Note: Descriptions are shown in the official language in which they were submitted.
CA 02317033 2000-08-29
TITLE: ARSENIC FILTERING MEDIA
FIELD OF THE INVENTION
The present invention relates to filtering media for removing arsenic
from ground water, and more particularly, the present invention relates to
arsenic filtering media that are renewable and that are efficiently usable for
filtering water at common drinking-water pHs.
BACKGROUND OF THE INVENTION
Arsenic is a naturally-occurring element found in ground and
surface water, and is present in high concentrations in many parts of the
world. Also, there are many locations where ground water has been
contaminated with arsenic from industrial activities such as mining
operations, waste pile run off and pesticide manufacturing.
It has long been known that arsenic is a highly toxic substance, a
suspected carcinogen, and can be deadly in pure form. The long-term
effects of consuming water with naturally occurring high levels of arsenic
have been the subject of numerous studies. It has been found that the so-
called chronic arsenic poisoning can cause thickening and discolouration
of the skin, cancers of the liver, kidney and skin, and loss of circulation in
the extremities causing a gangrenous-like condition known as blackfoot
disease.
1
CA 02317033 2000-08-29
Due to the toxic and carcinogenic nature of arsenic, government
agencies have established a maximum acceptable concentration of
allowable arsenic in drinking water. In Canada, Health and Welfare
Canada has set a maximum acceptable concentration of 25 ~,g/L in
drinking water. The World Health Organization has established a
maximum acceptable concentration of 10 ~g/L. Currently, the USA limit
is 50 ~g/L, but the USA's Environment Protection Agency is in the
process of revising this limit downward, perhaps to a standard as low as 10
~g/L. It is known that several States have already reduced the limit to 10
~g/L.
Several methods have been used in the past for removing arsenic
from water. Existing surface water treatment plants employing
conventional treatment trains such as lime softening, and coagulation/
flocculation/filtration have shown arsenic removal abilities as a side effect.
Advanced technologies such as ion exchange, activated alumina, and
membrane processes such as reverse osmosis, have had much less testing
but have shown good potential under certain conditions. However, it has
been demonstrated in previous research that the background water quality
matrix strongly influences arsenic removal. For examples, alkalinity affects
coagulation processes; sulfates affect ion exchange and membrane
processes; activated alumina performance declines with increasing pH and
fluoride concentration, and co-precipitation with iron is inhibited by high
chloride concentration.
In addition to the possible presence of inhibitory substances in
water, other difficulties associated with the removal of arsenic from
ground water include high cost, complexity, and method of use. For
examples, lime softening or coagulation/flocculation/filtration plants are
2
CA 02317033 2000-08-29
expensive and they require a relatively high degree of operator attention.
These plants are also known to create large quantities of residuals which
can pose disposal problems. Activated alumina and ion exchange media
are expensive to manufacture. Membrane filtration plants are also
expensive to build, can be technically challenging to operate, and often
result in wastage of over 50% of the water supply. In some cases it has
been found that for every 100 litres entering the plant, often less than 50
litres of high-purity water is produced, with the remaining being wasted.
Recently, research has been carried out regarding the use of iron-
oxide coated sand as filtering media. The iron-oxide coated sand has been
shown to efficiently remove arsenic from ground water. However, this
product has not been developed on a large scale, primarily due to the fact
that the existing formulation requires a complex and expensive preparation
including the baking of an iron-oxide coating onto the sand particles. To
date, this preparation procedure has been limited to production of small
quantities for laboratory experimentations.
Similar research has been carried out with respect to the use of iron-
oxide impregnated porous support materials as filtration media. For
example, Canadian Patent 1,067,627 issued to Gerald D. Lutwick on
December 4, 1979 teaches a method and apparatus for the removal of
arsenic from water by passing water containing arsenic over a porous
support material which is impregnated with ferric hydroxide. The Lutwick
patent provides two examples of filtering materials for removing arsenic
from ground water. In the first example the filtering material was
impregnated with 4.4% ferric ions as Fe(OH)3, and in the second example,
the filtering material was impregnated with 0.97% ferric ions as Fe(OH)3.
