protrusions 32 which
project into the wells 12 of the plate when the lid is closed on the plate.
The reagent may be
applied to the ends of these protrusions so that when the lid is placed on the
plate, the
protrusions project into the well and the reagent dissolves in the test
sample. The lid can be
separate from the lower plate or can be formed as an integral part. For
example, the lid portion
can be attached to the lower plate portion with a thin plastic "hinge
portion", which will allow
the lid to be closed onto the lower plate portion. In this exemplary
embodiment, each of the lid
protrusions has a cavity 34 in the tip, into which is deposited a reagent. The
inner surface of the
cavity can be corona treated to enhance adhesion of the reagent, and the
reagent can then be
dried onto the corona treated region. In use, the sample would be auto-
aliquoted into the wells,
then closure of the lid would bring the tips of the protrusions into contact
with the liquid in the
wells. This contact will result in dissolution of the reagent contained in the
cavities in the tips of
the protrusions into each of the sample aliquots. In the case where the
reagent is a bacterial
growth medium and the sample contains bacteria able to grow in that medium,
bacterial growth
can then occur in the wells in which there are viable bacteria.
2 0 Referring to Fig. 6A-6C, a rectangular incubation plate 10 is shown which
is similar in
construction to the plate of Figs. 1 A-1 C, except in having a handle portion
18. A user can grasp
the handle portion, thereby avoiding possible accidental contact with the
sample application area
of the plate. In addition, in applications in which the plate is dipped into a
liquid sample, by
grasping the handle portion, contact of the user's f ngers or a holding device
with the bulk
sample can be avoided, thereby reducing the chance of accidental contamination
of the sample.
The plate 10 of Figs. 6A-6C is also shown in Fig. 7A-7C, together with a lid
20. This
exemplary lid is formed to fully enclose the plate, and is thereby able to
prevent contamination
of the underside of a dipped plate, and to prevent contamination of bench
surfaces or other
objects with liquefied sample from the underside of a dipped plate. Thus, in
use, the well-
3 0 containing portion of the plate is dipped into a liquefied sample, excess
liquid is drained from
18
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/ZZ628
the plate, and the plate is inserted into the lid/container for incubation.
The lid/container 20 can
be held closed using friction-fit matching depressions 22 in the upper and
lower portions of the
lid. The wells of the plate may be chamfered.
CoatinE of Plate Surfaces with Reagent
A variety of different methods can be used for providing a reagent or reagents
within an
incubation plate. For example, a reagent could be provided so that the reagent
would be readily
and quickly dissolved on contact with a buffer or solution, preferably aqueous
solution. In such
an embodiment, the quantity of liquid added should be controlled so that the
resulting
1 o concentration of reagent distributed into the wells would be appropriate.
Thus, in this case, the
reagent could be placed inside the incubation plate in any manner and location
which would
retain the reagent during handling but would allow rapid dissolution on
addition of the liquid.
In this invention, however, the reagent is provided in a manner and/or
location in the
plate so that it is not necessary to measure the volume of liquefied sample
with which the plate is
contacted in order to obtain an appropriate concentration of reagent within
the sample aliquots
retained in the wells. This is achieved by providing a discrete quantity of
the reagent directly to
each well in which the presence of the reagent is desired. Such discrete
quantities can be
provided in a variety of ways, including the particular embodiments described
herein. These
embodiments either use reagent adhered to the well surface in such a manner
that the amount of
2 0 reagent dissolving during the auto-aliquoting process is negligible, or
provide reagent in a
location so that it does not come into contact with liquid until after the
auto-aliquoting process is
complete.
For example, the inner surface of one or more wells can be corona treated and
reagent
dried onto the treated surface, such as from a highly concentrated reagent
solution. Reagent
2 5 deposited in this manner will dissolve slowly, so that the amount lost
during the auto-aliquoting
process will be negligible. The majority of the reagent will then dissolve
during the initial part
of the incubation period. The dissolution rate can also be controlled by
selection of the media
components.
