Note: Descriptions are shown in the official language in which they were submitted.
CA 02312808 2000-06-02
A Device for Examining Respiratory Diseases,
and Diagnostic Agents
Specification
The invention relates to a device which, in associa-
tion with conventional analytical systems, can be used in
diagnosing the metabolic performance of the respiratory or-
gans. Using this device, it is possible to dose suitable
diagnostic agents in specific quantities into the inhalation
air and, following a defined period of time, collect metabo-
lites of the diagnostic agent and/or the remaining
diagnostic agent itself from the expired air. Using
conventional analytical systems (e.g., mass spectrometry,
gas chromatography, GC-MS-MS, HPLC-MS, isotope analysis),
these expired and collected substances can be identified and
determined quantitatively, thereby permitting conclusions as
to the health condition of the respiratory organs.
The invention is also directed to diagnostic agents
which can be used to determine the metabolic performance of
the respiratory tract epithelium - with particular advantage
also in association with the device according to the inven-
tion, and to an appropriate method for the chemical-analyti-
cal determination of the metabolic performance of the bron-
chial epithelium.
Referring to the prior art, it has been familiar for
a long time to collect volatile substances from expired air
by passing the air over a sorbent, adsorbing the substances
and determining them. In the mid-seventies, the so-called
Tenax, a polymer based on 2,6-diphenyl-p-phenylene oxide,
CA 02312808 2000-06-02
- 2 -
has been developed as a sorbent particularly suited for this
purpose (Krotoszynski et al., J. Chromatogr. Sci. 15, 239-
244, 1977; Krotoszynski et al., J. Analyt. Toxicol. 3, 225-
234, 1979).
More recent studies by Wallace et al., Environmental
Health Perspectives, Vol. 104, Supplement 5, pp. 861-869,
1996, deal with the utility of breath analysis in the deter-
mination of volatile organic substances in respiratory air
after exposing test persons to certain chemicals in their
environment (automobile exhaust gases, gas stations,
swimming pools, exposition to benzene and styrene during
active smoking).
Various methods and devices for collecting both
volatile and non-volatile substances from respiratory air
have become familiar.
Thus, WO 91/05255 Al describes a method wherein non-
volatile biopolymers such as proteins from the broncho-
alveolar boundary fluid are detected in respiratory air. To
this end, a test person breathes forced exhalations on a
sample carrier plate 1 cm2 in size and cooled to -196 C. The
sample then is freeze-dried on a cryo-table by applying an
ultra-high vacuum and subsequently analyzed.
DE 195 05 504 Al describes a method and a device for
collecting expired breath condensate. Therein, expired air
flows through a sample collector tube, being cooled to a
subfreezing temperature of below 0 C where liquid and
soluble components undergo condensation, freezing to the
interior wall of the sample collector tube which has a lower
temperature than the respiratory condensate.
In Applied Cardiopulmonary Pathophysiology 5, pp.
215-219, 1995, Gunther Becher et al. describe the determina-
tion of leukotriene B4 (LTB4) in respiratory condensate - a
CA 02312808 2000-06-02
~
- 3 -
mediator which, in the event of mucosa inflammation, is re-
leased from the inflammatory cells into the respiratory
tract. It has been shown that the amount of expired LTB4
correlates with the clinical stage of bronchial asthma. This
written document concludes that collection of the
respiratory condensate and biochemical analysis thereof is
well-suited for diagnosing inflammatory respiratory
diseases.
Being based on the measurement of a biochemical en-
dogenous component, the determination of LTB4 is the most
direct method at present. However, this method does not
offer any option for true quantification of the stage of
disease via standardizable reference values. On the other
hand, practical application in routine diagnostics is
limited by the necessity of accumulating and measuring
exceedingly low amounts of material, and these amounts are
subject to intra- and inter-individual variations which, in
their normal range, may be greater than e.g. the effect of
bronchial asthma.
Also, the problem is that in most of the cases one
does not know in which way the mediators present in the ex-
pired air, such as proteins, peptides, amino acids,. and
phospholipides, correlate with the stage of disease in the
respiratory tract or the lungs, so that diagnosis on this
basis is not possible as yet. Similarly, WO 91/05255 wherein
a method of diagnosing the health condition of lungs and
respiratory tract is claimed, merely demonstrates that non-
volatile biopolymers can be analyzed in human respiratory
air. WO 91/05255 does not reveal any conclusions as to the
health condition of the respiratory tract and, in
particular, the metabolic performance of the respiratory
epithelium.
