Note: Descriptions are shown in the official language in which they were submitted.
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
Method of and Apparatus for Disintegrating Biological Cells for Extraction and
Analysis of the Contents of the Cells
Field of the Invention
The present invention relates to a method of as well as an apparatus for
disinte-
grating biological cells for extraction and analysis of the contents of the
cells, par-
ticularly the nucleic acids.
For the analysis of biological cells and the analysis of the cell contents in
particular,
specifically proteins and nucleic acids (DNS and RNS), for instance for the
purposes
of medical diagnostics, the cell walls and membranes must be disintegrated in
order
to be able to extract the cell interior in a suitable form from the cells.
Prior Art
To this end a number of common methods are appropriate which are described in
a
general survey in the paper "Cell Fractionation" by Carl A. Schnaitman in:
Manual of
Methods for General Bacteriology, American Society for Microbiology,
Washington,
D.C., 2006, 1981, Chapter 5, pages 52 - 61. The methods described in this
paper
can be subdivided into different groups which are distinguished by their
specific type
of cell wall destruction or dissolution, respectively:
Mechanical methods are based on the generation of local pressure variations at
the
site of the cells to be disintegrated, which cause actually a break-up of the
cell walls
and membranes. Due to the strong pressure variations, which are required for
destroying the cell walls, however, devices with an appropriately stable
structure are
necessary such as those described in the paper "Modified Press for Disruption
of
Microorganisms" by J. R. Raper and E. A. Hyatt, in: J. of Bacteriology, vol.
85, pages
712 - 713. Other devices for generating such high pressure or shearing forces
are
so-called ball or pot mills and the so-called French press, which are
illustrated in
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
2
Chapter 9.3.6 on pages 103 - 104 in the "Manual of Industrial Microbiology and
Biotechnology", American Society for Microbiology, Washington, D.C., 1986.
The aforementioned devices require a lot of space in order to generate and
reliably
manage such high pressure forces, in addition to a very high expenditure in
engineering and mechanical terms, and are moreover difficult to automate.
Another
disadvantage of the known pressurising treatment of biological cells moreover
consists in the aspect that the cell contents - by which term the long-chain
DNS
molecules are to be understood in particular - are strongly fragmented, which
renders
a complete DNS analysis more difficult.
Apart therefrom, ultrasonic disintegration methods have become known wherein
the
cell walls and membranes are really torn up under the action of cavitation
effects so
that the cell interior may come out. In ultrasonic disintegration, however, a
substantial
sound energy is dissipated in the sample to be analysed, which involves an
unnecessary heating of the sample. Therefore cooling provisions are required.
As a
result of the occurrence of temperature variations, however, an process
management
with a fairly acceptable precision becomes more difficult. To this adds that
the known
apparatuses for application of the high-power ultrasound are difficult to
clean so that
the risk of inter-contamination cannot be precluded.
The disintegration of biological cells by exclusively chemical means requires
chemicals which are individually matched with the cells, which entails
difficulties in
the selection of the appropriate chemical in each case for the analysis of
unknown
cell types, as is the case, for instance, in the diagnosis of unknown
pathogenic
agents. One example of a cell disintegrating method with the exclusive
application of
purely chemical disintegrating reactions is described in "Molecular Cloning -
a
Laboratory Manual", second edition, Cold Spring Harbor Laboratory Press, 1989,
pages 1.25 - 1.31. The reaction times necessary to disintegrate cells with the
ex-
clusive application of chemicals are as long as several hours, despite high
con-
centrations of the used chemicals which may additionally produce interfering
effects
in the subsequent analytical steps, e. g. PCR reactions. For some micro-
organisms
the rate of disintegration is moreover insufficient; for instance it is
extremely difficult
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
3
with Micrococcus luteus to achieve a rate higher than 20% with application of
chemical processes.
