Note: Descriptions are shown in the official language in which they were submitted.
CA 02314356 2000-06-06
1
ANTI-COKING COATINGS FOR REFRACTORY ALLOYS USED IN THE
PETROLEUM FIELD
DESCRIPTION
Technical field
The object of the present invention is anti-coking
coatings for preventing the deposition of coke on
refractory alloy parts used in the petroleum field.
In petrol refineries, thermal power stations, polymer
production installations and elsewhere, there exist
numerous components which are in contact with hot
fluids containing hydrocarbons, at temperatures at
which degradation products form in the hydrocarbons and
may lead to deposits of coke on the components.
The coke has several origins:
- It may be a case of "pyrolitic" coke inherent in the
process. Thus, during the steam cracking of
hydrocarbons, the chemical reactions result from the
cracking of the carbon chains with the consequence of
creating the required light gases (ethylene and
propylene) and the formation of pyrolitic coke.
- It may be a question of "catalytic" coke due to the
presence, in large quantities, of elements reputed to
promote coking, such as nickel and iron, in the
B 13166.3 MDT
- CA 02314356 2000-06-06
2
components in contact with the hydrocarbons. These
elements (nickel and iron) cause the heterogeneous
catalysis of different chemical reactions giving rise
to the production of solid carbon deposited on the
surfaces in the form of filaments and/or amorphous
carbon.
Coke causes corrosion phenomena in the components, for
example petrochemical furnace tubes. It becomes
established on the walls of the tubes and,
preferentially, on the hottest parts. The different
problems related to the deposition of coke are as
follows:
1) A loss of heat transfer from outside the tube to
the inside since the coke acts as a thermal insulant,
which requires more energetic heating in order to
obtain, for example, the required steam cracking
temperature (approximately 850°C). There is then a
risk of damaging the refractory alloy in the tube and,
at the end of a certain time, the operator must
inevitably perform a decoking operation, that is to say
eliminate this coke by oxidising it by means of water
vapour and/or air.
2) A carbonising of the tubes. This is because,
although a protective layer containing chromium
generally forms on the surface of the tube, the latter
cracks easily because of compression stresses caused by
a difference in metal/oxide mesh as well as by the
conditions of use of the steam cracking tubes (thermal
shacks due to the coking/decoking cycle).
Thus there is a great advantage in finding the means of
preventing the deposition of coke on the refractory
B 13166.3 MDT
CA 02314356 2000-06-06
3
alloy surfaces in contact with hot fluids containing
hydrocarbons.
Prior art
The most widely used technique for preventing or
restricting the deposition of coke on metallic surfaces
is to apply an anti-coking coating to the latter.
Thus the document FR-A-2 662 704 [1] makes provision
for using, as an anti-coking coating, oxides, carbides,
nitrides and/or silicides such as titanium, zirconium
and niobium silicides, this coating being able to be
applied by conventional methods such as chemical vapour
deposition (CVD) or plasma assisted chemical vapour
deposition (PACVD). In this document, good results are
obtained with a metallic surface of nickel superalloy
with a deposit of alumina by impregnation by means of a
slurry, or a deposition of titanium carbide by chemical
vapour deposition.
The documents EP-A-0 607 651 [2] and EP-A-0 608 081 [3]
illustrate the use of coatings based on metallic
oxides, metallic fluorides or mixtures thereof in order
to prevent the deposition of coke on stainless steel
surfaces.
The document US-A-5,266,360 [4] illustrates the use of
coatings based on Si02, alumina or tungsten disulphide,
possibly comprising particles of metallic oxides such
as alumina, cerium oxide and cupric oxide for
inhibiting the formation of coke on gas turbine
components.
In documents [2] and [3], deposition is effected by CVD
whilst in document [4] the coatings of Si02 are
B 13166.3 MDT
CA 02314356 2000-06-06
4
obtained by immersion in an appropriate solution, using
the sol-gel technique.
Thus the most widely used coatings for preventing or
limiting the deposition of coke on metallic surfaces
are based on oxide, in particular Si02.
