Note: Descriptions are shown in the official language in which they were submitted.
CA 02314123 2005-06-02
-1-
METHOD OF TREATING SPENT POTLINER
MATERIAL FROM ALUMINUM REDUCTION CELLS
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates generally to processes for treating
spent potliner material from aluminum reduction cells in a manner in which
hazardous wastes are converted and recycled to useful, non-hazardous
substances. More specifically, the present invention relates to a process of
recovering, from spent aluminum potliner material, aluminum fluoride, reusable
1 o salts such as sodium sulfate and refractory material such as calcium
feldspar
which can be used to make brick products, for example. Further, large amounts
of energy can be recovered from the carbon, e.g., 8000 to 9000 BTU/Ib of
carbon.
2. Description of the Prior Art
15 The Hall-Heroult process for the production of metallic aluminum
dates from the 19t" Century. Many refinements to the process have been made,
but the basic Soderberg or pre-bake configurations using Hall-Heroult cells
remain the most common processes for aluminum production throughout the
world. In these processes, the bottom and internal walls of a cathode of an
2 o aluminum pot are formed with a liner of carbon blocks joined by conductive
carbonaceous binder and wrapped with refractory firebricks and insulating
bricks, the resulting combination being referred to as "potliner". The
insulating
bricks and firebricks are composed of material such as silica and alumina
(aluminum oxide).
2 5 During the production of aluminum, the aluminum reduction pot is
filled with a bath of alumina and molten salts. Over the three to seven year
life
span of an aluminum reduction pot bath, salts migrate into the potliner,
thereby
resulting in the deterioration and eventual failure of the utility of the
aluminum
cell as a cathode. During its life span, a cathodic potliner may absorb its
own
3 o weight in bath salt materials. The failed potliner material is referred to
as spent
potliner or SPL.
When an aluminum reduction cell is taken out of service, the SPL
is cooled and fractured to facilitate subsequent handling and disposal. The
CA 02314123 2005-06-02
-2-
fractured SPL is a non-homogenous material which contains carbon, silica
and/or alumina from the insulating brick and firebricks, aluminum, significant
quantities of sodium salts, aluminum salts and oxides, fluoride salts and
traces
of cyanides. On the average, a large aluminum smelter with a production
capacity of 175,000 tons of aluminum per year will produce about 6,000-12,000
tons of SPL per year. The quantity of SPL generated annually in the United
States alone has in recent years exceeded approximately 230,000 tons per year.
Because of its cyanide content, its high concentration of teachable
fluoride compounds, and the high volumes of SPL produced, SPL presents a
1 o significant environmental hazard and a major burden for aluminum
producers,
who remain ultimately liable for the proper disposal of SPL. The SPL has long
been listed as a hazardous waste by the U.S. Federal and State environmental
authorities. Current regulations require that SPL ultimately be treated to
explicitly remove the toxic cyanide, high concentration of teachable fluoride
s 5 compounds, and other characteristics which cause it to be listed as
hazardous
before it can be placed in a landfill disposal site.
Many different approaches have been tried over the years to
convert SPL to non-hazardous materials. One major technique includes
combustion or incineration of the SPL as exemplified in U.S. Patents
4,735,784;
20 4,927,459; 5,024,822; 5,164,174; 5,222,448 and 5,286,274. Unfortunately,
most
of these processes result in an end product consisting of a glassy slag
material
which still contains some hazardous, allegedly non-teachable, materials.
Another process includes chemical treatment to convert SPL to
non-hazardous materials. In these types of processes, as exemplified by
25 U.S. Patent 4,113,831, the initial SPL constituents are replaced with
compounds
which are less toxic, but which compounds are still above the hazardous
listing
levels established by various environmental authorities. Moreover, these
residues generally have a final volume which is comparable to the volume of
the
input.
3 o Another major technique of converting SPL to non-hazardous
materials includes pyrohydrolysis of the SPL material. This process generally
includes pyrolysis of the material in conjunction with the introduction of
water to
create an off-gas containing the fluoride materials as illustrated in U.S.
Patent
CA 02314123 2005-06-02
-3-
4,113,832. Such pyrohydrolysis techniques may also be used in conjunction
with fluidized bed reactors as disclosed in U.S. Patents 4,158,701 and
4,160,808. These processes also still tend to produce large volumes of waste
material which must be stored in landfills and which may contain allegedly non-
teachable hazardous waste. Thus, there is still a need for a process to
chemically treat SPL material from aluminum reduction cells, wherein the end
products of such a treatment process are all usable either within the process
itself or with other commercial processes as well as secondary end products
which are non-toxic to the environment and which do not include large volumes
of material for the landfill or for storage.
