Note: Descriptions are shown in the official language in which they were submitted.
CA 02318325 2000-07-18
WO 99/40465 PCTIUS98/13555
OPTICAL FIBER CONNECTOR USING PHOTOCURABLE ADHESIVE
Background of the Invention
Field of the Invention
The invention relates to an optical fiber connector and the use of radiation
in the
visible portion of the spectrum to cure a phatocurable adhesive in an optical
fiber
connector to bond the optical fiber, the reinforcing fibers and the optical
holder into a
unified structure, in order to provide an improved connection between two
optical fibers
or an optical fiber and an optoelectronic component.
Description of the Art
Optical fibers have replaced copper wire as the preferred medium for carrying
telecommunications signals. As with copper wire, it is necessary to provide
for the
interconnection of optical fibers during installation, repair or replacement
of the fibers,
and to terminate the fibers onto active optical devices. There are generally
two kinds of
interconnection devices, splices and connectors. The term "splice" usually
refers to a
device, which provides a permanent connection between a pair of optical
fibers. The term
"connector," in contrast, usually refers to a device, which may be engaged and
disengaged
repeatedly, often with a different plug or receptacle. A connector may also
refer to the
plug portion of a fiber termination, which is attached to an optical device.
Optical devices
include, for example, optical sensors {photoelectric diodes) and light sources
(LED's,
laser diodes). The termination of an optical fiber may be indirect, i.e., the
fiber may be
connected to some other (passive) optical device such as a beam splitter or
poiarizer,
before the light beam is directed to the active optical device. The present
invention is
generally directed to a connector, although this term should not be construed
in a limiting
sense since the present invention may inherently provide a permanent, as well
as
temporary connection or termination.
In the fiber optic connector described in U.S. Patent No. 5,381,498, the
connector
has a plug and a receptacle, the plug having a fiber-receiving, V-shaped
groove for each
fiber to be interconnected, with the end of the fiber terminating in the
middle of the
groove. The receptacle has a plate which retracts as the plug is inserted,
whereby another
-1-
CA 02318325 2000-07-18
WO 99/40465 PCT/US98/13555
fiber is lowered into the V-groove of the plug. Upon full insertion of the
plug, the two
fiber ends are in contact, and the fiber secured to the receptacle is
elastically deformed to
maintain a continuous compressive load between the terminal ends of the
fibers. The
connector provides for the quick disconnection and reconnection of a plurality
of optical
fiber pairs, without the use of ferrules or other alignment members. High
strength fiber
may be used to withstand repeated insertions and bowing of the fibers. The
exact lengths
of fibers (i.e., the relative locations of their terminal ends in the plug and
receptacle) are
not critical since tolerance is provided by the slack taken up in the bowed
receptacle fiber
(the terminal portion of the fiber secured to the plug does not bow, but
always remains
straight). The ends of the fibers may be prepared by simply cleaving and
beveling; the
end faces may optionally be cleaved at an angle (i.e., non-orthogonal to the
fiber axis) to
reduce signal reflections.
Many fiber optic splices employ plate elements having fiber-receiving grooves,
with mechanisms for clamping the terminal ends of the fibers in a common
groove. Some
of these devices are designed to interconnect a plurality of pairs of fibers,
such as the
splice shown in U.S. Patent No. 5,151,964. In U.S. Patent No. 4,028,162,
fibers approach
alignment grooves at a glancing angle and are held temporarily while a
connector plate is
adhered to the interconnected fibers. For other examples of techniques
involving bowed
fibers entering alignment grooves, see U.S. Patent Nos. 4,077,702, 4,148,559,
4,322,127
and 5,080,461, and French Patent Application No. 2;660,442. Some of the
connector
designs using the principle of bowing a fiber into a fiber-alignment groove
are rather
complex and require many parts, such as the designs seen in U.S. Patent Nos.
4,045,121,
4,218,113 and 4,767,180.
In order to provide a termination or interconnection with the required
strength
without damaging the system, the fibers must be secured to the connector body
to inhibit
or at least reduce the relative movement between the optical fiber and its
outer sleeve.
Such an attachment system may be mechanical, such as a clamp or set of clamps
or it may be a type of adhesive. A mechanical system may also include strength
members
such as layers of stranded steel wire, as disclosed in U.S. Patent No.
5,539,849.
Useful adhesives for termination must be capable of bonding to the outer
surface
of the fiber, which may be formed from materials such as glass, epoxy
silicones, and the
-2-
CA 02318325 2000-07-18
WO 99140465 PCTNS98I13555
like. It also must be capable of bonding to other materials used in fiber
optic cables and
their tenminations, such as polymeric coating layers, and strengthening fibers
used to
surround the optical fibers, and plastics from which the holder is formed. The
strengthening fibers are typically aromatic polyamide fibers derived from
S p-phenylenediamine and terephthaloyl chloride, available commercially as
Nomex~ or
Kevlar~.
U.S. Patent No. 4,699,462 discloses a method for foaming a termination between
a
fiber optic cable having a centrally positioned optical fiber, a plurality of
surrounding
reinforcements, and a component housing. An adhesive, preferably a heat
activated
adhesive, is applied within the termination and heat shrink tubing is applied
in order to
force the reinforcement fibers into adhesive engagement with the adhesive
layer. Bond
formation occurs primarily at the interface between the cladding on the
optical fiber core,
and reinforcement strands. The adhesive does not provide bonding to the heat
shrink
tubing; it is present to provide reinforcement to the termination.