Disclosure of the invention
The object of the present invention is novel coatings
and a novel method for preventing the deposition of
coke on a refractory alloy surface in contact with
fluids containing hydrocarbons.
According to the invention, the method for preventing
the deposition of coke on a refractory alloy surface in
contact with fluids containing hydrocarbons comprises
the following steps:
1) subjecting the refractory alloy surface to the
action of a gaseous plasma of oxygen and/or nitrogen at
low frequency, and
2) depositing on the surface thus treated a coating
based on silicon oxide, nitride or oxynitride by plasma
assisted chemical vapour deposition at low frequency,
using an organosilicic compound and a gas chosen from
amongst oxygen and nitrogen,
the two steps being performed consecutively and
continuously in the same installation without opening
to atmosphere between the two steps.
The first step in this method consists of diffusing
oxygen and/or nitrogen in the refractory alloy. This
diffusion treatment corresponds either to a nitriding,
or to an oxidation, or an oxynitriding. In the case of
a metallic refractory alloy surface including nickel
B 13166.3 MDT
CA 02314356 2000-06-06
and iron, this treatment repels the elements
facilitating coking such as nickel and iron, and thus
prepares the surface for receiving a deposit whilst in
addition promoting the adhesion of the deposit which
5 will then be formed in the second step.
According to the invention, the two steps of the method
are carried out in the same plasma assisted chemical
vapour deposition reactor, which has a system for
feeding the different gases used, such as oxygen,
nitrogen, the organosilicic compound and possibly
argon.
According to the invention, each of the steps is
performed using a low frequency, for example a
frequency situated in the range from 2 to 450 kHz.
Thus a low plasma density is obtained, along with more
homogeneous deposits able to cover parts of large size.
According to a preferred embodiment of the invention, a
coating based on silicon oxynitride is deposited in the
second step by PACVD.
In this case, it is possible to use, for the PACVD
deposition, an organosilicic compound containing
nitrogen, for example hexamethyldisilazane. The
mixture used for this deposition of silicon oxynitride
is a mixture of the nitrogenous organosilicic compound,
for example hexamethyldisilazane, and oxygen.
Preferably, in this case, a gaseous oxygen plasma is
used in the first step for oxidising the refractory
alloy surface.
The coating obtained under these conditions is a
silicon oxynitride coating of formula SiOXNy, in which x
and y are such that:
B 13166.3 MDT
CA 02314356 2000-06-06
6
1<x_<3 and
0.02<_y/x<_0.2.
According to a second embodiment of the invention, in
the second step a coating based on silicon nitride is
deposited. In this case, use is made in this second
step of a plasma formed from a mixture of nitrogen and
an organosilicic compound containing nitrogen such as
hexamethyldisilazane, and preferably the first step is
performed with a gaseous nitrogen plasma. In this
second embodiment of the invention, it is preferable to
first of all carry out an ionic pickling of the
refractory alloy surface under argon plasma before
performing the two steps of the method of the
invention.
In this second embodiment of the invention, it is also
possible to carry out a supplementary step of oxidation
of the coating obtained in the second step in order to
convert it at least partly into silicon oxynitride.
According to a third embodiment of the method of the
invention, in the second step a coating based on
silicon oxide is deposited, using a plasma formed from
a mixture of oxygen and an organosilicic compound
containing oxygen, such as hexamethyldisiloxane.
In this case, an oxygen plasma is preferably used in
the first step. In this third embodiment, the two
steps of the method can be supplemented by a third step
of treatment of the coating obtained with a nitrogen
plasma, in order to partly convert the SiOX coating
into silicon oxynitride.
In order to implement the different steps of the method
of the invention, using gaseous plasmas, the operations
B 13166.3 MDT
CA 02314356 2000-06-06
are carried out in the same PACVD installation using
very low pressures, for example 10 to 100 Pa.
The durations are chosen as a function of the gaseous
flow rates so as to obtain satisfactory coatings.
In the second step, the duration is preferably such
that a coating is obtained having a thickness of 0.1 to
100 Vim.
The gaseous flow rates are in the range from 10 to 500
cm3/min .
