Note: Descriptions are shown in the official language in which they were submitted.
CA 02314546 2000-07-26
P-4314 PATENT
Flow-Through Assay for Visually Detecting
the Presence of Influenza A and B
Field of the Invention
The present invention relates to a diagnostic test for detecting a human
influenza A and B antigen in a clinical specimen. The antigen is detected by
determining the presence of antibodies specific for influenza A or B. Antibody
A
and B are conjugated to a characteristic enzyme and, if antigen is present,
the
enzyme reacts with one or more substrates to produce a detectable reaction
prod uct.
Background of the Invention
The rapid diagnosis of viral infections is becoming an integral part of good
medical practice. Some viruses have definable antigens against which
antibodies
can be produced. These antibodies and antigens, i.e. immunoreactants, are
capable of binding with one another, thereby creating a highly specific
reaction
mechanism which can be used in vitro to determine the presence or
concentration
of that particular antigen in a biological sample. Therefore, immunoassays
have
been widely used for the measurement of the antigen and the subsequent
determination of the presence or absence of a virion.
There are several known immunoassay methods using immunoreactants,
wherein at least one of the immunoreactants is labeled with a detectable
component
so as to be analytically identifiable. For example, the "sandwich" or "two-
site"
i
CA 02314546 2000-07-26
technique may involve the formation of a ternary complex between an antigen
and
two antibodies. A convenient method of detecting complex formation in such a
technique is to provide one labeled antibody and an unlabeled antibody bound
to
a solid phase support such that the complex can readily be isolated. In this
example, the amount of labeled antibody associated with the solid phase is
directly
proportional to the amount of analyte in the test sample.
An alternative technique is the "competitive" assay. In one example of a
competitive assay, the capture mechanism again.may use an antibody attached to
an insoluble solid phase, but a labeled analyte (rather than a labeled
antibody)
competes with the analyte present in the test sample for binding to the
immobilized
antibody. Similarly, an immobilized analyte can compete with the analyte of
interest
for a labeled antibody: In these competitive assays, the quantity of captured
labeled
reagent is inversely proportional to the amount of analyte present in the
sample.
There are a number of assay devices and procedures wherein the presence
of an analyte is indicated by the analyte's binding to a labeled reagent or a
complementary binding member that is immobilized on a solid phase such as a
dipstick, teststrip, flow-through pad, paper, fiber matrix or other solid
phase material.
Such a specific binding reaction results in a distribution of the labeled
reagent
between that which is immobilized upon the solid phase and that which remains
free. Typically, the presence or amount of analyte in a test sample is
indicated by
the extent to which the labeled reagent becomes immobilized upon the solid
phase.
The use of porous teststrips in the performance of specific binding assays is
also well-known. In a sandwich assay procedure, a test sample is applied to
one
portion of the teststrip and is allowed to migrate through the strip material
by means
2
CA 02314546 2000-07-26
of capillary action. The analyte to be detected or measured passes through the
teststrip material and is transported into a detection zone on the teststrip
wherein
the analyte-specific binding member is immobilized. The extent to which the
analyte
becomes bound in the detection zone is then determined.
A variety of binding methods have been used to remove an analyte from a
test solution. U.S. Patent No. 4;020,151 describes a solid-phase assay for the
quantitation of antigens or antibodies in a test sample. The sample antigen or
antibody is adsorbed directly onto a solid support surface, such as anion
exchange
resin, and the support is then exposed to a labeled specific binding member
that is
immunologically reactive with the sample antigen or antibody.
Other assay methods involve the use of auxiliary specific binding members.
U.S. Patent No. 4,624,930 describes a process for determining the presence of
a
polyvalent antigen by incubating the antigen with three receptors; a first and
a third
receptor which bind to the antigen and a second receptor, bound to a solid
support,
which specifically binds to the first receptor.
Examples of devices utilizing some of the above principles are described in
U.S. Pat. Nos. 5,866,322, 5,663,055, 4,094,647, 4,235,601, 4,361,537,
4,366,241,
4,740,468, 4,879,215, 4,956,275, 4,059,407, 3,802,842, 3,915,639, 4,689,309,
4,168,146, 4,435,505, 4,594,327, 4,757,004, 4,956,302, 4,020,151, 4,145,406,
4,211,763, 4,362,697, 4,517,288, 4,624,930, 4,343,896, 4,880,751, 4,298,685,
4,935,147, 4,948,726, 4,959,303, 4,990,442, 4,839,231, 4,780,409, and
4,530,900,
which are incorporated herein by reference.
As will be appreciated by the foregoing discussion, there is significant
activity
3
CA 02314546 2000-07-26
in the field of diagnostics. There is an ever-growing demand for techniques
and
devices that are relatively simple and inexpensive to use.