U.S. Patent No. 5,058,984 discloses a fiber optic cable connector comprising a
plastic outer sleeve to be optically coupled to another optical fiber cable,
carrying at one
end, connection means for coupling, a tubular gripping member which adheres
the fiber to
the outer sleeve or holder, which is deformed by application of force so as to
grip the end
portion of the plastic outer sleeve and a female mounted within the other end
of the
connector body supporting an exposed end portion of an optical fiber. The
optical fiber is
adhered to the ferrule with adhesive material. The female is ceramic and the
exposed end
of the fiber is set with a light curable resin, generally blue light where the
female is
formed of zirconia. This allows a setting time to be reduced to about 60
seconds. It is
specifically disclosed that an irradiation curable adhesive might not adhere
sufficiently
strongly to the plastics outer sleeve; therefore the adhesive material is used
to secure the
end portion within the ferrule, and does not need to provide any adhesive to
the plastic
outer sleeve.
As can be seen, even with the use of adhesives, conventional fiber optic
connector
assemblies have required the use of additional positioning or bonding means in
order to
resist disruptive force. It would be very desirable to eliminate such means
and be able to
provide a unified system wherein the bond is foamed solely from an adhesive
which
-3-
CA 02318325 2000-07-18
WO 99/40465 PCTIUS98/13555
adheres the optical fiber to the outer holder of the connector, and adheres to
the fiber as
well as the coating and strengthening fibers without requiring additional
positioning
means such as heat shrink tubing, gripping members and the like.
U. S. Patent No. 5,525,648 discloses a treatment method for application to
dentin
and cervical enamel or adhering to hard tissue in a high humidity environment.
Primer
compositions disclosed bond strongly to dentin, and exhibit high shear
strength, and
include an acid and a film former, which are applied and then hardened. A wide
variety
of acids are useful, including organic, inorganic, solid and liquid acids.
Useful film
formers are water dispersible and may be selected from many polymers, monomers
and
mixtures. After standing time to achieve priming, the primer, optionally, with
an
additional layer of film former is then hardened by use of a polymerization
catalyst.
U.S. Patent No. 5,545,676 discloses a three component or ternary
photoinitiator
system for use in additional polymerization. A variety of acrylate monomers
are
disclosed. The system is disclosed to provide a combination of cure speed,
cure depth
and shelf life, and is disclosed to be useful in color profiling systems,
curable inks,
printing plates, photoresists, coated abrasives, photocurable adhesives and
composites,
e.g., for dentistry or autobody repair.
The present invention provides a fiber optic connector using a visible light
photocurable adhesive composition to bond the optical fiber(s), the
strengthening fibers
surrounding the fiber and a coating layer thereover into a unified structure.
The system
provides a fast cure, good depth of cure, and a safe, low energy means for
providing the
bond, which can be easily accomplished by a tradesperson.
Summary of the Invention
The invention provides an optical fiber connector comprising an optical fiber,
and
a termination or interconnection bonded in place solely by means of a visible
light
photocurable adhesive.
Specifically, the invention provides a fiber optic connector including
a) at least one fiber optic cable comprising at least one optical fiber
surrounded by a plurality of strengthening fibers, both of which are
surrounded by
at least one polymeric coating layer, a portion of said cable being stripped
in a
q._
CA 02318325 2000-07-18
WO 99140465 PGTIUS98I13555
layerwise manner such that an area of exposed fiber is succeeded by an area of
exposed strengthening fibers, succeeded by an area of coated cable;
b) a holder for the optical cable, and
c) a visible light curable adhesive injected into the holder,
S the optical fiber, the strengthening fibers and the holder being bonded into
a unified
structure by means of exposure to visible light for a period of up to 30
seconds.
Preferably, the adhesive comprises at least one acrylate monomer. The adhesive
is
curable by visible radiation having a wavelength between 400 and 700
nanometers;
preferably from 400 to about 600 nanometers, which includes a portion of the
blue and
the green area of the spectrum; most preferably between 500 and 600
nanometers. Curing
time is less than 30 seconds, preferably less than 25 seconds. The adhesive
bonds
strongly to the fiber, coating and holder to form a unified structure, such
that the structure
is not easily disrupted when force is applied to the connector, or to the
strengthening
fibers.
In an alternate embodiment, the adhesive is a photocurable adhesive which is
curable by exposure to radiation having wavelengths of from 700 nm to 1100 nm,
in the
near-infrared region.
Preferred optical fiber connector assemblies of the invention comprise an
adhesive
having a ternary photoinitiator system comprising an electron donor, a
sensitizer and a
diaryliodonium salt.
The invention also provides a method of bonding interconnections or
terminations
in optical fiber connectors and cables wherein the termination or
interconnection is
bonded in place solely by means of a visible light photocurable adhesive.
As used herein, these terms have the following meanings.
1. The term "visible light" means electromagnetic radiation having
wavelengths between 400 nm and 700 nm.
2. The term "near-infrared", abbreviated "nIR", means electromagnetic
radiation having wavelengths between 700 nm and 1000 nm.
3. The term "plug" means an article, which is present in a connector, for
retaining and selectively aligning the first optical fiber in a connector.
Plugs are
insertable into the receptacle to form a connection or termination.
_5_
CA 02318325 2000-07-18
WO 99/40465 PCT/US98/13555
4. The term "receptacle" means an article present in a connector, for
retaining
and selectively aligning the second optical fiber in a connection.
5. The term "holder" means that portion of the plug which holds the first
optical fiber in place.
6. The term "connector" means an article for forcing the end of a first
optical
fiber towards an end of a second optical fiber in contact in the end of the
first optical
fiber. A connector includes a plug and a receptacle.
7. The term "unified structure" as used herein refers to the condition wherein
components, including the optical fiber, plurality of reinforcing fibers and
the holder are
IO joined in a bonded, fixed relationship held together by photocurable
adhesive that has
been exposed to actinic radiation of suitable wavelength for adhesive
polymerization.
8. The terms "termination" and "connection" mean the point at which a first
optical fiber is forced into contact with either a second optical fiber or an
optoelectronic
device.
