Note: Descriptions are shown in the official language in which they were submitted.
CA 02313297 2007-10-03
LITHIUM SECONDARY BATTERY
The present invention relates to a lithium secondary battery using electrolyte
consisting of organic solvent containing lithium salt dissolved therein and
more
particularly to a lithium secondary battery using electrolyte the safety of
which is ensured
even if the battery is overcharged.
In recent years, a demand for a power source in the form of a small size and
light
weight battery has increased because of noticeable development of small size
and light
weight electronic appliances. Under such situations, a lithium secondary
battery such as a
lithium ion battery has been developed as a rechargeable small size and light
weight
battery of higher capacity for use in portable electronic appliances such as a
small size
video camera, a portable telephone, a note-book type personal computer and the
like.
In such lithium secondary batteries, a carbon material capable of absorbing
and
desorbing lithium ion is used as a negative electrode active material, a
lithium transition
metal oxide compound such as LiCoO2, LiNiO2, LiMn2O4 or the like is used as a
positive
electrode active material, and an organic solvent containing lithium salt
dissolved therein is
used as electrolyte. In the lithium secondary battery assembled with the
components,
lithium ions are desorbed from the positive electrode and absorbed into carbon
during an
initial charge of the battery so that the lithium secondary battery can be
used as a
rechargeable battery.
When the lithium secondary battery is overcharged, an excessive amount of
lithium
ions are extracted from the positive electrode and inserted into the negative
electrode in
accordance with an overcharged condition of the battery. This causes both the
positive and
negative electrodes to become thermally unstable, resulting in decomposition
of the
organic solvent of the electrolyte. As a result, the battery becomes over
heated by abrupt
exothermic reaction of the electrolyte, and the safety of the battery becomes
over heated by
abrupt exothermic reaction of the electrolyte, and the safety of the battery
deteriorates.
Particularly, such safety problems become very important when the energy
density of the
3
CA 02313297 2007-10-03
lithium secondary battery is increased.
To solve the safety problems, Japanese Patent Laid-open Publication No. 7-
302614
proposes a method for enhancing the safety of the battery by addition of a
small amount of
aromatic compound to the electrolyte. In the method proposed in the Japanese
Patent
Laid-open Publication No. 7-302614, a carbon material is used for the negative
electrode,
and an aromatic compound such as anisol derivative having a electron orbit at
an
reversible redox potential more noble than a positive electrode potential when
fully
charged in a molecular amount of less than 500 is added as an additive to the
electrolyte of
the battery. Such an aromatic compound is useful to prevent the battery from
overcharge
for protection of the battery.
Proposed also in Japanese Patent Laid-open Publication No. 9-106835
(corresponding with U.S. Patent No. 5,879,834) is a method for enhancing the
safety of the
battery by addition of an additive to the electrolyte of the battery. In the
method proposed
in the Japanese Patent Laid-open Publication No. 9-106835, a carbon material
is used for
the negative electrode, and a small amount of biphenyl, 3-R-thiophene, 3-
chlorothiophene,
furan at the like is used as the additive to the electrolyte of the battery so
that the internal
resistance of the battery is increased by polymerization of the components at
a higher
battery voltage than a maximum operating voltage of the battery for protection
of the
battery in an overcharged condition.
In the method proposed in the Japanese Patent Laid-Open Publication 7-302614,
the anisol derivative is effective to restrain overcharge of the battery but
deteriorates the
cycle characteristic and storage characteristic of the battery. The aromatic
compound is
decomposed by oxidation at an electric potential of about 4.5 V to generate
gas therefrom
and forms a polymerized substance for protection of the battery in an
overcharged
condition. However, in case the component of the electrolyte permits
dissolution of the
polymerized substance therein, the battery may not be protected in an
overcharged
condition. Consequently, the aromatic compound such as anisol derivative
having a
electron orbit does not effect to restrain overcharge of the battery.
4
CA 02313297 2000-06-30
In the method proposed in the Japanese Patent Laid-open Publication
No. 9-106835, the bipbenyl used as the additive to the electrolyte is lower ua
polarity and lower in solubili.ty. Accordingly, the additive is partly
deposited
during operation of the battery at a low temperature, resulting in
deterioration
of the battery performance. In addition, 3-chlorothiophene causes difficulty
in
its handling since it is stimulus and malodorous and is easily decomposed by
oxidationa Similarly, the furan is also easily decomposed by oxidation_ This
results in deterioration of the battery perfotmance.