In particular, the ratios of the oxygen or nitrogen
flow rates to the flow rate of organosilicic compound
range from 1 to 20.
According to the invention, the refractory alloy
subjected to the treatment is a refractory alloy like
the ones normally used in petrochemistry. The alloy
can in particular be a nickel-chromium-iron alloy
containing 0.10 to 0.600 carbon, 0.7 to 2% manganese
and 1 to 2°s silicon, and optionally minor additions of
niobium, titanium, tungsten and zirconium.
By way of example, this alloy can be of the Manaurite
type, in particular a Manaurite such as the ones whose
compositions are given in the accompanying Table 1.
Amongst these, preference is given to XM and XTM
Manaurites having the following compositions.
Manaurite XM comprising:
- 33 to 38% nickel,
- 23 to 28% chromium,
- 0.35 to 0.600 carbon,
- 1 to 1.5o manganese,
- 1 to 2% silicon,
B 13166.3 MDT
CA 02314356 2000-06-06
8
the remainder being iron with possibly minor additions
of Nb, Ti and Zr.
Manaurite XTM comprising:
- 34 to 37s chromium,
- 43 to 48~ nickel,
- 0.40 to 0.45% carbon,
- 1 to 2% manganese,
- 1 to 2o silicon,
the remainder being iron with possibly minor additions
of Nb and Ti.
Another object of the invention is a part made of
refractory alloy having a silicon oxynitride coating of
formula SiOxNy in which x and y are such that:
1<x<_3 and
0.02<_y/x<_0.2.
,The refractory alloy is in particular a nickel-
chromium-iron alloy, for example a Manaurite such as
the ones described above, and the coating can have a
thickness of 0.1 to 100 Vim.
Other characteristics and advantages of the invention
will emerge more clearly from a reading of the
following description given of course for purposes of
illustration and non-limitatively, with reference to
the accompanying drawing.
Brief description of the drawing
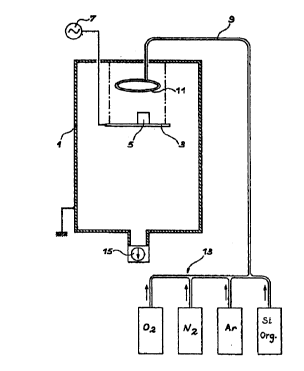
Figure 1 depicts schematically in vertical section a
reactor suitable for implementing the method of the
invention.
B 13166.3 MDT
CA 02314356 2000-06-06
9
Detailed disclosure of embodiments
In Figure 1, it can be seen that the deposition reactor
comprises an earthed enclosure (1), inside which there
is disposed a support (3) for the parts to be treated
(5), this support being connected to a low-frequency
generator (7). The support (3) can be a plate or rod
for suspending the parts to be treated.
The generator (7) comprising a system for adjusting the
power and frequency and a device for displaying the
self-bias voltage.
The gases necessary for the depositions are introduced
into the enclosure through a conduit 9 connected to a
gas diffuser 11 situated in the enclosure, which can be
cylindrical. The conduit 9 is connected to a gas
supply system 13 which comprises several lines through
which oxygen, nitrogen, argon and the organic silicon
compound can be brought to the gas diffuser 11. The
enclosure is also connected to a pumping unit 15 at its
lower part in order to produce and maintain the
required pressure in the enclosure.
A control console, not shown in the figure, has devices
for controlling pumping, gas flow rate and total
pressure, the pressure measurement being made by a
capacitive gauge. Each gas feed line is equipped with
an electronically regulated mass flow meter and stop
valve which are connected to the control cabinet
provided with devices for displaying the flow rate and
simultaneous adjustment.
All the steps of the method of the invention can be
implemented in this reactor.
B 13166.3 MDT
CA 02314356 2000-06-06
1
Thus, after having introduced the parts to be treated
into the chamber, it is possible to carry out first of
all if necessary an ionic pickling treatment by means
of an inert gas plasma, for example argon, using a
sufficiently low pressure to allow a powerful ion
bombardment, for example a biasing of -700 V.