The deficiency of all the previous solutions is
their incapability of reliably characterizing the
CA 02312808 2006-07-17
28751-7
- 4 -
physiological-chemical condition and the metabolic capacity
of the bronchial epithelium via the respiratory air.
It was therefore the object of the invention to
develop a method of determining the health condition of the
respiratory tract and, in particular, the metabolic activity
of cells on the boundary surface of the respiratory tract,
as well as the surface condition of the bronchial mucosa in
a reproducible, reliable, standarized and direct fashion
using chemical-analytical means, and to provide a simple
device for this purpose.
According to one aspect the invention provides a
device for examining respiratory diseases by dosing
diagnostic agents into the respiratory air, which agents are
capable of forming metabolites in the respiratory organs,
and collecting substances present in the expired air, the
device comprising a flow channel having a front end and an
opposite end and having a mouthpiece at its front end, an
inlet valve, an inhalation or injection unit for the
diagnostic agent, a cold trap to separate substances present
in the expired air, which is arranged at an angle relative
to the flow channel, and an adsorption vessel arranged
downstream of the cold trap at the opposite end of the flow
channel.
According to another aspect the invention provides
a method of determining the metabolic performance of the
bronchial epithelium, wherein diagnostic agents capable of
forming metabolites in the respiratory organs are dosed into
the respiratory air of a test person using the novel device,
the substances present in the expired air are collected
subsequently, and the metabolites and/or remaining amount of
diagnostic agent are subjected to analysis.
CA 02312808 2006-07-17
28751-7
- 5 -
According to the invention, a device is provided
by means of which a small, precisely defined amount of a
suitable diagnostic agent is inhaled during inspiration, and
the amount of said agent and/or its metabolites streaming
back during expiration is collected by successive, well-
defined cooling and adsorption and determined accurately.
A certain quantity of the diagnostic agent is
absorbed by the bronchial epithelium and optionally
converted in a biochemical reaction (metabolized) to give
well-defined resultant products. The level of absorption
and conversion depends on the health condition of the
epithelium tissue and thus, on the present metabolic
performance thereof. Consequently, it is possible to
determine the epithelium condition in a direct biochemical
way by measuring the quantity of expired diagnostic agent
relative to the inhaled amount and/or the corresponding
quantity of its defined metabolites.
The invention will now be described with reference
to the accompanying drawings, in which:
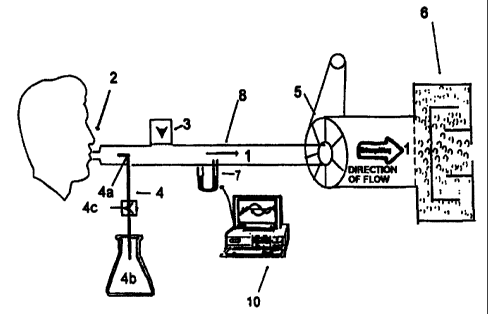
Fig. 1 shows a device according to the invention
having a fresh air valve 3 and a simple inhalation or
injection unit 4.
Fig. 2 shows a device according to the invention
having a fresh air valve 3 and an aerosol inhalation or
injection unit 4.
Fig. 3 shows a device according to the invention
having an antistatic reservoir bag.
Fig. 4 shows a part of the device according to the
invention including suction device 11, analytical unit 12,
cold trap 5, and adsorption section 6.
CA 02312808 2006-07-17
28751-7
- 5a -
Fig. 5 shows a reverse cold trap 5.
Referring to Figure 1, a device for examining
respiratory diseases consists of a flow channel 1 having a
mouthpiece 2 at its front end, an inlet valve 3, an
inhalation or injection unit 4 for the diagnostic agent, a
cold trap 5 to separate soluble substances present in the
expired air, which is arranged at an angle relative to the
flow channel 1, and an adsorption vessel 6 arranged down-
stream of the cold trap 5 at the opposite end of the flow
channel 1.
In a preferred embodiment of the invention, the
longitudinal axis of the cold trap is arranged at an angle
of about 450 relative to the flow channel, thereby achieving
an approximately uniform flow through the cold trap.
The inhalation or injection unit 4 for dosing the
diagnostic agent into the inspiration air can be designed in
various ways, but using per se known equipment or parts of
equipment. Thus, dosing can be effected using e.g. a dosing
pump or a piston syringe, or by simple suction of the
diagnostic agent in an appropriate dilution (cf., Fig. 1).