The tests were carried out at a water pH of 4.7 in the first case, and with
3
CA 02317033 2000-08-29
a water pH of 3.9 in the second case. The Lutwick patent further teaches
that arsenic removal is preferably carried out with the water at an optimum
pH of 4.4.
The Lutwick patent also teaches that in some applications, the
filtering material may be regenerated. The Lutwick patent is silent with
regard to a method of regeneration of the filtering material or whether
regeneration involves re-impregnation with Fe(OH)3 .
As such, it will be appreciated that there continues to be a need for
a filtering medium capable of use with well water as it comes out of the
ground, without pH adjustment before or after the filtration process. There
continues to be a need for a filtering medium from which arsenic can be
desorbed and washed out, and the adsorption capacity of which can be
restored to an effective level without having to re-impregnate ferric ions
therein.
Further, it is believed that there continues to be a need for an arsenic
filtering medium which is relatively inexpensive, effective over a wide
range of water chemistry and capable of being used on residential wells as
efficiently as in industrial, commercial and municipal installations.
SUMMARY OF THE INVENTION
The present invention provides for arsenic filtering media which
have a large arsenic adsorption capacity, which are particularly efficient in
removing arsenic from ground water at drinking-water pHs (6.5 - 8.0), and
which can be easily and inexpensively mass-produced. More importantly,
the filtering media of the present invention are renewable several times for
re-use after becoming saturated with arsenic.
4
CA 02317033 2000-08-29
Broadly, in a first aspect of the present invention, there is provided
an arsenic filtering medium consisting essentially of calcined diatomite
particles and ferric ions bonded to the calcined diatomite particles, and
which is produced by a method comprising the steps of: generating a
mixture of calcined diatomite particles and ferric chloride; allowing the
mixture to sit for 16 hours, thereby allowing ferric chloride to thoroughly
impregnate the diatomite particles, and slowly adding sodium hydroxide
to the mixture until the pH of the mixture reaches a value of at least 9.0,
for ensuring unhasty and full conversion of ferric chloride into ferric
hydroxide.
The filtering medium thus obtained has strong and durable bonds
between ferric ions and the diatomite particles. The filtering medium
according to this aspect of the invention is renewable several times with
minimum reduction to its arsenic adsorption capacity. Tests have shown
that a reduction in the arsenic adsorption capacity of the filtering medium
is limited to less than 10% each time the filtering medium is renewed.
Another characteristic of the filtering medium according to the
present invention is that arsenic is securely bonded to the filtering medium
to prevent leaching of arsenic downstream of a filter containing the
filtering medium saturated with arsenic.
It is believed that the advantageous features mentioned above are
obtained primarily from the strong and durable bonds generated during the
preparation of the filtering medium, between the ferric ions and the
diatomite particles.
It is also believed that the particularly high loading of ferric ions
onto the diatomite support material obtained by such preparation method
5
CA 02317033 2000-08-29
is another factor contributing to some degrees to the performance of the
filtering medium.
As such, in accordance with another aspect ofthe present invention,
the step of generating a mixture of calcined diatomite particles and ferric
chloride in the above method comprises the step of adding 4 ml of 2. I M
FeCl3 ~ 6H20 solution per gram of calcined diatomite having particles
ranging in sizes from 30 mesh to about 60 mesh, and the sodium hydroxide
used in the step of slowly adding sodium hydroxide to the mixture has a
concentration of 10 N. These additional limitations to the method for
preparing the filtering medium according to the present invention are
advantageous for providing a filtering medium that has ferric ions bonded
to the diatomite particles in a proportion of as much as 1.36 grams of ferric
hydroxide for each gram of diatomite particles. That is, for providing a
filtering medium having ferric ions therein in a proportion of as much as
30% by weight.