Next the first step of the method of the invention can
be carried out in this enclosure by means of an oxygen
or nitrogen plasma, for example at a frequency of 50
kHz. The deposition of the silicon-based coating is
then carried out, still in the same enclosure, by
modifying the gases introduced and the gaseous flow
rates and by adapting and changing the frequency of the
plasma, if necessary.
Thus the operating frequency can be chosen for each
operation in order to provide the treatment or growth
of the coating under the best possible conditions in
order to obtain the expected properties and good
adhesion of the coating.
The following examples, given as an indication and no
way limitatively, illustrate the method of the
invention.
In the following examples, a Manaurite XM substrate is
used, which has the composition given in Table 1.
Example 1
On the Manaurite XM substrate, an ionic oxidation
treatment is carried out using a pure oxygen plasma, at
a working pressure of around 16 Pa (0.16 mbar) with an
oxygen flow rate of 50 cm3/min and a frequency of 50
kHz. This treatment is carried out for 30 minutes.
B 13166.3 MDT
CA 02314356 2000-06-06
11
Next a coating based on silicon oxide is formed on the
substrate by means of an oxygen and
hexamethyldisiloxane plasma with respective flow rates
of 250 cm3/min for oxygen and 25 cm3/min for
hexamethyldisiloxane. The operation is carried out at
a pressure of 50 Pa (0.5 mbar) and at a frequency of 50
kHz, for 40 minutes.
In this way a coating based on silicon oxide is
obtained with a thickness of 1 ~tm.
The behaviour under coking of the Manaurite substrate
thus coated is tested by putting it in contact with a
flow of hydrocarbon (naphtha) at a temperature of 810°C
for 20 minutes. For comparison, the same test is
carried out on an untreated Manaurite XM substrate.
The improvement of the coking behaviour of the coated
substrate compared with the non-coated substrate is 6
to 10 0 .
Example 2
In this example, a Manaurite substrate is used
identical to the one of Example 1 and first of all an
ionic pickling treatment is carried out on it under
argon plasma for one hour using an argon flow rate of
50 cm3/min, a pressure of 10 Pa (0.1 mbar) and a
frequency of 50 kHz.
After this treatment, the substrate is subjected to the
action of a nitrogen plasma for 30 minutes at a total
pressure of 30 Pa (0.3 mbar) , operating at a frequency
of 50 kHz with a nitrogen flow rate of 50 cm3/min.
Next a coating based on silicon nitride is deposited
using a plasma formed from a gaseous mixture of
nitrogen and hexamethyldisilazane, with respective flow
B 13166.3 MDT
CA 02314356 2000-06-06
12
rates of 100 cm3/min for the nitrogen and 10 cm3/min for
the hexamethyldisilazane at a pressure of 30 Pa and a
frequency of 50 kHz, for 45 minutes.
After this coating formation step, the substrate is
subjected to oxidation in air for one hour at 1000°C.
In this way a 2 ~m thick coating based on silicon
oxynitride is obtained.
The substrate thus treated is subjected to the same
behaviour under coking test as in the Example 1. It is
thus found that it exhibits an improvement in behaviour
under coking of 9 to 10% compared with the uncoated
Manaurite substrate.
Example 3
In this example, a coating based on silicon oxynitride
is formed on a Manaurite substrate identical to the one
in Example 1.
For this purpose, the substrate is first of all
subjected to an ionic oxidation treatment by means of a
pure oxygen plasma at a working pressure of around 16
Pa (0.16 mbar) and at a frequency of 50 kHz with an
oxygen flow rate of 50 cm3/min for 30 minutes.
Next the deposition by PACVD is carried out, using a
mixture of oxygen and hexamethyldisiloxane with
respective flow rates of 100 cm3/min for oxygen and 10
cm3/min for hexamethyldisiloxane, at a pressure of 50
Pa (0.5 mbar) and at a frequency of 50 kHz, for 40
minutes.