9. The term "(meth)acrylate" includes both the acrylate and the methacrylate.
10. The term "GGP" fiber refers to a glass fiber ocverconated concentrically
with successive layers of glass cladding and polymeric, frequently epoxy
silicone coating
layers.
As used herein, all parts, percents, and ratios are by weight, unless
specifically
stated otherwise.
Brief Description of the Drawina~s
The invention will best be understood by reference to the accompanying
drawings,
wherein:
Figure 1 is a side view of a longitudinal section of one embodiment of the
present
invention, depicting a fiber optic connector including a plug and receptacle;
Figure 2 is a perspective view of the plug and receptacle of Figure 1, with a
partial
section revealing the bowed fibers in the plug interior.
Detailed Description of the Invention
Optical fiber assemblies of the invention comprise at least one optical fiber
in a
holder, adhered to the holder by means of a photocurable adhesive. While
previous
_6_
CA 02318325 2000-07-18
WO 99/40465 PCT/US98I13555
systems using adhesives have required other positioning devices such as heat
shrinkable
tubing, "gripping members" and the like, the assemblies of the invention
develop a strong
bond between the adhesive and the various portions of the connector such as
the holder,
the fiber optical cable coating, the strengthening members and the optical
fiber, forming a
unified structure.
Adhesive systems useful in assemblies of the invention are photocurable in the
visible area of the spectrum, and can be applied as one or two part adhesives.
The
adhesives may be provided already in the fiber assembly, or they may be
provided
separately, in syringe-like applicators, to enable application in the field.
A wide variety of monomers can be photopolymerized to form the connection or
termination in the connector of the invention.
Suitable monomers contain at least one ethylenically-unsaturated double bond,
and are capable of undergoing addition polymerization. The molecular weight
may vary,
and the "monomers" discussed may include oligomers.
One preferred monomer is formed by combining a dimethacrylate derived from
the reaction methacrylic acid and the diglycidyl ether of bisphenol A
(BISGMA)with a
hydrophilic monomer such as hydroxypropyl methacrylate, hydroxyethyl
methacrylate
(HEMA), or methacrylic acid. Such monomers include mono-, di-, or poly(meth)-
acrylates such as methyl (meth)acrylate, ethyl (meth)acrylate, isopropyl
(meth)acrylate,
n-hexyl (meth)acrylate, stearyl (meth)acrylate, allyl (meth)acrylate, glycerol
di(meth)acrylate, glycerol tri(meth)acrylate ethylene glycol di(meth)acrylate,
diethyleneglycol di(meth)acrylate, triethyleneglycol di(meth)acrylate,
diethyleneglycol
di(meth)acrylate, triethyleneglycol dimethacrylate, 1,3-propanediol
di(meth)acrylate,
trimethylolpropane tri{meth)acrylate, 1,2,4-butanetriol tri(meth)acrylate, 1,4-
cyclohexanediol diacrylate, pentaerythritol tetramethacrylate, sorbitol
hexa(meth)acrylate,
bis-1-(2-acryloxy)]-p-ethyoxyphenyldimethyl methane, bis[1-(3-acryloxy-2-
hydroxy)J-p-
propoxyphenyldimethylmethane, trihydroxyethyl-isocyanurate tri(meth)acrylate;
the bis-
acrylates and bis-methacrylates of polyethylene glycols having molecular
weights
between 200 and 500; copolymerizable mixtures of acrylate monomers such as
those
disclosed in U.S. Patent No. 4,652,274, and acrylated oligomers such as those
disclosed in
U.S. Patent 4,642,126, unsaturated amides such as methylene bis-acrylamide,
methylene
_7_
CA 02318325 2000-07-18
WO 99140465 PCTIUS98/13555
bis-methacrylamide, 1,6-hexamethylene bis-acrylamide, diethylene triamine tris-
acrylamide and beta-methyacrylaminoethyl methacrylate, and vinyl compounds
such as
styrene, diallyl phthalate, divinyl succinate, divinyl adipate and
divinylphthalate. The
adhesive may contain only one type of monomer or mixtures of two or more
monomers
may be used.
The photoinitiator system is one, which is capable of light absorption in the
visible
range, i.e., between 400 nm and 700 nm, or in the near-IR region of 700 to
1100 nm. In
preferred assemblies of the invention, the photoinitiator absorbs light
between 400 nm
and 600 nm, more preferably between 500 nm to 600 nm, in the green and a
portion of the
blue portion of the spectrum. Components in the photoinitiator system include
at least
one initiator and at least one sensitizes.
Useful sensitizers should be soluble in the monomer and are capable of light
absorption in the appropriate wavelengths. The sensitizes is also preferably
capable of
sensitizing 2-methyl-4,5-bis(trichloromethyl)-s-triazine, according to the
test procedure
described in U.S. Patent No. 3,729,313. Preferably, the sensitizes is also
shelf stable for
reasonable periods of time.
Suitable sensitizers are believed to include compounds in the following
categories:
ketones, coumarin dyes (e.g., keto-coumarins), xanthene dyes, acridine dyes,
thiazole
dyes, thiazine dyes, oxazine dyes, azine dyes, aminoketone dyes, porphyrins,
aromatic
polycyclic hydrocarbons, p-substituted aminostyryl ketone compounds,
aminotriaryl
urethanes, merocyanines, squarylium dyes and pyridinium dyes. Ketones (e.g.,
monoketones or alpha-diketones), ketocoumarins, aminoarylketones and p-
substituted
aminostyryl ketone compounds are preferred sensitizers. For applications
requiring high
sensitivity, it is preferred to employ a sensitizes containing a julolidinyl
moiety. For
applications requiring deep cure (e.g., where the coating or strengthening
fibers attenuate
radiation of similar wavelengths), it is preferred to employ sensitizers
having an
extinction coefficient below 1000, more preferably below 100, at the desired
wavelength
of irradiation for photopolymerization.
