Note: Descriptions are shown in the official language in which they were submitted.
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-1-
Wig: Method for the Prevention and Treatment of a Type I Hypersensitivity
Disorder
FIELDS1F INVENTION
The present invention relates to methods and compositions for treating a type
I
hypersensitivity disorder using Rh antibodies. In particular, Rho(D) immune
globulin
reduces the duration and/or intensity of exacerbations in asthmatic patients
and reduces the
dose requirement of concomitant or other therapeutics such as corticosteroids
in asthmatic
patients. Rho(D) immune globulin is also useful in preventing the onset and
reduce the
frequency of exacerbations or episodes in asthmatic patients.
~,~:KGROUND OF THE INVENTION
Hypersensitivity generally refers to an inappropriate or exaggerated immune
response to a particular antigen. There are four major classes of allergic or
hypersensitivity
reactions. Type I hypersensitivity results when an immunoglobulin E (IgE)
response is
mounted against an innocuous antigen causing mast cells to release mediators
which in tum
cause inflammation. Many conditions result from a type I hypersensitivity
response including
allergies, eczema, hayfever, urticaria, atopic dermatitis and asthma (see also
Gell-Coombs
classification: Coombs, R.R.A. and Gell, P.G.H.: Classification of allergic
reactions
responsible for clinical hypersensitivity and disease. In Clinical Aspects of
Immunology, eds.
Gell, P.G.H., Coombs, RR.A. and Lachmann, P J:, Blackwell Scientific
Publications, Oxford,
1975).
Asthma is. a chronic inflammatory illness characterized predominantly by
reversible airway obstruction and airway hyperresponsiveness. The major
clinical symptoms
of asthma include recurrent episodes of wheezing, breathlessness, chest
tightness and
coughing. Asthma is a common illness affecting 5~10% of the population, and it
is associated
with substantial mortality which is increasing in certain countries (Sears,
M.R. and Taylor,
D.R., Drug Safety 11: 259-283, X994; Page, GP., J. Asthma 30: 155-164, 1993;
Weiss, K.B.,
jAMA 264:1683-1687,1990; Gergen, P.J. and Weiss, K.B., jAMA 264: 1688-
1692,1990).
The pathogenesis of asthma is currently thought to be due, in large, to
inflammatory-based changes in the airway wall and to airway smooth muscle
dysfunction
(Hogg, J.C., APMIS 105: 735-745,1997). The basic mechanism underlying airway
narrowing or
broncoconstriction in asthma is typified by a type I immediate
hypersensitivity illness and is
believed to involve an activation of mast cells which release a large number
of inflammatory
mediators such as histamine, leucotrienes, cytokines and proteases. These
mediators exert
potent-effects to produce airway vasodilatation and increase vasopermeability.
Influx of
inflammatory cells including granulocytes (such ~as eosinophils), monocytes
(precursors of
macrophages) and lymphocytes into the brochial'tissue is also increased and
these cells in
tum secrete additional mediaboa ~of inflammation.vrhich further enhance
brochoconstriction,
mucus secretion and tissue inflammation (Serafin, W.E. In Goodman and Gilman's
The
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-2-
Pharmacological Basis of Therapeutics, Chap. 28, p. 659-682, 1996; Priel, LE.,
Med. Law 22:
351-361, 1993; Hughes, J.M., et. al., Clin. Exp. Allergy 23: 251-256, 1993).
Inappropriate
immune responses to airborne antigens by the respiratory T-lymphocyte system
have also
been attributed to play a part in the immunopathology of asthma (Holt, P.G. et
al., Eur.
Respir. J. 4 Suppl. 13: 6s-15s, 1991; Jansen, H.T. et al., Eur. Respir. J. 4
Suppl. 13: 3s-5s, 1991;
Holgate, S.T., Eur. Respir. J. 6: 1507-1520, 1993). T-lymphocytes overproduce
cytokines such
as interleukins to cause and maintain excessive infiltration and to upregulate
function of
eosinophils and mast cells. Mast cells are primarily responsible for the
secretion of the
vasoactive amine histamine while eosinophils have direct cytotoxic actions in
the bronchial
epithelium and are the major cause of bronchial tissue damage in asthma
(Karlen, S. et. al.,
Int. Rev. Immunol. 16: 227-247, 1998; Tonnel, A.B. et. al., Rev. Prat. 42:
2399-2404, 1992).
Classification or categorization of asthma can be based upon disease etiology
or severity (Fabbri, L.M. et al., In Allergy and Allergic Diseases, Chap. 87,
p. 1347-1359,
1997). From an etiology perspective, asthma may be categorized as extrinsic
(atopic) or
intrinsic. Extrinsic asthma is used to define asthma occurring in atopic
patients with an
increased amount of IgE against common environmental allergens. It is often
possible to
identify the immunogens associated with the onset of such an asthmatic attack
or
exacerbation, but an increasing number of non-allergenic agents that can also
provoke an
asthmatic attack has also been found. Identified environmental agents that
could instigate
asthmatic exacerbations are diverse and include, but are not limited to,
common allergens
(e.g. proteins found in ragweed, dust mite and pet dander), environmental
pollutants such as
sulphur dioxide as well as various pathogens in respiratory infections such as
for example
bacteria and viruses.
The category of intrinsic or non-atopic asthma is used to define a group of
patients for whom no extraneous or external causative agent can be identified.
A second classification system for asthma is based on the severity of the
illness. The overall severity of exacerbations among asthmatic patients can
vary
substantially in the clinical setting, and determination of the severity of
asthma is often
based primarily on a combination of symptom scores and spirometry measurements
{Sheffer,
A.L., Publication No. 95-3659, World Health Organization and National
Institute of
Health, 1995). The type and amount of therapeutic intervention required to
control the
illness is often correlated with illness severity (see below).
A majority of asthmatic patients have a self-limited or easily manageable
disease, but a subset of patients suffers from chronic, debilitating
intractable respiratory
difficulties. Major therapeutic treatment of asthma includes administration of
bronchodilators as physiological antagonists and anti-inflammatory agents to
counteract
airway vasodilatation and vasopermeation.
Examples of bronchodilators used in the treatment of asthma are
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99J00109
_3-
beta-adrenergic receptor agonists, xanthine derivatives and anticholinergics.
Beta-adrenergic receptor agonists (such as albuterol, terbutaline, pirbuterol,
salbutamol,
salrileterol and formoterol) provide rapid relief of acute bronchospasm and
are-also effective
bronchoprotective agents for prophylaxis in atopic asthma. Albuterol and
terbutaline are
available as nebulizer solutions and metered dose inhalers for pulmonary drug
delivery
(about 200-400 ug three to four times per day). Xanthine derivatives such as
theophylline
(given orally at about 12-16 mg per kg per day), enprofylline and
pentoxifylline, have longer
duration of action than beta-adrenergic receptor agonists thereby providing
adequate ~ w
protection against both early and late phase responses to an allergen
stimulus. However,
xanthines are relatively weaker bronchodilators and bronchoprotectors and
careful dose
titration and patient monitoring are required in view of their narrow
therapeutic margins.
The value of anticholinergic agents in asthma therapy is to date controversial
and their role
in combinational therapy is under investigation.
With respect to anti-inflammatory agents, two classes of agents are available
for the treatment of asthma which are namely cromolyn-based drugs and
corticosteroids, or
more specifically, glucocorticoids (Szefler, S.J., Clin. Allergy 76: 953-975,
1992). These
therapeutics may be given systemically by oral administration, injection or by
pulmonary
delivery such as aerosols or nebulizers. The latter approach has the advantage
of producing
high local drug concentration in the lungs thereby improving therapeutic ratio
and reducing
systemic side effects.
Cromolyn-like therapeutics block both the early and late phases of pulmonary
response in allergen or exercise-induced asthma and prevent the development of
airway
hyperresponsiveness. These compounds are relatively safe to use in all age-
groups and are
primarily efficacious in alleviating mild to moderate asthma. Their mechanisms
of action
are believed to involve the inhibition of antigen-induced bronchospasm as well
as autocoid
release through reduced calcium influx and altered protein phosphorylation in
bronchial
tissue and mast cells (Hoag and McFadden, 1991; Murphy and Kelly, 1987;
Shapiro and
Konig, 1985). Cromolyn sodium is usually given by inhalation as a solution or
powder at
about 2 mg doses four times per day while nedocromil is given also by
inhalation at about 4
mg doses twice per day.