Previous nebulization or atomization of the diagnostic agent
is also possible. Preferably, computer-controlled aerosol
generation using a pressurized, nozzle or ultrasonic
nebulizer or a centrifugal atomizer may also be employed
(cf., Fig. 2). In these cases, fresh air is sucked in via
inlet valve 3. For a person skilled in the art, however, it
would be no problem to provide other suitable inhalation or
injection units. Thus, for example, a previously prepared
air/diagnostic agent mixture can be provided in a reservoir
bag and inspired or dosed via inlet valve 3 (cf., Fig. 3).
According to the device of the invention, the
expired air to be analyzed, in order to collect the
CA 02312808 2006-07-17
28751-7
- 5b -
metabolites of the diagnostic agent present therein and/or
the remaining diagnostic agent itself, initially flows
through the cold trap 5 where the water present in the
expired air is condensed together with non-gaseous
substances that are present. To achieve optimum cooling
performance, the cold trap 5 can be a reverse cold trap, for
example, as illustrated in Fig. 5. However, any other
design is also possible, and the only point is that the
adjustable temperature range for cooling performance spans
at least from -5 C to -25 C. This can be done in a very
simple way by
CA 02312808 2006-07-17
28751-7
- 6 -
using a cooling jacket tube where water having a coolant
additive is passed through. When using a reverse cold trap
in accordance with Fig. 5, it may be connected to a
refrigerating aggregate. The cold trap 5 may be arranged in
a fashion enabling its removal from the device, so that the
respiratory condensate can be collected in frozen condition.
With a computer-controlled design of the deVice, however, it
is also possible to heat the cold trap 5 after collection of
the exhaled material is completed and suck the water
containing the substances to be determined directly into the
analytical unit 12 (cf., Fig. 4). According to the
invention, the device may also include multiple cold traps
arranged in series.
Downstream of cold trap 5, the gaseous components
and remaining residues of exhaled material pass through an
adsorption vessel 6. The adsorption vessel 6 includes a
sorbent commonly used to collect volatile substances in ex-
pired air, e.g. an organic polymer or active charcoal. Basi-
cally, the respiratory condensate will deposit on the
interior wall of cold trap 5.
As can easily be seen by someone skilled in the art,
the device is operable in two different ways, either by
means of mechanical valves or by using computer-controlled
valve switching. The passive flow control using a mechanical
valve 3 permits a purely mechanical separation of the
inspiratory and expiratory flow paths. The easy-running
valve 3 opens even at slightly reduced. pressure
(inspiration) within the mouthpiece, so that the influx of
the inspired volume invariably takes place via this inlet.
As soon as the vacuum declines or excess pressure develops
at the mouthpiece (no breath or expired air), the valve
closes automatically. The cooling and adsorption section is
remarkable for its system-inherent resistance, excluding
influx during inspiration. In case of small flow differences
(e.g., in measurements on children), an additional passive
CA 02312808 2006-07-17
28751-7
- 7 -
expi_ration valve is employed. When expiring, the only flow
path for the expired air is through the cooling and
adsorption section.
As an alternative to the passive variant, opening of
the cooling and adsorption section may be effected depending
on expiration volume and/or flow rate when using computer-
controlled valves.
Hence, by sucking off the frozen and re-thawed sub-
stances, the collected respiratory condensate can be fed
directly, i.e., on-line, into the analytical unit 12, e.g. a
usual combination of chromatograph and mass spectrometer. In
the event of a cold trap 5 having a removable design, the
respiratory condensate is removed manually from the interior
wall of the cold trap by thawing and subjected to analysis
or stored in a deep-frozen state until measurement.
In a particularly preferred embodiment of the inven-
tion, the patient is subjected to a cold air provocation
between two examinations, which is well-known in the diagno-
sis of respiratory diseases, so that a differential measure-
ment for type and amount of expired chemical substances
prior to and subsequent to mucosa irritation can be
performed. To effect cold air provocation, a per se kiiown
air cooling device is arranged upstream of inlet valve 3,
e.g. the RHES cold air provocation instrument by the company
Jager, Wurzburg (Germany) . In the device according to the
invention, this air cooling device may also be used to cool
the inner tube of cold trap 5. Owing to this differential
measurement, a substantial improvement in the reliability of
the analytical results is achieved.
Depending on the diagnostic agent employed, the in-
ventive analysis of diagnostic agent still present in the
expired material or metabolites thereof is performed using
conventional analytical methods, preferably mass-spectromet-
CA 02312808 2006-07-17
28751-7
- 8 -
ric and chromatographic investigations.