SUMMARY OF THE INVENTION
It is, accordingly, one object of the present invention to provide a
process for treating spent potliner material from aluminum reduction cells.
It is another object of the present invention to provide such a
process wherein aluminum fluoride, sodium compounds such as sodium sulfate,
calcium compounds and iron compounds and refractory materials such as
mullite which can be converted to brick or used as fuel or cement additive,
are all
recovered from the spent aluminum potliner material in a form which is
commercially usable.
2 o Still, it is another object of the present invention to provide a
process for treating SPL to selectively recover usable compounds such as
aluminum fluoride, sodium sulfate, chloride salts, mullite and other useful
materials therefrom.
Yet another object of the present invention is to provide a process
2 5 for the treating of spent potliner material from aluminum reduction cells
which
includes a total recycle of all by-products and elimination of all hazardous
wastes.
To achieve the foregoing and other objects and in accordance with
the purpose of the present invention, as embodied and broadly described
herein,
3 o a process of treating spent potliner material from aluminum reduction
cells and
recovering useful products is disclosed. In the process of the present
invention,
spent potliner material is introduced into an acid digester containing, for
example, sulfuric acid. As a result of this step, a gas component is produced
CA 02314123 2005-06-02
-4-
which includes hydrogen fluoride and hydrogen cyanide. Also, a slurry
component is produced which includes carbon, silica, alumina, sodium
compounds such as sodium sulfate, aluminum compounds such as aluminum
sulfate, iron compounds such as iron sulfate, magnesium and calcium
compounds such as magnesium and calcium sulfate. The slurry component
remains in the digester after the gas component is removed. The gas
component is recovered and heated an effective amount to convert or
decompose the hydrogen cyanide to a remaining gas component including C02,
H20, and nitrogen oxides, as well as HF gas. The remaining gas component is
s o directed through a water scrubber in which the HF gas is converted to
liquid
hydrofluoric acid. The hydrofluoric acid is then admixed with alumina
trihydrate
to form aluminum fluoride (a commercially useful end product) and water.
The slurry component is rinsed with water to separate a solid
fraction containing carbon, and refractory materials such as alumina and
silica
from a liquid fraction. The solid fraction may be admixed with an
alumina/silica
mixture and then used as fuel in cement or glass manufacturing. Alternatively,
the solid fraction can then be subjected to an elevated temperature in an
oxygen-rich atmosphere. This causes the carbon to oxidize to carbon dioxide
which itself has utility as a fuel, leaving a refractory material such as
mullite
2 o formed from silica and alumina which has commercial utility in forming
brick.
In one aspect of the invention, the remaining liquid portion of the
slurry is mixed with alcohol at a preferred ratio of about four parts alcohol
to
about one part liquid. This step removes in excess of 97% of the salts and
leaves a solution of sulfuric acid and alcohol. This solution is then
subjected to
2 5 distillation, with the volatile alcohol being recovered for reuse, and the
remaining
sulfuric acid available to be added back to the system digester to reduce acid
consumption. The filtered salts are then dissolved back in H20 and the pH
adjusted to a basic pH, e.g., about 12.0 to 12.5, with NaOH. This step holds
aluminum in solution as sodium aluminate and precipitates all other
impurities.
3 o The solution is filtered to remove the impurities containing calcium,
iron,
magnesium and silicates primarily. The clear solution is then further pH
adjusted
to an alkaline pH, e.g., about 7.0 to 8.0 pH, to remove AI(OH)3, and the
remaining solution is then admixed with alcohol to form and precipitate sodium
CA 02314123 2005-06-02
-5-
sulfate.
In another aspect of the invention, the remaining liquid portion of
the slurry may be treated to form soluble sodium aluminate by adjusting the
pH,
for example, of the liquid portion. Adjusting the pH causes insoluble salts
such
as calcium, iron and magnesium salts to form a precipitate which is removed
leaving a solution containing soluble sodium aluminate. The insoluble salts
are
then filtered and reused. The insoluble salts are further processed using acid
and heat to form a high purity calcium compound. Also, iron compounds are
precipitated and recovered from the remaining liquid portion. In addition,
s o magnesium salts are also precipitated and recovered from the remaining
liquid
portion. The solution remaining after calcium, iron and magnesium salts are
removed is added to the solution containing soluble sodium aluminate. The pH
of this solution is adjusted to form alumina trihydrate which can be removed
from
the solution. The solution remaining may be treated to remove residual AI(OH3)
15 before being added back to the digestion step.