After the formation of this coating, a post-treatment
is carried out in the same enclosure by means of a
nitrogen plasma using a nitrogen flow rate of 100
B 13166.3 MDT
CA 02314356 2000-06-06
13
cm3/min, a working pressure of around 30 Pa and a
frequency of 50 kHz, for 30 minutes.
The substrate thus treated is subjected to the same
behaviour under coking test as in Example 1. It is
thus found that it exhibits an improvement in behaviour
under coking of 16 to 21% compared with the uncoated
Manaurite XM substrate.
Example 4
In this example, a Manaurite substrate identical to the
10~ one in Example 1 is subjected to an ionic oxidation
treatment by means of a pure oxygen plasma, using an
oxygen flow rate of 50 cm3/min, a working pressure of
around 16 Pa (0.16 mbar) and a frequency of 50 kHz, for
30 minutes.
After this treatment, a coating of silicon oxynitride
is deposited by PACVD using a oxygen-
hexamethyldisilazane mixture with respective flow rates
of 24 cm3/min for the oxygen and 12 cm3/min for the
hexamethyldisilazane, at a pressure of 50 Pa (0.5 mbar)
and a frequency of 50 kHz, for 40 minutes.
The product obtained is subjected to the same behaviour
under coking test as in Example 1. The improvement
obtained is 39 to 54o compared with the uncoated
Manaurite XM substrate.
Example 5
Under conditions similar to those of Example 4, the
coating of Manaurite XM tubes with a length of 70 cm
and a diameter of 4 cm is carried out.
These tubes are then subjected to a behaviour under
coking test at 810°C, and a very significant
improvement in the behaviour under coking of the coated
B 13166.3 MDT
CA 02314356 2000-06-06
14
tube is observed compared with the uncoated tube, this
improvement being around 40~.
Extreme conditions of the coking oven are then tested,
that is to say a temperature of 980°C, and an
improvement of around 20°s is also obtained under these
conditions.
A third test is finally carried out on the same tubes
at a temperature of 810°C and it is found that the
coating still behaves effectively since the improvement
obtained is again 50 to 530.
References cited
[1] FR-A-2 662 704
[2] EP-A-0 607 651
[3] EP-A-0 608 081
[4] US-A-5,266,360
B 13166.3 MDT
CA 02314356 2000-06-06
15
Table 1
RefractoryThermalloyComposition Any
$
alloy (T) C Mn Si Ni Cr Fe additions
designation
ManauriteT-04 0.35/0.601.00/ 1.00/33/38 23/28remainderNb
36X 1.50 2.00
TX-63 0.35/0.501.00/ 1.00/33/38 23/28remainderTi, W
1.50 2.00
ManauriteMA-6300 0.35/0.601.00/ 1.00/33/38 23/28remainderNb, Ti,
XM 1.50 2.00 Zr
Manaurite 0.35/0.600.70/ 1.00/33/38 21/28remainderW + Nb
36X5 1.25 2.00
ManauriteT-63W 0.35/0.600.70/ 1.00/33/38 21/28remainderW
35-25W 1.25 2.00
Manaurite 0.35/0.501.00/ 1.00/39/90 20/27remainderNb
XA 2.00 2.00
T-58 0.40/0.600.70/ 1.00/37/90 18/21remainderNb
1.25 2.00
Manaurite 0.35/0.951.00/ 1.00/92/96 32/37remainderNb
XT 1.50 2.00
Manaurite 0.40/0.951.00/ 1.00/93/98 34/37remainderNb, Ti
XTM 2.00 2.00
ManauriteT-45 0.35/0.501.00/ 1.00/23.5/ 19/ remainderNb
29/29 1.50 2.00 26.5 26.5
Nb
ManauriteT-53 0.06/0.151.00/ 0.75/29/37 16/22remainderNb
900 1.50 1.50
ManauriteTX-53 0.10/0.181.00/ 1.00/33/37 24/27remainderNb
900B 1.50 1.50
ManauriteT-50 0.25/0.601.00/ 1.00/30/38 14/23remainderNb, Ti
35 1.50 2.00 and W
B 13166.3 MDT