Finally, Methods have become known wherein biological cells such as bacteria
or
fungi suspended in liquid media are exposed to a strong electrical field. For
instance,
electrical fields are applied to kill biological cells in a pasteurisation an
sterilisation
process, as is described,. for instance, in the US Patent 5 235 905. In an
attempt to
avoid an excessive amount of energy introduced into the sample and overheating
of
the sample pulsed electrical fields are applied to the sample with respective
pulse
lengths of a few micro seconds. With a biological cell consisting of
electrolytic and
therefore electrically conductive contents, from an electrical point of view,
which are
enclosed by an electrically insulating membrane, the carriers of electrical
charges
inside are displaced when an electrical field is applied externally. In this
manner local
potential differences are created between the interior of the cells and the
outside of
the cell, which result in the cell membrane being subjected to an electrical
break-
through so that it loses its semi-permeable characteristics whilst its
electrical
conductivity increases. With low external field strengths and short pulse
lengths the
modification of the electrical conductivity of the cell membrane is
reversible, a
phenomenon which plays a central role in electroporation (cf. in this context
"Electroporation and Electrofusion in Cell Biology" by E. Neumann, A. E.
Sowers, C.
A. Jordan, Plenum Press, New York, 1989). This effect is utilised, for
instance, to
introduce genetic material into the cells. The disadvantage of this
application of
electroporation resides in the aspect that the efficiency typically ranges
about 0.01 %,
which means that the material is actually introduced into a single cell out of
10,000
cells.
With stronger fields and sufficient pulse lengths, particularly with a
repeated
treatment of the biological cells, these modifications are permanent and
result in the
final death of the cell. This approach is used as a standard method for the
destruction
of micro-organisms in food items.
The German Patent DE 35 37 261 A1 discloses a method of field-induced transfer
of
macro molecules into living cells, which represents a process reciprocal to
the
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
4
selective transfer of cell contents out of cells and is hence subject to other
process
conditions. The transfer of particles into and out of cells can therefore not
be con-
sidered to be equivalent operations, specifically as this transfer is of an
asymmetrical
nature.
The asymmetrical or unidirectional transfer of various substances through the
cell
wall is rather part of the most important functions of the cell membrane. For
instance,
the cell membrane has, on the one hand, an asymmetrical structure and, on the
other
hand, the cell contents are substantially different from the external
environment of the
cell. The size of the transferred molecules is often comparable to the cell
size as
such, so that, not least as a result of this fact, the different transfer
mechanisms
which constitute the basis of transfer into and out of the cell, cannot be
described by
simple diffusion models - with simple diffusion not being a purely symmetrical
process either. The transfer mechanisms are complex, involve several steps and
are
highly specific of the respective direction of transfer.
The same applies also to the method of transformation of Bacillus
stearothermophilus
with a plasmid, which is disclosed by the Japanese Patent JP 04173093 A:
All the methods known for the disintegration of biological cells involve the
disad-
vantage that the processes cannot be realised in a fully automatic manner,
apart
from the partly very substantial expenditure in terms of apparatus and
technology. To
this adds that the cell disintegration rate is not always sufficiently high.
Brief description of the invention
The present invention is therefore based on the problem of improving a method
of
disintegrating biological cells for extraction and analysis of the cell
contents in such a
way that the method can be implemented in a fully automated manner without any
high expenditure in terms of apparatus and technology. In particular, the
extracted
fraction of the cell contents to be analysed should be high independently of
the cell
type, while the duration of the process time for extraction and isolation of
the cell
contents should be substantially reduced. Moreover, the invention intends to
provide
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
an apparatus which permits a low-cost, high-speed and reliable realisation of
the
method.
In accordance with the invention it has been found that the disadvantages
which
occur with the known methods of the exclusively chemical disintegration of
biological
cells can be avoided by a selective sequence or superimposition of electrical
and
chemical treatments of biological cells.
According to the present invention, the method of disintegrating biological
cells for
the extraction and analysis of the cell contents excels itself by the
provisions that the
cells are exposed to an electrical field and that the cells are contacted with
a
substance promoting the cell integration before or after the application of
the
electrical field.