In another example, reagent can be deposited onto protrusions from the surface
of the
3 o plate lid. The protrusions are positioned so that the tips of the
protrusions will project into
19
CA 02311059 2000-07-07
WO 99/22017 PC'TNS98/22628
individual wells when the lid is closed on the plate. Reagent can be deposited
on the outer
surface of protrusions, but preferably is deposited into cavities in the tip
of the protrusions. The
reagent can be deposited in any manner which will result in the reagent being
substantially
retained in position during pre-use handling of the plate. Thus, for example,
the reagent can be
retained by physical barrier means, but is preferably adhered to the surface
of the protrusion
and/or the surface of a cavity in the tip of the protrusion, such as by a
process involving corona
treatment and drying of the reagent onto the treated surface.
Corona Treatment and Reagent Deposition
Typical plastics are made up of long chains of linked subunits, for example,
polyethylene
1 o is composed of long chains of ethylene subunits. In general, aqueous
solutions and the solutes in
aqueous solutions are not attracted to interior chain subunits and therefore
will not bind
effectively to such locations. In contrast, there is often much greater
affinity between such
solutes and chain ends, particularly where the chain end has a relatively
polar character. As
understood by those skilled in the art, corona treatment is a method which
increases the
hydrophilicity of a treated plastic surface. The corona treatment process
generally entails
passing the plastic through an electric arc. The energy imparted by the
electric arc introduces a
significant number of chain breaks, thereby increasing the number of
relatively hydrophilic ends
available for interaction. In addition, the electric arc also generates ozone,
which, as a strong
oxidizing agent generates still additional chain breaks and polar side groups
by oxidizing bonds
2 0 within the polymer chains.
As indicated, this treatment process increases the binding of polar compounds,
such as
aqueous solution components. Therefore, for the use of corona treatment in
preparing plates of
this invention, following the corona treatment of the well surfaces, a
concentrated solution of
reagent is dispensed into the wells. As the Corona treated surfaces are now
wettable (e.g.,
hydrophilic) the liquid reagent forms a uniform coating over the surface of
the well. The plate is
then dried, generally in a drying oven, driving off the liquid water and
leaving a hard, coating of
solid reagent in the wells.
As noted above, different reagents can be delivered to different wells, and/or
some wells
may receive no reagent. Wells receiving no reagent would also usually not be
corona treated.
3 o Other methods known in the art can also be used to increase the adhesion
of reagent to the
CA 02311059 2000-07-07
wo ~nzo~ ~ rcrn3s9snZ6Zs
appropriate plate surfaces.
In use, a test sample can be liquefied or diluted with an appropriate sterile
buffer or
saline solution. A quantity of the liquefied sample can then be distributed
over the surface of the
plate. In a preferred mode of use, the liquefied sample is expected to contain
or is diluted to
contain about 1-40 units of the biological material to be quantified, e.g.,
viable bacterial cells.
An incubation plate 10 of for example Figs. lA-1C is dipped into the liquid
sample sufficiently
to fill the wells, withdrawn, and allowed to drain briefly by holding at about
90 degrees to the
horizontal. In this use mode, the plate provides both an auto-aliquoting
function and an auto-
dispensing function. Alternatively, in the case of a plate having a perimeter
wall, an excess of
the liquefied sample is placed in the incubation plate 10 and that liquid
swirled within incubation
plate 10 to distribute the inoculated liquid to each of wells 12. Incubation
plate 10 is then held at
an angle of approximately 90 degrees to allow excess liquid to drain from the
plate. As shown
in Figs. 3A-3C, a lid 14 may then be placed on the incubation plate and that
plate held in an
incubator for the appropriate length of time, for example 18-48 hours. After
that length of time,
the presence or absence of a positive result can be scored in each well 12 of
the plate.
