Note: Descriptions are shown in the official language in which they were submitted.
CA 02315877 2000-08-18
WO 93/16688 , PCT/US93/01806
-1-
FACILITATED OXYGEN DELIVERY IN CONJUNCTION WITH HEMODILUTION
BACKGROUND OF THE INVENTION
The present invention relates to improved medical
procedures in which addition of a synthetic oxygen carrier in
connection with autologous blood replacement (and, preferably,
in connection with hemodilution) is used to reduce or
eliminate the need for homologous blood.
More than 13 million units of blood are collected each
year in the United States alone, and about 10 million of these
units are transfused into 4 million recipients. Of the
transfused units, about two-thirds are used during surgical
procedures, and the remainder are used primarily for treating
severe anemia or in emergency indications. Experience from
clinical studies suggests that postoperative recovery can be
shortened if hemoglobin concentrations are not allowed to fall
to below 10 g/dL, the generally accepted indication for
transfusion (Zauder, Anesth. Clin. North Amen 8:471-80
(1990)). This criterion, however, is currently being
reevaluated due in part to a recent increase in awareness of
the risks associated with homologous blood transfusion (NIH
Consensus Conference JAMA 260:2700-2703 (1988)). This .ha.'
also resulted in a renewed interest in the use of autologous
blood transfusion techniques, in particular predonation and
acute normovolemic hemodilution (ANH).
Although autologous blood transfusion (i.e., reinfusion
of the patient's own blood) was first employed over 170 years
ago, it was not until the early 1970s that its use became more
widespread because of growing concerns about the transmissic~
of hepatitis. More recently, interest in autologous
transfusions on the part of both patients and physicians has
been stimulated by the emergence of AIDS. Despite an
increased awareness and acceptance of the benefits of
autologous blood transfusion, recent studies have revealed the
widespread underutilization of autologous predonation (which
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-2-
is estimated to represent only 2-5% of all units drawn
nationwide).
The outcome of some surgical procedures may be~improved
by reducing blood viscosity prior to surgery. This can be
accomplished with ANH at the start of an operation (Stehling
et al. Transfusion 31:857 (1991)). ANH is a procedure whereby
several units of blood are withdrawn from the patient at the
beginning of surgery and simultaneously replaced with either
a crystalloid or a colloid plasma volume expander. The basic
mechanism that compensates for most of the decreased oxygen
capacity of the diluted blood is the rise in cardiac output
and increased organ blood flow, factors that result from the
improved fluidity of blood (i.e., lower viscosity) at lower
hematocrit levels (Messmer et al Eur. Surg. Res. 18:254-263
(1986)).
Predonation typically involves withdrawal of several
units of a patient's blood during the six weeks prior to
surgery. To avoid excessive anemia, the amount of blood that
can be safely predonated in the weeks before surgery is
limited, as is the amount of blood that can be removed during
ANH.
One potential drawback of ANH and, to a lesser degree,
predonation, is the loss of oxygen carrying capacity of the
blood during surgery.
Quite apart from ANH and predonation, it has been
suggested that red cell substitutes, or blood substitutes,
could be used in place of homologous blood (i.e., blood from
other humans) during surgery. Extensive research in the field
of such blood substitutes over the past two decades has
resulted in several candidate compositions. These include
perfluorocarbon emulsions, such as FluosolT" (Green Cross
Corporation, Japan) and OxygentT" (Alliance Pharmaceutical
Corp., San Diego, USA), and hemoglobin compositions, such as
those derived from human, animal, or recombinant sources.
CA 02315877 2000-08-18
WO 93/16688 PCf/LJS93/018t16
-3-
Traditional thinking has been that a red cell substitute
would be given in volumes equal to the amount of whole blood
that would be used for the same purpose.
Unfortunately, the use of such blood substitutes to
replace blood used in transfusions has not been entirely
satisfactory. Early studies using Fluosol, for example, as a
blood substitute found that after blood loss, fluosal was
"unnecessary in moderate anemia and ineffective in severe
anemia." Gould, et al., NeW Engl. J. Med. 314:1653 (1986).
In this study, the average increase in arterial oxygen content
with the drug was only 0.7 ml/deciliter. Thus, it was
believed that use of such fluorocarbon emulsions as blood
substitutes would not provide a significant benefit in
severely anemic patients. Indeed, although the U.S. Food &
Drug Administration has now approved Fluosol for use as a
perfusion agent during percutaneous transluminal coronary
angioplasty (PTCA), it has to date refused to approve its use
as a blood substitute for general use.
Another problem in using fluorocarbon emulsions and
hemoglobin compositions as red cell substitutes or blood
substitutes to compensate for blood loss from surgery,
disease, or trauma is the relatively short half life of those
materials in vivo. Healthy humans typically require about two
weeks to manufacture new red cells and increase hematocrit to
2~ normal following blood loss. In contrast, the intravascular
half life of fluorocarbon emulsions and hemoglobin substitutes
in vivo is typically less than 72 hours, often less than 24
hours. Thus, even if sufficient quantities of a red cell
substitute are administered during and/or after surgery, for
example, to provide adequate oxygen delivery, the oxygen
carrying capacity will drop significantly long before the body
can compensate by making new red cells.
SUMMARY OF THE INVENTION
3~ The present invention includes a method for facilitating
autologous blood use by a patient facing a loss of blood,
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-4 -
comprising the steps of removing and preferably storing a
portion of the patient's blood, intravenously administering a
biocompatible liquid in sufficient quantity to substantially
maintain the patient's blood volume, wherein the liquid
comprises an effective oxygen-delivery enhancing amount of a
biocompatible oxygen carrier, after Which the patient
undergoes a loss of blood, and then readministering blood to
the patient, preferably the stored blood. In one embodiment,
- the biocompatible liquid further comprises a hemodiluent and
the hemodiluent is administered separately from the oxygen
carrier. Although the invention includes oxygen carriers
derived from human, animal, plant, or recombinant hemoglobin,
the preferred oxygen carrier is a fluorocarbon emulsion. In
that embodiment, the volume of the administered oxygen carrier
is advantageously less than about 50% of the volume of the
hemodiluent. The fluorocarbon emulsions may have
concentrations as low as 50 or 100, w/v, but preferably have
a concentration of at least 40% or 600, w/v. In some
embodiments, the hemodiluent is a crystalloid, a colloid, or
a combination thereof. A preferred aspect of the invention
includes administering oxygen breathing gas ~to the patient
during performance of the method. The blood loss contemplated
by the present invention includes blood loss from surgery,
trauma, or disease. Although the precise amount of
administered oxygen carrier will vary, general preferred
ranges are between about 0.5 and 10 ml/kg, based on the body
weight of the patient.