According to an aspect of the present invention there is provided a
method of treating spent potliner material from aluminum reduction cells,
which
spent potliner material includes at least one material from the group
consisting of
fluoride compositions, cyanide compositions, iron compositions, calcium
2 o compositions, magnesium compositions, alumina, carbon, silica and sodium
sulfate, the method comprising the steps of (a) introducing the spent potliner
material to an acid digester to produce a first gas component which includes
at
feast one material selected from the group consisting of hydrogen fluoride gas
and hydrogen cyanide gas, and a slurry component including at least one
25 material from the group consisting of carbon, silica, alumina, sodium
sulfate, iron
sulfate, calcium sulfate and magnesium sulfate, (b) recovering the gas
component from the digester and heating it to a temperature sufficient to
decompose hydrogen cyanide which has been produced, and thereby produce a
second gas component which is substantially free of cyanide and includes at
3 0 least one offgas from the group consisting of C02, H20, nitrogen and the
HF
gas, (c) combining the second gas component with water in a water scrubber to
form hydrofluoric acid from HF included in the second gas component, (d)
admixing the hydrofluoric acid with alumina trihydrate to form aluminum
fluoride,
CA 02314123 2005-06-02
-6-
(e) rinsing the slurry component with water and separating a first solid
fraction
including at least one of the group consisting of carbon, alumina and silica
from
a first liquid fraction, (f) adjusting the first liquid fraction to a first
basic pH
effective in forming an aluminate in solution while precipitating impurities
containing elements selected from the group consisting of Ca, Fe and Mg and
then filtering the impurities from liquid in the first liquid fraction to
provide a
filtered liquid fraction, (g) adjusting the filtered liquid fraction to a
second pH
effective in forming and precipitating alumina trihydrate from the filtered
liquid,
(h) admixing the first solid fraction with an alumina/silica mix to provide a
1 o mixture, and (i) subjecting the mixture to a temperature in the range of
1600°C to
2000°C in an oxygen-rich atmosphere to oxidize the carbon and vitrify
the
alumina and silica into refractory material.
According to another aspect of the present invention there is
provided a method of recovering alumina, carbon and silica from spent potliner
material from aluminum reduction cells, which spent potliner material includes
material from the group consisting of fluoride compositions, cyanide
compositions, iron compositions, calcium compositions, magnesium
compositions, alumina, carbon, silica and sodium sulfate, the method
comprising
the steps of (a) contacting the spent potliner with acid in an acid digester
to
2 o produce a gas component containing at least one gas selected from the
group
consisting of HF and HCN and a slurry component comprised of a solids and a
liquid solution, the solid comprised of carbon, silica, alumina and calcium
sulfate,
(b) maintaining the digester at less than atmospheric pressure to remove the
gases from the digester, (c) separating the liquid solution from the solids,
(d)
2 5 rinsing the solids at least once to remove residual acid from the solids,
(e)
reacting the calcium sulfate with ammonium chloride to form ammonium sulfate
and calcium chloride, and (f) separating the ammonium sulfate and calcium
. chloride to recover a mix of solids comprised of carbon, silica and alumina.
These and other objects of the present invention will become
3 o apparent to those skilled in the art from the following detailed
description,
showing the contemplated novel construction, combination, and elements as
herein described, and more particularly defined by the appended claims, it
being
understood that changes in the precise embodiments to the herein disclosed
CA 02314123 2005-06-02
-7-
invention are meant to be included as coming within the scope of the claims,
except insofar as they may be precluded by the prior art.
Brief Description of the Drawings
The accompanying drawings which are incorporated in and form a
part of the specification illustrate complete preferred embodiments of the
present
invention according to the best modes presently devised for the practical
application of the principles thereof, and in which:
Figure 1 is a flow diagram illustrating the various process steps and
by-products of the present invention.
1 o Figure 2 is a flow diagram illustrating steps in recovering AIF3 from
spent potliner.
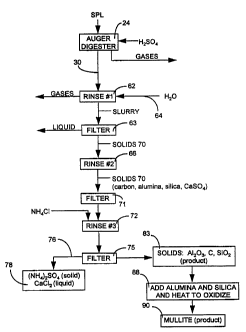
Figure 3 is a flow diagram illustrating steps in recovering mullite,
(NH4)2S04 and CaCl2 from spent potliner.