Example 1: Use of Incubation Plate For Bulk Testing
For total plate count, a plate as described above is used for the detection
and
quantification of the total bacterial concentration of food. It can be based
on a multiple enzyme
2 0 technology which correlates enzyme activity to the presence of viable
bacteria in food. It
utilizes multiple enzyme substrates that produce a blue fluorescent color when
metabolized by
bacteria. The multiple enzyme reagent is coated on interior surfaces of wells
of the plate. When
a liquefied prepared food sample is distributed into the wells of a plate as
described herein, the
total viable bacterial concentration of that food product can be determined
after 24 hours of
2 5 incubation. The actual medium used herein is not critical to the
invention, but is provided only
for illustrative purposes.
Storage and Disposal
Unused test plates are stored at room temperature (4° to 25°C)
away from the light.
After use, the incubation plate device will contain viable bacteria which must
be handled and
3 0 discarded appropriately.
21
CA 02311059 2000-07-07
WO 99122017 PCT/I1S98IZ2628
Test Procedure - Dig me~b~d sample ag ication
1. Obtain a sample by removing an appropriate quantity of a bulk material to
be
tested. If needed the sample is liquefied or diluted in a sufficient volume to
allow a plate to be
dipped into the sample.
2. Grasp an incubation plate without contacting the sample application surface
(preferably grasp the plate by the handle portion if the plate incudes such a
portion). Dip the
bottom portion of an opened incubation plate into the sample, withdraw the
plate immediately,
and hold at approximately 90 degrees to the horizontal to allow excess liquid
to drain from the
plate. Preferably the excess liquid is allowed to drain into a waste
receptacle rather than being
1 o allowed to drain back into the sample container in order to reduce the
possible deposition of
reagent into the sample container. Make sure that all liquid "cross bridges"
between wells are
removed by gently tapping the plate. Dispose of excess liquid appropriately.
3. Place the lid back on the plate (or close an attached lid).
4. Place the plate in an incubator for 24 hours. Plates can be inverted if
desired.
5. Count the number of fluorescent wells after 24 hours by placing a 6 watt
365nm
UV light within five inches of the plate. Do not read plate before 24 hours.
Results are stable to
48 hours.
6. Compare the number of fluorescent wells to an MPN chart to determine the
most
probable number of bacterial present in the plate.
2 0 Example 2: Use of Incubation Plate for Unit Dose Testin,E
The plate containing media described in Example 1 are used for this test.
Test Procedure
1. Add l Oml of sterile buffer (or saline) to a tube. If greater than O.lml of
food
sample is to be inoculated into the test, reduce the volume of sterile buffer
appropriately to
2 5 achieve a final volume of l Oml in the tube.
2. Inoculate the buffer with the food sample being tested.
3. Shake the tube several times to completely mix buffer and inoculated food
sample.
4. If the tube is of sufficient size, dip the plate into the sample or
alternatively pour
3 o the buffer/sample suspension over the sample application surface of an
incubation plate as
22
CA 02311059 2000-07-07
WO 99/22017 PCTILJS98/22628
appropriate, taking care that all the wells are filled. After sample
application, immediately hold
the plate at approximately 90 degrees to the horizontal to allow excess liquid
to drain from the
plate. Preferably the excess liquid is allowed to drain into a waste
receptacle. Make sure that all
liquid "cross bridges" between wells are removed by gently tapping the plate.
Dispose of excess
liquid appropriately.
5. Place the lid back on the plate (or close an attached lid).
6. Place the plate in an incubator for 24 hours. Plates can be inverted if
desired.
7. Count the number of fluorescent wells after 24 hours by placing a 6 watt
365nm
LTV light within f ve inches of the plate. Do not read plate before 24 hours.
Results are stable to
48 hours.
8. Compare the number of fluorescent wells to an MPN chart to determine the
most
probable number of bacterial present in the plate.
The patent documents and other references cited herein are each incorporated
by
reference to the same extent as if each had been separately incorporated by
reference in its
entirety.
Dip Stick Testing Device For Microorganism
Detection and Enumeration
Next referring to Figs. 8-13, the concept is to use small individual absorbent
materials
which contain lyophiiized or "dip-dried" reagent in a setting that allows
automatic sample
2 o distribution and target microorganism enumeration.