_g_
CA 02318325 2000-07-18
WO 99!40465 PGT/US98/13555
By way of example, a preferred class of ketone sensitizers has the formula:
ACO(X)bB
where X is CO or CR'R2, where R' and RZ can be the same or different, and can
be
hydrogen, alkyl, alkaryl or aralkyl, b is one ar zero, and A and B can be the
same or
different and can be substituted (having one or more non-interfering
substituents) or
unsubstituted aryl, alkyl, alkaryl, or aralkyl groups, or together A and B can
form a cyclic
structure which can be a substituted or unsubstituted cycioaliphatic, aromatic
heteroaromatic or fused aromatic ring.
Suitable ketones of the above formula include monoketones (b = 0) such as 2,2-
,
4,4- or 2,4-dihydroxybenzophenone, di-2-pyridyl ketone, di-2-furanyl ketone,
di-2-
thiophenyl ketone, benzoin, fluorones, quinones, e.g., chloroquinone, 2-aza-3-
carboxy-9-
fluorone, and the like, chalcone, Michler's ketone, 2-fluoro-9-fluorone, 2-
chlorothioxanthone, acetophenone, benzophenone, 1- or 2-acetonaphthone, 9-
acetylantracene, 2-, 3- or 9-acetylphenanthrene, 4-acetylbiphenyl,
propiophenone,
n-butyrophenone, valerophenone, 2-, 3- or 4-acetylpyridine, 3-acetylcoumarin
and the
like. Suitable diketones include aralkyldiketones such as anthraquinone,
phenanthrenequinone, o-, m- and p-diacetylbenzene, 1,3-, 1,4-, 1,5-, 1,6-, 1,7-
and 1,8-
diacetylnaphthalene, 1,5-, 1,8- and 9,10-diacetylanthracene, and the like.
Suitable
a-diketones (b = 1 and X = CO) include 2,3-butanedione, 2,3-pentanedione, 2,3-
hexanedione, 3,4-hexanedione, 2,3-heptanedione, 3,4-heptanedione, 2,3-
octanedione, 4,5-
octanedione, benzil, 2,2'-, 3,3'- and 4,4'-dihydroxylbenzil, furil, di-3,3'-
indolylethanedione, 2,3-bornanedione (camphorquinone), 1,2-cyclohexanedione,
1,2-
naphthaquinone, acenaphthaquinone, and the like.
Other preferred sensitizers include Rose Bengale, Fluorescein, Eosin Yellow,
Eosin Y, Ethyl Eosin, Eosin Bluish, Erythrosin Yellowish Blend, 4',5'-
Dibromofluorescein.
The photoinitiator system also includes an electron donor. A wide variety of
donors can be used; the donor should be soluble in the monomer, and have good
shelf
stability. Suitable donors are capable of increasing the speed of cure or
depth of cure of a
composition upon exposure to light of the desired wavelength. The donor has an
oxidation potential greater than zero, and less than ar equal to the oxidation
potential of p-
-9-
CA 02318325 2000-07-18
WO 99140465 PGTIUS98/13555
dimethoxybenzene. Preferable the oxidation potential is between about 0.5 and
1 volts vs.
A saturated calomel electrode (S.C.E.). Values may be measured experimentally
or
obtained from references such as N.L. Weinburg, Ed., Technique of
Electroorganic
Synthesis Part 11 Techniques of Chemistry, Vol. V (1975) and the like.
Preferred donors include amines (including aminoaldehydes and aminosilanes),
amides (including phosphoramides), ethers (;including thioether), areas
(including
thioureas), ferrocene, sulfinic acids and their salts, salts of ferrocyanide,
ascorbic acid and
its salts, dithiocarbamic acid and its salts, salts of xanthates, salts of
ethylene diamine
tetraacetic acid, and salts of tetraphenylboronic acid. The donor can be
unsubstituted or
substituted with one or more non-interfering substituents. Particularly
preferred donors
contain an electron donor atom such as a nitrogen, oxygen, phosphorus, or
sulfur atom,
and an abstractable hydrogen atom bonded to a carbon or silicon atom alpha to
the
electron donor atom.
Preferred amine donor compounds include alkyl-, aryl-, alkaryl- and aralkyl-
amines such as methylamine, ethylamine, propylamine, butylamine,
triethanolamine,
amylamine, hexylamine, 2,4-dimethylaniline, 2,3-dimethylaniline, o-, m- and p-
toluidine;
benzylamine, aminopyridine, N,N'-dimethylethylenediamine, N,N'-
diethylethylenediamine, N,N'-dibenzylethylenediamine, N,N'-diethyl-1,3-
pmpanediamine, N,N'-diethyl-2-butene-1,4-diamine, N,N'-dimethyl-1,6-
hexanediamine,
piperazine, 4,4'-trimethylenedipiperidine, 4,4'-ethylenedipiperidine, p-N,N-
dimethyl-
aminophenethanol and p-N-dimethylaminobenzonitrile; aminoaldehydes such as p-
N,N-
dimethyiaminobenzaldehyde, p-N,N-diethylaminobenzaldehyde, 9 julolidine
carboxaldehyde and 4-morpholinobenzaldehyde; and aminosilanes such as
trimethylsilylmorpholine, trimethylsilylpiperidine,
bis(dimethylamino)diphenylsilane,
tris(dimethylamino)methylsilane, N,N-diethylaminotrimethylsilane,
tris(dimethylamino)phenylsilane, tris(methylsilyl)amine,
tris(dimethylsilyl)amine,
bis(dimethylsilyl)amine, N,N-bis(dimethylsilyl)aniline, N-phenyl-N-
dimethylsiiylaniline
and N,N-dimethyl-N-dimethysilylamine. Tertiary aromatic alkylamines,
particularly
those having at least one electron-withdrawing group on the aromatic ring,
have been
found to provide especially good shelf stability. Good shelf stability has
also been
obtained using amines that are solids at room temperature.
