Note: Descriptions are shown in the official language in which they were submitted.
CA 02320105 2000-08-08
w0 99/42045 PGT/US99/03764
APPARATUS TO DETECT AND ELECTROSURGICALLY TREAT ABERRANT MYOELECTRIC ACTIVITY
This application is a continuation-in-part of U.S. Patent Application
Serial No. 08/731,372, filed October 11, 1996, which is a continuation-in-part
of U.S. Patent Application Serial No. 08/319,373, filed October 6, 1994, which
is a continuation-in-part of U.S. Application No. 08/286,862, filed August 4,
1994, which is a continuation-in-part of U.S. Patent Application Serial No.
08/272,162, filed July 7, 1994, which is a continuation-in-part of U.S. Patent
Application Serial No. 08/265,459, filed June 24, 1994, and is related to
concurrently filed Application entitled "GERD Treatment Apparatus and
Method" identified as Attorney Docket 14800-748, all with named inventor
Stuart D. Edwards, and all of which are incorporated herein by reference.
This invention relates generally to an apparatus to detect and treat
aberrant myoelectric activity, and more particularly the detection and
treatment
in a sphincter and/or stomach.
Gastroesophageal reflex disease (GERD) is a common gastroesophageal
disorder in which the stomach contents are ejected into the lower esophagus
due
to a dysfunction of the lower esophageal sphincter (LES). These contents are
highly acidic and potentially injurious to the esophagus resulting in a number
of
possible complications of varying medical severity. The reported incidence of
GERD in the U.S. is as high as 10% of the population (Castell DO; Johnston
-1-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
BT: Gastroesophageal RejTux Disease: Current Strategies For Patient
Management. Arch Fam Med, 5(4):221-7; (1996 April)).
Acute symptoms of GERD include heartburn, pulmonary disorders and
chest pain. On a chronic basis, GERD subjects the esophagus to ulcer
formation, or esophagitis and may result in more severe complications
including
esophageal obstruction, significant blood loss and perforation of the
esophagus.
Severe esophageal ulcerations occur in 20-30% of patients over age 65.
Moreover, GERD causes adenocarcinoma, or cancer of the esophagus, which is
increasing in incidence faster than any other cancer (Reynolds JC: Influence
Of
Pathophysiology, Severity, And Cost On The Medical Management Of
Gastroesophageal Rejlux Disease. Am J Health Syst Pharm, 53(22 Suppl
3):SS-12 (1996 Nov 15)).
One of the possible causes of GERD may be aberrant electrical signals
in the LES or the stomach. Such signals may cause a higher than normal
frequency of relaxations of the LES allowing acidic stomach contents to be
repeatedly ejected into the esophagus and cause the complications described
above. Research has shown that unnatural electrical signals in the stomach and
intestine can cause reflux events in those organs (Kelly KA, et al:
Duodenal-gastric Reflex and Slowed Gastric Emptying by Electrical Pacing of
the Canine Duodenal Pacesetter Potential. Gastroenterology. 1977 Mar; 72(3):
429-433). In particular, medical research has found that sites of aberrant
electrical activity or electrical foci may be responsible for those signals
(Karlstrom LH, et al.: Ectopic Jejunal Pacemakers and Enterogastric Reflex
after Roux Gastrectomy: E,j~''ect Intestinal Pacing. Surgery. 1989 Sep;
106(3):
486-495). Similar aberrant electrical sites in the heart, which cause
contractions
of the heart muscle to take on life threatening patterns or dysrhythmias, can
be
identified and treated using mapping and ablation devices as described in U.S.
Patent No. 5,509,419. However, there is no current device or associated
-2-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
medical procedure available for the electrical mapping and treatment of
aberrant
electrical sites in the LES and stomach as a means for treating GERD.
Current drug therapy for GERD includes histamine receptor blockers
which reduce stomach acid secretion and other drugs which may completely
block stomach acid. However, while pharmacologic agents may provide short
term relief, they do not address the underlying cause of LES dysfunction.
Invasive procedures requiring percutaneous introduction of
instrumentation into the abdomen exist for the surgical correction of GERD.
One such procedure, Nissen fundoplication, involves constructing a new "valve"
to support the LES by wrapping the gastric fundus around the lower esophagus.
