Note: Descriptions are shown in the official language in which they were submitted.
CA 02315256 2000-06-15
WO 99/312b2 PCTNS981Z6823
NEEDLE FREE INJECTION OF FORMULATED NUCLEIC ACID MOLECULES
Statement of Related Applications
This application is related to U.S. Patent Application entitled "NUCLEIC ACID
TRANSPORTERS FOR DELIVERY OF NUCLEIC ACIDS INTO A CELL" Serial
Nwnber 08/484,777, filed June 7,1995, International Patent Application
entitled
'~1TJCLEiC ACID TRANSPORTERS FOR DELIVERY OF NUCLEIC ACIDS INTO A
CELL" No. PCT/US96/05579 filed April 23, 1996 and U.S. Patent Application
"TRANSPORTERS FOR SPECIFIC DELIVERY OF MACROMOLECULES TO
CELLS" Serial Number 60/045,295, filed May 2, 1997 all of which are
incorpon~ted
herein by reference in their entirety, including any drawings.
Introduction
The present invention relates to products and methods useful for delivering
formulated nucleic acid molecules by needle-free injection.
The following information is presented solely to assist the understanding of
the
reader, and none of the information is admitted to describe or constitute
prior art to the
claims of the prescnt invention.
In the past, non-viral administration of nucleic acids ~n vivo has been
pursued by a
variety of methods. These include lipofectin/liposome fusion: Proc. Natl. Acad
Sci.,
Volume 84, pp. 7413-7417 (1993); polylysine condensation with and without
adenovirus
enhancement: Human Gene Therapy, Volume 3, pp. 147-154 {1992); and
..a
transferrinaraasferrin receptor delivery of nucleic acid to cells: Proc. Natl.
Acad Sci.,
Volume 87, pp. 3410-3414 (1990). The use of a specific composition consisting
of
polyacrylic acid has been disclosed in WO 94/24983. Naked DNA has been
administered
as disclosed in International Patent Publication No. WO 90/11092.
CA 02315256 2000-06-15
WO 99/31262 PCT/US98/Z6823
2
Since the introduction of the needle-syringe, injection technology has
remained
virtually unchanged. Once considered a simple and innocuous part of medical
therapy,
injections now take oa ominous consequences. Needlestick injuries can be a
life
threatening event. Jet injection, or the injection of proteins by aerosol
pressure, was
introduced into clinical use in 1947 (Military~l~Iedicine, June, S 16-524,
1963), and a base
of scientific data has validated the safety and effcacy of this method of
injection (New
Zealand Medical Journal, Vol. 95. p.815, 1983). Current aerosol pressure
injectors are
calibrated to allow the user to select the accurate depth of penetration, and
are available
with syringes designed for intramuscular, subcutaneous or epidermal
injections.
Use of aerosol pressure injections to immunize rabbits to HbsAg protein has
been
shown to be superior to classical syringe and needle methods, and resulted in
a nearly 4
fold increase in antibody production at eight weeks when compared with a
nornial syringe
and needle (Davis et al., Vaccine vo1.12(16):1503-91994). In the Davis et al.,
study the
DNA was suspended in endotoxin free Dulbeccos's PBS and the area to be
injected was
not pretreated.
The transfection of tissue by aerosol injection has been reported as being
superior
to needle injection methods (Kerr et al., Journal of Cellular Biochemistry
Supplement 0
(21A). 1995). It has also been shown that aerosol injection of rabbit
papilloma virus
genome into rabbit epithelium results in the induction of papillomas (Brandsma
et al.,
Proc. natl. Acad Sci. USA 88. 4816-4820. 1991). Aerosol injection has been use
to
introduce human cytomegalovirus immediate early gene i enhancer/ promoter
sequences,
bacterial chloramphenicol acetyltransferase, whey acidic protein promoter
sequences, and
the bacterial -galactosidase gene through the skin surface so as to transfect
skin, muscle,
fat and mammary tissue of living animals (Forth et al., Analytical
biochemistry, 205, 365-
368, 1992). It has been stated that suspending aerosol-injected DNA constructs
in
solutions which enhance cellular uptake of DNA may increase the number of
cells
transfected in a single injection (Forth et al., supra).
DNA bound by precipitation methods to inert particles have been delivered by
needle-free injection. Coating inert particles, such as gold microprojectiles,
and injecting.
them by needle-free devices offers direct injection into the cells due to the
projectile
carrier penetrating the cell. Tang et al., inoculated mice with DNA encoding
human
CA 02315256 2000-06-15
WO 99/31262 PCT/US98126823
3
growth hormone coated gold micropmjectiles and stated that this method was
superior to
needle injection of plasmids encoding human growth hormone. Tang et al,
reports that
this technique simplifies the procedure and shortens the time required to
produce
antibodies to particular proteins by eliminating steps for protein
purification and adjuvant-
administration. (Tang et ai., Nature, Vol. 356152-154 1992)
Summary Of the Invention
This invention features compositions and methods for enhancing the
administration to and uptake of nucleic acids in a mammal. An efficient
strategy for
enhancing needle-free delivery of nucleic acids in vivo is to protect the
nucleic acid from
degradation, thereby maintaining the administered nucleic acid at the target
site in order to
further increase its cellular uptake. The data presented herein demonstrates
that the
combinaxion of formulated nucleic acid molecules and needle free injection
methods are
synergistic, providing the desired antibody response to the resulting
expressed protein that
is unexpectedly high when compared with either needle-free injection of non-
formulated
nucleic acids or needle injection of formulated nucleic acids.
The invention provides a method to deliver formulated nucleic acid molecules
through and/or to the skin of a mammal by using an apparaxus configured and
arranged to
administer molecules by air, or mechanical pressure through and/or to the skin
of a
mammal. Thus, the present invention allows for superior delivery of nucleic
acid
molecules into cells in vivo by the combination of a needle free device and
formulated
nucleic acid molecules. Furthermore, the present invention also allows for
treatment of
diseases, vaccination, and treatment of muscle disorder and serum protein
deficiencies.
