Note: Descriptions are shown in the official language in which they were submitted.
CA 02312703 2000-06-OS
1
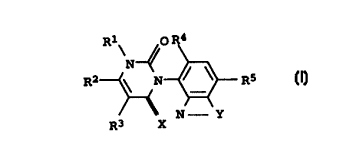
HERBICIDES 3-(BENZAZOL-4-YL)PYRIMIDINE-DIONE-DERIVATIVES
The present invention relates to novel
3-(benzazol-4-yl)pyrimidinedione derivatives of the formula I
R1~ O R4
N~ - ~ ~ 5
R2 ~ N R
Ir
N- Y
R3 X
where the variables have the following meanings:
X is oxygen or sulfur;
R1 is hydrogen, amino, C1-C6-alkyl or C1-C6-haloalkyl;
R2 is hydrogen, halogen, C1-C6-alkyl, C1-C6-haloalkyl,
C1-C6-alkylthio, C1-C6-alkylsulfinyl or C1-C6-alkylsulfonyl;
R3 is hydrogen, halogen or C1-C6-alkyl;
R4 is hydrogen or halogen;
RS is cyano, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy
or C1-C6-haloalkoxy;
=Y- is a group =N-N(R6)-, =C(ZR~)-N(R6)-, =C(ZR7)-O- or
=C(ZR7)-S-;
R6 is C1-C6-alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl,
C3-C6-alkenyl, C3-C6-alkynyl, C1-C6-alkylsulfonyl,
(C1-C6-alkyl)carbonyl, (C1-C6-haloalkyl)carbonyl,
(C1-C6-alkyl)thiocarbonyl, (C1-C6-alkoxy)carbonyl,
(C1-C6-alkoxy)thiocarbonyl or
C1-C6-alkyl which can be substituted by cyano, C1-C6-alkoxy,
C1-C6-alkylthio, (C1-C6-alkoxy)carbonyl,
(C1-C6-alkylamino)carbonyl, di(C1-C6-alkyl)aminocarbonyl or
(C1-C6-alkyl)carbonyloxy;
CA 02312703 2000-06-OS
0050/48647
2
Z is a chemical bond, oxygen, sulfur, -S(O)-, -S(O)2-, -NH- or
R~ and Re independently of one another are
C1-C6-alkyl, C1-C6-haloalkyl, hydroxy-C1-C4-alkyl,
cyano-C1-C4-alkyl, C1-C4-alkoxy-C1-C4-alkyl,
C1-C4-haloalkoxy-C1-C4-alkyl, C3-C4-alkenyloxy-C1-C4-alkyl,
C3-CQ-alkynyloxy-C1-C4-alkyl, C3-C$-cycloalkoxy-C1-C4-alkyl,
amino-C1-C4-alkyl, C1-C4-alkylamino-C1-C4-alkyl,
di(C1-C4-alkyl)amino-C1-C4-alkyl, C1-C4-alkylthio-C1-C4-alkyl,
C1-C4-haloalkylthio-C1-C4-alkyl, C3-C4-alkenylthio-C1-C4-alkyl,
C3-C4-alkynylthio-C1-C4-alkyl, C1-C4-alkylsulfinyl-C1-C4-alkyl,
Ci-C4-haloalkylsulfinyl-Cl-C4-alkyl,
C3-C4-alkenylsulfinyl-C1-C4-alkyl,
C3-C4-alkynylsulfinyl-C1-C4-alkyl,
C1-C4-alkylsulfonyl-C1-C4-alkyl,
C1-C4-haloalkylsulfonyl-C1-C4-alkyl,
C3-Ca-alkenylsulfonyl-C1-C4-alkyl,
C3-C4-alkynylsulfonyl-C1-C4-alkyl, C3-C6-alkenyl,
cyano-C3-C6-alkenyl, C3-C6-haloalkenyl, C3-C6-alkynyl,
cyano-C3-C6-alkynyl, C3-C6-haloalkynyl,
hydroxycarbonyl-C1-C4-alkyl,
(Ci-C4-alkoxy)carbonyl-C1-C4-alkyl,
(C1-C4-alkylthio)carbonyl-C1-C4-alkyl,
aminocarbonyl-C1-C4-alkyl,
(C1-C4-alkylamino)carbonyl-C1-C4-alkyl,
di(C1-C4-alkyl)aminocarbonyl-C1-C4-alkyl,
di(C1-C4-alkyl)phosphonyl-C1-C4-alkyl,
C3-Ce-cycloalkyl, C3-C8-cycloalkyl-C1-C4-alkyl, phenyl,
phenyl-C1-C4-alkyl, 3- to 7-membered heterocyclyl or
heterocyclyl-C1-C4-alkyl, it being possible for each
heterocyclyl ring to contain a carbonyl or thiocarbonyl ring
member,
and it being possible for each cycloalkyl, phenyl and
heterocyclyl ring to be unsubstituted or to have attached to
it one to four substituents, in each case selected from the
group consisting of cyano, nitro, amino, hydroxyl, carboxyl,
halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, C1-C4-alkylthio, C1-C4-haloalkylthio,
C1-C4-alkylsulfonyl, C1-C4-haloalkylsulfonyl,
(C1-C4-alkoxy)carbonyl, (C1-C4-alkyl)carbonyl,
(C1-C4-haloalkyl)carbonyl, (C1-C4-alkyl)carbonyloxy,
(C1_C4-haloalkyl)carbonyloxy and di(C1-C4-alkyl)amino,
' CA 02312703 2000-06-OS
0050/48647
3
or, if z is a chemical bond, R7 is, if desired, also
hydrogen, hydroxyl, cyano, mercapto, amino, halogen,
-CH(OH)-CH2-R9 , -CH(halogen)-CHz-R9, -CH2-CH(halogen)-R9,
-CH=CH-R9 or -CH=C(halogen)-R9, where
R9 is hydroxycarbonyl, (C1-C4-alkoxy)carbonyl,
(C1-CQ-alkylthio)carbonyl, aminocarbonyl,
(C1-CQ-alkylamino)carbonyl or di(C1-C4-alkyl)aminocarbonyl,
or R7 and R8 together are a 1,3-propylene, tetramethylene,
pentamethylene or ethyleneoxyethylene chain which can in each
case be unsubstituted or have attached to it one to four
C1-C4-alkyl groups or one or two (C1-C4-alkoxy)carbonyl
groups;
and to the agriculturally useful salts of the compounds I.
Moreover, the invention relates to
_ the use of the compounds I as herbicides,
- herbicidal compositions which comprise the compounds I as
active substances,
processes for the preparation of the compounds I and of
herbicidal compositions using the compounds I,
- methods of controlling undesirable vegetation with the
compounds I, and
- intermediates of the formulae III, IV and V for the preparation
of the compounds I.
WO 97/08170 describes certain 3-(benzox/benzothiazol-7-yl)-6-
(trifluoromethyl)uracils as herbicides. Other
3-(benzothiazol-7-yl)uracils and their use as herbicides and for
the desiccation/defoliation of plants are taught in WO 97/08171.
gubject-matter of WO 97/12886 are, inter alia, certain
3-benzisoxazol-7-yl-2,4-(1H,3H)pyrimidinediones, which are said
to have a herbicidal and desiccant action.
It is an object of the present invention to provide novel
herbicidally active uracil compounds which allow better targeted
control of undesirable plants than was possible with the known
compounds.
We have found that this object is achieved by the present
3-(benzazol-4-yl)pyrimidinedione derivatives of the formula I.
CA 02312703 2000-06-OS
0050/48647
4
There have furthermore been found herbicidal compositions which
comprise the compounds I and which have a very good herbicidal
action. Moreover, there have been found processes for the
preparation of these compositions and methods of controlling
undesirable vegetation with the compounds I.
Depending on the substitution pattern, the compounds of the
formula I can have one or more chiral centers, in which case they
exist as enantiomer or diastereomer mixtures. In the case of
compounds I having at least one olefinic radical, E/Z isomers may
also be possible. The present invention relates to the pure
enantiomers or diastereomers and also to mixtures of these.
The formula I only represents one of the possible versions for
some compounds according to the invention. Thus, for example,
those compounds I where R7 = hydroxyl may also be written as
tautomers I' [ -N=C ( OH ) -<---~-NH-CO- ]
\ O R4
R2 N
N R
I~
R3 X HN (O/S/N)
R6
O
Suitable amongst agriculturally useful salts are mainly the salts
of those cations or the acid addition salts of those acids whose
cations, or anions, respectively, do not adversely affect the
herbicidal action of the compounds I. Thus, suitable cations are,
in particular, the ions of the alkali metals, preferably sodium
and potassium, of the alkaline earth metals, preferably calcium,
magnesium and barium, and of the transition metals, preferably
manganese, copper, zinc and iron, and also the ammonium ion
which, if desired, can have attached to it one to four C1-C4-alkyl
substituents and/or a phenyl or benzyl substituent, preferably
diisopropylammonium, tetramethylammonium, tetrabutylammonium,
trimethylbenzylammonium, furthermore phosphonium ions, sulfonium
ions, preferably tri(C1-CQ-alkyl)sulfonium, and sulfoxonium ions,
preferably tri(C1-CQ-alkyl)sulfoxonium.
Anions of useful acid addition salts are, mainly, chloride,
bromide, fluoride, hydrogen sulfate, sulfate, dihydrogen
phosphate, hydrogen phosphate, phosphate, nitrate, hydrogen
carbonate, carbonate, hexafluorosilicate, hexafluorophosphate
CA 02312703 2000-06-OS
0050/48647
benzoate, and the anions of C1-CQ-alkanoic acids, preferably
formate, acetate, propionate and butyrate. They can be formed by
reacting I with an acid of the corresponding anion, preferably of
hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric
5 acid or nitric acid.
The organic moieties - like the meaning of halogen - which are
mentioned in the definition of the substituents R1 to R3 and R5 to
R9 or as radicals of cycloalkyl, phenyl or heterocyclic rings
represent collective terms for individual enumerations of the
individual group members. All carbon chains, ie. all alkyl,
haloalkyl, cyanoalkyl, hydroxyalkyl, aminoalkyl,
hydroxycarbonylalkyl, aminocarbonylalkyl, phenylalkyl,
heterocyclylalkyl, alkoxy, haloalkoxy, alkylthio, haloalkylthio,
alkylsulfinyl, haloalkylsulfinyl, alkylsulfonyl,
haloalkylsulfonyl, alkenyl, haloalkenyl, cyanoalkenyl,
alkenyloxy, alkenylthio, alkenylsulfinyl, alkenylsulfonyl,
alkynyl, haloalkynyl, cyanoalkynyl, alkynyloxy, alkynylthio,
alkynylsulfinyl and alkynylsulfonyl moieties, can be
straight-chain or branched. Halogenated substituents preferably
have attached to them one to five identical or different halogen
atoms. The meaning of halogen is in each case fluorine, chlorine,
bromine or iodine.
ether meanings are, for example:
- C1-C4-alkyl: CH3, CZHS, CHZ-CZHS, CH(CH3)Z, n-butyl, CH(CH3)-CZHS,
CH2-CH(CH3)2 Or C(CH3)3i
- C1-C4-haloalkyl: a C1-C4-alkyl radical as mentioned above which
is partially or fully substituted by fluorine, chlorine,
bromine and/or iodine, eg. CHZF, CHF2, CF3, CH2C1, CH(C1)Z,
C(C1)3, chlorofluoromethyl, dichlorofluoromethyl,
chlorodifluoromethyl, 2-fluoroethyl, 2-chloroethyl,
2-bromoethyl, 2-iodoethyl, 2,2-difluoroethyl,
2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl,
2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl,
2,2,2-trichloroethyl, C2F5, 2-fluoropropyl, 3-fluoropropyl,
2.2-difluoropropyl, 2,3-difluoropropyl, 2-chloropropyl,
3-chloropropyl, 2,3-dichloropropyl, 2-bromopropyl,
3-bromopropyl, 3,3,3-trifluoropropyl, 3,3,3-trichloropropyl,
CH2-CzFS, CFZ-C2F5, 1-(fluoromethyl)-2-fluoroethyl,
1-(chloromethyl)-2-chloroethyl, 1-(bromomethyl)-2-bromoethyl,
4-fluorobutyl, 4-chlorobutyl, 4-bromobutyl or nonafluorobutyl;
CA 02312703 2000-06-OS
0050/48647
6
- C1-C6-alkyl: a Cf-C4-alkyl radical as mentioned above or, for
example, n-pentyl, 1-methylbutyl, 2-methylbutyl, 3-methylbutyl,
2,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, 1,1-dimethylpropyl,
1,2-dimethylpropyl, 1-methylpentyl, 2-methylpentyl,
3-methylpentyl, 4-methylpentyl, 1,1-dimethylbutyl,
1,2-dimethylbutyl, 1,3-dimethylbutyl, 2,2-dimethylbutyl,
2,3-dimethylbutyl, 3,3-dimethylbutyl, 1-ethylbutyl,
2-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
1-ethyl-1-methylpropyl or 1-ethyl-2-methylpropyl, preferably
CH3. C2H5. CH2-C2H5, CH(CH3)2, n-butyl, C(CH3)3.
n-pentyl or n-hexyl;
- C1-C6-haloalkyl: a C1-C6-alkyl radical as mentioned above which
is partially or fully substituted by fluorine, chlorine,
bromine and/or iodine, ie. one of the radicals mentioned under
C1-C4-haloalkyl or 5-fluoro-1-pentyl, 5-chloro-1-pentyl,
5-bromo-1-pentyl, 5-iodo-1-pentyl, 5,5,5-trichloro-1-pentyl,
undecafluoropentyl, 6-fluoro-1-hexyl, 6-chloro-1-hexyl,
6-bromo-1-hexyl, 6-iodo-1-hexyl, 6,6,6-trichloro-1-hexyl or
dodecafluorohexyl;
- cyano-C1-C4-alkyl: CH2CN, 1-cyanoethyl, 2-cyanoethyl,
1-cyanoprop-1-yl, 2-cyanoprop-1-yl, 3-cyanoprop-1-yl,
1-cyanobut-1-yl, 2-cyanobut-1-yl, 3-cyanobut-1-yl,
4-cyanobut-1-yl, 1-cyanobut-2-yl, 2-cyanobut-2-yl,
3-cyanobut-2-yl, 4-cyanobut-2-yl, 1-(CHZCN)eth-1-yl,
1-(CHZCN)-1-(CH3)-eth-1-yl or 1-(CH2CN)prop-1-yl;
- hydroxy-C1-C9-alkyl: CH20H, 1-hydroxyethyl, 2-hydroxyethyl,
1-hydroxyprop-1-yl, 2-hydroxyprop-1-yl, 3-hydroxyprop-1-yl,
1-hydroxybut-1-yl, 2-hydroxybut-1-yl, 3-hydroxybut-1-yl,
4-hydroxybut-1-yl, 1-hydroxybut-2-yl, 2-hydroxybut-2-yl,
3-hydroxybut-2-yl, 4-hydroxybut-2-yl, 1-(CHzOH)eth-1-yl,
1-(CH20H)-1-(CH3)-eth-1-yl or 1-(CHZOH)prop-1-yl;
- amino-C1-C4-alkyl: CH2NH2, 1-aminoethyl, 2-aminoethyl,
1-aminoprop-1-yl, 2-aminoprop-1-yl, 3-aminoprop-1-yl,
1-amino-but-1-yl, 2-aminobut-1-yl, 3-aminobut-1-yl,
4-aminobut-1-yl, 1-aminobut-2-yl, 2-aminobut-2-yl,
3-aminobut-2-yl, 4-aminobut-2-yl, 1-(CH2NH2)eth-1-yl,
1-(CH2NH2)-1-(CH3)-eth-1-yl or 1-(CH2NH2)prop-1-yl;
- hydroxycarbonyl-C1-C4-alkyl: CH2COOH, 1-(COOH)ethyl,
2-(COOH)ethyl, 1-(COOH)prop-1-yl, 2-(COOH)prop-1-yl,
3-(COOH)prop-1-yl, 1-(COOH)but-1-yl, 2-(COOH)but-1-yl,
3-(COOH)but-1-yl, 4-(COOH)but-1-yl, 1-(COOH)but-2-yl,
2-(COOH)but-2-yl, 3-(COOH)but-2-yl, 4-(COOH)but-2-yl,
CA 02312703 2000-06-OS
0050/48647
7
1-(CH2COOH)eth-1-yl, 1-(CHZCOOH)-1-(CH3)-eth-1-yl or
1-(CHZCOOH)prop-1-yl;
- aminocarbonyl-C1-Cq-alkyl: CH2CONH2, 1-(CONHZ)ethyl,
2-(CONHZ)ethyl, 1-(CONH2)prop-1-yl, 2-(CONH2)prop-1-yl,
3-(CONH2)prop-1-yl, 1-(CONH2)but-1-yl, 2-(CONHZ)but-1-yl,
3-(CONH2)but-1-yl, 4-(CONHZ)but-1-yl, 1-(CONH2)but-2-yl,
2-(CONH2)but-2-yl, 3-(CONHZ)but-2-yl, 4-(CONHZ)but-2-yl,
1-(CH2CONH2)eth-1-yl, 1-(CH2CONHZ)-1-(CH3)-eth-1-yl or
1-(CH2CONH2)prop-1-yl;
- phenyl-C1-C4-alkyl: benzyl, 1-phenylethyl, 2-phenylethyl,
1-phenylprop-1-yl, 2-phenylprop-1-yl, 3-phenylprop-1-yl,
1-phenylbut-1-yl, 2-phenylbut-1-yl, 3-phenylbut-1-yl,
4-phenylbut-1-yl, 1-phenylbut-2-yl, 2-phenylbut-2-yl,
3-phenylbut-2-yl, 4-phenylbut-2-yl, 1-(benzyl)eth-1-yl,
1-(benzyl)-1-(methyl)eth-1-yl or 1-(benzyl)prop-1-yl,
preferably benzyl or 2-phenylethyl;
- heterocyclyl-C1-C4-alkyl: heterocyclylmethyl,
1-heterocyclylethyl, 2-heterocyclylethyl,
1-heterocyclylprop-1-yl, 2-heterocyclylprop-1-yl,
3-heterocyclylprop-1-yl, 1-heterocyclylbut-1-yl,
2-heterocyclylbut-1-yl, 3-heterocyclylbut-1-yl,
4-heterocyclylbut-1-yl, 1-heterocyclylbut-2-yl,
2-heterocyclylbut-2-yl, 3-heterocyclylbut-2-yl,
3-heterocyclylbut-2-yl, 4-heterocyclylbut-2-yl,
1-(heterocyclylmethyl)eth-1-yl,
1-(heterocyclylmethyl)-1-(methyl)eth-1-yl or
1-(heterocyclylmethyl)prop-1-yl, preferably heterocyclylmethyl
or 2-heterocyclylethyl;
- C1-C4-alkoxy: OCH3, OC2H5, OCH2-C2H5, OCH(CH3)2, n-butoxy,
OCH(CH3)-C2H5, OCH2-CH(CH3)z or C(CH3)3, preferably OCH3, OCZHS
or OCH(CH3)2:
- C1-C9-haloalkoxy: a C1-CQ-alkoxy radical as mentioned above
which is partially or fully substituted by fluorine, chlorine,
bromine and/or iodine, eg. OCHzF, OCHF2, OCF3, OCHZC1, OCH(C1)2,
pC(C1)3, chlorofluoromethoxy, dichlorofluoromethoxy,
chlorodifluoromethoxy, 2-fluoroethoxy, 2-chloroethoxy,
2-bromoethoxy, 2-iodoethoxy, 2,2-difluoroethoxy,
2,2,2-trifluoroethoxy, 2-chloro-2-fluoroethoxy,
2-chloro-2,2-difluoroethoxy, 2,2-dichloro-
2-fluoroethoxy, 2,2,2-trichloroethoxy, OC2F5, 2-fluoropropoxy,
3-fluoropropoxy, 2,2-difluoropropoxy, 2,3-difluoropropoxy,
2-chloropropoxy, 3-chloropropoxy, 2,3-dichloropropoxy,
CA 02312703 2000-06-OS
0050/48647
8
2-bromopropoxy, 3-bromopropoxy, 3,3,3-trifluoropropoxy,
3,3,3-trichloropropoxy, OCH2-CZFS, OCFZ-CZFS,
1-(CH2F)-2-fluoroethoxy, 1-(CHZC1)-2-chloroethoxy,
1-(CHZBr)-2-bromoethoxy, 4-fluorobutoxy, 4-chlorobutoxy,
4-bromobutoxy or nonafluorobutoxy, preferably OCHF2, OCF3,
dichlorofluoromethoxy, chlorodifluoromethoxy or
2,2,2-trifluoroethoxy;
- C1-C6-alkoxy: a C1-C4-alkoxy radical as mentioned above or, for
example, n-pentoxy, 1-methylbutoxy, 2-methylbutoxy,
3-methylbutoxy, 2,2-dimethylpropoxy, 1-ethylpropoxy, n-hexoxy,
1,1-dimethylpropoxy, 1,2-dimethylpropoxy, 1-methylpentoxy,
2-methylpentoxy, 3-methylpentoxy, 4-methylpentoxy,
1,1-dimethylbutoxy, 1,2-dimethylbutoxy, 1,3-dimethylbutoxy,
2,2-dimethylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethylbutoxy,
1-ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy,
1,2,2-trimethylpropoxy, 1-ethyl-1-methylpropoxy or
1-ethyl-2-methylpropoxy, preferably OCH3, OC2H5, OCH2-CZHS.
OCH(CH3)2, n-butoxy, OC(CH3)3, n-pentoxy or n-hexoxy;
- C1-C6-haloalkoxy: a C1-C6-alkoxy radical as mentioned above
which is partially or fully substituted by fluorine, chlorine,
bromine and/or iodine, ie. one of the radicals mentioned under
C1-C4-haloalkoxy or 5-fluoro-1-pentoxy, 5-chloro-1-pentoxy,
5-bromo-1-pentoxy, 5-iodo-1-pentoxy, 5,5,5-trichloro-1-pentoxy,
undecafluoropentoxy, 6-fluoro-1-hexoxy, 6-chloro-1-hexoxy,
6-bromo-1-hexoxy, 6-iodo-1-hexoxy, 6,6,6-trichloro-1-hexoxy or
dodecafluorohexoxy;
- C1-C4-alkylthio: SCH3, SCZHS, SCH2-CZHS, SCH(CH3)2, n-butylthio,
SCH(CH3)-CzHS, SCH2-CH(CH3)2 or SC(CH3)3, preferably SCH3 or
SCyHS;
C1-C4-haloalkylthio: a C1-C4-alkylthio radical as mentioned
above which is partially or fully substituted by fluorine,
chlorine, bromine and/or iodine, eg. SCHZF, SCHFz, SCF3, SCH2C1,
SCH(C1)2, SC(C1)3, chlorofluoromethylthio,
dichlorofluoromethylthio, chlorodifluoromethylthio,
2-fluoroethylthio, 2-chloroethylthio, 2-bromoethylthio,
2-iodoethylthio, 2,2-difluoroethylthio,
2,2,2-trifluoroethylthio, 2-chloro-2-fluoroethylthio,
2-chloro-2,2-difluoroethylthio, 2,2-dichloro-2-fluoroethylthio,
2,2,2-trichloroethylthio, SC2F5, 2-fluoropropylthio,
3-fluoropropylthio, 2,2-difluoropropylthio,
2,3-difluoropropylthio, 2-chloropropylthio, 3-chloropropylthio,
2,3-dichloropropylthio, 2-bromopropylthio, 3-bromopropylthio,
3,3,3-trifluoropropylthio, 3,3,3-trichloropropylthio,
CA 02312703 2000-06-OS
0050/48647
9
SCHZ-CzFs, SCF2-C2F5, 1-(CHZF)-2-fluoroethylthio,
1-(CH2C1)-2-chloroethylthio, 1-(CHZBr)-2-bromoethylthio,
4-fluorobutylthio, 4-chlorobutylthio, 4-bromobutylthio or
SCFZ-CF2-CZFS, preferably SCHF2, SCF3, dichlorofluoromethylthio,
chlorodifluoromethylthio or 2,2,2-trifluoroethylthio;
- C1-C6-alkylthio: a C1-C4-alkylthio radial as mentioned above or,
for example, n-pentylthio, 1-methylbutylthio,
2-methylbutylthio, 3-methylbutylthio, 2,2-dimethylpropylthio,
1-ethylpropylthio, n-hexylthio, 1,1-dimethylpropylthio,
1,2-dimethylpropylthio, 1-methylpentylthio, 2-methylpentylthio,
3-methylpentylthio, 4-methylpentylthio, 1,1-dimethylbutylthio,
1,2-dimethylbutylthio, 1,3-dimethylbutylthio,
2,2-dimethylbutylthio, 2,3-dimethylbutylthio,
3,3-dimethylbutylthio, 1-ethylbutylthio, 2-ethylbutylthio,
1,1,2-trimethylpropylthio, 1,2,2-trimethylpropylthio,
1-ethyl-1-methylpropylthio or 1-ethyl-2-methylpropylthio,
preferably SCH3, SCZHS, SCH2-CZHS, SCH(CH3)2. n-butylthio,
SC(CH3)3, n-pentylthio or n-hexylthio;
- C1-C4-alkoxy-C1-C4-alkyl: C1-C4-alkyl which is substituted by
C1-C4-alkoxy - as mentioned above -, eg. CHz-OCH3, CH2-OC2H5,
n-propoxymethyl, CH2-OCH(CH3)2, n-butoxymethyl,
(1-methylpropoxy)methyl, (2-methylpropoxy)methyl, CH2-OC(CH3)3.