The biological cells, which are, as a rule, suspended in a liquid medium, are
exposed
to an electrical field with typical field strengths between 5 and 100 kV/cm
which is
operated in pulsed mode with a pulse length of roughly 0.5 to 50 micro
seconds. The
cells may equally be present in cell unions such as tissue fragments or
applied and
immobilised on a support such as a filter.
Due to the action of the electrical field and the electrical break-through
pores are
created in the lipid layers whereof the cell membrane consists. With these
pores
being very small, however, and with certain cell types being able to obstruct
the
diffusion of large-size molecules such as those of nucleic acid, the treatment
of the
cells by means of an electrical field alone is not sufficient in many cases
for a release
of the cell contents.
In accordance with the invention it is proposed to utilise the creation of the
pores in
the cell membrane in order to promote and accelerate the action of chemicals
for the
disintegration of the cells.
According to one variant chemicals are used which produce such an effect on
the cell
contents and particularly on the molecules to be analysed that they are easier
and
more quickly to liberate. These chemicals diffuse into the cell interior
through the
pores which are created by the electrical treatment, and disintegrate, for
instance,
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
6
proteins and cut DNA molecules selectively into smaller fragments which,
however,
are a subsequent DNS analysis. The DNS fragments so created can then freely
diffuse through the pores to the outside. These processes are promoted by the
addition of a surface-reactive substance (detergent) such as sodium dodecyl
sulphate (SDS).
In another variant the fact is utilised that the pores created by the
electrical treatment
represent weak sites which substantially facilitate and accelerate the attack
by the
chemicals on the membrane. Chemicals appropriate for use in the complete
disintegration of the cell walls are, for instance, chaotropic reagents such
as urea,
guanidine hydrochloride or thiocyanate and others. Dimethyl sulfoxide =may be
employed as well.
With the biological cells suspended in a medium being exposed to an electrical
field
excessively high concentrations are not required for the admixture of the
additional
chemical substances, which can preclude negative effects which are due to the
presence of the chemical substance in the subsequent analytical steps.
For an optimisation and acceleration of the intended chemical effect the cells
contacted with the chemicals, e. g. in the form of a cell suspension, are
heated to a
temperature level between 35 and 100 °C.
In accordance with the invention an apparatus for realising the method is so
structured that a sample volume receiving cells or cell unions suspended in a
me-
dium is provided with two two-dimensionally configured and mutually opposing
electrodes, which can be occluded by a lid element on the upper side of the
sample
volume, which lid element has a convex configuration on the side facing the
sample
volume. Due to the convex configuration of the lid element the occlusion of
air
bubbles can be expediently avoided when the liquid medium is trapped within
the
sample volume, which bubbles would result in an inhomogeneous distribution of
the
electrical field and therefore possibly in undesirable electrical break-
through in the
treating apparatus.
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
7
Brief description of the drawing
The invention will be described in the following exemplary specification,
without any
restriction of the general inventive idea, by embodiments with reference to
the
drawing wherein:
Fig. 1 shows one embodiment of an apparatus for realising the method of
disinte-
grating biological cells,
Fig. 2 illustrates an alternative apparatus with a metal sheet on the lid
element,
Fig. 3 represents an alternative embodiment with a metal separating sheet
within the
sample volume, and
Fig. 4 shows an alternative embodiment with friction elements for comminution
of the
tissue.