The filtering medium according to this aspect of the present
invention is particularly appreciable for having an arsenic adsorption
capacity of about 1200 ~g/g, an efficient utilization with water at drinking-
water pHs and a renewability which is repeatable several times. The
filtering medium according to the present invention has also been found
efficient in the removal of other metals present with arsenic in the water to
be filtered. In tests, the filtering medium according to the present
invention has provided for over 99% removal of copper, 98% removal of
lead and over 98% removal of uranium. It also has been observed in many
cases that the filtering medium has the ability to remove iron from water.
Further, the filtering medium according to this aspect of the present
invention has shown no reduction in arsenic filtering efficiency with water
containing up to 250 mg/L of chloride or sulphate.
6
CA 02317033 2000-08-29
Although such high loading of ferric ions into the diatomite material
is preferable in most arsenic filtering installations, lighter loadings of
ferric
ions have nonetheless shown advantageous results and may be preferred
for use in less demanding applications for example. Therefore, in
accordance with a further aspect of the present invention, there are
provided arsenic filtering media consisting essentially of calcined
diatomite particles ranging in size from 30 mesh to 60 mesh, and between
5% and 30% by weight of ferric ions bonded to the calcined diatomite
particles. The arsenic filtering media according to this aspect of the
present invention are usable for filtering water at drinking-water pHs and
a restoration thereof is repeatable several times.
In accordance with yet another aspect of the present invention, there
is provided a method for renewing arsenic filtering media containing
calcined diatomite particles and ferric ions bonded to the calcined
diatomite particles, and being saturated with arsenic. This method
comprises the steps of slowly passing sodium hydroxide at a concentration
of between O.SN to 2.ON downward through the arsenic filtering media.
The abrasion of the arsenic filtering media is thereby reduced and a
performance thereof is substantively maintained. It has been found that
this method is efficient for desorbing at least about 82% of the arsenic
from the arsenic filtering media while retaining about 90% arsenic
adsorbing capacity ofthe arsenic filtering media. This method ofrenewing
filtering media has been found advantageous for not requiring a chemical
regeneration of the media such as desorbing arsenic therefrom and re-
impregnating ferric ions into the support material.
Still another feature of the invention is that the filtering media are
susceptible of a low cost of manufacture with regard to materials,
equipment and labour, and which accordingly are then susceptible of low
7
CA 02317033 2000-08-29
price of sale to the industry, thereby making such arsenic filtering media
economically available to the public.
Of considerable importance, the filtering media according to the
present invention have been certified for use in drinking water applications
by USA's National Sanitation Foundation (NSF International), under the
ANSI/NSF Standard 61 entitled: Drinking Water System Components -
Health Effects.
Other advantages and novel features of the invention will become
apparent from the following detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
A preferred embodiment of the present invention selected by way
of examples will now be described with reference to the accompanying
drawings in which:
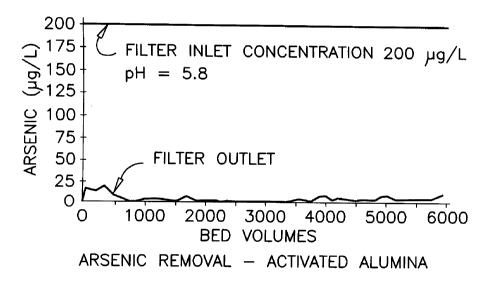
FIG.1 illustrates arsenic concentration in the inlet and outlet flows to and
from a bench-scale filtering column containing activated alumina;
FIG. 2 illustrates arsenic concentration in the inlet and outlet flows to and
from a bench-scale column filter containing a filtering medium
according to the present invention;
FIG. 3 illustrates arsenic concentration in the inlet and outlet flows to and
from a pilot filter containing a filtering medium according to the
present invention;
8
CA 02317033 2000-08-29
FIG. 4 illustrates arsenic concentration in the inlet and outlet flows to and
from a commercial filtering installation containing a filtering
medium according to the present invention;
FIG. 5 illustrates copper removal from water at water pH 8.0 using the
filtering medium according to the present invention in a pilot filter
installation.
