Note: Descriptions are shown in the official language in which they were submitted.
CA 02311903 2000-OS-26
WO 99/27885 PCT/S1;98/02146
TITLE
Multiple compartment container for medical solution
AREA OF INVENTION
The present invention relates to peritoneal dialysis, where
a dialysis solution is present in a multiple compartment
container provided with three or more compartments.
The invention relates particularly to such a container in
IS which the dialysis solution contains bicarbonate as buffer, as
well as glucose and calcium ions.
PRIOR ART
A container for peritoneal dialysis is disclosed in
WO 97/05852. The container contains conventional dialysis
solution with lactate as buffer. The container thus comprises
a first large compartment containing sodium lactate, sodium
chloride, calcium chloride and magnesium chloride, as well as
two small compartments containing glucose of high
concentration and low pH. The container is sterilized in an
autoclave with the solutions in situ in said compartments.
During use, either one or both of the compartments are
connected to the large compartment by frangible pins whereby
the dialysis solution contains a desired concentration of
glucose, such as 1.50, 2.50 or 40. Using a three compartment
container, it is possible to employ a single container for all
three degrees of concentration, which reduces costs for
logistics and storage.
3~ SUMMARY OF THE INVENTION
An object to the present invention is to adapt the
container according to WO 97/05852 for such type of peritoneal
dialysis in which the lactate buffer is entirely or partially
CA 02311903 2000-OS-26
WO 99/27885 PCT/SE98/02146
exchanged for a buffer consisting of bicarbonate.
As is known, bicarbonate cannot, however, be combined with
calcium or magnesium in the large compartment since this may
lead to precipitation of, for example, carbonates during long
term storage or at high pH. Accordingly, bicarbonate and
calcium/magnesium must be separated until shortly before use.
One option would be to include calcium in' the small
compartments containing glucose and bicarbonate in the large
compartment. Glucose and calcium/magnesium are largely
compatible, i.e. they do not precipitate. However, the
divalent ions catalyse glucose decomposition to a certain
extent. During mixing of the contents of the small compartment
with the contents of the large compartment shortly before use,
the risk of .precipitation of carbonates is low since the pH-
value of the entire solution is maintained well below
pH = 7.4.
A conventional peritoneal dialysis solution has a calcium
ion concentration of 1.75 mM (mmol/1) and a magnesium ion
concentration between 0.25-0.50 mM. The buffer may be solely
bicarbonate which thereby normally exists at a concentration
of 30-40 mM or a combination of bicarbonate and lactate, for
example 25 mM bicarbonate and 15 mM lactate.
In the following, only calcium ions will be considered
since they are of the greatest clinical significance, although
the principles of the invention is, of. course, equally
applicable to magnesium ions.
If the above solution were, however, to be used in a three
compartment container of the type which is described in
WO 97/05852, a problem arises if the calcium ions are included
in the small compartments in one and the same concentration.
When the contents of one of the small compartments are mixed
with the contents of the large compartment, a concentration of
1:5o glucose and, for example, 1.75 mM calcium is created,
which is regulated by the quantity of glucose and the
concentration of calcium in the first small compartment. If
the contents of the second small compartment are mixed with
the contents of the large compartment, a concentration of 2.50
glucose and 1.75 mM calcium results, which is regulated by the
CA 02311903 2000-OS-26
WO 99/27885 PCT/SE98/02146
3
quantity of glucose and the concentration of calcium in the
second small compartment. If, however, the third -glucose
concentration of 4% is to be obtained, the contents of both
the first and the second small compartments are mixed with the
contents of the large compartment, whereby a glucose
concentration of 4o is obtained, but the calcium concentration
becomes too great, in this case about 3.5 mM. It therefore does
not seem to be possible to provide a three compartment
container of this type offering the possibility of three
different glucose concentrations, since the calcium
concentration will be too high in the presence of the highest
glucose concentration. It would be neccesary to expand the
concept to a four compartment container, which, however, might
result in new problems of safety.
IS A further object of the present invention is to propose a
three compartment container of the above-mentioned type in
which the above-mentioned problem is solved.
The problematic issues with calcium for peritoneal
dialysis, as well as for haemodialysis, are complicated since
dialysis patients normally eat calcium carbonate to bind
phosphate which is present in food. A portion of the calcium
carbonate which is consumed together with food is absorbed in
the intestinal tract and increases the calcium concentration
in the blood. At the same time, the calcium carbonate binds
phosphate so that the phosphate load on the patient is
reduced. Phosphate is a molecule which is difficult to remove
from the body via dialysis since it does not pass easily
through membranes, whether they be synthetic membranes or
peritoneal membranes.
