Note: Descriptions are shown in the official language in which they were submitted.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
NEW USE OF LOCAL ANAESTHETICS AGAINST VASCULAR HEADACHES
Field of the invention
The present invention is directed to a new use of [(3-alkoxy-phenoxy)-ethyl]-
dialkylamine
derivatives. More particularly the invention is directed to the use of [(3-
alkoxy-phenoxy)-
ethylJ-dialkylamine derivatives as a medicament for use in the treatment of
vascular
headaches, and in particular as a medicament for the treatment of migraine.
~o
Back round and prior art
Migraine is a disorder that exhibits a spectrum of treatment responses in
afflicted
individuals. Some sufferers are fortunate and the therapy may be over-the-
counter remedies
~s or even non-drug regimens using behavior modification, acupuncture, and
hypnosis as
instruments of aborting the headache. Bed rest, a darkened room, and the use
of cold packs
applied to the temporal artery and its branches may modify the attack. Sleep
also has a
beneficial effect in ending an attack. Most patients, however, will require
prescription
drugs for relief from the migraine. The symptoms most in need of treatment are
the head
2o pain and gastrointestinal symptoms. To a lesser degree, photophobia and the
aura warrant
treatment; the latter may also be quite disturbing and require treatment
although its
duration is relatively brief. The oral absorption of agents is less than
optimal during acute
migraine because of the reduced gastrointestinal peristalsis. The more severe
the attack, the
greater is the absorption reduced. Furthermore, the presence of nausea and
frequent
2s vomiting will preclude oral administration of pharmacologic agents.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
2
During most of the 20th century, ergot alkaloids have been the cornerstone of
effective
therapy. Still many patients do not respond to ergots. They find the response
is incomplete,
the side effects encountered are frequent and disturbing, and the
contraindications preclude
their use in many migraineurs.
The exact pathogenesis of migraine is still unknown. Many theories have been
elaborated,
but none can account for all the clinical features or for all the
pathophysiological aspects
demonstrated in recent years. The pendulum has been swinging between vascular
(Wolff
io 1963) and neurogenic (Sicuteri 198 theories, with brief peripheral blood
excursions
(Hannington et al, 1981 ). In recent years, however, a general consensus has
been emerging
that in migraine both vascular and neural components are relevant and most
probably
interrelated {Lance et al, 1983; Welch 1987; Olesen 1991 ).
~s Recent epidemicologic data suggest that 17.6 percent of adult females and
5.7 percent of
adult males suffer from migraine (Stewart et al, 1992). The Center for Disease
Control
( 1991 ) recently reported that over the last decade the prevalence of
migraine has increased
60 percent. In addition, migraine is significantly under-diagnosed, with only
40 percent of
adult females and 30 percent of adult males suffering from migraine being
patient
Zo diagnosed (Lipton et el, 1992). Yet 80 percent of this population of
undiagnosed
migraineurs experience disability (Stewart et al, 1992), and most seek
periodic medical
care for other medical conditions.
Migraine is also under-treated. Only about 40 percent of females and 30
percent of males
zs utilize prescription drugs (Celentano et al, 1992). However, many of these
patients
discontinue prescription medication and rely on the over-the-counter remedies.
The most common drugs which at present are used for the treatment of migraine
and other
forms of vascular headaches, are inter alia triptanes,ergotamine, aspirin, and
NSAIDS.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
3
One major problem with the just mentioned drugs, is that they often have an
onset time of
from 60 minutes and up to 4 hours, which is a disadvantage in therapy of
vascular
headache conditions such as migraine. Furthermore, these drugs are often in
forms suitable
for oral administration, which often causes problems for the patient since
nausea and
vomiting is common among patients suffering from migraine, or other forms of
vascular
headache conditions.
Headache, 1995; 35: pp. 83-84, reports a study of lidocaine (4%) administered
intranasally
via a spray bottle. The results from this study shows that only 27 % of the
patients in the
~o study experienced moderate relief, and none of the patients in the study
experienced
excellent relief.
