Note: Descriptions are shown in the official language in which they were submitted.
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/26144
APPARATUS FOR REDUCING TISSUE VOLUMES BY THE USE OF ENERGY
This application is a continuation in part of U.S. Patent Application
No. 08/905,991, filed August S, 1997, which is a continuation-in-part of U.S.
Patent
Application No. 08/642,327, filed May 3, 1996, entitled "Method for Treatment
of
Airway Obstructions", which is in turn a continuation-in-part application of
U.S. Patent
Application No. 08/606,195, filed February 23, 1996, entitled "Method for
Treatment of
Airway Obstructions", which cross-references U.S. Patent Application No.
08/516,781,
filed August 18, 1995, entitled "Ablation Apparatus and System for Removal of
Soft
Palate Tissue", having named inventors Stuart D. Edwards, Edward J. Gough and
David L. Douglass, which is a continuation-in-part of U.S. Application No.
08/239,658,
filed May 9, 1994 entitled "Method for Reducing Snoring by RF Ablation of the
Uvula." This application is also related to an Application Serial No.
08/642,053, filed
5/3/96, entitled "Method and Apparatus for Treatment of Air Way Obstructions",
all
incorporated by reference herein.
Field of the Invention
This invention relates to an apparatus for the treatment of air way
obstructions,
and more particularly to an apparatus for creating selective cell necrosis in
interior
sections of selected head and neck anatomical structures of the human body
without
damaging vital structures.
Sleep-apnea syndrome is a medical condition characterized by daytime
hypersomnomulence, morning arm aches, intellectual deterioration, cardiac
arrhythmias,
snoring and thrashing during sleep. It is caused by frequent episodes of apnea
during
the patient's sleep. The syndrome is classically subdivided into two types.
One type,
termed "central sleep apnea syndrome", is characterized by repeated loss of
respiratory
effort. The second type, termed obstructive sleep apnea syndrome, is
characterized by
repeated apneic episodes during sleep resulting from obstruction of the
patient's upper
~1-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/Z6144
airway or that portion of the patient's respiratory tract which is cephalad
to, and does not
include, the larynx.
Treatment thus far includes various medical, surgical and physical measures.
Medical measures include the use of medications such as protriptyline,
medroxyprogesterone, acetazolamide, theophylline, nicotine and other
medications in
addition to avoidance of central nervous system depressants such as sedatives
or alcohol.
The medical measures above are sometimes helpful but are rarely completely
effective.
Further, the medications frequently have undesirable side effects and may be
contraindicated for some patients.
Surgical interventions have included uvulopalatopharyngoplasty, tonsillectomy,
surgery to correct severe retrognathia and tracheostomy. In one type of
surgical
intervention a standard LeFort I osteotomy is combined with a sagittal split
ramus
osteotomy to advance the maxilla, mandible and chin. Such a procedure may be
effective but the risk of surgery (e.g. morbidity and mortality) in these
patients can be
prohibitive and the procedures are often unacceptable to the patients.
Physical measures have included weight loss, nasopharyngeal airways, nasal
CPAP and various tongue retaining devices used nocturnally. These measures may
be
partially effective but are cumbersome, uncottifortable and patients often
will not
continue to use these for prolonged periods of time. Weight loss may be
effective but is
rarely achieved by these patients.
In patients with central sleep apnea syndrome, phrenic nerve or diaphragmatic
pacing has been used. Phrenic nerve or diaphragmatic pacing includes the use
of
electrical stimulation to regulate and control the patient's diaphragm which
is innervated
bilaterally by the phrenic nerves to assist or support ventilation. This
pacing is disclosed
in Direct Diaphragm Stimulation by J. Mugica et al. PACE vol. 10 Jan-Feb,
1987,
Part II; Prelimi»ary Test of a Muscular Diaphragm Pacing System o» Human
Patie»ts
by J. Mugica et al. from Neurostimulation: An Overview 1985, pp. 263-279; and,
Electrical Activation of Respiration by Nochomovitez IEEE Eng. in Medicine and
Biology, June, 1993.
However, it was found that many of these patients also have some degree of
obstructive sleep apnea which worsens when the inspiratory force is augmented
by the
pacer. The ventilation induced by the activation of the diaphragm also
collapses the
upper airway upon inspiration and draws the patient's tongue inferiorly down
the throat
choking the patient. These patients then require tracheostomies for adequate
treatment.
-2-
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
A physiological laryngeal pacemaker as described in Physiological Laryngeal
Pacemaker by F. Kaneko et al. from Trans Am Soc Artif Intern Organs, 1985, pp.
293-
296 senses volume displaced by the lungs and stimulates the appropriate nerve
to open
the patient's glottis to treat dyspnea. This apparatus is not effective for
treatment of
sleep apnea. The apparatus produces a signal proportional in the displaced air
volume
of the lungs and thereby the signal produced is too late to be used as an
indicator for the
treatment of sleep apnea. Also, there is often no displaced air volume in
sleep apnea
due to obstruction.
There are other surgical methods for the treatment of obstructive sleep apnea
but they also have medical drawbacks. Tracheostomy, while effective, carries
considerable morbidity and is aesthetically unacceptable to many patients.
Another
surgical procedure involves a standard Le Fort I osteotomy in combination with
a
sagittal split ramus osteotomy. However, this is a major surgical intervention
that
requires the advancement of the maxilla, mandible and china
Generally, there are two types of snoring. They are distinguished, depending
on
the localization of their origin. The first type of snoring, velar, is
produced by the
vibration of all of the structures of the soft palate including the velum, the
interior and
posterior arches of the tonsils and the uvula. Velar snoring results from a
vibration of
the soft palate created by the inspiratory flow of air, both nasal and oral,
which makes
the soft palate wave like a flag. The sound intensity of these vibrations is
accentuated
by the opening of the buccal cavity which acts as a sound box.
The second type, pharyngeal snoring, is a kind of rattle, including even horn
whistling. It is caused by the partial obstruction of the oropharyngeal
isthmus by the
base of the tongue with, now and again, its total exclusion by the tongue base
becoming
jammed against the posterior wall of the pharynx. This results in a sensation
of
breathing, apnea, which constitutes the sleep apnea syndrome. These two types
of
snoring may easily be combined in the same individual.
For some years there have been surgical techniques for correcting apnea.