-10-
CA 02318325 2000-07-18
WO 99/40ri65 PCTNS98I13555
Preferred amide donor compounds include N,N-dimethyiacetamide, N,N-
diethylacetamide, N-methyl-N-phenylacetamide, hexamethylphosphoramide,
hexaethylphosphoramide, hexapropylphosphoramide, trimorpholinophosphine oxide
and
tripiperidinophosphine oxide.
Suitable ether donor compounds include 4,4'-dimethoxybiphenyl, 1,2,4-
trirnethoxybenzene and 1,2,4,5-tetramethoxybenzene.
Suitable urea donor compounds include N,N'-dimethylurea, N,N-dimethylurea,
N',N'-diphenylurea, tetramethylthiourea, tetraethylthiourea, tetra-n-
butylthiourea, N,N-
di-n-butylthiourea, N,N'-di-n-butylthiourea, N,N-diphenylthiourea and N,N'-
diphenyl-
N,N'-diethylthiourea.
In one embodiment of the invention, the photoinitiator system is a ternary
system,
according to U.S. Patent No. 5,545,676. In such a three component system, the
additional
component is a diaryliodonium or suifonium salt. The salt should also be
soluble in the
monomer and be shelf stable when dissolved therein in the presence of the
sensitizer and
donor. Accordingly an election of a particular iodonium or sulfonium salt may
depend to
some extent on the monomers selected, and the other portions of the
photoinitiator
system. Such ternary system must contain these three parts; however, it may
contain
more than one sensitizer or electron donor, if desired.
Useful salts are those disclosed in U.S. Patent Nos. 3,729,313, 3,741,769,
3,808,006, 4,250,053, and 4,394,403.
Preferred iodonium salts include diphenyliodonium chloride, diphenyliodonium
hexafluorophosphate and diphenyliodonium tetrafluorborate.
Useful sulfonium complex salts are substituted with at least one, and
preferably
three, aromatic gmups. Representative groups are aromatic groups having 4 to
20 carbon
atoms and are selected from phenyl, thienyl and furanyl groups. These aromatic
groups
may optionally have one or more fused benzo rings (e.g., naphthyl and the
like;
benzothienyl, dibenzothienyl; benzofuranyl, dibenzofuranyl; etc.). Such
aromatic groups
may also be substituted, if desired, by one or more of the following groups,
or by other
groups which are essentially non-reactive with other components present in the
particular
composition in which the complex salt is to be used: halogen, vitro, aryl,
ester groups,
sulfoester groups, amido groups, carbamyl groups, sulfamyl groups, alkoxy
groups, aryl
-11-
CA 02318325 2000-07-18
WO 99/40465 PCTNS98/13555
groups, alkyl groups, aryloxy groups alkylsulfonyl groups, arylsulfonyl,
perfluoroalkyl
groups, and perfluoroalkylsulfonyl groups.
Examples of suitable aromatic sulfonium complex salt photoinitiators include:
triphenylsulfonium tetrafluoroborate, methyldiphenylsulfonium
tetrafluoroborate
dimethylphenylsulfonium hexafluorophosphate triphenylsulfonium
hexafluorophosphate,
triphenylsulfonium hexafluoroantimonate, 4-butoxyphenyldiphenylsulfonium
tetrafluoroborate, 4-chlorophenyldiphenylsulfonium hexafluoroantimonate,
tris(4-
phenoxyphenyl)sulfonium hexafluorophosphate, di(4-ethoxyphenyl)methylsulfonium
hexafluoroarsenate, 4-acetamidophenyldiphenylsulfonium tetrafluoroborate
dimethylnaphthylsulfonium hexafluorophosphate,
trifluoromethyldiphenylsulfonium
tetrafluoroborate, methyl(N-methylphenothiazinyl)sulfonium
hexafluoroantimonate
phenylmethylbenzylsulfonium hexafluorophosphate, 10-methylphenoxathiinium
hexafluorophosphate, 5-methylthianthrenium hexafluorophosphate, 10-phenyl-9,9-
dimethylthioxanthenium hexafluorophosphate, I0-phenyl-9-oxothioxanthenium
tetrafluoroborate, 5-methyl-10,10-ioxothianthrenium hexafluorophosphate.
The preferred salts are the triaryl-substituted salts such as
triphenylsulfonium
hexafluorophosphate. The triaryl-substituted salts are preferred because they
are more
thermally stable than the mono- and diaryl substituted salts and accordingly
may be used
in one-part curable systems where long shelf life is desired. The triaryl-
substituted
complex salts are also more amenable to dye sensitization in accordance with
this
invention.
The adhesive of the present invention may be used with a variety of different
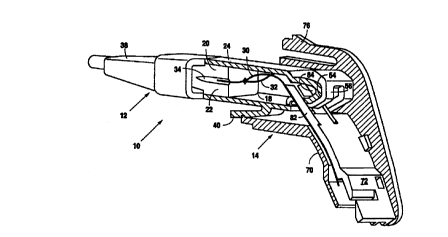
connector designs. Figures 1 and 2 illustrate one embodiment 10 of a fiber
optic
connector in accordance with the present invention. The connector 10 is
comprised of an
elongate plug 12 and a socket or receptacle 14. Figure 1 is a longitudinal
section of
connector 10 showing plug 12 fully inserted in receptacle 14, and receptacle
14 mounted
on a support surface or bulkhead 16. Figure 2 is a perspective view with
bulkhead 16
omitted, also with a partial longitudinal section to illustrate the interior
of the connector.