In a first aspect, the present invention features a method for delivering a
nucleic
acid molecule formulated with a tramsfection facilitating agent through and/or
to the skin
of a mammal by the use of a needle-free injection device. Preferably, the
needle-free
device is configured and arranged to cause aerosol delivery of the formulated
nucleic acid
through and/or to the skin of the marnmal.
By "delivery" or "delivering" is meant transportation of nucleic acid
molecules to
desired cells or any cells. The nucleic acid molecules will be delivered to
multiple cell
lines, including the desired target. Delivery results in the nucleic acid
molecules coming
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/268Z3
4
in contact with the cell surface, cell membrane, cell endosome, within the
cell membrane,
nucleus or within the nucleus, or any other desired area of the cell from
which transfection
can occur within a variety of cell lines which can include but are not limited
to; epithelial
cells, Langerhan cells, Langhans' cells, littoral cells, keratinocytes,
dendritic cells,
S macrophage cells, kupffer cells, lymphocytes and lymph nodes. Preferably,
the nucleic
acid molecule can be delivered thmugh and/or to the skin by aerosol pressure
and is not
significantly sheared.
The term "nucleic acid" as used herein refers to both RNA and DNA including:
cDNA, genomic DNA, plasmid DNA or condensed nucleic acid, nucleic acid
formulated
with cationic lipids, nucleic acid formulated with peptides, antisense
molecule, cationic
substances, RNA or mRNA. In a preferred embodiment, the nucleic acid
administered is
plasmid DNA which comprises a "vector". The nucleic acid can be, but is not
limited to, a
plasmid DNA vector with a eukaryotic promoter which expresses Human Growth
Hormone, such as in the example provided.
The term "formulated" as used herein means the process by which nucleic acid
molecules are combined with a transfection facilitating agent in a manner
which makes the
nucleic acid molecules more stable, protected, and therefore, more easily
transferable.
The term "transfection facilitating agent" as used herein refers to an agent
that
forms a complex with the nucleic acid. This molecular complex is associated
with nucleic
acid, molecule in either a covalent or a non-covalent manner. The transfection
facilitating
agent should be capable of h~ansporting nucleic acid molecules in a stable
state and of
releasing the bound nucleic acid molecules into the cellular interior. The
transfection
facilitating agent should also be capable of being bound to nucleic acid
molecules and
lyopholized or freeze dried and either rehydrated prior to needle-free
delivery or delivered
as a fine powder via needle-free delivery.
in addition, the transfection facilitating agent may prevent lysosomal
degradation
of the nucleic acid molccules by endosomal lysis. Furthermore, the
transfection
facilitating agent allows for efficient transport of the nucleic acid molecule
through the
cytoplasm of the cell to the nuclear membrane and into the nucleus and provide
protection.
In a preferred embodiment transfection facilitating agents are non-condensing
polymers, oils and surfactants. These may be suitable for use as compounds
which
CA 02315256 2000-06-15
WO 99/31262 PGT/US98I26823
prolong the localized bioavailability of a nucleic acid:
polyvinylpyrrolidones;
poiyvinylalcohols; propylene glycols; polyethylene glycols; polyvinylacetates;
poloxamers
(Pluronics)(block copolymers of propylene oxide and ethylene oxide, relative
amounts of
the two subunits may vary is different poloxamers); poloxamines (Tetronics);
ethylene
vinyl acetates; celluloses, including salts of c~rboxymethylcelluloses,
methylcelluloses,
hydroxypropylcelluloses, hydroxypropylmethylcelluloses; salts of hyalumnates;
salts of
alginates; heteropolysaccharides (pectins); dextrans; chitosans;
phosphatidylcholines
(lecithins); miglyols; polylactic acid; polyhydroxybutyric acid. Some of these
compounds
may be used as protective, interactive, non-condensing compounds and others as
sustained
release compounds, while some may be used in either manner under the
respectively
appropriate conditions.
In another embodiment cationic condensing agents such as cationic lipids,
peptides, or lipopetides may associate with the nucleic acid molecule and may
facilitate
transfection.
In a further embodiment some of these compounds may be covalently attached to
gold particles and thereby bind with nucleic acid molecules of the present
invention. Gold
particles coated with a polymer or polymers of the present invention can
deliver nucleic
acid molecules into cells by penetrating the cell when delivered by needle-
free injection
device.
The term "protects" or "protective" or "protected" as used herein refers to an
effect
of the interaction between such a compound and a nucleic acid such that the
rate of
degradation of the nucleic acid is decreased in a particular environment,
thereby
prolonging the localized bioavailability of the nucleic acid molecule. Such
degradation
may be due a variety of different of factors, which specifically include the
enzymatic
action of a nuclease. The protective action may be provided in different ways,
for
example, by exclusion of the nuclease molecules or by exclusion of water.
The compounds which protect the nucleic acid and/or prolong the localized bio-
availability of a nucleic acid may achieve one or more of the following
effects, due to their
physical, chemical or rheological properties: ( 1 ) Protect nucleic acid, for
example plasmid
DNA, from nucleases due to steric, viscosity, or other effects such as
shearing; (2) increase
the area of contact between nucleic acid, such as plastnid DNA, through
extracellular
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/Z6823
b
matrices and over cellular membranes, into which the nucleic acid is to be
taken up; (3)
concentrate nucleic acid, such as plasmid DNA, at cell surfaces due to water
cxclusion; (4)
indirectly facilitate uptake of nucleic acid, such as plasmid DNA, by
disrupting cellular
membranes due to osmotic, hydrophobic or lytic effects; and (5) indirectly
facilitate uptalo~
of nucleic acids by allowing diffusion of protected nucleic acid chains
through tissue at the
administration site.
By "prolonging the localized bioavailability of a nucleic acid" is meant that
a
nucleic acid when administered to an organism in a composition comprising such
a
compound will be available for uptake by cells for a longer period of time
than if
administered in a composition without such a compound, for example when
administered
in a saline solution. This increased availability of nucleic acid to cells
could occur, for
example, due to increased duration of contact between the composition
containing the
nucleic acid and a cell or due to protection of the nucleic acid fiom attack
by nucleases.
The compounds which prolong the localized bioavailability of a nucleic acid
are suitable
for internal administration.