To solve the problems discussed above, a primary object of the present
invention is directed to provide a lithium secondary battery in which an
additive effective for preventing overcharge of the battery is added to
electrolyte to ensure the safety of the battery without causing any
deterioration
of the cycle characteristic and storage characteristics at a low temperature.
According to the present invention, the object is accomplished by
providing a lithium secondary battczy which includes a cell casing provided
with a current interiupt device for cutting off a charge current of the
battery
when an internal gas pressure of the battery exceeds a predetermined value,
wherein the cell casing is filled with organic solvent containing an additive
such as alkylbenaene derivative or cycloalkylbenzene derivative having
tertiary
carbon adjacent a phenyl group. As the tertiary carbon adjacent the phenyl
group is active and higher in reaction, hydrogen atom is easily extracted from
the tertiary carbon in an overcharged condition of the batteiy. This causes
rapid decomposing reaction of the additive when the battery is overcharged.
As a result hydrogen gas generates and polymer of the additives is prodnced by
polymerization reaction of the additive. In the lithium secondary battery,
when the internal gas pressure of the battexy exceeds the predeterm.ined
value,
the current interrupt device is operated to cut off the charge current of the
battery. From the foregoing fact, it is presumed that addition of the additive
to
the organie solvent is effective to restrain decomposition of the electrolyte
thereby to ensure the safety of the battery- In this respect, it is noted that
the
polymor of the ddditiYGb aot5 a3 a re5i5tance SubStance in m ovcrchxged
CA 02313297 2000-06-30
condition of the battery and does not dissolve in the electrolyte. This is
useful
to effectively protect the battery against overcharge_
Since the additive added to the organic solvent is in a liquid state at
a room temperature, higher in porality and higher in dissolubility to the
electrolyte, the additive may not be deposited during operation of the battery
at
a low temperature and does not cause any deterioration of the battery
performance even if added to the electrolyte. Accordingly, in use of the
electrolyte added with the additive together with lithium salt dissolved in
the
organic solvent, the additive is useful to ensure the safety of the battery
without
causing any deterioration of the low tcmperature characteristic and storage
characteristics of the battery_
In a practical embodiment of the present i.nvention, it is desirable that
the alkylbenzene drivative is at least one of additives selected from the
group
consisting of isopropylbenzene (cumene), 1, 3-diisopropylbenzene, 1,
4-diisopropylbenzene, 1-znetlaylpropylbenzene, l, 3-bis(1-methylpropyl)
benzene and 1, 4-bis(1-methylpropyl) benzene. It is also desirable that the
cycloalkylbeuzene derivative is selected from either cyclohexylbenzene or
cyclopentylbenzerte.
For a better understanding of the present invention, and to show how
the same may be carried into effect, reference will now be made, by way of
example, to the accompanying drawings, in which:
Fig. 1 is a sectional view of a lithium secondary battery in accordance
with the present invention; and
Fig_ 2 is a partly broken sectional view of an electric current interrupt
device coupled with an opening of the cell casing shown in Fig. 1.
Hereinafter, an embodimezit of a Iithium secondary battery in
accordance with the present inventioz- will be described w7ith reference to
Figs.
1 and 2 of the drawings. Fig. 1 is a sectional view of the lithium secondary
battery in a coudifion wherc positive alld neg9tive eleetirode plates wound
6
CA 02313297 2000-06-30
tltrough a separator are contained in a cell casing, and Fig. 2 is a partly
broken
sectional view of an electric current interrupt device coupled with an opening
of the cell casing.
1_ Production of a negative electrode plate
A mixture of a negative electrode active material of natural graphite
(d = 3, 36 A) and a bonding agent of polyvinylidene fluoride (PVdF) was
dissolved in organic solvent of 1-methyl-2-pyrrolidone (NMP) to prepare a
slurry or paste. The slurry was uniformly coated on opposite entire surfaces
of a metal core in tire form of a copper foil of 20 m using a di.e-coater,
a doctor blade or the like to produce a negative electrode plate coated with
an
active material layer Similarly, the paste was uniformly coated on opposite
entire surfaces of the metal core by a roller coating method to produce a
negative electrode plate coated with an active material layer. The negative
electrode plate coated with the active material layer was passed througb a
dryer
to remove the organic solvent used for preparation of the slurry or paste.
Thereafter, the dried negative electrode plate was rolled under pressure by a
roller pressing machine to produce a negative electrode plate 10 of 0.14 mm in
thickness.