FIG. 6 illustrates lead removal from water at water pH 5.6 using the
filtering medium according to the present invention in a pilot filter
installation;
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
While this invention is susceptible of embodiments in many various
forms, there will be described in details herein a specific embodiment, with
the understanding that the present disclosure is to be considered as an
example of the principles of the invention and is not intended to limit the
invention to the embodiment illustrated.
A number a different filtering media were prepared and tested,
including some of the prior art media. However, for the clarity of the text,
only the test results for the filtering media according to the preferred
embodiment are included herein. In order to provide comparative
measures, the test results are compared to the results obtained with
activated alumina, which is believed to be the most common arsenic
adsorptive medium in use today. Also, for the clarity of the text, the
filtering media according to the preferred embodiment are referred to
herein as the Medium G2, or Media G2 TM.
9
CA 02317033 2000-08-29
The term Media G2 is used herein to indicate a filtering medium
according to the preferred embodiment and any possible variations to the
content and structure of this filtering medium, wherein the variations are
included within the ranges specified herein.
Preparation of the Activated Alumina
For reference purposes, the activated alumina media used in the
tests mentioned herein below were prepared from a standard 14 mesh
material, rinsed with tap water.
Preparation of the Media G2:
The preferred support material for preparing the Media G2 is
calcined diatomite having particles ranging in size from about 30 mesh
(0.85 mm) to about 60 mesh (0.42 mm). This support material is available
from Eagle-Picher Minerals Inc., a company from Reno, Nevada, USA,
under the designation MP 79.
The calcined diatomite was coated with 4 mL of 2.1 M FeCl3~6H20
solution per gram of diatomite. The solution was mixed into the diatomite
for 30 minutes using an end-over-end stirring apparatus running at 60 rpm.
The resulting slurry was then allowed to sit for 16 hours so that the ferric
chloride could soak into the diatomite particles. After soaking, all excess
solution was poured off of the media material and enough tapwater was
added to just submerge all of the media material. Then, 10 N NaOH was
slowly added over a 10 to 15 minute period to slowly bring the pH of the
slurry from about 1 up to a final value of about 9. Finally, the media
CA 02317033 2000-08-29
material was rinsed with tap water to remove excess ferric hydroxide not
bonded to the media particles. Rinsing was stopped when the
concentration of Fe was less than 0.1 mg/L in the outlet rinsing water.
The Media G2 were prepared in batches, wherein each batch
contained about 75 grams of calcined diatomite. Therefore, the mixing,
settling or reaction time periods specified above may be adjusted
accordingly with other batch sizes.
This method has been found particularly efficient in loading as
much ferric ions into the media as possible. It has been found that the
resulting filtering Media G2 contain as much as 1.36 grams of ferric
hydroxide adsorbed and bonded by ionic bonds to each gram of calcined
diatomite. In another form of expression, the Media G2 contain as much
as 0.71 g of ferric ions per gram of diatomite material, or 30% by weight
of ferric ions.
This particularly high loading of ferric ions to the calcined
diatomite is believed to be an important contributing factor in obtaining the
results and advantageous characteristics of the filtering Media G2 as
explained herein. Although this particularly high loading of ferric ions into
the media particles is believed to be a contributing factor for obtaining
arsenic filtering media that have great adsorption capacities, which are
efficient at drinking-water pH (6.5 - 8.0), and which are renewable several
times, it is believed that such high content of ferric ions is preferable but
not absolutely required for obtaining to a certain extent at least some of the
aforementioned characteristics.