By "suitable for internal administration" is meant that the compounds are
suitable
to be administered within the tissue of an organism, for example within a
muscle or within
a joint space, epidermally, intradermaily or subcutaneously. Properties making
a
compound suitable for internal administration can include, for example, the
absence of a
high level of toxicity to the organism as a whole.
The term "needle-free injection device" as used herein relates to an apparatus
that
is capable of injecting an aerosol through and/or to the skin of a marnmal
into the tissue by
air and/or mechanical pressure. It is understood that conventional devices of
this type are
calibrated to allow one of ordinary skill in the art to select and/or adjust
the desired
injection depth and therefore it is expected that future devices that perform
this function
will also be calibrated in the same manner. It is also understood that devices
of this type
may have a needle which is only used io collect a solution which is
subsequently
aerosolized, and delivered by needle-free means. The type of injection device
is not
considered a limiting aspect of the present invention. The primary importance
of a needle-
flee device is, in fact, the capability of the device to deliver an aerosol of
formulated
nucleic acid molecules through and/or to the skin of a mammal. The needle-flee
injection
CA 02315256 2000-06-15
WO 99/31262 PCT/US98/26823
7
device can include, for example, a Gene Gun or a Needle-Less Injector as
described in
U.S. Patent S,4$0,381 or a powder delivery device such as in PCT
W0/097/134652.
The term "apparatus" as used herein relates to the set of components that upon
combination allow the delivery of an aerosol through andlor to the skin of a
mammal. The;
S components can be a nozzle or needle-free syringe with which one can collect
and/or
administer the formulated nucleic acid molecules, and a pump or spring for
creating air
pressure which forcibly evacuates the formulated nucleic acid molecules from
the nozzle
or needle-free syringe in a manner that creates an aerosol capable of
penetrating the skin of
a mammal. To create air pressure the apparatus can employ gas pressure, gas
spring or
spring force.
Preferably, the apparatus is capable of being calibrated to allow selection of
depth
of delivery. Hence, delivery can occur through the skin or to the skin.
The term "aerosol" as used herein is a suspension of formulatcd nucleic acid
molecules in the form of a particulate mist. Aerosols have been defined as
colloidal
1 S systems consisting of very finely subdivided liquid or solid particles
dispersed in and
surrounded by a gas. The aerosol of the present invention can depend upon the
power of a
liquified or compressed gas or mechanical spring to generate the fine mist of
formulated
nucleic acid molecules. Particles of an aerosol can range from less than 1 to
SO m. The
particles are said to remain suspended in the air for relatively long periods
of time. The
size of the particles can be measured by conventional methods such as the
Milken Oil
Drop Experiment for measuring aerosol particle size, but the need to determine
specific
size is falling into disuse (Sciarra, et al., in Remington's Pharmaceutical
Sciences, 18th ed.
chapter 92, 1990). The aerosol can be a liquid, a powder, or a heterogeneous
mist
comprising both a liquid and solid phase.
2S The term "skin" refers to the outer covering of a mammal consisting of
epidermal
and den~rral tissue and appendages such as sweat ducts and hair folicles. Skin
can
comprise the hair of a mammal in cases where the mammal has an epidermis which
is
covered by hair. In mammals which have enough hair to be considered fiu or a
pelt it is
preferable to shave the hair, leaving primarily skin.
The team "mammal" refers to any warm blooded organism. Preferably the
mammal is a human.
CA 02315256 2000-06-15
WO 99131262 PCTNS98/26823
8
In preferred embodiments the method results in an immune response, preferably
a
humoral immune response targeted for the protein product encoded by the
nucleic acid
molecule, such as an antibody response that is preferably at least 3 times
greater than an
antibody response caused by needle injection of a protein product encoded by a
nucleic
acid molecule suspended in saline, and at lean 10 times greater than an
antibody response
caused by needle injection of a nucleic acid molecule formulated with a
transfection
facilitating agent. In other situations the immune response preferably is a
cytotoxic T-
lymphocyte response.
The term "immune response" as used herein refers to the mammalian natural
defense mechanism which can occur when foreign material is internalized. The
immune
response can be a global immune response involving the immune system
components in
their entirety. Preferably the immune response results from the protein
product encoded
by the formulated nucleic acid molecule. The immune response can be, but is
not limited
to; antibody production, T-cell proliferation /differentiation, activation of
cytotoxic T-
lymphocytes, and/or activation of natural killer cells. Preferably the immune
response is a
humoral immune response. However, as noted abave, in other situations the
immune
response, preferably, is a cytotoxic T-lymphocyte response.
The term "humoral immune response" refers to the production of antibodies in
response to internalized foreign material. Preferably the foreign material is
the protein
product encoded by a formulated nucleic acid molecule internalized by
injection with a
needle free device.
In an additional embodiment the needle-free device is selected from the group
consisting of Mediject, Bioject, Gent Gun, Mesoflash, Ped-O-ject and Powder-
Ject.
Generally, it is understood that such a device is accompanied by directions
for usage.
In another aspect the invention features a kit. The kit includes a provider
for
providing a nucleic acid molecule formulated with a transfection facilitating
agent and a
needle-free means for delivering the nucleic acid molecule through and/or to
the skin of a
The "provider" can be ions furnished to allow one of ordinary skill in the
art to make formulated nucleic acid molecules. The instructions will furnish
steps to make
the compounds used for formulating nucleic acid molecules. Additionally, the
instructions
CA 02315256 2000-06-15
WO 99/31262 PCT/US98I26823
9
will include methods for testing the formulated nucleic acid molecules that
entail
establishing if the formulated nucleic acid molecules are damaged upon
injection from the
needle-free device. The provider can also be the formulated nucleic acid
molecules
themselves.
The term "transfection" as used herein, refers to the process of introducing
DNA
(e.g., formulated DNA expression vector) into a cell, thcreby, allowing
cellular
transformation. Following entry into the cell, the transfected DNA may: ( 1 )
recombine
with that of the host; (2) replicate independently as a plasmid or temperate
phage; or (3) be
maintained as an episome without replication prior to elimination.
