Note: Descriptions are shown in the official language in which they were submitted.
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
5 LOW CURRENT DENSITY ELECTROLYTIC CELL
AND METHOD OF MANUFACTURING SAME
10
BACKGROUND OF THE INVENTION
15
1. Field of Invention
The present invention relates to an electrolytic cell of decreased
current density which allows for lower power consumption, and a method
20 for reconfiguring an electrolytic cell to allow for lower power consumption
while maintaining the designed current capacity of the cell.
2. Description of the Related Art
25 Electrolytic cells are used extensively on an industrial scale for the
production of metals and chemicals, e.g., for the electrowinning of
aluminum, copper, nickel and zinc and the production of chlorine, chlorides,
sodium hydroxide, sodium chlorate, hydrogen and oxygen. Of primary
importance in this invention are the chlor-alkali cells, and particularly
chlor-
30 alkali diaphragm cells.
Chior-alkali diaphragm cells were originally designed to operate at
relatively low current capacities of about 10,000 amperes. These cells had
CA 02316930 2000-07-04
WO 00/30187 PCTNS99/26107
2
relatively low production capacities by modern standards. Typical cells of
this type are the Hooker Type S cells which were developed by the Hooker
Chemical Corporation. This particular cell was subsequently improved upon
by increasing its designed current capacity to about 50,000 amperes with
5 corresponding increases in production capacity.
Subsequent cell designs have bean directed at increasing the
operating efficiency based on the electrical energy consumed, and to
increasing the production capacity of the cell to obtain a higher production
10 rate for a given cell room area. This has been achieved to a large extent
by
modifying or redesigning the cell to increase the designed current capacity.
This, in turn, directly increases the production capacity of the cell. As the
designed current capacity of the cell is increased, however, it is necessary
to maintain high operating efficiencies to maintain production economics.
15 Mere enlargement of the component parts of a cell initially designed to
operate at a relatively low current capacity for operation at a higher current
capacity will not achieve high operating efficiencies. Consequently,
modifications in the cell design as well as in the design of individual cell
components is often required to achieve this result. These design
20 improvements include the use of expandable anodes and cathode structures
of improved strength, current distribution and hydrogen gas release.
Modern electrolytic cells of this type are capable of operating at
current capacities of 165,000 amperes or more with production capacities
25 of 5 tons per day or more of chlorine or sodium hydroxide, and current
efficiencies exceeding 95%. In U.S. Patent 3,899,408, there is taught an
electrolytic cell designed for operation at such high current capacities. The
cell is provided with cathode structures comprised of cathode "fingers" in
the form of a rectangular box formed from foraminous conductive metal
CA 02316930 2000-07-04
WO 00/30187 PCTNS99/26107
3
plates. A corrugated conductive metal reinforcing member is enclosed
within the cathode finger and extends the length of the cathode. This
corrugated member is in electrical and physical contact with the cathode
finger, and serves to provide structural support for the cathode finger, to
5 maintain a uniform current distribution, and to provide a space for release
of
hydrogen gas.
It would still be desirable, however, to provide an electrolytic cell
design capable of operating at high current capacities and production rates,
10 yet provide decreased power consumption. It would also be desirable to
provide such an electrolytic cell and in doing so to modify only the internal
components of the cell and thereby utilize the existing cell structure and its
support equipment to maximum advantage.
15 SUMMARY OF THE INVENTION
In accordance with the present invention, a method for substantially
decreasing the power consumption of an electrolytic cell while maintaining
the designed current capacity is achieved by increasing the number of
20 cathodes and anodes within the cell body. This can be accomplished in
part by decreasing the lateral dimensions of the individual cathode tubes.
This modification helps to allow the use of substantially more cathodes and
anodes within the cell. It can be used with a reduction in the existing
spacing between anodes and cathodes. Moreover, sufficient space is
25 maintained within cathode tubes to allow for improved release of hydrogen
gas from the cathode. Subsequent assembly of a new cell in accordance
CA 02316930 2000-07-04
WO 00/30187 PCTNS99/Z6107
4
with the present invention, or reassembly of a cell on refurbishing of an
existing cell, results in a cell having a substantial reduction in power
consumption and a lower operating current density. In this manner, in one
aspect of the invention, the number of anodes within the cell can be
5 increased from 42 up to as many as 50 elements, while the number of
cathodes can be increased from 20 up to as many as 24 elements.