2-(methoxy)ethyl, 2-(ethoxy)ethyl, 2-(n-propoxy)ethyl,
2-(1-methylethoxy)ethyl, 2-(n-butoxy)ethyl,
2-(1-methylpropoxy)ethyl, 2-(2-methylpropoxy)ethyl,
2-(1,1-dimethylethoxy)ethyl, 2-(methoxy)propyl,
2-(ethoxy)propyl, 2-(n-propoxy)propyl,
2-(1-methylethoxy)propyl, 2-(n-butoxy)propyl,
2-(1-methylpropoxy)propyl, 2-(2-methylpropoxy)propyl,
2-(1,1-dimethylethoxy)propyl, 3-(methoxy)propyl, 3-(ethoxy)-
propyl, 3-(n-propoxy)propyl, 3-(1-methylethoxy)propyl,
3-(n-butoxy)propyl, 3-(1-methylpropoxy)propyl,
3-(2-methylpropoxy)propyl, 3-(1,1-dimethylethoxy)propyl,
2-(methoxy)butyl, 2-(ethoxy)butyl, 2-(n-propoxy)butyl,
2-(1-methylethoxy)butyl, 2-(n-butoxy)butyl,
2-(1-methylpropoxy)butyl, 2-(2-methylpropoxy)butyl,
2-(1,1-dimethylethoxy)butyl, 3-(methoxy)butyl, 3-(ethoxy)butyl,
3-(n-propoxy)butyl, 3-(1-methylethoxy)butyl, 3-(n-butoxy)butyl,
3-(1-methylpropoxy)butyl, 3-(2-methylpropoxy)butyl,
3-(1,1-dimethylethoxy)butyl, 4-(methoxy)butyl, 4-(ethoxy)butyl,
4-(n-propoxy)butyl, 4-(1-methylethoxy)butyl, 4-(n-butoxy)butyl,
4-(1-methylpropoxy)butyl, 4-(2-methylpropoxy)butyl or
4-(1,1-dimethylethoxy)butyl, preferably CHz-OCH3, CH2-OCZHS,
2-(OCH3)ethyl or 2-(OC2H5)ethyl;
CA 02312703 2000-06-OS
0050/48647
- C1-C4-haloalkoxy-C1-C4-alkyl: C1-C4-alkyl which is substituted
by C1-C4-haloalkoxy as mentioned above, eg. 2-(OCHF2)ethyl,
2-(OCF3)ethyl or 2-(OC2F5)ethyl;
5 - C1-C4-alkylthio-C1-C4-alkyl: C1-C4-alkyl which is substituted by
C1-C9-alkylthio - as mentioned above -, eg. CH2-SCH3, CH2-SC2H5,
n-propylthiomethyl, CH2-SCH(CH3)2, n-butylthiomethyl,
(1-methylpropylthio)methyl, (2-methylpropylthio)methyl,
CH2-SC(CH3)3, 2-(methylthio)ethyl, 2-(ethylthio)ethyl,
10 2-(n-propylthio)ethyl, 2-(1-methylethylthio)ethyl,
2-(n-butylthio)ethyl, 2-(1-methylpropylthio)ethyl,
2-(2-methylpropylthio)ethyl, 2-(1,1-dimethylethylthio)ethyl,
2-(methylthio)propyl, 2-(ethylthio)propyl,
2-(n-propylthio)propyl, 2-(1-methylethylthio)propyl,
2-(n-butylthio)propyl, 2-(1-methylpropylthio)propyl,
2-(2-methylpropylthio)propyl, 2-(1,1-dimethylethylthio)propyl,
3-(methylthio)propyl, 3-(ethylthio)propyl,
3-(n-propylthio)propyl, 3-(1-methylethylthio)propyl,
3-(n-butylthio)propyl, 3-(1-methylpropylthio)propyl,
3-(2-methylpropylthio)propyl, 3-(1,1-dimethylethylthio)propyl,
2-(methylthio)butyl, 2-(ethylthio)butyl, 2-(n-propylthio)butyl,
2-(1-methylethylthio)butyl, 2-(n-butylthio)butyl,
2-(1-methylpropylthio)butyl, 2-(2-methylpropylthio)butyl,
2-(1,1-dimethylethylthio)butyl, 3-(methylthio)butyl,
3-(ethylthio)butyl, 3-(n-propylthio)butyl,
3-(1-methylethylthio)butyl, 3-(n-butylthio)butyl,
3-(1-methylpropylthio)butyl, 3-(2-methylpropylthio)butyl,
3-(1,1-dimethylethylthio)butyl, 4-(methylthio)butyl,
4-(ethylthio)butyl, 4-(n-propylthio)butyl,
4-(1-methylethylthio)butyl, 4-(n-butylthio)butyl,
4-(1-methylpropylthio)butyl, 4-(2-methylpropylthio)butyl or
4-(1,1-dimethylethylthio)butyl, preferably CH2-SCH3, CH2-SC2H5,
2-(SCH3)ethyl or 2-(SC2H5)ethyl;
- C1-Ca-haloalkylthio-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C1-C4-haloalkylthio as mentioned above, eg.
2-(SCHF2)ethyl, 2-(SCF3)ethyl or 2-(SC2F5)ethyl;
- (C1-C4-alkyl)carbonyl: CO-CH3, CO-C2H5, CO-CH2-C2H5, CO-CH(CHg)2,
n-butylcarbonyl, CO-CH(CH3)-C2H5, CO-CH2-CH(CH3)2 or CO-C(CH3)3.
preferably CO-CH3 or CO-C2H5;
- (C1-C4-haloalkyl)carbonyl: a (C1-C4-alkyl)carbonyl radical - as
mentioned above - which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, eg. CO-CH2F,
CO-CHF2, CO-CF3, CO-CH2C1, CO-CH(C1)2, CO-C(C1)3,
chlorofluoromethylcarbonyl, dichlorofluoromethylcarbonyl,
CA 02312703 2000-06-OS
0050/48647
11
chlorodifluoromethylcarbonyl, 2-fluoroethylcarbonyl,
2-chloroethylcarbonyl, 2-bromoethylcarbonyl,
2-iodoethylcarbonyl, 2,2-difluoroethylcarbonyl,
2,2,2-trifluoroethylcarbonyl, 2-chloro-2-fluoroethylcarbonyl,
2-chloro-2,2-difluoroethylcarbonyl,
2,2-dichloro-2-fluoroethylcarbonyl,
2,2,2-trichloroethylcarbonyl, CO-CZFS, 2-fluoropropylcarbonyl,
3-fluoropropylcarbonyl, 2,2-difluoropropylcarbonyl,
2,3-difluoropropylcarbonyl, 2-chloropropylcarbonyl,
3-chloropropylcarbonyl, 2,3-dichloropropylcarbonyl,
2-bromopropylcarbonyl, 3-bromopropylcarbonyl,
3,3,3-trifluoropropylcarbonyl, 3,3,3-trichloropropylcarbonyl,
CO-CH2-C2F5, CO-CFZ-C2F5, 1-(CH2F)-2-fluoroethylcarbonyl,
1-(CH2C1)-2-chloroethylcarbonyl,
1-(CH2Br)-2-bromoethylcarbonyl, 4-fluorobutylcarbonyl,
4-chlorobutylcarbonyl, 4-bromobutylcarbonyl or
nonafluorobutylcarbonyl, preferably CO-CF3, CO-CH2C1 or
2,2,2-trifluoroethylcarbonyl;
- (Ci-Cs-alkyl)carbonyl: one of the abovementioned
(C1-CQ-alkyl)carbonyl radicals or, for example, n-pentyl-CO,
1-methylbutyl-CO, 2-methylbutyl-CO, 3-methylbutyl-CO,
2,2-dimethylpropyl-C0, 1-ethylpropyl-CO, n-hexyl-C0,
1,1-dimethylpropyl-CO, 1,2-dimethylpropyl-CO,
1-methylpentyl-C0, 2-methylpentyl-CO, 3-methylpentyl-CO,
4-methylpentyl-CO, 1,1-dimethylbutyl-CO, 1,2-dimethylbutyl-CO,
1,3-dimethylbutyl-C0, 2,2-dimethylbutyl-CO,
2,3-dimethylbutyl-C0, 3,3-dimethylbutyl-CO, 1-ethylbutyl-CO,
2-ethylbutyl-CO, 1,1,2-trimethylpropyl-CO,
12.2-trimethylpropyl-CO, 1-ethyl-1-methylpropyl-CO or
1-ethyl-2-methylpropyl-C0, preferably CO-CH3, CO-C2H5,
CO-CH2-CZHS, CO-CH(CH3)2, n-butyl-CO, CO-C(CH3)3, CO-(n-C5H11) or
CO-(n-C6H13):
- (C1-Cs-haloalkyl)carbonyl: a (C1-CS-alkyl)carbonylrest - as
mentioned above - which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, eg. CO-CHZF,
CO-CHF2, CO-CF3, CO-CH2C1, CO-CH(C1)z, CO-C(C1)3,
chlorofluoromethylcarbonyl, dichlorofluoromethylcarbonyl,
chlorodifluoromethylcarbonyl, 2-fluoroethylcarbonyl,
2-chloroethylcarbonyl, 2-bromoethylcarbonyl,
2-iodoethylcarbonyl, 2,2-difluoroethylcarbonyl,
2,2,2-trifluoroethylcarbonyl, 2-chloro-2-fluoroethylcarbonyl,
2-chloro-2,2-difluoroethylcarbonyl,
2~2-dichloro-2-fluoroethylcarbonyl,
2,2,2-trichloroethylcarbonyl, CO-C2F5, 2-fluoropropylcarbonyl,
3-fluoropropylcarbonyl, 2,2-difluoropropylcarbonyl,
2,3-difluoropropylcarbonyl, 2-chloropropylcarbonyl,
CA 02312703 2000-06-OS
0050/48647
12
3-chloropropylcarbonyl, 2,3-dichloropropylcarbonyl,
2-bromopropylcarbonyl, 3-bromopropylcarbonyl,
3,3,3-trifluoropropylcarbonyl, 3,3,3-trichloropropylcarbonyl,
CO-CHZ-C2F5, CO-CFZ-C2F5, 1-(CHZF)-2-fluoroethylcarbonyl,
1-(CH2C1)-2-chloroethylcarbonyl,
1-(CH2Br)-2-bromoethylcarbonyl, 4-fluorobutylcarbonyl,
4-chlorobutylcarbonyl, 4-bromobutylcarbonyl or
nonafluorobutylcarbonyl, preferably CO-CF3, CO-CHZC1 or
2,2,2-trifluoroethylcarbonyl;
- (C1-C4-alkyl)carbonyloxy: 0-CO-CH3, O-CO-C2H5, 0-CO-CH2-C2H5,
0-CO-CH(CH3)2, 0-CO-CHz-CHZ-C2H5, 0-CO-CH(CH3)-CZHS,
0-CO-CH2-CH(CH3)2 or 0-CO-C(CH3)3, preferably 0-CO-CH3 or
O-CO-CzHS;
- (C1-C4-haloalkyl)carbonyloxy: a (C1-C4-alkyl)carbonyl radical -
as mentioned above - which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, eg. O-CO-CH2F,
O-CO-CHF2, O-CO-CF3, 0-CO-CHZCl, O-CO-CH(C1)z, O-CO-C(C1)3,
chlorofluoromethylcarbonyloxy, dichlorofluoromethylcarbonyloxy,
chlorodifluoromethylcarbonyloxy, 2-fluoroethylcarbonyloxy,
2-chloroethylcarbonyloxy, 2-bromoethylcarbonyloxy,
2-iodoethylcarbonyloxy, 2,2-difluoroethylcarbonyloxy,
2,2,2-trifluoroethylcarbonyloxy,
2-chloro-2-fluoroethylcarbonyloxy,
2-chloro-2,2-difluoroethylcarbonyloxy, 2,2-dichloro-
2-fluoroethylcarbonyloxy, 2,2,2-trichloroethylcarbonyloxy,
0-CO-C2F5, 2-fluoropropylcarbonyloxy,
3-fluoropropylcarbonyloxy, 2,2-difluoropropylcarbonyloxy,
2.3-difluoropropylcarbonyloxy, 2-chloropropylcarbonyloxy,
3-chloropropylcarbonyloxy, 2,3-dichloropropylcarbonyloxy,
2-bromopropylcarbonyloxy, 3-bromopropylcarbonyloxy,
3,3,3-trifluoropropylcarbonyloxy,
3,3,3-trichloropropylcarbonyloxy, O-CO-CHy-CzFS, O-CO-CF2-C2F5,
1-(CH2F)-2-fluoroethylcarbonyloxy,
1-(CH2C1)-2-chloroethylcarbonyloxy,
1-(CHzBr)-2-bromoethylcarbonyloxy, 4-fluorobutylcarbonyloxy,
4-chlorobutylcarbonyloxy, 4-bromobutylcarbonyloxy or
nonafluorobutylcarbonyloxy, preferably O-CO-CF3, O-CO-CH2C1 or
2~2.2-trifluoroethylcarbonyloxy;
- (C1-C6-alkyl)carbonyloxy: one of the abovementioned
(C1-C4-alkyl)carbonyloxy radicals or, for example,
n-pentyl-COO, 1-methylbutyl-COO, 2-methylbutyl-COO,
3-methylbutyl-COO, 2,2-dimethylpropyl-COO, 1-ethylpropyl-COO,
n-hexyl-COO, 1,1-dimethylpropyl-COO, 1,2-dimethylpropyl-COO,
1-methylpentyl-COO, 2-methylpentyl-COO, 3-methylpentyl-COO,
CA 02312703 2000-06-OS
0050/48647
13
4-methylpentyl-C00, 1,1-dimethylbutyl-C00,
1,2-dimethylbutyl-COO, 1,3-dimethylbutyl-COO,
2,2-dimethylbutyl-COO, 2,3-dimethylbutyl-C00,
3,3-dimethylbutyl-COO, 1-ethylbutyl-COO, 2-ethylbutyl-COO,
1,1,2-trimethylpropyl-COO, 1,2,2-trimethylpropyl-C00,
1-ethyl-1-methylpropyl-COO or 1-ethyl-2-methylpropyl-COO,
preferably O-CO-CHg, O-CO-CZHS, O-CO-CHZ-CzHS, O-CO-CH(CH3)2.
n-butyl-COO, 0-CO-C(CH3)3, 0-CO-(n-C5H11) or 0-CO-(n-C6H13):
- (C1-C6-alkyl)thiocarbonyl: CS-CH3, CS-C2H5, CS-CH2-CZHS,
CS-CH(CH3)2, CS-(n-C4Hg), CS-CH(CH3)-CzHS, CS-CH2-CH(CH3)2.
CS-C(CH3)3, CS-(n-C5H11). CS-CH(CH3)-CHZ-CZHS.
CS-CHZ-CH(CH3)-C2H5, CS-CH2CHy-CH(CH3)2, CS-C(CHg)2-CZHS,
CS-CH(CH3)-CH(CH3)2, CS-CHZ-C(CH3)3, CS-CH(CZHS)-C2H5,
CS-(n-C6H13), CS-CH(CH3)-(n-CqHg), CS-CH2-CH(CH3)-CH2-C2H5,
CS-CHZCH2-CH(CH3)-CZHS, CS-CHZCHZCH2-CH(CH3)y,
CS-C(CH3)2-CHZ-C2H5, CS-CH(CH3)-CH(CH3)-C2H5,
CS-CH(CHg)-CH2-CH(CH3)Z, CS-CHZ-C(CH3)2-CZHS,
CS-CH2-CH(CH3)-CH(CH3)y, CS-CHZCH2-C(CH3)3, CS-CH(C2H5)-CHZ-CZHS,
CS-CHy-CH(C2H5)-C2H5, CS-C(CH3)2-CH(CH3)y, CS-CH(CH3)-C(CH3)3,
CS-C(CH3)(C2H5)-CZHS Or CS-CH(CzHS)-CH(CH3)2, preferably CS-CH3,
CS-CZHS, CS-CH2-C2H5, CS-CH(CH3)2 Or CS-(n-C4Hg);
- (C1-C4-alkoxy)carbonyl: CO-OCH3, CO-OC2H5, CO-OCH2-C2H5,
CO-OCH(CH3)2, n-butoxycarbonyl, CO-OCH(CH3)-C2H5,
CO-OCH2-CH(CH3)z or CO-OC(CH3)3, preferably CO-OCH3 or CO-OC2H5;
- (C1-C6-alkoxy)carbonyl: one of the abovementioned
(C1-C4-alkoxy)carbonyl radicals or, for example, n-pentoxy-CO,
1-methylbutoxy-CO, 2-methylbutoxy-CO, 3-methylbutoxy-CO,
2,2-dimethylpropoxy-CO, 1-ethylpropoxy-CO, n-hexoxy-CO,
1,1-dimethylpropoxy-CO, 1,2-dimethylpropoxy-CO,
1-methylpentoxy-CO, 2-methylpentoxy-CO, 3-methylpentoxy-CO,
4-methylpentoxy-CO, 1,1-dimethylbutoxy-C0,
1,2-dimethylbutoxy-CO, 1,3-dimethylbutoxy-CO,
2,2-dimethylbutoxy-CO, 2,3-dimethylbutoxy-CO,
3,3-dimethylbutoxy-CO, 1-ethylbutoxy-CO, 2-ethylbutoxy-CO,
1,1,2-trimethylpropoxy-CO, 1,2,2-trimethylpropoxy-C0,
1-ethyl-1-methylpropoxy-CO or 1-ethyl-2-methylpropoxy-CO,
preferably CO-OCH3, CO-OC2H5, CO-OCHZ-CZHS, CO-OCH(CH3)2,
n-butoxy-CO, CO-OC(CH3)3, n-pentoxy-CO or n-hexoxy-CO;
- (C1-C4-alkoxy)carbonyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by (C1-C4-alkoxy)carbonyl - as mentioned above -,
eg. CH2-CO-OCH3, CH2-CO-OCZHS, CHz-CO-OCH2-CZHS,
CHz-CO-OCH(CH3)2, n-butoxycarbonylmethyl,
CH2-CO-OCH(CH3)-C2H5, CH2-CO-OCH2-CH(CH3)2, CH2-CO-OC(CH3)3r
CA 02312703 2000-06-OS
0050/48647
14
I-(CO-OCH3)ethyl, 1-(CO-OC2H5)ethyl, 1-(CO-OCHZ-C2H5)ethyl,
I-[CH(CH3)2]ethyl, 1-(n-butoxycarbonyl)ethyl,
1-[1-methylpropoxycarbonyl]ethyl,
1-[2-methylpropoxycarbonyl]ethyl, 2-(CO-OCH3)ethyl,
2-(CO-OCZHS)ethyl, 2-(CO-OCH2-C2H5)ethyl, 2-[CO-OCH(CH3)Z]ethyl,
2-(n-butoxycarbonyl)ethyl, 2-[1-methylpropoxycarbonyl]ethyl,
2-[2-methylpropoxycarbonyl]ethyl, 2-[CO-OC(CH3)3]ethyl,
2-(CO-OCH3)propyl, 2-(CO-OC2H5)propyl, 2-(CO-OCH2-CZHS)propyl,
2-[CO-OCH(CH3)Z]propyl, 2-(n-butoxycarbonyl)propyl,
2-[1-methylpropoxycarbonyl]propyl,
2-[2-methylpropoxycarbonyl]propyl, 2-[CO-OC(CH3)3]propyl,
3-(CO-OCH3)propyl, 3-(CO-OCZHS)propyl, 3-(CO-OCH2-C2H5)propyl,
3-[CO-OCH(CH3)2]propyl, 3-(n-butoxycarbonyl)propyl,
3-[1-methylpropoxycarbonyl]propyl,
3-[2-methylpropoxycarbonyl]propyl, 3-[CO-OC(CH3)3]propyl,
2-(CO-OCHg)butyl, 2-(CO-OCZHS)butyl, 2-(CO-0CH2-C2H5)butyl,
2-[CO-OCH(CH3)Z]butyl, 2-(n-butoxycarbonyl)butyl,
2-[1-methylpropoxycarbonyl]butyl,
2-[2-methylpropoxycarbonyl]butyl, 2-[CO-OC(CH3)3]butyl,
3-(CO-OCH3)butyl, 3-(CO-OCzHS)butyl, 3-(CO-OCH2-C2H5)butyl,
3-[CO-OCH(CH3)2]butyl, 3-(n-butoxycarbonyl)butyl,
3-[1-methylpropoxycarbonyl]butyl,
3-[2-methylpropoxycarbonyl]butyl, 3-[CO-OC(CH3)3]butyl,
4-(CO-OCH3)butyl, 4-(CO-OC2H5)butyl, 4-(CO-OCHZ-C2Hg)butyl,
4-ICO-OCH(CH3)Z]butyl, 4-(n-butoxycarbonyl)butyl,
4-[1-methylpropoxycarbonyl]butyl,
4-[2-methylpropoxycarbonyl]butyl or 4-[CO-OC(CH3)3]butyl,
preferably CH2-CO-OCH3, CH2-CO-OCZHS, 1-(CO-OCH3)ethyl or
1-(CO-OC2H5)ethyl;
- (C1-C6-alkoxy)thiocarbonyl: for example CS-OCH3, CS-OC2H5,
CS-OCHZ-CZHS, CS-OCH(CH3)z, CS-O(n-C4H9), CS-OCH(CH3)-CZHS,
CS-OCH2-CH(CHg)2, CS-OC(CH3)3, CS-O(n-CSHii).
CS-OCH(CH3)-CHZ-C2H5, CS-OCH2-CH(CH3)-CZHS, CS-OCH2CH2-CH(CH3)2,
CO-OCH2-C(CH3)3, CS-OCH(CZHS)-C2H5, CS-O(n-C6H13).
CS-OC(CH3)2-CZHS, CS-OCH(CH3)-CH(CH3)2, CS-OCH(CH3)-(n-C4H9),
CS-OCHz-CH(CH3)-CHy-C2H5, CS-OCH2CH2-CH(CH3)-C2H5,
CS-OCH2CH2CH2-CH(CH3)Z, CS-OC(CH3)2-CH2-C2H5,
CS-OCH(CH3)-CH(CH3)-C2H5, CS-OCH(CH3)-CH2-CH(CH3)z,
CS-OCH2-C(CH3)2-C2H5, CS-OCH2-CH(CH3)-CH(CH3)2,
CS-OCHyCHy-C(CH3)3, CS-OC(CyHS)-CHy-C2H5, CS-OCH2-CH(CZHS)-C2H5,
CS-OC(CH3)Z-CH(CH3)2, CS-OCH(CH3)-C(CH3)3, CS-OC(CH3)(C2H5)-C2H5
or CS-OCH(C2H5)-CH(CH3)2, preferably CS-OCH3 or CS-OC2H5;
(Ci-C4-alkylthio)carbonyl: CO-SCH3, CO-SC2H5, CO-SCHZ-C2H5,
CO-SCH(CH3)Z, CO-SCHyCH2-C2H5, CO-SCH(CH3)-CZHS, CO-SCH2-CH(CH3)2
or CO-SC(CH3)3, preferably CO-SCH3 or CO-SC2H5:
CA 02312703 2000-06-OS
0050/48647
- (C1-CQ-alkylthio)carbonyl-Cl-C4-alkyl: C1-CQ-alkyl which is
substituted by (C1-C4-alkylthio)carbonyl - as mentioned
above -, ie. CHZ-CO-SCH3, CHZ-CO-SC2H5,
CH2-CO-SCH2-C2H5, CH2-CO-SCH(CH3)2, CHy-CO-SCH2CHy-C2Hg,
5 CHZ-CO-SCH(CH3)-CZHS, CH2-CO-SCHy-CH(CH3)2, CH2-CO-SC(CH3)3.
1-(CO-SCH3)ethyl, 1-(CO-SC2H5)ethyl, 1-(CO-SCH2-C2H5)ethyl,
1-[CO-SCH(CH3)2]ethyl, 1-(CO-SCH2CH2-CzHS)ethyl,
1-[CO-SCH(CH3)-C2H5]ethyl, 1-[CO-SCHZ-CH(CH3)2]ethyl,
1-[CO-SC(CH3)3]ethyl, 2-(CO-SCH3)ethyl, 2-(CO-SC2H5)ethyl,
10 2-(CO-SCHZ-C2H5)ethyl, 2-[CO-SCH(CH3)Z]ethyl,
2-(CO-SCH2CH2-C2H5)ethyl, 2-[CO-SCH(CH3)-CzHS]ethyl,
2-[CO-SCHz-CH(CH3)2]ethyl, 2-[CO-SC(CHg)3]ethyl,
2-(CO-SCH3)propyl, 2-(CO-SCZHS)propyl, 2-(CO-SCHZ-C2H5)propyl,
2-[CO-SCH(CH3)2]propyl, 2-(CO-SCHZCH2-C2H5)propyl,
15 2-ICO-SCH(CH3)-C2H5]propyl, 2-[CO-SCHZ-CH(CH3)Z]propyl,
2-[CO-SC(CH3)3]propyl, 3-(CO-SCH3)propyl, 3-(CO-SC2H5)propyl,
3-(CO-SCH2-CZHS)propyl, 3-[CO-SCH(CH3)z]propyl,
3-(CO-SCH2CH2-C2H5)propyl, 3-[CO-SCH(CH3)-CZHS]propyl,
3-[CO-SCH2-CH(CH3)z]propyl, 3-[CO-SC(CH3)3]propyl,
2-(CO-SCH3)butyl, 2-(CO-SC2H5)butyl, 2-(CO-SCHZ-C2H5)butyl,
2-[CO-SCH(CH3)2]butyl, 2-(CO-SCH2CH2-C2H5)butyl,
2-[CO-SCH(CH3)-C2H5]butyl, 2-[CO-SCHZ-CH(CH3)2]butyl,
2-[CO-SC(CH3)3]butyl, 3-(CO-SCH3)butyl, 3-(CO-SC2H5)butyl,
3-(CO-SCH2-CZHS)butyl, 3-[CO-SCH(CH3)2]butyl,
3-(CO-SCH2CH2-C2H5)butyl, 3-[CO-SCH(CH3)-CZH5]butyl,
3-[CO-SCHZ-CH(CH3)2]butyl, 3-[CO-SC(CH3)3]butyl,
4-(CO-SCH3)butyl, 4-(CO-SC2H5)butyl, 4-(CO-SCH2-C2H5)butyl,
4-[CO-SCH(CH3)2]butyl, 4-(CO-SCHZCH2-C2H5)butyl,
4-[CO-SCH(CH3)-C2H5]butyl, 4-[CO-SCHZ-CH(CH3)2]butyl or
4-[CO-SC(CH3)3]butyl, preferably CH2-CO-SCH3, CH2-CO-SCzHS,
1-(CO-SCH3)ethyl or 1-(CO-SCZH5)ethyl;
- C1-C6-alkylsulfinyl: a C1-CQ-alkylsulfinyl radical such as
SO-CH3, SO-CZHS, SO-CH2-CZHg, SO-CH(CH3)2, SO-(n-CqHg),
SO-CH(CH3)-CZHS, SO-CHz-CH(CH3)2 or SO-C(CH3)3, or, for example,
SO-(n-CSH11), 1-methylbutyl-SO, 2-methylbutyl-S0,
3-methylbutyl-S0, 2,2-dimethylpropyl-S0, 1-ethylpropyl-S0,
n-hexyl-S0, 1,1-dimethylpropyl-SO, 1,2-dimethylpropyl-SO,
1-methylpentyl-SO, 2-methylpentyl-SO, 3-methylpentyl-SO,
4-methylpentyl-S0, 1,1-dimethylbutyl-S0, 1,2-dimethylbutyl-SO,
1,3-dimethylbutyl-S0, 2,2-dimethylbutyl-SO,
2,3-dimethylbutyl-S0, 3,3-dimethylbutyl-SO, 1-ethylbutyl-SO,
2-ethylbutyl-SO, 1,1,2-trimethylpropyl-S0,
1,2,2-trimethylpropyl-SO, 1-ethyl-1-methylpropyl-SO or
1-ethyl-2-methylpropyl-S0, preferably SO-CH3, SO-C2H5,
SO-CH2-C2H5, SO-CH(CH3)y, SO-(n-C4Hg), SO-C(CH3)3, SO-(n-C5H11)
or SO-(n-C6Hls):
CA 02312703 2000-06-OS
0050/48647
16
- C1-C4-alkylsulfinyl-C1-C4-alkyl: C1-CQ-alkyl which is
substituted by C1-C4-alkylsulfinyl as mentioned above, eg.