2. Production of a positive electrode plate
A mixture of a positive electrode active material of LiCoOz,
a carbon conductive agent of acetylene black, graphite and the like and a
bonding agent of polyvinylidene fluoride (PVdF) were dissolved in organic
solvent of 1-methyl-2-pyrrolidone (NMP) to prepare a slurry or paste. The
slurry was uniformly coated on opposite entire surfaces of a metal core in the
form of an aluminum foil of 20 m using a die-coater, a doctor blade or the
like
to produce a positive electrode plate coated with an active material layer.
Similarly, the paste was uniforml.y coated on opposite entire surfaces of the
metal core by a roller coating method to produce a positive electrode plate
coated with the active material layer_ The positive electrode plate coated
with
the active material layer was passed through the dryer to remove the organic
solvent vsed for preparabion of the glltrly oz paste. Thereafter> the d~ed
7
CA 02313297 2007-10-03
positive electrode plate was rolled under pressure by the roller pressing
machine to
produce a positive electrode plate 20 of 0.17 mm in thickness.
3. Production of an Electrode Assembly
The negative electrode plate 10 and positive electrode plate 20 were
overlapped
through a micro porous membrane 30 of inexpensive polyolefin resin, preferably
through a
micro porous membrane (for example, of 0.025 mm in thickness) of polyethylene
in such a
manner that the center lines of the plates 10 and 20 in a width direction are
coincided with
each other. Thus, the overlapped electrode plates 10 and 20 were wound up
spirally with
the micro porous membrane by a winding machine and taped at their outermost
peripheries
to produce a spiral electrode assembly.
4. Preparation of Electrolyte
(1) Example 1
A solvent mixture of 40 part by weight ethylene carbonate (EC) and 60 part by
weight diethyl carbonate (DEC) was added to and mixed with LiPF6 of 1
mole/liter as
electrolyte salt. The solvent mixture was further mixed with 2% by weight
isopropylbenzene (cumene) represented by the following structural formula to
prepare an
amount of electrolyte [a] as an Example 1.
\ l
(2) Example 2
A solvent mixture of 40 part by weight ethylene carbonate (EC) and 60 part by
weight diethyl carbonate (DEC) was added to and mixed with LiPFs of 1
mole/liter and
further mixed with 2% by weight 1, 3-diisopropylbenzene represented by the
following
structural formula to prepare an amount of electrolyte [b] as an Example 2.
8
CA 02313297 2000-06-30
(3) Example 3
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weigh,t diethyl carbonate (DEC) was added to and mixed with LiPF6
of 1 mole/liter and ituther mixed with 2 % by weight of
1, 4-diisopropylbenzene represented by the following structural formula to
prepare an amount of electrolyte [c] as aa Example 3.
(4) Example 4
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weight diethyl carbonate (DEC) was mixed with LiPFs of 1
mole/liter as electrolyte salt and further mixed with 2 % by weight
1-methylpropyl benzene represented by the following structural formula to
prepare an amount of electrolyte [d] as an Example 4.
I~
(5) Example 5
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter as electrolyte salt and further mixed with 2 % by weight 1, 3-bis
(1 methylpropyl) benzene represented by the following structural formula to
prepare an amount of electrolyte [e] as an Example 5.
(6) Example 6
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
4o part by we4t igelyl carbonato (DEC) was mixed with LiPFs of 1
9
CA 02313297 2000-06-30
mole/liter as electrolyte salt and fuather mixed with 2 % by weight
1, 4-bis(l-methylpropyl) benzene represented by the following structural
formula to prepare an arciount of electrolyte [f] as an Example 6.
(7) Example 7
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter as electrolyte salt and further mixed with 2 % by weight
cyclohe:rylbenzene represented by the following structural formula to prepare
an amount of electrolyte [g] as an Example 7.
.~i
(8) Example 8
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter as electrolyte salt and further mixed with 2 % by weight
cyclopenthylbenzene represented by the following structural formula to prepare
an amount of electrolyte [h] as an Example 8_
(9) Comparative example 1
A solvent mixture of 40 part by weight ethylene carbonate (EC) and
60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
molelliter as electrolyte salt to prepare an amount of electrolyte [x] as
a Comparative example 1 without addition of any additive_
CA 02313297 2007-10-03
(10) Comparative example 2
A solvent mixture of 40 part by weight ethylene carbonate (EC) and 60 part by
weight diethyl carbonate (DEC) was mixed with LiPF6 of 1 mole/liter as
electrolyte salt
and further mixed with 2% by weight of biphenyl (C12H10) to prepare an amount
of
electrolyte [y] as a Comparative example 2.