Although the operation has a high rate of success, it is an open abdominal
procedure with the usual risks of abdominal surgery including: postoperative
infection, herniation at the operative site, internal hemorrhage and
perforation of
the esophagus or of the cardia. In fact, a recent 10 year, 344 patient study
reported the morbidity rate for this procedure to be 17% and mortality 1
(L7rschel, JD: Complications OfAntireflux Surgery, Am J Surg 166(1): 68-70;
(1993 July)). This rate of complication drives up both the medical cost and
convalescence period for the procedure and may exclude portions of certain
patient populations (e.g., the elderly and immuno-compromised).
Efforts to perform Nissen fundoplication by less invasive techniques
have resulted in the development of laparoscopic Nissen fundoplication.
Laparoscopic Nissen fundoplication, reported by Dallemagne et al. Surgical
Laparoscopy and Endoscopv, Vol. 1, No. 3, (1991), pp. 138-43 and by Hindler
et al. Surgical Laparoscopy and Endoscopy, Vol. 2, No. 3, (1992), pp. 265-272,
involves essentially the same steps as Nissen fundoplication with the
exception
that surgical manipulation is performed through a plurality of surgical
cannula
introduced using trocars inserted at various positions in the abdomen.
Another attempt to perform fundoplication by a less invasive technique
is reported in U.S. Patent No. 5,088,979. In this procedure an invagination
device containing a plurality of needles is inserted transorally into the
esophagus
-3-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
with the needles in a retracted position. The needles are extended to engage
the
esophagus and fold the attached esophagus beyond the gastroesophageal
junction. A remotely operated stapling device, introduced percutaneously
through an operating channel in the stomach wall, is actuated to fasten the
invaginated gastroesophageal junction to the surrounding involuted stomach
wall.
Yet another attempt to perform fundoplication by a less invasive
technique is reported in US Patent No. 5,676,674. In this procedure,
invagination is done by a jaw-like device and fastening of the invaginated
gastroesophageal junction to the fundus of the stomach is done via a transoral
approach using a remotely operated fastening device, eliminating the need for
an
abdominal incision. However, this procedure is still traumatic to the LES and
presents the postoperative risks of gastroesophageal leaks, infection and
foreign
body reaction, the latter two sequela resulting when foreign materials such as
surgical staples are implanted in the body.
While the methods reported above are less invasive than an open Nissen
fundoplication, some still involve making an incision into the abdomen and
hence the increased morbidity and mortality risks and convalescence period
associated with abdominal surgery. Others incur the increased risk of
infection
associated with placing foreign materials into the body. All involve trauma to
the LES and the risk of leaks developing at the newly created gastroesophageal
junction. None provide a means for detecting and treating aberrant electrical
sites causing abnormal LES relaxation and gastroesophageal reflux.
There is a need to provide an apparatus to detect and treat aberrant
myoelectric activity of a sphincter andlor a stomach. There is another need to
provide an apparatus to detect and treat an electrical foci of the aberrant
myoelectric activity of a sphincter and/or a stomach. There is a further need
to
detect and treat an electrically conductive pathway of the aberrant
myoelectric
activity of a sphincter and/or a stomach.
-4-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
SUMMARY OF THE INVENTION
Accordingly, an object of the invention is to provide an apparatus to
diagnose and treat sphincters and/or a stomach.
Another object of the invention is to provide an apparatus to diagnose
and treat GERD.
A further object of the invention is to provide an apparatus to detect and
treat aberrant myoelectric activity of a sphincter and/or a stomach.
Yet another object of the invention is to provide an apparatus to detect
and treat an electrical foci of aberrant myoelectric activity of a sphincter
and/or
a stomach.
Still a further object of the invention is to provide an apparatus to detect
and treat an electrically conductive pathway of aberrant myoelectric activity
of a
sphincter and/or a stomach.
Another object of the invention is to provide an apparatus to detect and
treat an electrical foci of aberrant myoelectric activity of a lower
esophageal
sphincter and/or a stomach.
A further object of the invention is to provide an apparatus to detect and
treat an electrically conductive pathway of aberrant myoelectric activity of a
lower esophageal sphincter and/or a stomach.
These and other objects of the invention are provided in an apparatus to
treat a sphincter that has a support member. A sphincter electropotential
mapping device includes a mapping electrode. The sphincter electropotential
mapping device is coupled to the support member and configured to detect one
of an aberrant neuroelectric or myoelectric activity of the sphincter or
stomach.