Glucocorticoids are more potent and more widely used than cromolyn
compounds and systemic corticosteroid therapy has remained as the mainstay of
treatment of
severe asthma (McFadden, E.R., Am. Rev. Resp. Dis. 147: 1306-1310, 1993;
Greenberger, P.A.,
Chest 101: 418S-4215, 1992). Examples of systemic glucocorticoid treatment
regimens are
prednisone given orally at 1-2 mg per kg patient body weight per day for 3-7
days and
methylprednisolone sodium succinate given intravenously at about 1 mg per kg
every 6 hours.
This form of therapy has proven to be efficacious in severe acute asthma and
status
asthmaticus, but the benefits of asthma control must be weighed against the
severe side
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-4-
effects of corticosteroids. Obesity, hirsutism and Gushing-like symptoms are
common
drawbacks, but more severe adversities such as growth suppression, diabetes
mellitus,
osteoporosis and intraocular hypertension are also encountered.
To reduce the risk of adversities associated with corticosteroid therapy in
patients with the more severe form of asthma, it is important to use the
lowest dose possible
to achieve therapeutic efficacy while minimizing the systemic drug
concentration. The
development of an inhalational/pulmonary route of administration for low-dose
corticosteroid therapy has improved dramatically its systemic toxicity profile
and has
allowed corticosteroids to be used in moderate asthma. In the United States,
beclomethasone
diproprionate (maxima of 0.4 and 0.8 mg per day for children and adults),
triamcinolone
acetonide (maxima of 1.2 and 1.6 mg per day for children and adults) and
flunisolide
(maxima of 1.0 and 2.0 mg per day for children and adults) are available as
metered dose
inhalers while budesonide diproprionate (about 1 mg per kg per day) and
fluticasone
proprionate are available in Europe. Nevertheless, serious side effects remain
prominent
with high dose inhalational therapy and chronic exposure to corticosteroids
can also result
in progressive pulmonary toxicities such as lung fibrosis.
A second means to reduce the dosage of corticosteroids required is
combinational therapy with other therapeutic agents. Certain anti-asthmatic
therapeutics
can complement or synergize with corticosteroids and are said to possess
"corticosteroid-sparing" effects. Simultaneous or sequential administration of
these agents
with corticosteroids allows the reduction of the dosages of corticosteroids to
achieve
equivalent efficacy.
A progressive treatment schedule for asthma therapy has been proposed by
Szefler, S.J. (Clin. Allergy 76: 953-975, 1992) which consists of four stages
accounting for the
severity of the illness and the corresponding aggressiveness of the treatment
regimen. The
first stage of the model contemplates the use of the short-acting beta-
adrenergic receptor
agonists to provide acute rapid relief of early onset asthmatic symptoms such
as sudden
episodic exacerbations precipitated by allergen exposure or respiratory
infections. The next
stage involves the implementation of prophylactic measures and the use of a
combination of a
bronchodilator (theophylline, beta-adrenergic receptor agonists) and an anti-
inflammatory
(cromolyn, inhalational glucocorticoid) as maintenance therapy. Symptoms in
stage 3 begin
to compromise the patient's quality of life. Systemic or high-dose
inhalational
glucocorticoid treatment is required to relieve acute exacerbations and
maintenance therapy
to control the frequency of exacerbations. Patients in stage 4 have
deteriorating pulmonary
function and suffer from frequent episodes of acute life-threatening
exacerbations. They are
poor-responders to conventional bronchodilator and glucocorticoid therapies
and require
immunomodulator therapy to counter the adverse effects of high-dose
bronchodilator and
glucocorticoid therapy. Patients in the latter stages can often befit from
alternative modes
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-5-
of therapy which currently include only a limited choice of drug candidates:
troleandomycin,
methotrexate, gold, hydroxychloroquine, dapsone, cyclosporine A and immune
globulin
products (Ruhl, R et al., Allergol. Immunopatitol. Madr. 21: 53-60, 1993).
Troleandomycin (TAO) was introduced originally as a macrolide antibiotic
against gram-positive bacteria and has more recently been studied for its
benefits in
infectious asthma (Wald, J.A. et al., J. Allergy Clin. Immunol. 78: 36-43,
1986; Zeiger, R.S. et
al., ]. Allergy Clin. Immunol. 66: 438-446, 1980; Fox, J.L., Penn. Med. J. 64:
634-635, 1961).
Administration of TAO to asthmatic patients produced significant clinical
improvements
particularly when given conjunctive to corticosteroid therapy. A steroid-
sparing effect was
recognized when TAO at 250 mg per day was combined with methylprednisolone in
steroid-dependent asthmatics. The underlying benefits of TAO include a
reduction in sputum
production and in airway inflammation. Conversely, TAO therapy has been
associated with
significant hepatotoxicity (contraindicated in patients with pre-existing
liver disease or
hypersensitivity to macrolides) and should only be used to treat very severe
forms of asthma.
Methotrexate inhibits dihydrofolate reductase and has been used with some
degree of success in the treatment of various immune disorders such as
rheumatoid arthritis
and psoriasis. More recent evidence indicates that methotrexate also reduces
the serum
levels of several subclasses of immune globulin and possesses anti-
inflammatory properties by
inhibiting C5a neutrophil chemotaxis and release of histamine and interleukin.
Its
usefulness in asthma therapy has been demonstrated mainly in steroid-dependent
asthmatics
in that patients treated with methotrexate were able to significantly reduce
their
corticosteroid dose requirements without changes in airway hyperresponsiveness
(Dyer, P.D.
et al., J. Allergy Clin. Immunol. 88: 208, 1991; Sorkness, C.A. et al., J.
Allergy Clin. Immunol.
87: 298, 1991; Shiner, R.J. et al., Lancet 336: 137, 1990; Mullarkey, M.F. et
al., N. Engl. J. Med.
318: 603-607, 1988; Mullarkey, M.F. et al., Ann. Allergy 56: 347-350, 1986).
Guidelines for
methotrexate therapy in the treatment of rheumatoid arthritis are applied in
general to
asthma therapy. It is given intramuscularly or orally in dosages of about 7-15
mg per week
and the parenteral route is preferred to maximize bioavailability.
Methotrexate therapy
should be limited to severe steroid-requiring asthmatic patients and this drug
is associated
with significant side effects including gastrointestinal disturbances such as
vomiting,
stomatitis, diarrhea, and hepatotoxicity.
Gold salts have been used effectively in the treatment of immune disorders
including rheumatoid arthritis and asthma (Foley, B. et al., J. Allergy Clin.
Immunol. 87:
298, 1991; Bernstein, D.I. et al., J. Allergy Clin. Immunol. 81: 6, 1988;
Muranaka, M. et al.,
Ann. Allergy 40: 132, 1978). Given weekly at dosages of about 10-100 mg as
intramuscular
injections, gold thiomalate produced significant clinical improvement in
patients with
extrinsic, but not intrinsic, asthma. More recent experience with an oral gold
compound,
auranofin (3 mg twice daily), suggest the ability of gold salts to decrease
bronchial
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-6-
hyperresponsiveness and to reduce maintenance dosages of corticosteroids
(steroid-sparing
effect). The mechanism of action for gold salts in asthma is not fully known
but is believed to
involve the inhibition of production and secretion of lyzosomal enzymes,
histamine,
chemokines and antibodies. Major adverse effects of gold therapy are
dermatitis,
proteinuria, stomatitis and diarrhea which are often reversible upon cessation
of treatment.
Numerous reports have also described the successful use of miscellaneous
therapeutics such as dapsone, hydroxychloroquine and cyclosporine A, in the
treatment of
severe corticosteroid-dependent asthma (Finnerty, N.A. and Sullivan, T.J., J.
Allergy Clin.