Further testings following the testing program described herein have
indicated that the strong and durable bonds generated during the
11
CA 02317033 2000-08-29
preparation of the Media G2, between the ferric ions and diatomite, and
the renewability feature associated therewith were found in some Media
G2 containing as low as 5% by weight of ferric ions. An efficient use at
drinking-water pHs was also found in some Media G2 containing about
5% by weight of ferric ions. Therefore, it is believed that the nature of the
bonds obtained during the preparation of the Media G2 by the above
method, between ferric ions and the diatomite support material, is as
important as the concentration of ferric ions in the diatomite support
material, for obtaining arsenic filtering media which are renewable several
times and which are efficient at drinking-water pHs.
Example 1: Bench-Scale Column Tests
Each column test was carried out using a filter comprising two
l5mm diameter, 150 mm long plastic columns connected in series. A
nominal quantity of 25 ml. (20g) of filtering Medium G2 was placed into
each of the columns, for a total of 50 mL of medium per filter. Tap water
spiked with 200 pg/L arsenic was pumped to each filter at a rate of 5
mL/min. using a metering pump. The empty bed contact time was therefore
5 minutes through the first column and 10 minutes through both columns.
Samples were collected at regular time intervals from sample points
located after the first and second columns.
Results of these tests are shown in FIGS. 1 and 2. As can be
appreciated the Medium G2 provided outstanding results, treating over
5000 bed volumes before the outlet concentration exceeded 25 ~g/L.
Medium G2 was still producing 2 ~g/L arsenic content until up to 4000
bed volumes. This performance was similar to that of activated alumina,
with both providing an arsenic adsorption capacity of over 1200 ~g/g.
12
CA 02317033 2000-08-29
Samples of the treated water from the column tests as described
above were subjected to a comprehensive metals scan and general
chemistry analysis. Typical results are presented in the following Tables
1 and 2. As can be seen in these tables, the Media G2 did not alter the
water quality in any negative way. Media G2, in addition to removing
arsenic, also provided 80% to 90% removal of copper and 98% to 99%
removal of lead. As a comparison, activated alumina provided 62%
removal of copper and 70% removal of lead. Further studies, as discussed
below, provided results to substantiate the ability of Media G2 to remove
copper and lead.
Table 1: Metal Analyses for Bench-Scale Column Tests
Conc entration
(~g/L)
Raw Activ. AluminaMedia G2
Aluminum 12 12 9
Antimony 0.1 <0.1 0.8
Arsenic 177 2 <1
Barium 129 112 115
Boron 23 24 26
Calcium 9550 9210 9750
Cobalt <0.1 0.2 0.4
Copper 890 340 196
Iron 30 <20 20
Lead 37.1 11.1 0.8
Lithium 1 1.2 1.1
Magnesium 2040 1930 2060
Manganese 8 6 6
Nickel 5 4 5
13
CA 02317033 2000-08-29
Concentration
(~,g/L)
Potassium 750 850 740
Sodium 5930 5880 5600
Strontium 54 56 58
Tin 1.3 0.9 0.4
Zinc 110 66 110
Table 2: General Chemistry Analyses, Bench-Scale Column Tests
C oncentration (mg/L)
Raw Activ. AluminaMedia G2
Ammonia (as N) <0.05 <0.05 <0.05
Alkalinity (as 15 13 14
CaC03)
Chloride 12.5 12.6 12.6
Sulfate 7.7 8.8 7,9
N03 + N02 (as N) 1.88 1.87 1.88
o-Phosphate (as 0.05 <0.01 <0.01
P)
r-Silica (as Si02)10.8 9.6 11.3
Total Organic Carbon<1 1 1
Turbidity (NTU) 0.2 0.2 0.3
Conductivity (~.s/cm)104 99 103
Hardness (as CaC03)32.2 30.9 32.8
Example 2: Pilot-Scale Testing
A nominal quantity of 18.2 Litres (10 kg) of Medium G2 was
placed in a pilot filter, which was operated on a continuous flow of
1.82L/min, with a water pH of 7. The results using this filter are shown in
FIG. 3. Breakthrough occurred after approximately 1500 bed volumes in
14
CA 02317033 2000-08-29
a first run, as indicated by the curve labelled as RUN 1. Breakthrough is
defined as when arsenic concentration at the filter outlet exceeds 25 ~g/L.