However, maxillary surgery to cure pharyngeal snoring requires major surgery,
with the
operation lasting several hours, and the uvula-palatopharnygoplasty procedure
to conrect
velar snoring is not without draw backs. This explains the popularity of
prosthesis and
other preventive devices.
More recently, portions of the soft palate have been removed by laser
ablation;
however, there are several limitations to this procedure. First, if too much
tissue is
-3-
CA 02315842 2000-06-21
WO 99/32041 PCTNS98/26144
removed, severe consequences result. Also, the degree of laser ablation is
difficult to
control and multiple treatments are usually required. Finally, patients have a
high
degree of soreness in their throats for many weeks.
U.S. Patent No. 4,423;812 discloses a loop electrode design characterized by a
bare active wire portion suspended between wire supports on an electrode
shaft. Tissue
striping is performed with a bare wire, that is connected to an electrode
shaft insulated
to.prevent accidental burns to the patient. This allows the physician to use
these
insulated parts to help position and guide the active wire portion during the
surgical
procedure. However, this requires that the physician shave off, during
multiple
procedures; successive thin superficial layers of the obstructing tissues to
avoid gross
resection and its adverse affects.
U.S. Patent No. 5,046,512 discloses a method for the treatment of snoring and
apnea. The method regulates air flow to the user to an extent comparable to
the volume
of air which flows through the users nasal passages. An associated apparatus
provides a
device having a body portion sufficiently wide to separate the users teeth. It
includes an
air passage comparable in area to the area of the user's nasal passages.
The use of oral cavity appliances has been proposed frequently for the
treatment
of sleep disorders. It has been recognized that movement of the mandible
forward
relative to the maxilla can eliminate or reduce sleep apnea and snoring
symptoms by
causing the pharyngeal air passage to remain open. Several infra-oral dental
appliances
have been developed which the user wears at night to fix the mandible in an
anterior
protruded position. Such dental appliances essentially consist of acrylic or
elastomeric
bit blocks, similar to orthodontic retainers or athletic mouth guards, which
are custom
fitted to a user's upper and lower teeth. The device may be adjusted to vary
the degree
of anterior protrusion.
U.S. Patent 4,901,737 discloses an infra-oral appliance while reducing snoring
which repositions the mandible in an inferior, open, and anterior, protrusive,
position as
compared to the normally closed position of the jaw. Once the dentist or
physician
determines the operative snoring reduction position for a particular patient,
an
appropriate mold is taken for the maxillary dentition and of the mandibular
dentition to
form an appliance template. This device includes a pair of V-shaped spacer
members
formed from dental acrylic which extend between the maxillary and mandibular
dentition to form a unitary mouthpiece.
CA 02315842 2000-06-21
WO 99/32041
PCTNS98/26144
While such dental appliances have proven effective in maintaining the mandible
in a protruded position to improve airway patency, they often result in
undesirable side
effects. One of the most common side effects is aggravation of the
tempromandibular
joint and related jaw muscles and ligaments, especially in individuals who
have a
tendency to grind their teeth during sleep. Aggravation of the
tempromandibutar joint
has be associated with a wide variety of physical ailments, including migraine
headaches. Accordingly, many individuals suffering from sleep apnea and
snoring
disorders are not able to tolerate existing anti-snoring dental appliances for
long periods
of time.
Opening of obstructed nasal airways by reducing the size of the turbinates has
been performed using surgical and pharmaceutical treatments. Examples of
surgical
procedures include anterior and posterior ethmoidectomy, such as those
described in
"Endoscopic Paranasal Sinus Surgery" by D. Rice and S. Schaefer, Raven Press,
1988;
the writings of M. Wigand, Messerklinger and Stamberger; and, U.S. Patent No.
5,094,233. The Wigand procedure, described in U.S. Patent No. 5,094,233,
involves
the transection of the middle turbinate, beginning with the posterior aspect,
visualization
of the sphenoid ostium and opening of the posterior ethmoid cells for
subsequent
surgery. In the sphenoidectomy step, the ostium of the sphenoid is identified
and the
anterior wall of the sinus removed. Following this step, the posterior ethmoid
cells may
be entered at their junction with the sphenoid and the fovea ethmoidalis can
be
identified as an anatomical landmark for further dissection. In anterior
ethmoidectomy,
the exenteration of the ethmoids is carried anteriorly to the frontal recess.
Complications, such as hemorrhage, infection, perforation of the fovea
ethmoidalis or
lamina papyracea, and scarring or adhesion of the middle turbinate, have been
reported
in connection with these procedures.
One of the problems encountered as a result of these procedures is
postoperative
adhesion occurring between the turbinates and adjacent nasal areas, such as
medial
adhesion to the septum and lateral adhesion to the lateral nasal wall in the
area of the
ethmoid sinuses. Otherwise successful surgical procedures may have poor
results in
these cases. Some surgeons have proposed amputation of a portion of the
turbinate at
the conclusion of surgery to avoid this complication, resulting in protracted
morbidity
(crust formation and nasal hygiene problems). The turbinate adhesion problem
detracts
from these endoscopic surgical procedures. Efforts have been made to reduce
the
-5-
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
complications associated with the surgical treatment of turbinate tissue, for
example by
the use of a turbinate sheath device. U.S. Patent No. 5,094,233.
U.S. Patent No. 3,901,241 teaches a cryosurgical instrument which is said to
be
useful for shrinking nasal turbinates. U.S. Patent No. 3,901,241.
Pharmaceuticals have also been developed for reducing the size of the
turbinates. However, pharmaceuticals are not always efficacious and generally
do not
provide a permanent reduction in turbinate size. Additionally, pharmaceuticals
can have
adverse side effects and are contraindicated for some patients.
Clearly, a medical need exists for a method and device for clearing obstructed
nasal passageways. It is preferred that the method and device be performable
with
minimal surgical intervention or post operative complications. It is also
preferred that
the method and device reduce the size of the turbinate structure without
involving
surgical cutting or the physical removal of tissue. It is also preferred that
the method
and device provide a reduction in turbinate structure size to increase air
flow in the nasal
passageway sufficiently impairing blood flow to the optic nerve and/or retina
and create
a permanent impairment of vision by the ablation.
It would be desirable to provide an ablation apparatus which eliminates the
need
for dental appliances for the treatment of snoring and sleep apnea disorders.
It would
also be desirable to provide a treatment device which is not an infra-oral
dental
appliance, and which can effectively and safely remove selected portions of
the soft
palate without providing the patient with undesirable side effects. Further,
it would be
desirable to provide a tissue ablation device which creates localized pressure
at an
electrode introduction tissue site to make it easier for electrode
introduction into tissue.