Immunol. 87: 297, 1991; Berlow, B.A. et al., J. Allergy Clin. Immunol. 87: 710-
715, 1991;
Charous, B.L., Ann. Allergy 65: 53-58, 1990). All agents were shown to
alleviate asthmatic
symptoms and to possess corticosteroid-sparing effects. The anti-neutrophilic
effects of
dapsone reduces inflammation, which may explain the efficacy of this compound.
Chloroquine-based drugs may elicit their effects through stabilization of
membranes and
inhibition of phospholipase A2. As an inhibitor of cytokine production,
cyclosporine A is a
well known immunosuppressor which may account for its anti-asthmatic effects.
Data from
larger scale controlled clinical studies are required to establish the
usefulness and value of
these agents in asthma.
The notion of immunomodulatory therapy for intervention in an immune
disorder such as asthma has been known and practised for many years. Active
immunotherapy with specific allergens effectively reduces symptoms of allergic
diseases and
decreases specific IgE levels in patients, while intravenous immune globulin
(IVIG) as an
immunotherapeutic has been used extensively to provide passive immunity in
humans since
the turn of the century.
Immune globulins (also known as immunoglobulins) are proteins produced by
lymphoreticular tissues. There are 6 known classes of immune globulin: IgG,
IgM, IgA, IgD,
IgE and secretory IgA. IgG (also known as gamma-globulin) is the most abundant
and the most
therapeutically relevant class of immune globulin. The primary function of
immune globulins
is to specifically recognize and bind antigens through reversible bonding
thereby facilitating
elimination of the antigens by the immune system.
IgG is a glycoprotein of approximately 150,000 Daltons consisting of 2 "heavy"
(gamma) chains and 2 "light" (kappa or gamma) chains held together by
disulphide as well
as weak covalent bonds. Within the IgG class, there are 4 subclasses: IgGl,
IgG2, IgG3 and
IgG4 comprising about 70%, 15%, 10% and 5% of total IgG in normal human serum,
respectively. These subclasses possess minor antigenic differences among their
"heavy"
chains resulting in distinct biological actions.
There are principally two types of immune globulins that are available as
therapeutic ag~ts: standard immune serum globulin preparations for general
use, and immune
globulin preparations that recognize specific antigens for use in specific
disorders.
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
_7_
Commercial examples of products in the latter category are hepatitis B immune
globulin,
varicella zoster immune globulin and Rh immune globulin. The primary
therapeutic basis for
immune globulins is passive immunity conferred to the recipient through the
direct
introduction of extraneous "ready-made" antibodies. The major clinical
utilities of immune
globulins are prophylaxis and/or treatment of antigen-associated disorders.
It is well known in the art that immune globulins may be prepared by isolation
of natural immune globulins from mammalian serum or by recombinant DNA
technology. In
the first instance, they may be isolated using the Cohn cold ethanol
fractionation method
(see Huchet, J. et al., Rev. Fr. Transfus. 13:231, 1970; Chown, B. et al.,
Can. Med. Assoc. J.
100:1021, 1969; Barandun, S. et al., Vox Sang. 7: 157-174,1962). Immune
globulin prepared by
this method suffers from relatively low yield and purity, and the resultant
product contain
aggregated polymers of the protein and cannot be administered by intravenous
injection or
infusion. Intravenous injection or infusion is preferred in the clinical
setting due to its instant
bioavailability and rapid onset of therapeutic protection as compared to other
parenteral
routes in which the immune globulin is partially lost due to proteolysis
and/or incomplete
absorption.
Improved methods have been developed to provide purer monomeric proteins
and/or to substantially increase product yield. Examples of these methods
include: utilizing
acid treatment (see Jouvenceaux, A. et al., Rev. Fr. Transfus. 12 (suppl.):
341, 1969),
ion-exchange chromatography e.g. using DEAE-Sephadex columns {see Canadian
Patent
number 1,168,152; Canadian Patent number 1,201,063; Cunningham, C.J. et al.,
Biochem. Soc.
Trans. 8: 178, 1980; Hoppe, H.H. et al., Vox. Sang. 25: 308, 1973; Hoppe, H.H.
et al., Munch.
Med. Wochenschr. 109: 1749, 1967; Baumstark, J.S. et al., Arch. Biochem.
108:514, 1964),
ultracentrifugation, or treatment with pepsin, plasmin, a sulfitolytic agent
or
beta-propriolactone (see U.S. Patent No. 4,160,763; Barandun, S. et al.,
Monogr. Allergy 9:
39-60, 1975; Stephan, Vox Sang. 28: 422-437, 1975; Wells, J.L.V. et al.,
Austr. Ann. Med. 18:
271, 1969; Baumgarten, W. et al., Vox Sang. 13: 84, 1967; Merler, E. et al.,
Vox Sang. 13: 102,
1967; Sgouris, J.T. et al., Vox Sang. 13: 71,1967; Barandun, S. et al., Vox
Sang. 7: 157-174, 1962;
Nisonoff, A. et al., Science 132: 1770-1771,1960). Immune globulin prepared by
such improved
processes may be administered by parenteral means including intravenous
injection.
Monoclonal immune globulins can be produced using recombinant and
hybridoma techniques (see Canadian Patent number 1,303,534; Canadian Patent
number
1,303,533; European Patent Application 87302620.7 published as EP 239,400;
European Patent
Application 93102609.0 published as EP 557,897; Fletcher, A. and Thompson, A.,
Transfus.
Med. Rev. 9: 314-326,1995; Aping-Mees, M. et al., Strat. Mol. Biol. 3:1-
9,1990; Huse, W.D. et
al., Science 246: 1275-1281, 1989; Sastry, L. et al., Proc. Natl. Acad. Sci.
LISA 86: 5728-5732,
1989). Similarly, binding partners or domains may also be constructed using
recombinant
DNA techniques to incorporate the variable regions of a gene encoding a
specific antibody
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
_g_
(see PCT Patent Application PCT/GB93/00605 published as WO 93/19172; PCT
Patent
Application PCT/GB93/02492 published as WO 94/13804; PCT Patent Application
PCT/EP90/01964 published as WO 91/07492; Bird et al., Science 242: 423-
426,1988).
The clinical value of MG in asthma therapy has been postulated for many
years but few controlled studies have been conducted to establish this mode of
therapy. Use
of IVIG in the clinical setting for this purpose remains relatively limited.
Previous reports describing the benefits of IVIG therapy include the ability
of
IVIG to reduce recurrent wheezing in asthmatic patients with
immunodeficiencies (Smith,
T.F., J. Asthma 26: 5-13, 1989; Smith, T.F. et al, Monogr. Allergy 23: 188,
1988; Page, R. et al.,
J. Pediatr. 112:126-131,1988) and to reduce the number/frequency of asthmatic
exacerbations
and improve pulmonary function (forced expiratory volume per second, thoracic
gas volume,
airway resistance, specific conductance) in steroid-dependent asthmatic
patients without
immunodeficiencies {Gelfand, E.W. et al., CIin. Exp. Immunol. 104 Suppl. 1: 61-
66, 1996;
Smiley, J.D. and Talbert, M.G., Am. J. Med. Sci. 308: 295-303, 1995; Silk,
H.J., ]. Asthma 31:
231-241, 1994; Schuster, A. And Wahn, V., infusionsther. Transfusionsmed. 20
Suppl. 1: 141-
144, 1993; Alvarez, J.M. and Szefler, S.J., J. Asthma 29: 3-11, 1992; Fireman,
P. And Friday, G.,
Clin. Rev. Allergy 10: 135-142, 1992; Levinson, A.L, J. Allergy Clin. Immunol.
88: 552-554,
1991; Mazer, B.D. and Gelfand, E.W., J. Allergy Clin. Immunol. 87:976-983,
1991; Mazer, B.D.
et al., Clin. Immunol. Immunopathol. 53: 5156-S163, 1989). The dosages of
glucocorticoid
required by these patients for illness control were also significantly reduced
during the I VIG
treatment period. Unlike other alternative agents discussed above, side-
effects observed
with IVIG therapy in asthmatic patients were relatively less severe at doses
of up to 2 g/kg
every 4 weeks.