The invention further includes use of a non-blood oxygen
carrier in the preparation of a medicament for use in the
foregoing method, or for use during hemodilution of a patient,
particularly when the hemodilution and administration of the
oxygen carrier is followed by transfusion of whole blood or
red cells, preferably an autologous transfusion.
CA 02315877 2001-08-07
-4a-
According to an aspect of the invention, there is
provided, use of a biocompatible, synthetic, oxygen-
carrying liquid for enhancing the oxygen-delivery
capacity of a pat:ient's blood, for use in a medical
procedure, involving:
removing a portion of a patient's blood;
using said biocompatible liquid by either
i) admiraistering said biocomptible liquid to
the patient and mairataining the patient's blood volume,
or
ii) administering said biocompatible liquid
and a hemodiluent to the patient; said patient undergoing
a loss of blood subsequent to the administration of the
biocompatible liquid;
said patient undergoing an administration of a
breathing gas enriched with a concentration of 50~ to
100 oxygen to said patient during the procedure; and
said patient undergoing a readministration of the
removed blood.
According to another aspect of the invention, a
composition for facilitating autologous blood use by a
patient facing a loss of blood, for use in a medical
procedure involving:
removing and storing a portion of the patient's
blood;
intravenously administering a biocompatible
liquid in sufficient: quantity to maintain the patient's
blood volume;
having said patient undergo a loss of blood
subsequent to the administration of said biocompatible
liquid;
CA 02315877 2000-08-18
-4b-
administering breathing gas enriched with a
concentration of 50~ to 100 oxygen to said patient
during said loss of blood; and
readministering said stored blood to said patient,
wherein said composition comprises an effective oxygen-
delivery enhancing amount of a biocompatible synthetic
oxvaen carriPr_
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/41846
_5_
BRIEF DESCRIPTION OF THE DRAWINGS
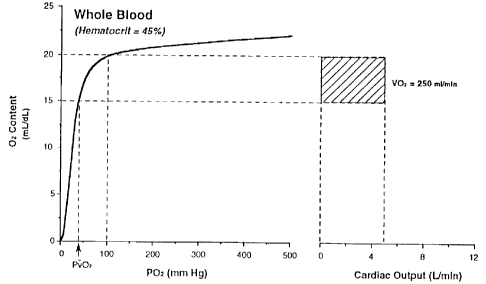
Figure 1 is a graph showing the relationship between the
OZ delivery from hemoglobin in blood and the cardiac output
under normal conditions (hematocrit - 45%). Total OZ
utilization (or consumption; V02) is equal to the product of
cardiac output times the arterial-venous OZ content
difference, and has been indicated by the cross-hatched area.
OxyHb dissociation curves were generated from data provided by
the model developed by Winslow, Int. J. Clin. Monitor Comp.
2:81-93 (1985)).
Figure 2 is a graph showing oxygen delivery and total OZ
consumption following acute normovolemic hemodilution (to a
hematocrit of 250) and injection of 3.0 mL/kg BW of a 90% w/v
perflubron emulsion (Ox~~gent HT) while breathing 100% OZ . The
potential contributions to total Oz consumption (VOz) by the
individual compartments (i.e., the red cells, plasma, and the
perflubron emulsion) is shown by the different cross-hatched
areas. At a cardiac output of 10 L/min, (and an arterial pOZ
of 500 mmHg) the total amount contributed to VOZ by the
plasma and perflubron phase alone approximately equals normal
V02 .
Figure 3 is a graph showing the cardiac output in
anesthetized dogs during and following acute crystalloid
hemodilution.~ Data are Means ~ SEM.
Figure 4 is a graph showing mixed venous POZ in
anesthetized dogs during and following severe crystalloid
hemodilution. * Indicates a significant difference between
the two groups. Data are Means ~ SEM.
Figure 5 is a graph showing the percent of oxygen
delivery and total oxygen consumption contributed only by the
OZ dissolved in the perflubron emulsion in anesthetized dogs
following severe crystalloid hemodilution. * Indicates a
significant difference between the two groups. Data are Means
~ SEM.
Figure 6 is a graph showing perflubron levels in the
blood as a function of time following injection of perflubron
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-6-
emulsion.
Figure 7 is a graph showing the percent of total oxygen
consumption contributed only by the OZ bound to hemoglobin in
anesthetized dogs following severe crystalloid hemodilution.
* Indicates a significant difference between two groups. Data
are Means ~ SEM.
DETAILED DESCRIPTION OF THE INVENTION
A. Overview of the Invention
10. The invention described below combines the use of limited
intravascular half-life oxygen carriers (blood substitutes)
with autologous blood transfusion strategies, including in
particular, a combination of predonation and perioperative
hemodilution. In patients who have donated blood prior to
surgery (predonation) and for whom concerns about adequate
oxygen-carrying capacity remain, an oxygen carrier can be
infused. An additional margin of safety with. respect to
enhanced oxygen delivery will be provided with such a
supplementation. Small amounts of the substitute, typically
not approaching one-to-one (i.e. not equal volume)
replacement, would be effective in providing~this margin of
safety.
As an alternative, an oxygen-carrying blood substitute
with limited intravascular persistence can be used as a
2~ partial replacement formulation during perioperative
hemodilution. As above, this supplementation need not be a
one-to-one replacement for the volume of blood withdrawn
during or after hemodilution, but is rather to supplement the
oxygen-carrying capacity during or after hemodilution with
3D crystalloid and/or colloid-based solutions. In this clinical
situation an additional margin of safety is afforded to the
hemodiluted patient, by augmenting total oxygen delivery.
Two unique features of the present invention are of
particular importance. First, the invention represents a
35 departure from use of blood substitutes as replacements for
blood in acute or chronic anemia on a one-to-one basis.