In another embodiment, an apparatus to treat a stomach has a support
member. A stomach electropotential mapping device includes a mapping
electrode. The stomach electropotential mapping device is coupled to the
support member and configured to detect and treat one of an aberrant
neuroelectric or myoelectric activity of the stomach.
-5-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
Additionally, the electropotential mapping device can also include one
or more treatment electrodes.
FIG. 1 is an illustrated lateral view of the upper GI tract including the
esophagus and lower esophageal sphincter and the positioning of the sphincter
mapping and treatment apparatus of the present invention in the lower
esophageal sphincter.
FIG. 2 is a lateral view of the present invention illustrating the energy
delivery device, power source, controllers, map, display device, and the
mapping assembly in an expanded and contracted state.
FIG. 3 depicts a lateral view of the present invention that illustrates
components on the flexible shaft including a proximal fitting, connections and
proximal and distal shaft segments.
FIG. 4A illustrates a lateral view of the basket assembly used in an
embodiment of the invention.
FIG. 4B is a lateral view that illustrates placement of the mapping
electrodes on the basket assembly and their electrical connections to the
controller.
FIG. SA is a lateral view of the basket assembly that illustrates the range
of camber in the basket assembly.
FIG. SB is a perspective view illustrating a balloon coupled to the basket
assembly.
FIG. 6A is a lateral view of the junction between the basket arms and the
shaft illustrating the pathway used for advancement of the movable wire or the
delivery of fluids.
FIG. 6B is a frontal view of a basket arm in an alternative embodiment
of the invention illustrating a track in the arm used to advance the movable
wire.
-b-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
FIG. 7 is a cross-sectional view of a section of the basket arm illustrating
stepped and tapered sections in basket arm apertures.
FIG. 8 is a lateral view of the basket assembly illustrating the placement
of the radial supporting member.
FIG. 9A is a lateral view of the sphincter mapping and treatment
apparatus illustrating the mechanism used in one embodiment of the invention
to increase the camber of the basket assembly.
FIG. 9B is a similar view to 9A showing the basket assembly in an
increased state of camber.
FIG. 10 is a lateral view of the sphincter mapping and treatment
apparatus illustrating the deflection mechanism.
FIG. 11 is a lateral view illustrating the use of electrolytic solution to
create an enhanced RF electrode.
FIG. 12 is a lateral view of the basket assembly illustrating the use of
needle electrodes.
FIG. 13 is a lateral view illustrating the use of an insulation segment on
the needle electrode to protect an area of tissue from RF energy.
FIG. 14 is a lateral view illustrating the placement of needle electrodes
into the sphincter wall by expansion of the basket assembly.
FIG. 15 is a lateral view illustrating placement of needle electrodes into
the sphincter wall by advancement of an electrode delivery member out of
apertures in the basket arms.
FIG. 16 is a cross sectional view illustrating the configuration of a basket
arm aperture used to select and maintain a penetration angle of the needle
electrode into the sphincter wall.
FIG. 17 is a lateral view illustrating placement of needle electrodes into
the sphincter wall by advancement of an electrode delivery member directly out
of the distal end of the shaft.
_7_
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
FIG. 18A is a lateral view illustrating a radial distribution of electrodes
on the expandable mapping assembly of the invention.
FIG.18B is a lateral view illustrating a longitudinal distribution of
electrodes on the expandable mapping assembly of the invention.
FIG. 18C is a lateral view illustrating a spiral distribution of electrodes
on the expandable mapping assembly of the invention.
FIG. 18D is a lateral view illustrating a radial-longitudinal distribution
of electrodes on the expandable mapping assembly of the invention.
FIG. 19 is a flow chart illustrating a sphincter treatment method.
FIG. 20 is a lateral view of sphincter smooth muscle tissue illustrating
electromagnetic foci and pathways for the origination and conduction of
aberrant electrical signals in the smooth muscle of the lower esophageal
sphincter or other tissue.
FIG. 21 is a lateral view of a sphincter wall illustrating the infiltration of
tissue healing cells into a lesion in the smooth tissue of a sphincter
following
treatment with the sphincter treatment apparatus of the present invention.