WO 98/38998 discloses the use of levobupivacaine and ropivacaine in the
treatment of
migraine. These local anaesthetics are however structurally distinct from the
local
is anaesthetics used in accordance with the present invention.
In order to achieve satisfactory pain relief for patients suffering from
vascular headache,
the drug used to treat the vascular headache condition should preferably have
a fast onset of
action, and also as few side effects as possible.
Thus, the problem underlying the present invention is to find a new way of
therapy for
vascular headache conditions, and in particular migraine. A further problem
underlying the
present invention, is to find a new way of therapy providing a fast onset of
action, i.e. a fast
pain relief as well as relief of other symptoms associated a vascular headache
condition, to
is the patient suffering from the vascular headache condition.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
4
s
Outline of the invention
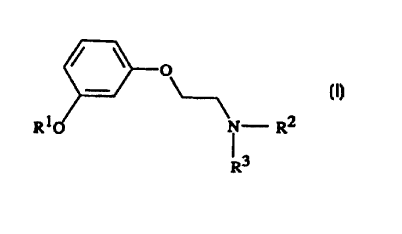
The present invention is directed to the use of a compound of the formula I
O
I
R10 N~ R2
1 3
R
wherein
RI represents C3_5 alkyl; and
R2 and R3 independently represent CI_3 alkyl; or a pharmaceutically acceptable
salt thereof ;
io
for the manufacture of a medicament for use in the treatment of a vascular
headache
condition.
A further aspect of the present invention is the use of a compound of the
formula I above,
~s for the manufacture of a medicament for use in the treatment of migraine.
Still a further aspect of the present invention is a method for the treatment
of vascular
headache conditions, in particular migraine, comprising administering to a
subject
suffering from said vascular headache condition, an effective amount of a
medicament
2o comprising a compound of the formula I as active agent.
A person skilled in the art will appreciate the various kinds of headache
conditions falling
under the definition vascular headache. However, the following brief
explanations may be
given.
2s
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
S
The wording "vascular headache" is intended to include any kind of vascular
headaches, in
particular migraine, cluster headache, post-traumatic headache, tension
headache, muscular
headache and headaches due to vascular diseases.
The wording "migraine" should be interpreted according to The International
Classsification of Migraine, Headache Classification Committee 1988. However,
the
specified vascular headache conditions are not to be considered as limiting
the invention in
any way.
~o
"Cluster headache" is most typically defined as the temporal clustering of
attacks during
periods usually lasting between 2 weeks and 3 months, separated by remissions
of at least
14 days, but usually several months - this type of cluster headache is also
called "episodic
cluster headache". "Chronic cluster headache" is characterised by absence of
remissions of
is at least 14 days for more than one year (Textbook of pain, p.504,
3'° edition, 1994).
"Post-traumatic headache" is headache caused as a result of some head trauma
(Textbook of pain, p.504, 3'~ edition, 1994).
Zo "Tension headache" and "muscular headache" belong to the group of headaches
formerly
described as "muscle contraction", "psychogenic", "stress" or "essential"
(Textbook of
pain, p. 502, 3rd edition 1994).
The compounds according to formula I above, may also preferably be used for
surgery in
is the nasal area.
By the wording "fast onset of action" is intended that a therapeutic or
prophylactic effect
shall preferably have been achieved within 15 minutes from the time of
administration.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
6
Patients suffering from vascular headache conditions such as migraine, often
get a feeling
that an attack of headache is approaching. In accordance with the present
invention, when a
patient starts to feel that an attack of a vascular headache condition is
going to afflict him
s or her, this patient may administer, preferably intranasally, a dosage of a
compound of the
farmula I . Since the active agent of the formula I has a fast onset of
action, the attack of
approaching headache will be. blocked before it has developed into a painful,
vascular
headache condition.
io The use in accordance with the present invention also encompasses the
administration of a
compound of the formula I as an acute rescue medicine. This means that the use
of a
compound of the formula I is also effective for therapy of fully developed
vascular
headache conditions such as migraine.