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-
Instead, the present invention increases the margin of safety
of existing autologous transfusion technologies, preferably
through less that one-to-one replacement that is, by smaller
volume infusion of an oxygen carrier (blood substitute). The
present invention includes the discovery that a small volume
of an oxygen carrier will be efficacious by providing the
benefit of enhanced oxygen delivery, particularly when used in
combination with autologous transfusion techniques. This
hypothesis has been confirmed in a dog model of acute
hemodilution.
Second, the combined use of autologous and blood
substitute infusion technologies to avoid homologous
transfusion is emphasized. The present invention contemplates
use of both predeposit and perioperative autologous
technologies with preferably less that one-to-one infusions of
various oxygen-carrying blood substitute formulations. This
invention includes use of any or all of these technologies in
whatever order or of whatever magnitude they may be clinically
useful in the perioperative clinical setting described.
B. Materials
A large number of materials suitable for use in the
present invention are already known in the art. Without
limiting the'scope of the invention, certain representative
materials are discussed below.
Several compositions have been proposed or demonstrated
to function as intravenous oxygen carriers. These include
fluorocarbon emulsions, including but not limited to
. perfluorocarbon emulsions. Such emulsions are typically
fluorocarbon-in-water emulsions having a discontinuous
fluorocarbon phase and a continuous aqueous phase. The
emulsions typically include emulsifying agents and osmotic
agents, together with buffers and electrolytes.
The fluorocarbon emulsion may be selected from a wide
range of suitable emulsions. Advantageously, it is a
fluorocarbon-in-water emulsion, having a preferred
fluorocarbon concentration of about 5o to about 1250, w/v.
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
_g_
Fluorocarbons are fluorine substituted hydrocarbons that
have been used in medical applications as imaging agents and
as blood substitutes. U.S. Patent No. 3,975,512 to Long uses
fluorocarbons, including brominated perfluorocarbons, as a
contrast enhancement medium in radiological imaging.
Brominated fluorocarbons and other fluorocarbons are known to
be safe, biocompatible substances when appropriately used in
medical applications.
It is additionally known that oxygen, and gases in
general, are highly soluble in some fluorocarbons. This
characteristic has permitted investigators to develop
emulsified fluorocarbons as blood substitutes. For a general
discussion of the objectives of fluorocarbons as blood
substitutes and a review of the efforts and problems in
achieving these objectives see "Reassessment of Criteria for
the Selection of Perfluorochemicals for Second-Generation
Blood Substitutes: Analysis of Structure/Property
Relationship" by Jean G. Riess, Artificial Organs 8:34-56,
1984.
The fluorocarbon, in one preferred embodiment, is a
perfluorocarbon or substituted perfluorocarbon: Fluorocarbon
molecules used in these emulsions may have various structures,
including straight or branched chain or cyclic structures, as
described in Fiiess, J., Artificial Organs 8(1):44-56 (1984).
These .molecules may also have some degree of unsaturation, and
may also contain bromine or hydrogen atoms, or they may be
amine derivatives. The fluorocarbons may be present in the
emulsion in any useful concentration, but usually range from
_ about 5o to 125% weight per volume (w/v). As used throughout,
concentrations defined as weight/volume are understood to
represent grams/ml and o weight per volume to represent
grams/100 ml.
Although concentrations as low as 5%, w/v are
contemplated, in a preferred embodiment the concentrations are
at least 25% or 300, preferably at least 40%, 500, 550, 600,
75% or 80% w/v. Emulsions of 85%, 900, and 1000 are
particularly preferred. Preferred fluorocarbon emulsion
CA 02315877 2000-08-18
-g-
formulations are those disclosed in U.S. Patent Nos.
4,865,836; 4,987,154; and 4,927,623.
There are a number of fluorocarbons that are contemplated
for use in the present invention. These fluorocarbons include
bis (F-alkyl) ethanes such as C~F9CH=CH4CF9 (sometimes
designated "F-44E") , i-C3F9CH=CHC6F~3 ("F-i36E") , and
C6F~3CH=CHC6F~3 ( "F-66E" ) ; cyclic fluorocarbons, such as C10F18
("F-decalin", "perfluorodecalin" or "FDA"), F-adamantane
("FA"), F-methyladamantane ("FMA"), F-1,3-dimethyladamantane
("FDMA"), F-di-or F-trimethylbicyclo[3,3,1]nonane ("nonane");
perfluorinated amines, such as F-tripropylamine("FTPA") and F-
tri-butylamine ~("FTBA"), F-4-methyloctahydroquinolizine
("FMOQ"), F-n-methyl-decahydroisoquinoline ("FMIQ"), F-n-
methyldecahydroquinoline ("FHQ"), F-n-cyclohexylpurrolidine
("FCFiP") and F-2-butyltetrahydrofuran ("FC-75"or "RM101").
Other. suitable fluorocarbons may be selected from
brominated perfluorocarbons, such as 1-bromo-heptadecafluoro
octane (C8F»Br,.sometimes designated perfluorooctylbromide or
. "PFOB", now known by the U.S. Adopted Name "perflubron"), 1
bromopenta-decafluoroheptane (C~F~SBr), and 1-
bromotridecafluorohexane (C6F~3Br, sometimes known as
perfluorohexylbromide or "PFHB"). Other brominated
fluorocarbons~are disclosed in US Patent. No. 3,975,512 to
Long. Also contemplated are fluorocarbons having nonfluorine
substituents, such as perfluorooctyl chloride, perfluorooctyl
hydride, and similar compounds having different numbers of
carbon atoms, e.g., 6-12 carbon atoms.
Additional fluorocarbons contemplated in accordance with
this invention include perfluoroalkylated ethers or
polyethers, such as (CF3) ZCFO (CF2CFZ) 20CF (CF3) Z, (CF3) zCFO
(CFZCF2)30CF(CF3) , (CF3)CFO(CFZCFZ)F, (CF3)2CF0(CFZCF2)2F,
(C6F~3)z0. Further, fluorocarbon-hydrocarbon compounds, such
as, for example compounds having the general formula C~FZrt,~
C~,Fz~,"~, C~Fzrt,iOCn,F2n,,~, or C~FZ~,~CF=CHC~,Fz~,,~, where n and n' are
the same or different and are from about 1 to about 10 (so
long as the compound is a liquid at room temperature). Such
CA 02315877 2000-08-18
WO 93!16688 PCT/US93/01806
-10-
compounds, for example, include C$F~7CZH5 and C6F~3 CH=CHC6H~3.