(11) Comparative example 3
A solvent mixture of 40 part by weight ethylene carbonate (EC) and 60 part by
weight diethyl carbonate (DEC) was mixed with LiPF6 of 1 mole/liter as
electrolyte salt
and further mixed with 2% by weight 4-chloroanisol to prepare an amount of
electrolyte
[z] as a Comparative example 3.
5. Manufacture of a Lithium Secondary Battery
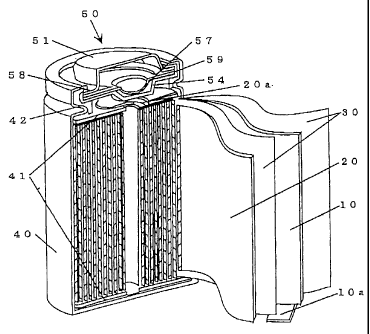
As shown in Fig. 1, a pair of insulation plates 41 were attached to opposite
ends of
the electrode body assembly and contained in a cylindrical cell casing 40
which was made
of a sheet metal and cylindrically formed by a pressing process. The cell
casing 40 was
used as a negative terminal of the battery. Subsequently, a current collector
l0a extended
from the negative electrode plate 10 of the battery was welded to an internal
bottom
portion of the cell casing 40, while a current collector 20a extended from the
positive
electrode plate 20 was welded to a bottom surface of a sealing cap with
current interrupt
device 50.
As shown in Fig. 2, the sealing cap with current interrupt device 50 is
composed of
a positive cap 51 of stainless steel formed in a reversed dish plate and a
bottom plate 54 of
stainless steel formed in a dish plate. The positive cap 51 has a convex
portion 52
protruded upward and an annular flat flange portion 53 forming a bottom part
of the
convex portion 52. The convex portion 52 is forrned at its corner with a
plurality of
circumferentially equally spaced vent holes 52a. The bottom plate 54 has a
concave
portion 55 protruded downward and an annular flat flange portion 56. The
concave portion
55 of bottom plate 54 is formed at its corner with a plurality of
11
CA 02313297 2000-06-30
circumferentially equally spaced vent holes 55a.
Disposed in a space between the positive electrode plate 51 and bottom
plate 54 is an el ectric conduction plate 57 which is deformed when an
internal
pressure of the battery exceeds a predetermined value. The electric
conduction plate 57 was made of aluminum foil of 0.2 mm in thickness formed
with uneven surfaces, which electric conduction plate 57 has a concave portion
57a and an annular flat flange portion 57b. The concave portion 57a of
electric conduction plate 57 is placed in contact with the upper surface of
concave portion 55 of bottom plate 54, and the flange portion 57b of electric
conduction plate 57 is disposed between the flange portion 53 of positive
electrode cap 51 and the fl.ange portion 56 of bottom plate 53 and fixed in
place
in a liquid-tight manner through an insulation gasket 59 of polypropylene
(PP).
Provided on the :flange portion 57b of electric conduction plate 57 is
a PTC (Positive Temperature Coefficient) thermistor element 58 the resistance
value of which is increased in the occurrence of abnonmal heat generation
caused by flow of an electric cuuent in the battery_ When an internal gas
pressure of the battery exceeds the predetermined value, the concave portion
57a of electric conduction plate 57 is deformed to disconnect the electric
conduction plate 57 from the concave portion 55 of bottom plate 57 thereby to
cut off flow of an electric current or short-circuit current in the battery.
The cell casing 40 was supplied with each electrolyte [a] -[h] aud [x],
[y], [z] and closed in a liquid-tigltt manner by means of the current i-
nterrupt
device 50 fixedly coupled therewith through an insulation gasket 42 of
polypropylene. Thus, eleven kinds of cylindrical lithium secondary batteries
A - H and )~ Y, Z were manufactured._ Each nominal capacity of the lithium
secondary batteries was 1350 mAh. The lithium secondary batteries A - H
were supplied with eacb electrolytes [a] -[h], and the batteries X, Y, Z were
supplied with each electrolyte [x], [y], [z].