As used herein, "transformation" relates to transient or permanent changes in
the
characteristics (expressed phenotype) of a cell induced by the uptake of a
vector by that
cell. Genetic material is introduced into a cell in a form where it expresses
a specific gene
product or alters the expression or effect of endogenous gene products.
Transformation of the cell may be associated with production of a variety of
gene
products including protein and RNA. These products may function as
intracellular or
extracellular structural elements, ligands, hormones, neurotransmitters,
growth regulating
factors, enzymes, chemotaxins, serum proteins, receptors, carriers for small
molecular
weight compounds, drugs, immunomodulators, oncogenes, cytokines, tumor
suppressers,
toxins, tumor antigens, antigens, antisense inhibitors, triple strand forming
inhibitors,
ribozymes, or as a ligand recognizing specific structural determinants on
cellular structures
for the purpose of modifying their activity. This list is only an example and
is not meant
to be limiting.
In another aspect, the invention features a method for making a kit.
Preferably the
method involves the step of combining a provider for providing a nucleic acid
formulated
with a transfection facilitating agent and a needle-free means for delivering
the nucleic
acid to a mammal. The provider for providing a nucleic acid can be
instructions for
formulating nucleic acid molecules or simply the formulated nucleic acid
molecules.
In yet another aspect, the invention also features a method for treating a
mammal
that is suffering from a disorder conventionally treated by administering
human growth
hormone. The method requires administering a nucleic acid molecule encoding
human
CA 02315256 2000-06-15
WO 99131262 pCT/US98/26823
gmwth hormone and formulated with a transfection facilitating agent through
and/or to the
skin of the mammal by use of a needle-free device.
In another aspect, the invention features a method for treating a mammal that
is
suffering from a cancer by administering a nucleic acid molecule encoding the
appropriate;
5 cancer antigen. The method requires administering a nucleic acid molecule
encoding a
cancer antigen and formulated with a transfection facilitating agent thmugh
and/or to the
skin of the mammal by use of a needle-free device.
Preferably, the cancer is melanoma and the appropriate cancer antigen is MAGE
1.
In yet another aspect, the invention also features a method for treating a
mammal
10 that is suffering from an infectious disease by administering a nucleic
acid molecule
encoding an antigen for the infectious disease. The method requires
administering a
nucleic acid molecule encoding an antigen for the infectious disease and
formulated with a
transfection facilitating agent through andlor to the skin of the mamznal by
use of a needle-
free device.
In a preferred embodiment the infectious disease is chronic hepatitis and the
antigen is HBV core antigen.
Administration as used herein refers to the mute of introducing the formulated
nucleic acid molecules of the invention into the body of cells or organisms.
Administration includes the use of aerosol pressure as provided by a needle
free device to
targeted areas of the mammalian body such as the muscle and the lymph nodes.
Prior to administration, the nucleic acid molecules of the invention can be
formulated with at least one other type of molecule. For example, the
molecular
complexes can be formulated with other molecules such as polyvinyl-pyrrolidone
as
described herein. Formulation techniques are provided herein by example.
In a prefen~ed embodiment administration is directed through the epiden~nis,
intradermis, and subcutaneous layer to the muscle tissue. The present
invention
administers formulated nucleic acid molecules in a manner which causes contact
with
various mammalian cell types which are not contacted by conventional needle
injection
techniques during one injection. Cell lines contacted by injection with the
present
invention are, but are not limited to, epithelial cells, langerhan cells,
keratinocytes,
dendritic cells, macrophage cells, kupfer cells, lymphocytes and lymph nodes.
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/26823
11
Another aspect of the present invention features a mcthod for modifying inert
particles by first washing the inert particles in fuming nitric acid solutions
or in solutions
of nitric acid mixed with sulfuric acid, and then combining the inert
particles with cysteine
terminated cationic DNA binding peptides to form a monolayer peptide coating.
Preferably, the DNA binding peptide recognizes and binds a specific nucleic
acid sequence
of a designed plasmid. Hence, one molecule of DNA binding peptide binds with
an inert
particle and a plasmid, therefore reducing the amount of crosslinking between
inert
particles. The inert particles can be but are not limited to gold particles.
Preferably, the
inert particles are combined with nucleic acid molecules and delivered through
the skin of
a mammal to cells by a needle-free device.
The term "inert particles" refers to biologically inactive particles which can
be but
are not limited to particles from the group consisting of, ferrite crystals,
gold particles or
beads, tungsten spheres, other metals or biologically inactive compounds which
are of a
density of roughly I S - 20g/cm3 and a size of mughly 1-3 m. Generally, the
optimum
particle is small enough to produce minimal cell damage. The size of particles
is not a
limiting aspect. It is known by those of ordinary skill in the art that the
particle must be
large enough to acquire sufficient momentum to penetrate the cell: momentum
being a
function of size density and velocity, and by convention particle size should
be roughly 10
times smaller than the target cell.
A further aspect of the invention features a method for delivering a nucleic
acid
molecule formulated with a modified inert particle through and/or to the skin
of a mammal
by the use of a needle-free injcction device. Preferably, the needle-free
device is
configured and arranged to cause aerosol delivery of the formulated nucleic
acid through
and/or to the skin of the mammal.
. The summary of the invention described above is not limiting and other
features
and advantages of the invention will be apparent from the Following detailed
description of
the invention and from the claims.
Brief Descri 'on c~f'Ifie Drawings
The drawings will herein briefly be described.
CA 02315256 2000-06-15
WO 99/31262 PCTlUS98/26823
12
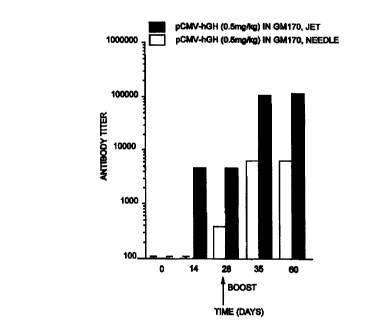
Figure 1 shows the humoral immune response to hGH in dogs after needle and
needle-free injection of transfection facilitating agent formulated pCMV-hGH
in dogs.
The antibody titer for each data point is an average for two dogs.