The calcium concentration in the peritoneal dialysis
solution which is generally recommended today is 1.75 mM. This
would seem to be a suitable compromise which corresponds to
the normal physiological concentration of free calcium in
blood, since calcium is present in blood both freely as well
as bound to protein, particularly albumin. If the calcium
concentration in the patient is increased, a removal of
calcium takes place, whereas if the concentration is too low,
calcium is supplied to the body.
CA 02311903 2000-OS-26
WO. 99/27885 PCT/S 698/02146
4
Should a surplus of calcium arise, hypercalciemia, the
following symptoms, among others, result: vomiting, dizziness,
reduced muscle function, ECG-disorders and necrosis
calcification.
Hypercalciemia is usually rectified by t:he patient avoiding
consumption of calcium carbonate for several days, whereupon
the calcium concentration quickly reduces.
On the other hand, hypocalciemia results in i.a. the
following symptoms: cramps, hyper-reflexes, spasms, ECG
changes and hyperparatyroidism.
Hypocalciemia is normally counteracted by increased intake
of calcium carbonate, while the calcium concentration in the
dialysis solution is normally kept constant.
It is recognized that the problematics with calcium are
difficult since the above-mentioned symptoms are difficult to
diagnose.
More recently, it has been proposed to further reduce the
calcium concentration in dialysis solution to 1.35, 1.25 or
even 1.0 mM. The reason is that the oral intake of calcium
carbonate can thereby be increased and further reduce the
phosphate charge on the patient. The risk for hypocalciemia
increases therewith and increased awareness for diagnosis and
treatment is necessary.
Earlier trials have shown that the transport of calcium
ions across the peritoneal membrane is affected by the glucose
concentration in the peritoneal dialysis solution. This effect
is, however, rarely discussed.
According to the present invention, however, this discovery
is used to solve the above-mentioned problem in a three
compartment container of the type which is described in
WO 97/05852 to thereby adapt this container to a buffer
comprising bicarbonate.
Our experiments with simulations of calcium transport
across the peritoneal membrane indicate the following
unexpected results. If a peritoneal dialysis solution is to be
neutral (in- and outflow the same) with regard to the calcium
transport during a four hour period, it should comprise about
1.2 mM calcium at 1.5o glucose, about 1.6 mM at 2.5% glucose
CA 02311903 2000-OS-26
WO 99/27885 PCT/SE98/02146
and, finally, about 2.3 mM calcium at 4.Oo glucose, i.e. the
calcium concentration should be substantially proportional to
the glucose concentration in the final solution.
This can be achieved in a three compartment container by
5 means of calcium being present in substantially the same
concentration in the two small glucose compartments. The final
glucose concentration in the mixed solution is determined by
the volume of the small compartments since the glucose
concentration is identical in the small glucose compartments,
for example 50%. This results in that the calcium
concentration will always remain proportional to the glucose
concentration. The higher the glucose concentration (greater
volume), the more ultrafiltration and thus also higher calcium
concentration in the mixed solution. The calcium concentration
is may in that respect be selected so that the patient either has
a net loss, net gain or neutral calcium balance irrespective
of the ultra- filtration and the glucose concentration.
A further object in the present invention is to provide a
three compartment container of the above-mentioned type with
bicarbonate in the large compartment and glucose together with
calcium in the two small compartments, whereby the increase in
the calcium concentration at the highest glucose concentration
results in better calcium control than previously. This
invention is completely different from previously known
methods in which the same calcium concentration has always
been used irrespective of the glucose concentration.
By using a peritoneal dialysis solution in which the
calcium concentration is proportional to the glucose
concentration, it is possible to use one and the same solution
in the small compartments, i.e. the equipment which is used to
fill the three compartment container need only produce
solutions having two different compositions, one for the large
compartment and one for the two small compartments. In this
manner, the manufacturing costs are reduced.
Thus, there is provided according to the invention a
container containing a medical solution, particularly for
peritoneal dialysis, consisting of a large compartment having
a volume which is sufficiently large to contain the finally
CA 02311903 2000-OS-26
W0,99/27885 PCT/SE98/02146
6
prepared medical solution, and at least two small compartments
which contain partial quantities of the medical solution which
are incompatible for long term storage with the contents of
the large compartment. The large compartment comprises
bicarbonate ions and the small compartments comprises glucose
and calcium ions.