It will be appreciated that esters, thioethers, and other
variously modified mixed fluorocarbon-hydrocarbon compounds
are also encompassed within the broad definition of
"fluorocarbon" materials suitable for use in the present
invention. Mixtures of fluorocarbons are also contemplated.
Additional "fluorocarbons" not listed here, but having those
properties described in this disclosure that would lend
themselves to use in vivo in accordance with the present
invention are also contemplated.
Emulsifying agents used in the emulsions of this
invention may be anionic, cationic or non-ionic surfactants or
combinations thereof as are well known to those in the
chemical arts or they may be mixtures of synthetic compounds
such as Pluronic F-68, a condensate of ethylene oxide with
propylene glycol, as used in U.S. Patent No. 4,073,879 to
Long. Fluorosurfactants, such as those described by J. Riess
et al. Intel Symposium on Blood Substitutes, Montreal, May,
1987, are particularly suitable can also be -used.
20- Emulsifying agents may also be mixtures of the above agents.
Particularly suitable emulsifiers may include natural
amphipathic compounds such as phospholipids, particularly
phosphatidylcholine, wherein combined hydrophilic and
hydrophobic properties enable the molecule to interface with
both aqueous and fluorocarbon systems, thereby forming the
emulsion droplets. There are various species of each class of
phospholipids, such as the phospholipid cholines, comprising
various pairings of saturated and unsaturated fatty acids in
the glycerol structures. Phosphatidylcholine is an abundant
natural material (lecithin) which may be purified from egg
yolk, or may be produced synthetically (Avanti Polar Lipids,
Pelham, ALA). Phospholipid emulsifiers, particularly egg yolk
phospholipid and lecithin, are particularly preferred.
The phospholipid emulsifying agent should be included in
3~ the range of from 2 to 14% w/v, usually increasing the
phospholipid concentration with increasing fluorocarbon
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-11-
concentration. The preferred amount for an emulsion
comprising 75% w/v bromofluorocarbon is 2.5 to 5% w/v and 3.5
to 10% w/v of phospholipid for an emulsion with~100% w/v
bromofluorocarbon. In a preferred embodiment, the
phospholipid comprises at least 2% w/v of the emulsion.
Emulsification requires large amounts of energy to
convert a two-phase immiscible systLm into a suspension of
discontinuous small droplets of hydrophobic fluid in an
aqueous continuous phase. Fluorocarbon emulsification may be
carried out generally by either of two general processes which
provide energy to the system to break up the fluorocarbon
volume into small droplets. In sonication emulsification, a
probe is inserted into the mixture of fluorocarbon,
emulsifier, and aqueous phase, and bursts of energy are
released from the tip of the probe. In a mechanical
emulsification process, such as that performed by a
Microfluidizer~' apparatus (Microfluidics, Newton, MA 02164),
streams of the mixed emulsion components are directed through
the apparatus at high velocity and under high pressure (e. g.
15, 000 psi) , and the high shear forces or cavitation resulting
from the mechanical stress applied to the fluid produce the
emulsion.
The aqueous phase of the emulsion may have components
dissolved therein which give the emulsion desirable
properties. For example, it may comprise an osmotic agent to
bring the emulsion to physiological isotonicity. The osmotic
agent may be sodium chloride, or it may be a polyhydroxyl
compound, such as a sugar or mannitol. The aqueous phase will
also contain soluble buffering agents.
The lipid phase of the emulsion may also have components
dissolved therein. For example, a phosphatidyl choline
emulsifier may have glycerol, phosphatidyl glycerol, other
phospholipids or cholesterol admixed, and further contain an
antioxidant substance, such as a tocopherol, to protect
against lipid oxidation.
Several fluorocarbon emulsions have been produced
commercially for use as intravascular oxygen carriers. These
CA 02315877 2000-08-18
-12-
include a mixed decalin emulsion sold by Alpha Therapeutics
Corp. under the trademark FLUOSOL and perflubron emulsions
produced by Alliance Pharmaceutical Corp. of San Diego,
California.
One exemplary perflubron emulsion is a 90% (w/v)
perflubron emulsion referred to as Oxygent~"HT having the
following Formula I:
FORMULA I P~RFLUBRON EMULSION
. Component Percent (w/v)
Perflubron 90.000
Egg Yolk Phospholipid 4.000
NaHZP04 H20, USP 0 . 052
Na2HP04 7H20, USP 0 . 355
~ NaCl, USP 0.280
EDTA, USP 0.020
d-Q-tocopherol, USP 0.002
Water for injection, 48:400
Hemoglobin compositions contemplated for use in the
present invention are well known. Such compositions are
disclosed, for example, in the following U.S. Patents:
U.S. Patent Nos. 4,911,929; 4,861,867; 4,857,636;
4,777,244; 4,698,387; 4,600,531; 4,526,715; 4,473,494;
and 4,301,144.
Various materials have been used_successfully as plasma
expanders in connection with hemodilution procedures. These
include the well-known categories of crystalloid compositions
(exemplified by Ringers-lactate and saline (0.9%) both from
Baxter Healthcare Corp., Deerfield, IL) and colloid
compositions. Colloid compositions include (1) modified fluid
gelatins, such as those sold under the following trademarks:
Plasmagel~ (R. Bellon Lab., Neuilly-sur Seine, France),
Gelifundolm (Biotest, Frankfurt,. Germany), Haemacel~ (Hoechst-
Roussel Pharmaceutical Inc., Sommerville, NJ); (2) dextran
solutions, such as those sold under the trademarks Macrodex~
CA 02315877 2000-08-18
WO 93!16688 PCT/US93/01806
-13-
(dextran-70) and Rheomacrodexm (dextran-40) both from
Pharmacia, Piscataway, NJ); (3) albumin solutions, such as
those sold under the trademark Albutein° (Alpha Therapeutics,
Los Angeles, CA) and human serum albumin (5%) from Abbott
Labs, North Chicago, IL; (4) starch solutions such as
Hetastarch (Hycroxyethylstarch) Hespan~ (DuPont, Willmington,
DE). These are administered in various volumes to maintain
the patient's blood volume in the normal range and to
encourage the increase in cardiac output that accompanies
hemodilution procedures. In general, crystalloid-based
solutions need to be given in volume ratios of 2:1 or 3:1 to
blood withdrawn; colloids are usually given in lesser amounts.