Figure 2 shows a comparison between pCMV-hGH suspended in saline and
transfection facilitating agent formulated pCI~V-hGH for the elicitation of
humoral
immune response to hGH in dogs after needle-free injection. The antibody titer
for each
data point is an average for two dogs.
Figure 3 shows the amounts of expressed Luciferase in conditions where a
plasmid
encoding CMV-Luciferase is formulated with modified gold particles that are
coated or
uncoated with Cys-Tyr-Lys-ala- (Lys)g -Trp-Lys (CK8). The formulations are
then loaded
on a Kapton carrier membrane in ethanol, water or precipitated with Ca2+ and
then loaded
in ethaaol.
fled Description of the Preferred Embodiments
The delivery of formulated nucleic acid molecules by the use of a needle free
device represents a novel approach to gene delivery. The present invention
offers a
nucleic acid delivery apparatus that provides an increased immune response
when
compared to previous methods. The invention provides the advantage of allowing
the
uptake of formulated nucleic acid molecules by a wide variety of cell types
simultaneously. Injecting formulated nucleic acid molecules by needle free
device results
in the formulated nucleic acid molecules directly contacting many more cell
types than in
conventional needle injection. Thus, the present invention provides an
enhanced delivery
of nucleic acid molecules and also provides a more e~cient gene delivery
system which
can be used to generate an immune response.
Needle-free delivery of formulated nucleic acid molecules through and/or to
the
skin of a mammal, depends on several factors which are discussed below,
including
transfection efficiency and the composition of the formulated nucleic acid
molecule.
I. DNA Infection Variables
The intensity of an immune response and the level of gene delivery and
expression
achieved with the present invention can be optimized (>5-fold effect over
controls) by
CA 02315256 2000-06-15
WO 99/31262 PCT/US98/268Z3
13
altering the following variables. The variables are: the formulation
(composition, plasmid
topology), the technique and protocol for injection (angle of injection, state
of muscle),
and, the pretreatment of the muscle with myotoxic agents. An immune response
can be
measured by, but is not limited to, the amount of antibodies produced for a
protein
encoded and expressed by the injected nucleic acid molecule.
Other injection variables that can be used to significantly effect the levels
of
antibodies and/or cytotoxic T-lymphocytes produced in response to the protein
encoded by
the formulated nucleic acid molecule provided by the needle-free injection
method of the
present invention are the state of the muscle being injected and injection
technique.
Examples of the variables include muscle stimulation, muscle contraction,
muscle
massage, delivery angle, and apparatus manipulation. Massaging the muscle may
force
plasmid out of the muscle either directly or via lymphatic drainage. By
altering the depth
of penetration and/or the angle at which the needle-flee device is placed in
relation to
muscle fibers the present invention improves the plasmid distribution
throughout the
injection area which subsequently increases the antibody response to the
protein which is
encoded and expressed by the plasmid.
Needle flee injection systems pmvide an attractive method for administration
of
plasmid DNA for the purpose of intramuscular immunization. Not only do they
provide
the general benefit of avoiding needle-stick injury, but they may produce
better
distribution of injected substances in the muscle. Direct gene transfer was
first
demonstrated on mammary tissue using the Ped-O jet system, but this apparatus
was
designed largely for intradennal or subcutaneous injections (Forth et al.,
Anal. bioclrem.
205:365-368., 1992).
We have found that needle-free injection of formulated nucleic acids can be
superior to classical needle injection in a dog model. We injected a plasmid,
suspended in
saline, containing the gene for Human Growth Hormone by needle-free and needle
injection to dog muscle and measured antibody production. We found that with
needle
free injection at 14 and 28 days and prior to boost resulted in a measurable
antibody titer
versus needle injection which resulted in an almost undetectable titer.
CA 02315256 2000-06-15
WO 99/31262 PCTIUS98/26823
14
II. Nucleic acid based vaccines
The present invention can be used to deliver nucleic acid vaccines in a more
efficient manner than is conventionally done at the present time. Nucleic acid
vaccines, or
the use of plasmid encoding antigens or therapeutic molecules such as Human
Growth
Hormone, has become an area of intensive research and development in the last
half
decade. Comprehensive reviews on nucleic acid based vaccines have been
published
[M.A. Liu, et al.(Eds.), 1995, DNA Vaccines: A new era in vaccinology, Vol.
772, Ann.
NY. Acad. Sci., New York; Kumar, V., and Sercarz, E., 1996, Nat. Med. 2:857-
859;
Ulmer, J.B., et al., (Fds.) Current Opinion in Immunology; 8:531-536. Vol.
772, Ann. NY.
Acad. Sci., New York]. Pmtective immunity in an animal model using plasmid
encoding
a viral protein was first observed in 1993 by Ulmer et al. [Ulmer, J.B., et
al., 1993, Science
259:1745-1749]. Since then, several studies have demonstrated protective
immunity for
several disease targets and human clinical trials have been started. Many
disease targets
have been investigated. Examples include antigens of Borrelia burgdorferi, the
tick-borne
infectious agent for Lyme disease (Luke et al., J. Infect. Dis. 175:91-97,
1997), human
immunodeficiency virus-1, (Letvin et al., Proc. Nat. Acod Sci. USA 94:9378-
9383, 1997),
B cell lymphoma (Syrengelas et al., Nature Medicine. 2:1038-41, 199, Herpes
simplex
virus (Bourne et al., J. Infectious dis. 173:800-807, 1996), hepatitis C virus
(Tedeschi et
al., Hepatology 25:459-462, 1997), rabies virus (Xiang et al., virology,
209:569-579,
1995), Mycobacterium tuberculosis (Lowrie in Genetic Vaccines and
Immunotherapeutic
Strategies CA Thibeault, ed. Intl Bus Comm, Inc., southbomugh, MA 01772 pp. 87-
122,
1996), and Plasmodium falciparum (Hoffman et al., Vaccine 15:842-845, 1997).
An important goal of gene therapy is to effect the uptake of nucleic acid by
cells,
thereby causing an immune response to the pmtein encoded by the injected
nucleic acid.