In addition to the foregoing advantages, the new electrolytic cell has
the following additional advantage of improved anodic efficiency as a result
10 of lower current density producing smaller chlorine gas bubbles at the
anode which are more easily removed from the anode surface. Similarly,
the hydrogen disengagement of the cathode is also improved, resulting in
less hydrogen in the chlorine which is a significant product improvement.
In addition, the lower current density also results in a lower cell liquor
15 temperature which reduces the corrosion of metal parts and extends the life
of the plastic cell top and rubber anode blanket.
In one aspect, the invention is directed to an electrolytic cell having a
walled enclosure of a size containing from about 42 to about 46 anodes
20 and from about 20 to about 22 cathodes, the cathodes comprising spaced
apart conductive metal cathode tube members of foraminous conductive
metal plates forming the exterior of a cathode tube structure, the
improvement in the cell comprising an increased number of up to about 25
cathode tube members of reduced cathode-to-cathode spacing and an
25 increased number of up to about 52 anodes, of reduced anode-to-anode
spacing, the cell having a cathode area increase of up to about 20 percent
and an anode area increase of up to about 20 percent.
CA 02316930 2000-07-04
WQ 00/3018? PCT/US99/2610?
In a related aspect, the invention is directed in a manner related to
the foregoing, but for a cell having three times the number of anodes than
cathodes, plus three additional anodes, wherein there is provided an
increased number of anodes from about 87 to about 102, and increased
5 number of cathodes from about 28 to about 33.
In another aspect, the invention is directed to a method of decreasing
the power consumption of an electrolytic cell, the cell having a walled
enclosure of a size containing from about 42 to about 46 anodes and from
about 20 to about 22 cathodes, with there being a proportion of two times
the number of anodes than cathodes, plus two additional anodes, each
cathode comprising spaced apart foraminous conductive metal plates
forming the exterior of a cathode tube structure, wherein a cell top is
removed from the electrolytic cell and the walled enclosure of a size is
separated from a cell base, the method comprising the steps of:
(a) removing the cathode tube structures from the cell;
(b) replacing the cathode tube structures within the cell with an
increased number of up to about 24 cathode tube structure members of
reduced cathode-to-cathode spacing;
20 (c) providing an increased number of up to about 52 anodes to
the cell to correspond in the aforesaid proportion with the increased number
of cathode tube structure members, with the cathode tube structure
members being positioned between anodes;
(d) reattaching the cathode walled enclosure of a size to the cell
base, whereby the cell size is maintained while the anodes and cathodes
are increased; and
(e) replacing the cell top.
CA 02316930 2000-07-04
Wa00/30187 PCT/US99/26107
6
In a related aspect, the above method is directed in a related manner
to a cell having about 87 anodes and about 28 cathodes, where there is
provided an increase to about 102 anodes as well as to about 33 cathodes.
5 In yet another aspect, the invention is directed to a method for
providing an electrolytic cell of reduced power consumption, said cell
having a walled enclosure sized for containing from about 42 to about 46
anodes and from about 20 to about 22 cathodes, with there being a
proportion of two times the number of anodes than cathodes, plus two
10 additional anodes, each cathode comprising spaced apart conductive
foraminous metal plates forming the exterior of a cathode tube structure,
wherein the cell comprises a cell top, the walled enclosure, and a cell base,
the method comprising the steps of:
la) inserting in the cell an increased number from greater than
15 about 20 up to about 25 cathode tube structure members of. reduced
cathode-to-cathode spacing;
(b) providing an increased number of up to about 52 anodes to
the cell to correspond in the aforesaid proportion with the increased number
of cathode tube structure members, with the cathode tube structure
20 members being positioned between anodes;
(c) attaching the cathode walled enclosure of the size to the cell
base; and
(d) attaching the cell top.
25 Again in a related manner, the foregoing method can be directed to a
cell having about 87 anodes and about 28 cathodes, where there is
provided an increase to about 102 anodes and about 33 cathodes.