The exact mechanism of action underlying the action of IVIG in asthma
remains to be defined. At low doses, IVIG administration may produce its
effects through an
increase in patient serum antibody level thereby conferring passive protection
to individuals
with impaired immunity to infections (Barlan, LB. et al., J. Allergy, Clin.
Immunol. 92:
353-355, 1993; Buckley, R.H., JAMA 258: 2841-2849, 1987). It is possible that
IVIG improves
asthma by alleviating any persistent infection in the airways that could be
etiologic in some
asthmatic exacerbations. In addition, therapy with IVIG may provide broadly
reacting
anti-idiotypic antibodies thereby attenuating hyperreactive responses in
asthmatic patients
(Busse, W.W. et al., Am. J. Respir. Crit. Care Med. 154: S70-S72,1996). At
higher doses, IVIG
may act as an immunomodulator to regulate the immune system. 1VIG may
interrupt the
cascade of inflammatory events that underlie asthma and modulate the
regulation of IgE
antibody production.
Conversely, conflicting reports exist to indicate the null-effect of IVIG
therapy
in adult or pediatric asthmatic patients (Fontana, V., J. Pediutr. 62: 80-84,
1963; Abernathy,
R. et al., Pediatr. 21: 980-993, 1958). A more recent study by Jakobsson, T.
et al. (Allergy 49:
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-9-
413-420, 1994) revealed that the benefits of IVIG in moderately severe
asthmatic patients
were only slight and transient and the investigators did not recommend the
general use of
IVIG in asthma. To date, the role of IVIG in asthma therapy is reserved as an
experimental-type alternative agent (Montanaro, A., J. Asthma 31: 227-229,
1994). This may
be attributed, in part, to its relatively high cost, the invasiveness of
therapy, and unproven
efficacy compared to existing commercially available anti-asthmatic agents.
An increasing understanding of the pathogenesis of asthma has led to the
implication of diverse immune mediators in asthma etiology which have become
the targets
for anti-asthmatic immunotherapy. Specific immune globulin products targeting
these
mediators have been designed and tested, examples of which are listed as
follows.
United States Patent Nos. 5,449,760 and 5,342,924 and PCT Patent Application,
published as WO 97/33616 describe a humanized murine anti-IgE antibody and its
therapeutic potential in treating allergic diseases including asthma. Such
anti-IgE
antibodies are also documented previously by Kolbinger, F. et al. (Protein
Eng. 6: 971-980,
1993; Kolbinger, F. et al., Biotechnol. 94-112: 49-51, 1994).
Zhou, C.Y. et al. (J. Asthma 34: 195-201, 1997) demonstrated the ability of
anti- interleulcin-4 antibodies to inhibit IgE production and proposed their
usefulness in the
prophylaxis of asthma.
United States Patent No. 5,670,626 describes monoclonal IgA antibodies
specific against major allergenic proteins found in ragweed, dust mites and
animal dander.
These antibodies are potentially useful in recognizing and neutralizing major
allergens
associated with the etiology of extrinsic asthma.
European Patent Publication Nos. 528,931, 528,951 and 551,501 and United
States Patent No. 5,324,510 describe humanized IgG antibodies specific against
intercellular
adhesion molecule (ICAM)-1, endothelial-leucocyte adhesion molecule (ELAM)-1
or CD18
which are implicated in the mediation of airway abnormalities in asthma.
Interference of
the functions of adhesion molecules is a plausible approach for asthma
therapy.
Other mediator-specific immune globulins that are potentially useful in
asthma are anti-CD4 antibodies targeting T-lymphocytes (PCT Patent
Application,
published as WO 92/08474); anti-endothelia antibodies for the treatment of
vasoconstrictive
illnesses (European Patent publication No. 384,144); humanized antibodies
specific against
alpha-4 integrin (PCT Patent Application, published as WO 97/18838; Lobb, R.R.
et al., Eur.
Respir. J. 9 Suppl. 22: 104s-108s, 1996); antibodies specific against platelet
activating factor
{PAF) (European Patent publication No. 761,232); and humanized anti-VLA-4 IgG
or IgA
antibodies (European Patent publication Nos. 678,122 and 626,861).
The present inventor has surprisingly found that antibodies specific against
Rh antigens are effective in the prophylaxis and treatment of asthma in
mammals with or
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-10-
without immunodeficiency. These lth antibodies and their use are directed
toward antigenic
targets that are clearly different than those recognized by antibodies of the
prior art.
The present inventor has found that Rh antibodies are useful for the
preventing and treating type I hypersensitivity disorders. Accordingly,
broadly stated the
present invention relates to a method for preventing and treating asthmatic
exacerbations or
episodes in an animal comprising administering an effective amount of lth
antibodies or
anti-1Zh immunoglobulin to an animal in need thereof. In one embodiment, the
type I
hypersensitivity disorder is asthma. In preferred embodiments, an Rh positive
human
subject is treated with anti-lZha(D) immunoglobulin and an Rh negative subject
is treated
with anti-c immunoglobulin.
In another aspect of the invention, a process is provided for preparing an
anti-Rh immunoglobulin fraction comprising contacting an aqueous animal plasma
fraction
containing IgG with two different chromatographic separation columns to
produce a purified
IgG-rich fraction; and treating said purified IgG fraction with a
solvent/detergent process.
In a further aspect, the present invention also relates to a pharmaceutical
composition for use in preventing and treating a type I hypersensitivity
disorder comprising
Rh antibodies or anti-Rh immunoglobulin in admixture with a suitable diluent
or carrier. In
one embodiment, the composition for preventing the onset and reducing the
severity or
duration of asthmatic exacerbations comprises an anti-Rh immunoglobulin,
preferably human
anti-Rh immunoglobulin, in admixture with a suitable diluent or carrier.
In yet another aspect, the invention provides the use of anti-Rh
immunoglobulin for preventing or treating a type I hypersensitivity disorder,
such as
asthmatic exacerbations or episodes.
In a further aspect, the present invention provides the use of anti-lth
immunoglobulin for manufacturing a medicament for preventing or treating a
type I
hypersansitivity disorder such as anti-asthma therapy or prophylaxis.
Other features and advantages of the present invention will become apparent
from the following detailed description and attached drawings. In addition,
reference is
made herein to various publications, patents and patent applications which are
hereby
incorporated by reference in their entirety. It should be understood, however,
that the
detailed description and the specific examples while indicating preferred
embodiments of
the invention are given by way of illustration only, since various changes and
modifications
within the spirit and scope of the invention will become apparent to those
skilled in the art
from this detailed description.
DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates a treatment schedule for WinRho SI7~ in asthma and the
efficacy of WinRho SD~ in preventing asthmatic episodes.
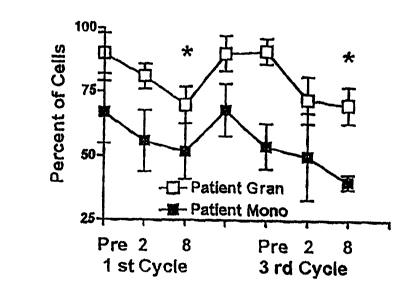
Figure 2 illustrates the efficacy of WinRho SD~ in decreasing patient
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-11-
granulocyte and monocyte counts.
Figure 3 illustrates the efficacy of WinRho SD~ in reducing the immune
responsiveness of Peripheral Blood Mononuclear Cells.
DETAILED DESCRIPTION OF THE INVENTION
As hereinbefore mentioned, the present inventor has found that Rh antibodies,
in particular anti-Rho(D) immunoglobulin, are useful in preventing and
treating type I
immediate hypersensitivity reactions including asthmatic exacerbations.
Accordingly,
broadly stated the present invention relates to a method for preventing and
treating a type I
hypersensitivity disorder in an animal comprising administering an effective
amount of lZh
antibodies or anti-Rh immunoglobulin to an animal in need thereof. In one
embodiment, the
type I hypersensitivity disorder is asthma.
The term "effective amount" means an amount effective, at dosages and for
periods of time necessary to achieve the desired result.
The term "animal" means all members of the animal kingdom including
humans.
The term "Rh antibodies" means antibodies specific for antigens of the Rh
blood group system, or epitopes thereof (see Rh Blood Group System, in Blood
Transfusion in
Clinical Medicine, ed. Mollison, P.L. et al., chapter 8, page 328, for a
review of the Rh blood
group antigens, which is incorporated in its entirety herein by reference).