CHZSOCH3, CHySOC2H5, n-propylsulfinylmethyl, CHZSOCH(CH3)2,
n-butylsulfinylmethyl, (1-methylpropylsulfinyl)methyl,
(2-methylpropylsulfinyl)methyl, (1,1-dimethylethylsulfinyl)-
methyl, 2-methylsulfinylethyl, 2-ethylsulfinylethyl,
2-(n-propylsulfinyl)ethyl, 2-(1-methylethylsulfinyl)ethyl,
2-(n-butylsulfinyl)ethyl, 2-(1-methylpropylsulfinyl)ethyl,
2-(2-methylpropylsulfinyl)ethyl,
2-(1.1-dimethylethylsulfinyl)ethyl, 2-(SOCH3)propyl,
3-(SOCH3)propyl, 2-(SOC2H5)propyl, 3-(SOCZHS)propyl,
3-(propylsulfinyl)propyl, 3-(butylsulfinyl)propyl,
4-(SOCH3)butyl, 4-(SOCZHS)butyl, 4-(n-propylsulfinyl)butyl or
4-(n-butylsulfinyl)butyl, in particular 2-(SOCH3)ethyl;
- Cl-CQ-haloalkylsulfinyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C1-C4-haloalkylsulfinyl as mentioned above, eg.
2-(2,2,2-trifluoroethylsulfinyl)ethyl;
- C1-C4-alkylsulfonyl: SOZ-CH3, S02-C2H5, SOZ-CHZ-CZHS,
S02-CH(CH3)2, n-butylsulfonyl, S02-CH(CH3)-CZHS, S02-CHz-CH(CH3)2
or SOZ-C(CH3)3, preferably S02-CH3 or S02-CZHS;
- C1-C4-haloalkylsulfonyl: a C1-C4-alkylsulfonyl radical - as
mentioned above - which is partially or fully substituted by
fluorine, chlorine, bromine and/or iodine, eg. S02-CH2F,
SOZ-CHFy, S02-CF3, SOZ-CHyCl, S02-CH(C1)2, SOz-C(C1)3,
chlorofluoromethylsulfonyl, dichlorofluoromethylsulfonyl,
chlorodifluoromethylsulfonyl, 2-fluoroethylsulfonyl,
2-chloroethylsulfonyl, 2-bromoethylsulfonyl,
2-iodoethylsulfonyl, 2,2-difluoroethylsulfonyl,
2,2,2-trifluoroethylsulfonyl, 2-chloro-2-fluoroethylsulfonyl,
2-chloro-2,2-difluoroethylsulfonyl,
2,2-dichloro-2-fluoroethylsulfonyl,
2,2,2-trichloroethylsulfonyl, S02-C2F5, 2-fluoropropylsulfonyl,
3-fluoropropylsulfonyl, 2,2-difluoropropylsulfonyl,
2,3-difluoropropylsulfonyl, 2-chloropropylsulfonyl,
3-chloropropylsulfonyl, 2,3-dichloropropylsulfonyl,
2-bromopropylsulfonyl, 3-bromopropylsulfonyl,
3,3,3-trifluoropropylsulfonyl, 3,3,3-trichloropropylsulfonyl,
S02-CH2-CZFS. S02-CF2-C2F5.
1-(fluoromethyl)-2-fluoroethylsulfonyl,
1-(chloromethyl)-2-chloroethylsulfonyl,
1-(bromomethyl)-2-bromoethylsulfonyl, 4-fluorobutylsulfonyl,
4-chlorobutylsulfonyl, 4-bromobutylsulfonyl or
CA 02312703 2000-06-OS
0050/48647
17
nonafluorobutylsulfonyl, preferably S02-CH2C1, S02-CF3 or
2,2,2-trifluoroethylsulfonyl;
- C1-C6-alkylsulfonyl: a C1-C4-alkylsulfonyl radical as mentioned
above or, for example, S02-(n-CSH11), 1-methylbutyl-S02,
2-methylbutyl-502, 3-methylbutyl-S02, 2,2-dimethylpropyl-S02,
1-ethylpropyl-SOz, n-hexyl-502, 1,1-dimethylpropyl-S02,
1,2-dimethylpropyl-502, 1-methylpentyl-S02, 2-methylpentyl-S02,
3-methylpentyl-S02, 4-methylpentyl-S02, 1,1-dimethylbutyl-S02,
1,2-dimethylbutyl-S02, 1,3-dimethylbutyl-S02,
2,2-dimethylbutyl-S02, 2,3-dimethylbutyl-S02,
3,3-dimethylbutyl-S02, 1-ethylbutyl-S02, 2-ethylbutyl-502,
1,1,2-trimethylpropyl-S02, 1,2,2-trimethylpropyl-502,
1-ethyl-1-methylpropyl-S02 or 1-ethyl-2-methylpropyl-S02,
preferably S02-CH3, S02-C2H5, S02-CH2-C2H5, S02-CH(CH3)2.
S02-(n-CqHg), S02-C(CH3)3, S02-(n-C5H11) Or S02-(n-C6H13)1
- C1-C4-alkylsulfonyl-C1-CQ-alkyl: C1-C4-alkyl which is
substituted by C1-C4-alkylsulfonyl as mentioned above, eg.
CH2S02-CH3, CH2S02-C2H5, CH2S02-CH2-C2H5, CH2S02-CH(CH3)2,
CH2S02-CH2CH2-C2H5, (1-methylpropylsulfonyl)methyl,
(2-methylpropylsulfonyl)methyl, CH2S02-C(CH3)3, CH(CH3)S02-CH3,
CH(CH3)SOz-C2H5, CH2CH2S02-CH3, CH2CH2S02-C2H5, CH2CHzS02-CH2-C2H5.
CH2CH2S02-CH(CH3)2, CH2CH2S02-CHyCH2-C2H5,
2-(1-methylpropylsulfonyl)ethyl,
2-(2-methylpropylsulfonyl)ethyl, CH2CH2S02-C(CH3)3.
2-(S02-CH3)propyl, 2-(S02-C2H5)propyl, 2-(S02-CH2-C2H5)propyl,
2-[S02-CH(CH3)2]propyl, 2-(S02-CH2CH2-C2H5)propyl,
2-(1-methylpropylsulfonyl)propyl,
2-(2-methylpropylsulfonyl)propyl, 2-[S02-C(CH3)3]propyl,
3-(S02-CH3)propyl, 3-(S02-C2H5)propyl, 3-(S02-CH2-C2H5)propyl,
3-[S02-CH(CH3)2]propyl, 3-(S02-CH2CH2-C2H5)propyl,
3-(1-methylpropylsulfonyl)propyl,
3-(2-methylpropylsulfonyl)propyl, 3-[S02-C(CH3)3]propyl,
2-(S02-CH3)butyl, 2-(S02-C2H5)butyl, 2-(S02-CH2-C2H5)butyl,
2-[S02-CH(CH3)2]butyl, 2-(S02-CH2CH2-C2Hg)butyl,
2-(1-methylpropylsulfonyl)butyl,
2-(2-methylpropylsulfonyl)butyl, 2-[S02-C(CH3)3]butyl,
3-(S02-CH3)butyl, 3-(S02-C2H5)butyl, 3-(S02-CHZ-C2Hg)butyl,
3-[S02-CH(CH3)2]butyl, 3-(S02-CH2CH2-C2H5)butyl,
3-(1-methylpropylsulfonyl)butyl,
3-(2-methylpropylsulfonyl)butyl, 3-[S02-C(CH3)3]butyl,
4-(S02-CH3)butyl, 4-(S02-C2H5)butyl, 4-(S02-CH2-C2H5)butyl,
4-[S02-CH(CH3)2]butyl, 4-(S02-CHZCH2-C2H5)butyl,
4-(1-methylpropylsulfonyl)butyl,
4-(2-methylpropylsulfonyl)butyl or 4-[S02-C(CH3)3]butyl, in
particular CH2CH2S02-CH3 or CH2CH2S02-C2H5;
CA 02312703 2000-06-OS
0050/48647
18
- C1-C4-haloalk~ylsulfonyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C1-C4-haloalkylsulfonyl as mentioned above, eg.
2-(2,2,2-trifluoroethylsulfonyl)ethyl;
- C1-C4-alkylamino-C1-C4-alkyl: C1-C4-alkyl which is substituted
by C1-Cq-alkylamino such as H3C-NH-, H5C2-NH-, n-propyl-NH-,
1-methylethyl-NH-, n-butyl-NH-, 1-methylpropyl-NH-,
2-methylpropyl-NH- and 1,1-dimethylethyl-NH-, preferably
H3C-NH- or H5C2-NH-, ie., for example, CHZCHZ-NH-CH3,
CH2CH2-N(CH3)2, CH2CHy-NH-CyHS or CHZCH2-N(CZHS)2;
- (C1-CQ-alkylamino)carbonyl: CO-NH-CH3, CO-NH-CZHS,
n-propylamino, CO-NH-CH(CH3)Z, CO-NH-CH2CHy-CZHS,
CO-NH-CH(CH3)-CzHS, CO-NH-CH2-CH(CH3)z or CO-NH-C(CH3)3.
preferably CO-NH-CH3 or CO-NH-CZHS;
- (C1-C6-alkylamino)carbonyl: one of the
(C1-C4-alkylamino)carbonyl radicals mentioned above or, for
example, CO-NH-(n-CSH11), 1-methylbutyl-NHCO-,
2-methylbutyl-NHCO-, 3-methylbutyl-NHCO-,
2,2-dimethylpropyl-NHCO-, 1-ethylpropyl-NHCO-, CO-NH-(n-C6H13).
1,1-dimethylpropyl-NHCO-; 1,2-dimethylpropyl-NHCO-,
1-methylpentyl-NHCO-, 2-methylpentyl-NHCO-,
3-methylpentyl-NHCO-, 4-methylpentyl-NHCO-,
1,1-dimethylbutyl-NHCO-, 1,2-dimethylbutyl-NHCO-,
1,3-dimethylbutyl-NHCO-, 2,2-dimethylbutyl-NHCO-,
2,3-dimethylbutyl-NHCO-, 3,3-dimethylbutyl-NHCO-,
1-ethylbutyl-NHCO-, 2-ethylbutyl-NHCO-,
1,1,2-trimethylpropyl-NHCO-, 1,2,2-trimethylpropyl-NHCO-,
1-ethyl-1-methylpropyl-NHCO- or 1-ethyl-2-methylpropyl-NHCO-,
preferably CO-NH-CH3, CO-NH-CZHS, CO-NH-CHZ-CZHS, CO-NH-CH(CH3)Z,
CO-NH-(n-C4H9), CO-NH-C(CH3)3, CO-NH-(n-C5H11) or
CO-NH-(n-C6H13)f
- (C1-C4-alkylamino)carbonyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted [lacuna] (C1-C4-alkylamino)carbonyl as mentioned
above, preferably by CO-NH-CH3 or CO-NH-CZHS, as [sic] eg.
CHZ-CO-NH-CH3, CH2-CO-NH-C2H5, CH2-CO-NH-CHy-CzHS,
CH2-CO-NH-CH(CH3)2, CHZ-CO-NH-CH2CHz-CzHS,
CHZ-CO-NH-CH(CH3)-C2H5, CH2-CO-NH-CHZ-CH(CH3)p,
CHy-CO-NH-C(CH3)3, CH(CH3)-CO-NH-CH3, CH(CH3)-CO-NH-CzHS,
2-(CO-NH-CH3)ethyl, 2-(CO-NH-CZHS)ethyl,
2-(CO-NH-CHZ-C2H5)ethyl, 2-[CH2-CO-NH-CH(CH3)2]ethyl,
2-(CO-NH-CHZCHZ-CzHS)ethyl, 2-[CO-NH-CH(CH3)-CZHS]ethyl,
2-[CO-NH-CHZ-CH(CHg)2Jethyl, 2-[CO-NH-C(CH3)3]ethyl,
2-(CO-NH-CH3)propyl, 2-(CO-NH-C2H5)propyl,
2-(CO-NH-CHZ-C2H5)propyl, 2-[CHZ-CO-NH-CH(CH3)z]propyl,
CA 02312703 2000-06-OS
0050/48647
19
2-(CO-NH-CH2CH2-CzHS)propyl, 2-[CO-NH-CH(CH3)-C2H5]propyl,
2-[CO-NH-CHZ-CH(CH3)2]propyl, 2-[CO-NH-C(CH3)3]propyl,
3-(CO-NH-CHg)propyl, 3-(CO-NH-CZHS)propyl,
3-(CO-NH-CHZ-CZHS)propyl, 3-[CHy-CO-NH-CH(CH3)z]propyl,
3-(CO-NH-CHyCH2-C2H5)propyl, 3-[CO-NH-CH(CH3)-C2Hg]propyl,
3-[CO-NH-CH2-CH(CH3)y]propyl, 3-(CO-NH-C(CH3)3]propyl,
2~(CO-NH-CH3)butyl, 2-(CO-NH-C2H5)butyl,
2-(CO-NH-CHZ-CZHS)butyl, 2-[CH2-CO-NH-CH(CH3)2]butyl,
2-(CO-NH-CHZCH2-CyHS)butyl, 2-[CO-NH-CH(CH3)-C2H5]butyl,
2-[CO-NH-CH2-CH(CH3)2]butyl, 2-[CO-NH-C(CH3)3]butyl,
3-(CO-NH-CH3)butyl, 3-(CO-NH-C2H5)butyl,
3-(CO-NH-CH2-C2H5)butyl, 3-[CHz-CO-NH-CH(CH3)y]butyl,
3-(CO-NH-CHZCHZ-CZHS)butyl, 3-[CO-NH-CH(CH3)-CZHS]butyl,
3-[CO-NH-CHZ-CH(CH3)2]butyl, 3-[CO-NH-C(CH3)3]butyl,
4-(CO-NH-CH3)butyl, 4-(CO-NH-CZHS)butyl,
4-(CO-NH-CH2-C2H5)butyl, 4-[CH2-CO-NH-CH(CH3)2]butyl,
4-(CO-NH-CH2CH2-C2H5)butyl, 4-[CO-NH-CH(CH3)-CZHS]butyl,
4-[CO-NH-CH2-CH(CH3)2]butyl or 4-[CO-NH-C(CH3)3]butyl,
preferably CHZ-CO-NH-CH3, CHZ-CO-NH-C2H5, CH(CH3)-CO-NH-CH3 or
CH(CHg)-CO-NH-C2H5;
- di(C1-C4-alkyl)amino: N(CH3)Z, N(C2H5)2, N,N-dipropylamino,
N,N-di(1-methylethyl)amino, N,N-dibutylamino,
N,N-di(1-methylpropyl)amino, N,N-di(2-methylpropyl)amino,
N.N-di(1,1-dimethylethyl)amino, N-ethyl-N-methylamino,
N-methyl-N-propylamino, N-methyl-N-(1-methylethyl)amino,
N-butyl-N-methylamino, N-methyl-N-(1-methylpropyl)amino,
N-methyl-N-(2-methylpropyl)amino,
N-(1,1-dimethylethyl)-N-methylamino, N-ethyl-N-propylamino,
N-ethyl-N-(1-methylethyl)amino, N-butyl-N-ethylamino,
N-ethyl-N-(1-methylpropyl)amino,
N-ethyl-N-(2-methylpropyl)amino,
N-ethyl-N-(1,1-dimethylethyl)amino,
N-(1-methylethyl)-N-propylamino, N-butyl-N-propylamino,
N-(1-methylpropyl)-N-propylamino,
N-(2-methylpropyl)-N-propylamino,
N-(1,1-dimethylethyl)-N-propylamino,
N-butyl-N-(1-methylethyl)amino,
N-(1-methylethyl)-N-(1-methylpropyl)amino,
N-(1-methylethyl)-N-(2-methylpropyl)amino,
N-(1,1-dimethylethyl)-N-(1-methylethyl)amino,
N-butyl-N-(1-methylpropyl)amino,
N-butyl-N-(2-methylpropyl)amino,
N-butyl-N-(1,1-dimethylethyl)amino,
N-(1-methylpropyl)-N-(2-methylpropyl)amino,
N_(1,1-dimethylethyl)-N-(1-methylpropyl)amino or
CA 02312703 2000-06-OS
0050/48647
N-(1,1-dimethylethyl)-N-(2-methylpropyl)amino, preferably
N(CH3)2 or N(CzHS)27
- di(C1-C4-alkyl)amino-C1-C4-alkyl: C1-C4-alkyl which is
5 substituted by di(C1-C4-alkyl)amino as mentioned above, eg.
CH2N(CH3)2, CH2N(CZHS)Z, N,N-dipropylaminomethyl,
N,N-di[CH(CH3)Z]aminomethyl, N,N-dibutylaminomethyl, N,N-di-
(1-methylpropyl)aminomethyl, N,N-di(2-methylpropyl)aminomethyl,
N,N-di[C(CH3)3]aminomethyl, N-ethyl-N-methylaminomethyl,
10 N-methyl-N-propylaminomethyl, N-methyl-N-[CH(CH3)2]aminomethyl,
N-butyl-N-methylaminomethyl,
N-methyl-N-(1-methylpropyl)aminomethyl,
N-methyl-N-(2-methylpropyl)aminomethyl,
N-[C(CH3)3]-N-methylaminomethyl, N-ethyl-N-propylaminomethyl,
15 N-ethyl-N-[CH(CH3)2]aminomethyl, N-butyl-N-ethylaminomethyl,
N-ethyl-N-(1-methylpropyl)aminomethyl,
N-ethyl-N-(2-methylpropyl)aminomethyl,
N-ethyl-N-[C(CH3)3]aminomethyl,
N-[CH(CH3)Z]-N-propylaminomethyl, N-butyl-N-propylaminomethyl,
20 N-(1-methylpropyl)-N-propylaminomethyl,
N-(2-methylpropyl)-N-propylaminomethyl,
N-[C(CH3)3]-N-propylaminomethyl, N-butyl-N-(1-methylethyl)-
aminomethyl, N-[CH(CH3)2]-N-(1-methylpropyl)aminomethyl,
N-[CH(CH3)2]-N-(2-methylpropyl)aminomethyl, N-[C(CH3)3]-N-
[CH(CH3)Z]aminomethyl, N-butyl-N-(1-methylpropyl)aminomethyl,
N-butyl-N-(2-methylpropyl)aminomethyl, N-butyl-N-[C(CH3)3]-
aminomethyl, N-(1-methylpropyl)-N-(2-methylpropyl)aminomethyl,
N-[C(CH3)3]-N-(1-methylpropyl)aminomethyl, N-[C(CH3)a]-
N-(2-methylpropyl)aminomethyl, N,N-dimethylaminoethyl,
N~N-diethylaminoethyl, N,N-di(n-propyl)aminoethyl,
N,N-di[CH(CH3)2]aminoethyl, N,N-dibutylaminoethyl,
N,N-di(1-methylpropyl)aminoethyl,
N,N-di(2-methylpropyl)aminoethyl, N,N-di[C(CH3)3]aminoethyl,
N-ethyl-N-methylaminoethyl, N-methyl-N-propylaminoethyl,
N-methyl-N-[CH(CH3)Z]aminoethyl, N-butyl-N-methylaminoethyl,
N-methyl-N-(1-methylpropyl)aminoethyl,
N-methyl-N-(2-methylpropyl)aminoethyl,
N-[C(CH3)3]-N-methylaminoethyl, N-ethyl-N-propylaminoethyl,
N-ethyl-N-[CH(CH3)2]aminoethyl, N-butyl-N-ethylaminoethyl,
N-ethyl-N-(1-methylpropyl)aminoethyl,
N_ethyl-N-(2-methylpropyl)aminoethyl,
N-ethyl-N-[C(CH3)3]aminoethyl, N-[CH(CH3)2]-N-propylaminoethyl,
N-butyl-N-propylaminoethyl,
N-(1-methylpropyl)-N-propylaminoethyl,
N-(2-methylpropyl)-N-propylaminoethyl,
N-[C(CH3)3]-N-propylaminoethyl, N-butyl-N-[CH(CH3)2]aminoethyl,
N-[CH(CH3)Z]-N-(1-methylpropyl)aminoethyl,
N-[CH(CH3)Z]-N-(2-methylpropyl)aminoethyl,
CA 02312703 2000-06-OS
0050/48647
21
N-[C(CH3)3]-N-[CH(CH3)z]aminoethyl,
N-butyl-N-(1-methylpropyl)aminoethyl,
N-butyl-N-(2-methylpropyl)aminoethyl,
N-butyl-N-[C(CH3)3]aminoethyl,
N-(1-methylpropyl)-N-(2-methylpropyl)aminoethyl,
N-[C(CH3)3]-N-(1-methylpropyl)aminoethyl or
N-[C(CH3)3]-N-(2-methylpropyl)aminoethyl, in particular
N,N-dimethylaminoethyl or N,N-diethylaminoethyl;
- di(C1-C9-alkyl)aminocarbonyl: CO-N(CH3)z, CO-N(CzHs),
CO-N(CHZ-CZHS)z, CO-N[CH(CH3)zlz. N,N-dibutylaminocarbonyl,
CO-N[CH(CH3)-C2H5]z, CO-N[CHz-CH(CH3)z]z, CO-N[C(CH3)3]2.