C. Procedures
Autologous blood use virtually eliminates the possibility
of contracting blood-borne diseases associated with
transfusions. Autologous blood for use in subsequent
transfusions can be obtained in a number of ways, including
one or more of the following: predeposit; perioperative
isovolemic hemodilution; intraoperative salvage; and
postoperative salvage.
Predeposit :equires that the surgery be planned well in
advance of the =.::~ual date. Blood is donated by the patient
during the wee:.:. and months bef ore surgery , and is stored f or
subsequent adr.inistration to the patient. Phlebotomies of
350-400 ml are typically performed at 2-7 day intervals, with
the last collection more than 72 hours before surgery. The
blood may be stored in the liquid state as whole blood, or it
may be divided into red cells and plasma which can be frozen
to preserve labile components.
Perioperative isovolemic hemodilution is the process of
collecting blood immediately before a surgical procedure with
the concomitant replacement by a sufficient volume of
crystalloid or colloid solution. This practice decreases
blood viscosity during surgery, thereby reducing the work load
on the heart and increasing microcirculation. Typically,
sufficient blood is removed to reduce the hematocrit from a
typical normal value of approximately 0.45 to about 0.20 to
CA 02315877 2000-08-18
-14-
0.35, preferably about 0.25 to about 0.30. This blood is
stored for readministration to the patient during or after
surgery. After removal of some of the blood, or
simultaneously with the removal, a crystalloid or colloid
plasma expander (or both) is administered to the patient to
maintain blood volume at a desired value, typically at the
normal value.
Intraoperative blood. salvage involves collecting blood
lost from a wound or body cavity during surgery, processing
it, and reinfusing the processed blood into the same patient.
This procedure is safe and effective if certain basic
precautions are followed to ensure against contamination of
the blood with bacteria or other pathogens, or malignant
cells. Autotransfusion devices for collecting, filtering, and
reinfusing the blood are commercially available. Also, some
devices separate and wash the red blood cells, thereby
avoiding, administration of blood contaminated by debris,
irrigating solutions, activated factors, anticoagulants, and
free hemoglobin. Suitable devices of this type are
exemplified by the Haemonetics Cell Separation and Cell
Washer, Haemonetics Corp., Braintree, MA.
Postoperative salvage and autotransfusion involves the
recovery of blood drained from the.surgical wound during the
hours following the operation. If basic precautions are taken
to insure the sterility of the collected blood,.the procedure
is safe and well tolerated. The same commercial devices can
be used for this procedure as for intraoperative blood
salvage.
Detailed reviews of autologous blood procedures and acute
isovolemic or normovolemic hemodilution are found, for
example, in Stehling, et al., Transfusion 31:857 (1991) and
Mercuriali, et al, Autologous Hlood, Transmedica Europe
Limited, Eastbourne, United Kingdom (1991),
In the practice of the present invention, autologous
blood procedures (preferably 'involving perioperative
hemodilution) are combined with administration of non-blood
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-15-
oxygen carriers, including hemoglobin compositions and, more
preferably, fluorocarbon emulsions. The invention below
combines the use of dose-limited and short intravascu~lar half-
life oxygen carrying drugs with autologous blood transfusion
techniques, including in particular, predonation and
perioperative hemodilution techniques. In patients who have
donated blood prior to surgery (predonation) an oxygen-
carrying drug can be infused during surgery to support
adequate oxygen delivery, thereby conserving the autologous
blood for definitive correction of anemia at the end of
surgery or post operatively. Small amounts of the oxygen
carrying drug (less that 50% of blood volume), are effective
in providing this margin of safety during the surgery period
when cardiac output elevation occur due to lower blood
viscosity. This method further reduces or eliminates the need
for administering homologous blood to the patient.
Similarly, a dose-limited oxygen-carrying drug with short
intravascular persistence which does not cause adverse
hemodynamic effects, can be used effectively as an additive to
standard perioperative hemodilution. As outlined above, the
oxygen supplementation provided by the drug would provide a
margin of safety during the actual surgery. In this clinical
setting, the additional margin of safety is afforded to the
hemodiluted patient, by augmenting total oxygen delivery
during surgery and conserving autologous blood for the
definitive correction of anemia at the end of surgery or post
operatively. The need for homologous blood is thereby reduced
or eliminated.
In particular, one embodiment of the invention involves
removal of a portion of the patient's blood, and
administration of an intravenous fluid to reduce the
hematocrit from about 0.45 to between 0.20 to about 0.35,
preferably from about 0.25 to about 0.30. This removal is
usually deliberate, although the invention may also be used
with trauma victims or other patients suffering involuntary
blood loss. With deliberate removal, the blood is stored for
readministration to the patient at a later time.
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-16-
Either simultaneously with or after removal of the blood,
sufficient intravenous fluid is administered to permit
regulation of cardiac output in order to maintain oxygen
delivery at a level at least approximately equivalent to
levels prior to removal of the patient's blood, in a manner
well known in the hemodilution art. This intravenous fluid
includes an oxygen carrier other than red blood cells,
preferably a biocompatible fluorocarbon emulsion of the type
previously discussed, although hemoglobin compositions are
also contemplated, as are other oxygen carriers. In addition,
the intravenous fluid preferably includes a plasma expander,
such as a colloid or crystalloid.
Advantageously, the volume of intravenous fluid
administered to the patient is at least about equal to 750,
preferably at least about 100% of the volume of blood removed
from the patient. More preferably, the volume of intravenous
fluid is between about 150% and 3000 of the volume of blood
removed, depending on whether the fluid is predominantly a
colloid or a crystalloid. Alternatively, the volume of
intravenous fluid administered to the patient is adequate to
reduce the hematocrit of the patient to the levels discussed
above:
In one embodiment of the invention, the intravenous fluid
comprises a major portion of a plasma expander and a minor
portion of oxygen carrier. The volume ratio of administered
expander to an oxygen carrier will range from 1:1 to at least
10:1, depending on whether the fluid is a crystalloid or a
colloid, and on the composition of the oxygen carrier, the
concentration of the oxygen carrier, POZ and cardiac output.