It would yet further desirable to provide an ablation apparatus with a safety
stop that
reduces surface ablation at an electrode introduction tissue site.
CRY OF . n~tvFNTTO~
Accordingly, an object of the invention is to provide an apparatus for the
treatment of obstructed nasal and upper respiratory passage ways through the
use of
selective cell necrosis at a selected site of different head and neck
anatomical structures.
Another object of the invention is to provide an apparatus to treat airway
obstructions.
Yet another object of the invention is to provide an ablation apparatus that
provides controlled cell necrosis of upper airway anatomical structures.
-6-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/2G144
A further object of the invention is to provide an ablation apparatus that
applies
localized force at an electrode tissue introduction site.
Still another object of the invention is to provide an ablation apparatus that
minimizes surface cell necrosis at an energy delivery device tissue
introduction site.
These and other objects of the invention are achieved in a cell necrosis
apparatus to reduce the volume of a selected site of an anatomical structure.
An energy
delivery device is coupled to a distal portion of a handpiece. The energy
delivery device
has a tissue piercing distal end. A pressure plate is positioned at an
exterior of the
energy delivery device and a cable is coupled to the energy delivery device.
In another embodiment, a cell necrosis apparatus includes a handpiece and an
energy delivery device coupled to a distal portion of the handpiece. A safety
stop is
positioned at an exterior of the energy delivery device. A cable is coupled to
the energy
delivery device.
In yet another embodiment, An apparatus to reduce a volume of a selected site
1 S in an interior of an anatomical structure includes an introduces. An
energy delivery
device is at least partially positionable in the interior of the introduces. A
pressure plate
is positioned at an exterior of the introduces and a cable is coupled to the
energy
delivery device.
In still a further embodiment, an advancement member is coupled to the energy
delivery device.
In still yet another embodiment, an infusion lumen in the energy delivery
device
is coupled to medicinal solutions, irrigating solutions electrolyte solutions,
contrast
media and disinfectants via a disinfectant medium introduction member.
BRIEF DhSCRPTION OF T FI tIIRFc
Figures IA-B illustrate lateral view of the oral cavity and the positioning of
the
cell necrosis apparatus of the present invention in the oral cavity.
Figure 1 C depicts a lateral view of the oral cavity illustrating the
repositioning
of the tongue following treatment.
Figures 2A-B illustrate front and side perspective views of the pressure plate
shown in Figures IA-C.
Figures 3A-3B illustrate the creation of various cell necrosis zones.
Figure 4 illustrates the introduction of fluids into the necrosis zone using a
multi
aperture hollow energy delivery device.
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
Figure S illustrates the introduction of boluses solution to tissue.
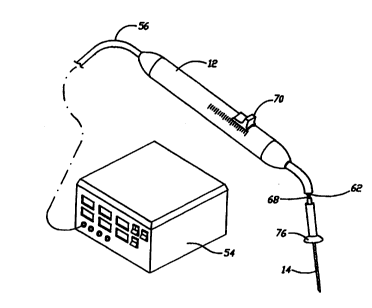
Figure 6A illustrates a perspective view of the cell necrosis apparatus of the
present invention coupled to an energy source.
Figure 6B illustrates a close up cross-sectional view of a hollow energy
delivery
device of the invention utilized to create a cell necrosis zone below a tissue
surface.
Figure 7 illustrates a cross-sectional view of the distal end of the energy
delivery
device of Figure 6B.
Figure 8 illustrates a cross-sectional view of the hollow energy delivery
device
with a sealing plug to control fluid flow.
Figure 9 illustrates the creation of cell necrosis zones in the uvula and the
repositioning of the uvula in the oral cavity.
Figure 10 illustrates the creation of cell necrosis zones in the turbinates
and the
repositioning of the turbinates in the nasal cavity.
Figure 11 illustrates a cross-sectional view of the arteries of the nasal
cavity.
Figure 12 depicts a cross-sectional view of the head illustrating the arteries
of
the nasal cavity.
Figure 13 depicts a cross-sectional view of the head taken laterally through
the
nasal cavity illustrating a shrinkage of the turbinates following treatment
with the cell
necrosis apparatus of the present invention.
Figure 14 depicts a close up cross-sectional view of Figure 13.
Figure 15 depicts a cross-sectional view of the head illustrating the creation
of
cell necrosis zones in the soft palate structure.
Figure 16 depicts a cross-sectional view of the soft palate structure of
Figure I 5
illustrating the repositioning of the soft palate structure following creation
of the cell
necrosis zones.
Figure 17A is a perspective view of an embodiment of the present invention
with the pressure plate positioned at an exterior of an introducer.
Figure 17B depicts a perspective and cross sectional view of the distal tip of
the
introducer.
Figure 17C is an enlarged view of the distal tip of the energy delivery
device.
Figure 17D depicts the flow path between the cell necrosis apparatus and the
infusion fluid reservoir.
Figure I 8 depicts a block diagram of the feed back control system that can be
used with the cell necrosis apparatus as shown in Figures lA-C.
_g_
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
Figure 19 depicts a block diagram of an analog amplifier, analog multiplexer
and microprocessor used with the feedback control system of Figure 19.
Figure 20 depicts a block diagram of the operations performed in the feedback
control system depicted in Figure 18.
I~ETAIL1;D D -.S('~RIpTION
Referring now to Figures lA-C, a cell necrosis apparatus 10 is used to reduce
the volume of a selected site in an interior of a head and neck structure, and
more
particularly to a structure that is associated with an airway passage.
Suitable anatomical
structures include but are not limited to the tongue, uvula, soft palate
tissue, tonsils,
adenoids, turbinate structures and the like. In Figures lA-C, cell necrosis
apparatus 10
is shown as including a handpiece 12 coupled to an energy delivery device 14.
1t will be appreciated that although the term "energy delivery device" in
includes but is not limited to a device for the delivery of electromagnetic
energy such as
RF, microwave and optical energy; a device for the delivery of acoustical
energy such as
ultrasonic energy; a device for the delivery of a thermal liquid jet; and, a
device for
performing resistance heating. The preferred energy source is an RF source and
electrode 14 is an RF electrode operated in either bipolar or monopolar mode
with a
ground pad electrode. In a monopolar mode of delivering RF energy, a single
electrode
14 is used in combination with an indifferent electrode patch that is applied
to the body
to form the other contact and complete an electrical circuit. Bipolar
operation is
possible when two or more electrodes 14 are used. Multiple electrodes 14 may
be used.