N-ethyl-N-methylaminocarbonyl, N-methyl-N-propylaminocarbonyl,
N-methyl-N-[CH(CH3)2]aminocarbonyl,
N-butyl-N-methylaminocarbonyl,
N-methyl-N-(1-methylpropyl)aminocarbonyl,
N-methyl-N-(2-methylpropyl)aminocarbonyl,
N-[C(CH3)3]-N-methylaminocarbonyl,
N-ethyl-N-propylaminocarbonyl,
N-ethyl-N-[CH(CH3)z]aminocarbonyl,
N-butyl-N-ethylaminocarbonyl,
N-ethyl-N-(1-methylpropyl)aminocarbonyl,
N-ethyl-N-(2-methylpropyl)aminocarbonyl,
N-ethyl-N-[C(CH3)3]aminocarbonyl,
N-[CH(CH3)z]-N-propylaminocarbonyl,
N-butyl-N-propylaminocarbonyl,
N-(1-methylpropyl)-N-propylaminocarbonyl,
N-(2-methylpropyl)-N-propylaminocarbonyl,
N-[C(CH3)3]-N-propylaminocarbonyl,
N-butyl-N-[CH(CH3)z]aminocarbonyl,
N-[CH(CH3)Z]-N-(1-methylpropyl)aminocarbonyl,
N-[CH(CH3)z]-N-(2-methylpropyl)aminocarbonyl,
N-[C(CH3)3]-N-[CH(CH3)z]aminocarbonyl,
N-butyl-N-(1-methylpropyl)aminocarbonyl,
N-butyl-N-(2-methylpropyl)aminocarbonyl,
N-butyl-N-[C(CH3)3]aminocarbonyl,
N-(1-methylpropyl)-N-(2-methylpropyl)aminocarbonyl,
N-[C(CH3)3)-N-(1-methylpropyl)aminocarbonyl or
N-[C(CH3)3]-N-(2-methylpropyl)aminocarbonyl, preferably
CO-N(CH3)z or CO-N(CZHS)2i
- di(CI-C6-alkyl)aminocarbonyl: one of the abovementioned
di(C1-C4-alkyl)aminocarbonyl radicals or, for example,
N(CH3)-(n-C5H11)r N(C2H5)-(n-C5H11)r N(CH2-C2H5)-(n-C5H11)r
N(n C4H9) (n C5H11)r N(n-C5H11)-(n-C5H11)r N(n-C6H13)-(n-C5H11)r
N(CH3)-(n-C6H13)r N(C2H5)-(n-C6H13). N(CHz-CzHS)-(n-C6H13).
N(n-C4H9)-(n-C6H13)r N(n-C5H11)-(n-C6H13) Or N(n-C6H13)2i
CA 02312703 2000-06-OS
0050/48647
22
- di(C1-C4-alkyl)aminocarbonyl-C1-C4-alkyl: Ci-C9-alkyl which is
substituted by di(C1-C4-alkyl)aminocarbonyl as mentioned above,
preferably by CO-N(CH3)2 or CO-N(C2H5)2, eg. CHZ-CO-N(CHg)2,
CH2-CO-N(C2H5)Z, CH(CH3)-CO-N(CH3)Z or CH(CH3)-CO-N(CzHS)2.
preferably CHZ-CO-N(CH3)2 or CH(CH3)-CO-N(CH3)27
- di(C1-C4-alkyl)phosphonyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by di(C1-C4-alkyl)phosphonyl such as
-PO(OCH3)2, -PO(OCZHS)2, N,N-dipropylphosphonyl,
N,N-di(1-methylethyl)phosphonyl, N,N-dibutylphosphonyl,
N,N-di(1-methylpropyl)phosphonyl,
N,N-di(2-methylpropyl)phosphonyl,
N,N-di(l,l-dimethylethyl)phosphonyl,
N-ethyl-N-methylphosphonyl, N-methyl-N-propylphosphonyl,
N-methyl-N-(1-methylethyl)phosphonyl,
N-butyl-N-methylphosphonyl, N-methyl-N-(1-methylpropyl)-
phosphonyl, N-methyl-N-(2-methylpropyl)phosphonyl,
N-(1,1-dimethylethyl)-N-methylphosphonyl,
N-ethyl-N-propylphosphonyl,
N-ethyl-N-(1-methylethyl)phosphonyl, N-butyl-N-ethylphosphonyl,
N-ethyl-N-(1-methylpropyl)phosphonyl,
N-ethyl-N-(2-methylpropyl)phosphonyl,
N-ethyl-N-(1,1-dimethylethyl)phosphonyl,
N-(1-methylethyl)-N-propylphosphonyl,
N-butyl-N-propylphosphonyl,
N-(1-methylpropyl)-N-propylphosphonyl,
N-(2-methylpropyl)-N-propylphosphonyl,
N-(1,1-dimethylethyl)-N-propylphosphonyl,
N-butyl-N-(1-methylethyl)phosphonyl,
N-(1-methylethyl)-N-(1-methylpropyl)phosphonyl,
N-(1-methylethyl)-N-(2-methylpropyl)phosphonyl,
N-(1,1-dimethylethyl)-N-(1-methylethyl)phosphonyl,
N-butyl-N-(1-methylpropyl)phosphonyl,
N-butyl-N-(2-methylpropyl)phosphonyl,
N-butyl-N-(1,1-dimethylethyl)phosphonyl,
N-(1-methylpropyl)-N-(2-methylpropyl)phosphonyl,
N-(1,1-dimethylethyl)-N-(1-methylpropyl)phosphonyl or
N-(1,1-dimethylethyl)-N-(2-methylpropyl)phosphonyl, preferably
by -PO(OCH3)2 or
-PO(OC2H5)2, eg. CH2-PO(OCH3)Z, CH2-PO(OC2H5)2, CH(CH3)-PO(OCH3)2
or CH(CH3)-PO(OCZHS)2:
- C3-C6-alkenyl: prop-1-en-1-yl, allyl, 1-methylethenyl,
1-buten-1-yl, 1-buten-2-yl, 1-buten-3-yl, 2-buten-1-yl,
1-methylprop-1-en-1-yl, 2-methylprop-1-en-1-yl,
1-methylprop-2-en-1-yl, 2-methylprop-2-en-1-yl, n-penten-1-yl,
n-penten-2-yl, n-penten-3-yl, n-penten-4-yl,
1-methylbut-1-en-1-yl, 2-methylbut-1-en-1-yl,
CA 02312703 2000-06-OS
0050/48647
23
3-methylbut-1-en-1-yl, 1-methylbut-2-en-1-yl,
2-methylbut-2-en-1-yl, 3-methylbut-2-en-1-yl,
1-methylbut-3-en-1-yl, 2-methylbut-3-en-1-yl,
3-methylbut-3-en-1-yl, 1,1-dimethylprop-2-en-1-yl,
1,2-dimethylprop-1-en-1-yl, 1,2-dimethylprop-2-en-1-yl,
1-ethylprop-1-en-2-yl, 1-ethylprop-2-en-1-yl, n-hex-1-en-1-yl,
n-hex-2-en-1-yl, n-hex-3-en-1-yl, n-hex-4-en-1-yl,
n-hex-5-en-1-yl, 1-methylpent-1-en-1-yl,
2-methylpent-1-en-1-yl, 3-methylpent-1-en-1-yl,
4-methylpent-1-en-1-yl, 1-methylpent-2-en-1-yl,
2-methylpent-2-en-1-yl, 3-methylpent-2-en-1-yl,
4-methylpent-2-en-1-yl, 1-methylpent-3-en-1-yl,
2-methylpent-3-en-1-yl, 3-methylpent-3-en-1-yl,
4-methylpent-3-en-1-yl, 1-methylpent-4-en-1-yl,
2-methylpent-4-en-1-yl, 3-methylpent-4-en-1-yl,
4-methylpent-4-en-1-yl, 1,1-dimethylbut-2-en-1-yl,
1,1-dimethylbut-3-en-1-yl, 1,2-dimethylbut-1-en-1-yl,
1,2-dimethylbut-2-en-1-yl, 1,2-dimethylbut-3-en-1-yl,
1,3-dimethylbut-1-en-1-yl, 1,3-dimethylbut-2-en-1-yl,
I.3-dimethylbut-3-en-1-yl, 2,2-dimethylbut-3-en-1-yl,
2,3-dimethylbut-1-en-1-yl, 2,3-dimethylbut-2-en-1-yl,
2,3-dimethylbut-3-en-1-yl, 3,3-dimethylbut-1-en-1-yl,
3,3-dimethylbut-2-en-1-yl, 1-ethylbut-1-en-1-yl,
1-ethylbut-2-en-1-yl, 1-ethylbut-3-en-1-yl,
2-ethylbut-1-en-1-yl, 2-ethylbut-2-en-1-yl,
2-ethylbut-3-en-1-yl, 1,1,2-trimethylprop-2-en-1-yl,
1-ethyl-1-methylprop-2-en-1-yl, 1-ethyl-2-methylprop-1-en-1-yl
or 1-ethyl-2-methylprop-2-en-1-yl;
- C3-C6-haloalkenyl: C3-C6-alkenyl as mentioned above which is
partially or fully substituted by fluorine, chlorine, bromine
and/or iodine, eg. 2-chloroallyl, 3-chloroallyl,
2,3-dichloroallyl, 3,3-dichloroallyl, 2,3,3-trichloroallyl,
2,3-dichlorobut-2-enyl, 2-bromoallyl, 3-bromoallyl,
2~3-dibromoallyl, 3,3-dibromoallyl, 2,3,3-tribromoallyl or
2,3-dibromobut-2-enyl;
- cyano-C3-C6-alkenyl: for example 2-cyanoallyl, 3-cyanoallyl,
4-cyanobut-2-enyl, 4-cyanobut-3-enyl or 5-cyanopent-4-enyl;
- C3-C6-alkynyl: prop-1-yn-1-yl, prop-2-yn-1-yl, n-but-1-yn-1-yl,
n-but-1-yn-3-yl, n-but-1-yn-4-yl, n-but-2-yn-1-yl,
n-pent-1-yn-1-yl, n-pent-1-yn-3-yl, n-pent-1-yn-4-yl,
n-pent-1-yn-5-yl, n-pent-2-yn-1-yl, n-pent-2-yn-4-yl,
n-pent-2-yn-5-yl, 3-methylbut-1-yn-3-yl, 3-methylbut-1-yn-4-yl,
n-hex-1-yn-I-yl, n-hex-1-yn-3-yl, n-hex-1-yn-4-yl,
n-hex-1-yn-5-yl, n-hex-1-yn-6-yl, n-hex-2-yn-1-yl,
CA 02312703 2000-06-OS
0050/48647
24
n-hex-2-yn-4-yl, n-hex-2-yn-5-yl, n-hex-2-yn-6-yI,
n-hex-3-yn-1-yl, n-hex-3-yn-2-yl, 3-methylpent-1-yn-1-yl,
3-methylpent-1-yn-3-yl, 3-methylpent-1-yn-4-yl,
3-methylpent-1-yn-5-yl, 4-methylpent-1-yn-1-yl,
4-methylpent-2-yn-4-yl and 4-methylpent-2-yn-5-yl, preferably
prop-2-yn-1-yl;
- C3-C6-haloalkynyl: C3-C6-alkynyl as mentioned above which is
partially or fully substituted by fluorine, chlorine, bromine
and/or iodine, eg. 1,1-difluoroprop-2-yn-1-yl,
4-fluorobut-2-yn-1-yI, 4-chlorobut-2-yn-1-yl,
1,1-difluorobut-2-yn-1-yl, 5-fluoropent-3-yn-1-yl or
6-fluorohex-4-yn-1-yl;
- cyano-C3-C6-alkynyl: for example 3-cyanopropargyl,
4-cyanobut-2-yn-1-yl, 5-cyanopent-3-yn-1-yl and
6-cyanohex-4-yn-1-yl;
- C3-Cq-alkenyloxy-C1-Cq-alkyl: C1-Cq-alkyl which is substituted
by C3-Cq-alkenyloxy such as allyloxy, but-I-en-3-yloxy,
but-1-en-4-yloxy, but-2-en-1-yloxy, 1-methylprop-2-enyloxy or
2-methylprop-2-enyloxy, ie., for example, allyloxymethyl,
2-allyloxyethyl or but-1-en-4-yloxymethyl, in particular
2-allyloxyethyl;
- C3-Cq-alkynyloxy-C1-Cq-alkyl: C1-Cq-alkyl which is substituted
by C3-Cq-alkynyloxy such as propargyloxy, but-1-yn-3-yloxy,
but-1-yn-4-yloxy, but-2-yn-1-yloxy, 1-methylprop-2-ynyloxy or
2-methylprop-2-ynyloxy, preferably propargyloxy, ie., for
example, propargyloxymethyl or 2-propargyloxyethyl, in
particuar 2-propargyloxyethyl;
- C3-Cq-alkenylthio-C1-Cq-alkyl: C1-Cq-alkyl which is substituted
by C3-Cq-alkenylthio such as allylthio, but-1-en-3-ylthio,
but-1-en-4-ylthio, but-2-en-1-ylthio, 1-methylprop-2-enylthio
or 2-methylprop-2-enylthio, ie., for example, allylthiomethyl,
2-allylthioethyl or but-1-en-4-ylthiomethyl, in particular
2-(allylthio)ethyl;
- C3-Cq-alkynylthio-C1-Cq-alkyl: C1-Cq-alkyl which is substituted
by C3-Cq-alkynylthio, such as propargylthio, but-1-yn-3-ylthio,
but-1-yn-4-ylthio, but-2-yn-1-ylthio, 1-methylprop-2-ynylthio
or 2-methylprop-2-ynylthio, preferably propargylthio, ie., for
example, propargylthiomethyl or 2-propargylthioethyl, in
particular 2-(propargylthio)ethyl;
CA 02312703 2000-06-OS
0050/48647
- C3-C4-alkenylsulfinyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C3-C4-alkenylsulfinyl, such as allylsulfinyl,
but-1-en-3-ylsulfinyl, but-1-en-4-ylsulfinyl,
but-2-en-1-ylsulfinyl, 1-methylprop-2-enylsulfinyl or
5 2-methylprop-2-enylsulfinyl, ie., for example,
allylsulfinylmethyl, 2-allylsulfinylethyl or
but-1-en-4-ylsulfinylmethyl, in particular
2-(allylsulfinyl)ethyl;
10 - C3-CQ-alkynylsulfinyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C3-C4-alkynylsulfinyl, such as
propargylsulfinyl, but-1-yn-3-ylsulfinyl,
but-1-yn-4-ylsulfinyl, but-2-yn-1-ylsulfinyl,
1-methylprop-2-ynylsulfinyl or 2-methylprop-2-ynylsulfinyl,
15 preferably propargylsulfinyl, ie., for example,
propargylsulfinylmethyl or 2-propargylsulfinylethyl, in
particular 2-(propargylsulfinyl)ethyl;
- C3-C4-alkenylsulfonyl-C1-C4-alkyl: C1-C4-alkyl which is
20 substituted by C3-C4-alkenylsulfonyl, such as allylsulfonyl,
but-1-en-3-ylsulfonyl, but-1-en-4-ylsulfonyl,
but-2-en-1-ylsulfonyl, 1-methylprop-2-enylsulfonyl or
2-methylprop-2-enylsulfonyl, ie., for example,
allylsulfonylmethyl, 2-allylsulfonylethyl or
25 but-1-en-4-ylsulfonylmethyl, in particular
2-(allylsulfonyl)ethyl;
- C3-C4-alkynylsulfonyl-C1-C4-alkyl: C1-C4-alkyl which is
substituted by C3-C4-alkynylsulfonyl, such as
propargylsulfonyl, but-1-yn-3-ylsulfonyl,
but-1-yn-4-ylsulfonyl, but-2-yn-1-ylsulfonyl,
1-methylprop-2-ynylsulfonyl or 2-methylprop-2-ynylsulfonyl,
preferably by propargylsulfonyl, ie., for example,
propargylsulfonylmethyl or 2-propargylsulfonylethyl, in
particular 2-(propargylsulfonyl)ethyl;
- C3-C6-cycloalkyl: cyclopropyl, cyclobutyl, cyclopentyl or
cyclohexyl;
- C3-CB_cycloalkyl: cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl or cyclooctyl;
- C3-CB-cycloalkyl-C1-C6-alkyl: for example cyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl,
cycloheptylmethyl, cyclooctylmethyl, 2-(cyclopropyl)ethyl,
2-(cyclobutyl)ethyl, 2-(cyclopentyl)ethyl, 2-(cyclohexyl)ethyl,
2-(cycloheptyl)ethyl, 2-(cyclooctyl)ethyl,
CA 02312703 2000-06-OS
0050/48647
26
3-(cyclopropyl)propyl, 3-(cyclobutyl)propyl,
3-(cyclopentyl)propyl, 3-(cyclohexyl)propyl,
3-(cycloheptyl)propyl, 3-(cyclooctyl)propyl,
4-(cyclopropyl)butyl, 4-(cyclobutyl)butyl,
4-(cyclopentyl)butyl, 4-(cyclohexyl)butyl,
4-(cycloheptyl)butyl, 4-(cyclooctyl)butyl,
5-(cyclopropyl)pentyl, 5-(cyclobutyl)pentyl,
5-(cyclopentyl)pentyl, 5-(cyclohexyl)pentyl,
5-(cycloheptyl)pentyl, 5-(cyclooctyl)pentyl,
6-(cyclopropyl)hexyl, 6-(cyclobutyl)hexyl,
6-(cyclopentyl)hexyl, 6-(cyclohexyl)hexyl, 6-(cycloheptyl)hexyl
or 6-(cyclooctyl)hexyl;
- C3-Ce-cycloalkyloxy-C1-C4-alkyl: cyclopropyloxymethyl,
1-cyclopropyloxyethyl, 2-cyclopropyloxyethyl,
1-cyclopropyloxyprop-1-yl, 2-cyclopropyloxyprop-1-yl,
3-cyclopropyloxyprop-1-yl, 1-cyclopropyloxybut-1-yl,
2-cyclopropyloxybut-1-yl, 3-cyclopropyloxybut-1-yl,
4-cyclopropyloxybut-1-yl, 1-cyclopropyloxybut-2-yl,
2-cyclopropyloxybut-2-yl, 3-cyclopropyloxybut-2-yl,
3-cyclopropyloxybut-2-yl, 4-cyclopropyloxybut-2-yl,
1-(cyclopropyloxymethyl)eth-1-yl,
1-(cyclopropyloxymethyl)-1-(CH3)eth-1-yl,
1-(cyclopropylmethyloxy)prop-1-yl, cyclobutyloxymethyl,
1-cyclobutyloxyethyl, 2-cyclobutyloxyethyl,
1-cyclobutyloxyprop-1-yl, 2-cyclobutyloxyprop-1-yl,
3-cyclobutyloxyprop-1-yl, 1-cyclobutyloxybut-1-yl,
2-cyclobutyloxybut-1-yl, 3-cyclobutyloxybut-1-yl,
4-cyclobutyloxybut-1-yl, 1-cyclobutyloxybut-2-yl,
2-cyclobutyloxybut-2-yl, 3-cyclobutyloxybut-2-yl,
3-cyclobutyloxybut-2-yl, 4-cyclobutyloxybut-2-yl,
1-(cyclobutyloxymethyl)eth-1-yl,
1-(cyclobutyloxymethyl)-1-(CH3)eth-1-yl,
1-(cyclobutyloxymethyl)prop-1-yl, cyclopentyloxymethyl,
1-cyclopentyloxyethyl, 2-cyclopentyloxyethyl,
1-cyclopentyloxyprop-1-yl, 2-cyclopentyloxyprop-1-yl,
3-cyclopentyloxyprop-1-yl, 1-cyclopentyloxybut-1-yl,
2-cyclopentyloxybut-1-yl, 3-cyclopentyloxybut-1-yl,
4-cyclopentyloxybut-1-yl, 1-cyclopentyloxybut-2-yl,
2-cyclopentyloxybut-2-yl, 3-cyclopentyloxybut-2-yl,
3-cyclopentyloxybut-2-yl, 4-cyclopentyloxybut-2-yl,
1-(cyclopentyloxymethyl)eth-1-yl,
1-(cyclopentyloxymethyl)-1-(CH3)eth-1-yl,
1-(cyclopentyloxymethyl)prop-1-yl, cyclohexyloxymethyl,
1-cyclohexyloxyethyl, 2-cyclohexyloxyethyl,
1-cyclohexyloxyprop-1-yl, 2-cyclohexyloxyprop-1-yl,
3-cyclohexyloxyprop-1-yl, 1-cyclohexyloxybut-1-yl,
2-cyclohexyloxybut-1-yl, 3-cyclohexyloxybut-1-yl,
CA 02312703 2000-06-OS
0050/48647
27
4-cyclohexyloxybut-1-yl, 1-cyclohexyloxybut-2-yl,
2-cyclohexyloxybut-2-yl, 3-cyclohexyloxybut-2-yl,
3-cyclohexyloxybut-2-yl, 4-cyclohexyloxybut-2-yl,
1-(cyclohexyloxymethyl)eth-1-yl,
1-(cyclohexyloxymethyl)-1-(CH3)eth-1-yl,
1-(cyclohexyloxymethyl)prop-1-yl, cycloheptyloxymethyl,
1-cycloheptyloxyethyl, 2-cycloheptyloxyethyl,
1-cycloheptyloxyprop-1-yl, 2-cycloheptyloxyprop-1-yl,
3-cycloheptyloxyprop-1-yl, 1-cycloheptyloxybut-1-yl,
2-cycloheptyloxybut-1-yl, 3-cycloheptyloxybut-1-yl,
4-cycloheptyloxybut-1-yl, 1-cycloheptyloxybut-2-yl,
2-cycloheptyloxybut-2-yl, 3-cycloheptyloxybut-2-yl,
3-cycloheptyloxybut-2-yl, 4-cycloheptyloxybut-2-yl,
1-(cycloheptyloxymethyl)eth-1-yl,
1-(cycloheptyloxymethyl)-1-(CH3)eth-1-yl,
1-(cycloheptyloxymethyl)prop-1-yl, cyclooctyloxymethyl,
1-cyclooctyloxyethyl, 2-cyclooctyloxyethyl,
1-cyclooctyloxyprop-1-yl, 2-cyclooctyloxyprop-1-yl,
3-cyclooctyloxyprop-1-yl, 1-cyclooctyloxybut-1-yl,
2-cyclooctyloxybut-1-yl, 3-cyclooctyloxybut-1-yl,
4-cyclooctyloxybut-1-yl, 1-cyclooctyloxybut-2-yl,
2-cyclooctyloxybut-2-yl, 3-cyclooctyloxybut-2-yl,
3-cyclooctyloxybut-2-yl, 4-cyclooctyloxybut-2-yl,
1-(cyclooctyloxymethyl)eth-1-yl,
1-(cyclooctyloxymethyl)-1-(CH3)eth-1-yl or
1-(cyclooctyloxymethyl)prop-1-yl, in particular
C3-C6-cycloalkoxymethyl or 2-(C3-C6-cycloalkoxy)ethyl.
3- to 7-membered heterocyclyl is to be understood as meaning not
only saturated, partially or fully unsaturated, but also
aromatic, heterocycles having one to three hetero atoms selected
from a group consisting of
- one to three nitrogen atoms,
- one to two oxygen and
_ one or two sulfur atoms.
Examples of saturated heterocycles which can contain a carbonyl
or thiocarbonyl ring member are:
oxiranyl, thiiranyl, aziridin-1-yl, aziridin-2-yl,
diaziridin-1-yl, diaziridin-3-yl, oxetan-2-yl, oxetan-3-yl,
thietan-2-yl, thietan-3-yl, azetidin-1-yl, azetidin-2-yl,
azetidin-3-yl, tetrahydrofuran-2-yl, tetrahydrofuran-3-yl,
tetrahydrothiophen-2-yl, tetrahydrothiophen-3-yl,
pyrrolidin-1-yl, pyrrolidin-2-yl, pyrrolidin-3-yl,
1,3-dioxolan-2-yl, 1,3-dioxolan-4-yl, 1,3-oxathiolan-2-yl,
1,3-oxathiolan-4-yl, 1,3-oxathiolan-5-yl, 1,3-oxazolidin-2-yl,
CA 02312703 2000-06-OS
0050/48647
28
1,3-oxazolidin-3-yl, 1,3-oxazolidin-4-yl, 1,3-oxazolidin-5-yl,
1,2-oxazolidin-2-yl, 1,2-oxazolidin-3-yl, 1,2-oxazolidin-4-yl,
1,2-oxazolidin-5-yl, 1,3-dithiolan-2-yl, 1,3-dithiolan-4-yl,
pyrrolidin-1-yl, pyrrolidin-2-yl, pyrrolidin-5-yl,
tetrahydropyrazol-1-yl, tetrahydropyrazol-3-yl,
tetrahydropyrazol-4-yl, tetrahydropyran-2-yl,
tetrahydrogyran-3-yl, tetrahydropyran-4-yl,
tetrahydrothiopyran-2-yl, tetrahydrothiopyran-
3-yl, tetrahydropyran-4-yl, piperidin-1-yl, piperidin-2-yl,
piperidin-3-yl, piperidin-4-yl, 1,3-dioxan-2-yl, 1,3-dioxan-4-yl,
1,3-dioxan-5-yl, 1,4-dioxan-2-yl, 1,3-oxathian-2-yl,
1,3-oxathian-4-yl, 1,3-oxathian-5-yl, 1,3-oxathian-6-yl,
1,4-oxathian-2-yl, 1,4-oxathian-3-yl, morpholin-2-yl,
morpholin-3-yl, morpholin-4-yl, hexahydropyridazin-1-yl,
hexahydropyridazin-3-yl, hexahydropyridazin-4-yl,
hexahydropyrimidin-1-yl, hexahydropyrimidin-2-yl,
hexahydropyrimidin-4-yl, hexahydropyrimidin-5-yl, piperazin-1-yl,
piperazin-2-yl, piperazin-3-yl, hexahydro-1,3,5-
triazin-1-yl, hexahydro-1,3,5-triazin-2-yl, oxepan-2-yl,
oxepan-3-yl, oxepan-4-yl, thiepan-2-yl, thiepan-3-yl,
thiepan-4-yl, 1,3-dioxepan-2-yl, 1,3-dioxepan-4-yl,
1,3-dioxepan-5-yl, 1,3-dioxepan-6-yl, 1,3-dithiepan-2-yl,
1,3-dithiepan-2-yl, 1,3-dithiepan-2-yl, 1,3-dithiepan-2-yl,
1,4-dioxepan-2-yl, 1,4-dioxepan-7-yl, hexahydroazepin-1-yl,
hexahydroazepin-2-yl, hexahydroazepin-3-yl, hexahydroazepin-4-yl,
hexahydro-1,3-diazepin-1-yl, hexahydro-1,3-diazepin-2-yl,
hexahydro-1,3-diazepin-4-yl, hexahydro-1,4-diazepin-1-yl and
hexahydro-1,4-diazepin-2-yl.