In order to ensure good tolerability of the
diagnostic agent, formulations including endogenous or
related substances are preferably used, e.g. amino acids or
higher alcohols in high dilution. These compounds were
found to be suitable diagnostic agents for the present
method of investigation.
To ensure high specificity of diagnosis and
optimize the sensitivity of determination, these substances
are administered in a stable isotope labelled (i.e., non-
radioactive) form. Only in this way it is possible to
identify the expired diagnostic agent or a specific
conversion product thereof as part of the inspired
diagnostic agent.
Thus, the quantity of the stable isotope labelled
diagnostic agent (or a defined resultant product thereof)
recovered within a specific period of time is a reliable
measure for the actual absorptive and metabolic performance
of the epithelium.
The invention is also directed to a novel use of
pharmaceutically tolerable higher alcohols or amino acids in
a stable isotope labelled form in the diagnosis of
respiratory diseases and to a method for the chemical-
analytical determination of the metabolic performance of the
bronchial epithelium using the device according to the
invention. The test persons inhale diagnostic agents
capable of forming metabolites in the respiratory organs and
subsequently, the expired air is collected and the
metabolites and/or remaining amount of diagnostic agent are
determined after being recovered by freezing and adsorption.
CA 02312808 2006-07-17
28751-7
- 9 -
In a preferred variant, a test person inhales a
stable isotope labelled, pharmaceutically tolerable higher
alcohol as diagnostic agent. Following an exposure period
of about 1 to 3 hours, preferably 1 to 2 hours, the expired
air is collected and the expired diagnostic agent or
metabolites thereof are frozen out. Subsequently, the
amount and/or the isotope content of the substances are
determined and compared to the isotope basic value in the
respiratory air zero sample of the test person.
Preferred diagnostic agents are higher alcohols,
preferably C8 alcohols and more preferably having at
least 13 C atoms, e.g. hexadecanol-1, which preferably
is 13C-labelled.
In another preferred variant, stable isotope
labelled amino acids are used as diagnostic agents. The
expired air is collected over a time period of at
least 30 minutes from the first expiration event on and
analyzed specifically for gaseous metabolites of the
diagnostic agent or N-labelled nitrous gases using per se
known methods. A preferred diagnostic agent is
a 15N-labelled amino acid, and particularly preferred is the
amino acid L-arginine.
In another preferred variant, a cold air
provocation on the test person is carried out after
collecting the expired air, and the diagnostic agent is
administered once more as an inhalation. The determination
of the expired air is repeated, and these measured values
are correlated with the values measured prior to
provocation, thereby making it possible to determine the
sensitivity for the bronchial surface and establish the
severity of an inflammation.
CA 02312808 2006-07-17
28751-7
- 10 -
Example 1
liters of air in a sealed vessel is mixed with
defined quantities of between 1 and 100 mg of hexadecanol-1
labelled with the stable isotope 13C (and containing more
5 than 95 atom-% of 13C in the CH2OH group) . According to the
invention, [1-13C] -hexadecanol serves as diagnostic agent for
measuring the permeability of the bronchial epithelium.
Prior to applying the 13C-hexadecanol-1 formulation, a
respiratory air sample is collected from the individual to
be examined ("zero sample") in order to measure the actual
natural 13C basic value of expired carbon dioxide of said
person. The basic value is related to the so-called PDB
standard value, which is 1.1112328 atom-% 13C. From the
second digit behind the decimal point on, this value is
subject to deviation in each of the individuals, and this
deviation can be measured with high precision. To this end,
a mass spectrometer or a special respiratory gas 13C
measuring instrument is used in a per se known fashion
(e.g., the FANci2 instrument supplied by the company
Fischer Analysen Instrumente, Leipzig (Germany)).
Breathing ten times, the prepared air/hexadecanol
mixture then is inspired completely using the array of
Fig. 3 according to the invention. After a waiting period
of 1 and 2 hours, respectively, the expired air of 50
exhalations is collected each time, using the array of the
invention. Expired [1-13C]-hexadecanol and carbon dioxide
are separated by fractionated freezing. Amount and 13C
content of
CA 02312808 2006-07-17
28751-7
- 11 -
both substances are determined.
Now, the 13C value of carbon dioxide in the expired
air exhibits a characteristic deviation from the one of the
zero sample: The more [1-13C]-hexadecanol has permeated the
bronchial epithelium, the higher the increase of this value
will be -(e.g. from 1.11127 to 1.2467... atom-% 13C) ; because,
only after passing this barrier, the hexadecanol can enter
the blood circulation to be degraded to carbon dioxide in
the liver. Accordingly, the amount of 13C isotope derived
from [1-13C] -hexadecanol and recovered in the COz of the
expired air is a reliable measure for the permeability of
the bronchial epithelium.