Examples of the Rh
antibodies include anti-D (also known as anti-Rho, and also referred to herein
as
anti-Rho(D)); anti-C (also known as anti-rh'); anti-E (also known as anti-
rh"); anti-c (also
known as anti-hr') and anti-e (also known as anti-hr"). The Rh antibodies of
the present
invention may be preparations from plasma enriched for Rh antibodies,
polyclonal
antibodies, monoclonal antibodies, antibody fragments (e.g. Fab, and F(ab')2),
and those
produced by recombinant DNA technology.
In one embodiment, the invention provides a method for treating or preventing
asthma comprising administering an effective amount of Rh antibodies to an
animal in need
thereof. In particular, the present inventor has found that asthmatic patients
who have
been treated with anti-Rho(D) immunoglobulin suffer from fewer and less severe
asthmatic
exacerbations. In patients with more severe form of the disease or
corticosteroid-dependent
asthma, lower corkicosteroid dosages are required for disease control when
said patient are
treated with anti-Rho(D) immunoglobulin. While not wishing to be bound by a
particular
theory, the Rh antibodies act by modulating the body's immune system such as
the
attenuation of inappropriate immune cell function and the cytokine cascade
system. For
example, an inhibition of the cytokine and chemokine cascades would reduce the
influx of
inflammatory cells into the brochial tissue thereby reducing
brochoconstriction, mucus
secretion and tissue inflammation.
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-12-
Generally Rh ant~odies for use in the invention are selected depending on the
Rh antigens present/absent on the red cells of the subject to be treated. Anti-
Rho(D) is
preferably used to treat Rh-positive (i.e. D-positive) subjects, and anti-c is
preferably used to
treat Rh-negative (i.e. D-negative) subjects.
Preparations with a high Rh antibody content may be isolated as an immune
globulin fraction from plasma, preferably human plasma, using conventional
techniques. For
example, they may be isolated using: (a) the Cohn cold ethanol fractionation
method or
modifications thereto (see Huchet, J. et al., Rev. Fr. Transfus. 13:231, 1970;
Chown, B. et al.,
Can. Med. Assoc. J. 100:1021, 1969; Jouvenceaux, A. et al., Rev. Fr. Transfus.
12 (suppl.): 341,
1969; Barandun, S. et al., Vox Sang. 7: 157-174, 1962); (b) ion-exchange
chromatographic
methods ( e.g. using DEAE-Sephadex) and modifications thereto may be used to
produce Rh
antibodies of higher product yield and quality (Cunningham, C.J. et al.,
Biochem. Soc. Traps.
8: 178, 1980; Hoppe, H.H. et al., Vox. Sang. 25: 308, 1973; Hoppe, H.H. et
al., Munch. Med.
Wochenschr. 109: 1749, 1967; Baumstark, J.S. et al., Arch. Biochem. 108:514,
1964); or (c)
anion-exchange chromatographic method as taught in Canadian Patent No.
1,201,063, and
modifications thereto. Commercially available anti-RhoD immune globulin
preparations
may also be used in the methods. For example, anti-RhoD preparations including
WinRho~
or WinRho SD~ (Cangene Corporation), HyplZho-D~ (Miles Canada Inc.) or RhoGAM~
or
MICRhoGAIVI~ (Ortho Diagnostics) may be used in the present invention.
In an embodiment of the invention, an anti-RhoD immune globulin fraction is
prepared by contacting an aqueous plasma fraction containing IgG with one or
more
chromatographic separation columns to produce a purified IgG-rich fraction.
The aqueous
plasma fraction used in the process may be normal non-immunized plasma from an
animal
source, preferably a human source, or hyperimmune plasma such as plasma from
Rh
alloimmunized donors. For example, the RhoD antigen is used to immunize the
animal
through intramuscular, subcutaneous, intraperitoneal, or intraocular
injection, with or
without an adjuvant such as Freund's complete or incomplete adjuvant. With the
option of
booster immunizations, samples of serum are collected and tested for
reactivity to the antigen
in standard assays (described below). Particularly preferred polyclonal
antisera will give a
signal on one of the assays that is at least three times greater than
background. Once the
titre of the animal has reached a plateau in terms of antigen reactivity,
larger quantities of
the antisera may be obtained readily either by periodic bleeding or by
exsanguinating the
animal.
Human anti-RhoD immune globulin may also be produced in human volunteers.
For example, an anti-RhoD immune globulin preparation may be obtained from a
subject who
is naturally immunized (e.g. from an Rh incompatible pregnancy) or
artificially immunized
using Rh-positive blood cells or RhoD antigen.
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-13-
Anti-RhoD immune globulin-containing plasma collected from animal or
human is modified to the ionic strength and pH of the initial buffer used with
the
chromatographic separation column. In an embodim~t of the invention, the
aqueous animal
plasma fraction is contacted with one or more, preferably one to two, anionic
exchangers to
produce a purified IgG-rich fraction.
By way of example, aqueous animal or human plasma fraction is applied to an
anion exchange column which may contain an agarose cross-linked anionic
exchange resin
such as DEAE-Sepharose CL6B or DEAE-Biogel, and an IgG-rich fraction is
obtained by
eluting with an equilibrating buffer. The IgG-rich fraction may be
concentrated for example
by ultrafiltration. The concentrated IgG-rich fraction is then applied to a
second different
anion exchange column such as DEAE-Biogel or DEAE-Sephadex A-50. A purified
IgGrich
fraction is isolated by elution with an appropriate equilibrating buffer which
may be further
purified using ultrafiltration.
The purified IgG protein may optionally be treated with a solvent and
detergent to inactivate lipid envelope viruses. Suitable solvents and
detergents which may
be used include Triton X-100 and tri(n-butyl) phosphate (Horowitz, B., Curr.
Stud. Hematol.
Blood Transfus. 56: 83-96, 1989). After the process, said solvents and
detergents may be
removed using conventional methods such as reverse phase chromatography.
Monoclonal immune globulins may also be produced readily using recombinant
and hybridoma techniques (see Canadian Patent number 1,303,534; Canadian
Patent number
1,303,533; European Patent Application 87302620.7 published as EP 239,400;
European Patent
Application 93102609.0 published as EP 557,897; Fletcher, A. and Thompson, A.,
Transfus.
Med. Rev. 9: 314-326,1995; Alting-Mees, M. et al., Strat. MoI. Biol. 3: 1-
9,1990; Huse, W.D. et
al., Science 246: 1275-1281, 1989; Sastry, L. et al., Proc. Natl. Acad. Sci.
USA 86: 5728-5732,
1989). Similarly, binding partners or domains may be constructed using
recombinant DNA
techniques to incorporate the variable regions of a gene encoding a specific
antibody (see PCT
Patent Application PCT/GB93/00605 published as WO 93/19172; PCT Patent
Application
PCT/GB93/02492 published as WO 94/13804; PCT Patent Application PCT/EP90/01964
published as WO 91/07492; Bird et al., Science 242: 423-426,1988). It will be
apparent to one
skilled in the art that the fractionation and recombinant approaches may be
applied to
diverse types of immune globulins. For example, specific monoclonal immune
globulins
against different antigens may be generated by techniques based on the same
principle of
recombinant DNA technology.
Anti-Rh immunoglobulin produced by the processes above may be formulated
with a wetting agent (non-ionic surface active agents) such as polysorbate 80,
also known as
TWEEN 80~. The immune globulin preferably is at least about 95% pure, more
preferably
about 99.5% pure and the wetting agent reduces the amount of fragmentation
over extended
periods of time to provide a highly stable preparation enriched for Rh
antibodies. Non-ionic
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-14-
surface agents such as sorbitan esters or polyoxyethylene sorbitan esters of
fatty acids
(TWEEN~ or SPAN~ type surface active agents) may be prepared by methods well
known in
the art and added to a final concentration of about 0.005% to about 0.5%. Said
surface active
agents may also be obtained commercially from j.T. Baker Inc. (Phillipsburg,
New jersey,
USA), ICI Atkemix (Brantford, Ontario, Canada), Van Waters and Rogers Ltd.