Figure 4 is a flow diagram illustrating steps in recovering AI(OH)3,
Na2S04 and metal chlorides from spent potliner.
Figure 5 is a flow diagram showing an alternate method of treating
calcium, iron and magnesium compounds in spent potliner.
Detailed Description of the Preferred Embodiments
The process of the present invention for the treatment of spent
2o potliner (SPL) waste materials is shown diagrammatically by Figure 1, which
process is generally identified by the reference numeral 10. The input
material
12 consists of SPL as its major ingredient, but may also include any other
waste
stream with similar chemical make-up. One preferred operation is described
below, although it will be apparent to one skilled in the art that many of the
steps
2 5 are optional.
In preferred operations, input material 12 is pulverized by a crusher
14 to a particulate feed size of 16 mesh or less, although larger particles
may be
used. One preferred form of crusher operation is a two-stage process in which
an initial crusher hopper 14 reduces the SPL material to approximately two-
inch
3 o size pieces, with the resulting two-inch size pieces then being sent to a
second
crusher 15 which reduces them to about 16 mesh or less in size. The
particulate
material from the crushers 14 and 15 is then sent to a magnetic separator 16
which removes iron and any other ferromagnetic particulate metal 17, and in
CA 02314123 2005-06-02
_$-
particular iron, from the particulate feed. A 16 mesh classifier 18 returns
any
particulate material which is greater than 16 mesh to crusher 14 through a
return
loop 19 in order to reduce the size of that material to 16 mesh or less, since
particulate material larger than 16 mesh is not recommended or preferred.
The resulting particulate feed 20 may be directed initially into a
soak tank 22 for a sufficient time, e.g., about 24 hours, and temperature to
remove gases such as ammonia, acetylene and methane gases, the soak tank
preferably containing neutral H20 and waste water from caustic scrubber 58
used in the polishing step. The feed 23 is then directed into an acid digester
24
1 o containing preferably, sulfuric acid; however, other acids which liberate
HF or
HCN gases may be used singly or in combination with sulfuric acid. Particulate
feed 23 is preferably fed into digester 24 by a sealed, variable drive, heated
screw. The auger digester 24 is preferably maintained under a negative
pressure in order to assist in removing gases which are generated within
digester 24. In preferred operations, the digester 24 is maintained at an
elevated temperature, for example, up to 300°C and typically
100° or 135° to
250°C. The speed of the preferred input and output augers are adjusted
to allow
for an approximately 30-60 minute retention time of the particulate feed
material
23 within the digester 24 with longer times not found to be detrimental.
Shorter
2o times can be used at higher temperatures. In the digester 24, the SPL and
other
materials react with the acid, e.g., sulfuric acid, causing any fluoride and
cyanide
material to be converted to HF and HCN gas, respectively, which is
continuously
removed from the digester 24 in a gas stream or gas component 28. The
remaining solid material is removed from digester as a solid component 30.
In preferred operations, concentrated or strong acid 32, e.g.,
sulfuric acid (approximately 93% by weight) is added to the digester 24 at a
rate
of approximately 0.8 Ibs H2S04 to one pound particulate material, depending to
some extent on the soluble portion of the spent potlining. The ratio of acid
to
particulate material by weight can range from 0.2 to 2.1, and in particular
from 0.8
3o to 1.1, for H2S04 acid. While, as noted, H2S04 is the preferred acid for
the
digester 24, it should be understood that other acids such as HC104, HCI,
HN03,
H3(P04) and oleum, or combinations thereof, may also be utilized. The
different
acids may produce different effluent salts. However, the process can be
adjusted to
CA 02314123 2005-06-02
_g_
accommodate the different materials. Water is continuously added to the soak
tank 22 at a rate to maintain the soak tank level and to maintain
approximately
20% moisture content within the digester 24. The moisture or liquid content in
the digester can range from 5 to 100 wt.% water to spent potliner. In
preferred
operations, the water added to the digester is purge water 36 from caustic
scrubber 58, as described in greater detail below. By thus recycling the purge
water, any fluoride salts captured from other parts of the process are
recovered,
and the water thus provided is at a temperature in the range of from about
ambient to 120°F, thereby saving heating energy. The use of purge water
also
s o eliminates the need to dispose of the waste stream from caustic scrubber
58.