Uptake of nucleic acid by cells is dependent on a number of factors, one of
which is the
length of time during which a nucleic acid is in proximity to a cellular
surface. The
present invention provides formulations which increase the length of time
during which a
nucleic acid is in proximity to a cellular surface, and penetrate the cell
thereby delivering
nucleic acid molecules into the cell.
Nucleic acid based vaccines are an attractive alternative vaccination strategy
to
subunit vaccines, purified viral protein vaccines, or viral vector vaccines.
Each of the
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/2b823
traditional approaches has limitations that are overcome if the antigens) is
expressed
directly in cells of the body. Furthermore, these traditional vaccines are
only protective in
a strain-specific fashion. Thus, it is very difficult, and even impossible
using traditional
vaccine approaches to obtain long lasting immunity to viruses that have
several sera types~r
5 or viruses that are prone to mutation.
Nucleic acid based vaccines offer the potential to produce long lasting
immunity
against viral epitopes that are highly conserved, such as with the
nucleoprotein of viruses.
Injecting plasmids encoding specific proteins by the present invention
results.in increased
immune responses, as measured by antibody production. Thus, the present
invention
10 includes new methods of providing nucleic acid vaccines by delivering a
formulated
nucleic acid molecule with a needle-free device as described herein.
The efficacy of nucleic acid vaccines is enhanced by one of at least three
methods:
(1) the use of delivery systems to increase the stability and distribution of
plasmid within
the muscle, (2) by the expression (or delivery) of molecules to stimulate
antigen
15 presentation/tcansfer, or (3) by the use of adjuvants that may modulate the
immune
response.
Polvmeric and non- ,pQlvmeric formulations for ~asmid del~verv to muscle
The present invention provides polymeric and non-polymeric formulations which
address problems associated with injection of nucleic acids suspended in
saline. Plasmids
suspended in saline have poor bioavailability in muscle due to rapid
degradation of
plasmid by extracellular nucleases. One possible approach to overcome the poor
bioavailability is to protect plasmid from rapid nuclease degradation by
condensing the
plasmid with commonly used cationic complexing agents. However, due to the
physiology of the muscle, the use of rigid condensed particles containing
plasmid for
efficient transfection of a larger number of muscle cells has not been
successful to date.
Cationic lipid and polylysine plasmid complexes do not cross the external
lamina to gain
access to the caveolae and T tubules [Wolff, J.A., et al., 1992, J. Cell. Sci.
103:1249-1259].
Thus, the strategy identified for increasing the bioavailability of plasmid in
muscle
was to: protect plasmid from rapid extracellular nuclease degradation,
disperse and retain
CA 02315256 2000-06-15
WO 99131262 PCTNS98/26823
16
intact plasmid in the muscle, and facilitate the uptake of plasmid by muscle
cells. Two
specific methods of accomplishing this, which preferably are used in
conjunction with
needle-free delivery, are (a) the use of protective, interactive, non-
condensing systems (b)
the use of modified gold particles.
Delivery and expression of nucleic adds is limited due to degradation of the
nucleic acids by components of organisms, such as nucleases. Thus, protection
of the
nucleic acids when delivered in vivo can greatly enhance the resulting
expression, thereby
enhancing a desired pharmacological or then~peutic effect. It was found that
certain types
of compounds which interact with a nucleic acid (e.g., DNA) in solution but do
not
condense the nucleic acid provide ~n vivo protection to the nucleic acid, and
correspondingly enhance the expression of an encoded gene product. A detailed
description of the formulations that can be used in the present invention can
be found in
PCT Application No. PCT/US96/05679 which is hereby incorporated as a reference
in its
entirety including any drawings. As noted above, preferably such formulations
are
delivered by using a needle free device as described herein.
Inert particles coated with DNA have been used to deliver genes to cells. An
advantage of coating inert particles, for instance gold beads, with nucleic
acids is that the
particle carrier actually penetrates the cell. Hence, the nucleic acid is
delivered to the
interior of the cell and should become incorporated and expressed in a more
efficient
manner. However, the conventional procedure for preparation of DNA-coated gold
particles results in heterogeneous distribution of DNA from particle to
particle, which
produces variable expression from cell to cell (Butow et al., Meth Enzymol.
264:265-278,
1996).
It is undesirable to create nucleic acid vaccines which pmduce variable
results from
sample to sample. Thus, the present invention provides gold particles
uniformly coated
with DNA by covalently attaching cysteine terminated DNA binding peptides to
the gold
surface. Furthermore, the invention enhances the intracellular transport of
DNA released
from the gold particles by non-covalent association of high affinity DNA
binding peptides,
which contain nuclear localization sequences, with the plasmid. The
formulation provides
the most reproducible amount of plasmid on gold particles, independent of the
diameter of
CA 02315256 2000-06-15
WO 99/31262 PCT/US9$/26823
17
the particle. Preferably such modified gold particle formulations are
delivered by using a
needle-free device as described herein.
VI Diseases and Conditions for Intramuscutar p,~ De]ivg~y
The present invention described herein can be utilized for the delivery and
expression of many different coding sequences. In particular, the demonstrated
effectiveness for the PINC systems (PCT Application No. PCT/US96/05679) for
delivery
to muscle indicate that such formulations are effective for delivery of a
large variety of
coding sequences to muscle by needle free injection. Specific suggestions for
delivery of
coding sequences to muscle with the needle free device of the present
invention include
those summarized in Table 1 below.
Table 1: Applications for Plaamid-Based Gene Therapy
by Intramnscalar Injection
Muscle and nerve dborden References are numbered as they are cited in U.S.
Application No. PCT/US96N5679, which hu beeo
incorporated by reference in its entirety.