These ranges are most desirable when using a high
concentration fluorocarbon emulsion, having at least about
40%, preferably at least about 50% or 60% fluorocarbon, w/v.
In one preferred embodiment, where a fluorocarbon
emulsion such as perflubron emulsion is used as the oxygen
carrier, the amount of actual perfluorocarbon administered to
the patient is advantageously from about 0.5 g/kg to about 10
g/kg, preferably 2-6 g/kg, based on the weight of the patient.
CA 02315877 2000-08-18
WO 93!16688 PCT/LJS93/01806
-17-
When a 90% w/v or 1000 w/v fluorocarbon emulsion is used, the
volume of emulsion necessary to deliver the desired dosage is
about 0.5 or 0.55 ml/kg to about l0 or 11 ml/kg, preferably
about 2 to 6 ml/kg. Simple calculation provides the preferred
volume of emulsion when different concentrations of
fluorocarbon are used.
The hemodiluted patient is then administered a breathing
gas enriched in oxygen, preferably at least 50-60%, and most
preferably 100% oxygen. The effects of the enriched breathing
gas, increased cardiac output due to hemodilution, the oxygen
carrier, and the dissolved oxygen in the aqueous portion of
the circulating intravascular fluid all combine to supply
enhanced levels of oxygen to the patient. The collective
contributions of these factors to oxygen delivery in the
patient are discussed in more detail in sections D. and E.
below.
During or after the surgical procedure (or other
condition resulting in blood loss), the autologous blood
removed from the patient (or the red cell portion thereof) can
be readministered to the patient. The oxygen carrier,
meanwhile, is cleared from the circulation .in a relatively
short time, and its oxygen-carrying function is supplanted by
the autologous transfusion of red cells, if required.
D. OxyQen Delivery to Tissues
Although not intending to be bound to any particular
theory of operation, the following discussion provides a
framework for understanding the physical and physiological
mechanisms contributing to the function of the present
invention.
Oxygen transport to tissues can be considered to occur
via two processes. The first, is the connective (bulk)
delivery of Oz to tissues, and the second is the delivery of
OZ to tissues via a diffusive process.
(1) Connective Oxygen Delivery
The first process, connective Oz delivery, is described
by the Fick equation shown below, where VOZ - oxygen
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-18-
consumption, C.O. - cardiac output, and (a - v)OZ - the
arterial-venous 02 content difference.
VOz=[ (C.O. ) ]x( (a-v)OZ]
Although the Fick equation is quite straightforward, a
number of physiological variables of importance are imbedded
in it. For example, the arterial-venous differential in
oxygen content [(a - v)OZ] is determined by the Oz content of
l0 both arterial (CaOZ) and venous (CvO2) blood, respectively,
which, in turn, is directly related to the hemoglobin (Hb)
concentration and the OZ saturation. Oxygen saturation is
determined by the POZ and by the position of the oxyHb
(oxygenated form of Hb) dissociation curve. The POZ is
determined by the Oz tension in the inspired air and the
capacity of the lung to oxygenate pulmonary capillary blood.
Finally, the position of the oxyHb dissociation curve is
determined by 2,3-diphosphoglycerate (2,3-DPG) as well as pH
and pCOZ, which differ between arterial and venous blood.
2o Similarly, cardiac output (C. O.) is controlled by many
factors, including heart rate, the left ventricular filling
volume (i.e., stroke volume), and the demand for OZ in tissues
(i.e., oxygen consumption, vo2). Assuming a constant blood
volume and under stable hemodynamic conditions, the left
ventricular filling volume is proportional to the blood
viscosity, which, in normal humans, is primarily a function of
the hematocrit (percent of red cells in blood).
Some of these complex relationships can be shown
graphically (see Figure 1). In Figure 1, Oz content is
plotted against OZ tension, POz. Figure 1 presents data for
a normal, 70 kg man at rest with a hemoglobin concentration of
14.4 g/dl (hematocrit - 450). The data for the oxyHb
dissociation curve used to create this graphic representation
CA 02315877 2000-08-18
WO 93/16688 PCT/h'S93/01806
-19-
were generated by the model developed by WinSlow (1980, which
calculates the total OZ contents dissolved in the plasma and
bound to hemoglobin. For a given arterial and venous POz of
100 and 40 torn, respectively, the arterial to venous oxygen
content difference (Ca02 - CvOZ) is 5 mL/dL. At a normal
cardiac output of 5 L/min, the OZ consumption (vO2, represented
by the cross-hatched area) is approximately 250 mL/min or 5
mL/kg/min.
Normally, more 02 is delivered to tissue than is
utilized, providing a "margin of safety." When the connective
(bulk) delivery of OZ decreases below a certain critical
point, tissue function may be compromised, with various
consequences such as tissue hypoxia, production of lactic
acid, infarction, necrosis, etc. once this critical oxygen
delivery level is reached (i.e., when OZ delivery is severely
limited), then VOZ (oxygen consumption) will be supply
limited. The actual value for the critical oxygen delivery
level is very difficult to specify, since there are likely to
be different values for different organs or different
capillary beds.
When OZ consumption is not supply-limited, changes in Oz
content of the arterial blood can be compensated for by other
normal physiological mechanisms. For example, in anemia, the
cardiac output becomes elevated (see below) , as does the level
of red cell 2, 3-DPG. The latter serves to shift the oxyHb
dissociation curve to the right (reduced affinity, increased
PSO~the POZ at which hemoglobin is 50% saturated with
A similar compensatory mechanism (with respect to the
cardiac output) occurs during acute normovolemic hemodilution
(Messmer et al. Res. Exp. Med. 159:152-56 (1986)). As the
hematocrit decreases during the hemodilution, blood viscosity
also decreases significantly, which allows the cardiac output
to increase without any significant changes in the work load
on the heart. In this way, total oxygen consumption can be
maintained. This is illustrated in Figure 1, where it can be
seen that the amount of oxygen consumption from hemoglobin
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-2 0-
(i.e., total area of lighter shading) is the same in both
Figure 1 (before hemodilution) and Figure 2 (after
hemodilution).