Example 2
The apparatus is used as in Example 1. In addition,
however, a cold air provocation is performed after
collecting the expired air, wherein a per se known RHES air
cooling device by the company Jager, Wurzburg, is used which
may be arranged upstream of valve 3 in the array according
to the invention. 20 minutes after the cold air provocation,
the steps of admixing, inspiring, expiring, and measuring
illustrated in Example 1 are repeated in the same fashion
once again. The 13C carbon dioxide values measured after cold
air provocation are correlated with the values measured
prior to provocation. In this way, an intra-individually
standardized and thus, inter-individually comparable measure
for the sensitivity of the bronchial surface is obtained.
Example 3
Between 1 and 100 mg of a 15N-labelled amino acid,
preferably L- [guanino-15N2] arginine, dissolved in a
physiological sodium chloride solution, is mixed into the
storage air container. This commercially available 'SN-
labelled arginine contains e.g. 95 atom-% 15N in its guanino
CA 02312808 2006-07-17
28751-7
- 12 -
group. Breathing up to 20 times, the prepared air/aerosol
mixture is inspired completely using the inventive array of
Fig. 3, and, according to the invention, the expired air
from the first expiration event on is passed via valve 8
across the cold trap and adsorber section, namely, over a
total time of 60 minutes, with cold trap and adsorber being
exchanged after 30 minutes. After this time, the contents of
the cold traps and adsorber sections is subjected to GC-MS
analysis. The substances frozen out together with expired
moisture and the substances that underwent adsorption were
specifically examined for gaseous metabolites of
[15Nz] arginine, i.e., [15N] ammonia and 15N-labelled nitrous
gases. In those cases where virtually no labelled ammonia or
nitric oxide was found, the metabolic activity of the
bronchial epithelium was normal, i.e., relatively low. In a
condition of stress or inflammation, however, the metabolic
activity is massively increased. This is seen in a
comparatively large amount of 15N-labelled gaseous substances
in the expiration condensate, which can only be derived from
'5N-labelled arginine. Consequently, the amount of 15N isotope
recovered in the condensate is a reliable measure for the
metabolic activity and the inflammation level of the
bronchial epithelium. In addition, urine may also be
examined for metabolites containing 15N.
Example 4
The apparatus is used as in Example 3. After
completing the one-hour collection of expiration condensate
and 15N-labelled gases, cold air provocation in a per se
known manner is performed as in Example 2. In this way, it
is possible for the first time to perform a biochemical
measurement of the bronchial epithelium sensitivity to cold
air provocation - and indeed, a,non-invasive and in vivo one
in the form of a reduced or increased amino acid metabolism.
Also, the severity of an inflammation can be determined from
the ratio of the amount of 15N in the expired gases derived
CA 02312808 2006-07-17
28751-7
- 13 -
from inspired arginine prior to and subsequent to cold air
provocation.
CA 02312808 2006-07-17
28751-7
- 14 -
Reference list
Fig. 1:
1 Flow channel
2 Mouthpiece
3 Inlet valve for fresh air
4 Inhalation or injection unit
4a Supply pipe
4b Vessel for diagnostic agent
4c Valve
Cold trap
6 Adsorption vessel
7 Flow meter
8 Valve
Computer control
Fig. 2:
1 Flow channel
2 Mouthpiece
3 Inlet valve for fresh air
4 Inhalation or injection unit
4a Supply pipe
4b Vessel for diagnostic agent
4c Valve
4d Device for nebulizing diagnostic agent
5 Cold trap
6 Adsorption vessel
7 Flow meter
8 Valve
10 Computer control
11 Suction device
12 Analytical unit
CA 02312808 2006-07-17
28751-7
- 15 -
Fig. 3..
1 Flow channel
2 Mouthpiece
3 Inlet valve for air/diagnostic agent mixture
4 Reservoir bag including air/diagnostic agent
mixture
=5 Cold trap
6 Adsorption vessel
7 Flow meter
8 Valve
Computer control
11 Suction device
12 Analytical unit
Fig. 4:
1 Flow channel
5 Cold trap
6 Adsorption vessel
11 Suction device
12 Analytical unit
Fig. 5:
1 Flow channel
5 Cold trap
13 Internal tube
14 External body with cooling fins