Examples of unsaturated heterocycles which can contain a carbonyl
or thiocarbonyl ring member are:
dihydrofuran-2-yl, 1,2-oxazolin-3-yl, 1,2-oxazolin-5-yl,
1,3-oxazolin-2-yl;
Preferred amongst the heteroaromatic rings are the 5- and
6-membered rings, eg.
furyl such as 2-furyl and 3-furyl, thienyl such as 2-thienyl and
3-thienyl, pyrrolyl such as 2-pyrrolyl and 3-pyrrolyl, isoxazolyl
such as 3-isoxazolyl, 4-isoxazolyl and 5-isoxazolyl, isothiazolyl
such as 3-isothiazolyl, 4-isothiazolyl and 5-isothiazolyl,
pyrazolyl such as 3-pyrazolyl, 4-pyrazolyl and 5-pyrazolyl,
oxazolyl such as 2-vxazolyl, 4-oxazolyl and 5-oxazolyl, thiazolyl
such as 2-thiazolyl, 4-thiazolyl and 5-thiazolyl, imidazolyl such
as 2-imidazolyl and 4-imidazolyl, oxadiazolyl such as
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl and
1,3,4-oxadiazol-2-yl, thiadiazolyl such as 1,2,4-thiadiazol-3-yl,
CA 02312703 2000-06-OS
0050/48647
29
1,2,4-thiadiazol-5-yl and 1,3,4-thiadiazol-2-yl, triazolyl such
as 1,2,4-triazol-1-yl, 1,2,4-triazol-3-yl and 1,2,4-triazol-4-yl,
pyridinyl such as 2-pyridinyl, 3-pyridinyl and 4-pyridinyl,
pyridazinyl such as 3-pyridazinyl and 4-pyridazinyl, pyrimidinyl
such as 2-pyrimidinyl, 4-pyrimidinyl and 5-pyrimidinyl,
furthermore 2-pyrazinyl, 1,3,5-triazin-2-yl and
1,2,4-triazin-3-yl, in particular pyridyl, pyrimidyl, furanyl and
thienyl.
p,ll phenyl, carbocyclic and heterocyclic rings are preferably
unsubstituted.
Preferred for the use of the 3-(benzazol-4-yl)pyrimidinedione
derivatives I as herbicides are those compounds I where the
variables have the following meanings, in each case alone or in
combination:
X is oxygen;
R1 is hydrogen, amino or C1-C6-alkyl, in particular hydrogen or
C1-C4-alkyl, especially preferably hydrogen or methyl;
R2 is hydrogen, halogen, C1-C6-alkyl, C1-C6-haloalkyl or
C1_C6-alkylsulfonyl, in particular trifluoromethyl;
R3 is hydrogen;
R4 is hydrogen, fluorine or chlorine;
R5 is cyano or halogen, in particular chlorine;
=Y- is =N-N(R6)- or =C(ZR7)-5-, in particular =N-N(R6)-;
R6 is C1-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl,
C1-C6-alkylsulfonyl, (C1-C6-alkyl)carbonyl,
(C1-C6-alkyl)thiocarbonyl, (C1-C6-alkoxy)carbonyl or
C1-C6-alkyl which can be substituted by cyano,
(C1-C6-alkoxy)carbonyl, di(C1-C6-alkyl)aminocarbonyl or
(C1-C6-alkyl)carbonyloxy, in particular C1-C6-alkyl,
C3-C6-alkynyl, C1-C6-alkylsulfonyl or (C~-C6-alkoxy)carbonyl.
Especially preferred are also the 3-(benzazol-4-yl)pyrimidine-
dione derivatives Ia f~-- I where X = oxygen, R1 = methyl,
RZ = trifluoromethyl, R3 = hydrogen, R4 = fluorine, R5 = chlorine
and =Y- - =C(ZR7)-N(CH3)-}
~
CA 02312703 2000-06-OS
0050/48647
H3C O F
N
F3C ~ N ~ ~ C1
Ia,
5
O N\ 'N-CH3
IZ R7
10 in particular the compounds Ia.l to Ia.272 which follow:
Table 1
No . -ZR7
15Ia . l -H _ _
Ia.2 -CH3
Ia.3 -C3H5
Ia.4 -n-C3H~
20Ia.S -CH(CH3)2
Ia.6 -n-C4H9
Ia.7 -CH2-CH(CH3)2
Ia.8 -CH(CH3)-CyHS
25Ia.9 -C(CH3)s
Ia.IO -CH2-CH=CHz
Ia.ll -CH2-CH=CH-CH3
Ia.l2 -CH2-CHy-CH=CH2
Ia.l3 -CH2-C=CH
30Ia.l4 -CH2-OCH3
Ia.lS -CHZ-CH2-OCH3
Ia.l6 -CH2-CN
Ia.l7 -CHy-CH2F
35Ia.l8 -CH2-CF3
Ia.l9 -CHZ-CH2C1
Ia.20 -CH2-CO-OCH3
Ia.21 -CH2-CO-OC2H5
40Ia.22 -CH2-CO-N(CH3)3
Ia.23 -cyclobutyl
Ia.24 -cyclopentyl
Ia.25 -cyclohexyl
Ia.26 -phenyl
45
Ia.27 -CHZ-cyclobutyl
Ia.28 -CH2-cyclopentyl
CA 02312703 2000-06-OS
0050/48647
31
No . -ZR7
Ia.29 -CH2-cyclohexyl
Ia.30 -CHz-phenyl
Ia.31 -N02
Ia.32 -CN
Ia.33 -F
Ia.34 -C1
Ia.35 -Br
Ia.36 -OCH3
Ia.37 -OC2H5
Ia.38 -O(n-C3H7)
Ia.39 -OCH(CH3)2
Ia.40 -0(n-C4H9)
Ia.41 -OCH2-CH(CH3)2
Ia.42 -OCH(CH3)-C2H5
Ia.43 -OC(CH3)a
Ia.44 -OCH2-CH=CH2
Ia.45 -OCHZ-CH=CH-CH3
Ia.46 -OCH2-CHZ-CH=CHZ
Ia.47 -OCH(CH3)-CH=CHZ
Ia.48 -OCH2-C=CH
Ia.49 -OCH(CH3)-C = CH
Ia.50 -OCHZ-OCH3
Ia.51 -OCH2-CH2-OCH3
Ia.52 -OCH2-CN
Ia.53 -OCH2-CHZF
Ia.54 -OCH2-CF3
Ia.55 -OCH2-CO-OCH3
Ia.56 -OCHZ-CO-OCZHS
Ia.57 -OCHz-CO-N(CH3)2
Ia.58 -O-cyclobutyl
Ia.59 -O-cyclopentyl
Ia.60 -O-cyclohexyl
Ia.61 -0-phenyl
Ia.62 -OCH2-cyclobutyl
Ia.63 -OCH2-cyclopentyl
Ia.64 -OCHZ-cyclohexyl
Ia.65 -OCHZ-phenyl
Ia.66 -CHZ-OH
Ia.67 -CH2-OCH3
CA 02312703 2000-06-OS
0050/48647
32
No. -ZR~
Ia.68 -NHz
Ia.69 -NH-CH3
Ia.70 -N(CH3)z
Ia.71-.. -NH-C2H5
Ia.72 -N(C3H5)2
Ia.73 -NH-(n-C3H7)
Ia.74 -N(n-C3H~)2
Ia.75 -NH-(n-C4H9)
Ia.76 -N(n-CqH9)z
Ia.77 -NH-CH(CH3)z
Ia.78 -N[CH(CH3)212
15Ia,7g -NH-CH2-CH(CH3)z
Ia.80 -N(CHZ-CH(CH3)z)2
Ia.81 -NH-CHz-CH=CHz
Ia.82 -N(CHz-CH=CHz)2
20Ia.83 -NH-CHz-C = CH
Ia.84 -N(CHz-C=CH)z
Ia.85 -CHZ-N(CH3)2
Ia.86 -SH
25Ia.87 -SCH3
Ia.88 -SCZHS
Ia.89 -S-(n-C3H7)
Ia.90 -S-(n-CqH9)
Ia.91 -SCH(CH3)z
30Ia.92 -SCHZ-CH(CH3)z
Ia.93 -SCH(CH3)-C2H5
Ia.94 -SC(CH3)3
Ia.95 -SCHZ-CH=CHz
35Ia.96 -SCHz-CH=CH-CH3
Ia.97 -SCHZ-CHz-CH=CHz
Ia.98 -SCH(CH3)-CH=CHz
Ia.99 -SCHz-C=CH
40Ia.100 -SCH(CH3)-C = CH
Ia.101 -SCHz-OCH3
Ia.102 -SCHZ-CHz-OCH3
Ia.103 -SCHz-CN
Ia.104 -SCHZ-CH2F
45
Ia.105 -SCHZ-CF3
Ia.106 -SCHZ-CH2C1
CA 02312703 2000-06-OS
0050/48647
33
No . -ZR~
Ia.107 -SCH2-CO-OCH3
Ia.108 -SCH2-CO-OC2H5
Ia.109 -SCHZ-CO-N(CH3)2
Ia.110 -S-cyclobutyl
Ia.lll -S-cyclopentyl
Ia.112 -S-cyclohexyl
Ia.113 -S-phenyl
Ia.114 -SCH2-cyclobutyl
Ia.115 -SCH2-cyclopentyl
Ia.116 -SCH2-cyclohexyl
Ia.117 -SCH2-phenyl
Ia.118 -CH2-SCH3
Ia.119 -SO-CH3
Ia.120 -SO-CyHs
Ia.121 -SO-(n-C3H7)
Ia.122 -SO-(n-CqH9)
Ia.123 -SO-CH(CH3)2
Ia.124 -SO-CH2-CH(CH3)2
Ia.125 -SO-CH(CH3)-CZH5
Ia.126 -SO-C(CH3)3
Ia.127 -SO-CHZ-CH=CHz
Ia.128 -SO-CHy-CH=CH-CH3
Ia.129 -SO-CH2-CH2-CH=CH2
Ia.130 -SO-CH(CH3)-CH=CH2
Ia.131 -SO-CH2-C=CH
Ia.132 -SO-CH(CH3)-C=CH
Ia.133 -SO-CHz-OCH3
Ia.134 -SO-CHy-CH2-OCH3
Ia.135 -SO-CH2-CN
Ia.136 -SO-CHy-CH2F
Ia.137 -SO-CH2-CF3
Ia.138 -SO-CHZ-CH2C1
Ia.139 -SO-CH2-CO-OCH3
Ia.140 -SO-CH2-CO-OC2H5
Ia.141 -SO-CHz-CO-N(CH3)2
Ia.142 -SO-cyclobutyl
Ia.143 -SO-cyclopentyl
Ia.144 -SO-cyclohexyl
Ia.145 -SO-phenyl.
CA 02312703 2000-06-OS
0050/48647
34
No . -ZR~
Ia.146 -SO-CHZ-cyclobutyl
Ia.147 -SO-CHZ-cyclopentyl
Ia.148 -SO-CH2-cyclohexyl
Ia.149 -SO-CH2-phenyl
Ia.150 -CHZ-SO-CH3
Ia.151 -SOz-CH3
Ia.152 -SOZ-C2H5
Ia.153 -SOZ-(n-C3H7)
Ia.154 -SOZ-(n-C4H9)
Ia.155 -S02-CH(CH3)2
Ia.156 -SOZ-CHZ-CH(CH3)2
Ia,157 -SOz-CH(CH3)-C2H5
Ia.158 -SOz-C(CH3)3
Ia.159 -S02-CHZ-CH=CHZ
Ia.160 -SOy-CHZ-CH=CH-CH3
Ia.161 -S02-CH2-CH2-CH=CHZ
Ia.162 -SOZ-CH(CH3)-CH=CHZ
Ia.163 -SOZ-CHZ-C=CH
Ia.164 -SOZ-CH(CH3)-C=CH
Ia.165 -SOZ-CHZ-OCH3
Ia.166 -SOz-CHZ-CHy-OCH3
Ia.167 -S02-CH2-CN
Ia.168 -SOZ-CH2-CHZF
Ia.169 -S02-CHZ-CF3
Ia.170 -S02-CHz-CHZCl
Ia.171 -S02-CHy-CO-OCH3
Ia.172 -S02-CHZ-CO-OC2H5
Ia.173 -SOZ-CHZ-CO-N(CH3)2
Ia.174 -SOz-cyclobutyl
Ia.175 -SOy-cyclopentyl
Ia.176 -SOz-cyclohexyl
Ia.177 -S02-phenyl
Ia.178 -SOz-CH2-cyclobutyl
Ia.179 -SOZ-CHZ-cyclopentyl
Ia.180 -S02-CH2-cyclohexyl
Ia.181 -S02-CHZ-phenyl
Ia.182 -CH2-S02-CH3
Ia.183 -CHZ-CH(C1)-CO-OH
Ia.184 -CH2-CH(C1)-CO-OCH3
CA 02312703 2000-06-OS
0050/48647
No. -ZR7
Ia.185 -CH2-CH(Cl)-CO-OC2H5
Ia.186 -CH2-CH(Cl)-CO-0(n-C3H7)
5 Ia.187 -CH2-CH(C1)-CO-0(n-C4Hg)
Ia.188 -CH2-CH(C1)-CO-OCH(CH3)2
Ia.189 -CH2-CH(Cl)-CO-OCH2-CH(CH3)2
Ia.190 -CH2-CH(Cl)-CO-OCH(CH3)-C2H5
Ia.191 -CH2-CH(C1)-CO-OC(CH3)3
10
Ia.192 -CH2-CH(Hr)-CO-OH
Ia.193 -CH2-CH(Br)-CO-OCH3
Ia.194 -CH2-CH(Br)-CO-OC2H5
Ia.195 -CH2-CH(Br)-CO-O(n-C3H7)
15 Ia,196 -CH2-CH(Br)-CO-O(n-CQHg)
Ia.197 -CH2-CH(Br)-CO-OCH(CH3)2
Ia.198 -CH2-CH(Br)-CO-OCH2-CH(CH3)2
Ia.199 -CH2-CH(Br)-CO-OCH(CH3)-C2H5
20 Ia.200 -CH2-CH(Br)-CO-OC(CH3)3
Ia.201 -CH=CH-CO-OH
Ia.202 -CH=CH-CO-OCH3
Ia.203 -CH=CH-CO-OC2H5
25 Ia.204 -CH=CH-CO-0(n-C3H7)
Ia.205 -CH=CH-CO-0(n-C4Hg)
Ia.206 -CH=CH-CO-OCH(CH3)2
Ia.207 -CH=CH-CO-OCH2-CH(CH3)2
Ia.208 -CH=GH-CO-OCH(CH3)-C2H5
30 Ia.209 -CH=CH-CO-OC(CH3)3
Ia.210 -CH=C(Cl)-CO-OH
Ia.211 -CH=C(C1)-CO-OCH3
Ia.212 -CH=C(C1)-CO-OC2H5
35 Ia.213 -CH=C(C1)-CO-0(n-C3H7)
Ia.214 -CH=C(C1)-CO-0(n-C4Hg)
Ia.215 -CH=C(C1)-CO-OCH(CH3)2
Ia.216 -CH=C(C1)-CO-OCH2-CH(CH3)2
Ia.217 -CH=C(C1)-CO-OCH(CH3)-C2H5
Ia.218 -CH=C(C1)-CO-OC(CH3)3
Ia.219 -CH=C(Br)-CO-OH
Ia.220 -CH=C(Br)-CO-OCH3
Ia.221 -CH=C(Br)-CO-OC2H5
Ia.222 -CH=C(Br)-CO-O(n-C3H7)
Ia.223 -CH=C(Br)-CO-O(n-C4Hg)
CA 02312703 2000-06-OS
0050/48647
36
No . -2R7
Ia.224 -CH=C(Br)-CO-OCH(CH3)z
Ia.225 -CH=C(Br}-CO-OCHZ-CH(CH3}z
Ia.226 -CH=C(Br)-CO-OCH(CH3)-C2Hs
Ia.227 -CH=C(Br}-CO-OC(CH3)3
Ia.228 -CHz-CH(C1)-CO-NHz
Ia.229 -CHZ-CH(C1)-CO-NH-CH3
Ia.230 -CHZ-CH(C1)-CO-N(CH3)z
Ia.231 -CHz-CH(C1)-CO-NH-CyHs
Ia.232 -CHz-CH(C1)-CO-N(C2HS)z
Ia.233 -CHz-CH(C1)-CO-NH-(n-C3H~)
Ia.234 -CHz-CH(C1)-CO-N(n-C3H7)z
Ia,235 -CHz-CH(C1)-CO-NH-(n-C4Hg)
Ia.236 -CHz-CH(C1)-CO-N(n-C4Hg)z
Ia.237 -CHz-CH(Br)-CO-NHz
Ia.238 -CHz-CH(Br)-CO-NH-CH3
Ia.239 -CHZ-CH(Br)-CO-N(CH3)z
Ia.240 -CHz-CH(Br)-CO-NH-C2Hs
Ia.241 -CHz-CH(Br)-CO-N(C2H5)z
Ia.242 -CHz-CH(Br)-CO-NH-(n-C3H7)
Ia.243 ~CH2-CH(Br)-CO-N(n-C3H7)z
Ia.244 -CHz-CH(Br)-CO-NH-(n-C4Hg)
Ia.245 -CHz-CH(Br)-CO-N(n-C4Hg)z
Ia.246 -CH=CH-CO-NHz
Ia.247 -CH=CH-CO-NH-CH3
Ia.248 -CH=CH-CO-N(CH3)2
Ia.249 -CH=CH-CO-NH-C2Hs
Ia.250 -CH=CH-CO-N(CzHs)z
Ia.251 -CH=CH-CO-NH-(n-C3H7)
Ia.252 -CH=CH-CO-N(n-C3H7)z
Ia.253 -CH=CH-CO-NH-(n-C4Hg)
Ia.254 -CH=CH-CO-N(n-C4Hg)z
Ia.255 -CH=C(C1)-CO-NHz
Ia256 -CH=C(C1)-CO-NH-CH3
Ia.257 -CH=C(C1)-CO-N(CH3)2
Ia.258 -CH=C(C1)-CO-NH-CZHs
Ia.259 -CH=C(C1)-CO-N(CZHs)2
Ia.260 -CH=C(C1)-CO-NH-(n-C3H7)
Ia.261 -CH=C(C1)-CO-N(n-C3H7)z
Ia.262 -CH=C(Cl)-CO-NH-(n-C4H9}
CA 02312703 2000-06-OS
0050/48647
37
No~. -ZR7
Ia.263 -CH=C(C1)-CO-N(n-C4Hg)Z
Ia.264 -CH=C(Br)-CO-NH2
Ia.265 -CH=C(Br)-CO-NH-CH3
Ia.266 -CH=C(Br)-CO-NH(CH3)2
Ia.267 -CH=C(Br)-CO-NH-C2H5
Ia.268 -CH=C(Br)-CO-N(C2H5)2
Ia.269 -CH=C(Br)-CO-NH-(n-C3H7)
Ia.270 -CH=C(Br)-CO-N(n-C3H~)Z
Ia.271 -CH=C(Br)-CO-NH-(n-C4Hg)
Ia.272 -CH=C(Br)-CO-N(n-CqH9)2
Furthermore, especially preferred 3-(benzazol-4-yl)-
pyrimidinedione derivatives are those of the formulae Ib to Ih,
in particular
- the compounds Ib.l - Ib.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R4 is hydrogen:
H3C O
N
F3C ~ N ~ ~ C1
Ib
O N~N-CH3
~ZR~
- the compounds Ic.l - Ic.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R1 is hydrogen:
H O F
N
F3C ~ N- ~ ~ C1
Ic
O N ~ N- CH3
ZR7
- the compounds Id.l - Id.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R1 and R4 are hydrogen:
CA 02312703 2000-06-OS
0050/48647
38
H 0
N
FgC ~ N- ~ ~ C1
Id
'O N~ N-CH3
ZR7
_ the compounds Ie.l - Ie.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that Y
is =C(ZR7)-N(S02CH3)-.
H3C 0 F
N-
F3C ~ N C1
Ie
0 N~N-S02-CH3
~ZR7
- the compounds If.l - If.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R4 is hydrogen and Y is =C(ZR7)-N(SOZCH3)-.
H3C O
N
F3C ~ N- ~ ~ C1
If
O N\ 'N-SOZ-CH3
IZ R7
- the compounds Ig.l - Ig.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R1 is hydrogen and Y is =C(ZR7)-N(SOZCH3)-.
H O F
N-~ - / \
F3C ~ N C1
Ig
'O N~ N-~-S02-CH3
ZR7
CA 02312703 2000-06-OS
0050/48647
39
- the compounds Ih.l - Ih.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
R1 and RQ are hydrogen and Y is =C(ZR7)-N(S02CH3)-.
H O
\
N
F3C ~ N ~ ~ C1
Ih
O NYN-S02-CH3
Z R7
- the compounds Ii.l - Ii.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
=Y- is =C(ZR7)-O-:
H3C O F
N
F3C ~ N ~ ~ C1
Ii
O N\ 'O
IZ R7
- the compounds Ik.l - Ik.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that
=Y- is =C(ZR7)-O- and R4 is hydrogen:
H3C O
\
N
F3C ~ N- ~ ~ C1
Ik
'O N ~ O
ZR~
- the compounds Im.l - Im.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that Y
is =C(ZR7)-O- and R1 is hydrogen:
CA 02312703 2000-06-OS
0050/48647
H O F
N
F3C ~ N ~ ~ C1
Im
5 '0 N ~ O
ZR7
10 _ the compounds In.l - In.272, which differ from the
corresponding compounds Ia.l - Ia.272 only by the fact that Y
is =C(ZR~)-0- and R1 and R4 are hydrogen:
H 0
15 N--~ - /
F3C ~ N C1
In
O N\ '0
20 IZR~
The 3-(benzazol-4-yl)pyrimidinedione derivatives of the formula I
can be obtained in various ways, for example by one of the
25 following processes:
Process A~:
Reaction of a 3-(benzazol-4-yl)pyrimidinedione derivative I where
R1 is hydrogen with a compound II in a manner known per se:
H O R4 R1 O R4
\N~ L1-alkyl or \N-
3 5 R2 \ N / \ RS + R2 N ~ ~ R5
L1-haloalkyl
R3 X N - Y R3 X N - Y
I (Rl = H) II I (Rl ~ H, NHZ)
L1 is a customary leaving group such as halogen, preferably
chlorine, bromine or iodine, (halo)alkylsulfonyloxy,
preferably methylsulfonyloxy or trifluoromethylsulfonyloxy,
arylsulfonyloxy, preferably toluenesulfonyloxy, and
alkoxysulfonyloxy, preferably methoxysulfonyloxy or
ethoxysulfonyloxy.
CA 02312703 2000-06-OS
0050/48647
41
The process is normally carried out in an inert organic
solvent, for example in a protic solvent such as the lower
alcohols, preferably in methanol or ethanol, if desired as a
mixture with water, or in an aprotic solvent, for example in
an aliphatic or cyclic ether such as methyl tert-butyl ether,
1,2-dimethoxyethane, tetrahydrofuran and dioxane, in an
aliphatic ketone such as acetone, diethyl ketone and ethyl
methyl ketone, in an amide such as dimethylformamide and
N-methylpyrrolidone, in a sulfoxide such as dimethyl
sulfoxide, in a urea such as tetramethylurea and
1,3-dimethyltetrahydro-2(1H)-pyrimidinone, in a carboxylic
acid ester such as ethyl acetate, or in a halogenated
aliphatic or aromatic hydrocarbon such as dichloromethane,
dichloroethane, chlorobenzene and the dichlorobenzenes.
If desired, the process can be carried out in the presence of
a base, suitable bases being inorganic bases, eg, carbonates
such as sodium carbonate and potassium carbonate, hydrogen
carbonates such as sodium hydrogen carbonate and potassium
hydrogen carbonate or alkali metal hydrides such as sodium
hydride and potassium hydride, and also organic bases, eg.
amines such as triethylamine, pyridine and
N,N-diethylaniline, or alkali metal alkoxides such as sodium
methoxide, sodium ethoxide and potassium tert-butoxide.
The amount of base and alkylating agent II is preferably in
each case 0.5 times to twice the molar amount, based on the
amount of starting compound I (where R1 = hydrogen).
In general, the reaction temperature is from 0°C to the
boiling point of the reaction mixture, in particular from 0
to 60~C.
A preferred process variant consists in alkylating the salt
of I, which has been obtained by cyclizing IV where R1 = H or
V where R1 = H in accordance with process D) without
isolating it from the reaction mixture, which may still
contain excess base, eg. sodium hydride, sodium alkoxide or
sodium carbonate.