A number of reagent-containing absorbent (hydrophilic) materials ("reagent
islands") are
immobilized onto a support structure made of a hydrophobic material to form
individual reagent
islands. The reagent islands may be embedded in the support structure, for
example. However,
this should not be construed as limiting as any form of securing the reagent
island to the support
2 5 structure may be used as long as it does not interfere with the assay. The
liquid absorbing rate of
each reagent island in this embodiment is the same because the reagent islands
are made of the
same material and have the same size. Further configuration can be made so
there are two or
more groups of reagent islands in different sizes to form a setting that can
be used to achieve
higher microorganism quantification without serial dilution based on the
principles of the Most
3 o Probable Number (MPN) method.
23
CA 02311059 2000-07-07
WO 99/22017 PCT/US98122628
In a preferred embodiment, the reagent is one developed to test for the
presence of target
microorganisms in a sample using, but not limited to, defined substrate
technology (DST) and/or
Multiple Enzyme Technology (MET). A positive detection of target
microorganisms in such
reagents will cause a color change of the reagent and/or cause the emission of
a fluorescent
signal from the reagent when viewing under a long-wave IJV lamp. Examples of
such reagents
are Colilert~, EnterolertTM, Simplate TPCT"', and Simplate CEcTM.
Sample inoculum is pre-calculated based on the maximum absorbing rate
(saturation
point) of a reagent island and the total number of reagent islands on a
device. When the pre-
determined amount of liquid sample (i.e. water, milk, juice and food
homogenous) is inoculated
on to this device, each reagent island absorbs the same amount of sample.
Sample can be
distributed to each reagent island by simply moving the device in back-forth
or circular motion.
The area between each reagent island is hydrophobic and when each reagent
island absorbs
sample to its saturation point, there will be no sample left between the
reagent islands and cross
contamination between reagent island will be prevented. Liquid sample absorbed
by the reagent
islands also re-hydrolyzes the reagent in the reagent islands to support the
growth of
microorganisms in the sample.
After incubating the sample-containing device at a pre-determined temperature,
reagent
islands containing target microorganisms from the sample will have a change in
color and/or
emit fluorescent signals (positive); reagent islands lacking target
microorganisms from the
2 0 sample will exhibit no color change and emit no fluorescent signals (a
negative result). Target
microorganism concentration in the sample under testing can be calculated
based on the number
of positive and negative reagent islands observed using the Most Probable
Numbers (MPN)
method.
Another application of this concept is to have reagent islands lyophilized
with different
types of reagents. Each reagent is designed to test one specific aspect of a
sample, such as
microorganisms, chemicals, or any other detectable analyte of interest.
Combination of these
reagent islands in the same device forms a test kit providing a one-step test
for multiple
chemicals, analytes, or biological materials.
Specifically referring to Figs. 8 and 9, there is shown another aspect of the
invention which
3 0 comprises a dip stick 100 containing a reagent island 1 O 1. The stick
will generally be made of
24
CA 02311059 2000-07-07
WO 99/22017 PCT/US98/126Z8
plastic, but its composition is not critical and may be constructed of any
hydrophobic material
which will not leach into the test sample or interfere with the assay. Figures
8 and 9 depict the
stick with a single reagent island.
Figure 10 depicts a preferred embodiment of the dip stick containing multiple
reagent
islands 102. Zones of reagent islands may be set up on the dip stick with each
zone having
reagent islands that contain different reagents or different combinations of
reagents than the
other zones, thereby enabling multiple assays to be performed on a single dip
stick, each zone
testing for a different analyte or microorganism. Figure 11 depicts the dip
stick assay device of
Fig. I O which has undergone the assay procedure and illustrates reagent
islands showing both
positive results (dark reagent islands) and negative results (white reagent
islands).
Figure 12 shows a preferred embodiment of this aspect of the invention. There
is shown
the dip stick 100 with multiple reagent islands 101 which is folded and
inserted into a test tube
103. In this embodiment, the test tube also has a cap 104 which further
protects the reagent
islands from environmental factors. Of course, the device can be placed in
other types of
containers such as a plastic sleeve or any container which serves to protect
the device. The
reagent placed on the reagent island can be any reagent or combination of
reagents which can be
distributed onto or into the material of the reagent island.