The depicted embodiment provides for the interconnection of two pairs of
fibers, but
those skilled in the art will appreciate that the inventive concepts described
herein extend
to single pair interconnection as well as interconnection of a multiplicity of
pairs.
-12-
CA 02318325 2000-07-18
WO 99I404S5 PCTNS981I3555
The plug 12 includes a fiber holder 18 which may be constructed of two
clamping
elements or blocks 20 and 22, and a plug body or shroud 24 which is attached
to fiber
holder 18. Optical fibers 30 and 32 which are to be interconnected or
terminated pass
through holder 18 and into the hollow interior of shroud 24. The terminal
portions of the
fibers are bare, that is, they are not affixed to any alignment member such as
a female.
The shroud 24 therefore serves not only to assist in physically locating plug
12 in
receptacle 14, but also to provide protection for the otherwise exposed
terminal portions
of the fibers (the shroud could be made retractable to fully expose the fiber
tips, if
required). The holder 18 has fiber-receiving grooves 34 formed in the adjacent
surfaces
of blocks 20 and 22; these two components may be identical parts.
The optical fibers are secured to holder 18 by use of the adhesive heretofore
described. The adhesive is injected through holes in the connector for that
purpose, and
cured. The adhesive should adhere to the cable coating, which is typically
epoxy silicone,
polyolefm or polyvinylchloride, where such is still present as well as the
strengthening
fibers, e.g., Kevlar~, in those areas where the outer coating has been
stripped away, to an
inner polymeric layer, which is likewise typically epoxy silicone, polyolefin
or
polyvinylchloride, and finally to the fiber itself to hold the fiber in
position.
The holder 18 may have an extension 36 surrounding the fibers for additional
strain relief and clamping. A boot 38 may be provided for further strain
relief and
capturing of the strengthening fibers in the fiber cable (KEVLAR~ strands),
and to assist
in handling plug 12. The strengthening fibers need not be crimped, but they
may be
adhered into the unified structure of the holder by means of the same adhesive
as used to
secure the fiber to the holder, as described supra. Strain relief of the
strengthening
members is attained by a force fit of straightwall section of the optical
fiber holder within
the boot 38. This is dependent upon the choice of materials used for the boot
38 and the
optical fiber holder 18, and yields a design that does not require a crimp
ring and which
assists in ease of manufacture and reduces the number of needed components.
"One-way"
barbs on the surface of the optical fiber holder assist in the attachment of
the boot 38 to
the optical fiber holder 18, which also assists in the attachment of the cable
to the plug 12.
A latch 40 is integrally molded onta one side of shroud 24 to releasably
secure
plug 12 to receptacle 14. The latch 40 may also impart mechanical polarization
to the
-13-
CA 02318325 2000-07-18
wo mao46s pcrius9m3sss
plug, that is, it may be inserted into receptacle 14 only in one orientation.
Plug 12 may be
biased in the interconnected position, e.g., by a springboard (a flexible
cantilever} formed
inside receptacle 14, to be pushed back against latch 40 to minimize the
effect of
manufacturing tolerances.
S The receptacle 14 includes a body or housing 70 and another fiber holder 72.
The
housing 70 may also have appropriate features (such as latch arms 76) allowing
it to be
releasably mounted to bulkhead 16 which may be, for example, a patch panel or
workstation outlet (wall box faceplate). The latch mechanism may provide for
mounting
from the front of the panel, to allow all preparatory work to be done at the
front side of
the panel, or may provide for mounting from the rear of the panel, to allow
all preparatory
work to be done at the back side of the panel. Additional mechanisms may be
provided,
such as the fiber hold-down, to retain the fibers firmly in the grooves. The
fibers do not
extend to the very tips of fingers 82 and 84 but rather terminate a su~cient
distance from
the tips to allow proper support of the portion of the optical fibers in the
plug when the
connector is in use. If the fiber-to-fiber contact occurs very near the tips
of the V-grooves
(or if the plug is inserted too far}, the fiber portion in the plug can bend
beyond the groove
and lifted away from the apex, breaking the connection.
The receptacle 14 may have as many of these fingers with fiber-alignment
grooves
as there are fibers in plug I2. Fingers 82 and 84 are shaped to project into
slots s4 and
56, respectively, of shroud 24 when plug 12 is fully inserted into receptacle
14. Fingers
82 and 84 enter shroud 24 at an oblique (nonzero) angle with respect the plug
axis, i.e.,
the axis defined by either of the optical fibers 30 or 32 when they are
extending straight
within shroud 24. This angle is preferably about 42°, which balances
concerns regarding
fiber end face contact pressure, fiber forces directed into the V-groove, the
effects of
friction, and the desired tolerance window (a larger angle increases
tolerances}. Since the
receptacle fibers are not directed toward opening 74, there is no danger of
escaping light
injuring a user's eyes. Receptacle fiber holder 72 is pivotally attached to
housing 70 by
providing posts on the first end of holder 72 which snap into cutouts or hooks
88 formed
at one end of receptacle housing 70. The holder 72 releasably locks into place
using
bumps or studs formed on the side of the holder, which engage holes 89 in
receptacle
housing 70. An alternative design for the receptacle fiber holder may be used
in which
-14-
CA 02318325 2000-07-18
WO 99140465 PCTNS98/13555
the holder is molded as a single piece with a breakaway top or cover plate
that can snap
onto its base, the base having the fiber-positioning grooves.
All of the components of connector 10 (except plug boot 38) may be formed of
any durable visable light or infrared radiation transmitting material,
preferably an
injection moldable polymer such as polycarbonate, VALOX (a polyester sold by
General
Electric), or RADEL (a polyarylsulfone sold by Amoco). The material may
include
conductive fillers to render the components semiconductive in order to
minimize
triboelectric charging which can induce fiber end contamination, so long as
such fillers do
not unduly attenuate the radiation during curing. The boot 38 is preferably
formed of low
modulus copolyester elastomer, such as that available from RTP of Winona,
Minnesota,
under material number 1559X67420B.
