Note: Descriptions are shown in the official language in which they were submitted.
CA 02318657 2004-12-29
3
GLYPHOSATE FORMULATIONS CONTAINING ETHERAMINE
SURFACTANTS
s FIELD OF THE INVENTION
Novel agriculturally acceptable formulations of the herbicide N-
phosphonomethylglycine (glyphosate) comprising tertiary or quaternary
etheramine or etheramine oxide surfactants are provided.
BACKGROUND OF THE INVENTION
Glyphosate is well known as a highly effective and commercially
important herbicide useful for combating the presence of a wide variety of
unwanted vegetation, including agricultural weeds. Glyphosate is
conventionally applied as a formulated product dissolved in water to the
foliage
of annual and perennial grasses and broadleaf plants and the like, is taken up
over a period of time into the leaves, and thereafter translocates throughout
the
plant.
Usually, glyphosate is formulated in commercial compositions in the form
of a water-soluble salt. Salts in commercial use include the ammonium salt,
alkylamine salts, such as the isopropylamine salt, alkali metal salts, such as
the
sodium salt, and the trimethylsulfonium salt. However, formulations of
glyphosate in its acid form are also used. Typical glyphosate salt
formulations
include aqueous concentrates, requiring simple dilution and distribution in
water
for application by the end-user, and water-soluble or water-dispersible dry
formulations, especially granules, requiring dissolution or dispersion in
water
prior to application.
CA 02318657 2004-12-29
4
Under most application conditions, the herbicidal efficacy of glyphosate
can be significantly enhanced by including one or more surfactants in the
composition to be applied. It is believed that such surfactants act partly by
facilitating the penetration of glyphosate, a relatively hydrophilic compound,
through the rather hydrophobic cuticle which normally covers the external
above-ground surfaces of higher plants.
Wyrill and Burnside, Weed Science, Vol. 25 (1977), pp. 275-287,
conducted a wide-ranging study of different classes of surfactants as agents
for
lo enhancing the herbicidal activity of glyphosate, applied as the
isopropylamine
salt. They demonstrated that the choice of surfactant has a pronounced effect
on the herbicidal performance of a glyphosate formulation, but beyond showing
a general tendency for surfactants having high values of hydrophile-lipophile
balance (HLB) to be more efficacious than surfactants of the same class having
low HLB values, they did not observe any predictive relationship between
efficacy and surfactant chemical class. Some of the most effective surfactants
identified in the Wyrill and Burnside study were ethoxylated tertiary and
quaternary alkylamines.
Commercial formulations of glyphosate have frequently used ethoxylated
tertiary alkylamine surfactants, for example an ethoxylated tallowamine having
an average of about 15 moles of ethylene oxide (EO) per mole of tallowamine.
Monsanto Company of St. Louis, Missouri has for many years sold, under the
trademark Roundup herbicide, glyphosate formulations containing various
concentrations of such an ethoxylated tallowamine surfactant.
European Patent No. 0 290 416 to Forbes et al. discloses compositions
of glyphosate salts comprising ethoxylated tertiary alkylamine surfactants
having less than 15 moles of EO. For example a composition is disclosed
comprising the isopropylamine salt of glyphosate and an ethoxylated
CA 02318657 2004-12-29
cocoamine surfactant having an average of 5 moles of EO. It is taught by
Forbes et al. that certain herbicidal efficacy advantages are obtainable with
such compositions by comparison with compositions where the EO level in the
surfactant is around 15 moles.
5
European Patent No. 0 274 369 to Sato et al. discloses glyphosate
compositions comprising ethoxylated quaternary alkylamine surfactants.
Several examples are shown wherein the surfactant is an ethoxylated N-methyl
cocoammonium chloride surfactant having 2 moles of EO.
A drawback of ethoxylated tertiary alkylamine surfactants of prior art is
that when included in concentrate formulations at levels consistent with good
herbicidal performance, they tend to be irritant to eyes. In some, but not
all,
cases, eye irritancy can be reduced by converting the tertiary alkylamine to
the
corresponding quaternary (N-methyl) alkylamine. U.S. Patent No. 5,317,003 to
Kassebaum discloses that a glyphosate composition containing as the
surfactant an ethoxylated N-methyl cocoammonium chloride surfactant having
15 moles of EO is less irritant to eyes than an otherwise identical
composition
wherein the surfactant is an ethoxylated tertiary cocoamine surfactant having
15 moles of EO.
An alternative solution to the eye irritancy problem is suggested in U.S.
Patent No. 5,118,444 to Nguyen, wherein ethoxylated tertiary alkylamine
surfactants are converted to their N-oxides. Examples are shown of glyphosate
compositions wherein the surfactant is an ethoxylated tallowamine oxide
surfactant having 10, 15 or 20 moles of EO.
A further drawback of ethoxylated tertiary alkylamine surfactants of the
prior art is that when water is added to them, they tend to form a stiff gel
which
3o adds to the complexity and expense of manufacturing formulations containing
CA 02318657 2004-12-29
6
such surfactants, by making it difficult to clean vessels and pipes. In
practice,
this problem is ameliorated by adding an anti-gelling agent, such as
polyethylene glycol, to the surfactant.
Never previously disclosed as components of concentrate glyphosate
formulations are alkoxylated tertiary or quaternary etheramine or etheramine
oxide surfactants. United Kingdom Patent No. 1,588,079 to Texaco
Development Corporation discloses examples of ethoxylated
alkyloxyisopropylamine and alkylpoly (isopropyl) amine surfactants and
io methods of preparing them, and suggests they are useful as detergents,
dispersants, wetting agents and emulsifiers. Surfactants disclosed have the
representative chemical structure
(CHZCH2O)X -H
1
Rl-(OCH2CH)m -N
I I
CH3 (CH2CH2O)y -H
wherein R, is Ca-C18 alkyl, m is a number from 1 to 5, and x and y are average
2o numbers such that x+y is in the range from 2 to 20.