Brief description of one embodiment
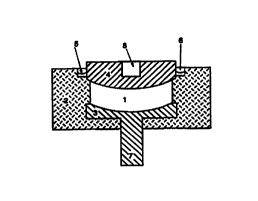
Fig. 1 illustrates an expedient apparatus for realising the method of
disintegrating
biological cells, showing a treatment cell including a synthetic housing 2
into which a
lower electrode 3 is incorporated by casting and providing for an upper
electrode 4
which is the lid element at the same time. The biological cells to be
disintegrated,
which are suspended in a solution, are present inside the sample volume 1
between
the electrodes. In an alternative the cells may be immobilised on a support
such as a
filter, with the residual volume of the apparatus being topped up with an
electrolyte
solution. On account of the convex curvature of the surface of the lid element
4 it is
ensured that when the lid element is placed on top air bubbles will not be
trapped in
the sample volume 1. The excessive suspension 5, which is urged out through
the lid
element 1 when the lid element is placed on top of the sample volume 1,
accumulates in a recess 6 provided in the synthetic housing 2 circularly
around the lid
element 4. The lower electrode 3 opposite the lid element 4, by contrast, has
a
concave shape so that the distance between both electrodes is chosen to be
constant. Moreover, the configuration of the electrode shape facilitates the
filling of a
liquid into the sample volume 1 without air bubbles and furthermore a largely
complete removal of the sample after completion of the cell disintegration
process.
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
8
For contacting the electrodes 4 and 3 for conveying and fixing the parts of
the
treatment cell in an automatic device in particular, the lower electrode 3 is
provided
with a plug 7 whilst the upper electrode 4 has a recess 8.
The electrode spacing, which is preferably set in this embodiment, amounts to
2 to 5
mm while the electrode diameter is roughly 1 cm. With these dimensions and
with a
voltage of approximately 20 kV, which is still comparatively easy to manage,
the
strength of the electric field ranges between 40 and 100 kV/cm, which is a
field
intensity which is well suitable for cell disintegration.
The electrode surface may preferably be made of aluminium because this
rriaterial is
biologically insert and has a sufficient chemical stability. Moreover, it is
easy to
process and inexpensive and therefore suitable for disposable articles in
particular.
After the treatment with the electrical field the chemical treatment may be
continued
in the same volume by adding the chemicals, or alternatively the cell
suspension may
be transferred by means of a pipette into another vessel.
Even though the embodiment illustrated in Fig. 1 may be troublesome for
removal of
the sample due to the structural design of the lid element because the lid
element
must be removed before the sample volume 1 is emptied and a substantial part
of
the solution may adhere thereto as a drop. This, in turn, could result in the
contamination of the environment and hence in the inter-contamination of
further
samples, which should, however, be avoided particularly in case of an
automated
handling of such samples.
To facilitate the removal of the samples particularly in an automatic
apparatus and in
order to ensure avoidance of inter-contamination the apparatus according to
the
embodiment of Figure 2 provides for a two-piece configuration of the lid
element 4.
The lid element 4 is provided with a central bore 9 and is enclosed by an
electrically
conductive sheet 10 which wraps the lid element 4 and is preferably made of
aluminium. After completion of the inventive method a pipette is introduced
through
the passage 9 to pierce the sheet 10 so that the suspension contained in the
sample
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
9
volume 1 can be exhausted by suction. Even though in this embodiment the sheet
must be replaced each time this approach appears to be yet expedient
specifically
since the treatment cell as a whole may be configured as disposable article in
order
to avoid inter-contamination.
Figure 3 shows a further embodiment which comprises a lid element 4 comparable
to
that of the embodiment according to Fig. 1. However, this design provides for
a metal
sheet 11 separating the sample volume 1, which sheet constitutes at the same
time
the lower electrode. A cavity 12, which is connected to a passage 7, is
provided
underneath the electrode 11. Like in the embodiment according to Fig. 1 the
treatment cell is filled with a suspension of biological cells while a high-
voltage
treatment can be performed with chemical additives for cell disintegration.
Upon completion of the application of an electrical field the cavity 12 is
evacuated
through the passage 7. Due to the difference in pressure the sheet 11 is torn
open so
that the processed suspension can be exhausted by suction through the passage
7
for further analysis.
This variant is particularly suitable for a fully automatic realisation of the
method,
wherein the risk of inter-contamination can be completely precluded.