Assembly and installation of connector 10 is straightforward. Plug 12 is
typically
assembled in the factory, although it may easily be assembled in the field. To
place the
adhesive into the assembly, simply place adhesive into a syringe like
applicator (if
desirable, the adhesive may be provided in such an applicator), over the
opening in the
holder and inject the adhesive into the holder 18. The adhesive is then cured
by placing a
light having the required wavelength radiation above the connector for a
period of 25
seconds to a few minutes. Useful lights include the Model XL3000, available
from 3M,
which uses a 75 Watt tungsten source, either unfiltered or filtered to pass
blue light.
It is also understood that plug 12 or receptacle 14 could be mounted on a
jumper
cable or patch cord with any kind of optical connector at the other end of the
fibers. It is
. recommended that fibers be used which have a longer Hfe awhen exposed to
indoor
environments, such as the high-strength fibers available from Minnesota Mining
and
Manufacturing Co. Those fibers have a conventional core and cladding which is
surrounded by a novel three-layer construction, as discussed in U.S. Patent
No. 5,381,504,
disclosed herein by reference. Those skilled in the art will also appreciate
that the
connector of the present invention can accommodate discrete optical fibers or
multifiber
ribbons, as well as both single-mode and mufti-mode fibers.
Fibers which are to be pre-terminated to either plug 12 or receptacle 14
should be
stripped, cleaved and cleaned. If the fibers are in the form of a ribbon which
is part of a
bundled group of ribbons in a cable, then a portion of the cable jacket must
first be cut
-15-
CA 02318325 2000-07-18
WO 99/40465 PCTNS98I13555
back to reveal the ribbons. Most cables have several protective layers, and
each of these
layers must be removed to provide access to the fiber ribbons. Similar steps
must be
taken to remove the protective layers of a cable having a single discrete
fiber. After the
fibers have been removed from the protective cable jacket, they are stripped.
The stripped
fibers are then ready for cleaving which may be accomplished using any one of
several
commercially available fiber cleavers, such as that shown in U.S. Patent No.
5,024,363.
The cleave length for attachment of the fibers to plug 12 is the distance from
fiber holder
18 which, in the preferred embodiment, is about 23 mm. For attachment of
fibers to
receptacle 14, the cleave length is the distance from fiber holder 72 which,
in the
preferred embodiment, is about 15 mm.
Once the craftsperson is satisfied that each of the fibers has an acceptable
end
face, the fibers may be removed from the cleaver. The fibers may further
optionally be
provided with an asymmetric treatment, like cleaving so as to impart an angled
end face,
as taught in U.S. Patent No. 5,048,908. For the plug, fiber preparation may be
done ailer
the fiber cable has been threaded through boot 38.
Final assembly of plug 12 comprises the simple steps of placing the fibers in
the
V-grooves of holder 18 and snapping shroud 24 onto holder 18. An assembly
fixture may
be used to guide shroud 24 onto the fiber holder so as to avoid damaging the
fibers as they
are inserted into the shroud. The ends of the fibers should terminate in the
plug about 0.5
mm from the end of the shroud. Completion of receptacle 14 is also simple.
Fiber holder
72 is attached to housing 70, first by pushing the pivot posts into cutouts
88, and then
snapping the studs into holes 89. Care should be taken during placement of the
fibers in
the V-grooves and attachment of the holder to the receptacle to not
contaminate the fiber
tips.
Installation of connector 10 is equally straightforward. Receptacle 14 is
optionally
mounted to any desired surface by convenient means, such as latching arms 7b
(other
constructions could be molded into housing 70 for custom mounting). Several
receptacles
could also be mounted in a single module, and they can be designed for front
or rear
loading, or sliding from the side. After receptacle 14 is mounted, the
connection is
completed by simply inserting plug 12 into opening 74. Plug 12 is released
from
receptacle 14 by latch 40.
-16-
CA 02318325 2000-07-18
WO 99/40465 PCTIUS98/13555
The dimensions of the various components of connector 10 may vary considerably
depending upon the desired application. The following approximate dimensions
are
considered exemplary. Plug 12 has an overall length of 57 mm, a width of 12
mm, and a
thickness of 8 mm, and fiber holder 18 provides clamping grooves that are 13
mm long.
Plug shroud 24 extends 25 mm beyond holder 18, providing an interior space
which is 24
mm long, 10 mm wide and 6 mm high. Opening 74 of receptacle 14 is 12 mm x 10
mm.
Its overall height and depth are 38 mm and 36 mrn. Receptacle fiber holder 72
is 20 mm
long (from the end where the fibers are clamped to the tips of forgers 82 and
84), 12 mm
wide and 1.5 mm thick. The fiber-alignment grooves in fingers 82 and 84 are
11.5 mm
long and have a maximum depth of 2 mm which suitably accommodates most
conventional optical fibers. The interior angle of the V-grooves should not be
too narrow
since this might result in excess friction with the fibers, but it also should
not be too wide
since this would not keep the fibers guided properly. A 90° interior
angle is believed to
be a good compromise.
Although the invention has been described with reference to specific
embodiments, this description is not meant to be construed in a limiting
sense. Various
modifications of the disclosed embodiment, as well as alternative embodiments
of the
invention, will become apparent to persons skilled in the art upon reference
to the
description of the invention. Variations are possible in the plug, receptacle
and holder.
For detailed discussion of the mechanical construction of a useful connector,
see U.S.