12
CA 02313297 2000-06-30
6. Test
(1) Overcharge test
The eleven kinds of lithium secondary batteries A - H and X, Y, Z each
were charged by a charge current of 1350 mA (1C) until the battery voltage
becomes 4_ X V Thereafter, the secondary batteries each were fu11y charged at
a constant voltage of 4.1 V for three hours and overcharged by a charge
current
of 2700 mA (2C) to measure each cut-off time of electric current in the
batteries and each maximum temperature of the batteries_ A sesult of the
measurement is listed in the followi.ng table 1_
(2) Low temperature characteristic
The lithium secondary batteries A - H and 7, Y, Z were charged by a
charge current of 1350 mA (iC) at a room temperature (25 'C) until the battery
voltage becomes 4.1 V. Subsequently, the secondary batteries were charged at
the constant voltage of 4.1 V for three hours and rested for three hours at
the
room tempera.ture_ Thereafter, the secondary batteries were discharged by a
discbarge current of 1350 mA (1C) at 0 C until the 6.nal voltage becomes 2.75
V to measure each discharge capacity (mAh) of the batteries at the room -
temperature.
On the other hand, the lithium secondary batteries A - H and X, Y, Z
each were charged by a charge current of 1350 mA (1C) until the battery
voltage becomes 4.1 V Subsequently, the secondary batteries were fully
charged at the constant voltage of 4_ 1 V for three hours and rested at 0 C
for
three hours. Thereafter, the secondary batteries were discharged by
a discharge current of 1350 mA (1 C) at 0 C until the final voltage becomes
2.75 V to measure each discharge capacity (xpAh) of the batteries at a low
temperature.
Based on each discharge capacity of the batteries described above,
a ratio of the discharge capacity (mAh) at the low temperature to the
discharge
capacity at the room temperature was calculated by the followi.ng formula (1)
as a low te~~llperatare oharacteri5tic of tbe respecfive secondary batterieg.
13
CA 02313297 2000-06-30
Low temperature characteristic
(Discharge capacity at low temperature / Discharge capacity at
room temperature) x 100 % .. (1)
A result of the calculation is listed in the following table 1.
(3) Storage characteristics
The lithium secondary batteries A - H and X, Y, Z each were charged
by a charge current of 1350 mA (1C) at a room temperature (25 'C) until the
battery voltage becomes 4.1 V. Subsequently, the secondary batteries were
fully charged at the constant voltage of 4.1 V for three hours and stored in
an atmosphere of 60 C for twenty days. Thereafter, the secondary batteries
were discharged by the discharge current of 1350 mA (1C) until the final
battery voltage becomes 2_75 V to measure each discharge capacity of the
batteries after storage at a high temperature. In addition, a ratio of the
discharge capacity after storage at the high temperature to the discbarge
capacity at the room temperature was calculated by the following formula (2)
as a storage characteristic of the respective batteries.
Storage characteristics
(Discharge capacity after storage at high temperature /
Ihiscbarge capacity at room temperature) x 100 %..... (2)
A result of the calculation is shown in the foUowing table 1.
14
CA 02313297 2000-06-30
Table i
Kind of Electrolytc (Added Cnrrent M=znuaa Lorv Storage
Baftery amountoCdissolved cut-off Temp. tenmperature eharaetcristic
substance) wt.% timc ( C) characteristic (%)
Kind of Additive (Ivlin.) (%)
A N 15 74 83 91
LiPF6(EC_DEC--F:6)2%
Cumene
B TM 17 81 83 92
LiPFo(EC:DEC=4:6)2%
1,3-diiso ro ylbenzene
C im 17 83 84 89
L,iPF6(EC:DEC=4:6)2%
1,4-diiso r ylbenzene
D 1N1 17 78 83 90
LiPF6(EC:DEC=4:6)2%
1-meth ! ro lbenzene
E IM ls 80 81 89
LiPFo(EC:DEC=4:6)2%
] , 3 -b i s (1-methylpropyl)
benzeue
F 1m 19 82 83 91
LiPFfi(EC:DEC=4:6)2%
1,4-bis(1-methylpropyl)
benzene
G ~ 15 72 85 92
Lil'Fs(EC:DEC=4:6)2%
olohe. lbcnzene
H 17 79 84 90
Li.PF6(EC:DEC=4:6)2"/o
cyclopcntylbe%ene
X 1M 32 Burst 85 93
LiPF6(EC:DEC~-4:6)2%
without any additive
y IM 20 88 60 85
LiPF4(EC:DE .C1F:6)2%
bi henyl
z ~ 21 90 77 65
LiPFb(EC:DEC=4:6)2%
4-chlorotiaisol
As is understood from the table 1, the battery X using the electrolyte
[x] of the Comparative example 1 prepared without addition of any additive
burst upon lapse of thirty two minutes after overcharged, but the low
temperature characteristics and storage characteristics of the battery X were
excellent. In the battery Y using the electrolyte [y] of the Comparative
example 2 prepared with addition of biphenyl, a charge current was cut off
WoIl lap5f of tWgIltiy minuteb aft r tho battery waS overoharged. At the time,
CA 02313297 2000-06-30
the maximum temperature of the battery Y was 88 C, and the low tempcrature
characteristics and storage characteristics of the battery Y were
deteriorated_
In the battery Z using the electrolyte [z] of the Comparative example 3
prepared with addition of 4- chloroanisol, a charge current w-as cut off upon
lapse of twenty one minutes after the battery was overcharged. At the time,
the maximum temperature of the battery Z was 90 C, and the low temperature
characteristics and storage characteristics of the battery Z were
deteriorated.