In all cases described in the foregoing a subsequent chemical treatment is
necessary
in order to transfer the cell contents efficiently to the outside. The
chemicals may be
added to the solution prior to the application of the electrical field; as
they are
electrolytes in many cases, however, or produce optimum effects in
electrolytic
buffers they should be added to the cell suspension only after completion of
application of the electrical field.
In a variant of the chemical treatment a mixture of 15 different restriction
endo-nu-
cleases (3 U/ml of each) are added to the cell suspension and the suspension
is then
incubated at 37 °C for a period of 10 to 30 minutes approximately. The
regents
diffuse through the membrane pores, which are created by the application of
the
electrical field, into the cell interior and selectively cut the DNS strands
to fragments
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
including some thousands of base pairs which may be used later on for a PCR
reaction. After the treatment proteinase K in a concentration of 1 Ng/ml is
added to
the suspension, and the temperature level is set to 60 °C. Proteinase K
equally
diffuses through the pores and causes the lysis of both cell proteins and the
enzymes
added in the previous step so that any interfering influence on the subsequent
PCR
reactions is precluded. After incubation at 60 °C for 10 to 30 minutes
the temperature
is raised to 95 °C. In this step the proteinase K is denatured and the
DNS diffuses
through the pores in the membrane to the outside. With such a treatment the
PCR
inhibitors - both the added ones and those inherent to the cell - are largely
disintegrated.
In another variant of the chemical treatment, upon completion of the
application of
the electrical field, guanidine hydrochloride or thiocyanate (GdnHCI or
GdnSCN) are
added to the cell suspension so as to adjust a concentration of 0.3 to 3 M.
The
suspension is heated to a temperature of 60 to 100 °C and is incubated
for 10 to 30
minutes. With such a treatment the cell walls and membranes are destroyed and
the
DNS is consequently liberated. This method is simpler than the approach
described
before but it entails the disadvantage that guanidine compounds, which are
here
used in a concentration lower than that common for a purely chemical
disintegration
of cells, may inhibit the subsequent PCR reaction and must therefore be
eliminated
from the solution.
In the embodiment according to Fig. 4 the surface on the electrodes 13 and 3
is
roughened so that the tissue fragments 16 introduced into the sample volume 1
may
be comminuted.
In correspondence with the embodiment according to Fig. 4 the upper electrode
13
and the housing 2 are so configured that the electrode is axially guided for
rotational
movement. The residual volume of the sample volume 1 is topped up with a
liquid 17
and the tissue sample 16 is compressed between the electrodes by pressure
exerted
onto the upper electrode 13. In this case the liquid is only required for
precluding
electrical discharges between the electrodes because the sample as such
contacts
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
11
the electrodes directly. The liquid introduced between the electrodes is
therefore not
definitely electrically conductive so that that pure water or even oil may be
used.
Following the high-voltage treatment and the addition of appropriate chemical
ad-
ditives the tissue sample 16 is crushed by the application of a high pressure
on the
upper electrode 13, possibly in combination with rotational movements. The
upper
electrode 13 and the housing 2 with the lower electrode 3 hence play the role
of a die
and a female mould. The electrode surfaces should preferably have a rough
profile
17 so as to render the tissue disintegration more efficient.
The liquid liberated in this step or the paste so formed, respectively, which -
contains
the nucleic acids, rises in the gap 14 between the upper electrode 13 and the
housing 2 and can be exhausted by suction through the passage 15 for further
analysis.
CA 02311429 2000-OS-24
F97R51 PCT PCTlDE 98/0297
12
LIST OF REFERENCE NUMERALS
1 processing space (is filled with the biological material to be treated
in the appropriate medium (electrolyte solution))
2 housing / capsule
3 lower electrode
4 lid
excess medium
6 recess / groove therefor
7 plug
8 bore for electrode connection
9 passage in the lid
metal sheet on the lid
11 lower electrode made of metal sheet
12 cavity
13 upper electrode
14 gap
passage in the housing
16 tissue sample
17 liquid