Tomah Products, Inc. of Milton, Wisconsin in a brochure titled
"Ethoxylated Amines", dated August 22, 1994, disclose, together with a series
of ethoxylated tertiary alkylamines, a number of ethoxylated tertiary
etheramine
surfactants having the representative chemical structure
(CH2CH2O)x -H
I
Ri -OCH2CH2CH2 -N
I
(CH2CH2O)y -H
wherein R, is C1o - C26 alkyl and x and y are average numbers such that x+y is
in the range from 2 to 15. Suggested uses of the Tomah ethoxylated amines
CA 02318657 2004-12-29
7
include "agricultural adjuvants", a well-known application of the ethoxylated
tertiary alkylamines listed. No suggestion is made that the ethoxylated
tertiary
etheramines included in the list would have advantages over the ethoxylated
tertiary alkylamines as agricultural adjuvants, nor is there any teaching
relevant
to the making of concentrate glyphosate compositions with ethoxylated tertiary
etheramines.
Another brochure from Tomah Products titled "Quaternaries", dated
September 1, 1994, includes in a list of quaternary amine surfactants a number
io of ethoxylated quaternary etheramines having the representative chemical
structure
(CH2CH2O)X -H
I
R1-OCH2CH2CH2 -N+ -CH3 CI"
i5 1
(CH2CH2O)y -H
wherein R, is an aliphatic group exemplified by isodecyl or isotridecyl and
x+y is
2. The list of suggested uses for Tomah's quaternaries does not include
2o agricultural adjuvants.
Another brochure from Tomah Products titled "AO-14-2", dated August
24, 1994, discloses an ethoxylated etheramine oxide which can be deduced to
have the representative chemical structure
(CH2CH2O), -H
I
Ri -OCH2CH2CH2 -N->O
1
(CH2CH2O)y -H
wherein R, is an aliphatic group exemplified by isodecyl and x+y is 2. The
list
of suggested uses for AO-14-2 does not include agricultural adjuvants.
CA 02318657 2004-12-29
8
It is an object of the present invention to provide novel compositions of
glyphosate herbicide containing an etheramine surfactant which imparts good
herbicidal efficacy, yet having low irritancy to eyes.
It is a further object of the present invention to provide the commercial
formulator of glyphosate with an alternative to ethoxylated alkylamine
surfactants that (1) allows elimination or substantial reduction of the need
for
the use of an anti-gelling agent, (2) is soluble in aqueous formulations
having
io higher glyphosate acid equivalent loadings than prior art formulations
having
comparable efficacy, and (3) provides herbicidal efficacy superior to that
obtainable with comparable amounts of said ethoxylated alkylamine surfactants
having similar degrees of ethoxylation.
It is a further object of the present invention to provide concentrate liquid
and dry formulations of glyphosate with an etheramine surfactant having good
storage stability.
These and other objectives are satisfied by the compositions disclosed
2o herein.
SUMMARY OF THE INVENTION
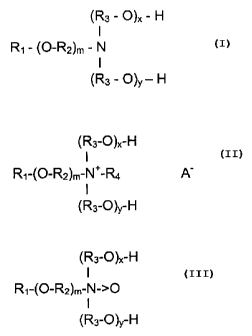
There are provided new herbicidal compositions comprising glyphosate
or a salt thereof and an etheramine surfactant, defined as an amine surfactant
in which the hydrophobe is connected to the amine group via a series of up to
about 10 oxyalkylene groups.
Specifically, the etheramine surfactant may be a tertiary amine having
the representative chemical structure
CA 02318657 2004-12-29
9
(R3-O)x-H
I
Rl-(O-R2)R, -N
1
(R3-O)y-H
wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from 1 to about 10, R2 in each of the m(O-R2)
groups is independently C, - C4 alkylene, R3 groups are independently C, - C4
lo alkylene, and x and y are average numbers such that x+y is in the range
from 2
to about 60.
Alternatively, the etheramine surfactant may be a quaternary amine
having the representative chemical structure
(R3 - O)X -H
Ri -(O-R2)m -N+ - R4 A'
(R3 -O)y -H
wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from 1 to about 10, R2 in each of the m(O-Rz)
groups is independently C, -Ca alkylene, R3 groups are independently C, -C4
alkylene, R4 is Cl -C4 alkyl, x and y are average numbers such that x+y is in
the
range from 0 to about 60, and K is an agriculturally acceptable anion.
As a third possibility, the etheramine surfactant may be an amine oxide
having the representative chemical structure
(R3-O)X-H
Ri -(O-R2)m -N->O
(R3 -O)y -H
CA 02318657 2004-12-29
wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from 1 to about 10, R2 in each of the m(O-R2)
groups is independently C, - C4 alkylene, R3 groups are independently C, -C4
5 alkylene, and x and y are average numbers such that x+y is in the range from
2
to about 60.
Compositions of the invention may be prepared on site by the end-user
shortly before application to the foliage of vegetation to be killed or
controlled,
io by mixing in aqueous solution a glyphosate containing composition and a
composition comprising a surfactant having a chemical structure encompassed
by those represented immediately above. Such compositions of the invention
are referred to herein as "tank-mix" compositions.
Alternatively, compositions of the invention may be provided to the end-
user already formulated, either at the desired dilution for application
("ready to
use" compositions) or requiring dilution, dispersion or dissolution in water
by the
end-user ("concentrate" compositions). Such preformulated compositions of
the invention are storage-stable and may be liquid or dry.
A method of use of compositions of the invention to kill or control weeds
or other unwanted vegetation is also provided.
DETAILED DESCRIPTION OF THE INVENTION
Compositions of the invention may contain glyphosate in its acid form.
However, because of the relatively low solubility of glyphosate acid in water,
more soluble salts of glyphosate are generally preferred. As in commercial
compositions of prior art, an especially preferred salt for aqueous
compositions
of the invention is the isopropylamine salt of glyphosate, while an especially
CA 02318657 2004-12-29
11
preferred salt for dry compositions of the invention is the ammonium salt.