Duehonne's muscular dystrophy Acsadi 1991 [5], Karpati 1993 [6J, Miller 1993
[7j
Myotrophic disorders (IGF-I) Coleman 1997 [8], Alila 1997 [9]
Neumtrophic disorders (IGF-I) Alila 1997 [9], Rabinovsky 1997 [10]
2S Secretion of a:preaod protein
into the systemic circulation
Hemophiliac A and B Anwer 1996 [I1], Kuwahara-Rundell
1994 [12], Miller 1994
[131
30 l.rythropoictin-responsive Tripathy 1996 [14]
Pituitary dwarfism Anwer 1996 [l l], Dahler 1994
[!5]
1-Antittypsin defiaency Levy 1996 [I6]
Autoimmune and Inflammatory Raz 1993 [17]
diseases
Hypercholesterolema Fazio 1994 [18J
35 Hypotension Ma 1995 [191
Hypertension Xiong 1995 [20]
CA 02315256 2000-06-15
WO 99/31262 PCT/US98/2b823
18
Nncleieweid vaccines
Herpes Simplex Virus Manickan 1995 [21], Ghiasi 1995
[22], McClements 1996
[23], Kriesel 1996 [24]
S Hepatitis B Virus Davis 1993 [25], Davis 1994 [2b],
Davis 1996 [27]
Influenza Virus Donnelly 1995 [28], Ulmer 1993
[29], Uhner 1994 [30]
Tuberculosis Ld~vtie 1994 [31], Tascon, 1996
[32]
Human immunodeflciency Virus Shiver 1995 [33], Coney 1994
[34], Wang 1993 [35]
Cancer Raz 1993 [I7], Russell 1994 [36]
1 U Maleria Hoffman 1995 [37j, Sedegah 1994
[38]
Hepatitis C virus Major 1995 [39], Lagging 1995
[40]
Flavivirus Phillpotts ! 996 [41 ]
C:ytom~alovirus Parade 1995 [42]
Salmonella typhi Lopez-Macias 1995 [43]
1$ Mycoplasma puhnonis Lai 1995 [44]
Rabies virus Xiang 1995 [45]
]~~CS
20 The following examples are offered by way of illustration and are not
intended to
limit the scope of the invention in any manner. One of ordinary skill in the
art would
recognize that the various molecules and/ or amounts disclosed in the examples
could be
adjusted or substituted by larger amounts (for larger scaled experiments) or
by inclusion of
a different Transfection Facilitating Agent.
Ezample 1
Demonstration of Transfection Facilitatinsz Agent Plasmid DNA Complex
Formation
~paration of PyP Formulated Nucleic Acid Molecules
Concentrated pDNA stock solutions were made by lyophilizing and rehydrating
pDNA with water to a final pDNA concentration of 3-Smg/ml. Formulations were
made
by aliquoting appropriate volumes of sterile stock solutions of pDNA, SM NaCL,
and
polymer to obtain a final pDNA concentration in an isotonic polymer salution.
Stock
solutions were added in the following order: water, plasmid, polymer, and SM
NaCI. The
plasmid and polymers were allowed to incubate at room temperature for 15
minutes prior
CA 02315256 2000-06-15
WO 99/31262 PCT/US98I268Z3
19
to adding salt or lactose for ionicity adjustments. Likewise, Na-citrate
buffers in 0.9%
NaCI were added after incubating the plasmid and polymers for 15 minutes at
room
temperature. The osmotic pressure of selected formulations was measured (n=3)
using a
Fiske One-Ten Micro-Sample Osmometer. The pH of all formulations was measured
using,
an Accumet Model 15 pH Meter and the viscosity of all formulations was musing
a Progrannmable Rheometer Model DV-III.
Dynamic dialysis was used with various interactive polymer formulations to
measure binding between PVP and plasmid DNA. One ml of formulations and
corresponding controls were place in prewashed dialysis sacs. The dialysis
sacs were
closed and suspended in stsrred saline solutions (100 ml) at 25°C. One
ml aliquots were
taken from the acceptor compartment over time and replaced with fresh media.
The
concentration of PVP in the diffused samples collected over time was measured
spectroscopically at 220 nm.
In all cases, the rate of PVP diffusion through the dialysis membrane was d
in the presence of plasmid DNA, indicating complex formation between PVP and
plasmid
DNA. The reduction in the diffusion rate for PVP in the presence of plasmid
DNA was
directly proportional to the initial amount of PVP in the dialysis sac. It was
also
determined that the sac volume remained constant during the duration of the
experiment
and that adherence of PVP to the membrane was negligible.
~~xration of Nucleic Acid Molecules Formulated With Modified Gold Particles
100 mg of 2.3 m gold particles were place in fuming nitric acid overnight at
23°C.
The particles were then washed exhaustively with 18 S2 deionized water, which
had been
degassed under vacuum. A solution of Cys-Tyr-Lys-Ala- (Lys)8-Trp-Lys (CK8) was
treated for 1 hr with l OmM dithiothreitol and then passed over a desalting
column
prepared in degassed water. Su~cient peptide to make the final solution 1mM
CK8 was
added to the cleaned gold particles and allowed to sit under N2 overnight at
23°C. The
particles were then washed with degassed water until there was no absorbance
at 280 nm
and then lyophilized. One pg of CMV-luciferase was added in water to 0.45 mg
of
modified gold particles, and then dried in a desiccator. Binding of the DNA to
the CK8
modified particles was shown by eluting with 1.5 mM phosphate, and visualizing
the
CA 02315256 2000-06-15
WO 99/31262 PCT/US98/26823
eluted relaxed and supercoiled plasmid on an agarose gel with ethidium
bromide. As a
control, DNA was precipitated with Cap''', dried and added to the center of
the Kapton
cagier membrane in 100% ethanol. DNA formulated with modified gold particle
was
transferred to the Kapton membrane in water and in 100% ethanol. (Fig. 3)
5 Significant iuciferase expression in vivq.was observed with DNA formulated
with
modified gold particles when the formulation was transferred to the Kapton
membrane in
both 100~/o ethanol and, more importantly, in water (fig 3). Although the
average
expression was lower using water as the transfer solvent, this result suggests
that it will be
possible to include proteins such as transcription factors with the DNA.
Ezample 2
N 1'
Device.
Formulated plasnuds were prepared as follows in a sterile manner in 2m1 single
dose
vials.