Unless the salts of those compounds I where R1 is hydrogen
cannot be prepared directly by the cyclization under basic
conditions, which has been described as method D), they can
also be obtained in a manner known per se from the process
products of methods C) to F). To this end, for example, the
aqueous solution of an inorganic or organic base is treated
CA 02312703 2000-06-OS
0050/48647
42
with the 3-(benzazol-4-yl)pyrimidinedione derivative I where
Ri is hydrogen. Here, salt formation is normally sufficiently
rapid at as little as 20 to 25~C.
It is especially advantageous to prepare the sodium salt by
dissolving the 3-(benzazol-4-yl)pyrimidinedione derivative I
where Ri = hydrogen in an aqueous sodium hydroxide solution
at 20 to 25~C, approximately equivalent amounts of
3-(benzazol-4-yl)pyrimidinedione derivative I (Where Ri = H)
and sodium hydroxide being employed. The corresponding salt
of the 3-(benzazol-4-yl)pyrimidinedione derivative I can then
be isolated for example by precipitation with a suitable
inert solvent or by evaporating the solvent.
Salts of the 3-(benzazol-4-yl)pyrimidinedione derivatives I
whose metal ion is other than an alkali metal ion can usually
be prepared by double decomposition of the corresponding
alkali metal salt in aqueous solution, and ammonium,
Phosphonium, sulfonium and sulfoxonium salts by means of
ammonia or phosphonium, sulfonium or sulfoxonium hydroxides.
Process B~
Reaction of a 3-(benzazol-4-yl)pyrimidinedione derivative of the
formula I where Ri is hydrogen with an electrophilic aminating
reagent in the presence of a base:
\ O R4 H2 \ O R9
R2 N~ / \ R5 aminating reagent 2 N ' / \ R5
N ~ R N
\ base \
R3 X N - Y R3 X N - Y
I ~Ri = H} I ~R1 = NH2}
An aminating reagent which has proved itself especially to
date is 2,4-dinitrophenoxyamine, but it is also possible to
use, for example, hydroxylamine-O-sulfonic acid (HOSA), which
is already known from the literature as an aminating reagent
(cf., for example, E. Hofer et al., Synthesis 1983, 466; W.
Friedrichsen et al., Heterocycles 20 (1983) 1271; H. Hart et
al., Tetrahedron Lett. 25 (1984) 2073; B. Vercek et al.,
Monatsh. Chem. ~4 (1983) 789; G. Sosnousky et al., Z.
Naturforsch. 38 (1983) 884; R.S. Atkinson et al., J. Chem.
Soc. Perkin Trans. 1987, 2787).
CA 02312703 2000-06-OS
0050/48647
43
The amination can be carried out in a manner known per se
(see, for example, T. Sheradsky, Tetrahedron Lett. 1968,
1909; M.P. Wentland et al., J. Med. Chem. 27 (1984) 1103 and
in particular EP-A 240 194, EP-A 476 697 and EP-A 517 181,
which teach the amination of uracils).
The reaction is normally carried out in a polar solvent, for
example in dimethylformamide, N-methylpyrrolidone, dimethyl
sulfoxide or in ethyl acetate, which has proved to be
especially suitable to date.
Suitable bases are, for example, alkali metal carbonates such
as potassium carbonate, alkali metal alkoxides such as sodium
methoxide and potassium tert-butoxide or alkali metal
hydrides such as sodium hydride.
The amount of base and aminating agent is preferably in each
case 0.5 times to twice the molar amount, based on the amount
of starting compound.
Process C~
Sulfurization of a 3-(benzazol-4-yl)pyrimidinedione derivative of
the formula I where X is oxygen:
Ri O R4 R1 0 R4
\N~ ~ \ 5 sulfurization 2 \N--~
R \ N R R \ N RS
\\ _ \\ N - Y
R3 O N Y R3 S
I (X = O) I (X = S)
As a rule, sulfurization is effected in an inert solvent or
diluent, for example in an aromatic hydrocarbon such as
toluene and the xylenes, in an ether such as diethyl ether,
1,2-dimethoxyethane and tetrahydrofuran, or in an organic
amine such as pyridine.
Especially suitable sulfurization reagents are phosphorus(V)
sulfide and 2,4-bis(4-methoxyphenyl)-1,3,2,4-
dithiadiphosphetane-2,4-dithione ("Lawesson reagent").
CA 02312703 2000-06-OS
0050/48647
44
Normally, once to five times the molar amount, based on the
starting compound to be sulfurized, will suffice for an
essentially complete reaction.
The reaction temperature is normally from 20 to 200~C,
preferably 40~C to the boiling point of the reaction mixture.
Process D~
CYclization of an arylurea of the formula III or of an
arylanilide of the formula IV in the presence of a base:
\ 0 R4 \ O
z N p' NH ~ ~ 5 2 N ' OLz
R ~ / R or R ~ / O R4
R3 OLz N Y R3 NH ~ ~ RS
base N - Y
III IV
Ri 0 R4
\
N
Rz ~ ~ ~ 5
N R
R3 '~ N - Y
I (X = O)
Lz is lower alkyl, preferably Ci-C4-alkyl, or phenyl.
As a rule cyclization is effected in an inert organic solvent
or diluent which is aprotic, for example in an aliphatic or
cyclic ether such as 1,2-dimethoxyethane, tetrahydrofuran and
dioxane, in an aromatic such as benzene or toluene, or in a
Polar solvent such as dimethylformamide or dimethyl
sulfoxide. Mixtures of polar solvent and a hydrocarbon such
as n-hexane are also suitable. Depending on the starting
compound, water may also be suitable as diluent.
Suitable bases are, preferably, alkali metal alkoxides, in
particular the sodium alkoxides, alkali metal hydroxides, in
particular sodium hydroxide and potassium hydroxide, alkali
~
CA 02312703 2000-06-OS
0050/48647
metal carbonates, in particular sodium carbonate and
potassium carbonate, and metal hydrides, in particular sodium
hydride. When using sodium hydride as the base, it has proved
advantageous to carry out the process in an aliphatic or
5 cyclic ether, in dimethylformamide or in dimethyl sulfoxide.
Normally, 0.5 times to twice the molar amount of base, based
on the amount of IV or V, will suffice for successfully
carrying out the reaction.
In general, the reaction temperature is from (-78)~C to the
boiling point of the reaction mixture in question, in
particular (-60) to 60~C.
If Ri in formula III or IV is hydrogen, the process product
is obtained as a metal salt, the metal corresponding to the
cation of the base used. The salt can be isolated and
purified in a manner known per se or, if desired, converted
by means of acid to give the free compound I where Ri =
hydrogen.
Process E~
Treatment of a substituted 2-aminoaniline Va with nitrous acid
R1 O R4 Ri O R4
\N~ ~ ~ "HNO2..
R ~ N R5 R2 \ N ~ ~ R5
R3 X NHy NHR6 R3 X N\ ~
N R6
Va
I {=y- _ =N-N(R6)-}
The cyclization reaction can be carried out by processes
known per se (cf., for example, Houben-Weyl, Methoden der
organischen Chemie [Methods in Organic Chemistry], Georg
Thieme Verlag Stuttgart, Vol. EBd, Ist Edition 1994, pp.
409-415).
The reaction is preferably carried out in acidic aqueous
media, but lower carboxylic acids such as acetic acid are
also suitable diluents. Suitable acidic aqueous solvents are,
in particular, dilute mineral acids, for example 10~ strength
hydrochloric acid.
~
CA 02312703 2000-06-OS
0050/48647
46
The nitrous acid is preferably prepared in situ by adding an
alkali metal nitrite - in substance or in aqueous solution -
to the reaction mixture, which is composed of the
diaminobenzene in acidic aqueous solution or in a carboxylic
acid.
A suitable reaction temperature is, in particular, 0 to 20~C,
very especially approximately 5~C.
The starting materials are expediently employed in
approximately stoichiometric amounts, or the process is
carried out with an excess of the theoretically expected
amount of nitrous acid, which is not more than 10 mold.
The following intermediate can be cyclized in a similar manner:
R4 R4
R5 --~,. ~ ~ R5
H2N NHR6 N~N~N~
R6
VI VII
Process F~
Condensation of a substituted 2-aminophenol, 2-aminothiophenol or
2-aminoaniline (V) with carbonic acid derivatives or carboxylic
acid derivatives:
O R4 carbonic acid R1 O R4
N // derivative or ~N~
scarboxylic acid
R ~ N R derivative R ~ N R5
X N (O/S/I )
R3 X NH2 (O/S/I )-H R3
R6 ZR7 R6
V
I (Z = chemical bond, O, NH;
R7 = H, unsubst. or subst.
alkyl, alkenyl or aryl)
The condensation reaction of the bifunctional benzenes V with
the carbonic acid derivatives or carboxylic acid derivatives
are carried out in a manner known per se (cf., for example,
Houben-Weyl, Methoden der organischen Chemie [Methods in
Organic Chemistry], Georg Thieme Verlag Stuttgart, Vol. EBc,
' CA 02312703 2000-06-OS
0050/48647
47
1st Edition 1994, pp. 247-284; Vol. EBb, 1st Edition 1994,
pp. 881-901; Vol. EBa, 1st Edition 1993, pp. 1032-1078).
Preferred carbonic acid derivatives or carboxylic acid
derivatives are the corresponding anhydrides, acid chlorides,
ortho esters, diimides, nitriles, trichloromethyl-substituted
compounds, isocyanates and their thio analogs.
Suitable solvents/diluents are, in particular, organic
solvents, for example aromatic hydrocarbons such as benzene,
toluene and o-, m-, p-xylene, halogenated hydrocarbons such
as methylene chloride, chloroform and dichloroethane, lower
alcohols such as methanol and ethanol, aliphatic or cyclic
ethers such as dimethoxyethane, tetrahydrofuran and dioxane,
carboxylic esters such as ethyl acetate or aprotic polar
solvents such as dimethylformamide and ~dimethyl sulfoxide.
If desired, the reaction can be accelerated by adding
catalytic amounts of an acid. Suitable acids are, in
Particular, mineral acids such as hydrochloric acids [sic] or
sulfonic acids such as p-toluenesulfonic acid. The amounts of
acid are preferably 0.1 to 5 mol percent, based on the amount
of V.
The reaction temperatures are preferably from 20~C to reflux
temperature of the reaction mixture in question, in
particular 60~C to reflux temperature.
The carbonic acid derivative or carboxylic acid derivative is
employed either in an approximately stoichiometric amount or
in an excess. In suitable cases, a very large excess may also
be employed, or else the process can be carried out in the
absence of a solvent. Approximately stoichiometric amounts or
an excess of up to 10 mole equivalents, based on the amount
of V, are preferred.
The substituted 2-aminophenols, -thiophenols and -anilines
(V) are expediently obtained by reducing the corresponding
2-nitrophenols, -thiophenols or -anilines VIII (cf., for
example Houben-Weyl, Methoden der organischen Chemie [Methods
in Organic Chemistry], Georg Thieme Verlag Stuttgart, Vol.
XI/1, 4th Edition 1957, p. 431 et seq.):
CA 02312703 2000-06-OS
0050/48647
48
Rl O R4 R1 O R4
2 \N ~ / \ \N
R \ N R5 reduction RZ \ N / \ R5
R3 X NOy (O/S/I )-H R3 X NH2 (0/S/I )-H
6
VIII R6 V R
Suitable reducing agents are, in particular,
- elemental metals such as iron, tin and zinc,
- hydrogen in the presence of suitable catalysts such as
palladium or platinum on charcoal or Raney nickel, or
- complex hydrides such as LiAlH4 and NaBH4, in the
presence or absence of catalysts.
Depending on the reducing agent, suitable solvents are
normally carboxylic acids such as acetic acid or propionic
acid, alcohols such as methanol and ethanol, ethers such as
diethyl ether, methyl tert-butyl ether, tetrahydrofuran and
dioxane, aromatics such as benzene and toluene, and mixtures
of these.
The reactions can be carried out at from (-100)~C to the
boiling point of the reaction mixture in question.
The starting compounds are normally employed in approximately
stoichiometric amounts; however, in individual cases an
excess of one or the other component of up to approximately
10 mold may also be advantageous.
The 2-nitrophenols, -thiophenols and -anilines VIII, in turn, can
be set free from the corresponding protected nitro compounds IX:
R1 O R4 R1 0 R4
N N
z ~ / \ 5 elimination 2 / \ s
R N R R N R
\ reagent \
R3 X N02 (0/S/N)-protection R3 X N02 (0/S/I )-H
IX 6 VIII R6
~
CA 02312703 2000-06-OS
0050/48647
49
Protection = customary protective group which protects the
phenols, or thiophenols, as ethers or the amino group as an
amide.
The protective groups can be eliminated by processes known
per se (cf., for example, Greene/Wuts: Protective Groups in
Organic Synthesis, John Wiley & Sons, Inc., 2nd Edition 1991,
p. 145 et seq. and p. 279 et seq.).
Suitable elimination reagents are, in particular:
- for alkylphenols: trimethylsilyl iodide, boron
tribromide, boron trichloride, aluminum trichloride,
lithium chloride or hydrogen bromide;
- for unsubstituted or substituted benzylphenols or
-thiophenols: boron trifluoride, hydrofluoric acid or
hydrogen/catalyst, preferably noble metal catalysts such
as palladium or platinum.
The solvent/diluent is preferably chosen in such a way that
it is inert to the elimination reagent in question. When
using the halides trimethylsilyl iodide, boron tribromide,
boron trichloride or aluminum trichloride, halogenated
solvents such as dichloromethane, chloroform, carbon
tetrachloride and dichloroethane are especially preferred.
Hydrogen bromide is preferably used in aqueous solution, very
especially preferably as a 48~ strength solution; lithium
chloride is preferably employed in polar solvents such as
lower alcohols, dimethyl sulfoxide and dimethylformamide;
hydrogenolytic methods are preferably carried out in lower
alcohols or carboxylic acids, without or with the addition of
a hydrogen transfer agent such as cyclohexene and
cyclohexadiene.
The temperature for the elimination reaction is preferably
from O~C to the boiling point of the reaction mixture in
question.
The elimination reagent is preferably employed in
approximately stoichiomeric amounts or in an excess. The
excess is especially preferably between one and ten mole
equivalents, based on the amount of IX.
Finally, the protected nitro compounds IX can be obtained in a
manner known per se by nitrating (protected) phenols, thiophenols
or anilines X (cf., for example, Houben-Weyl, Methoden der
CA 02312703 2000-06-OS
0050/48647
organischen Chemie [Methods in Organic Chemistry], Georg Thieme
Verlag Stuttgart, VoI. 10/1, 1971, p. 479 et seq.):
R1 O R4 R1 0 R4
5 2 ~N~ ~ ~ ~N~
R ~ N R5 R2 ~ N ~ ~ 5
R
nitration
R3 X (O/S/N)-protection R3 X NOZ (0/S/N)-pro-
10 I tection
Rs R6
X IX
15 suitable nitration reagents are, in particular, nitric acid,
as a mixture with sulfuric acid or acetic anhydride, or
nitronium salts, specifically nitronium tetrafluoroborate.
The mixture composed of nitric acid and sulfuric acid can be
composed of any desired weight ratios of the two components;
20 Preferred are those mixtures where the sulfuric acid
predominates greatly or acts as the solvent. Similarly, this
is also true for the mixture of nitric acid and acetic
anhydride.
Nitronium tetrafluoroborate is preferably employed in aprotic
25 Polar solvents, eg. in acetonitrile or nitromethane.
The reaction temperature is generally from (-80) to 80~C, in
particular (-20)~C to 30~C.
30 When using the reagent nitric acid in the nitration
reactions, the process is preferably carried out with an
approximately equimolar amount or especially preferably with
an excess of nitration reagent. The excess can be many times
the amount of X. Nitronium tetrafluoroborate is preferably
35 employed in equimolar amounts relative to the substrate, or
in a small excess of between 1.1 and 1.5 mole equivalents.
The following intermediates may also be nitrated in a similar
manner:
45
CA 02312703 2000-06-OS
0050/48647
51
R4 R4
nitration
RS pzN - ~ ~ R5
N- Y
N- Y
XI XII
3-(genzazol-4-yl)pyrimidinedione derivatives of the formula I
with one or more chiral centers are normally obtained as
enantiomer or diastereomer mixtures which, if desired, can be
resolved to give the essentially pure isomers by the methods
conventionally used for this purpose, for example by means of
crystallization or chromatography on an optically active
adsorbate. Pure optically active isomers can be prepared
advantageously from corresponding optically active starting
materials.
Those 3-(benzazol-4-yl)pyrimidinedione derivatives of the formula
I where R1 is hydrogen can be converted into their salts in a
manner known per se (see, in this context, what has been said for
process A)).
30
40
The arylureas of the formula III are novel. They can be prepared
by methods known per se, for example by one of the following
processes:
' CA 02312703 2000-06-OS
0050/48647
52
Process G~
Reaction of a (3-ketocarboxylic ester XIII with a urea XIV:
R1 0 R4
O /N~ NH ~ ~ 5
R2 0 + H R
OL2 N
R3
XIII XIV
R1. 0 R4
(Cat.) N
---~~. R2 ~ NH ~ ~ 5
R
R3 OLZ N - Y
III
LZ is lower alkyl, preferably C1-C4-alkyl, or phenyl.
The process is preferably carried out under essentially
anhydrous conditions in an inert solvent or diluent,
especially preferably in the presence of an acidic or basic
catalyst.
Suitable solvents or diluents are, in particular, organic
solvents which are miscible with water to give azeotropic
mixtures, for example aromatics such as benzene, toluene and
o-, m-, p-xylene, halogenated hydrocarbons such as methylene
chloride, chloroform, carbon tetrachloride and chlorobenzene,
aliphatic and cyclic ethers such as 1,2-dimethoxyethane,
tetrahydrofuran and dioxane, or cyclohexane, but also
alcohols such as methanol and ethanol.
Suitable acidic catalysts are, preferably, strong mineral
acids such as sulfuric acid and hydrochloric acid,
phosphorus-containing acids such as orthophosphoric acid and
polyphosphoric acid, organic acids such as p-toluenesulfonic
acid, and acidic cation exchangers such as "Amberlyst 15"
(Fluka).
CA 02312703 2000-06-OS
0050/48647
10
53
Examples of suitable basic catalysts are alkali metal
hydrides such as sodium hydride and, especially preferably,
alkali metal alkoxides such as sodium methoxide and sodium
ethoxide.
XIV and the ~-ketocarboxylic ester XIII are expediently
employed in approximately stoichiometric amounts, or else the
process is carried out with a small excess of one or the
other component, of up to approximately 10 mold.
An amount of 0.5 to 2 molg of catalyst, based on the amount
of one of the starting compounds, will normally suffice.
The reaction is generally effected at from 60 to 120~C so as
rapidly to eliminate water which forms, preferably at the
boiling point of the reaction mixture.
Process H~
Reaction of an enol ether XV with a urea XVI:
R1 O R4
OL3 \ N-
RZ O + H NH ~ \ R5
\ OL2 N -~ Y
R3
XV XVI
R1 O R4
N
NH ~ \ 5
R \ ~ R
R3 OL2 N - Y
III
LZ and L3 are in each case lower alkyl, preferably
C1-C4-alkyl, or phenyl.
The reaction is preferably carried out in an inert organic
solvent which is miscible with water, eg. an aliphatic or
cyclic ether such as 1,2-dimethoxyethane, tetrahydrofuran and
dioxane, or a lower alcohol, in particular ethanol, the
CA 02312703 2000-06-OS
0050/48647
54
reaction temperature normally being from 50 to 100~C,
preferably the boiling point of the reaction mixture.
However, the reaction can also be carried out in an aromatic
diluent such as benzene, toluene and o-, m-, p-xylene, in
which case the addition of either an acidic catalyst such as
hydrochloric acid and p-toluenesulfonic acid or of a base,
for example an alkali metal alkoxide such as sodium methoxide
and sodium ethoxide, is recommended. In this process variant,
again, the reaction temperature is normally from 50 to 100~C,
but preferably 60 to 80~C.
As regards the ratios by weight, what has been said for
method G) also applies here.
Process J~
Reaction of an et~aminoester XVII with an isocyanate XVIII:
R1 Rq R1 O R4
\ NH OLZ OCN ~ ~ R5 ~,. R2 N p _ NH ~ ~ 5
R + ~ ~ R
R3 \p N Y R3 OL2 N - Y
XVII XVIII III
L2 is lower alkyl, preferably C1-C4-alkyl, or phenyl.
The reaction is expediently carried out in the presence of an
essentially anhydrous aprotic organic solvent or diluent, for
example an aliphatic or cyclic ether such as diethyl ether,
1,2-dimethoxyethane, tetrahydrofuran and dioxane, an
aliphatic or aromatic hydrocarbon such as n-hexane, benzene,
toluene and o-, m-, p-xylene, a halogenated aliphatic
hydrocarbon such as methylene chloride, chloroform, carbon
tetrachloride, 1,2-dichlorethane and chlorobenzene, an
aprotic polar solvent such as dimethylformamide, hexamethyl
phosphoric triamide and dimethyl sulfoxide, or a mixture of
these.
If desired, the process can also be carried out in the
presence of a metal hydride base such as sodium hydride and
potassium hydride, or an organic tertiary base such as
~
CA 02312703 2000-06-OS
0050/48647
triethylamine and pyridine, it being possible for the organic
base to act simultaneously as the solvent.
The starting materials are expediently employed in
5 stoichiometric amounts, or else the process is carried out
with a small excess of one or the other component, of up to
approximately 10 mol%. If the process is carried out in the
absence of a solvent in the presence of an organic base, the
latter will be present in a larger excess.
The reaction temperature is preferably from (-80) to 50~C, in
particular (-60) to 30~C.
In an especially preferred embodiment, the resulting enamine
ester III is converted directly (ie. "in situ") with an
excess of base in accordance with process D) to give the
corresponding product of value I.
Process K~
Reaction of an enaminoester XVII with a urethane XIX:
R1 O R4 R1 O R4
~ L40- ~
NH OL2 NH ~ ~ RS RZ N o'NH
R
R -I-
(base
N - Y N -- Y
R3 0 R3 OL2
XVII XIX III
L2 and L4 independently of one another are lower alkyl,
preferably C1-C4-alkyl, or phenyl.
This reaction is expediently carried out in an aprotic polar
solvent or diluent such as dimethylformamide, 2-butanone,
dimethyl sulfoxide and acetonitrile, advantageously in the
presence of a base, for example an alkali metal alkoxide or
alkaline earth metal alkoxide, in particular a sodium
alkoxide such as sodium methoxide, an alkali metal carbonate
or alkaline earth metal carbonate, in particular sodium
carbonate, or an alkali metal hydride such as lithium hydride
and sodium hydride.
CA 02312703 2000-06-OS
0050/48647
56
Once to twice the molar amount of base, based on the amount
of XVII or XIX, will normally suffice.
The reaction temperature is generally from 80 to 180~C,
preferably the boiling point of the reaction mixture.
As regards the weight ratio of the starting compounds, what
has been said for method G) also applies here.
In an especially preferred embodiment, a sodium alkoxide is
used as the base, and the alcohol which is formed in the
course of the reaction is distilled off continuously. The
resulting enaminoesters IV can be cyclized in accordance with
process D), without being isolated from the reaction mixture,
to give a salt of the substituted benzothiazoles I (where
R1 = H).
The urethanes XIX, in turn, can be prepared, for example, from
the carbonyl chlorides XX and anilines XXI:
R4 O R4
O + H N ~ ~ Rs base La ~ N ~ ~ Rs
2
L40 C 1 N - Y N - Y
XX ' XXI XIX
To avoid an excess of aniline, it is necessary, as a rule, to add
an auxiliary base such as triethylamine, pyridine or the alkali
metal carbonates to scavenge the hydrogen chloride which is
formed during the reacton. Pyridine is especially suitable since
it can be used simultaneously as the solvent.
Suitable solvents/diluents other than pyridine are, in
Particular, aromatic hydrocarbons such as benzene, toluene and
o-, m-, p-xylene, halogenated hydrocarbons such as methylene
chloride, chloroform and dichloroethane, lower alcohols such as
methanol and ethanol, aliphatic or cyclic ethers such as
dimethoxyethane, tetrahydrofuran and dioxane, carboxylic esters
such as ethyl acetate or aprotic polar solvents such as
dimethylformamide and dimethyl sulfoxide.
~
CA 02312703 2000-06-OS
0050/48647
57
The reaction temperature is generally from O~C to reflux
temperature of the reaction mixture in question.
The starting materials are expediently employed either in
5 approximately stoichiometric amounts, or else an excess of
carbonyl chloride of not more than 10 mol percent is chosen.
The auxiliary base is normally used in an approximately equimalar
10 ~°unt - based on the amount of XX or XXI - or in an excess of up
to approx. twice the molar amount. When using pyridine as the
auxiliary base, an even larger excess is recommended, in which
case the process can be carried out without an additional
solvent.
Process L~
Reaction of an isocyanate XXII with an aniline derivative XXI:
R4 H O R4
\
NCO
O H N ~ ~ 5 ~ Rz N~ NH ~ ~ 5
Rz~ .f. 2 R ~ ~ R
~~ OL2 N - Y N - Y
R3. R3 OL2
XXII XXI III (R1 = H)
Lz is lower alkyl, preferably C1-C4-alkyl, or phenyl.