As can be appreciated, the results are inferior to those obtained during
bench scale column testing described in Example 1. These differences are
believed due to the water pH, which was 7.0, as compared to 5.8 for the
bench scale column test.
After breakthrough, the Medium G2 was renewed in situ by passing
a small volume of 2N NaOH through it, as will be explained later. The
filter was put back on line and immediately produced water with an arsenic
concentration below 2 ~g/L. Interestingly, the performance ofthe Medium
G2 after restoration, as shown as RUN 2 in FIG. 3, was much better than
during the first run with the same water pH of 7Ø A possible explanation
for this increase in performance is that the sodium hydroxide used in the
restoration procedure may have effectively oxidized some ferric sites on
the medium which were not oxidized during the original medium
preparation procedure. During this second round of tests RUN 2, more
than 2800 bed volumes were passed through the filter, and the outlet
arsenic concentration in the outlet water remained less than 2 ~g/L.
In addition to daily arsenic testing, a complete metal scan and
general chemistry analyses were conducted on the pilot filter inlet and
outlet water. Results are presented in Tables 3 and 4. The results
presented in these tables demonstrate that, as with the bench-scale column
filters, the water chemistry is not negatively impacted in any way by the
Media G2 and that significant copper and lead removal is obtained; 94%
and 84% respectively.
CA 02317033 2000-08-29
Table 3: Metal Analyses For Pilot-Filter Tests Using Media G2
C oncentration L)
(~,g/
Filter Inlet Filter Outlet
Aluminum 19 7
Antimony <0.1 0.3
Arsenic 203 12
Barium 125 4
Boron 35 32
Calcium 8370 14200
Cobalt 0.1 0.4
Copper 177 10
Iron <20 <20
Lead 6.7 1.1
Lithium 1 O,g
Magnesium 1780 2960
Manganese 6 32
Nickel 1 2
Potassium 760 1060
Sodium 128000 105000
Strontium 44 88
Tin 0.3 <0.1
Zinc 23 78
16
CA 02317033 2000-08-29
Table 4: Chemistry Analyses For Pilot-Filter Tests Using Media G2
Concen tration (mg/L)
Filter InletFilter Outlet
Ammonia (as N) <0.05 <0.05
Alkalinity (as 271 240
CaC03)
Chloride 10 10.2
Sulfate 8.9 10.9
N03 + N02 (as N) 1.58 1.54
o-Phosphate (as 0.11 <0.02
P)
r-Silica (as Si02)10 10.7
Total Organic Carbon<1 <1
Turbidity (NTU) <0.1 0.1
Conductivity (~s/cm)521 496
Hardness (as CaC03)28.2 47.6
Example 3: Commercial Installation Testing
A filter containing Medium G2 was used to remove arsenic from the
water supply to a commercial installation at Rose Hill Centre in Holly,
Michigan, USA. The inlet and outlet arsenic concentrations to and from
the filter were monitored by USA's Environment Protection Agency
(EPA), and by personnel from the University of Michigan (UM). The
filter size was determined according to an expected duration of two years
at a continuous water flow of 60 gal./min., and to an arsenic adsorption
capacity of 1200 ~g/g. Test results are available for a period of almost 6
months at the design flow rate, during which time, the Medium G2 has
shown no sign of saturation nor no need for restoration. Test results for
this commercial installation are shown in FIG. 4.
17
CA 02317033 2000-08-29
Example 4: Effect of pH
Both the Media G2 and activated alumina were tested to determine
their arsenic adsorption capacities at a water pH ranging from 6.0 to 8Ø
Results are shown in Table 5 below.
Table 5: Effect of Water pH on Arsenic Adsorption Capacity
pH range Variation in
Adsorption Capacity
Activated Alumina6.0 - 8.0 58
Media G2 6.0 - 8.0 34
As can be appreciated, performance of both media declined
significantly as pH increased. However, Media G2 were more consistent
over the pH range. The adsorption capacity of Media G2 declined by 34%
as water pH was increased from 6.0 to 8.0 while activated alumina
performance decreased by 58% over the same pH range.