Summary of the Invention
The present invention is directed to a method for diagnosing human
influenza A or B comprising the steps of a) providing a test sample suspected
of
containing influenza A or influenza B virus; b) contacting the test sample
with a solid
phase to which viral antigen binds; c) contacting the solid phase with anti-
influenza
A antibodies coupled to a first enzyme and anti-influenza B antibodies coupled
to
a second enzyme; d) contacting the solid phase with substrates for the first
and
second enzymes; and e) determining the presence or amount of viral antigen by
visibly detecting a colored precipitate deposited on the solid phase by action
of at
least one of the enzymes.
Brief Description of the Drawings
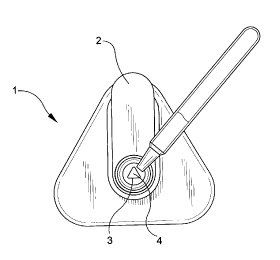
FIGS. 1 through 4 generally illustrate an assay according to the present
invention.
FIG. 1 shows a sample being applied to a flow controller of a Direc6c~en~
(Becton
Dickinson) influenza detection device.
FIG. 2 shows a wash solution being applied to the flow controller.
FIG. 3 shows a positive test result indicating the presence of influenza A
antigen.
FIG. 4 shows a negative test result indicating no presence of influenza A
antigen.
4
CA 02314546 2000-07-26
Detailed Description of the Invention
The present invention may be used to detect the presence of human
influenza A and B in a clinical specimen. The clinical samples that are tested
in the
invention typically will be nasal, pharyngeal, nasopharyngeal or respiratory
secretions collected as wash, expectorate, aspirate or swab specimens.
Reagents which can be utilized in the present invention can be prepared as
described in the Direcfi ecLn~ Flu A products .insert, incorporated herein by
reference, except that in a preferred embodiment, thimerasol can be
substituted for
sodium azide.
An example of the kinds of reagents which can be used in a preferred
embodiment of the invention, and how they can be used, is set forth below as
follows.
Reagent A (extraction reagent) -- as in DirectiqenO Flu kit, but 0.2%
thimerasol can be used in place of sodium azide.
Extraction, 1.6% mucolytic agent and 6.4% detergent, with 0.2%
thimerasol (preservative).
Reagent 1 -- no change (no azide)
Wash, 150 mM citric acid.
Reagent 2 -- as in Directig~en~ Flu kit, but 0.2% thimerasol can be used in
place of sodium azide.
Wash, 50 mM Tris and rabbit IgG with 0.2% thimerasol (preservative).
s
CA 02314546 2000-07-26
Reagent 3 -- Anti-influenzaA monoclonal antibodies (2) coupled to alkaline
phosphatase and anti-influenza B monoclonal antibody (1) coupled to
horseradish peroxidase (Pierce 31497) in Tris buffered diluent.
Reagent 4 -- as in Direcfiqen~ Flu kit but 0.2% thimerasol can be used in
place of sodium azide.
Wash, 5% butanol, 2M urea and 100 mM Hepes with 0.2% thimerasol
(preservative).
Reagent 5 -- as in Directiqen~ Flu kit but 0.2% thimerasol can be used in
place of sodium azide.
Wash, 50 mM Tris and 150 mM NaCI with 0.2% thimerasol
(preservative).
Reagent 6 -- Alkaline phosphatase substrate (Moss, Inc. Product No. INTH
- 1000)
Reagent 7 -- KPL HRP substrate (50-77-00) TMB membrane peroxidase
substrate system.
In a preferred embodiment, antigens which can be utilized in the present
invention are: for Flu B, B Lee 40 strain; and for Flu A, Flu A virus of the
subtype
H1N1.
Solid phase devices utilized in the present invention can be selected, for
example, from Direcfiqen~ test kits.
6
CA 02314546 2000-07-26
It is well-known to those skilled in the art that anti-influenza A and anti-
influenza B antibodies may be coupled with enzymes. Such enzymes include, for
example, peroxidase, urease, galactosidase, glucose oxidase, alkaline
phosphatase
and beta-glucuronidase.
S
Referring to the FIGURES, the assay of the present invention is performed
according to the following scheme:
1. Once the sample is extracted, it is applied to the flow controller (2) of
a DirectigenO influenza detection device (1 ). The flow controller (2) is
provided with
a triangular opening(3) and the sample flows through the opening (3) into a
nylon
membrane (4). The nylon membrane (4) is supported on an absorbent material,
thus, the sample, which flows through the nylon membrane (4) permitting
antigen
to bind non-specifically to the membrane (4) in the shape of a triangle, also
flows
into the absorbent material.