When the energy source is RF, an RF energy source may have multiple
channels, delivering separately modulated power to each electrode 14. This
reduces
preferential heating that occurs when more energy is delivered to a zone of
greater
conductivity and less heating occurs around electrodes 14 which are placed
into less
conductive tissue. If the tissue hydration or blood infusion in the tissue is
uniform, a
single channel RF energy source may be used to provide power for the treatment
of cell
necrosis zones relatively uniform in size.
Handpiece 12 can be a proximal portion of energy delivery device 14 that is
suitably configured to enable placement and removal of cell necrosis apparatus
to and
from a selected anatomical structure and may include, in one embodiment, a
proximal
portion of energy delivery device 14 that is insulated. A pressure plate I S
can be
positioned on an exterior surface of energy delivery device 14. Pressure plate
15
-9-
CA 02315842 2000-06-21
W0 99/32041
PCT/US98126144
includes a tissue interface surface 17 which can include all of a portion of
the indicated
surface depending on the amount of contact between the anatomical structure
surface
and tissue interface surface 17 which may be dependent on the amount of force
applied
to the surface of the anatomical structure.
Handpiece 12 and energy delivery device 14 are sized and of a suitable
geometry to be maneuverable in an oral cavity 16, pierce a tongue surface 18
and
advance into an interior 20 of a tongue 22 a sufficient distance 24 to a
tissue site 26.
Another embodiment of pressure plate 15 is as a safety stop such that depth of
tissue
penetration of energy delivery device 14 is controlled by pressure plate 15.
Electromagnetic energy is delivered to tissue site 26 to create cell necrosis
at zone 28
without damaging a main branch of the hypoglossal nerve. A cable 30 is coupled
to
energy delivery device 14. For purposes of this disclosure, the main branches
of the
hypoglossal nerve are those branches which if damaged create an impairment,
either
partial or full, of speech or swallowing capabilities. As shown in Figure 1 C,
the treated
structure of tongue 22 is repositioned in oral cavity 16. With this cell
necrosis, the back
of the tongue 22 moves in a forward direction (as indicated by the arrow) away
from the
air passageway. The result is an increase in the cross-sectional diameter of
the air
passageway.
Handle 14 is preferably made of an electrical and thermal insulating material.
When energy delivery device 14 is an electrode, the electrode can be made of a
conductive material such as stainless or a shaped memory metal, such as
Nitinol (a
nickel titanium alloy), commercially available from Raychem Corporation (Menlo
Park,
California) as well as numerous other companies. In one embodiment, only a
distal end
of electrode 14 is made of the shaped memory metal in order to effect a
desired
deflection.
Cell necrosis apparatus 10 can include visualization capability including but
not
limited to a viewing scope, an expanded eyepiece, fiber optics, video imaging,
and the
like.
Energy delivery device 14 can include an insulator 32 which can be adjustable
in length and in a surrounding relationship to an exterior surface of energy
delivery
device 14. Insulator 32 serves as a barrier to thenmal or RF energy flow.
Insulator 32
can be in the form of an sleeve that may be adjustably positioned at the
exterior of
energy delivery device 14. In one embodiment insulator can be made of a
polyamide
material and be a 0.002 inch shrink wrap. The polyamide insulating layer is
semi-rigid.
-10-
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
That portion of energy delivery device 14 which is not insulated is an energy
delivery
surface 33.
I-Iandpiece 12 can have a reduced diameter at a distal portion 34 to
facilitate
positioning, maneuverability, provide easier access to smaller openings and
improve the
visibility in the area where energy delivery device 14 is to penetrate.
To use cell necrosis apparatus 10 in oral cavity 16, a topical and then a
local
anesthetic is applied to tongue 22. After a suitable period for the anesthesia
to take
effect, the physician may grasp the body of tongue 22 near the apex, using a
gauze pad
for a better grip. Tongue 22 is then drawn forward, bringing the body and the
root of
tongue 22 further forward for improved accessibility. Grasping handpiece 12,
the
physician positions a distal portion of energy delivery device 14 at tongue
surface 18.
The position of energy delivery device 14 in Figures I A-C illustrates cell
necrosis zone
28 below a mucosal surface 36 providing a protected zone 38. An insulated
portion 40
of energy delivery device 14 prevents delivery of energy to a main branch of a
hypoglossal nerve and/or to mucosal surface 36.
Energy delivery device 14 can have an angle 42 at a bend zone 44 which is
lateral to a longitudinal axis of handpiece 12. Energy delivery device 14 can
be
malleable to create different bend zones, depending on the anatomical
structure and the
insertion position of the anatomical structure. With the use of a bending
fixture, not
shown, the arc of angle 42 can be adjusted by the physician as needed at the
time of
treatment.
One or more sensors 46 can be included and positioned at a distal end of
energy
delivery device 14, at a distal end of insulator 32, as well as at other
positions of cell
necrosis apparatus 10. Sensor 46 is of conventional design, including but not
limited to
thermistors, thermocouples, resistive wires, and the like. Suitable sensors
that may be
used for sensor 46 include: thermocouples, fiber optics, resistive wires,
thermocouple IR
detectors, and the like. Suitable thermocouples for sensor 46 include: T type
with
copper constantene, J type, E type and K types.
Energy delivery device 14 can experience a steep temperature gradient as
current moves outward through the energy delivery device 14. This causes the
tissue
that is immediately adjacent to energy delivery device 14 to reach
temperatures of 100
degrees C or more while tissue only 5 to 10 mm away may be at or near body
temperature. Because of this temperature gradient, it is often necessary to
position
energy delivery device 14 several times at the intended insertion site or use
a plurality of
-11-
CA 02315842 2000-06-21
WO 99/32041
PCTNS98/26144
energy delivery devices 14 to create a cell necrosis zone 28 of the desired
volume.
Because of the aggressive heating immediately proximal of energy delivery
device 14
desiccation of tissue adjacent to energy delivery device 14 may result. When
the fluid
within the tissue is desiccated, no electrical current flows through the
tissue and the
heating is then terminated. This problem can be solved by using lower energy
delivery
rates (e.g. power) to energy delivery device 14, in turn reducing the rate of
temperature
increase in adjacent tissue. This solution requires extended treatment
periods.