(Richmond,
British Columbia, Canada), or Nikkol Co. (Japan). Other stabilizers such as
sodium
chloride (final concentration of up to about 0.9%), mannitol, and/or L-glycine
or L-histidine
(final concentration of about 0.025M to 0.05M) can also be used to achieve or
further
stabilization of such anti-Rh immunoglobulin preparation, and the pH of the
fraction may be
adjusted within the range of 4.0 to 5.4. The resulting preparation may be
sterilized, for
example, by filtration. If desired the preparation may be freeze-dried, and
reconstituted
using a suitable solution such as sterile Water For Injection (USP) or 0.9%
sodium chloride.
By way of example and reference, currently available intravenously injectable
immune globulin preparations commonly consist of an immune globulin
distributed in a
physiologically compatible medium. Said medium may be sterile water for
injection (WFI)
with or without isotonic amounts of sodium chloride. For example, the
recommended diluent
for reconstitution of Iveegam~, Gammagard~, or, Venoglobulin~, is sterile WFI.
Sandoglobulin~ is supplied with 0.9% (w/v) sodium chloride solution as diluent
(see Gahart,
B.L. & Nazareno, A.R., Intravenous Medications: a handbook for nurses and
allied health
professionals, p. 516-521, Mosby, 1997). WinlZho SD~, an anti-RhoD immune
globulin
produced by Cangene Corporation, is reconstituted in 0.9% sodium chloride
solution for
intravenous injection. The immune globulin product by Schura (cited above) is
formulated as a
solution of 165 mEq/L sodium ion and 120 mEq/L chloride ion with a final pH of
6.7. The
Miles' intravenous immune globulin preparation, Gammimune~, when constituted,
has an
osmolality of 278 mOsm/L and a pH of 4.0-4.5. U.S. patent Nos. 4,396,608 and
4,499,073 also
disclose a low pH (3.5-5.0) and low ionic strength 00.001) immune globulin
formulation for
intravenous injection. The globulin protein concentration in the above
preparations ranges
from 0.5% to 20%.
A preferred preparation obtained using the processes described above for the
present invention has the following characteristics: 2-3% human
immunoglobulin, no or very
low level buffer, essentially no ionic strength, 10 ppm polysorbate 80 and 10%
sorbitol, pH
4Ø
It will be apparent to one skilled in the art that the preparations used in
the
present invention may contain more than one type of Rh antibody. For example,
a
preparation may contain both anti-Rho(D) and anti-c. Such compositions are for
intravenous,
intramuscular, subcutaneous, oral, enteral, intranasal, intrapulmonary or
inhalational use. In
particular, those forms for intramuscular or subcutaneous administration are
used, or forms for
infusion or intravenous injection are used, which can be prepared as solutions
of the antibodies
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/OOI09
-15-
or as powders of the antibodies to be mixed with one or more pharmaceutically
acceptable
excipients or diluents, suitable for the aforesaid uses and with an osmolarity
which is
compatible with the physiological fluids. For example, as described herein, an
lth antibody
preparation may be formulated with a wetting agent and/or stabilized by
addition of a
stabilizer. Preferably, the preparations are in a form suitable for
intravenous or
intramuscular administration (e.g. WinRho SDI and WinRho SDFT"', Cangene
Corporation,
Winnipeg, Canada). When administering the compositions/preparations of the
invention by
injection, the administration may be by continuous infusion or by single or
multiple bolus
injections.
In an embodiment of the invention, forms for intravenous injection or infusion
are selected to maximize drug bioavailability, reduce dosage, and to elicit
faster
pharmacodynamic action. For example, lth negative subjects were injected with
adult and
fetal lth-positive red blood cells and subsequently WinRho SD~ (e.g. 120 ug)
was
administered by intravenous or intramuscular injection. Peak plasma levels of
WinRho SD~
were achieved immediately after intravenous injection but were only achieved
24 after
intramuscular injection. Intravenous injection also produced two-fold higher
peak plasma
levels than intramuscular injection. Clearance of Rh-positive red blood cells
was complete
within 8 hours of intravenous administration, and 24 hours of intramuscular
administration
(Bowman, J.M. et al., CMA Journal 123: 1121-1125,1980). In the present
invention, a faster red
blood cell clearance may correlate with a faster onset of action.
The compositions of the invention may contain one or more Rh antibodies
together with one or more other active substances. Examples of active
substances which may
be used in the compositions/preparations include bronchodilators such as beta-
adrenergic
receptor agonists (albuterol, terbutaline, pirbuterol, salbutamol, salmeterol,
formoterol) and
xanthine derivatives {theophylline, enprofylline, pentoxifylline), anti-
inflammatory
agents such as cromolyn-like drugs {cromolyn sodium, nedocromil) and
glucocorticoids
(prednisone, methylprednisolone sodium succinate, beclomethasone
diproprionate,
triamcinolone acetonide, flunisolide, budesorude dipmprionate, fluticasone
proprionate), and
miscellaneous therapeutics such as troleandomycin, methotrexate, gold,
hydroxychloroquine,
dapsone and cyclosporine A. The Rh antibodies and active substances may be
administered
by any conventional means available for the use in conjunction with
pharmaceuticals, either
as individual separate dosage units administered simultaneously or
concurrently, or in
physical combination of each component in a single or combined dosage unit.
The lth
antibodies and active substances can be administered alone, but are generally
administered
with a pharmaceutical carrier selected on the basis of the chosen route of
administration and
standard pharmaceutical practice as described herein.
The combination of Rh antibodies and active substances may result in a
synergistic action which enhances the effects of the Rh antibodies, or
enhances the effects of
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-16-
the active substances. The doses of Rh antibodies and active substances may be
each selected
so that the Rh antibodies and active substances alone would not show a full
effect.
The compositions of the invention can be intended for administration to human
or animals. An appropriate preparation may be selected for a particular
subject based on the
presence/absence of lth antigens on the surface of the red blood cells of the
subject. Said
compositions/preparations are intended to provide to the recipient subjects an
amount of Rh
antibodies sufficient to prevent or treat asthmatic exacerbations. More
specifically, the
amount is said to be sufficient if the dosage, route of administration, etc.,
of the Rh
antibodies are sufficient to reduce the intensity, duration and/or frequency
of asthmatic
exacerbations or if the required dosages of other medications that the
recipient subject may
receive can be reduced without compromising therapeutic effectiveness.
Dosages of anti-lZhoD immune globulin in the formulations of the present
invention depend on individual needs, on the desired effect in a particular
therapeutic
indication, and on the chosen route of drug administration. Daily dosages to
humans by
intramuscular or intravenous injection generally vary between about 10 ug (50
lU) to 400 (2,000
lU) ug per kg body weight. For intramuscular injection, the preferred dosage
is about 20 ug
(100 lU) to 400 ug (2,000 ILJ) per kg body weight. For intravenous injection,
the preferred
dosage is about 10 ug (50 II)7 to 200 ug (1,000 IU) per kg body weight,
preferably 50 ug (250 lU)
per kg body weight. These dosages are significantly lower than those suggested
for
intravenous immune globulin (IVIG) treatment of asthmatic patients. The lower
dosages
provided in the present invention also reduce the risk of adverse reactions
such as
cardiovascular and thromboembolic events.
The compositions and preparations described herein can be prepared by per se
known methods for preparing pharmaceutically acceptable compositions which can
be
administered to human subjects, such that an effective quantity of the active
substance is
combined in a mixture with a pharmaceutically acceptable vehicle. Suitable
vehicles are
described, for instance, in Remington's Pharmaceutical Sciences (Remington's
Pharmaceutical
Sciences, Mack Publishing Company, F.aston, PA, USA,1985). On this basis, the
compositions
include, albeit not exclusively, solutions of Rh antibodies in association
with one or more
pharmaceutically acceptable vehicles or diluents, and contained in buffered
solutions with a
suitable pH and isoosmotic with the physiological fluids.
Pharmaceutical techniques may also be employed to control the duration of
action of the compositions/preparations of the present invention. Control
release
preparations may be prepared through the use of polymers to complex,
encapsulate, or absorb
the Rh antibodies.