Also, the recycling of the purge water provides for more favorable economics
in
the process.
The gas component 26 from the soak tank 22 and the gas
component 28 leaving the digester 24 will normally contain hydrogen cyanide
(HCN) and hydrogen fluoride (NF). The gas components 26 and 28 are then
heated at heater or oxidizer 38. In preferred operations, heater or oxidizer
38 is
in the form of an art known as electric converter/oxidizer which is designed
to
heat the gas component 28 to a temperature sufficiently high to oxidize the
hydrogen cyanide, for example, to approximately 400° to 1000°
and in particular
750°-850°C, in the presence of air. At this temperature,
hydrogen cyanide is
oxidized and converted or decomposed into a residual gas component 40
including H20, C02 and N02, NOx or N2, while the HF gas remains unreacted.
The residual gas component 40 is then preferably cooled in waste heat recovery
boiler 42. Typically, the temperature of the gases is reduced to less than
150°-
200°C. The cooled residual gas component is then directed into a water
scrubber 44. The heat recovered in the boiler 42 is redirected to other stages
of
the process 10, as desired, to thereby save energy and enhance the efficiency
of
the process.
In the water scrubber 44, hydrogen fluoride in the residual gas
3 o component 40 is converted to liquid hydrofluoric acid 46 which is directed
to an
alumina trihydrate reaction tank 48 in which it reacts with the alumina
trihydrate
to form aluminum fluoride and water. Alumina trihydrate 136 is introduced into
CA 02314123 2005-06-02
-10-
the reaction tank 48 from another portion of the process as described below.
Alumina trihydrate as used herein is meant to include AI203~3H20 or AI(OH)3
and may be referred to as aluminum hydroxide, aluminum hydrate, hydrated
alumina or hydrated aluminum oxide. The reaction tank 48 is heated to a
temperature to effect reaction between hydrofluoric acid and the aluminum
hydroxide to form aluminum fluoride. The temperature can be in the range of
100° to 300° and preferably, the temperature is in the range of
135° to 250°C
with a typical temperature being about 200°F for about three hours. The
aluminum fluoride is then filtered at 49 and directed to a dryer 50 where the
1 o residual solids are heated to less than 10% moisture. These dried solids
are
then directed to a calciner or dryer 51 where the solids are flash heated to a
temperature of about 700°C forming aluminum fluoride 52. Water vapor 53
is
redirected from the dryer 50 and reaction calciner or dryer 51 back to the
water
scrubber 44, thereby eliminating a waste stream at this point of the process.
Gases 56 from the water scrubber 44, from which HF has been removed are
then passed to a caustic scrubber 58 as a polishing step before release to the
atmosphere 60. In preferred form, the caustic scrubber 58 utilizes NaOH to
reach an alkaline or basic, e.g., a preferred, pH in the range of about 7.0 to
8Ø
In broader aspects, it will be understood that the pH can range from 6.5 to
10.
2 o Other alkali or alkaline earth metal hydroxides may be used such as KOH
and
Ca(OH)2, or combinations thereof. Sodium hydroxide is preferred because it
causes less complications in other liquid streams of the over-all process. As
described above, purge water 36 from the caustic scrubber 58 is redirected
back
to soak tank 22 for use therein. This eliminates another waste stream in the
2 5 overall process and also recaptures any residual fluorides which were
unreacted
with the water scrubber 58.
The aluminum fluoride 52 which is thus produced, is the first
primary solid end product of the process 10 of the present invention, and may
be
utilized commercially in any number of applications. For example, the aluminum
3 o fluoride 52 may be used as a bath additive for bath ratio corrections in
the cell.
This substantially eliminates any environmental problems caused by the
fluoride
materials in the SPL, and, as detailed above, provides a substantial cost
benefit
CA 02314123 2005-06-02
-11-
and savings.