ARoiication of Test Samgle to Dip Stick Device
The methods of applying sample to the device are varied. The dip stick can be
dipped
2 0 into the test sample, and left in contact long enough for the reagent
islands to absorb a
predetermined amount of fluid. This will generally be less than 3 seconds, but
may be more
depending on what material the reagent islands are constructed from. In a
preferred
embodiment, the reagent islands are constructed from cellulose. However, they
may also be
constructed of any material which is capable of absorbing and holding a volume
of fluid. The
2 5 test sample may also be pipetted from the test solution onto the reagent
islands with a transfer
pipette.
The support structure may also comprise a box made of plastic or other
suitable
hydrophobic material. The reagent islands may be positioned inside of the box
and the box can
be opened and test solution applied using a pipette or by pouring, or any
suitable means. The
3 0 box may have a lid to further protect the device secured thereon or as a
separate piece.
CA 02311059 2000-07-07
wo ~n2om prrnJS9sn26zs
Referring now to Figure 13, the support structure may also comprise a center
support 104
to which "leaves"105 are attached and support the reagent islands 106. The
leaves can be
arranged in a three-dimensional structure as depicted in Figure 13, thereby
maximizing the
number of leaves which can be accommodated on one device. The device can be
placed into a
circular container 108 and a cap 107 placed thereon to provide further
protection for the device
before, during, and after development of the assay.
~igette Device
Referring now to Figure 14, in another aspect, the invention provides a device
comprising a support structure 109 which is contained within a laboratory
pipette 111. Multiple
reagent islands 110 are contained on or within the support structure 109.
Preferably, the support
structure 109 has more than one side with reagent islands 110 thereon. The
support structure
109 is most preferably formed of a synthetic polymer, such as a plastic, and
is inserted into a
laboratory pipette 111 which is preferably comprised of glass or plastic and
has a tapered end for
sample uptake. Sample may be merely drawn into the pipette by an asperation
apparatus known
in the art, allowed to remain for a time sufficient for the reagent islands to
absorb a
predetermined volume of test sample, and expelled from the device. The device
is then
incubated for a time sufficient to develop the assay, and the results
determined. The pipette
itself comprises both a simple means of applying sample to the test device, as
well as a
protective function.
2 0 xa le 1
A Bacterial Detection System for coliforms and
E. coli in Milk Using the Dip Stick Device
The following is an example of how the present invention provides a method of
coliform
detection in milk that is easy, does not comprise many steps, and provides
results that are easy to
2 5 interpret. The assay was conducted using a test device with multiple
reagent islands, each of
which absorbed .033 ml of milk. The milk was added to the test device and was
auto-aliquoted
to all the reagent islands. The sample was incubated for 24 hours and provided
the number of
coliforms present by examining how many of the reagent islands change color.
The number of
coliforms present is determined based on the MPN chart. The data obtained from
the assay are
3 o set out in Table 1. The flexibility of the test device's uptake ability is
emphasized since volume
26
CA 02311059 2000-07-07
WO 99/12017 PCTNS98I22628
uptake can be easily adjusted by adjusting the number and/or sizes of the
reagent islands. The
device also has the capability to have multiple tests on the same device, by
impregnating reagent
islands with different media.
all
Milk Sam, Dipstick Device (cfu/ml)
#7197A 10.3
#7197B 11.8
#7197C 16.4
#710 56.3
#713 18
#3B 1 0
#3B2 37
#2B2 0
#2B 1 9.3
#618 69.1
#618A All +
#622A 12
#622B 36
2 #622C 3
0
#7697A 56
#7697B 22
#7697C 16
#7697D 31
#811 3
#815 16
#813 6.2
Mean: 23.41
Std. Deviation 20.75
27
CA 02311059 2000-07-07
WO 99/22017 - PCT/US98lZ2628
The invention has been described in detail with particular reference to
certain preferred
embodiments thereof, but it will be understood that variations and
modifications can be effected
within the spirit and scope of the invention.
28