The therapeutic effects of the present invention may be obtained by providing
to a patient any of the above described Rh antibody compositions or
preparations. The
compositions and preparations may be provided to patients who are suffering
from, or are
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-17-
susceptible to, episodes of asthmatic exacerbations. The compositions and
preparations may
also be provided to patients who are undergoing therapy with another mode of
asthma
intervention such as corticosteroid therapy.
Having generally described the invention, the same will be more readily
understood through reference to the following examples which are provided by
way of
illustration, and are not intended to be limiting of the present invention.
This example illustrates a schedule for treating asthmatic patients and
preventing asthmatic exacerbations. Rho(D)-positive asthmatic patients are
treated with
multiple courses of intravenous WinRho SD~ (Cangene's brand of anti-RhpD
immunoglobulin). The courses are given at 3-week intervals, and each course
consists of
intravenous injections of about 25 to 50 microgram (ug) per kilogram (kg) per
day WinRho SI7~
for 2 consecutive days for a total of about 50 to 100 ug per kg per course.
The total dose of .
WinRho SD~ may be combined and give on a single occasion, and throughout the
treatment
period, the dosage of WinRho SD~ may be adjusted according to the individual
patient's
clinical response. Efficacy of WinRho SD~ treatment may be monitored by:
1. monitoring asthmatic symptoms in patients and the number of exacerbations
or
episodes per time period;
2. assessing patient symptom scores (0, no symptom; l, mild symptoms but no
discomfort; 2,
moderate symptoms with no change in daily routine; 3, severe symptoms
interfering
with sleep or activity; 4, very severe incapacitating symptoms);
3. assessing patient pulmonary function by spirometry and body plethysmography
to
measure lung volume, resistance, specific conductance and flow rates;
4. performing skin prick testing (SPT) using different dilutions of commercial
antigens
(Greer Laboratories, N.Y. or Hollister-Stier Laboratories, Utah) to assess
patient
immune reactivity with histamine as positive control.
Intravenous WinRho SD~ therapy at this dosage should reduce the frequency,
intensity and duration of asthmatic exacerbations in asthmatic patients.
Patient symptom
score (e.g. average monthly symptom score) and pulmonary function (e.g. lung
volumes, flow
rates such as FEVl and FVC) should improve with therapy and a progressive
diminution in
skin test reactivity (increase in tolerance to higher antigen concentrations
and decrease in end
point titration) should become evident within 6 months of WinRho SD~ therapy.
This example illustrates the synergistic utility of anti-RhoD immunoglobulin
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-18-
with other anti-asthma therapeutic agents. WinRho SD~ is administered
intravenously to
patients receiving glucocorticoid therapy. Dosage regimentation of intravenous
WinRho SD~
is substantially as described in Example 1. Effective therapeutic dosages of
glucocorticoids
may constitute an average oral dose of 1 to 2 mg per kg patient body weight of
prednisone on
alternate days. Alternatively, an effective inhalational dose of a
glucocorticoid is 0.4 mg to
0.8 mg beclomethasone diproprionate per day administered via a metered dose
inhaler.
Combinational therapy of a glucocorticoid with anti-lZhoD immunoglobulin
should provide greater and more sustained effect in preventing and treating
asthmatic
exacerbations than monotherapy. Consequently, escalation of glucocorticoid
dosage
(commonly required in more severe asthmatic patients) could be avoided and the
maintenance
dosage of the glucocorticoid (e.g. average daily, weekly or monthly dose of
glucocorticoid)
could be reduced significantly within 6 months of WinRho SD~ therapy so to
minimize
glucocorticoid-associated side effects.
WinRho S1)~ '
A female ltho(D)-positive patient of 38 years of age presented with a history
of mild to moderate asthma for over 3 years was treated with WinRho SD~ in
1996. The
patient received repeated courses of intravenously administered WinRho SD~ at
about 50 to
100 ug per kg body weight every two to three weeks for 14 weeks. The exact
treatment
schedule is presented Figure 1. All adverse events were monitored and recorded
during and
after the treatment period.
During the entire duration of the WinItho SD~ treatment period (for over 100
days), the patient did not experience any asthmatic episodes. Conversely, upon
cessation of
WinRho SD~ therapy, recurrence of asthma was evidenced by two asthmatic
episodes
reported on Days 183 and 225. The patient was again treated with WinRho SD~ on
Days 225
and 280 and all symptoms of asthmatic episodes subsided thereafter. The
present crossover
data clearly indicate the ability of anti-RhaD immunoglobulin in preventing
the onset of and
reducing the frequency of asthmatic episodes.
EXA~LB.4
Corticosteroid-S~a,",n,'~ng effects of Win ho S1D~ in Aster
A second Rho(D)-positive asthmatic male patient of 53 years of age who is
~iIV-positive and has significant lymphopenia was treated with WinRho SD~.
This
patient has a long history of asthma of over 44 years received intermittent
WinRho SD~
treatment over a period of 6 months in 1992. Approximately 50 ug per kg
patient weight (3000
ug) WinRho SD~ was administered to the patient on days 1, 17, 25, 96, 145 and
173 of the
study, and all adverse events and concurrent medications were monitored and
recorded
throughout the study.
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-19-
During the entire course of this 6-month study, this patient experienced only
2
asthmatic episodes and required 10 mg oral prednisone on the each occasion.
The intensity of
corticosteroid intervention for asthma control observed during the WinRho SD~
treatment
period was significantly lower than the 1 to 2 mg prednisone per kg patient
body weight per
day conventionally required. The 10 mg dosages of prednisone administered to
the patient
are equivalent to about 0.2 mg per kg patient body weight and_represent only
10-20% of the
recommended dosage of prednisone for asthma control.
WLnR_h_p DecrPa~ G~rLO~~~nd Monoc~~e CoLnts
Airway hyperresponsiveness and clinical asthma are at least in part caused by
the inflammation generated by cell mediated immune mechanisms involving T
cells,
monocytes, granulocytes and mast cells. This example illustrates the ability
of anti-Rho(D)
immunoglobulin (WinRho SD~, Cangene Corporation, Winnipeg, Canada) to decrease
the
number of circulating granulocytes and monocytes in Rh-positive patients in
vivo and thereby
reduce cell-mediated immune hyperreactivity.
In a crossover study, 5 patients were randomized to receive 25 ug/kg or 50
ug/kg
WinRho SD~ as a single dose by intravenous infusion in the first cycle of
treatment. All
patients were examined prior to drug administration on day 1 and subsequently
at 1 and 7
days after drug administration (days 2 and 8). Subsequently, 3 further cycles
of WinRho SD~
treatment were given to the patients at alternating high and low dosage
regimens for a total
of 4 courses ( i.e. 25 ug/kg - 50 ug/kg - 25 ug/kg - 50 ug/kg or 50 ug/kg - 25
ug/kg - 50 ug/kg - 25
ug/kg).
At the time of examination, venous blood samples (5 mL each) were collected
by venipuncture into Vacutainer~ tubes containing sodium heparin (15 U/mL) as
anticoagulant. Peripheral blood smears stained and spread by routine technique
(using
Wright's-Giemsa stain and a centrifugal peripheral smear device) were prepared
in
duplicates from the collected blood and were examined visually for granulocyte
and monocyte
content by conventional microscopic methods. It should be readily apparent to
a person
skilled in the art that the differential white blood cell count may also be
performed using
automated instrumentation such as the Coulter S-Plus IV differential cell
counter (Coulter
Electronics, Hialeah, FL) (Cox, C.J. et al., Am. ]. Clin. Pathol. 84: 297-306,
1985) or an optical
flow cytometer (Hellma GmbH & Co., Mullheim/Baden, Federal Republic of
Germany)
(Terstappen, L.W. et al., Cytometry 9: 39-43, 1988).
Administration of WinRho SD~ at 25 ug/kg or 50 ug/kg rapidly reduced the
number of circulating granulocytes and monocytes (day 2 data) and a
statistically significant
reduction at the level of p < 0.05 was observed by day 8 {Figure 2). Upon
cessation of WinRho
SD~ therapy, patient granulocyte and monocyte counts returned to baseline
levels. Repeated
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-20-
administration of WinRho SD~ at 25 ug/kg or 50 ug/kg in subsequent treatment
cycles
produced similar dramatic decreases in patient granulocyte and monocyte counts
which again
reached statistically significant levels by day 8 (course 3 data shown).