~s Thus, the present invention is intended not only to encompass therapeutic
treatment of
vascular headache conditions, but also prophylactic treatment of vascular
headache
conditions.
The medicament comprising a compound of the formula I above as the active
agent may be
Zo administered in form of a pharmaceutical formulation, further comprising
pharmaceutically
acceptable carriers, diluents or adjuvants.
The compound according to formula I above may be administered nasally or
intravenously.
Preferably the medicament is administered nasally in form of a spray. The
wording "spray"
is will be appreciated by a person skilled in the art, but includes a solution
which is
distributed such as to increase the contact area with the nasal mucosa.
Administration of
the medicament in form of drops and powders is a further useful alternative.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
7
The dose of the active agent of the formula I above will vary depending on the
formulation
used, as well as on the severity of the vascular headache condition to be
treated.
A preferred dose is 0.01-200 mg active agent/!cg body weight, more preferably
s 0.1-2 mg active agent/kg body weight. Similar ranges apply if a compound of
the formula I
is used for prophylactic treatment.
Preferred compounds for use as active agents in accordance with the present
invention, are
io compounds of the formula I selected from anyone of
isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine;
ethyl-isopropyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine;
diisopropyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine;
~s diethyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine;
isopropyl-methyl-[2-(3-n-butoxy-phenoxy)-ethyl]-amine;
ethyl-isopropyl-[2-(3-n-butoxy-phenoxy)-ethyl]-amine;
diisopropyl-[2-(3-n-butoxy-phenoxy)-ethyl]-amine;
ethyl-isopropyl-[2-(3-n-pentoxy-phenoxy)-ethyl)-amine; and
zo diisopropyl-[2-(3-n-pentoxy-phenoxy)-ethyl)-amine.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
8
Particularly preferred compounds of the formula I for use in accordance with
the present
invention are
(i) isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine, which is a
compound of the
formula II
O
I I
O N
~o (ii) ethyl-isopropyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine, which is a
compound of the
formula III
O
II! ,
O N
and
~s (iii) diisopropyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine, which is a
compound of the
formula IV
o
IV
CA 02314452 2000-06-12
WO 99/32103 PCT1SE98/02299
9
Biological evaluation
Neurogenic inflammation within cephalic tissue, involving vasodilation and
plasma protein
extravasation, has been proposed as a mechanism in headache pathogenesis
(Michael A.
Moscowitz, Neurology 43, (Suppl 3), June 1993).
In order to determine the ability of the compounds of the formula I above at
inhibiting
neurogenic inflammation, the following test model was used.
~o
The effect of isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine on
neurogenic
inflammation was examined in the hindpaws of rats.
is Animal preparation
Six male Sprague Dawley rats (260 - 290 g) were anesthetized with 2 %
isoflurane in a
nitrous oxide/oxygen mixture (2/3 - 1/3) and artificially ventilated after
insertion of a
tracheal cannula. The jugular vein was cannulated and the animals were
paralyzed by an
i.v. injcetion of 0.06 mg/kg pancuronium bromide (Pawlori ). The levels of
isoflurane,
Zo 02, N20 and the end-tidal C02 (3.5 - 4.5 %) were monitored during the
entire
experimental period. Core temperature was maintained at 38 t 0.5 °C
with a homeothermic
blanket system.
The animals were prepared to enable electrical stimulations {3 mA, 2ms, 2 Hz
for 5 min) of
2s the sciatic nerve of both legs. 10 - 20 p,l of isopropyl-methyl-[2-(3-n-
propoxy-phenoxy)-
ethyl)-amine (2 %) in a gel formulation (batch TARO-83-2) or, when needed, the
vehicle
(batch 106-10-2) were applied on the sciatic nerve distal to the stimulation
electrodes 10
min prior to stimulation. The nerves were then sectioned proximal to the
electrodes, and
Evans blue dye ( 10 mg/ml, kg) was given intravenously 2 min prior to
stimulation.