In the batteries A - H respectively using the electrolytes [a] -[h] of the
Examples 1- 8, a charge current was cnt off upon lapse of fifteen to nineteen
minutes after the batteries were overcharged respectively. At the time, the
maximum temperature of the respective batteries was 72 - 83 C, and the low
temperature chaxacteristic and storage characteristics of the respective
batteries
were excellent.
It is presumed that the above result was obtained for the following
reason. When the batteries A- H are overcharged after charged at 4.1 V,
additives such as isopropylbenzene (cunaene), 1, 3-diisopropylbenzene, 1,
4-diisopropylbenzene, 1-methylpropylbenzene, 1,3-bis(1-methylpropyl)
benzene, 1,4-bis(1-methylpropyl)benzene, cyclohexylbenzene and
cyclopentylbenzene are decomposed to generate gas therefrom and
polymerized to generate polymerization heat. When the batteries are ftztther
overcharged in such a condition described above, the amount of gas increases,
and the current interrupt device 50 is operated after lapse of fifteen to
nineteen
minutes to cut off an overcharge current so that the temperature of the
respective batteries gradually lowers_
In. comparison of the batteries A - H, it has been fouud that addition of
the additives does not cause any significant difference in the characteristics
of
the batteries. From these facts, it is desirable that at least one of
additives
selected from the group consisting of isopropylbenzene (cumene), 1,
3-diisopropylbenzene, 1, 4-diisopropylbenzene, 1-methylpropylbenzene, 1,
3=bis(lmmethylpropyl) bemIle, 1, 4-bis(1-methyipropyl) ben2ene,
16
CA 02313297 2000-06-30
cyclohexylbenzene and cyclopentylbenzene is added to the electrolyte.
Particularly, cyclohexylbenzene is useful to enhance the lo* temperature
characteristics and storage characteristics of the litMoan secondary battery
7. Discussion of the sort of electrolyte
The influence caused by the sort of electrolyte was discussed as
described below.
(1) Example 9
A solvent mixture of 40 part by weight ethylene carbonate (F;C)
and 60 part by weight dimethyl carbonate (DMC) was mixed with LiPF6 of 1
mole/liter and further mixed with 2 % by weight cyclohexylbenzene to prepare
an amount of electrolyte [i] as an Example 9.
(2) Example 10
A solvent mixture of 40 part by weight ethylene carbonate (EC) and 60
part by weight methyl ethyl carbonate (MEC) was mixed with LiPF6 of 1
mole/iiter and furtlaer mixed with 2 % by weight cyclohexylbenzene to prepare
an amount of electrolyte [j] as an Example 10.
(3) Example 11
A solvent mixture of 40 part by weight ethylene carbonate (EC ), 30
part by weight dimethyl carbonate (DEC) and 30 part by weight dimetlhyl
carbonate (DMC) was mixed with LiPF6 of 1 mole/liter and furtber mixed with
2 % by weight cyclohexylbenzene to prepare an amount of electrolyte [k] as an
Example 11.
(4) Example 12
40 part by weight ethylene carbonate (EC) and 60 part by weight
diethyl carbonate (DEC) was mixed with LiPFG of 0.5 mole/liter and LiBF4 of
0.5 mole/liter as electrolytic salt and further mixed with 2 % by weight
cyclohexylbenzene to prepare an amount electrolyte [1] as an Example 12_
17
CA 02313297 2007-10-03
The electrolytes [i] - [1] each were stored in the cell casing 40, and the
current interrupt
device 50 was coupled with the opening of cell casing 40 and caulked to close
the cell casing in a
liquid-tight manner. Thus, lithium secondary batteries I - L using the
electrolytes [i] - [1] were
manufactured. The lithium secondary battery I was filled with the electrolyte
[i], the lithium
secondary battery J was filled with the electrolyte [j], the lithium secondary
battery K was filled
with the electrolyte [k], and the lithium secondary battery L was filled with
the electrolyte [1].