Plesmid/iriposomes (DOTMA:DOPE 1:1 m/m) 1:3 -/+ 20 g DNAImI
Plasmid/K8/TTS-1 1:3:1 -/+/- 20 g DNA/ml
pVP 5% w/v 20 g DNA/ml
PEG 10% w/v 20 g DNA/ml
Control:
DNA suspended in Saline 20 g DNAImI
DNA formulations were injected by needle-free device into SOmI polypropylene
tubes indicative of a "high impact" worst case scenario. Additionally the
needle free
device was set at maximum penetration as described in the product instruction
manual.
DNA stability was determined by agarose gel electrophoresis of the samples
before
and after injection. Measurement of stability was determined by the amount of
visible
degradation, i.e. smearing, and by quantitating supercoiled and open circular
DNA. Our
data indicates that even in this extreme example the formulated DNA samples
only exhibit
slight degradation.
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/26823
21
Eznmple 3
Demono~ of Formulated Nucleic Acid Stabili~,y Upgn_Ir~iection Through Variops
Needle Free Device Deliverv Nozzles
Formulated nucleic acid molecules were prepared as follows in a sterile
manner.
0.1 mg pCT0129/mL in DOTMA:Chol (1:1 ~m) 1:3 (-/+); 10%lactose
3.0 mg pCT0129/mL in S% PVP (50 kDa); 150 mM NaCI
Non-formulated samples:
0.1 mg pCT0129/mL in 150 mM NaCL
3.0 mg pCT0129/mL in 150 mM NaCL
Each of these formulations were injected through different size needle-free
injection
nozzles. A non-injected formulation was used as a control for each experiment.
The
formulation was injected into a 50mL conical tube. Each sample was agarose gel
electrophoresed after injection.
Measurement of stability was determined by the amount of visible degradation,
i.e.,
smearing, and by quantitating supercoiled and open circular DNA. Our data
indicates that
even in this example which uses the most extreme injection parameters the
nozzle size did
not effect the state of the formulated DNA.
Example 4
Elicitation of Immune Res~~onse Following~tramuscular Iniection~f plasmid.
In this study, a Human Growth Hormone {hGH) expression plasmid is injected
into
the muscle of dogs and pigs to examine the production of anti-hGH
antibodies.(figs 1,2).
The expression plasmid was injected via a needle free device or by a needle to
examine if the magnitude of the immune response is affected by the injection
method or
formulation.
For elicitation of immune response, pCMV-hGH plasmid was suspended in saline
or
formulated with PVP and injected into biceps femoris and semitendinosus
muscles of dogs
using a needle free device or 22 gauge needle. Blood was collected before
plasmid
injection and once a week after the injection. Blood samples were kept
overnight at 4° C,
centrifuged at 2000 g for 15 min and serum was collected for detection of anti-
hGH
antibodies by ELISA.
CA 02315256 2000-06-15
WO 99/31262 PCT/US98I26823
22
Significant levels of hGH antibodies were detectable in jet (aerosol) injected
animals 14 days after a single i.m. dose of hGH plasmid. The levels of hGH
antibodies
increased over time reaching a plateau by 21 days and remained elevated
throughout the
112 day pre-boost periods. Repeating the plasmid dose on day 112 augmented the
antibody response by 100 fold.
In comparison, the needle injection of hGH plasmid formulated with PVP did not
pmduce significantly detectable levels of hGH antibodies. A booster dose was
required to
achieve significant antibody response from needle injection. Peak antibody
levels from
aerosol injections were about 20 times higher than from needle injections.
Control
animals received CMV-CAT plasmid formulated with PVP without the hGH gene, and
the
animals did not produce anti-hGH antibodies.
Needle-free injection of hGH plasmid suspended in saline also leads to the
production of anti-hGH antibodies. A single dose of naked DNA was su~cient to
achieve
significant levels of hGH antibodies when administered via the needle-free
device.
I S In comparison, multiple doses were required to elicit antibody response
with needle
injection. The antibody response from needle-free injection was 5 times higher
compared
to needle injection.
In the same experiment, needle-fi~ee injection gave 15-20 times better immune
response compared to needle injection when the plasmid was formulated with
PVP.
One skilled in the art would readily appreciate that the present invention is
well
adapted to carry out the objects and obtain the ends and advantages mentioned,
as well as
those inherent therein. The molecular complexes and the methods, procedures,
treatments,
molecules, specific compounds described herein are presently representative of
preferred
embodiments are exemplary and are not intended as limitations on the scope of
the
invention. Changes therein and other uses will occur to those skilled in the
art which are
encompassed within the spirit of the invention are defined by the scope of the
claims.
It will be readily apparent to one skilled in the art that varying
substitutions and
modifications may be made to the invention disclosed herein without departing
from the
scope and spirit of the invention.
CA 02315256 2000-06-15
WO 99/31262 PCTNS98/26823
23
All patents and publications mentioned in the specification are indicative of
the
levels of those skilled in the art to which the invention pertains. All
patents and
publications are herein incorporated by reference to the same extent as if
each individual
publication was specifically and individually indicated to be incorporated by
reference.
The invention illustratively described h'grein suitably may be practiced in
the
absence of any element or elcments, limitation or limitations which is not
specifically
disclosed herein. Thus, for example, in each instance herein any of the teens
"comprising", "consisting essentially of and "consisting of may be replaced
with either
of the other two teens. The terms and expressions which have been employed are
used as
terms of description and not of limitation, and there is no intention that in
the use of such
terms and expressions of excluding any equivalents of the features shown and
described or
portions thereof, but it is recognized that various modifications are possible
within the
scope of the invention claimed. Thus, it should be understood that although
the present
invention has been specifically disclosed by prefen~ed embodiments and
optional features,
modification and variation of the concepts herein disclosed may be resorted to
by those
skilled in the art, and that such modifications and variations are considered
to be within the
scope of this invention as defined by the appended claims.
In addition, where features or aspects of the invention are described in teens
of
Markush groups, those skilled in the art will recognize that the invention is
also thereby
described in terms of any individual member or subgroup of members of the
Markush
group. For example, if X is described as selected from the group consisting of
bromine,
chlorine, and iodine, claims for X being bromine and claims for X being bmmine
and
chlorine are fully described.
Those references not previously incorporated herein by reference, including
both
patent and non-patent references, are expressly incorporated herein by
reference for all
purposes. Other embodiments are within the following claims.