Example 5: Effect of Sulfate and Chloride
Two filters containing Media G2 were operated for three days,
corresponding to 430 bed volumes, with a feed water containing 200 ~g/L
arsenic and either one of the following elements and proportions; (a) 500
mg/L sulfate; (b) 500 mg/L chloride; (c) 250 mg/L chloride, or (d) 250
mg/L sulfate. Outlet arsenic concentrations at the end of this period are
shown in Table 6.
18
CA 02317033 2000-08-29
Table 6: Effect of Sulfate and Chloride on Media G2
Inlet Water Contaminant Outlet Arsenic Concentration
(in addition to 200~g/L (~.g/L)
As)
500 mg/L Chloride 11
500 mg/L Sulfate 10
250 mg/L Chloride <2
250 mg/L Sulfate <2
At the 500 mg/L concentration, both chloride and sulfate had a
noticeable effect, although outlet arsenic concentration was still only 10-11
gg/L after 430 bed volumes. At the 250 mg/L concentration, both chloride
and sulfate had no effect. The 250 mg/L testing was then continued a
further six days, for a total of 1150 bed volumes, and still no effect was
observed.
Example 6: Copper Removal
Testing of the Media G2 was carried out at a feed water pH of 5.6,
with an inlet concentration of copper of 3580 mg/L. Removal of copper
was 80% after one day of operation, but quickly declined to less than 50%.
When the filter inlet pH was increased to 8.0, with inlet concentration of
5520 ~g/L, the outlet concentration did not exceed 10 ~,g/L in over 600
bed volumes of operation. This represents a removal efficiency of 99.8 %.
This is illustrated in FIG. 5.
Example 7: Lead Removal
Testing for lead removal was initially carried out at a feed water pH
of 5.6, and filter inlet concentration of lead of 100 ~g/L. Removal
efficiencies were such that the filter outlet concentration did not exceed 3
19
CA 02317033 2000-08-29
~g/L, as shown in FIG. 6. Removal efficiencies declined markedly at
pH 8.0, to as low as 50% after 750 bed volumes of flow.
Consequently, where the Media G2 are being used to remove
copper, the filter inlet water pH should be adjusted to 8.0 whereas, water
containing lead should be adjusted to pH 5.6. A preferred approach to the
situation where both metals are present in the water to be treated, would
include passing the water with a pH of 5.6 over the Medium G2 for
removing lead first, followed by pH adjustment to a drinking water level,
and effecting a second pass over the same medium or over a second bed of
Medium G2 in series with the first one for removing copper.
Example 8: Uranium Removal
Testing has also been carried out to remove uranium from water.
The feed water pH was adjusted to 6.5, with a filter inlet concentration of
uranium of 120 ~g/L. Removal results provided an outlet concentration not
exceeding 4 ~ug/L.
Media G2 Renewability
The terms renewable, renewability and restoration are used herein
to explain the desorption and the rinsing off of the arsenic from a filtration
medium, and the restoring or reviving of its filtration efficiency to a level
which is approaching the new condition of the medium. The terms
renewable, renewability and restoration are also used herein to differentiate
from the word "regenerate" which implies according to an unabridged
definition, to generate or produce anew. The filtering media according to
the present invention are renewed without having to re-impregnate ferric
ions therein.
CA 02317033 2000-08-29
Restoration of the Media G2 is preferably effected in situ by slowly
passing NaOH downward through the media. The in situ restoration
method is preferred to avoid the safety considerations and procedures
associated with the manipulation of arsenic-saturated filtering media and
for being more appropriate for realization in an automatic desorption and
rinsing system. Renewing of arsenic-saturated Media G2 using 0.5 N,
1.ON, and 2.ON NaOH was carried out, and the percentage of arsenic
recovered in each case was measured. Results are presented in Table 7.