This reaction is expediently carried out in an essentially
anhydrous aprotic organic solvent or diluent, for example in
the presence of an aliphatic or cyclic ether such as diethyl
ether, 1,2-dimethoxyethane, tetrahydrofuran and dioxane, an
aliphatic or aromatic hydrocarbon such as n-hexane, benzene,
toluene and o-, m-, p-xylene, a halogenated aliphatic
hydrocarbon such as methylene chloride, chloroform, carbon
tetrachloride, 1,2-dichloroethane and chlorobenzene, an
aprotic polar solvent such as dimethylformamide, hexamethyl
phosphoric triamide and dimethyl sulfoxide, or a mixture of
these.
If desired, the process can be carried out in the presence of
a metal hydride base such as sodium hydride and potassium
hydride, an alkali metal or alkaline earth metal alkoxide
such as sodium methoxide, sodium ethoxide and potassium
tert-butoxide, or of an organic nitrogen base such as
' CA 02312703 2000-06-OS
0050/48647
58
triethylamine and pyridine, it being possible for the organic
base to act simultaneously as the solvent.
The starting materials are expediently employed in
approximately stoichiometric amounts, or else one of the
components is used in an excess, of up to approximately
20 mol%. If the process is carried out in the absence of a
solvent in the presence of an organic base, the latter will
advantageously be present in an even larger excess.
The reaction temperature is generally from (-80) to 150~C,
preferably (-30)~C to the boiling point of the reaction
mixture in question.
The arylanilides of the formula IV are also novel; they, too, can
be prepared in a manner known per se, for example by reacting an
amide XXIII with a urethane XXIV
in accordance with process M~:
R4 R1 O ~ 0
O
R2 ~ ~ ~ ~ ~OL2 R2 N~OLZ RQ
HN g5
XXIV
H
R3 O N - Y R3 NH ~ ~ RS
N- Y
XXIII IV (R1 = H)
Lz is lower alkyl, preferably C1-C4-alkyl, or phenyl.
The reaction is advantageously carried out in an essentially
anhydrous solvent/diluent under atmospheric pressure,
especially preferably in the presence of an acidic catalyst.
To prepare enamine carboxylates IV where R1 = amino, it is
recommended to employ compounds XXIV with protected amino
group (for example as hydrazone).
Suitable solvents/diluents are, in particular, organic fluids
which can be mixed with water to give an azeotropic mixture,
for example aromatics such as benzene, toluene and o-, m-,
CA 02312703 2000-06-OS
0050/48647
59
p-xylene, or halogenated hydrocarbons such as carbon
tetrachloride and chlorobenzene.
Suitable catalysts are, in particular, strong mineral acids
such as sulfuric acid, organic acids such as
p-toluenesulfonic acid, phosphorus-containing acids such as
orthophosphoric acid and polyphosphoric acid, or acidic
cation exchangers such as "Amberlyst 15" (Fluka).
In general, a reaction temperature from approximately 70 to
150~C is sufficient; however, to remove the resulting water
of reaction rapidly, the process is expediently carried out
at the boiling point of the reaction mixture in question.
XXIII and XXIV are normally employed in approximately
stoichiometric amounts; preferably, XXIV is used in a slight
excess of up to approximately 20 mol%.
The amide XXIII can be prepared as follows (Process N~):
R4
O
O
~ '~ H2N / \ R5
O \ O N - Y ---~ XXI I I ( R3 = H )
O~ CH3
H3C
XXV XXI
The reaction is preferably carried out in an anhydrous inert
aprotic solvent, for example in a halogenated hydrocarbon
such as methylene chloride, chloroform, carbon tetrachloride
and chlorobenzene, an aromatic hydrocarbon such as benzene,
toluene and o-, m-, p-xylene, or an aliphatic or cyclic ether
such as diethyl ether, dibutyl ether, 1,2-dimethoxyethane,
tetrahydrofuran and dioxane.
The reaction temperature is generally from approximately 70
to 140~C, in particular from 100 to 120~C.
XXV and XXI are normally employed in approximately
stoichiometric amounts, or else one of the components is used
in an excess of up to approximately 10 mold.
CA 02312703 2000-06-OS
0050/48647
The isocyanates XVIII can be obtained, for example, from the
aniline derivatives XXI in accordance with process O~:
R4 R4
5
HzN ~ ~ R5 + "COC12" -! OCN ~ ~ R5
N- Y N- Y
10 XXI XVIII
The process can be carried out in an inert, essentially
anhydrous solvent or diluent or in the absence of solvents,
15 the aniline derivatives XXI preferably being reacted with
phosgene, a "phosgene equivalent", such as diphosgene,
triphosgene and carbonyldiimidazole, or with trichloromethyl
chloroformate.
20 Suitable solvents or diluents are, in particular, aprotic
organic solvents, for example dimethylformamide or aromatics
such as toluene and o-, m-, p-xylene, halogenated
hydrocarbons such as methylene chloride, chloroform,
1,2-dichloroethane and chlorobenzene, aliphatic or cyclic
25 ethers such as 1,2-dimethoxyethane, tetrahydrofuran and
dioxane, or esters such as ethyl acetate, and mixtures of
these.
The starting materials are expediently employed in
30 approximately stoichiometric amounts, or else one of the
components is employed in an excess of up to approx.
200 mol%.
Depending on the aniline derivative XXI employed, it may be
35 advantageous to add a base such as triethylamine, for example
in 0.5 times to twice the molar amount, based on the amount
of XXI.
40 The reaction temperature is generally from (-20)~C to the
reflux temperature of the solvent or reaction mixture in
question.
The aniline derivatives XXI, in turn, can be obtained in a manner
45 known per se (cf., for example, Houben-Weyl, Methoden der
organischen Chemie [Methods in Organic Chemistry], Georg Thieme
CA 02312703 2000-06-OS
0050/48647
61
Verlag Stuttgart, Vol. XI/1, 4th Edition 1957, p. 431 et seq.) by
reducing the corresponding nitro derivatives XXVI:
R4 R4
02N ~ ~ R5 - reduction H N ~ ~ 5
2 ~R
N -Y N- Y
XXVI XXI
As regards reducing agents, solvents, reaction temperatures and
weight ratios, reference may be made to what has been said above
for process F~.
The compounds XXVIII and XXI can also contain one or more chiral
centers, in which case they are normally obtained as enantiomer
or diastereomer mixtures. If desired, the mixtures can be
resolved into the essentially pure isomers by the methods
conventionally used for this purpose, for example by means of
crystallization or chromatography on an optically active
adsorbate. Pure optically active isomers can also be prepared,
for example, from corresponding optically active starting
materials.
Unless otherwise specified, all the processes described above are
expediently carried out under atmospheric pressure or under the
inherent pressure of the reaction mixture in question.
In general, the reactants are employed in a molar ratio of 0.95:1
to 5:1.
As a rule, the reaction mixtures are worked up by methods known
per se, for example by diluting the reaction solution with water
and subsequently isolating the product by means of filtration,
crystallization or solvent extraction, or
by removing the solvent, partitioning the residue in a mixture of
water and a suitable organic solvent and working up the organic
phase to give the product.
The compounds I and their agriculturally useful salts are
suitable as herbicides, both in the form of isomer mixtures and
in the form of the pure isomers. The herbicidal compositions
which comprise I effect very good control of vegetation on
non-crop areas, especially at high rates of application. In crops
such as wheat, rice, maize, Soya and cotton, they act against
broad-leaved weeds and grass weeds without inflicting substantial
' CA 02312703 2000-06-OS
0050/48647
62
damage to the crop plants. This effect is observed mainly at low
rates of application.
Depending on the application method in question, the compounds I,
or herbicidal compositions comprising them, can also be employed
in a further number of crop plants for eliminating undesirable
plants. Examples of suitable crops are the following:
Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spec. altissima, Beta vulgaris spec.
rapa, Brassica napus var. napus, Brassica napus var.
napobrassica, Brassica raps var, silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifoliurn),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spec., Manihot
esculenta, Medicago sativa, Musa spec., Nicotiana tabacum
(N.rustica), Olea europaea, Oryza sativa, Phaseolus lunatus,
Phaseolus vulgaris, Picea abies, Pinus spec., Pisum sativum,
Prunus avium, Prunus persica, Pyrus communis, Ribes sylvestre,
Ricinus communis, Saccharum officinarum, Secale cereale, Solanum
tuberosum, Sorghum bicolor (s. vulgare), Theobroma cacao,
Trifolium pratense, Triticum aestivum, Triticum durum, Vicia
faba, Vitis vinifera and Zea mays.
In addition, the compounds I can also be used in crops which, by
means of breeding, including genetic engineering methods, have
been made tolerant to the action of herbicides.
The compounds I, or the herbicidal compositions comprising them,
can be used, for example, in the form of directly sprayable
aqueous solutions, powders, suspensions, also highly concentrated
aqueous, oily or other suspensions or dispersions, emulsions, oil
dispersions, pastes, dusts, materials for spreading or granules,
by means of spraying, atomizing, dusting, spreading or pouring.
The use forms depend on the intended purposes; in any case, they
should guarantee the finest possible distribution of the active
ingredients according to the invention.
Suitable inert auxiliaries are essentially: mineral oil fractions
of medium to high boiling point such as kerosine and diesel oil,
furthermore coal tar oils and oils of vegetable or animal origin,
aliphatic, cyclic and aromatic hydrocarbons, eg. paraffins,
' CA 02312703 2000-06-OS
0050/48647
63
tetrahydronaphthalene, alkylated naphthalenes and their
derivatives, alkylated benzenes and their derivatives, alcohols
such as methanol, ethanol, propanol, butanol and cyclohexanol,
ketones such as cyclohexanone, strongly polar solvents, eg.
amines such as N-methylpyrrolidone, and water.
Aqueous use forms can be prepared from emulsion concentrates,
suspensions, pastes, wettable powders or water-dispersible
granules by adding water. To prepare emulsions, pastes or oil
dispersions, the substrates [sic], as such or dissolved in an oil
or solvent, can be homogenized in water by means of wetter,
tackifier, dispersant or emulsifier. Alternatively, it is
possible to prepare concentrates composed of active substance,
wetter, tackifier, dispersant or emulsifier and, if appropriate,
solvent or oil, and these concentrates are suitable for dilution
with water.
Suitable surfactants are the alkali metal salts, alkaline earth
metal salts and ammonium salts of aromatic sulfonic acid, eg,
ligno-, phenol-, naphthalene- and dibutylnaphthalenesulfonic
acid, and of fatty acids, of alkyl- and alkylarylsulfonates, of
alkyl sulfates, lauryl ether sulfates and fatty alcohol sulfates,
and salts of sulfated hexa-, hepta- and octadecanols, and of
fatty alcohol glycol ethers, condensates of sulfonated
naphthalene and its derivatives with formaldehyde, condensates of
naphthalene or of the naphthalenesulfonic acids with phenol and
formaldehyde, polyoxyethylene octylphenol ethers, ethoxylated
isooctyl-, octyl- or nonylphenol, alkylphenyl polyglycol ethers,
tributylphenyl polyglycol ether, alkylaryl polyether alcohols,
isotridecyl alcohol, fatty alcohol/ethylene oxide condensates,
ethoxylated castor oil, polyoxyethylene alkyl ethers or
polyoxypropylene alkyl ethers, lauryl alcohol polyglycol ether
acetate, sorbitol esters, lignin-sulfite waste liquors or
methylcellulose.
Powders, materials for spreading and dusts can be prepared by
mixing or binding the active substances together with a solid
carrier.
Granules, eg. coated granules, impregnated granules and
homogeneous granules, can be prepared by binding the active
ingredients to solid carriers. Solid carriers are mineral earths
such as silicas, silica gels, silicates, talc, kaolin, limestone,
lime, chalk, bole, loess, clay, dolomite, diatomaceous earth,
calcium sulfate, magnesium sulfate, magnesium oxide, ground
synthetic materials, fertilizers such as ammonium sulfate,
CA 02312703 2000-06-OS
0050/48647
64
ammonium phosphate, ammonium nitrate, ureas, and products of
vegetable origin such as cereal meal, tree bark meal, wood meal
and nutshell meal, cellulose powders, or other solid carriers.
The concentration of the active ingredients I in the ready-to-use
preparations can be varied within wide ranges. In general, the
formulations comprise approximately from 0.001 to 98% by weight,
preferably from 0.01 to 95% by weight, of at least one active
ingredient. The active ingredients are employed in a purity of
from 90% to 100%, preferably 95% to 100% (in accordance with NMR
spectra).
The formulation examples which follow illustrate the preparation
°f such products:
I. 20 parts by weight of Compound No. 1 are dissolved in a
mixture composed of 80 parts by weight of alkylated
benzene, 10 parts by weight of the adduct of 8 to 10 mol of
ethylene oxide to 1 mol of oleic acid N-monoethanolamide, 5
parts by weight of calcium dodecylbenzenesulfonate and
5 parts by weight of the adduct of 40 mol of ethylene oxide
to 1 mol of castor oil. Pouring the solution into 100,000
parts by weight of water and finely distributing it therein
gives an aqueous dispersion which comprises 0.02% by weight
of the active ingredient.
II. 20 parts by weight of Compound No. 2 are dissolved in a
mixture composed of 40 parts by weight of cyclohexanone,
30 parts by weight of isobutanol, 20 parts by weight of the
adduct of 7 mol of ethylene oxide and 1 mol of
isooctylphenol and 10 parts by weight of the adduct of
mol of ethylene oxide and 1 mol of castor oil. Pouring
the solution into 100,000 parts by weight of water and
35 finely distributing it therein gives an aqueous dispersion
which comprises 0.02% by weight of the active ingredient.
III. 20 parts by weight of Active Ingredient No. 4 are dissolved
in a mixture composed of 25 parts by weight of
40 cyclohexanone, 65 parts by weight of a mineral oil fraction
of boiling point 210 to 280~C and 10 parts by weight of the
adduct of 40 mol of ethylene oxide and 1 mol of castor oil.
Pouring the solution into 100,000 parts by weight of water
and finely distributing it therein gives an aqueous
dispersion which comprises 0.02% by weight of the active
ingredient.
~ CA 02312703 2000-06-OS
0050/48647
IV. 20 parts by weight of Active Ingredient No. 6 are mixed
thoroughly with 3 parts by weight of sodium
diisobutylnaphthalene-a-sulfonate, 17 parts by weight of
the sodium salt of a lignosulfonic acid from a sulfite
5 waste liquor and 60 parts by weight of pulverulent silica
gel, and the mixture is ground in a hammer mill. Finely
distributing the mixture in 20,000 parts by weight of water
gives a spray mixture which comprises 0.1~ by weight of the
active ingredient.
V. 3 parts by weight of Active Ingredient No. 8 are mixed with
97 parts by weight of finely divided kaolin. This gives a
dust which comprises 3~ by weight of the active ingredient.
VI. 20 parts by weight of Active Ingredient No. 12 are mixed
intimately with 2 parts by weight of calcium
dodecylbenzenesulfonate, 8 parts by weight of fatty alcohol
polyglycol ether, 2 parts by weight of the sodium salt of a
phenol/urea/formaldehyde condensate and 68 parts by weight
of a paraffinic mineral oil. This gives a stable oily
dispersion.
VII. 1 part by weight of Compound No. 14 is dissolved in a
mixture composed of 70 parts by weight of cyclohexanone,
20 parts by weight of ethoxylated isooctylphenol and 10
parts by weight of ethoxylated castor oil. The mixture can
subsequently be diluted with water to give the desired
concentration of active ingredient. This gives a stable
emulsion concentrate.
VIII. 1 part by weight of Compound No. 19 is dissolved in a
mixture composed of 80 parts by weight of cyclohexanone and
20 parts by weight of Wettol~ EM 31 (= nonionic emulsifier
based on ethoxylated castor oil; BASF AG). Then, the
mixture can be diluted with water to give the desired
concentration of active ingredient. This gives a stable
emulsion concentrate.
The active ingredients I, or the herbicidal compositions, may be
applied pre- or post-emergence. If the active ingredients are
less well tolerated by certain crop plants, application
techniques may be used in which the herbicidal compositions are
sprayed, with the aid of the spraying equipment, in such a way
that they come in as little contact as possible, if any, with the
leaves of the sensitive crop plants while reaching the leaves of
CA 02312703 2000-06-OS
0050/48647
66
undesirable plants which grow underneath, or the bare soil
surface (post-directed, lay-by).
Depending on the control target, the season, the target plants
and the growth stage, the rates of application of active
ingredient I are from 0.001 to 3.0, preferably 0.01 to 1.0, kg/ha
active substance (a.s.).
To widen the spectrum of action and to achieve synergistic
effects, the 3-(benzazol-4-yl)pyrimidinedione derivatives I may
be mixed with a large number of representatives of other groups
of herbicidal or growth-regulating active ingredients and applied
jointly. Suitable components for mixtures are, for example,
12,4-thiadiazoles, 1,3,4-thiadiazoles, amides, aminophosphoric
acid and its derivatives, aminotriazoles, anilides,
aryloxy/heteroaryloxyalkanoic acids and their derivatives,
benzoic acid and its derivatives, benzothiadiazinones,
2-(hetaroyl/aroyl)-1,3-cyclohexanediones, heteroaryl aryl
ketones, benzylisoxazolidinones, meta-CF3-phenyl derivatives,
carbamates, quinolinecarboxylic acid and its derivatives,
chloroacetanilides, cyclohexane-1,3-dione derivatives, diazines,
dichloropropionic acid and its derivatives, dihydrobenzofurans,
dihydrofuran-3-ones, dinitroanilines, dinitrophenols, diphenyl
ethers, dipyridyls, halocarboxylic acids and their derivatives,
areas, 3-phenyluracils, imidazoles, imidazolinones, N-phenyl-
3,4,5,6-tetrahydrophthalimides, oxadiazoles, oxiranes, phenols,
aryloxy- and heteroaryloxyphenoxypropionic esters, phenylacetic
acid and its derivatives, 2-phenylpropionic acid and its
derivatives, pyrazoles, phenylpyrazoles, pyridazines,
pyridinecarboxylic acid and its derivatives, pyrimidyl ethers,
sulfonamides, sulfonylureas, triazines, triazinones,
triazolinones, triazolecarboxamides and uracils.
Furthermore, it may be advantageous to apply the compounds I,
alone or in combination with other herbicides, also as a mixture
with other crop protection agents, for example with pesticides or
agents for controlling phytopathogenic fungi or bacteria. Also of
interest is the miscibility with mineral salt solutions which are
employed for remedying nutritional and trace-element
deficiencies. Nonphytotoxic oils and oil concentrates may also be
added.
CA 02312703 2000-06-OS
0050/48647
67
Preparation examples:
Example 1
3-[7-Chloro-5-fluoro-1-methyl-1H-benzotriazol-4-yl]-1-methyl-6-
trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp. 2)
0.12 g of methyl iodide was added dropwise at 20~C to a mixture of
0.25 g of 3-[7-chloro-5-fluoro-1-methyl-1H-benzotriazol-4-
Y1]-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione, 0.12 g of
potassium carbonate and 20 ml of absolute dimethylformamide. The
reaction mixture was subsequently stirred for a further 18 hours,
whereupon it was treated with 50 ml of water. Then, the mixture
was extracted three times using in each case 20 ml of ethyl
acetate. The combined organic phases were washed with water,
dried over sodium sulfate and finally concentrated. The crude
product was purified by chromatography on silica gel (eluent:
cyclohexane/ethyl acetate = 1:1). Yield: 0.07 g;
1H NMR (270 MHz, in CDC13): 8 [ppm] = 7.45 (d,lH), 6.45 (s,lH),
4.55 (s,3H), 3.60 (s,3H).
Example 2
3-[7-Chloro-5-fluoro-1-methyl-1H-benzotriazol-4-yl]-6-
trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp. 1)
0.43 g of ethyl 3-amino-4,4,4-trifluorobut-2-enoate in 3 ml of
dimethylforinamide was added at O~C in the course of 15 minutes
under a nitrogen atmosphere to 0.13 g of sodium methoxide in 7 ml
of absolute dimethylformamide. The mixture was first stirred for
one and a half hours at 10~C, whereupon a solution of 0.57 g of
ethyl 7-chloro-5-fluoro-1-methyl-1H-benzotriazol-4-ylcarbamate in
20 ml of dimethylformamide was added dropwise to the reaction
mixture in the course of 15 minutes. The mixture was subsequently
heated to 20~C and stirring was continued for 5 minutes. The
mixture was then heated to 60~C, and 0.35 g of
1,8-diazabicyclo[5.4.0]undec-7-
ene (DBU) was added. Finally, stirring was continued for 4 hours
at 120~C and for 18 hours at 20~C.
For working-up, the mixture was poured into 100 ml of a 10~ by
weight aqueous potassium carbonate solution. The product of value
was extracted by means of diethyl ether (twice 50 ml) and, after
the aqueous phase which remained had been brought to a pH of 1
with hydrochloric acid, by means of ethyl acetate (three times
30 ml).
CA 02312703 2000-06-OS
0050/48647
68
The combined organic phases were then washed with approx. 20 ml
of saturated aqueous sodium chloride solution and 30 ml of 10% by
weight aqueous lithium chloride solution, then dried over sodium
sulfate and finally concentrated. Yield: 0.25 g;
1H NMR (250 MHz, in CDC13): b [ppm) = 10.25 (br,lH), 7.45 (d,lH),
6.30 (s,lH), 4.60 (s,3H).
Step 2.1
2-Chloro-4-fluoro-N-trifluoroacetylaniline
144.3 g of trifluoroacetic anhydride in 150 ml of diethyl ether
were added dropwise at 0°C to 100 g of 2-chloro-4-fluoroaniline in
800 ml of absolute diethyl ether. After the mixture had been
heated to 20°C, 500 ml of water were added. The organic phase was
separated off and washed three times with water, then dried over
sodium sulfate and finally concentrated. Yield: 151.5 g;
1H NMR (270 MHz, in CDC13): b [ppm] = 8.35 (br,lH), 8.25 (dd,lH),
7.20 (dd,lH), 7.05 (dt,lH).
Step 2.2
3-Chloro-5-fluoro-2N-trifluoroacetylaminonitrobenzene
375 ml of 98% strength nitric acid were slowly added dropwise at
(-5)°C to 75 g of 2-chloro-4-fluoro-N-trifluoroacetylaniline in
753 ml of acetic anhydride. The mixture was then stirred for one
hour at (-5)°C, whereupon it was heated to 20°C. The course of
the
reaction was monitored by means of high-pressure liquid
chromatography on an RP1~-18 column (eluent: acetonitrile/water
- 7:3). As soon as starting material was no longer detectable,
the reaction mixture was poured into an ice-cold saturated
aqueous sodium chloride solution. The solid product of value was
subsequently separated off, washed with water and dried for
several hours in a drying oven under reduced pressure at 20°C.
Yield: 62.3 g;
1H NMR (270 MHz, in CDC13): b [ppm] = 8.50 (br,lH), 7.80 (dd,lH),
7.60 (dd,lH).
45
1) reversed phase, on silica gel
CA 02312703 2000-06-OS
0050/48647
69
Step 2.3
3-Chloro-5-fluoro-2-(N-methyl-N-trifluoroacetylamino)-
nitrobenzene
12.0 g of methyl iodide were added to a mixture of 16.1 g of
3-chloro-5-fluoro-2N-trifluoroacetylaminonitrobenzene, 11.6 g of
potassium carbonate and 100 ml of absolute dimethylformamide. The
reaction mixture was subsequently stirred for 18 hours at 20~C,
whereupon 500 ml of water were added. Then, the mixture was
extracted three times with 100 ml of ethyl acetate in each case.
The combined organic phases were dried over sodium sulfate and
finally concentrated.
Yield: 16.3 g;
1H NMR (270 MHz, in CDC13): b [ppm] = 7.80 (m,lH), 7.60 (m,lH),
3.40 (s,3H).
Step 2.4
3-Chloro-5-fluoro-2-methylaminonitrobenzene
173 ml of a 1-normal sodium hydroxide solution were added to a
solution of 16.3 g of 3-chloro-5-fluoro-2-(N-methyl-N-trifluoro-
acetylamino)nitrobenzene in 173 ml of ethanol. The mixture was
subsequently stirred for 1 hour, whereupon it was diluted with
500 ml of water. Then, it was extracted three times with 80 ml of
ethyl acetate. The combined organic phases were washed with
water, dried over sodium sulfate and finally concentrated. Yield:
10.1 g;
1H NMR (270 MHz, in CDC13): b [ppmj = 7.70 (dd,lH), 7.35 (dd,lH),
6.70-6.50 (br,lH), 3.10 (d,3H).
Step 2.5
2-Amino-6-chloro-4-fluoro-N-methylaniline
55.94 g of tin dichloride dehydrate were added to 10.1 g of
3-chloro-5-fluoro-2-methylaminonitrobenzene in 207 ml of absolute
ethanol. The mixture was subsequently heated at 50~C, and 0.94 g
of sodium boranate in 55 ml of absolute ethanol was added
dropwise in such a way that the temperature of the mixture did
not exceed 60~C. For working-up, the mixture was poured into 1 1
of ice-water, whereupon the pH was brought to 14 with sodium
hydroxide solution. Then, the mixture was extracted three times
with 100 ml of tert-butyl methyl ether. The combined organic
phases were washed with water, dried over sodium sulfate and
finally concentrated. Yield: 7.5 g;
CA 02312703 2000-06-OS
0050/48647
1H NMR (270 MHz, in CDC13): b [ppm] = 6.50 (dd,lH), 6.35 (dd,lH),
3.90-3.60 (br,3H), 2.65 (s,3H).