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
Assessment of neurogenic inflammation
The neurogenic inflammation was assessed 5 min after the end of the
stimulation by rating
the localized extravasation of Evans Blue in the skin area of the hindpaw
innervated by the
sciatic nerve, using a 4-point scale as shown in Table 1 below. In a
preliminary experiment
s the inability of the vehicle to block the development of the neurogenic
inflammation was
checked.
Table 1
Score Extravasation of Evans Blue
0 No evidence of blue s ots on the skin
1 < 25 % of the sciatic nerve innervation area tintedin
blue
2 25 - 75 % of the sciatic nerve innervation area tinted
in blue
3 > 75 % of the sciatic nerve innervation area tinted
in blue
io
protocol
The following gel formulation was used in the tests performed below.
In redient ,_ uanti~ m
isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine20
H drox ro lmeth lcellulose 4000 c s 20
H drochloric acid 2 M 44.
Sodium h droxide 2 M, to H 4.0-5.5 .s.
Water, urified to 1.00 .s.
is The gel was prepared by mixing isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-
ethyl]-amine
and the hydrochloric acid, whereafter a major part of the water was added. The
mixture was
stirred until all of the isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-
amine had
dissolved. pH was adjusted to about 4 and the rest of the water was added. The
obtained
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
11
solution was heated to 70 °C, whereafter the
hydroxypropylmethylcellulose was added
while vigorous stirring. The jelly was finally cooled while stirring, pH was
measured and,
if necessary, adjusted to 4.0 - 5.5.
s Three rats received a 2 %gel formulation as described above on the sciatic
nerve on one
side and the vehicle on the other side. Both sciatic nerves were stimulated
simultaneously
by the same constant-current stimulator. The vehicle-treated side received
isopropyl-
methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine (2 %) in a gel formulation gel
and the
stimulating procedure, while the other side was neither treated nor stimulated
and then
io served as a sham control (no neurogenic inflammation). As is shown in Table
2 below,
isopropyl-methyl-[2-(3-n-propoxy-phenoxy)-ethyl]-amine (2 %) applied topically
on the
sciatic nerve, blocked the development of the neurogenic inflammation in the
rat as asessed
by the extravasation of Evans Blue.
~s Table 2
Treatment Score: 0 1 2 3
isopropyl-methyl-[2-(3-n-propoxy- 5 1
phenoxy)-ethyl]-amine (n=6)
Vehicle (n=3)
Sham (n=3) 3
CA 02314452 2000-06-12
WO 99/32103 PCT/SE98/02299
12
II. Clinical model
A compound of the formula I above is administered to migraine patients having
moderate
to severe pain according to the 4 point verbal rating scale (VRS): none, mild,
moderate and
s severe respectively. The route of administration is two different types of
nasal
administration, one anterior application and one dorsonasal application. Each
type of
administration is having its own placebo control. The patient's visual
analogue scale (VAS)
score is recorded at baseline.
~o Each patient receives 1 puff in each nostril every 2 minutes for a 10
minute period. Thus,
each patient receives a total of 10 puffs (5 puffs in each nostril) of a
compound of the
formula I above
After administration of the first puffs ( 1 per nostril), patient satisfaction
is recorded every
~s 5 minutes up to 2 hours. Migraine pain according to the 4 point VRS and VAS
scales is
recorded every 15 minutes up to 2 hours, and thereafter every hour up to 24
hours
following the initial does. Other symptoms such as nausea, vomiting,
photophobia and
phonophobia, are recorded to the same schedule as theat for VRS and VAS.
2o Adverse events are monitored throughout the treatment period as well as at
a follow-up
visit after administration of the study drug.