Referring now to Figures 2A and 2B, pressure plate 1 S has an exterior
geometry
section selected from a planar surface, a curved surface, a concave surface, a
convex
surface and combinations thereof. One embodiment is a convex curved shape,
including hemispherical in order to minimize trauma to tissue adjacent to the
tissue
insertion site. Pressure plate 15 will also have an aperture for the
advancement and
retraction of electrode 14. The preferred planar geometry of pressure plate 15
is
circular. The preferred tissue contact surface area of pressure plate 1 S is
between 0.005
to 0.250 inches. Pressure plate 15 will also be made of an electrically non-
conductive
material in order to electrically isolate tissue insertion site from all
sources of electrical
current other than that delivered by energy delivery device 14. Tissue
interface surface
17 applies a force to energy delivery device insertion site of the anatomical
structure.
This force can compress and/or immobilize the tissue at the energy delivery
device
insertion site to facilitate a penetration of the energy delivery device 14
into the
anatomical structure. Pressure plate 15 can be adjustably mounted on an
exterior of
energy delivery device 14. Pressure plate can be configured to allow the
advancement
and retraction of the energy delivery device 14. Pressure plate 15 can be
positioned
against the tongue, uvula, soft palate, tonsils and turbinate in order to
facilitate entry of
energy device 14 into the interior of each structure with minimal trauma to
surface
tissue.
Figures 3A and 3B illustrate cell necrosis zones 28 and insulator 32 in
greater
detail. Referring now to Figure 4, an embodiment of the invention is disclosed
where
energy delivery device 14 includes a hollow lumen 48 and a plurality of
apertures
through which a fluid medium can flow. Suitable fluid mediums include but are
not
limited to cooling and heating fluids, electrolytic solutions, chemical
ablation medium, a
disinfectant medium and the like.
A suitable electrolytic solution is saline, solutions of calcium salts,
potassium
salts, and the like. Electrolytic solutions enhance the electrical
conductivity of the
-12-
CA 02315842 2000-06-21
WO 99/32041
PCT/US98/26144
tissue. When a highly conductive fluid is infused into tissue, the electrical
resistance is
reduced and the electrical conductivity of the infused tissue is increased.
With this
condition there will be little tendency for tissue surrounding energy delivery
device 14
to desiccate and the result is a large increase in the capacity of the tissue
to carry RF
energy. A zone of tissue which has been heavily infused with a concentrated
electrolytic
solution can then become so conductive as to actually act as an electrode. The
effect of
the larger (fluid) electrode is that greater amounts of current can be
conducted, making it
possible to heat a much greater volume of tissue in a given time period.
In addition to the larger electrode area that results from infusion of an
electrolytic solution it is then possible to inject one or more boluses 50 of
electrolytic
solution as shown in Figure 5. RF current 52 can then flow through the infused
tissue
surrounding electrode 14 and follow the course of least electrical resistance
into the
infused tissue of the neighboring bolus.
By placing the injections of electrolytic solution according to the need for
thermal tissue damage, a single electrode 14 may deliver heating to a large
volume of
tissue and the shape of cell necrosis zone 28 created may be placed to create
cell
necrosis in exactly the area desired. This simplifies the control of cell
necrosis zone 28
generation and allows the physician to produce larger lesions in a brief
session.
Additionally, the conductivity of the injected electrolytic solution can be
decreased. While the advantages of avoiding desiccation adjacent to electrode
14 are
maintained, higher electrical resistance is encountered in the infused tissue.
This results
in greater heating in the tissue closer to electrode 14. Varying the
electrical conductivity
of the infused tissue can be used to adjust the size of cell necrosis zone 28
and control
the extent of thermal damage.
Disinfectant mediums can also be introduced through energy delivery device
14. Suitable disinfectant mediums include but are not limited to Peridex, an
oral rinse
containing 0.12% chlorhexidine glucinate (I, I'-hexanethylenebis[5-(p-
chlorophenyl)
biganide) di-D-gluconate in a base containing water, 11.6% alcohol, glycerin,
PEG 40
sorbitan arisoterate, flavor, dosium saccharin, and FD&C Blue No. 1. The
disinfectant
medium can be introduced prior to, during and after cell necrosis.
Referring now to Figures 6 through 8, energy delivery device 14 may include
hollow lumen 48 that is in fluid communication with a control unit 54 which
controls
the delivery of the fluid via a conduit 56 configured to receive a cooling or
heating
-I3-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/26144
solution. All of only a portion of a distal portion 14' of energy delivery
device 14 is
cooled or heating.
The introduction of a cooling fluid reduces cell necrosis of surface layers
without the use of insulator 32. This preserves surface mucosal and/or
epidermal layers
as well as protects a tissue site in the vicinity or in cell necrosis zone 28
from receiving
sufficient energy to cause cell necrosis. For instance, it may be desirable to
insert
energy delivery device 14 into an organ in a position which is adjacent to, or
even
within, some feature that must be preserved while treating other areas
including but not
limited to blood vessels, nerve bundles, glands and the like. The use of
cooling permits
the delivery of thermal energy in a predetermined pattern while avoiding
heating critical
structures.
A sealing plug 58 may be positioned in hollow energy delivery device 14 and
used to determine the length of energy delivery device 14 that receives the
cooling fluid.
Sealing plug 58 can include one or more sealing wipers 60 positioned on an
outer
diameter of sealing plug 50. A fluid tube 62 is coupled to a proximal portion
of sealing
plug 58 and positioned adjacent to the proximal surface of sealing plug 58. A
plurality
of fluid distribution ports 64 are formed in fluid tube 62. Cooling fluid,
which may be a
saline solution or other biologically compatible fluid, is fed from control
unit 54 through
a small diameter dual lumen tube positioned in conduit 56. Cooling fluid flows
through
fluid tube 62 to the most distal end where it exits through fluid distribution
ports 64
arranged about the outer diameter of fluid tube 62. Cooling fluid then flows
within
hollow lumen 48 and is in direct contact with the wall structure of energy
delivery
device 14, which is typically metallic and provides a highly efficient heat
transfer.
Cooling fluid flows to the proximal end of energy delivery device protected a
14 and
through the second lumen of the fluid tube 62, then to control unit 54 which
includes
both a supply reservoir and a return reservoir to catch and retain the used
cooling fluid.
Energy delivery device 14 may have one or more sensors 46 for sensing the
temperature of the tissue. This data is fed back to control unit 54 and
through an
algorithm is stored within a microprocessor memory of control unit 54.