The lithium secondary batteries I - L were overcharged in the same manner as
described
above to measure a lapse of time during which the current interrupt device 50
is operated after the
battery was overcharged and to measure each maximum temperature of the
batteries. A result of
the measurement is listed in the following table 2. Similarly, the temperature
characteristics and
storage characteristics of the respective batteries were measured. A result of
the measurement is
listed in the following table 2.
Table 2
Kind of Electrolyte (Added amount of Current Maximum Low Storage
Battery dissolved substance) wt.% Low cut- Temp. Temp. characteristic
Kind of Additive off time ( C) characteristic (%)
(Min.) (%)
I IM 15 73 84 92
LiPF6(EC:DMC= 4:6)2%
cyclohexylbenzene
J IM 16 74 85 91
LiPF6 (EC:MEC=4:6)2%
cyclohexylbenzene
K IM LiPF6 16 76 84 91
(EC:DEC:DMC=4:3:3)2%
cyclohexylbenzene
L 0.5MLiPF6 + 0.5MLiBF4 16 75 82 87
(EC:DEC=4:6)2%
cyclohexylbenzene
As is understood from the table 2, it has been found that substitution of the
sort of
organic solvent or dissolved substance in the electrolyte does not cause any
significant difference in the current cut-off time, maximum temperature, low
temperature
characteristics and storage characteristics of the batteries. It
18
CA 02313297 2000-06-30
is, therefore, noted that the additive comprised of cyclobexylbenzene is
effective irrespectively the sort of electrolyte_ Although the effect of an
additive other than cyclohexylbenzene is not shown in the table 2, the same
result as in use of cyclohexylbenzene was obtained in use of an additive
selected from the group consisting of isopropylbenzene (cumene), 1,
3-diisopropylbenzene, 1, 4-diisopropylbenzene, 1-methylpropylbenzene, 1,
3-bis(1 methylpropyl) benzene, 1, 4-bis(1-methylpropyl) benzene,
cyclohexylbenzene and cyclopentylbenzene,
8.. Discussion of an addition amount of additives
An influence caused by an addition amount of additives was discussed
as described below.
(1) Example 13
A solvent mixture of 40 part by weight ethylene carbonate (EC)
and 60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter and further mixed with 1% by weight cyclohexylbenzene added as
an additive to prepare an amount of electrolyte [m] as an Example 13.
(2) Example 14
A solvent mixture of 40 part by weight ethylene carbonate (EC)
and 60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter and further mixed with 3 % by weight cyclohexylbenzene added as
an additive to prepare an amount of electrolyte [n] as an Example 14.
(3) Example 15
A solvent mixttue of 40 part by weight ethylene carbonate (EC)
and 60 part by weight diethyl carbonate (DEC) was raixed LiPf6 of 1 mole/liter
added thereto and fi.uther mixed with 5 % by weight cyclohexylbenzene added
thereto as an additive to prepare an amouut of electrolyte [o] as an Example
15.
19
CA 02313297 2000-06-30
(4) Example 16
A solvent mixture of 40 part by weight ethylene carbonate (EC)
and 60 part by weight diethyl carbonate (DEC) was mixed with LiPF6 of 1
mole/liter and further mixed with 10 % by weight cyclohexylebenzene added
thereto as an additive to prepare an amount electrolyte [p] as an Example 16.
The electrolytes (m] -[p] each were stored in the cell casing 40,
and the current interrupt device 50 was coupled the opening of cell casing 40
and caulked to close the cell casiag 40 in a liquid-tight mannet Thus,
lithiuum
secondary batteries M-- P using the electrolyte [m] -[p] were manafactared.
The lithiam second battery M was -511ed with the electrolyte [m], the lxtbuum
secondary battery N was filled witb the electrolyte [n], the lithium secondary
battery 0 was filled with the electrolyte [o], and the lithium secondary
battery P
was filled with the electrolyte [p].