CA 02316930 2000-07-04
W(3 OOI30187 PCT/US99/26107
7
In a still further aspect, the invention is directed to a cathode tube
structure member comprising spaced apart foraminous conductive metal
plates having a tube width of about 1 5/64 inches and a tube length of
about 50 inches.
5
In another aspect, the invention is directed to a method of operating
an electrolytic cell having from greater than 20 up to about 24 cathodes
and from greater than 42 up to about 50 anodes, which method comprises:
(a) carrying out the electrolytic cell operation at a current density
10 below 1.5 amps/inchz;
(b) operating the electrolytic cell at a current capacity within the
range of from about 50,000 amperes to about 100,000 amperes; and
(c) conducting the electrolytic cell operation at a cell voltage
below about 3.4.
15
BRIEF DESCRIPTION OF THE DRAWINGS
Further features of the present invention will become apparent to
those skilled in the art to which the present invention relates from reading
20 the following specification with reference to the accompanying drawings, in
which:
Fig. 1 is a perspective view of the exterior of an electrolytic cell used
for employing the present invention.
Fig. 1 A is a side elevation, in section of a type of cathode sidewall
25 utilized for the electrolytic cell of Fig. 1.
Fig. 2 is an enlarged partial sectional and elevation view depicting
internal components including cathode tube structures, as well as a portion
of the walled enclosure of the electrolytic cell of Fig. 1.
Fig. 2A is a magnified view of a portion of the cathode tube
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
8
structures and anodes of Fig. 2 highlighting the cathode-to-cathode spacing
(CS) and the cathode tube width (TW).
5 DESCRIPTION OF THE PREFERRED EMBODIMENTS
Electrolytic cells employing the present invention can typically be
useful for the electrolysis of a dissolved species contained in a bath, such
as in electrolyzers employed in a chlor-alkali cell to produce chlorine and
10 caustic soda, or in an electrolysis process producing chlorate.
The cathode and cathode assembly elements can be made of any
electrically conductive metal resistant to attack by the catholyte in the
cell.
Steel and stainless steel can be advantageously used, as well as nickel and
15 valve metals such as titanium may be utilized. The cathodes can be any of
those as are conventionally used in a cell including activated cathodes.
Referring then, to Fig. 1, there is shown an electrolytic cell 1 which
can be adapted in accordance with the present invention. Principal
20 elements of the cell 1 include the cell top 2, which can be formed from a
corrosion-resistant plastic material, the cathode walled enclosure or "cell
can" 3, and cell base 5 (Fig. 2). Part of the cathode walled enclosure 3 is
positioned behind a sidewall busbar 4 (Fig. 1 A), as will be more particularly
described hereinbelow. The cell top 2 is fastened to the cathode walled
25 enclosure 3 which is, in turn, fastened to a cell base 5. The fastening
means allow ease of removal of the cell top 2, cathode walled enclosure 3
and cell base 5.
CA 02316930 2000-07-04
WQ 00/30187 PCT/US99/26107
9
Referring then to Fig. 1 A, there is depicted the interface bonded
structure of walled enclosure 3 and sidewall busbar 4. This bonded
structure extends the full length from an edge of the cell top 2 downwardly
to a cell base 5. The sidewall busbar 4 can be a unitary, monolithic and
5 planar busbar 4 that, for the particular cell of the figure, is as high as
the
cathode walled enclosure 3 and can be longer than the walled enclosure 3
to which it is bonded. The busbar 4 may thus be actually larger than the
walled enclosure 3. The extra length of the sidewall busbar 4 and its
adjacent walled enclosure 3 together form one wall of the cell 1. In
10 assembly, the cathode busbar 4, being typically a copper busbar 4, can be
interface bonded to the cathode walled enclosure 3 such as by explosion
bonding, brazing or roll bonding. A cathode busbar 4 of this type is
described in U.S. Patent 5,137,612, the disclosure of which is incorporated
herein by reference.
15
It is contemplated that for purposes of the present invention,
however, the cathode busbar structure 4 may comprise other
configurations, e.g., a plurality of copper busbar strips of varying
dimensions. These busbar strips can be attached to the cathode walled
20 enclosure 3 by any suitable manner, as by welding. A cathode busbar
structure of this type is described in U.S. Patent 3,904,504, the disclosure
of which is incorporated herein by reference.