Many
other salts may be used either in aqueous or in dry formulations, including
but
not restricted to alkylamine, such as dimethylamine and n-propylamine,
alkanolamine, such as monoethanolamine, alkylsulfonium, such as
trimethylsulfonium, and alkali metal, such as sodium and potassium, salts of
glyphosate. Regardless of whether acid or a salt is used, it is generally
preferred to refer to the amount of glyphosate applied or contained in a
formulation in terms of glyphosate acid equivalent, conventionally abbreviated
as "a.e.".
Tank-mix and ready to use compositions of the invention are aqueous
solutions comprising from about 1 to about 50 g glyphosate a.e./I,
occasionally
more. A preferred range for tank-mix and ready to use compositions is from
about 5 to about 20 g a.e./l.
Concentrate compositions of the invention may be aqueous solutions
comprising from about 50 to about 500 g glyphosate a.e./I or more, preferably
from about 200 to about 500 g. a.e./I and most preferably from about 350 to
about 500 g a.e./l. An example of an especially preferred aqueous concentrate
composition of the invention contains the isopropylamine salt of glyphosate at
about 360 g a.e./l, the same level as is present in commercial compositions
being sold as Roundup herbicide by Monsanto Company.
A surprising advantage of aqueous compositions of the invention over
prior art compositions is that the glyphosate concentration can be increased
to
very high levels, for example from about 450 to about 500 g a.e./I, yet the
surfactant concentration is still adequate to give excellent herbicidal
performance without the end-user requiring to add more surfactant in the spray
tank. Many such highly concentrated compositions have remarkably good
storage stability under a wide range of temperature conditions.
CA 02318657 2004-12-29
12
Alternatively, concentrate compositions of the invention may be dry
formulations, presented for example in the form of powders, pellets, tablets
or,
preferably, granules, to be dispersed or dissolved in water prior to use.
Typically no water-insoluble ingredients are present at substantial levels in
such
compositions and the formulations are therefore fully water-soluble. Dry water-
soluble or water-dispersible compositions of the invention comprise from about
20% to about 80% weight/weight glyphosate a.e., preferably from about 50% to
about 76%, and most preferably from about 60% to about 72%. An example of
lo an especially preferred water-soluble granular composition of the invention
contains the ammonium salt of glyphosate at about 72% weight/weight, the
same level as is present in commercial compositions being sold as Scout
herbicide by Monsanto Company.
In dry compositions of the invention glyphosate may itself provide the
support for other formulation ingredients, or there may additionally be
present
one or more inert ingredients providing such support. An example of an inert
support that may be used is ammonium sulfate. The term "dry" as used herein
does not imply that dry compositions are totally free of water; typically dry
compositions of the invention comprise from about 0.5 to about 5 percent by
weight, preferably less than about 1 percent by weight water.
Dry water-soluble or water-dispersible granular formulations of the
invention can be made by any process known in the art, including but not
restricted to spray drying, fluid-bed agglomeration, pan granulation, or
extrusion. In dry formulations, glyphosate may be present as a salt, for
example the sodium or ammonium salt, or as the acid. Formulations containing
glyphosate acid may optionally contain an acid acceptor such as an ammonium
or alkali metal carbonate or bicarbonate, ammonium dihydrogen phosphate or
the like, so that upon dissolution or dispersion in water by the end user a
water
CA 02318657 2004-12-29
13
soluble salt of glyphosate is produced.
What distinguishes compositions of the invention from all previously
described glyphosate compositions is the presence therein of an alkoxylated
tertiary or an alkoxylated or non-alkoxylated quaternary etheramine or an
alkoxylated etheramine oxide surfactant having the representative chemical
structure
(a)
(R3 -O)X -H
Ri -(O-R2)m -N
(R3 -O)y -H
wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from I to about 10, R2 in each of the m(O-R2)
groups is independently C, -C4 alkylene, R3 groups are independently C, -C4
alkylene, and x and y are average numbers such that x+y is in the range from 2
to about 60;
or(b)
(R3-O)X-H
R, -(O-Rz)n, -N+ -R4 A"
I
(R3-O)y-H
wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from 1 to about 10, R2 in each of the m(O-R2)
groups is independently C, -C4 alkylene, R3 groups are independently C, -C4
alkylene, R4 is Cl -C4 alkyl, x and y are average numbers such that x+y is in
the
CA 02318657 2004-12-29
14
range from 0 to about 60, and A" is an agriculturally acceptable anion;
or (c)
(R3 -O), -H
1
Ri -(O-R2)m -N->0
I
(R3-O)y -H
io wherein R, is a straight or branched chain C6 to about C22 alkyl, aryl or
alkylaryl
group, m is an average number from 1 to about 10, R2 in each of the m(O-R2)
groups is independently C, -C4 alkylene, R3 groups are independently C, -C4
alkylene, and x and y are average numbers such that x+y is in the range from 2
to about 60.
Alkylamine or alkylamine oxide surfactants in glyphosate compositions of
prior art have no (O-R2) groups, in other words m = 0. We have found that
surprisingly improved properties can be imparted to glyphosate compositions
when from 1 to about 10 (O-R2) groups are inserted in the surfactant structure
2o between the R, group and the nitrogen atom.
Aryl groups, if present in R,, have 5-7, preferably 6, carbon atoms and
may or may not be substituted with moieties. The alkyl portion in any
alkylaryl
group comprising R, has 1-16 carbon atoms. An example of such an alkylaryl
group is alkylphenyl, for example nonylphenyl.
However, in preferred surfactants of the invention R1 is a straight or
branched chain alkyl group having about 8 to about 18, for example about 10-
15, carbon atoms, and are derived from the corresponding alcohol. For
3o example, the alkyl group may be of natural derivation, such as from coconut
or
tallow, or may be derived from a synthetic alcohol such as isodecyl,
isotridecyl,
CA 02318657 2004-12-29
linear C12 -C14 or octadecyl alcohols.