Work by Guyton et al. Cardiac Output and its Regulation,
2nd Ed. Saunders, Philadelphia (1973)) has shown that over a
broad range, the cardiac output varies inversely with
hematocrit, with an "optimum hematocrit" in approximately the
range of 40 to 45% for normal, resting humans. When
hematocrit values exceed 450, blood viscosity limits cardiac
output such that there is little beneficial effect from the
additional OZ carrying capacity of the increased number of
circulating red cells. When the hematocrit is less than about
40%, the lower viscosity results in a decreased total
peripheral resistance to blood flow which allows cardiac
output to increase in order to maintain normal oxygen
delivery.
It should be noted that augmenting 02 transport by
administration of a cell-free oxygen carrier differs from
simple transfusion in several important ways. A key point in
understanding the value of a low-dose acellular "blood
substitute" is that plasma OZ is increased, rather than red
cell OZ, as is the case with transfusion of blood.
Transfusion of red cells will increase bulk blood viscosity,
which can cause a decrease in cardiac output and therefore may
no.t increase the bulk OZ delivery.
Addition of a cell-free Oz carrier, on the other hand,
will increase bulk Oz delivery by elevating the Oz content of
the plasma and potentially increasing the cardiac output
(since overall blood viscosity would be reduced). Figure 2
illustrates the increase in potential oxygen consumption which
can be achieved by elevating the Oz content of blood by
breathing 100% OZ.
This additional contribution to VOZ is primarily due to
an increased amount of OZ dissolved in the plasma compartment.
Theoretically, V02 can be further increased by addition of a
low dose of a 90o w/v perflubron emulsion under these
conditions which would provide an even greater margin of
CA 02315877 2000-08-18
WO 93/16688 PCT/L)S93/01806
-21-
safety. Figure 2 illustrates the additional increase in
potential oxygen consumption which can be achieved with a
overall dose of a 90% w/v perflubron emulsion.
(2) Diffusion oxygen Delivery
Oxygen transport to tissue also occurs via diffusion.
There are a series of diffusion boundaries through which OZ
must pass on its way from the red cell to the tissues. Fick's
law of diffusion states that the overall rate of diffusion of
a gas from one compartment to another is governed by the
diffusion gradient, the difference between the gas
concentrations (P~-P2) within the two compartments, and a
diffusion constant, Kd, which is a lumped-sum reflection of
many factors including properties of the boundary layers,
1~ temperature, etc.
d (Oz)
_~ ( P~ _Pt )
The process of OZ diffusion can be simply illustrated by
considering the movement of water through holes in a wall
separating a' higher elevation reservoir and a lower level
reservoir. Water is supplied initially at one elevation (P~),
and flows to a second lower level (Pz). The hydrostatic
pressure driving this movement is the vertical difference in
height between the two reservoirs. The total rate of water
2~ movement is also limited by the cross-sectional area of the
holes in the barrier which provide resistance to flow from
compartment 1 to 2. In this analogy, the two water levels
correspond to the two 02 pressures (P~ and P2) in Fick' s law of
diffusion, shown above, and the cross-sectional area of the
holes in the barrier (through which the water flows) would be
represented by the diffusion constant, Kd.
Experimental work has shown that there are probably two
barriers to diffusion of Oz from the red cell to the tissues:
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
-22-
the layer of unstirred plasma surrounding the red blood cell,
and the collective membranes separating the plasma space from
the cellular cytosol of adjacent tissue. Raising the level of
Oz in the plasma will have the effect of increasing the rate
of diffusion into tissues, since the plasma represents an
"intermediate level reservoir" in the preceding analogy. In
fact, if there is not a limiting supply of OZ in red cells,
then the rate of movement of 02 from plasma to tissues will be
proportional to this plasma reservoir. This represents the
essence of the proposed use of low-dose 02 carriers to reduce
the need to transfuse homologous blood.
The proposed mechanism assumes that a small reduction of
the reservoir of available Oz (e.g., hemodilution) will not
appreciably change the overall rate of diffusion because it is
assumed that the barrier to diffusion represented by the
membranes between the plasma and tissue cytosol space is
rate-limiting. Experimental evidence exists to support this
assumption.
Increasing the diffusive delivery of OZ to tissue is
sometimes called "diffusion facilitation", and could increase
OZ delivery to tissues under conditions where OZ delivery might
be otherwise supply-limited. In other words, increasing the
dissolved (plasma) Oz concentration is expected to decrease
the level at which critical Oz delivery occurs and thereby
increase the margin of safety in terms of prevention of tissue
hypoxia. Experimental evidence suggests that this is, in
fact, the case. In a recent study by Faithfull & Cain (J.
Crit. Care 3:14-18 (1988)), dogs were initially hemodiluted
with either 6o dextran (average molecular weight 70,000, in
Tyrode's solution), or the perfluorocarbon emulsion, Fluosol,
and then progressively hemorrhaged to determine the critical
OZ extraction ratios. Fluosol-treated dogs had lower mixed
venous POZ levels and higher OZ extraction fractions at the
critical OZ delivery point. This indicated that
perfluorochemicals in Fluosol may have promoted diffusion of
Oz into the tissues. This effect was very evident in these
Fluosol studies since these dogs likely had a compromised
CA 02315877 2000-08-18
1~'O 93/16688 PCf/US93/01806
-23-
microcirculation due to the severe capillary flow
inhomogeneity that occurs in dogs immediately following
injection of only 1 to 2 mL of the Fluosol emulsion (Faithfull
et al. (Microvasc. Res. 33:183-93 (1987)).
It should be noted that transfusion of red cells will not
affect 02 diffusion in the same manner as described. In fact,
an additional physiological effect described by Federspiel et
al. Microvasc. Res. 32:164-89 (1986)), refers to the fact
that in normal capillary beds, red cells are separated by
considerable distances as they individually traverse the
capillary network. The OZ would be expected to transfer from
red cells to tissue predominantly across the area where the
red cell is closely in contact with the endothelial cells
lining the vasculature. Addition of a cell-free OZ carrier
might increase the rate of Oz transfer, simply on the basis
that more Oz. would be in contact with the endothelial cells.