The lithium secondary batteries M - P were overcharged in the
same rnanner as described above to measure a lapse of time dnring which the
current i.ntemipt device 50 is operated after the battery was overcharged and
to
measure each maximum tempeiature of the batteries. A result of the
measurement is listed in the following table 3. Similarly, the temperature
cbaracteristics and storage characteristics of the respective batteries were
measured. A result of the measurement is listed in the following table 3. In
the table 3, a measurement result of the battery added with 2 % by weight
cyclohexylbenzene is also listed_
CA 02313297 2000-06-30
Table 3
Kin.d of Electrolyte (Added amount Current Maximum Low Storage
Battery of dissolved substance) cut-off Temp. temperature charaeteristic
wt_% time ( C) characteristic (%)
Kind of Additivc (Min (%)
M 1M 16 75 85 92
LiPF6(EC:DEC=4:6) J %
c clohe Lbenzene
G JM 15 72 85 92
LiPF6(ECD1rC=4:6)2%
c clohex lbenzene
N rM 15 72 85 92
LiPF6(EC:DEC=4:6)3%
cloh lbenzene
0 1M 15 72 84 91
LiE'F6(EC: DEC=4: 6)5 %
c cloh benzene
p IM 15 71 80 88
LiPF6(EC:DEC-4:6)1Q%
c clohe. ibenzene
As is understood from the table 3, it has beev found that addition of
the additives in an extent of 1 to 10 % by weight does not cause any
significant
difference in the current cut-off time, maxi.mum temperature, low temperature
cbaracteristics and storage characteristics of the batteries. It is,
therefore,
desirable that the addition amoimt of the additive is deteznaa.ned in an
extent of
1 to 10 % by weight, preferably ixa an extent of I to S% by weight. Although
the effect of an additive other than cyclohexylbenzene is not shown in the
table
3, the same result as in use of cyclohexylbenzene was obtained in use of an
additive selected from the group consisting of isopropylbenzene (cumene), 1,
3-diisopropylbenzene, 1, 4-diisopropylbenzene, 1-naethylpropylbenzene, 1,
3-bis(1-methylpropyl) benzene, 1, 4-bis(1-met'hylpropyi) benzene,
cyclohexylbenzene, and cyclopentylbenzene.
From the above fact, it bas been conSrmed that addition of an
additive comprised of alkylbenzene derivative having tertiaiy carbon adjacent
a pbenyl group such as isopropylbenzene (cumene), 1, 3-diisopropylbenzene, 1,
4-diisopropylbenzene, 1-methylpropylbenzene, 1, 3-bis(1-methylpropyl)
benzene, and 1, 4-bis(1-methylpropyl) benzene or cycloalkylbenzene derivative
21
CA 02313297 2007-10-03
having tertiary carbon adjacent a phenyl group such as cyclohexylbenzene or
cyclopentylbenzene is useful to ensure the safety of the battery against
overcharge without
causing any deterioration in cycle characteristics and storage characteristics
of the battery
at a low temperature.
0
Although in the foregoing embodiments, natural graphite (d = 3.36 A) was used
as
the negative electrode active material, it is desirable that carbon material
capable of
absorbing and desorbing lithium ion such as carbon black, coke, glassy carbon,
carbon
fiber or sintered material thereof are used as the negative electrode active
material.
Although in the foregoing embodiments, LiCoO2 was used as the positive
electrode
active material, it is desirable that lithium containing transition metal
oxide compound
capable of receiving lithium ion as a guest such as LiNiO2i LiCoXNi(i_X)02,
LiCrO2, LiVO2,
LiMnO2, a LiFeO2, LiTiO2, LiScO2, LiYO2, LiMn2O4 and the like is used as the
positive
electrode active material. Particularly, it is preferable to use either LiNiO2
or
LiCoxNi(1_X)02 or a mixture of LiNiO2 and LiCoxNi(1_X)02 as the positive
electrode active
material. The term "x" in the compound LiCo,,Ni(1_X)O2 is "0<x<1".
As the electrolyte, inexpensive ion conductive substance containing lithium
salt
dissolved in organic solvent, higher in ion conduction rate, chemically and
electrochemically stable to the positive and negative electrodes, usable in a
wide
temperature range and higher in safety can be used in the battery. For
example, at least one
of solvents selected from the group consisting of ethylene carbonate (EC),
diethyl
carbonate (DEC), dimethyl carbonate (DMC), methyl ethyl carbonate (MEC),
propylene
carbonate (PC), sulfolane (SL), tetrahydrofuran (THF) and v -butyrolactone
(GBL) is used
as the organic solvent, and at least one of Lithium salts selected from the
group consisting
of LiPF6, LiBF4, LiC1O4, LiAsF6, LiCF3SO3, Li(CF3SO2)2N, Li(C2F5SO2)2N and
LiC4F9SO3, is used as the lithium salt.
22