The R2 substituent closest to the nitrogen atom (the proximal R2 group)
is in preferred examples a linear propylene (-CH2CH2CH2-), isopropylene
5 (-CH2CH (CH3)-) or ethylene (-CH2CH2-) group. Preferred examples where the
proximal R2 group is linear propylene have m = 1. Where the proximal R2
group is isopropylene or ethylene, m is preferably in the range from 1 to 5,
most
preferably from 2 to 3, and all R2 groups are preferably the same.
10 R3 substituents in preferred examples are independently selected from
isopropylene and ethylene. In especially preferred examples all R3 groups are
ethylene. In tertiary etheramines and etheramine oxides of the invention it is
preferred that x+y is in the range from 2 to about 20. In quaternary
etheramines of the invention it is preferred that x+y is in the range from 0
to
15 about 20. A particularly preferred range for x+y in tertiary and quaternary
etheramines and etheramine oxides of the invention is from 2 to about 10, more
particularly from 2 to about 5.
In quaternary etheramines of the invention R4 is preferably methyl and A'
is preferably a halide, for example chloride or bromide, a phosphate or a
sulfate
ion, or alternatively may be a glyphosate ion or may be contributed by an
anionic surfactant included with the etheramine in the formulation. It will be
recognized by those skilled in the art that at low pH, such as may well exist
in a
glyphosate formulation, tertiary etheramines will most likely be protonated at
the
nitrogen atom and may be associated with a counterion; in such cases the
tertiary etheramine can be represented by the chemical structure shown above
for a quaternary etheramine, except that R4 is hydrogen. The counterion A" in
a
low pH glyphosate formulation comprising a tertiary etheramine is most likely
glyphosate itself.
CA 02318657 2004-12-29
16
One especially preferred surfactant useful in compositions of the
invention is a tertiary etheramine having Ri = C12 -C14 alkyl, R2 =
isopropylene,
m = 2, R3 = ethylene and x+y = 5.
Another especially preferred surfactant useful in compositions of the
invention is a tertiary etheramine having R, = C12 -C14 alkyl, R2 = ethylene,
m = 3, R3 = ethylene and x+y = 5.
Two other especially preferred surfactants useful in compositions of the
lo invention are tertiary etheramines having R1 = isodecyl, R2 = linear
propylene,
m = 1, R3 = ethylene and x+y = 2 or 5 respectively.
Another series of especially preferred surfactants useful in compositions
of the invention is a tertiary etheramine having R, = coco alkyl, R2 = linear
propylene, m= 1, R3 = ethylene and x+y = a number in the range from 2 to 10.
Two other especially preferred surfactants useful in compositions of the
invention are tertiary etheramines having R, = isotridecyl, R2 = linear
propylene,
m = 1, R3 = ethylene and x+y = 2 or 5 respectively.
Two other especially preferred surfactants useful in compositions of the
invention are quaternary etheramines having R, = isodecyl or isotridecyl
respectively, R2 = linear propylene, R3 = ethylene, R4 = methyl, m = 1 and
x+y = 2.
Any convenient and effective herbicidal activity enhancing amount of the
etheramine surfactant can be used in compositions of the invention. In tank-
mix and ready to use formulations very high levels of surfactant are
achievable,
for example up to 5% weight/volume or even higher, but for reasons of
3o economy it will be more normal to use a concentration in the range from
about
CA 02318657 2004-12-29
17
0.125% to about 2% weight/volume. One of ordinary skill in the art will be
able
to determine from tests on different plant species an appropriate level of
etheramine surfactant to include for any particular glyphosate application.
In concentrate liquid or dry compositions of the invention, the etheramine
surfactant is preferably included at a weight/weight ratio to glyphosate a.e.
in
the range from about 1:20 to about 1:1, most preferably from about 1:10 to
about 1:2, for example about 1:6.
Long-term storage stability is an important commercial attribute of
concentrate formulations. In the case of aqueous concentrate formulations of
glyphosate salts, it is particularly important that surfactants in the
formulation do
not separate from the other ingredients as a distinct phase. Many aqueous
concentrates made with surfactants of prior art show a tendency for phase
separation at high temperatures. It is a feature of the etheramine surfactants
herein disclosed that they show good compatibility with glyphosate salts,
particularly the isopropylamine salt, as evidenced by relatively high cloud
points
even in aqueous solutions having high glyphosate concentrations. In general
for most applications, a cloud point higher than about 50 C is desirable.
In addition to glyphosate or a salt thereof and the etheramine surfactant,
any of a variety of further ingredients or adjuvants may be included in
formulations of the present invention, as long as such added materials are not
significantly antagonistic to the glyphosate herbicidal activity. Examples of
such
added materials illustratively include anti-gelling agents, antifreezes,
thickeners,
dyes, antimicrobial preservatives or additives to further enhance herbicidal
activity, such as ammonium sulfate or fatty acids.
A second surfactant of a class other than etheramines, for example a
primary or secondary alcohol ethoxylate, an alkyl ester of sucrose or
sorbitan,
CA 02318657 2004-12-29
18
or an alkyl polyglucoside, may also be included. When such a second
surfactant is present, it is preferable that the weight/weight ratio of
etheramine
to the second surfactant is greater than about 1:1, most preferably greater
than
about 2:1, for example around 4:1.
Preferably when a second surfactant is included in a highly concentrated
glyphosate formulation of the invention, for example one containing about 450
to about 500 g a.e./I, the etheramine surfactant comprises at least about 75%
by weight of the total surfactant present.
Mixtures of glyphosate with other herbicides are also within the scope of
the present invention if an etheramine surfactant is included in the
formulation.
Examples of such other herbicides include bialaphos, glufosinate, 2,4-D,
MCPA, dicamba, diphenylethers, imidazolinones and sulfonylureas.
Methods of use of glyphosate formulations are well known to those of
skill in the art. Aqueous concentrate formulations of the invention are
diluted in
an appropriate volume of water and applied, for example by spraying, to the
weeds or other unwanted vegetation to be kiiied or controlled. Dry concentrate
formulations of the invention are dissolved or dispersed in an appropriate
volume of water and applied in the same way. For most purposes,
compositions of the invention are applied at glyphosate a.e. rates in the
range
from about 0.1 to about 5 kg/ha, occasionally more. Typical glyphosate a.e.
rates for control of annual and perennial grasses and broadleaves are in the
range from about 0.3 to about 1.5 kg/ha. Compositions of the invention may be
applied in any convenient volume of water, most typically in the range from
about 50 to about 1000 I/ha.