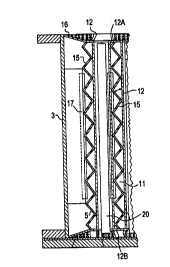
In Fig. 2 there is then illustrated, for a representative cell, the
25 cathode walled enclosure 3 and the individual cathode tube structure
members 11 . These representative cathode tube structure members 11 are
formed from pairs of adjacent parallel conductive metal screens or
perforated plates 12. These two plates 12, with their top 12A and bottom
12B, collectively form a cathode tube. The plates 12 of an individual tube
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
10
11 for the representative cell of the figure to be refurbished are usually
spaced apart approximately 1 3/16 inches. The cathode tube structure
members 11 further include cathode tube reinforcing means 15. Disposed
at each end of the electrolytic cell 1 and within the walled enclosure 3 are
5 cathode end tube structures 17. These end tube structures 17 are
somewhat identical to the cathode tubes 11, however, the end tube
structures have only one conductive metal screen or perforated plate 12
forming the end of the cathode tube structure.
10 In Fig. 2A, there is then shown an expanded view of the bottom
portion of Fig. 2, in which the cathode tube structure member width,
usually referred to herein just as the "tube width" (TW) is illustrated. This
tube width is the distance measured between the outside surfaces of the
two plates 12 of a cathode tube. The cathode-to-cathode spacing (CS),
15 then, is the distance measured between cathodes. This can be the spacing
between adjacent cathodes or, as depicted in the figure, the spacing
between a cathode end tube structure 17 and the next adjacent cathode
tube structure member 11. In the representative old cell 1 to be
refurbished, the spacing (CS) will generally be 2 1 /4 inches.
20
In operation, the cell 1 is filled with an electrolytic medium,
preferably a brine solution, and current is supplied to the cell 1 through
external connections. The products of the cell 1 are removed through
outlets situated on the side of the cell 1. These products for a chlor-alkali
25 cell include sodium hydroxide, chlorine and hydrogen gas.
In refurbishing an electrolytic cell 1 in accordance with the present
invention, the initial step is to drain the cell 1 of electrolytic solution
and
disconnect the external electrical connections to the cell 1. The cell top 2
CA 02316930 2000-07-04
WO-00/30187 PCT/US99/26107
11
is then removed from the cell by disconnecting it from the cathode walled
enclosure 3. After removal of the cell top 2, the cathode walled enclosure
3 is disconnected and removed from the cell base 5. The cathode walled
enclosure 3 can be removed from the cell base 5 using any convenient
5 means, such as by a crane or hoist.
After detachment and removal of the cathode walled enclosure 3
from the cell base 5, the cathode tube structure members 1 1, usually 20 in
number for this representative cell, are removed from the cathode walled
10 enclosure 3. This is accomplished by cutting the cathode rim screen 16
(Fig. 2) around the internal periphery of the cathode walled enclosure 3 and
then cutting any connection points of the cathode tube structure members
11 to the cathode walled enclosure 3. The existing cathode tube structure
members 1 1 are then removed from the enclosure 3.
15
New cathode tube structure members 11 similar in appearance and
which can be at least generally similar in their elements of construction to
the original cathode tube structure members 11 are then installed into the
cathode walled enclosure 3. The new cathode tube structure members 11,
20 however, for the representative cell of the figures, have a narrower
lateral
dimension or "tube width" (TW) of approximately 1 5/64 inches, again as
measured between the outside surfaces of adjacent plates 12 in the same
structure 11. This width permits the accommodation of up to 24 cathode
tube structures 11 in the refurbished cathode walled enclosure, providing a
25 cathode area increase of up to approximately 20 percent. Generally, such
cathode area increase of from about 15 to about 20 percent can be realized
for the type of cell as represented by the cell of the figutes. In order to
accommodate this narrower structure, any corrugated conductive metal
reinforcing means 15 which may be used will also be narrower, i.e., have a
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
12
narrower width, than the original reinforcing means. For length, the new
cathode tube structure members 11 can, by way of example, have a tube
length of about 50 inches.