Instructions are
sent to an electronically controlled micropump (not shown) to deliver fluid
through the
fluid lines at the appropriate flow rate and duration to provide control of
tissue
temperature.
The reservoir of control unit 54 may have the ability to control the
temperature
of the cooling fluid by either cooling the fluid or heating the fluid.
Alternatively, a fluid
-14-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/Z6144
reservoir of sufficient size may be used in which the cooling fluid is
introduced at a
temperature at or near that of the normal body temperature. Using a thermally
insulated
reservoir, adequate control of the tissue temperature may be accomplished
without need
of refrigeration or heating of the cooling fluid.
Cooling zone 66 is adjustable in size and location by moving sealing plug 58
using a stylet 68 which is controlled by a slider 70 position at handpiece 12.
In this
manner, the position of cooling zone 66 can be moved along the length of
energy
delivery device 14 and the area which is cooled is then proximal of sealing
plug 58. In
the event it is desirable to have cooling zone 66 within a length of energy
delivery
device 14 then a second sealing plug 58 can be positioned at a distance
proximal of the
first or distal sealing plug 58 and the cooling fluid then re-enters the
second lumen of
fluid tube 62 at proximal sealing plug 58. The distance between the two
sealing plugs
58 determines the length of cooling zone 66. In this example, the distal and
proximal
sealing plugs 58 move together when activated by stylet 68, re-positioning
cooling zone
66.
In another embodiment, the distal and proximal sealing plugs 58 are adjusted
individually. This provides the ability to both change the position and length
of cooling
zone 66.
In typical use, cooling zone 66 is positioned so that a predetermined
thickness
of mucosal or epidermal tissue 72 on the surface of the tissue to be treated
74 is
protected as indicated at 75 while the desired cell necrosis zone 28 is
formed.
An alternative feature is the ability to indicate to the physician the amount
of
energy delivery device 14 length that is inserted into the tissue and the
depth of
protected area 32. To accomplish this, a portion of cell necrosis apparatus 10
comes in
contact with mucosal or epidermal surface 72. This can be achieved with a
contact
collar 76 or with a larger surface that is contoured to fit against the organ
or anatomical
feature to be treated. The dimensional relationship between contact collar 76
and
handpiece 12 is maintained by a sleeve 78 through which energy delivery device
14,
fluid tube 62 and stylet 68 all pass. With this dimensional relationship
maintained, it is
then possible to indicate with indexing pointers on handpiece 12 the distance
of energy
delivery device 14 distal of contact collar 76 or the surface of cell necrosis
apparatus 10.
The distance of cooling zone 66 is then positioned distal of contact collar 76
or the
surface cell necrosis apparatus 10. Because all cooling is within energy
delivery device
-15-
CA 02315842 2000-06-21
WO 99132041 PCTNS98/Z6144
14 and external insulator 32 is not used, energy delivery device 14 penetrates
easily
through the tissue without drag or resistance that is present when insulator
32 is present.
In another embodiment, sealing plugs 58 and direct flow of cooling fluid are
replaced with a slidable inner cooling plug which may be constructed of a
material with
efficient heat transfer characteristics. Suitable cooling plug materials
include but are not
limited to copper, beryllium copper, silver and aluminum alloys. Cooling plug
is sized
to fit intimately against the inner surface of needle 14. This allows transfer
of heat from
energy delivery device 14 to cooling the plug. In this embodiment, cooling
plug has
interior passageways through which cooling fluid passes. This draws heat from
the
cooling plug.
Although this embodiment does not provide the highly efficient cooling
available by having the cooling fluid in direct contact with the inner surface
of energy
delivery device 14, a more thorough isolation of the cooling fluid from the
body is
provided. This results from reducing the possibility of experiencing some
leakage past
sealing plug 58 of the other embodiment.
In yet another embodiment of cooling, heat pipe technology is used. A sealed
compartment contains a mixture of gases which have the ability to rapidly
vaporize and
condense at temperatures which facilitate the transfer of heat with high
efficiency. In
this embodiment, a cooling module within handpiece 12 cools the proximal end
of the
tubular heat pipe and heat is conducted from cooling zone 6b to the cooling
module.
Cell necrosis apparatus 10 can be used to create cell necrosis in other
structures
that affect airway passages including but not limited to the uvula, turbinate
structures,
soft palate structures and tonsils.
As shown in Figure 9, cell necrosis apparatus 10 is used to create one or more
cell necrosis zones 28 in uvula 80. Energy delivery device 14 is configured to
be
maneuverable in oral cavity 16, pierce an uvula exterior surface, advance into
an interior
of the uvula a sufficient distance 84 to a tissue site, deliver
electromagnetic energy to the
tissue site and create controlled cell necrosis. The creation of cell necrosis
zones 28
repositions the treated uvula 80 in oral cavity 16 (as indicated by the
arrows) while
substantially preserving an uvula mucosal layer 82 at an exterior of uvula 80.
Cell
necrosis zones 28 are created in uvula 80 without creating an ulceration line
at a tip 86
of uvula 80. Controlled cell necrosis tightens and reshapes uvula 80.
In creating uvula 80, energy delivery device 12 can have a variety of
geometric
configurations and may include a curved distal end. The different cell
necrosis zones 28
-16-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/26144
can be stacked in one or more treatment sessions. This permits the physician
to control
the amount of tissue treated and to assess the results of each session before
proceeding
with additional procedures. Because exterior mucosal tissue is spared, the
patient
experiences little pain or discomfort.
Referring now to Figure 10, cell necrosis apparatus 10 is used to create cell
necrosis zones 28 in a turbinate structure 88, which can include the interior
nasal
conchs, the middle nasal conchs, the superior nasal conchs, and combinations
thereof.
Energy delivery device 14 is configured to be maneuverable in a nostril,
pierce a
turbinate structure surface 90 advance into an interior of turbinate structure
88 a
sufficient distance to a tissue site, deliver electromagnetic energy to the
tissue site and
create controlled cell necrosis of turbinate structure 88 to increase the size
of a nasal
passageway 90.
Sufficient electromagnetic energy is delivered to the tissue site to create
controlled cell necrosis of the turbinate structure without sufficiently
limiting blood flow
to the optic nerve and/or the retina (Figure 11 ). As shown in Figure 12
disruption of the
blood flow to the optic nerve and/or retina can sufficiently damage the optic
nerve
and/or retina and create a permanent impairment of vision.