The present invention is illustrated by but not limited to the following
3o Examples.
CA 02318657 2004-12-29
19
EXAMPLES
Cloud point was determined for certain liquid compositions of the
Examples as follows. A sample of the composition in a test tube was heated in
a water bath until it became cloudy. The test tube was then removed from the
water bath and the sample stirred with a thermometer until it became clear.
The temperature at which the sample became clear was recorded as the cloud
point of the composition.
to Percentages expressed as "%" in the following Examples are by
weight/weight unless otherwise indicated.
Example 1
is The surfactant used in Example 1 is a tertiary etheramine having the
chemical structure represented above in which R, is C12 - C14 alkyl, m is 3,
x+y
is 5 and R2 and R3 are each ethylene.
The aqueous concentrate composition of Example 1 was prepared by
20 mixing the following ingredients in the order given:
(1) aqueous solution of glyphosate isopropylamine salt containing
46% glyphosate a.e., 67.4 g.
(2) surfactant as defined above, 10.0 g.
25 (3) deionized water, 22.6 g.
The composition can be calculated to contain 31 % glyphosate a.e. and
10% surfactant. Specific gravity of the composition at 20/15.6 C was
determined to be 1.1628. Cloud point of the composition was >90 C.
CA 02318657 2004-12-29
Example 2
The surfactant used in Example 2 is the same as that used in Example
1. The aqueous concentrate composition of Example 2 was prepared by mixing
5 the following ingredients in the order given:
(1) aqueous solution of glyphosate isopropylamine salt containing
46% glyphosate a.e., 1348 g.
(2) surfactant as defined above, 110 g.
10 (3) deionized water, 542 g.
The composition can be calculated to contain 31 % glyphosate a.e. and
5.5% surfactant. Specific gravity of the composition at 20/15.6 C was
determined to be 1.1630. Cloud point of the composition was >90 C.
The composition of Example 2 was submitted for eye irritation testing
according to the standard procedure prescribed in U.S. Environmental
Protection Agency (EPA) Publication 540/9-82-025, November 1982, entitled
Pesticidal Assessment Guidelines, Subdivision F, Hazard Evaluation: Human
2o and Domestic Animals. The study was conducted in compliance with EPA
Good Laboratory Practice (GLP) standards. Results were obtained placing the
composition in toxicity category III, indicating low irritancy to eyes.
Example 3
The surfactant used in Example 3 is a tertiary etheramine having the
chemical structure represented above in which R, is C12 -C14 alkyl, m is 2,
x+y
is 5, R2 is isopropylene and R3 is ethylene.
The aqueous concentrate composition of Example 3 was prepared by
CA 02318657 2004-12-29
21
mixing the following ingredients in the order given:
(1) aqueous solution of glyphosate isopropylamine salt containing 46%
glyphosate a.e., 1348 g.
(2) surfactant as defined above, 200 g.
(3) deionized water, 452 g.
The composition can be calculated to contain 31 % glyphosate a.e. and
10% surfactant. Specific gravity of the composition at 20/15.6 C was
io determined to be 1.1618. Cloud point of the composition was >90 C.
Example 4
The surfactant used in Example 4 is the same as that used in Example
3. The aqueous concentrate composition of Example 4 was prepared by mixing
the following ingredients in the order given:
(1) aqueous solution of glyphosate isopropylamine salt containing
46% glyphosate a.e., 1348 g.
(2) surfactant as defined above, 110 g.
(3) deionized water, 542 g.
The composition can be calculated to contain 31% glyphosate a.e. and
5.5% surfactant. Specific gravity of the composition at 20/15.6 C was
determined to be 1.1617. Cloud point of the composition was >90 C.
The composition of Example 4 was submitted for eye irritation testing
according to the standard procedure prescribed in EPA Publication 540/9-82-
025, November 1982 cited above. The study was conducted in compliance
with GLP standards. Results were obtained placing the composition in toxicity
CA 02318657 2004-12-29
22
category III, indicating low irritancy to eyes.
Examples 5-7
The surfactants used in Examples 5-7 are tertiary etheramines having
the chemical structure represented above in which R, is C12 -C14 alkyl, m is 3
and R2 and R3 are each ethylene. The value of x+y is varied as shown in the
table below.
lo Aqueous concentrate compositions containing 31% glyphosate a.e. in
the form of the isopropylamine salt and 11 % surfactant were prepared by a
procedure similar to that of Examples 1-4. Cloud point of each composition
was determined as shown in the table below.
Example x+r Cloud point ( C)
5 5 >95
6 10 81
7 15 66
2o Examples 8-11
The surfactants used in Examples 8-11 are tertiary etheramines having
the chemical structure represented above in which R, is C12 - C14 alkyl, m is
2,
R2 is isopropylene and R3 is ethylene. The value of x+y is varied as shown in
the table below.
Aqueous concentrate compositions containing 31 % glyphosate a.e. in
the form of the isopropylamine salt and 11 % surfactant were prepared by a
procedure similar to that of Examples 1-4. Cloud point of each composition
was determined as shown in the table below.
CA 02318657 2004-12-29
23
Example x y Cloud point ( C)
8 2 76
9 5 >95
10 >95
5 11 15 71
Example 12
Comparative herbicidal efficacy was determined in a field test at
io Jerseyville, Illinois. Treatments were applied post-emergence to plants
which
had grown from seeds planted mechanically in rows. A randomized block
design with three replicates was used. Applications were made with a
backpack sprayer with multiple nozzles giving an overlapping spray pattern to
maximize uniformity of application. Herbicidal efficacy was evaluated as
is percent control estimated visually by comparison with untreated plots.