Now returning to the process of the present invention at digester
24, the solid component 30 from the digester 24 is directed to a first rinse
housing 62 which receives input water 64, and thence through filter 63 to a
second rinse housing 66 with additional input water 65. The first rinse 62
removes water soluble salts from the input slurry 30. In the preferred
process,
the slurry 68 from the first rinse housing 62 passes through the filter press
63,
and then the solids 69 are introduced to the second stage water rinse housing
66 for polishing. The solid stream or fraction 70 from the second water rinse
66
1 o includes carbon and refractory materials such as alumina, silica, and,
generally,
a relatively high concentration of calcium sulfate salt. Due to this high
concentration of calcium sulfate level, the solid stream 70 passes through a
filter
71 and into a third rinse 72 which is used in the preferred processes to
remove
the soluble calcium sulfate salts from the solids. In preferred operations
wherein
mullite is a desired end product, ammonium chloride is reacted with the
calcium
sulfate to form ammonium sulfate and calcium chloride as indicated by the
reaction formula
CaS04 + 2NH4CI -~ (NH4)2S04 + CaCl2
The aluminum chloride may be introduced as a solution 74 at approximately 20
2 o wt.% and introduced with rinse 72. It will be appreciated that other
concentrations may be used, e.g., from 15 to 50 wt.% NH4C1. The solution
containing these two remaining salts (ammonium sulfate and calcium chloride)
are filtered at 75 and carried by stream 76 to a storage unit 78 wherein they
may
later be recovered or reused as a calcium chloride liquid and an ammonium
2 5 sulfate solid. Regardless of their later use, both of these salts are non-
toxic and
present no substantial environmental problem.
The solids 80 which remain after the rinses 62, 66 and 72 are
filtered at 75 and are preferably directed to a mixer dryer 82 and include
alumina, silica and carbon. In the alternative, the solids 80 may be directed
to a
3 o storage unit 83 wherein they may be sold and readily used in cement
manufacture or in the glass and ceramics industry. In another example, to the
alumina 84 and silica 85 mix at mixer dryer 82, may be added alumina and/or
silica to provide a ratio within mixer dryer 82 at a ratio of about 70% to
30%, by
CA 02314123 2005-06-02
-12-
weight, alumina to silica, respectively. The alumina to silica ratio in solids
80
may be adjusted by the addition of alumina and/or silica. The alumina to
silica
ratio may be adjusted by adding alumina and/or silica to provide 40 to 90 wt.%
alumina, the remainder silica on an alumina and silica basis. This alumina to
silica mix 86 is then passed into a high temperature vessel 88 in which it is
subjected to an elevated temperature to oxidize carbon in the mix. Typically,
the
temperature is in the range of about 1,600° to about 2,000°C in
an oxygen-rich
atmosphere. This causes any carbon remaining therein to be oxidized to carbon
dioxide, while simultaneously vitrifying the alumina and silica into a fused
1 o composition of alumina and silica. Typical of the fused composition is
mullite 90
which can be of high purity. Mullite 90 is a second major solid end product of
the
process of the present invention. The mullite may be utilized to make furnace
brick for use within aluminum reduction cells or for use for other commercial
purposes.
15 In the preferred method, solids 86 are transferred to a high
temperature vessel 88 and subjected to an elevated temperature in the presence
of an oxygen-rich atmosphere. This causes remaining carbon to oxidize to
carbon dioxide thereby providing 8000 to 9000 BTU/Ib energy and a usable
refractory material 90,
2 o e.g., mullite.
In preferred processes, the oxygen-rich atmosphere within the
vessel 88 is maintained by introducing oxygen, preferably in the form of air
92, to
the vessel 88. Carbon dioxide and heat as well as small amounts of gases, HF
and particulates, are removed from the vessel 88 in the form of a heated gas
25 stream 93 and are then directed through a heat recovery boiler 94 to a bag
house 95. In the bag house 95, the particulates are removed and redirected as
bag house catch 96 to the soak tank 22, while the gases 97 are directed to the
caustic scrubber 58 and then back to the soak tank 22. Thus, the carbon in the
SPL is used for useful purposes within the process 10 of the present invention
3 o as a fuel source to lower energy costs of the system, rather than
remaining as a
useless landfill material typical of prior SPL treatment processes or systems.
The liquid fraction 98 form the first and second rinse housings 62
and 66, respectively, having been filtered at 63 is then directed to an
alcohol
CA 02314123 2005-06-02
-13-
separator 100. In the separator 100, alcohol, for example methanol or ethanol
102, is admixed with the liquid 98 in a volume ratio of approximately 4:1
alcohol
to liquid fraction, for example. The ratio of alcohol to liquid fraction can
range
from 10:1 to 1:5, for example, depending on the liquid fraction. This step is
capable of separating about 97% or more of the salts in the liquid fraction 98
which are filtered out of slurry stream 103 at filter 104. The liquid stream
106
from the filter 104 includes the alcohol and excess acid from the digester 24
and
is directed through a recovery evaporation still 108 wherein alcohol is
separated
and returned to the alcohol storage source 102. The remaining sulfuric acid is
1 o stored at 110 and eventually returned along line 22a to soak tank 22
(Figure 1 )
for reuse in the digester 24. In this manner, the use of sulfuric acid and
sodium
hydroxide in the process 10 can be reduced, while alcohol is recovered and
reused, thus enhancing the economics of the process 10 as compared to prior
art systems.