5 Additionally, it is contemplated that conductive rods (not shown)
may be placed between the corrugations of the reinforcing means 15 and
form a part of the cathode tube structure members 11. These rods can be
metal rods consisting of copper, brass, or bronze and can serve to conduct
electrical current more efficiently along the cathode tubes. The space
10 between the reinforcing means 15 and the perforated plates 12 is
approximately the same as provided in the original design. This space
defines a hydrogen gas channel within the cathode tube structure member
11. While the tube width is decreased for the cell of the figures, this helps
allow for an increased number of tubes, thereby providing adequate space
15 for release of the hydrogen gas from the cathode tube structure member
11. The same number of electrical contact points is also more than
adequate in the new member since the reconfigured cell has a somewhat
lower current density than the original cell. For instance, the refurbished
cell of the figures may have a current density of as low as approximately
20 1.2 amperes per square inch. This compares to a current density of 1.5
amperes per square inch for the conventional cell. Both of these current
densities are based on equivalent design current capacity of 84,000
amperes. Design current capacity for commercial cells will often be within
the range from about 50,000 amperes to about '100,000 amperes.
25
The new cathode tube structure members 1 1 are then reinstalled in
the cathode walled enclosure 3 in a similar manner as the original cathode
tube structure members 11. A new cathode rim screen 16 is also installed
around these members 11 and the sidewall of the cathode walled enclosure
CA 02316930 2000-07-04
WO 00/30187 PCTNS99/26107
13
3. By reduction of the cathode-to-cathode spacing ICS) from a
conventional 2 1 /4 inches to 1 7/8 inches, additional anodes 20 can be
inserted in the new configuration, boosting the total from 42 anodes to a
total of up to 50 anodes, in the refurbished representative cell of the
5 figures. This represents an anode area increase of up to approximately
twenty percent. It will, hence, be understood that the type of cell as
represented by the cell of the figures has a proportion of anodes to
cathodes that is two times the number of anodes than cathodes, plus two
additional anodes. With the improved cells of this type there can generally
10 be obtained an anode area increase of from about 15 to about 20 percent.
The anodes 20 (Fig. 2) are positioned between adjacent cathode tube
structure members 11. All cathodes, including additional cathodes, are
attached to the cell base 5 in any suitable manner, such as by bolting or
welding. Also, in a cell refurbishing, the anode-to-anode spacing can be
15 reduced from 3 5/8 inches to 3 1 /8 inches. As for the cathodes, this
anode spacing is the width within the cell 1 between adjacent anodes 20.
While the representative invention cell of the figures has herein been
described as containing 24 cathodes and 50 anodes, it is also contemplated
20 that the cell could contain from 21 to 23 cathodes. In this regard, there
would then be from 44 to 48 anodes, there being two times as many
anodes as cathodes in the cell, plus two additional anodes.
If the invention cell is an improved MDC-55, such cell can have three
25 times the number of anodes than cathodes, plus three additional anodes.
Thus, an original cell of 28 cathodes and 87 anodes may have, as an
improved cell, 33 cathodes and 102 anodes. For this type of cell the anode
area increase, as well as the cathode area increase, can also be on the
order of about 20 percent. For this cell the anode-to-anode spacing may be
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
14
reduced by about 0.8 inch. Moreover, where the invention cell could be an
improved MDC cell of the type MDC-29 that has twice the number of
anodes than cathodes, plus two additional anodes, the cell could contain 25
cathodes, as opposed to 22 for the original cell, and as an improved cell
5 have 52 anodes, as opposed to 46 for the original cell. These original MDC
types of cells ace in commercial use and have been depicted in Kirk-Othmer,
4'" edition, Volume No. 1 at page 967.
The cathode walled enclosure 3 can then be reattached to the cell
10 base 5. The cell top 2 is then reinstalled and attached to the cathode
walled enclosure 3. Following connection of the electrical contacts, the cell
1 can be placed into service.
The outer surfaces of the individual cathode plates 12 are covered by
15 a separator. The separator for the cell will be a diaphragm, e.g., an
asbestos diaphragm, which may sometimes be referred to herein as a
"diaphragm porous separator". For the diaphragm in the electrolytic cell 1,
a synthetic, electrolyte permeable diaphragm can also be utilized. The
synthetic diaphragms generally rely on a synthetic polymeric material, such
20 as polytetrafluoroethylene fiber as disclosed in U.S. Patent 5,606,805 or
expanded polytetrafluoroethylene as disclosed in U.S. Patent 5,183,545.