Referring to Figure 10, energy delivery device 14 creates cell necrosis zones
28
to reduce the size turbinate structure 88 by removing only so much of
turbinate structure
88 to increase the size of the nasal passageway but insufl'icient to create a
permanent,
(i) dysosmic state, (ii) dry nose condition, (iii) atrophic rhinitis state,
(iv) a loss of ciliary
function or (v) damage to the nerves of nasal cavity creating a permanent loss
of nasal
and facial structure activity. The creation of the ablation zones in turbinate
structure 88
repositions turbinate structure 88. In one embodiment, no more than 33% of the
mucosal layer of the lower turbinate is removed. Further removal may create
the
dysosmic state, a permanent dry nose condition and/or a loss of ciliary
function.
As illustrated in Figures 13 and 14, cell necrosis apparatus 10 provides
controlled ablation of turbinate structures 88 and the resulting turbinate
structure is
repositioned in the nasal cavity, and can "open up" the nasal cavity for
allergy sufferers
and the like. Pressure plate 15 is positioned against desired turbinate to
facilitate entry of
energy delivery device into tissue to reach necrosis site 92.
In another embodiment, cell necrosis apparatus 10 reduces a volume of a
selected site in an interior of a soft palate structure 94 (refer to Figures
15 and 16).
-17-
CA 02315842 2000-06-21
W0 99/32041 PCT/US98l26144
Energy delivery device 14 is configured to be maneuverable in oral cavity 16,
pierce a
soft palate structure surface 96, advance a sufficient distance to a tissue
site, deliver
electromagnetic energy to the tissue site, create controlled cell necrosis
zones 28 and
reposition soft palate structure 94. in oral cavity 16 with reduced necrosis
of an exterior
mucosal surface 98 of soft palate structure 94. The creation of cell necrosis
zones 28
repositions soft palate structure 94 and tightens the interior tissue of soft
palate structure
as illustrated by the arrows.
Referring now to Figures 17A, 17B, 17C, and 17D an embodiment of cell
necrosis apparatus 100 is illustrated where energy delivery device 114 is at
least
partially positionable in an introduces 102 coupled to handpiece 112 and
pressure plate
115 is positionable on an exterior surface of introduces 102. Pressure plate
115 includes
a tissue interface surface 117.
One or more energy delivery devices 114 can extend from different ports
formed along an exterior surface of introduces 102. Introduces 102 can also be
the same
as handpiece 112. An energy delivery device advancement device 104 may be
provided, although in various embodiments with introduces 102 one is not
necessary.
Energy delivery device advancement device 104 can include guide tracks or
tubes 106
positioned in the interior of introduces 102. Energy delivery devices 114 may
be
positioned in guide tracks 106 and advanced from the guide tracks 106 into the
interior
of the anatomical structwe. Cabling is coupled to energy delivery devices 114.
Introduces i 02 and handpiece 112 may be one device. Pressure plate 115 can
also be
positioned at a distal portion of the introduces 102. A second energy delivery
device
114 can be coupled to a second introduces 102 coupled to the handpiece.
Similarly a
second pressure 115 can be positioned at an exterior of the second introduces
102.
A disinfectant medium introduction member 108 can be coupled to cell necrosis
apparatus 100 either in an interior or at an exterior. Disinfectant medium
introduction
member 108 may be slidably positioned in introduces 102 or at its exterior.
Alternatively, disinfectant medium introduction member 108 can be an optical
fiber
coupled to a light energy source. Disinfection medium introduction member can
be
coupled to infusion fluid reservoir 111 (see figure 17D).
Energy delivery devices 114 are at least partially positioned in an interior
of
introduces 102. Each energy delivery device 114 can be advanced and retracted
through
a port 109 formed at a distal end or along a side of introduces 102. Energy
delivery
devices 114 can be hollow to receive a variety of different infusion fluids,
including
-18-
CA 02315842 2000-06-21
WO 99/32041 PCTNS98/26144
medicinal solutions, electrolyte solutions, irrigation solutions and contrast
media. This
is accomplished by coupling energy delivery device 114 to infusion fluid
reservoir 111
through energy delivery device advancement device 104 (see figure 17D).
Introducer 102 includes a temperature control medium conduit 111 that can
extend through an interior of introducer 102. The depth of tissue penetration
of energy
delivery device 114 is controlled by pressure plate 115.
An energy delivery surface 133 of energy delivery device 114 can be adjusted
by inclusion of an adjustable or non-adjustable insulation sleeve 132.
Insulation sleeve
132 can be advanced and retracted along the exterior surface of energy
delivery device
114 in order to increase or decrease the length of energy delivery surface
133.
Introducer 102 can be malleable. A soft metal member may be enclosed or
encapsulated within a flexible outer housing to form a malleable introducer
I02.
In another embodiment, handpiece 112 is conformable or deflectable. This can
be achieved mechanically or with the use of shape memory metals. A steering
wire, or
other mechanical structure, can be attached to either the exterior or interior
of a distal
end of introducer 102. In one embodiment, a deflection knob (not shown)
located on
handpiece 112 is activated by the physician causing a steering wire to tighten
(not
shown). This imparts a retraction of the distal end of introducer 102. It will
be
appreciated that other mechanical devices can be used in place of the steering
wire. The
deflection may be desirable for tissue sites with difficult access.
Energy delivery devices 114 can be spring loaded. When energy delivery
device advancement device 104 is.moved back, springs cause selected energy
delivery
devices 114 to advance out of introducer 102.
One or more sensors 146 may be used to measure temperatures. One or more
sensors 146 may be positioned on an interior or exterior surface of energy
delivery
device 114, insulation sleeve 132, or be independently inserted into the
interior of the
anatomical structure.
Cell necrosis apparatus 100 can include visualization capability including but
not limited to a viewing scope, ultrasound, an expanded eyepiece, fiber
optics, video
imaging, and the like.
Additionally, an ultrasound transducer 116 can determine the size and position
of the created lesion. In one embodiment, two ultrasound transducers 116 are
positioned on opposite sides of introducer 102 to create an image depicting
the lesion in
-19-
CA 02315842 2000-06-21
W0 99/32041 PCTNS98/261d4
the anatomical structure. Each ultrasound transducer 116 is coupled to an
ultrasound
source (not shown).