2. The flow controller (2) with its triangular opening (3), is removed from
the device (1). The device (1) with nylon membrane (4) is provided with a
circular
opening(6).
3. A wash solution is then applied to the nylon membrane (4) of the
device (1 ).
4. A tris-buffered diluent containing anti-influenza antibodies conjugated
to enzymes is then applied. If the influenza antigen is present, the
antibodies will
bind thereto in the shape of a triangle.
5. Wash solutions are applied and then a substrate appropriate for the
CA 02314546 2000-07-26
enzymes is applied. If influenza antigen is present, a colored triangle (7)
will be
visible.
Preferably, the assay will detect influenza A and influenza B by a
S differentiating test. At step 5 above, the solution is provided with
influenza A and
influenza B antibodies conjugated to different enzymes. The presence of a
specific
antigen would be detected by the color of the triangle produced.
The differentiating test might utilize alkaline phosphatase conjugated to
influenza A antibody and horseradish peroxidase conjugated to influenza B
antibody. At step 5 above, a substrate for alkaline phosphatase would be added
and if influenza A antigen is present, an orange triangle will result. In a
preferred
embodiment the substrate mixture for alkaline phosphatase can be
bromochloroindolyl phosphate and p-iodonitrotetrazolium. If no influenza A
antigen
is present, the nylon membrane will remain uncolored. A substrate for
horseradish
peroxidase would then be added. In a preferred embodiment, the substrate
mixture
for horseradish peroxidase can be 3, 3', 5, 5'-tetramethyl-benzidine and
hydrogen
peroxide. If influenza B antigen is present, a blue triangle will result. If
no influenza
B antigen is present, the nylon membrane will remain uncolored. Most
preferably,
influenza A and influenza B substrates are combined as a single reagent
thereby
avoiding the need for sequential addition of reagents.
Example 1
This example illustrates the differentiated method of detecting the presence
of
human influenza A or human influenza B according to the assay of the present
invention with the following steps:
s
CA 02314546 2000-07-26
1. Devices from a DirectiqenO Flu kit were used in the experiments.
2. Antigen preparations consisted of a preparation of a Flu A virus of the
subtype H1N1,and a preparation of Flu B Lee 40.
The negative control was detergent-treated egg fluid (non-infected) with
0.2% sodium azide.
3. Reagent solutions were prepared as for the DirectiaenQ Flu A kit except
that
sodium azide was replaced with thimerasol. The solutions were dispensed
into dropper vials in the volumes detailed in the Product Insert from the
DirecticLenO Flu A kit.
4. The negative control, Flu A antigen, and Flu B antigen were mixed
separately
with extraction reagentA as described in the DirecficLenO Product Insert; four
(4) drops each of antigen and negative control were dispensed into a tube
and followed by eight (8) drops of reagent A.
5. Extracted Flu A antigen was dispensed dropwise into the device and allowed
to absorb completely.
6. Reagent 1 was added to the device until the well was filled and allowed to
absorb completely.
7. The flow controller was removed and Reagent 2 [four (4) drops] was applied
to the device membrane. The reagent was allowed to absorb completely.
8. Four (4) drops of Reagent 3 (anti-Flu A/alkaline phosphatase and anti-Flu
9
CA 02314546 2000-07-26
B/peroxidase) were dispensed onto the membrane and the device was
allowed to stand for two (2) minutes after the reagent had absorbed.
9. Reagent 4 was dispensed until the device well was filled and allowed to
absorb completely.
10. Reagent 5 [four (4) drops] was dispensed onto the well and allowed to
absorb.
11. Reagent 6 [four (4) drops], the substrate for alkaline phosphatase, were
dispensed onto the membrane and the device allowed to stand for five (5)
minutes. An intense orange triangle was observed.
12. Reagent 7 [four (4) drops], the peroxidase substrate was dispensed, and
the
device allowed to stand for five (5) minutes. There was no change in the
color of the triangle.
13. Reagent 5 [four (4) drops] was dispensed onto the membrane and allowed
to absorb completely.
Example 2
The experiment was repeated as in Example 1 (with steps 1-13), but with Flu
B antigen utilized in place of Flu A antigen (at step 5). In this case, no
triangle was
observed at step 11. A dark blue triangle was observed at step 12.
The experiment was repeated with negative control solution at step 5. In this
io
CA 02314546 2000-07-26
case, no triangle was observed at step 11 or step 12.
While this invention is satisfied by embodiments in many different forms,
there is described in detail preferred embodiments of the invention. It is to
be
understood that the present disclosure is to be considered exemplary of the
principles herein, it is not intended to limit the invention.
a