Such synthetic diaphragms can contain a water insoluble inorganic
particulate, e.g., silicon carbide, or zirconia, as disclosed in U.S. Patent
4,606,805. Of particular interest for the diaphragm is the generally non-
25 asbestos fiber diaphragm containing inorganic particulates as disclosed in
U.S. Patent 4,853,101. The teachings of this patent are incorporated
herein by reference.
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
15
Broadly, this diaphragm of particular interest comprises a non-
isotropic fibrous mat wherein the fibers of the mat comprise 5-70 weight
percent organic halocarbon polymer fiber in adherent combination with
about 30-95 weight percent of finely divided inorganic particulates
5 impacted into the fiber during formation. The diaphragm has a weight per
unit surface area of between about 3 to about 12 kilograms per square
meter. Preferably, the diaphragm has a weight in the range of about 3-7
kilograms per square meter. A particularly preferred particulate is zirconia.
Other metal oxides, i.e., titanic, can be used, as well as silicate,
aluminates,
10 ceramics, cermets, carbon, and mixtures thereof. Especially for this
diaphragm of particular interest, the diaphragm may be compressed, e.g., at
a compression of from about one to about 6 tons per square inch.
It is contemplated that the anodes 20 will be coated with an
15 electrochemically active coating. As representative of the
electrochemically
active coatings for the foraminous metal anode are those provided from
platinum or other platinum group metals or they can be represented by
active oxide coatings such as platinum group metals, magnetite, ferrite,
cobalt spinal or mixed metal oxide coatings. Such coatings have typically
20 been developed for use as anode coatings in the industrial electrochemical
industry. They may be water based or solvent based, e.g., using alcohol
solvent. Suitable coatings of this type have been generally described in one
or more of the U.S. Patent Nos. 3,265,526, 3,632,498, 3,711,385 and
4,528,084. The mixed metal oxide coatings can often include at least one
25 oxide of a valve metal with an oxide of a platinum group metal including
platinum, palladium, rhodium, iridium, and ruthenium or mixtures of
themselves and with other metals. Further coatings include tin nxidR
manganese dioxide, lead dioxide, cobalt oxide, ferric oxide, platinate
coatings such as MxPT304 where M is an alkali metal and x is typically
CA 02316930 2000-07-04
WO 00/30187 PCT/US99/26107
16
targeted at approximately 0.5, nickel-nickel oxide and a mixture of nickel
and lanthanum oxides, such an lanthanum nickelate.
it has been observed that a loss of current efficiency occurs in the
5 present invention. This decrease in current efficiency results from the
reduction in power consumption and identical feed brine temperature and
ambient conditions of a conventional cell. To counter effect the hss ir,
current efficiency, it is contemplated that the operating temperature of the
present invention can be increased by either raising the feed brine
10 temperature or by insulating the electrolytic cell. This is typically done
by
wrapping the cathode with an insulation blanket.
While the foregoing discussion has been directed to refurbishing of a
conventional electrolytic cell, it is also contemplated that the present
7 5 invention can be directed to the construction of a new electrolytic cell.
Results of the cell operation presented in the following table illustrate
the improved performance of the representative new cell of the figures as
compared to the conventional, or comparative cell. Both cells operate at an
20 equivalent design current capacity of 84,000 amperes and also produce the
same amount of cell product. However, the new cell has a significantly
reduced power consumption as well as a reduced current density and
operates at a cell voltage below 3.4.
25
CA 02316930 2000-07-04
W4 00/30187 PCT/US99/26107
17
TABLE 1
Comparative Cell Invention Cell
Current efficiency 95.6 95.3
Average Cell Voltage 3.48 3.30
Power (KWHDC/metric ton CI2) 2751 2617
Cell Liquor Temperature 96.5 93.4
Anolyte Temperature 92.5 90
4
Cell Liquor Strength (g/I NaOH) 145 ~ .
145'"
Brine Feed (g/I NaCI) 325 325
Current Density (amperes/in2) 1.5 1.26
15 "The cells can be operated at lower caustic content in the cell liquor.
This will result in greater current efficiencies.
25
35