15 The salts 112 from the filter 104 are redissolved in a water bath
114 and then pH adjusted in tank 116 to a basic pH, for example, preferably
using sodium hydroxide 118 to a pH of about 12.0 to 12.5. It will be
appreciated
that any basic pH can be used that is effective in forming a soluble
aluminate,
e.g., sodium aluminate and insoluble impurities such as metal hydroxides. For
2 o example, the pH can range from 9 to 14 and in particular from 11.8 to 13.
A pH
of 12 to 12.5 is an example of a pH which is effective. Also, sodium hydroxide
is
an example of a metal hydroxide which can be used. However, any alkali or
alkaline earth metal hydroxide may be used and is effective in forming a
soluble
aluminate and insoluble metal hydroxides. For example, KOH and Ca(OH)2 may
2 5 be used. Thus, this step forms a slurry 120 containing soluble sodium
aluminate
and insoluble impurities including calcium, iron and magnesium compounds
such as calcium hydroxide, iron hydroxide and magnesium hydroxide. The
insoluble impurities are filtered at 122 and directed via solids stream 124 to
the
storage tank 78.
3 o HCI 125 can be introduced to the tank 78 to react with the metal
hydroxides and produce metal chlorides, for example, to produce a mixture 127
of calcium chloride, iron chloride and magnesium chloride, which mixture 127
is
a useful product for use in industrial water treatment.
CA 02314123 2005-06-02
-14-
The liquid fraction 126 from the filter 122 is directed to a second pH
correction tank 128 wherein an acid 130, such as sulfuric acid, is added to
lower
the pH, for example, to about 7.0 to 8.0 to precipitate alumina trihydrate
although
the pH can range from about 6.5 to 10. This step forms a slurry 132 containing
soluble sodium sulfate and alumina trihydrate precipitate. It will be
understood
that other acids may be used to lower the pH. Further, the pH used is a pH
which enables separation of the sulfate from the hydroxide.
The alumina trihydrate may be removed from the solution in
another way. That is, alumina trihydrate may be precipitated between the range
of 11.8 to 125 by slowly adjusting the pH of the solution with acid such as
sulfuric
acid down to pH 11.8 and thereafter allowing the pH to adjust upwardly. This
procedure is repeated until the pH will not rise above the pH of 11.8. This
precipitates the crystal form of alumina trihydrate instead of the gel form.
This is
the preferred method for recovering alumina trihydrate.
The slurry 132 is then filtered and rinsed at 134, and the alumiria
trihydrate solids 136 are polished at 138 and then redirected as the alumina
trihydrate stream 54 to the reaction tank 48 to form aluminum fluoride as
previously discussed. The sodium sulfate containing liquid stream 140 from the
filter 134 is directed to a second alcohol separation tank 142 wherein alcohol
2 0 144, as noted earlier, either methanol or ethanol, is mixed with the
liquid stream
in a volume ratio of approximately 4:1 alcohol:liquid stream to precipitate
sodium
sulfate. The ratio of alcohol to liquid stream can range from 10:1 to 1:5, for
example. The precipitated sodium sulfate is filtered at 146 and is then
directed
to a dryer 148 and then storage 150, wherein the resultant sodium sulfate is
approximately 99.0% pure. The liquid portion 152 is directed from the filter
146
to an alcohol recovery still 154 wherein alcohol is separated and directed via
stream 156 back to storage unit 144 for reuse in the process, while the water
stream 158 is directed to water recycle storage unit 160 for reuse within the
process 10, such as at 114.
3 o Alternatively, as shown in Figure 5, liquid fraction 98 resulting from
the first and second rinse housings 62 and 66, respectively, having been
filtered
at 63 is directed to tank 116 where the pH is adjusted. As noted, the pH is
adjusted to form soluble sodium aluminate and insoluble impurities, e.g.,
calcium
CA 02314123 2005-06-02
-15-
hydroxide, iron hydroxide and magnesium hydroxide. The insoluble impurities
are filtered and directed to tank 204 where the pH of the liquid in tank 204
is
adjusted. In tank 204, the pH is lowered and the tank heated to precipitate
calcium compounds, e.g., calcium sulfate. Typically, the pH is adjusted to a
pH
less than 1 by the addition of an acid such as H2SO4. Also, typically the tank
is
heated to a temperature in the range of 80° to 110°C. The
calcium compounds,
e.g., calcium sulfate, are filtered at 205 and then stored in storage tank
206.