In general, improvement of blood flui~ity by hemodilution
has been shown to increase mean tissue F.2 in various organs
(Messmer et al. Res. Exp. Med. 159:152-56 (1973)). This
increase in tissue POZ was attributed to more even flow
distribution at the microcirculatory level and was interpreted
as improved tissue oxygenation. On the other hand, Homer
Microvasc. Res. 2 :308-23 (1981)) argued that in acute anemia
there may be.large differences between red blood cell POZ and
the plasma P02. This would occur as a result of OZ diffusion
from the red cell being slowed by passage through the plasma
(which has very low OZ solubility characteristics). With
hemodilution, the spacing between red blood cells in tissue
capillaries is increased so that outward diffusion of OZ from
red cells is slowed further by the increased diffusional
barrier of plasma. The resultant gradient for P02 may not be
resolved (i.e., not all the oxygen has time to unload) during
the short time that the red cell dwells in the capillary and
02 extraction may be diminished accordingly (Gutierrez,
Respirat. Physiol. 63:79-96 (1985)).
The presence of an additional OZ carrier such as a
perfluorochemical in the plasma will increase the total
CA 02315877 2000-08-18
WO 93!16688 PCT/US93/01806
-24-
content in the plasma compartment of blood and may facilitate
the diffusion of 02 from the red cell into the tissues.
According to the model (see Figure 2), the addition of a
relatively small dose (3 mL [2.7 g perflubron]/kg BW) of a
highly concentrated 90 % w/v perflubron emulsion will result in
a significant increase in the total OZ content in the plasma.
When performed during respiration with 100% OZ and in the
presence of acute normovolemic hemodilution (to a hematocrit
of 25%), the net result would represent a doubling of the
oxygen consumption. Normal oxygen consumption would come
preferentially from the perflubron and the plasma, since this
02 is physically~dissolved and therefore readily available
(compared to the Oz that is chemically bound to hemoglobin as
a ligand) . The remaining 02 carried by the red cells would
therefore represent an available reservoir of extra 02 that
would supply additional oxygen, when needed, to prevent
certain sensitive tissues from reaching a critical level of OZ
delivery.
In summary, a low-dose cell-free oxygen carrier is
2D superior, in terms of tissue oxygenation, to additional red
cell transfusion. Such an oxygen carrier is used for the
temporary enhancement of oxygen delivery during the acute
phase of surgery. None of the currently available oxygen
carriers can' be considered effective "blood substitutes"
because of their short retention time in the circulation
(hours) compared to red cells (months). With routine use,
especially in uncomplicated elective surgery combined with
acute normovolemic hemodilution procedures, the need for
transfusion (i.e., "transfusion trigger") can be reduced.
3D This can eliminate the need for transfusion of homologous red
blood cells in many cases and, thereby, significantly reduce
the risk of transfusion-borne disease.
CA 02315877 2000-08-18
WO 93/16688 PCT/US93/01806
_25_
E. Examples
ERAMPLE 1
Enhancement of Oz Delivery B~ Perfluorocarbon Emulsion
Followincr Acute Hemodilution in Dons
This example was designed to determine the efficacy of
oxygen delivery by 90% w/v perflubron emulsion formulation to
Formula I in anesthetized mongrel dogs (n=9) subjected to
acute normovolemic hemodilution. Four control animals
(injected with 3.3 mL Ringer's-lactate/kg body weight) were
included in this study. A bolus injection of epinephrine was
used in all dogs to contract the spleen and release
sequestered red blood cells prior to hemodilution. Following
this injection, and prior to beginning the hemodilution
process, baseline measurements were obtained for cardiac
output, mean arterial pressure, heart rate, pulmonary artery
pressure, pulmonary wedge pressure, arterial and venous blood
gases, hematocrit, and total blood oxygen content. During
hemodilution (breathing room air) to ~a hematocrit of
approximately 250, each aliquot of blood removed was
immediately replaced with 3 volumes of Ringers-lactate (R-L).
Following this, blood samples were collected~for measurement
of all variables. Dogs were then ventilated with 100% oxygen
and further hemodiluted to a hematocrit of approximately 10-
120. During' this second hemodiluted procedure, the blood
volume removed was replaced with 1-1.5 volumes of colloid,
consisting of the dog's plasma (collected during the first
hemodilution) and supplemented with an albumin solution (50
HSA in R-L). Following this, blood samples were again drawn
for measurement of all variables. A 90% w/v perflubron
emulsion having the composition of Formula I was injected to
a total dose of 3.3 mL [3.0 g perflubron]/kg body weight at a
rate of approximately 20-30 mL/min, and blood samples were
drawn at various intervals for a total of 3 hours.
As expected, cardiac output rose significantly following
hemodilution, primarily because of the reduction in blood
viscosity at the lower hematocrits (Figure 3), and was able to
reach even higher levels in the perflubron emulsion-treated
CA 02315877 2000-08-18
VVO 93/16688 PCT/LJS93/01806
-26-
dogs. Mixed venous POZ was significantly higher following
infusion of the 90% w/v perflubron emulsions compared to
controls at all timepoints during the 3 hour post-injection
monitoring period (Figure 4).
The percent of total oxygen delivery (DOZ) contributed by
the perflubron emulsion-dissolved OZ was approximately 8% to
10%, while total oxygen consumption (VOz) contributed by the
perflubron emulsion-dissolved Oz was approximately 25-300
(Figure 5). These values decreased slowly to approximately 60
and 22%, respectively, by 3 hours due to clearance of the
perflubron emulsion from the circulation (Ti~Z = 5 hr, Figure
6). Calculation of hemoglobin saturation (based on blood pH,
temperature. PCO2, and POZ levels), demonstrated that the
percent of total oxygen consumption (VOZ) contributed by the
hemoglobin-carried oxygen was significantly higher in the
control dogs that in the perflubron emulsion-treated dogs
(Figure 7), indicating that the presence of the perflubron
emulsion had a sparing effect on the reserve of OZ still
available in the red cells.
Although the invention has been described with reference
to particular preferred embodiments, the scope of the
invention is defined by the following claims and should be
construed to include reasonable equivalents.