According to the invention, it is an advantage if the
glucose concentration is substantially proportional to the
calcium ion concentration in the finally prepared medical
solution.
To obtain such proportional concentration, the two small
compartments may comprises solutions having the same
concentration of glucose and calcium, though having different
volumes, or the first of the small compartments contains
IS glucose and calcium ions with such a concentration that the
glucose concentration when mixing the contents of said first
small compartment with the contents of the large comparment
obtains a first predetermined value, such as 1.50, and the
calcium ion concentration obtains a second predetermined
value, such as 1.0 mM, while the second of the small
compartments contains glucose and calcium ions having such a
concentration so that the glucose concentration in the finally
prepared solution when mixing the contents of said second
small compartment with the contents of the large comparment
obtains a third predetermined value, such as 2.5%, and the
calcium ion concentration obtains a fourth predetermined
value, such as 1.6 mM, and finally the contents of the first
and the second of the small compartments may be mixed with the
contents of the large compartment to obtain a finally prepared
solution with higher concentrations of glucose and calcium
ions, such as 4% glucose concentration and 2.5 mM calcium.
The small compartments may further comprise magnesium ions,
for example in a concentration such that the resulting
concentration in the finally prepared medical solution is
about 0.25-0.75 mM.
The invention also relates to a method for preparing a
medical solution, particularly for peritoneal dialysis, in a
container consisting of a first large compartment having a
CA 02311903 2000-OS-26
WO 99/27885 PCT/SE98/02146
7
volume which is sufficiently large to contain the finally
prepared medical solution, and a plurality of small
compartments which contain partial quantities of the medical
solution which are not compatible for long term storage with
the contents of the large compartment. The large compartment
comprises bicarbonate ions and the small compartments comprise
glucose and calcium ions. The contents of one or more of the
small compartments are mixed with the contents of the large
compartment to produce a medical solution in which the
concentration of glucose is substantially proportional to the
concentration of calcium ions.
Finally, the invention also relates to a use of a container
for preparation of a peritoneal dialysis solution in which the
concentrations of glucose and calcium ions are substantially
IS proportional.
BRIEF DESCRIPTION OF THE DRAWINGS
Further objects, problems, solutions and features will be
apparent from the following detailed description of the
invention with reference to the drawings describing several
embodiments of the invention.
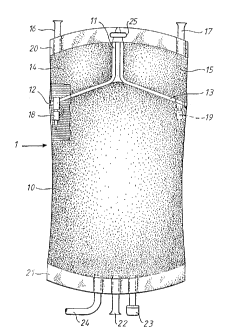
Fig. 1 is a plan view of a container which may be used in
the present invention.
Figs. 2, 3 and 4 are computer-simulated diagrams which show
the calcium balance across the peritoneal membrane.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Fig. 1 discloses a three compartment container which may be
used in the present invention. The container 1 is of the type
which is sealed at both ends with weld seams 20 and 21. The
container is divided into a large compartment 10 and two small
compartments 14 and 15 by weld seams 11, 12 and 13.
The small compartment 14 and/or 15 may communicate with the
large compartment 10 by means of frangible pins 18, 19 which
are normally sealed but can be manually opened. When the
frangible pin 18 and/or 19 is opened, the contents in the
compartments 14 and/or 15 flow down into the large compartment
10 and mix with the contents therein.
CA 02311903 2000-OS-26
WO~ 99/27885 PCT/SE98/02146
8
During manufacture of the container, suitable solution is
supplied to the various compartments 10, 7_4 and 15 via inlet
tubes 16, 17 and 22 extending through the weld seams 20 and
21. A sample and/or infusion port 23 extending through the
weld seam 21 permits sampling from, and infusion to, the
compartment 10.
The solution in the compartment 10 is supplied to a patient
via a conduit 24. The container is placed on a stand by means
of an opening 25 in the upper weld seam 20. The bag described
so far is substantially identical to the one disclosed in
WO 97/05852.
The container preferably has a size such that two litres of
peritoneal dialysis solution are accommodated in the large
compartment 10 in a ready-mixed state. Larger or smaller
containers are of course feasible according to the invention.
We have studied the transport of calcium ions across the
peritoneal membrane as a function of time, during treatment
with a peritoneal dialysis solution comprising glucose as well
as different concentrations of calcium ions. Figs. 2, 3 and 4
show the results for glucose concentrations of 1.50, 2.5o and
40, respectively. It is apparent that the calcium transport is
not only dependent on the concentration gradient across the
peritoneal membrane, but also ultrafiltration contributes. A
normal CAPD-treatment requires that the patient carries a PD-
solution for four hours (240 minutes) and then exchanges it
for a fresh PD-solution. If such a PD-solution should result
in zero transport of calcium across the peritoneal membrane
during the 240 minute period, calcium concentrations of 1.2
mM, 1.6 mM and 2.3 mM respectively are required.