Plant species on which herbicidal efficacy was evaluated were Japanese
millet (Echinochloa crus-galli, ECHCF), broadleaf signalgrass (Brachiaria
platyphylla, BRAPP), prickly sida (Sida spinosa, SIDSP), redroot pigweed
(Amaranthus retroflexus, AMARE), hemp sesbania (Sesbania exaltata,
SEBEX), morningglory (Ipomoea sp., IPOSS) and velvetleaf (Abutilon
theophrasti, ABUTH).
All glyphosate formulations in this test were aqueous concentrates
diluted in water to give an application volume of 93 I/ha. Dilutions were made
so as to give three glyphosate application rates of 314, 628 and 840 g a.e./ha
for each formulation.
Standard treatments used for reference in this field test were made with
concentrate formulations A and B containing glyphosate isopropylamine salt at
360 g a.e./I and respectively 15.4% and 7.7% of MON 0818, a surfactant based
CA 02318657 2004-12-29
24
on ethoxylated tallowamine having an average of 15 moles of EO.
Concentrate formulations C-J of the invention contained glyphosate in
the form of the isopropylamine salt at 360 g a.e./I. Concentrate formulations
C-
F further contained, as sole surfactant, a tertiary etheramine having the
chemical structure represented above in which R, is C12 -C14 alkyl, m is 2,
x+y
is 5, R2 is isopropylene and R3 is ethylene. Surfactant contents in
concentrate
formulations C, D, E and F were respectively 3.5%, 5.5%, 7.5% and 10%.
Concentrate formulations D and F are essentially identical to the compositions
io of Examples 4 and 3 above, respectively. Concentrate formulations G-J
contained, as sole surfactant, a tertiary etheramine having the chemical
structure represented above in which R, is C12 -C14 alkyl, m is 3, x+y is 5,
and
R2 and R3 are each ethylene. Surfactant contents in concentrate formulations
G, H, I and J were, respectively, 3.5%, 5.5%, 7.5% and 10%. Concentrate
is formulations H and J are essentially identical to the compositions of
Examples 2
and 1 above respectively.
Concentrate formulations K and L of the invention contained glyphosate
in the form of the isopropylamine salt at 420 g a.e./I and a tertiary
etheramine
20 surfactant having the chemical structure represented above in which R, is
C12 -
C14 alkyl, m is 3, x+y is 5, and R2 and R3 are each ethylene. Etheramine
surfactant contents in concentrate formulations K and L were respectively 5.5%
and 3.5%. Concentrate formulation L additionally contained 3.9% of an alkyl
polyglucoside surfactant having a C9 -C>> alkyl chain and an alkyl/glucose
molar
25 ratio of 1:1.6.
Results of the field test are tabulated below.
CA 02318657 2004-12-29
Percent Control
Formu- g a.e.
lation /ha ECHCF BRAPP SIDSP AMARE SEBEX IPOSS ABUTH
5 A 314 97 96 70 96 99 59 59
B 314 97 98 81 95 98 52 52
C 314 100 100 68 98 100 49 50
D 314 98 98 73 96 100 48 50
to E 314 100 100 52 95 100 46 50
F 314 100 100 75 96 100 58 59
G 314 100 100 66 94 99 53 52
H 314 99 100 50 88 98 53 55
15 I 314 97 98 65 97 97 60 63
J 314 98 100 74 95 97 60 56
K 314 92 96 76 93 98 54 53
20 L 314 97 98 77 95 97 55 56
A 628 100 100 96 100 100 73 75
B 628 100 100 95 99 99 68 66
25 C 628 100 100 94 99 100 71 70
D 628 100 98 95 100 100 85 78
E 628 100 100 98 99 99 77 77
F 628 100 100 93 100 100 81 79
3o G 628 100 100 99 99 100 87 84
H 628 100 100 97 98 100 74 72
I 628 100 100 97 100 100 83 78
J 628 100 100 98 99 100 77 70
K 628 100 98 92 99 99 72 73
L 628 100 100 94 99 100 79 81
A 840 100 100 97 99 100 90 78
4o B 840 100 100 99 99 100 87 81
C 840 100 100 100 100 100 90 90
D 840 100 98 100 100 100 89 83
E 840 100 100 98 100 100 91 86
F 840 100 100 99 99 100 90 88
CA 021318657 2004-12-29
26
G 840 100 100 96 100 100 86 83
H 840 100 100 100 100 100 86 83
1 840 100 100 96 98 100 88 80
J 840 100 100 98 100 100 92 82
K 840 100 100 100 100 100 92 92
L 840 100 100 99 99 99 88 86
Example 13
The surfactant used in Example 13 is the same as that used in
Example 1. The dry water-soluble granular composition of Example 13 was
prepared by adding to a small food-processor bowl the following ingredients:
(1) powdered ammonium glyphosate containing 86.6% glyphosate
a.e., 37.5 g.
(2) surfactant as defined above, 12.5 g.
(3) water, 2.5 g.
2o No gelling of the surfactant was observed. The ingredients were mixed,
forming homogeneous small granules. The granules were dried in a fluid-bed
dryer at 65 C for 15 minutes.
The composition can be calculated to contain, on a dry weight basis,
25% surfactant. Glyphosate assay was determined to be 61.8% a.e.
Example 14
The surfactant used in Example 14 is the same as that used in
3o Example 3. The dry water-soluble granular composition of Example 14 was
prepared by adding to a small food-processor bowl the following ingredients:
CA 02318657 2004-12-29
27
(1) powdered ammonium glyphosate containing 86.6% glyphosate
a.e., 37.5 g.
(2) surfactant as defined above, 12.5 g.
(3) water, 2.5 g.
No gelling of the surfactant was observed. The ingredients were mixed,
forming homogeneous small granules. The granules were dried in a fluid-bed
dryer at 65 C for 15 minutes.
to The composition can be calculated to contain, on a dry weight basis,
25% surfactant. Glyphosate assay was determined to be 66.2% a.e.
Example 15
For comparative greenhouse testing of herbicidal efficacy, aqueous
concentrate compositions containing 31 % glyphosate a.e. in the form of the
isopropylamine salt and 5.5% surfactant were prepared by a procedure similar
to that of Examples 1-4.