The present study provides empirical evidence to demonstrate that
anti-Rho(D) immunoglobulin significantly reduces the number of cells that are
involved in
the immune hyper-responsiveness in asthma and other type I hypersensitivity
disorders. In
this respect, anti-lRho(D) immunoglobulin is useful for the correction of the
exaggerated
smooth muscle dysfunction and profound inflammation by intervening at the
mechanistic
level.
This example illustrates further effects of anti-ltho(D) immunoglobulin on
immune cell function. Type I hypersensitivity reactions occurs when antigenic
material
which is not in itself noxious (e.g. grass pollen, certain foodstuff and
drugs) evokes an
exaggerated immune response resulting in profound inflammation. Airway
hyperresponsiveness to antigen and bronchial inflammation are the major
symptoms of
clinical asthma.
An antigen-induced immune reaction is mediated through a cascade of
cooperative events and a foreign antigen alone is not sufficient to elicit a
full scale immune
response. Activation of naive T-lymphocytes requires a co-stimulatory signal
delivered by a
professional antigen presenting cell (APC) and the three major types of APCs
are
B-lymphocytes, macrophages and dendritic cells. Upon recognition of an
antigen, the APC
internalizes, processes and displays the antigen (or fragments thereof) on an
appropriate
major histocompatibility complex (MHC) with a co-stimulatory molecule on the
cell surface.
Once the antigen-MHC is presented to the T-lymphocyte receptor, the
T-lymphocyte becomes activated and is able to elicit both cell-mediated and
humoral
immune responses including the activation of macrophages to effect lysozyme
fusion and the
activation of B-lymphocytes to produce specific antibodies against the
antigen. The primary
means by which T- lymphocytes exert their actions is through the secretion of
cytokines such
as interleukin (IL-2). Secreted interleukin-2 (IL-2) also promotes T-cell
clonal expansion and
differentiation and cause excessive infiltration and upregulation of the
function of
eosinophils and mast cells. In asthma, the excessive release of cytokines and
the IgE type
antibodies produced by activated B-lymphocytes both stimulate masts cells to
secrete
histamine to produce bronchial inflammation.
In the crossover study of Example 5, also illustrated is the ability of
anti-lZhp(D) immunoglobulin (WinlZho SD~ to suppress the responsiveness of
immune cells
and to reduce the magnitude of immune response mounted against a foreign
antigen. To
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
-21-
reiterate, 5 patients were randomized to receive 25 ug/kg or 50 ug/kg WinRho
SD~ as a single
dose by intravenous infusion in the first cycle of treatment. All patients
were examined prior
to drug administration on day 1 and subsequently at 1 and 7 days after drug
administration
(days 2 and 8}. Subsequently, 3 further cycles of Winltho SD~ treatment were
given to the
patients at alternating high and low dosage regimens for a total of 4 courses.
At the time of examination, venous blood samples (10 mL each) were collected
by venipuncture into Vacutainer~ tubes containing sodium heparin (15 U/mL) as
anticoagulant. Peripheral blood mononuclear cells (PBMCs} were obtained from
the collected
blood by standard techniques using centrifugation (1,2008 for 30 minutes at
20°C) through a
1.077 g/mL Percoll (Pharmacia LKB, Baie d'Urfe, Quebec, Canada) gradient
(Semple, J.W.
and Freedman, J., Blood 78: 2619-2625, 1991).
The resulting cell sample contained approximately 18% DR+
antigen-presenting cells (APC) (9% CD19+ B cells and 9% CD14+
monocytes/macrophages),
approximately 55% T cells and 25% CD3-CD56+ natural killer (NK) cells. T
lymphocyte and
monocyte enrichment was optionally performed using a modified method of
Gutierrez et al.
(J. Immunol. Methods 29: 57, 1979). Briefly, the washed PBMCs were layered
onto a
discontinuous Percoll gradient consisting of the following densities: 1.091
g/mL (70%), 1.060
g/mL (50%), 1.050 g/mL (40%), and 1.030 g/mL (30%); and then centrifuged at
1,2008 for 30
minutes at 20°C. The PBMC band at the 30%/40% Percoll interface were
enriched for DR+
APCs [approximately 46% DR+ APC (14% CD19+ and 32% CD14+)] while the band at
the
50%/70% Percoll interface were enriched for T cells [approximately 65% T-
lymphocytes].
All in vitro assays were performed in RPMI 1640 medium (GIBCO Laboratories,
Grand Island,
IVY) containing 5% pooled inert human group AB serum, 2 mmol/L L-glutamine
(GIBCO),100
g/mL perucillin/streptomycin (GIBCO), and 5 x 10'5 mol/L 2-mercaptoethanol
(cRPMI).
Patient immune cell responsiveness was examined by a seven-day PBMC assay
using allogeneic platelets as stimulus (Sample, J.W. et al., Blood 78: 2619-
2625, 1991; Sample,
J.W. et al., Blood 78: 474, 1991). Enriched DR+ APC and T-cell enriched PBMCs
(4:1 T:DR+
ratio) were cultured in 96-well round-bottom tissue culture plates in cRPMI.
Allogeneic
platelets were prepared by conventional methods (Sample, J, et al., Blood 87:
4245-4254, 1996;
Freedman, J. and Hornstein, A., Am. J. Hematol. 38: 414, 1991) and were
titrated into the
cultures (about 2 x 105 PBMC) and incubated for 6 days at 37°C. On day
6, 50 L of culture
medium was removed from each well and tested for IL-2 content by stimulation
of
proliferation of a murine IL-2-dependant cell line, CTLL (Sample, J.W. et al.,
Blood 86:
805-812, 1995; Sample, J.W. et al., Blood 78: 474, 1991). 3[H]-thymidine (1
uCi) was then
added to each well, and the plates were incubated for 24 hours at 37°C.
The cells were then
harvested with a Skatron cell harvester onto filter discs and incorporated
radioactivity
(reflecting proliferative activity) was counted in an LKB Rack Beta counter.
PBMC
CA 02320104 2000-08-08
WO 99/40939 PCT/CA99/00109
supernatant IL-2 levels were calculated based on recombinant human IL-2
standards
(GIBCO-BRL, Gaithersburg, MD).
Human allogeneic platelets showed no in vitro stimulatory activity on T-cell
enriched cultures without DR+ APCs. However, the addition of allogeneic
platelets to DR+
APC and T- cell enriched culture (collected prior to WinRho SIB therapy first
cycle)
demonstrated significant immune reaction as evidenced by the increase in CTLL
cell
proliferation caused by the high levels of IL-2 secreted by APC-activated T-
lymphocytes
into the PBMC culture medium (Figure 3). Treatment of the patient with 25
ug/kg or 50 ug/kg
WinlZho SD~ dramatically inhibited T lymphocyte activation by the antigenic
stimulus and
the level of IL-2 secretion by T-lymphocytes in culture decreased virtually to
baseline level
by day 8 after the first treatment. Surprisingly, the effect of WinRho SD~ was
sustained
beyond day 8 and the inhibition of IL-2 release remained apparent at the time
of the second
treatment. Thereafter, the level of IL-2 secretion remained at baseline
throughout the
subsequent 3 cycles of therapy.
The present study provides further evidence to support the view that
anti-Rho(D) immunoglobulin is useful for correcting the exaggerated immune
response
underlying type I hypersensitivity disorders by significantly reducing the
inherent immune
responsiveness of T-lymphocytes, through APC mediation, to an artificial
antigenic stimulus.
Inhibition of cytokine release by T-lymphocytes would in turn prevent
downstream
cell-mediated and humoral immune responses that cause tissue inflammation and
other
clinical symptoms of a type I hypersensitivity disorder.
Having illustrated and described the principles of the invention in a
preferred
embodiment, it should be appreciated by those skilled in the art that
invention can be
modified in arrangement and detail without departure from such principles. We
claim all
modifications coming within the scope of the following claims.