Table 7: Restoration Efficiencies of Media G2
NaOH As Adsorbed As Desorbed % Desorbed
ConcentrationOnto Media by NaOH
~~g) ~l~g)
0.5 N 1996 1515 76
1.0 N 2016 1662 82
2.0 N 1970 1503 76
As can be appreciated, all three NaOH concentrations performed
very similarly in terms of desorbing arsenic from the filtering media
samples.
Media G2 were also tested for multiple restorations. Results of tests
using multiple restorations of Media G2 and their uses in pilot filters are
presented in Table 8. In this table, the renewability of Media G2 is
compared to the renewability of activated alumina.
It has been found that between the first and fifth renewing cycles,
Media G2's adsorption capacity decreased by 33%, while the performance
21
CA 02317033 2000-08-29
of activated alumina decreased much more significantly, that is by 54%.
This equals a reduction in performance of less than 10% per cycle for
Media G2 and 18% per cycle for the activated alumina.
Table 8: Adsorption After Multiple Restorations
After 1 st DesorptionAfter 5th Desorption
Media G2 580 ~g/g 390 ~g/g
Activated Alumina982 ~g/g 453 ~,g/g
Accordingly, the restoration of the filtering efficiencies of Media
G2 is achieved by using from at least as low as 0.5 N NaOH to at least as
high 2.0 N NaOH. Slower decline in performance have been observed
when the restoration is carried out in-situ using a circulation of sodium
hydroxide and rinsing with water, wherein the sodium hydroxide was
passed slowly downward through the filtering media.
Applicability of the Media G2 in the Industry
As may be appreciated, the Media G2 are simple to prepare and
highly effective in removal of arsenic from water. In side-by-side column
testing under identical conditions, they provided an arsenic removal
capacity similar to that of activated alumina. A total of 5000 bed volumes
of water containing 200 pg/L of arsenic was treated before the outlet
concentration of the filter exceeded 25 ~.g/L. This is equivalent to an
arsenic adsorption capacity of over 1200 ~g/g of medium. With a 10
minutes of empty bed contact time, this means a continuous operation time
of over 830 hours or 35 days before washing and restoration would be
required.
22
CA 02317033 2000-08-29
Washing and restoration of a filter containing about 10 kg of
filtering media for example, require approximately 2 hours, meaning a
down time of only 0.2 % and volume of waste production of about 0.1
of the treated water volume
While the above-described tests used a very high raw water arsenic
concentration of 200 ~g/L, it is known that 95 % of North American water
supplies have less than SO~g/L of arsenic. Run times between washing
and restoration would theoretically be four times longer with such water.
In residential applications, a filtering cartridge as known in the art,
containing the Medium G2 is preferably installed in a water supply system
such that the flow of water through the filter is downward. The volume of
the filter cartridge is preferably selected such that a water retention time
through the Medium G2 is at least about 10 minutes. Desorbing, washing
and restoration of the Medium G2 is preferably effected in-situ using
piping systems, pumps and timers that are known to those skilled in the art
of water softeners for example.
In the larger applications, the filtering Media G2 are preferably
installed in filter beds or in large filter reservoirs as also known in the
art.
Again, the quantity of Media G2 and the size of the bed or tank are
selected to provide a water retention time through the Media G2 of about
10 minutes.
As to additional details related to the manufacturing, installation
and use of the filtering media of the present invention, the same should be
23
CA 02317033 2000-08-29
apparent from the above description, and accordingly further discussion
relative to the manner of making, using and renewing the Media G2 would
be considered redundant and is not provided.
While one embodiment of the present invention has been described
herein above, it will be appreciated by those skilled in the art that various
modifications, alternate compositions, alternate methods and equivalents
may be employed without departing from the true spirit and scope of the
invention. Therefore, the above description and illustrations should not be
construed as limiting the scope of the invention which is defined by the
appended claims.
24