Formulations C1 and C2 were made using tertiary alkylamine
surfactants of prior art. The surfactant in Cl is an ethoxylated cocoamine
having 5 moles of EO. If the representative chemical structure shown for a
tertiary etheramine of the invention is applied to the surfactant of Cl, it
will be
seen that R, is coco alkyl of average carbon chain length about 12, m is 0, R3
is
ethylene and x+y is 5. The surfactant in C2 is an ethoxylated tallowamine
having 5 moles of EO. If the representative chemical structure shown for a
tertiary etheramine of the invention is applied to the surfactant of C2, it
will be
seen that R, is tallow alkyl of average carbon chain length about 18, m is 0,
R3
is ethylene and x+y is 5.
CA 02318657 2004-12-29
28
Formulations E1-E9 were made using tertiary or quaternary N-methyl
etheramine surfactants of the invention. The chemical structures of these
surfactants can be deduced from the following table, by reference to the
representative structures shown above.
Formu- Surfactant
lation Type Rl R2 R3 m x y A"
El tertiary C12_14alkyl CH2CH (CH3) CH2CH2 2 5
io E2 tertiary coco alkyl CH2CH2CH2 CH2CH2 1 10
E3 tertiary isodecyl CH2CH2CH2 CH2CH2 1 2
E4 tertiary isodecyl CH2CH2CH2 CH2CH2 1 5
E5 tertiary isotridecyl CH2CH2CH2 CH2CH2 1 2
E6 tertiary isotridecyl CH2CH2CH2 CH2CH2 1 5
E7 tertiary C15 aikyl CH2CH2CH2 CH2CH2 1 2
E8 quaternary isodecyl CH2CH2CH2 CH2CH2 1 2 CI"
E9 quaternary isotridecyl CH2CH2CH2 CH2CH2 1 2 CI-
For greenhouse testing, seeds of velvetleaf (Abutilon theophrasti,
2o ABUTH) and Japanese millet (Echinochloa crus-galli, ECHCF) were planted in
10.2 cm square pots of soil with added fertilizer. Plants were allowed to grow
until the desired growth stage or size (3-leaf stage for ABUTH, 20-25 cm
height
for ECHCF) for spraying. Pots were selected for uniformity before treatment
and four replicate pots were assigned to each treatment, including an
untreated
control. Spray solutions were prepared by dilution of the concentrate
glyphosate formulations in water. Spraying was performed with a device which
simulates agricultural field spraying equipment, delivering a fine spray at a
pressure of about 207 kilopascals. Speed of travel of the spray device over
the
plants was adjusted to give the desired spray volume of 187 I/ha. For
logistical
3o reasons, all four replicates of each treatment were sprayed together. After
spraying, the plants were returned to the greenhouse. Herbicidal efficacy was
evaluated by visual assessment 16 days after treatment and recorded as
"percent control" on an arbitrary scale by comparison with untreated plants.
On
this scale 0 means no visible effect and 100 means death of all plants. In the
CA 02318657 2004-12-29
29
table below, percent control values given are the means of four replicates.
Percent Control
ABUTH ECHCF
q a.e./ha: 112 224 336 448 112 224 336 448
Formulation
C 1 0 1 60 50 6 30 61 50
C2 0 6 53 79 0 6 53 79
El 1 13 58 78 10 55 78 86
E2 3 15 49 65 3 15 49 91
E3 0 9 50 60 9 53 69 89
E4 0 4 35 65 10 53 55 78
E5 0 0 n.d. 53 5 50 n.d. 95
E6 0 6 55 65 15 55 71 81
E7 0 5 50 71 6 55 75 86
E8 0 5 40 73 10 61 76 97
E9 3 13 71 70 5 58 73 75
2o n.d. = no data
Example 16
To determine compatibility of surfactants of the invention with very
concentrated aqueous formulations of glyphosate, aqueous concentrate
compositions containing 480 g/l glyphosate a.e. in the form of the
isopropylamine salt and 80 g/l surfactant were prepared by a procedure similar
to that of Examples 1-4. The compositions of Example 16 are approximately
one-third more concentrated in respect of glyphosate a.e. than those of
3o Example 15.
Formulations C3 and C4 were made using tertiary alkylamine
surfactants of prior art. The surfactant in C3 is the same as that in
formulation
Cl of Example 15. The surfactant in C4 is the same as that in formulation C2
of Example 15.
CA 02318657 2004-12-29
Formulations E10, E11, E12 and E13 were made using the same
tertiary or quaternary N-methyl etheramine surfactants of the invention as are
present in formulations El, E6, E8 and E9 respectively of Example 15.
5
Surfactant compatibility was determined by measuring cloud point
of the formulations, as shown in the table below.
Formulation Cloud point ( C)
10 C3 >90
C4 >90
E10 >90
E11 >90
15 E12 >90
E13 >90
Example 17
20 Compositions of the invention were prepared by a procedure
similar to that of Examples 1-4 to illustrate the incorporation of a nonionic
surfactant together with the etheramine surfactant in a highly concentrated
aqueous glyphosate formulation. In formulations E14-E25 glyphosate is
present as its isopropylamine salt at 480 g a.e./I and the total surfactant
25 (etheramine plus nonionic surfactant) concentration is 80 g/l. In all cases
the
weight/weight ratio of etheramine to the nonionic surfactant is 4:1.
The etheramine surfactant in formulation E14 is the same as that
in formulation El of Example 15. The nonionic surfactant in formulation E14 is
3o an ethoxylated C14 -C16 linear primary alcohol having an average of 7 moles
of
EO.
CA 02318657 2004-12-29
31
The etheramine surfactant in formulation E15 is the same as that
in formulation El of Example 15. The nonionic surfactant in formulation E15 is
an ethoxylated C12 -C13 linear primary alcohol having an average of 5 moles of
EO.
The etheramine surfactant in formulation E16 is the same as that
in formulation El of Example 15. The nonionic surfactant in formulation E16 is
an ethoxylated Cõ linear primary alcohol having an average of 7 moles of EO.