Step 2.6
5 7-Chloro-5-~luoro-1-methylbenzotriazole
A solution of 3.25 g of sodium nitrite in 19 ml of water was
added at 5°C to 7.5 g of 2-amino-6-chloro-4-fluoro-N-methylaniline
10 in 117 ml of 10% strength hydrochloric acid. After the reaction
mixture had been stirred for one hour at 5°C, it was diluted with
200 ml of water. Then, the solids were separated off, washed with
3x50 ml of water and dried in a vacuum drying oven at 20°C. Yield:
7.2 g;
15 1H NMR (270 MHz, in CDC13): b [ppm] = 7.60 (dd,lH), 7.30 (dd,lH),
4.55 (s,3H).
Step 2.7
20 7-Chloro-5-fluoro-1-methyl-4-nitrobenzotriazole
0.65 ml of 98% strength nitric acid were slowly added dropwise at
(-20)°C to 1.5 g of 7-chloro-5-fluoro-1-methylbenzotriazole in
28 ml of concentrated sulfuric acid. Then, the mixture was
25 stirred for one hour at 0°C, whereupon it was heated to 20°C.
Stirring was subsequently continued for 18 hours, and the
reaction mixture was then poured into 500 ml of ice-water. The
solids were separated off, washed with water and dried in a
vacuum drying oven at 20°C. Yield: 1.66 g;
30 1H NMR (270 MHz, in CDC13): b [ppm] = 7.50 (d,lH), 4.65 (s,3H).
Step 2.8
4-Amino-7-chloro-5-fluoro-1-methylbenzotriazole
1.66 g of 7-chloro-5-fluoro-1-methyl-4-nitrobenzotriazole were
reduced with tin dichloride/sodium boranate by a method similar
to what has been said for step 2.5. Yield: 1.27 g;
1H NMR (250 MHz, in (CD3)2S0): b [ppm] = 7.45 (d,lH), 6.25
(br,2H), 4.45 (s,3H), 4.30 (q,2H), 1.35 (t,3H).
CA 02312703 2000-06-OS
0050/48647
71
Step 2.9
Ethyl 7-chloro-5-fluoro-1-methyl-1H-benzotriazol-4-ylcarbamate
2.28 g of ethyl chloroformate were slowly added dropwise to 13 ml
of absolute pyridine at O~C, whereupon the mixture was stirred at
this temperature for 15 minutes. Then, 1.27 g of
4-amino-7-chloro-5-flu4ro-1-methylbenzotriazole in 20 ml of
pyridine were added dropwise at O~C. Stirring was subsequently
continued, first for 30 minutes at O~C, and the mixture was then
heated at 20~C and again stirred for 18 hours. Finally, the
reaction mixture was poured into 100 ml of 10~ strength
hydrochloric acid. Then, the mixture was extractd three times
with 50 ml of tert-butyl methyl ether. The combined organic
phases were washed with 100 ml of water and then concentrated.
50 ml of diethyl ether were added to the residue. The undissolved
component was separated off and washed with 3x30 ml of diethyl
ether. The combined ether phases were concentrated. Yield:
0.37 g;
1H NMR (250 MHz, in CDC13): 8 [ppm] = 7.35 (d,lH), 6.90 (br,lH),
4.55 (s,3H).
Example 3
3-[7-Chloro-5-fluoro-1-methyl-2-trifluoromethyl-1H-benzimidazol-
4-yl]-1-methyl-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione
(Comp. I.5)
2.46 g of 3-[7-chloro-5-fluoro-1-methyl-2-trifluoromethyl-1H
benzimidazol-4-yl]-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione
were alkylated with methyl iodide by a method similar to the one
described in Example 1. The crude product was purified by
chromatography on silica gel (eluent: cyclohexane/ethyl acetate =
2:1). Yield: 1.4 g;
1H NMR (250 MHz, in CDC13): b [ppm] = 7.35 (d,lH), 6.40 (s,lHj,
4.30 (s,3H), 3.55 (s,3H).
Example 4
3-[7-Chloro-5-fluoro-1-methyl-2-trifluoromethyl-1H-benzimidazol-
4-yl]-1-amino-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp.
6)
0.25 g of 2,4-dinitro-O-aminophenol was added to a mixture of
0.5 g of 3-[7-chloro-5-fluoro-1-methyl-2-trifluoromethyl-1H-
benzimidazol-4-yl]-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione,
2.35 g of potassium carbonate and 5 ml of ethyl acetate. After
CA 02312703 2000-06-OS
0050/48647
72
the mixture had been stirred for 18 hours at 20~C, it was diluted
with 50 ml of ethyl acetate. The resulting mixture was washed
with 3x30 ml of water, dried over sodium sulfate and finally
concentrated. The crude product was purified by means of
medium-pressure liquid chromatography (MPLC; eluent:
cyclohexane/ethyl acetate = 2:1). Yield: 0.4 g;
1H NMR (250 MHz, in CDC13): b [ppm] = 7.35 (d,lH), 6.30 (s,lH),
4.65 (s,2H), 4.25 (s,3H).
Example 5
3-[7-Chloro-5-fluoro-1-methyl-2-trifluoromethyl-1H-benzimidazol-
4-yl]-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp. 4)
4.31 g of ethyl 3-amino-4,4,4-trifluorobut-2-enoate in 20 ml of
dimethylformamide were added dropwise at 0 bis 5~C to 0.82 g of
sodium hydride in 50 ml of absolute dimethylformamide. The
mixture was then stirred for one hour at the same temperature,
whereupon 7-chloro-5-fluoro-4-isocyanato-1-methyl-2-
trifluoromethylbenzimidazole (from step 5.4) in 40 ml of
dimethylformamide were added at (-30)~C. Stirring was subsequently
continued for one hour at (-30)~C and for a further hour at 20~C.
For working-up, the reaction mixture was carefully poured into
200 ml of ice-water. Acidification with 10~ strength hydrochloric
acid gave a solid which was filtered off, washed with water and
dried in a vacuum drying oven at 20~C. After purification by flash
chromatography (eluent: cyclohexane/ethyl acetate = 2:1), 5.08 g
of product of value were obtained.
After the solids had been separated off, product of value which
still remained in the filtrate (2.46 g) was isolated by
extracting the filtrate three times with 200 ml of tert-butyl
methyl ether, washing the combined ether phases, drying them over
sodium sulfate and concentrating them.
Total yield: 7.54 g;
1H NMR (250 MHz, in CDC13): b [ppm] = 7.40 (d,lH), 6.30 (s,lH),
4.30 (s,3H).
Step 5.1
7-Chloro-5-fluoro-1-methyl-2-trifluoromethylbenzimidazole
15.5 g of 3-chloro-5-fluoro-2-(N-methyl-N-trifluoroacetyl-
amino)nitrobenzene (step 2.3) were reduced with tin
dichloride/sodium boranate without intermediate isolation by a
method similar to what has been said for step 2.5 to give the
CA 02312703 2000-06-OS
0050/48647
73
corresponding amino compound which then underwent spontaneous
cyclization, with the elimination of water, to give the product
of value. Yield: 9.32 g;
1H NMR (250 MHz, in CDC13): b [ppm] = 7.45 (d,lH), 7.20 (d,lH),
4.25 (s,3H).
Step 5.2
7-Chloro-5-fluoro-1-methyl-4-nitro-2-trifluoromethylbenzimidazole
46.3 ml of 98~ strength nitric acid were slowly added dropwise at
O~C to 9.65 g of 7-chloro-5-fluoro-1-methyl-2-trifluoromethyl-
benzimidazole in 96.5 ml of acetic anhydride. After the mixture
had been stirred for one hour at O~C, it was heated carefully to
20~C. (In the event that an exothermal reaction did commence, the
temperature was kept below 25~C by means of an ice bath.) The
reaction mixture was subsequently first stirred for another two
hours at 20~C and then poured into ice-cold saturated aqueous
sodium chloride solution. The solids formed were separated off,
washed with water and dried at 20~C in a vacuum drying oven.
Yield: 8.5 g;
1H NMR (400 MHz, in CDC13): 8 [ppm] = 7.40 (d,lH), 4.35 (s,3H).
Step 5.3
4-Amino-7-chloro-5-fluoro-1-methyl-2-trifluoromethylbenzimidazole
8.71 g of 7-chloro-5-fluoro-1-methyl-4-nitro-2-trifluoromethyl-
benzimidazole were reduced by means of tin dichloride/sodium
boranate by a method similar to what has been said for step 2.5.
Yield: 6.3 g;
1H NMR (270 MHz, in CDC13): 8 [ppm] = 7.05 (d,lH), 4.45 (br,2H),
4.20 (s,3H).
Step 5.4
7-Chloro-5-fluoro-4-isocyanato-1-methyl-2-trifluoromethyl-
benzimidazole
23.32 g of diphosgene were added to 6.3 g of 4-amino-7-chloro-5-
fluoro-1-methyl-2-trifluoromethylbenzimidazole in 100 ml of
absolute toluene. The mixture Was subsequently refluxed for
6 hours. After the reaction mixture had been stirred for a
further 18 hours at 20~C, it was concentrated. The crude product
obtained was reacted directly, without purification, to give the
end product I.4.
CA 02312703 2000-06-OS
0050/48647
74
Example 6
3-[7-Chloro-1,2-dimethyl-5-fluoro-IH-benzimidazol-4-yl]-1-methyl-
6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp. 8)
0.33 g of 3-[7-chloro-1,2-dimethyl-5-fluoro-1H-
benzimidazol-4-yl]-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione
was alkylated with methyl iodide by a method similar to what has
been said for Example 1. Yield: 0.04 g;
1H NMR (250 MHz, in CDC13): 8 [ppm] = 7.10 (d,lH), 6.40 (s,lH),
4.00 (s,3H), 3.55 (s,3H), 2.55 (s,3H).
Example 7
3-[7-Chloro-1,2-dimethyl-5-fluoro-1H-benzimidazol-4-yl]-6-
trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Comp. 7)
7-Chloro-1,2-dimethyl-5-fluoro-4-isocyanatobenzimidazole, from
step 7.4, was reacted with ethyl 3-amino-4,4,4-trifluorobut-2-
enoate by a method similar to what has been said for Example 5.
The crude product was purified by means of medium-pressure liquid
chromatography (eluent: ethyl acetate/methanol = 15:1). Yield:
0.7 g;
iH NMR (270 MHz, in CDC13): b [ppm] = 7.15 (d,lH), 6.20 (s,lH),
4.05 (s,3H), 2.55 (s,3H).
Step 7.I
7-Chloro-1,2-dimethyl-5-fluorobenzimidazole
100 ml of 10~ strength hydrochloric acid were added to 6.96 g of
2-amino-6-chloro-4-fluoro-N-methylaniline (from step 2.5) in
4.1 g of acetic anhydride. The mixture was subsequently refluxed
for 4 hours. After cooling, the mixture was taken up in
ice-water. It was then neutralized carefully with an aqueous
sodium carbonate solution. The solid crude product which had
formed was separated off, washed with water and dried in a vacuum
drying oven at 20~C. Yield: 7.92 g;
1H NMR (270 MHz, in CDC13): b [ppm] = 7.25 (dd,lH), 6.95 (dd,lH),
4.00 (s,3H), 2.55 (s,3H).
CA 02312703 2000-06-OS
0050/48647
Step 7.2
7-Chloro-1,2-dimethyl-5-fluoro-4-nitrobenzimidazole
5 98% strength nitric acid was added dropwise at from (-5) to not
more than O~C to 7.92 g of 7-chloro-1,2-dimethyl-5-
fluorobenzimidazole in 139 ml of concentrated sulfuric acid,
during which process the course of the reaction was monitored by
means of high-performance liquid chromatography (HPLC) on an
10 RP-18 column (eluent: acetonitrile/water = 1:1). As soon as
starting material was no longer detectable, the reaction mixture
was poured into ice-water, whereupon the pH was brought to 14 by
means of sodium hydroxide solution. The solids were separated
off, washed with water and dried at 20~C in a vacuum drying oven.
15 The two regioisomeric nitro compounds which had formed were
separated by means of flash chromatography on silica gel (eluent:
.ethyl acetate; the product which eluted first was the desired
regioisomer). Yield: 5.6 g;
1H NMR (400 MHz, in CDC13): 8 [ppm] = 7.05 (d,lH), 4.10 (s,3H),
20 2~65 (s,3H).
Step 7.3
4-Amino-7-chloro-1,2-dimethyl-5-fluorobenzimidazole
5.6 g of 7-chloro-1,2-dimethyl-5-fluoro-4-nitrobenzimidazole were
reduced by means of tin dichloride/sodium boranate by a method
similar to what has been said for step 2.5. The crude product
obtained was employed directly, without purification, in step
~'4~ Yield: 4.1 g.
Step 7.4
7-Chloro-1,2-dimethyl-5-fluoro-4-isocyanatobenzimidazole
4,1 g of 4-amino-7-chloro-1,2-dimethyl-5-fluorobenzimidazole were
reacted with diphosgene by a method similar to what has been said
for step 5.4. The crude product obtained was reacted directly,
again without purification, to give the end product I.7.
Example 8
3-[7-Chloro-2-dimethylamino-5-fluorobenzoxazol-4-yl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione (Comp. 19)
1.0 g of 3-[2-amino-4-chloro-6-fluoro-3-hydroxyphenyl]-1-
methyl-6-trifluoromethyl-2,4-(1H,3H)-pyrimidinedione and 0.5 g of
dichloromethyleneimmonium chloride were mixed in 100 ml of
~
CA 02312703 2000-06-OS
0050/48647
76
1,2-dichloroethane, whereupon the mixture was filled into a glass
holder for pressurized containers and heated for 5 hours at 120°C
in a sealed pressurized container. During this process, the
inherent pressure of the container climbed to approx. 5 bar. The
container was subsequently cooled. The clear product solution was
washed with dilute aqueous potassium carbonate solution.
The organic phase was dried over sodium sulfate and finally
concentrated. The crude product was purified by flash
chromatography using a short column (eluent:
cyclohexane/tert-butyl methyl ether = 8:2). Yield: 0.5 g;
1H NMR (270 MHz, in CDC13): 8 [ppm) = 6.85 (d,lH), 6.4 (s,lH),
3.6 (s,3H), 3.25 (s,6H).
Step 8.1
3-[4-Chloro-6-fluoro-3-methoxy-2-nitrophenyl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione
Nitrating acid, composed of 20.4 ml of concentrated sulfuric acid
and 25.5 ml of 98~ strength nitric acid, was slowly added
dropwise with cooling to (-20)~C to 51.0 g of
3-[4-chloro-6-fluoro-3-methoxyphenylj-1-methyl-6-trifluoromethyl-
2,4-(1H,3H)-pyrimidinedione in 1 1 of concentrated sulfuric acid.
After the addition had ended, stirring was continued for
30 minutes at (-20)~C. The reaction mixture was then stirred into
1 1 of ice-water. The solids formed were separated off, washed
with water and dried in a vacuum drying oven at 20~C. Yield:
57.0 g;
1H NMR (270 MHz, in CDC13): 8 [ppmj = 7.55 (d,lH), 6.35 (s,lH),
4.05 (s,3H), 3.55 (s,3H).
Step 8.2
3-[4-Chloro-6-fluoro-3-hydroxy-2-nitrophenyl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione
19.0 g of lithium chloride were added to 57.0 g of
3-[4-chloro-6-fluoro-3-methoxy-2-nitrophenyl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione in approx. 500 ml of
absolute dimethylformamide. The mixture was subsequently stirred
for 3 hours at 80-90~C. After cooling, 1 1 of water was added to
the reaction mixture. The product of value was extracted with
3x200 ml of methyl tert-butyl ether. The ether phase was
repeatedly washed with water and then dried and finally
concentrated. Yield: 46.1 g;
CA 02312703 2000-06-OS
0050/48647
77
1H NMR (250 MHz, in CDC13): 8 [ppm] = 7.65 (d,lH), 6.35 (s,lH),
3.60 (s,3H).
Step 8.3
3-[2-Amino-4-chloro-6-fluoro-3-hydroxyphenyl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione
34 g of iron powder were added at 65~C, a little at a time, to
46.0 g of 3-[4-chloro-6-fluoro-3-hydroxy-2-nitrophenyl]-1-
methyl-6-trifluoromethyl-2,4-(1H,3H)-pyrimidinedione in 423 ml of
water and 36.8 ml of concentrated hydrochloric acid. The mixture
was subsequently refluxed for 3 hours. After cooling, the mixture
was shaken with 500 ml of ethyl acetate. The organic phase was
freed from the remaining inorganic material by means of
filtration on Celite~ (Manville Corporation). The filtrate was
dried over sodium sulfate and finally concentrated. Yield: 37.5
g:
1H NMR (270 MHz, in CDC13): 8 [ppm] = 6.65 (d,lH), 6.40 (s,lH),
3.60 (s,3H).
Example 9
3-[7-Chloro-5-fluorobenzoxazol-4-yl]-1-methyl-6-trifluoromethyl-
2'4-(1H,3H)-pyrimidinedione (Comp. 12)
0.5 g of trimethyl orthoformate was added to a solution of 0.5 g
of 3-[2-amino-4-chloro-6-fluoro-3-hydroxyphenyl]-1-
methyl-6-trifluoromethyl-2,4-(1H,3H)-pyrimidinedione (from step
8.3) in 30 ml of absolute methanol. The mixture was then refluxed
for 20 hours. Solvent and excess ortho ester were subsequently
removed under reduced pressure. The residue was dissolved in
ethyl acetate. The organic phase was washed with water and then
dried over sodium sulfate and finally concentrated. The crude
Product was purified by means of flash chromatography (eluent:
cyclohexane/tert-butyl methyl ether = 3:1). Yield: 0.26 g;
1H NMR (250 MHz, in CDC13): 8 [ppm] = 8.20 (s,lH), 7.40 (d,lH),
6.40 (s,lH), 3.60 (s,3H).
45
CA 02312703 2000-06-OS
0050/48647
78
Example 10
3-[7-Chloro-5-fluoro-2-methoxybenzoxazol-4-yl]-1-methyl-6-
trifluoromethyl-2,4-(1H,3H)-pyrimidinedione (Comp. 14)
1.0 g of 3-[2-amino-4-chloro-6-fluoro-3-hydroxyphenyl]-1-
methyl-6-trifluoromethyl-2,4-(1H,3H)-pyrimidinedione (from step
8.3) was reacted with tetramethyl orthocarbonate by a method
similar to what has been said for Example 9. Yield: 0.7 g;
1H NMR (250 MHz, in CDC13): 8 [ppm] = 7.10 (d,lH), 6.40 (s,lH),
4.20 (s,3H), 3.60 (s,3H).
In addition to the 3-(benzazol-4-yl)pyrimidinedione derivatives
of the formula I which have been described above, others which
were, or can be, prepared in a similar manner are listed in
Table 2 which follows:
25
35
45
CA 02312703 2000-06-OS
0050/48647
79
U
O d' N l0
N M M
e1 O rl 01 I~r-1
I !~ 1 l0 ~Di
W rl N I O r1O 1 I tl1n-1
r-I N 00~-~IrlM I~ II1M rl
O ~ l0v-iO ri \O 1D-1O
U
II
u,
P4
x
II
y fh t~1M M t~fM M t'f N
U U
1 I i U V U U U U x U
M
W
U
II
N
II
x
H .f.~ ~ .i'.~ ~ G C ~ G'.
O O O O O O O O O O
N 1 1 I ,!a,.QJa ,~.G .O.sa,~.i7.fa
U
I i I I 1 I
x x x x x x
I I I z z z z z z o 0 0 0
~ I I I I
o x x x ~ ~ '. ~ ~ ";!
o U U U ~ 0 ~ " ~ ~
_ 4 P4 fx GY,G4R:
z z z N N N N N N N N N N
z ~ I I I 1 ~ v v v v v ~
/ U U U U U U U U U U
2 2
U
M
W
a rl
R:W W W W W W W W W U W W W
H1N f"1N Y~7N C~'1M M M
x x x x x x x x x x
x x U z x U z x U z U U U U
N
O
r~
b O O riN M
z '-1N M C~ lf7l0 I~00 01ri ~-i'-1ri
CA 02312703 2000-06-OS
0050/48647
O
N ~D N I~ M
r1 ~' .-1O O
ri ri r-1r-1 r-i
1 1 1 I I
C~O rl.-ILC1r-IO N rl rlN '-1rl rl O
e-1rlrl c!rlri O rl rlO riri rl (~'~
E ~ O O ~ O ~ ~ O O ~'-~ O O O N
x
N
U
O
N
I
x ~ '"
N
tI
U
x O O
U "
,
U
x
c~a, - O
~
~
~
M U U ~ x M U ii ~ x x M M M M
n x I I .ON x >,x x x U N x x x x x x
G~U C C CLU U U V U V U U tl1U U U I I I O
_I
M
x
'>~'d 'C'dU 1 'C7'L3"~ 'Lf 'CT1 TJ'C 'O
a a a a - x ~ a c s~ a s~ a a a
~ ~
N O .C,fa~7.OI .L7.L7.C7.CaO V1 .O.O .L7.~ i I I ,La
I I I
M M M
I I I I I r i I I I I I I I I I I x x x I
O O O O O O O O O O O O O O cn v7v7 U U U O
I I I I I I I I I I I I I I I I I N N N I
......-..-..-..-.~....n O O O ...
n n n n n n n n n n n n n n n n n Ulf!7V1 n
P4 ~'P4 GGP4'fx W'W' W'f~ fYP:P: RSCYOR.'C4'~.-...
N N N N N N N N N N N N N N N N N ~..,,'t.,"~,N
V U U U U U U U U U U U U U U U U 2 Z 2 U
IIII ItII IIIIII IIII IIII flIIII IIII IIII IIIIII ll
PdW W W W W W W W fs.~W W W W W U U U W W W W
M M M M M M M M M M M M M M M N M M M
~.x x x x x x x x x x x x x x x x x x x
G:U U U U U U U U U U U U U U U x z x U U U
O d' u7~O l~CD01 O r-1N ch d t11t0 I~fb C1O ~-1N M er
'Zr~"~~ ~ ~ '~~ N N N N N N N N N M (~"1c'~M M M
CA 02312703 2000-06-OS
0050/48647
81
U
O
,r
t3.y l ~-1r-Irlr-Ir-IO
O rlr1 .-Ir-1-rirWC
O O O O O O O
N x
0 o x
itr~ r
V 5r
()N ~ M ~ Gi
M N x
~
r w x U N ~ x
x U U U a +~ z a~
b ~ ~ b b b
~ a c a a s~
0 0 0 0 0 o x
N .17.Ja.L~tl1.G7.O ..L7z
I
x
U
U
1
N
x
U
1 I
I 1 I 1 I
z o 0 0 0 0 0 0
I I t I 1 1 I 1
., ..., ~ ~... .,
r n r n n r . n
n
P4 t~R: OG4I:Ri A.'fh'
N N N N N N N N
I
v ~ v v .r v v
U U U U U U U U
x w w w w w w w w
M M M M M
x x x x x
x U U U x x x U U
O ~7 l0t~ CO01O riN
z M M M M c~"1~ ch
' CA 02312703 2000-06-OS
0050/48647
82
Use examples
The herbicidal action of the 3-(benzazol-4-yl)pyrimidinedione
derivatives I was demonstrated by the following greenhouse
experiments:
The culture containers used were plastic flowerpots containing,
as substrate, loamy sand with approx. 3.0% of humus. The seeds of
the test plants were sown in separately for each species.
In the case of the pre-emergence treatment, the active
ingredients which were suspended or emulsified in water were
applied directly after sowing by means of finely distributing
nozzles. The containers were irrigated gently to promote
germination and growth and subsequently covered with translucent
plastic hoods until the plants had rooted. This cover caused
uniform germination of the test plants, unless this was adversely
affected by the active ingredients.
For the post-emergence treatment, the test plants were first
grown to a height of 3 to 15 cm, depending on the plant habit, and
only then treated with the active ingredients which were
suspended or emulsified in water. The test plants for this
purpose were either sown directly and grown on in the same
containers, or they were raised separately as seedlings and
transplanted into the test containers a few days prior to
treatment. The rate of application for the post-emergence
treatment was 15.6 or 7.8 g/ha a.s. (active substance).
The plants were kept at 10 - 25~C or 20 - 35~C, depending on the
species. The test period extended over 2 to 4 weeks. During this
time, the plants were tended, and their response to the
individual treatments was evaluated.
Evaluation was done on a scale of from 0 to 100. 100 means no
plant emergence, or complete destruction of least the aerial
parts of the plant, and 0 means no damage, or normal course of
growth.
The plants used in the greenhouse experiments belonged to the
following species:
CA 02312703 2000-06-OS
0050/48647
83
Scientific name English name
Amaranthus redroot pigweed
retroflexus
Chenopodium album lambsquarters
(goosefoot)
Galium aparine catchweed bedstraw
Solanum nigrum black nightshade
veronica species speedwell species
At rates of application of 15.6 and 7.8 g/ha a.s. post-emergence,
Compound No. 18 had a very good herbicidal effect against the
abovementioned weeds.
20
30
40