The calculations presuppose certain properties of i.a. the
peritoneal membrane. We have concluded that a calcium
concentration which is substantially proportional to the
glucose concentration results in a substantially calcium-
neutral treatment, i.e. the calcium transport across the
peritoneal membrane will be substantially the same,
independent of the selected glucose concentration.
In accordance with the present invention, the container 1
is to be used to prepare a peritoneal dialysis solution in
CA 02311903 2000-OS-26
WO~ 99/27885 PCT/SE98/02146
9
which the buffer consists of bicarbonate. Accordingly, the
solutions in the various compartments may be the following.
EXAMPLE 1
The compartment 10 contains 1900 ml with the composition:
sodium bicarbonate 40 mM
sodium chloride 132 mM
Compartment 14 contains 100 ml of the composition:
glucose 30%
calcium chloride 20 mM
magnesium chloride 5 mM
sodium chloride 132 mM
Compartment 15 contains 100 ml of the composition:
glucose 50%
calcium chloride 33 mM
magnesium chloride 8 mM
sodium chloride 132 mM
By mixing the contents of compartment 14 and compartment
10, a peritoneal dialysis solution is obtained with the
following composition:
glucose 1.50
calcium 1.0 mM
bicarbonate 38 mM
sodium 132 mM
magnesium 0.25 mM
Mixing the contents of compartment 15 and compartment 10
results in a dialysis solution having the composition:
CA 02311903 2000-OS-26
W O. 99/27885 PCT/S E 98/02146
10
glucose 2.50
calcium 1.65 mM
bicarbonate 38 mM
sodium 132 mM
magnesium 0.4 mM
By mixing the contents of both compartments l4~and 15 with
the contents of compartment
10, a dialysis solution is
obtained having the followi ng composition:
glucose 4.0%
calcium 2.5 mM
bicarbonate 36 mM
sodium 132 mM
magnesium 0.6 mM
In a second example of the invention, the contents of each
of the small compartments have the same composition according
to the following:
EXAMPLE 2 '
Compartment 10 contains 1950 ml of the composition:
sodium bicarbonate 25 mM
sodium lactate 15 mM
sodium chloride 132 mM
Compartment 14 contains 60 ml of the composition:
glucose 500
calcium chloride 33 mM
magnesium chloride 8 mM
sodium chloride 132 mM
CA 02311903 2000-OS-26
WO '99/27885 PCT/SE98/02146
Compartment 15 contains 100 ml of the composition:
glucose 500
calcium chloride 33 mM
magnesium chloride 8 mM
sodium chloride 132 mM
In a further alternative embodiment of the present
invention, solutions are employed with the following
compositions in the various compartments 10, 14 and 15.
EXAMPLE 3
Compartment 10 contains 1850 ml of the composition:
IS sodium bicarbonate 27 mM
sodium lactate 16 mM
sodium chloride 132 mM
Compartment 14 contains 150 ml of the composition:
glucose 200
calcium chloride 13 mM
magnesium chloride 3.2 mM
sodium chloride 132 mM
Compartment 15 contains 260 ml of the composition:
glucose 200
calcium chloride 12.5 mM
magnesium chloride 3.1 mM
sodium chloride 132 mM
Because of the effect of dilution, the calcium
concentration will be somewhat lower than in the first two
examples.
To avoid problems with high pH-values, the pH-value in the
large compartment is normally adjusted to a pH = 7.2. The pH-
value in the small compartments is normally low, about 3.0, in
CA 02311903 2000-OS-26
WO 99127885 PCT/SE98/02146
12
order to prevent glucose decomposition during autoclaving.
When a patient uses a container according to the present
invention, he will experience a calcium inflow or outflow
which is determined by the concentration of free calcium in
the blood and the concentration of calcium in the finally
prepared dialysis solution as well as the glucose
concentration. In the case with the highest a1»~~.~P
concentration, the net effect will be approximately the same
as with the two lower concentrations and the use of the
container according to the invention is, therefore, easy and
simple for the patient.
The invention has been described above with reference to
preferred embodiments of the invention. A skilled person will
recognize that further combinations are possible.
is Modifications which are appearant to a skilled person are
intended to be incorporated within the scope of the invention.
The invention is limited only by the appended claims.