Liquid from filter 205 is directed to tank 207 where the pH is
adjusted to precipitate iron compounds such as iron hydroxide. Typically, the
pH
1 o is adjusted upwardly to a pH in the range of 4.5 to 5.5. The precipitate
is filtered
at 208 and stored in storage tank 209.
Liquid from filter 208 is directed to tank 210 where the pH is again
adjusted to precipitate magnesium compounds such as magnesium hydroxide.
The magnesium compounds are precipitated by adjusting the pH to a pH in the
range of 10.5 to 12. Thereafter, the magnesium precipitate is removed at
filter
211 and stored in tank 212. Then, liquid stream 213 from filter 211 is
directed to
tank 116 to enhance aluminum recovery. The three compounds recovered, e.g.,
calcium sulfate, iron hydroxide and magnesium hydroxide, are relatively pure
and thus have good commercial value.
2 o As the result of the above process 10, spent potliner material is
reduced and recycled into commercially useful ingredients, that is, aluminum
fluoride; mullite raw brick material; AI203, C and Si02 useful in cement or
glass
manufacture. Sodium sulfate, calcium sulfate, magnesium hydroxide and iron
hydroxide are also recovered.
2 5 EXAMPLE
Sixty tons per day of SPL feed, including caked materials and
sweepings, is continuously introduced to the crusher 14 and is processed
through the steps of the process 10 as described above. Utilizing this
process,
the 60 tons/day SPL input 12 yields approximately 13 tons/day aluminum
3 o fluoride end product, approximately 10 tons/day of a refractory material,
and
approximately 50 tons/day of reusable salts, e.g., sodium sulfate, for a total
of
about 73 tons of recycled solid materials, with the balance of the starting
materials being converted to harmless gases and salts. In processing this 60
CA 02314123 2005-06-02
-16-
tons/day of SPL input 12, substantially all of the cyanides contained therein
are
destroyed, and substantially all of the fluorides are converted to aluminum
fluoride as a useful end product. Thus, these highly environmentally damaging
materials are either eliminated or converted to useful products.
As can be seen from the above, the present invention provides a
highly efficient process for not only treating the significantly hazardous
spent
potliner material from aluminum reduction cells, but also serves to convert
the
components of the SPL to useful end products. Moreover, there are no
significant amounts of solid waste material from the process of the present
1 o invention which must be subsequently disposed of in landfills or stored,
as
previously required in other processes and practices for treating spent
potliner
material. In addition, the process of the present invention efficiently
recycles
water and heat and produces refractory material which can be used in the
fabrication of new aluminum reduction cells, thereby providing a highly
efficient
15 and economic process without a liquid or noxious gas waste stream. The
primary end products of aluminum fluoride, refractory material and sodium
sulfate are all usable, either in the actual manufacture of aluminum reduction
cells or in other commercial endeavors. The stored impurities of calcium
sulfate,
iron hydroxide, magnesium hydroxide, ammonium sulfate and calcium chloride
2 o are all benign, and are all treatable in accordance with conventional
processes
and may be reclaimed for a wide variety of commercial uses since they include
no environmentally hazardous materials, such as for water treatment to recover
fluoride and solids. As a result, it is seen that the present invention is a
highly
efficient process and very economical in both its operation as well as its
yield,
2 5 and that it avoids having to deposit fused solid material containing
environmentally hazardous component into landfills or storage.
The foregoing exemplary descriptions and the illustrative preferred
embodiments of the present invention have been explained in the drawings and
described in detail, with varying modifications and alternative embodiments
3 o being taught. While the invention has been so shown, described and
illustrated,
it should be understood by those skilled in the art that equivalent changes in
form and detail may be made therein without departing from the true spirit and
scope of the invention. It should be further understood that the scope of the
CA 02314123 2005-06-02
-17-
present invention is to be limited only to the claims except as precluded by
the
prior art. Moreover, the invention as disclosed herein may be suitably
practice in
the absence of the specific elements or steps which are disclosed herein.