Patent 5,381,498, and copending U.S.S.N.'s 08/577,740, and 08/801,058. For
example,
although only two fiber pairs are shown connected in the figures, connector 10
could
accommodate practically any number of fibers (or just a single pair). It is
therefore
contemplated that such modifications can be made without departing from the
spirit or
scope of the present invention as defined in the appended claims.
Test Methods
Kevlar~ Pull Test
Cables were inserted into the plug with no Kevlar~ fanout. The jacket was
placed
about .010 cm behind the rear injection ports. The adhesive was injected by
hand using a
3cc syringe, being sure that the cavity was completely filled.
-17-
CA 02318325 2000-07-18
WO 99140465 PCT/US98/13555
Each plug was then cured with two 5 second exposures, one on the top and one
on
the bottom with the appropriate light sources (Dymax adhesive UV, or visible.
with blue
filter, adhesive of the invention with white light).
Each cable was cut in half and split until two independent cables protruded
from
the plug. For the Kevlar~ pulls, the plug was placed into a fixture that was
attached to a
chattillon DFM100 scale. One at a time, the cables consisting of jacket,
Kevlar~, and
GGP fiber, were wrapped around a 6.25 cm diameter mandrel and clamped into
place.
They were pulled at a rate of 1.25 cm per minute until failure.
Fiber Pull Test
For three fiber pulls, after the plug was placed into the fixture as described
for the
Kevlar~ pull test, the jacket was striped off about four inches from the plug,
exposing the
Kevlar~ and GGP fiber. The GGP fiber was wrapped around the 6.25 cm mandrel
and
clamped in place. They were pulled at a rate of 1.25 cm per minute until
failure.
Next, the Kevlar~ was wrapped around the 6.25 cm mandrel and pulled at the
same rate until failure.
All pull test were accurate to 0.1 pound, or 2.2 kilograms.
Glo~_ ssarv
CPQ Camphorquinone
EDMAB Ethyl-p-dimethylaminobenzoate
DDPIPF6 diphenyliodonium hexafluorophosphate
Bis GMA Reaction product of Bis Phenol A diglycidyl ether and 2 equivalents of
methacrylic acid
TEGDMA Triethyleneglycoldimethacrylate
DHEPT dihydroxyethyl paratoluidine
Examples
Example 1
Samples of optical fiber assemblies were made by stripping the outer jacket of
the
cable, trimming the Kevlar~ fibers and inserting the optical fiber into a dual
termination
connector.
-18-
CA 02318325 2000-07-18
WO 99/40465 PCTNS98/13555
Before bonding the fibers were cleaned with a lint free pad which had been
wetted
with isopropyl alcohol, then the fibers were- inserted into the fitting. Two
drops of
adhesive were then added to each fiber's channel in the dual termination
fixture, and the
connector was bonded by exposing it for 25 seconds to a 3M Model XL 3000
curing light
having a 75 watt tungsten source filtered to pass blue light.
In Examples C 1 and C2, the adhesives used were commercially available UV
curable adhesives from Dymax Corp, Torrington CT, as Dymax 3072 and Dymax
3095,
respectively. The recommended curing light, Model 3000-EC was also purchased
from
Dymax Corp. The adhesive of the invention has the following composition
(Example 1 ).
INGREDIENT PERCENT
HEMA w/ 10 ppm MEHQ 37.08%
BISGMA resin 61.79%
EDMAB 0.50%
CPQ 0.25%
DHEPT 0.38%
The pull force needed to remove the fiber from the connector is shown in Table
1.
Table 1
Example Kevlar~Pull forceFiber Pull force UV cure time
(Newtons.) (Newtons.) (sec.)
C 1 67 6.6 40
C2 289 6.6 - 30
1 334 13 15
Examples 2-X and Comparative Examples
Samples were made according to Example 1 and tested for strength of the
unified
structure. In Examples 2 and 3; an adhesive of the invention having a
formulation as
described below was used. These connectors were then bonded by exposing them
to 25
seconds to a 3M Model XL 3000 curing light having a 75 watt tungsten source.
For Comparative Examples C4 and C5, commercially available adhesives were
used; C6 was used as purchased, C4 was modified by the addition of the ternary
-19-
CA 02318325 2000-07-18
WO 99/404b5 PCTNS98/13555
photoinitiator system used in Examples 2 and 3, i.e., the CPQ, EDMAB, and
DDPIPF6 in
the same amounts. Example CS included this modification, and further contained
0.1%
rose bengal.
Example 2
INGREDIENT PARTS BY WEIGHT
Bis GMA 50.0
TEGDMA 50.0
CPQ O.I7
EDMAB 1.00
DPIPF6 0.60
Tinuvin~ P 1.00
Example 3
INGREDIENT PARTS BY WEIGHT
Bis GMA 50.0
TEGDMA 50.0
CPQ 0.17
Rose Bengal 0.10
EDMAB 1.00
DPIPF6 0.60
Tinuvin~ P 1.00
-20-
CA 02318325 2000-07-18
WO 99/40465 PCT/US9$/13555
Ta le 2
UV Blue White
Example
Kevlar~ Fiber Kevlar~ Fiber Kevlar~ Fiber
Pull Pull Pull forcePull Pull Pull
force force ~''l~) force force force
(N.) (N.) ~.) (N.)
C4 207.1 3.41 302.3 3.85 298.4 2.96
CS 252.8 3.$5 311.5 3.41 15.96 3.11
C6 48.45 3.26 8.668 3.41 15.96 3.11
2 320.3 7.26 342.6 8 343.7 N/A
3 345.9 5.93 334.3 8.59 351.5 9.63
As can be seen, the commercially available adhesive does not produce the same
type of unified structure as adhesives of the invention, even when a ternary
photoinitiator
and additional sensitizers are added. This is true whether ultraviolet light
is used to cure
the adhesives, visible light across a broad spectrum, or visible light
filtered to pass only
the blue area of the spectrum.
-21-