The etheramine surfactant in formulation E17 is the same as that
in formulation E1 of Example 15. The nonionic surfactant in formulation E17 is
an ethoxylated C>> -C12 linear primary alcohol having an average of 6 moles of
EO.
The etheramine surfactant in formulation E18 is the same as that
in formulation El of Example 15. The nonionic surfactant in formulation E18 is
an ethoxylated C12 -C,5 secondary alcohol having an average of 9 moles of EO.
The etheramine surfactant in formulation E19 is the same as that
in formulation El of Example 15. The nonionic surfactant in formulation E19 is
an alkyl polyglucoside having a C8 -ClQ alkyl chain and an average of 1.7
moles
of glucose.
The etheramine surfactant in formulation E20 is the same as that
in formulation E6 of Example 15. The nonionic surfactant in formulation E20 is
an ethoxylated C14 -C,6 linear primary alcohol having an average of 7 moles of
EO.
The etheramine surfactant in formulation E21 is the same as that
in formulation E6 of Example 15. The nonionic surfactant in formulation E21 is
CA 02318657 2004-12-29
32
an ethoxylated C12 -C15 secondary alcohol having an average of 9 moles of EO.
The etheramine surfactant in formulation E22 is the same as that
in formulation E8 of Example 15. The nonionic surfactant in formulation E22 is
an ethoxylated C14 -C16 linear primary alcohol having an average of 7 moles of
EO.
The etheramine surfactant in formulation E23 is the same as that
in formulation E8 of Example 15. The nonionic surfactant in formulation E23 is
an ethoxylated C12 -C15 secondary alcohol having an average of 9 moles of EO.
The etheramine surfactant in formulation E24 is the same as that
in formulation E9 of Example 15. The nonionic surfactant in formulation E24 is
an ethoxylated C14 -C16 linear primary alcohol having an average of 7 moles of
EO.
The etheramine surfactant in formulation E25 is the same as that
in formulation E9 of Example 15. The nonionic surfactant in formulation E25 is
an ethoxylated C12 -C15 secondary alcohol having an average of 9 moles of EO.
Cloud points of formulations E14 - E25 are shown in the table
2o below.
Formulation Cloud point ( C)
E14 79
E15 73
E16 63
E17 71
E18 77
E19 >90
E20 >90
E21 >90
E22 >90
E23 >90
E24 >90
E25 >90
CA 02318657 2004-12-29
33
An attempt was made to make formulations similar to E14 - E25,
but containing, in place of the etheramine component, an ethoxylated tertiary
alkylamine surfactant of prior art. Whether a cocoamine with 5 moles of EO or
a tallowamine with 5 moles of EO was used, the prior art surfactant was found
s to be incompatible with any of the ethoxylated primary or secondary alcohol
surfactants used in formulations E14 - E25, at equal glyphosate, amine
surfactant and nonionic surfactant loadings as in these same formulations.
Thus, a further unexpected advantage of etheramine surfactants of the present
invention over alkylamine surfactants of prior art is their relatively good
io compatibility with nonionic surfactants in highly concentrated aqueous
glyphosate formulations.
Example 18
is Further to the compositions of Example 15, aqueous concentrate
compositions containing 31 % glyphosate a.e. in the form of the isopropylamine
salt and 5.5% surfactant were prepared by a procedure similar to that of
Examples 1 - 4.
20 Formulations Cl and C2 are as in Example 15.
Formulations C3 and C4 were likewise made using tertiary
alkylamine surfactants of prior art. The surfactant in C3 is an ethoxylated
cocoamine having 2 moles of EO. The surfactant in C4 is an ethoxylated
25 tallowamine having 2 moles of EO.
Formulation C5 was made using a quaternary alkylamine
surfactant of prior art, which is N-methyl cocoammonium chloride having 2
moles of EO.
CA 02318657 2004-12-29
34
Formulation C6 was made using an alkylamine oxide surfactant of
prior art, supplied by Tomah Products, Inc. as "AO-728 Special". It is an
alkylamine N-oxide having 2 moles of EO; the chain length or origin of the
alkyl
chain is not disclosed by Tomah.
Formulations El - E9 are as in Example 15.
Formulation E26 was made using an etheramine oxide surfactant
lo of the invention. Its chemical structure can be deduced from the following
table,
by reference to the representative structures shown above.
Formu- Surfactant
lation Type R1 R2 R3 m L+A A
E26 N-oxide isodecyl CH2CH(CH3) CH2CH2 1 2
Greenhouse testing was conducted exactly as described in Example
15, except that herbicidal efficacy was evaluated 17 days after treatment.
Average percent control is shown in the table below.
Percent Control
ABUTH ECHCF
g. a.e./ha: 224 336 448 672 224 336 448 672
Formulation
C1 33 58 69 83 41 69 66 95
C2 44 58 74 78 39 60 63 95
C3 44 49 85 84 50 64 83 91
C4 41 51 65 85 54 66 84 88
C5 31 41 66 76 51 71 86 93
C6 34 38 60 69 46 64 65 81
El 46 65 83 91 48 71 91 99
CA 02318657 2004-12-29
E2 50 59 75 85 40 60 71 93
E3 46 56 68 73 39 55 66 95
E4 39 55 78 80 49 59 79 89
E5 40 51 73 75 51 63 79 86
5 E6 49 60 83 86 51 63 80 94
E7 31 39 75 75 46 63 75 95
E8 43 55 73 80 46 58 73 93
E9 48 50 84 85 51 60 85 98
E26 48 59 73 83 48 61 84 94
While the illustrative embodiments of the invention have been
described with particularity, it will be understood that various other
modifications
will be apparent to and can be readily made by those skilled in the art
without
departing from the spirit and scope of the invention. Accordingly, it is not
intended that the scope of the claims appended hereto be limited to the
examples and descriptions set forth hereinabove but rather that the claims be
construed as encompassing all the features of patentable novelty which reside
in the present invention, including all features which would be treated as
equivalents thereof by those skilled in the art to which the invention
pertains.