In one embodiment, cell necrosis apparatus 100 is coupled to an open or closed
loop feedback system. Referring now to Figure 18, an open or closed loop
feedback
system couples sensor 346 to energy source 392. In this embodiment, energy
delivery
device 314 is one or more RF electrodes 314.
The temperature of the tissue, or of RF electrode 314 is monitored, and the
output power of energy source 392 adjusted accordingly. Additionally, the
level of
disinfection in the oral cavity can be monitored. The physician can, if
desired, override
the closed or open loop system. A microprocessor can be included and
incorporated in
the closed or open loop system to switch power on and off, as well as modulate
the
power. The closed loop system utilizes a microprocessor 394 to serve as a
controller;
monitor the temperature, adjust the RF power, analyze at the result, refeed
the result,
and then modulate the power.
With the use of sensor 346 and the feedback control system a tissue adjacent
to
RF electrode 31.4 can be maintained at a desired temperature for a selected
period of
time without impeding out. Each RF electrode 314 is connected to resources
which
generate an independent output. 'The output maintains a selected energy at RF
electrode
314 for a selected length of time.
Current delivered through RF electrode 314 is measured by current sensor 396.
Voltage is measured by voltage sensor 398. Impedance and power are then
calculated at
power and impedance calculation device 400: These values can then be displayed
at
user interface and display 402. Signals representative of power and impedance
values
are received by a controller 404.
A control signal is generated by controller 404 that is proportional to the
difference between an actual measured value, and a desired value. The control
signal is
used by power circuits 406 to adjust the power output in an appropriate amount
in order
to maintain the desired power delivered at respective RF electrodes 314.
In a similar manner, temperatures detected at sensor 346 provide feedback for
maintaining a selected power. Temperature at sensor 346 is used as a safety
means to
interrupt the delivery of energy when maximum pre-set temperatures are
exceeded. The
actual temperatures are measured at temperature measurement device 408, and
the
temperatures are displayed at user interface and display 402. A control signal
is
generated by controller 404 that is proportional to the difference between an
actual
-20-
CA 02315842 2000-06-21
WO 99/32041 PCT/US9$/26144
measured temperature and a desired temperature. The control signal is used by
power
circuits 406 to adjust the power output in an appropriate amount in order to
maintain the
desired temperature delivered at the sensor 346. A multiplexer can be included
to
measure current, voltage and temperature, at the sensor 346, and energy can be
delivered to RF electrode 314 in monopolar or bipolar fashion.
Controller 404 can be a digital or analog controller, or a computer with
software. When controller 404 is a computer it can include a CPU coupled
through a
system bus. On this system can be a keyboard, a disk drive, or other non-
volatile
memory systems, a display, and other peripherals, as are known in the art.
Also coupled
to the bus is a program memory and a data memory.
User interface and display 402 includes operator controls and a display.
Controller 404 can be coupled to imaging systems, including but not limited to
ultrasound, CT scanners, X-ray, MRI, mammographic X-ray and the like. Further,
direct visualization and tactile imaging can be utilized.
The output of current sensor 396 and voltage sensor 398 is used by controller
404 to maintain a selected power level at RF electrode 314. The amount of RF
energy
delivered controls the amount of power. A profile of power delivered can be
incorporated in controller 404 and a preset amount of energy to be delivered
may also be
profiled.
Circuitry, soRware and feedback to controller 404 result in process control,
and
the maintenance of the selected power setting that is independent of changes
in voltage
or current, and used to change, (i) the selected power setting, (ii) the duty
cycle (on-off
time), (iii) bipolar or monopolar energy delivery and (iv) fluid delivery,
including flow
rate and pressure. These process variables are controlled and varied, while
maintaining
the desired delivery of power independent of changes in voltage or current,
based on
temperatures monitored at sensor 346.
Referring to Figure I9, current sensor 396 and voltage sensor 398 are
connected
to the input of an analog amplifier 410. Analog amplifier 410 can be a
conventional
differential amplifier circuit for use with sensor 346. The output of analog
amplifier
410 is sequentially connected by an analog multiplexer 406 to the input of AID
converter 408. The output of analog amplifier 410 is a voltage which
represents the
respective sensed temperatures. Digitized amplifier output voltages are
supplied by A/D
converter 408 to microprocessor 394. Microprocessor 394 may be a type 68HCII
available from Motorola. However, it will be appreciated that any suitable
-21-
CA 02315842 2000-06-21
WO 99/32041 PCT/US98/26144
microprocessor or general purpose digital or analog computer can be used to
calculate
impedance or temperature.
Microprocessor 394 sequentially receives and stores digital representations of
impedance and temperature. Each digital value received by microprocessor 394
corresponds to different temperatures and impedances.
Calculated power and impedance values can be indicated on user interface and
display 402. Alternatively, or in addition to the numerical indication of
power or
impedance; calculated impedance and power values can be compared by
microprocessor
394 with power and impedance limits. When the values exceed predetermined
power or
impedance values, a warning can be given on user interface and display 402,
and
additionally, the delivery of RF energy can be reduced, modified or
interrupted. A
control signal from microprocessor 394 can modify the power level supplied by
energy
source 392.
Figure 20 illustrates a block diagram of a temperature/impedance feedback
I S system that can be used to control temperature control fluid flow rate
through introducer
102. Energy is delivered to RF electrode 314 by energy source 392, and applied
to
tissue site 424. A monitor 416 ascertains tissue impedance, based on the
energy
delivered to tissue, and compares the measured impedance value to a set value.
If the
measured impedance exceeds the set value, a disabling signal 412 is
transmitted to
energy source 392, ceasing further delivery of energy to electrode 314. If
measured
impedance is within acceptable limits, energy continues to be applied to the
tissue.
During the application of energy sensor 346 measures the temperature of tissue
and/or
electrode 314. A comparator 420 receives a signal representative of the
measured
temperature and compares this value to a pre-set signal representative of the
desired
temperature. Comparator 420 sends a signal to a flow regulator 422
representing a need
for a higher temperature control fluid flow rate, if the tissue temperature is
too high, or
to maintain the flow rate if the temperature has not exceeded the desired
temperature.
The foregoing description of a preferred embodiment of the invention has been
presented for purposes of illustration and description. It is not intended to
be exhaustive
or to limit the invention to the precise forms disclosed. Obviously, many
modifications
and variations will be apparent to practitioners skilled in this art. It is
intended that the
scope of the invention be defined by the following claims and their
equivalents.
What is claimed is:
-22-