FIG. 22 is a view similar to that of FIG. 21 illustrating shrinkage of the
lesion site caused by cell infiltration.
FIG. 23 is a lateral view of the esophageal wall illustrating the preferred
placement of lesions in the smooth muscle layer of a esophageal sphincter.
FIG. 24 is a lateral view illustrating the ultrasound transducer,
ultrasound lens and power source of an embodiment of the present invention.
FIGS. 25A-D are lateral views of the sphincter wall illustrating various
patterns of lesions created by the apparatus of the present invention.
FIG. 26 is a lateral view of the sphincter wall illustrating the delivery of
cooling fluid to the electrode-tissue interface and the creation of cooling
zones.
FIG. 27 depicts the flow path, fluid connections and control unit
employed to deliver fluid to the electrode-tissue interface.
_g_
CA 02320105 2000-08-08
WO 99/42045 PCT1US99/03764
FIG. 28 depicts the flow path, fluid connections and control unit
employed to deliver fluid to the RF electrodes.
FIG. 29 is an enlarged lateral view illustrating the placement of sensors
on the expansion device or basket assembly.
FIG. 30 depicts a block diagram of the feed back control system that can
be used with the sphincter mapping and treatment apparatus.
FIG. 31 depicts a block diagram of an analog amplifier, analog
multiplexes and microprocessor used with the feedback control system of FIG.
30.
FIG. 32 depicts a block diagram of the operations performed in the
feedback control system depicted in FIG. 30.
DETAILED DESCRIPTION
Referring now to FIGS. 1 and 2, one embodiment of sphincter mapping
and treatment apparatus 10 that is used to map the myoelectric activity of and
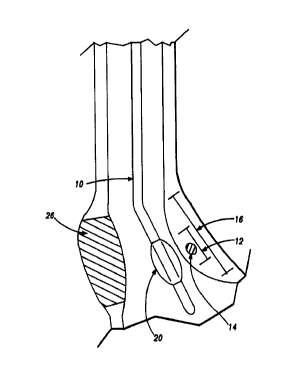
deliver energy to a treatment site 12 to produce lesions 14 in a sphincter 16,
such as the lower esophageal sphincter (LES), comprises a flexible elongate
shaft 18, also called shaft 18, coupled to an expandable mapping assembly 20,
in turn coupled with one or more mapping electrodes 22.
Expandable mapping assembly 20 establishes a three dimensional array
of mapping electrodes 22. In use, the expandable mapping assembly 20 records
the activation times, the distribution, and the waveforms of the myoelectricwl
and neuroelectrical action potentials in sphincter 16, which can include the
LES,
and adjoining structures that trigger aberrant relaxation of muscle tissue in
sphincter I 6. Suitable materials for mapping electrodes 22 include gold,
platinum and other metals known to those skilled in the art.
Mapping electrodes 22 are configured to be coupled to a controller 24.
Controller 24 receives and processes the potentials recorded by the mapping
electrodes 22 on expandable mapping assembly 20 and produces an
electropotential map 27, also called a map, of the myoelectric and
neuroelectric
-9-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
activity in sphincter 16. Controller 24 and electropotential map 27 are used
by
the physician to diagnose abnormalities and pathologies within sphincter 16
and
adjoining structures which will be further discussed herein. Controller 24 can
be coupled to an output or display device 29 that can include a cathode ray
tube,
S liquid crystal display, passive or active matrix flat screen display or
printer and
the like.
Expandable mapping assembly 20 has a central longitudinal axis 28 and
is moveable between contracted and expanded positions substantially there
along. This can be accomplished by a ratchet mechanism as is known to those
skilled in the art and by the use of other mechanisms disclosed herein. The
expandable mapping assembly 20 is further configured to be positionable in a
sphincter 16 such as the LES or adjacent anatomical structure, such as the
cardia
of the stomach. Once positioned within the desired sphincter 16, the operating
physician causes expandable mapping assembly 20 to expand to an expanded
stationary position within the sphincter so that mapping electrodes 22 thereof
engage sphincter wall 26 for sensing and detecting electrical energy or
impulses
therefrom. At least portions of sphincter mapping and treatment apparatus 10
maybe sufficiently radiopaque in order to be visible under fluoroscopy and/or
sufficiently echogenic to be visible under ultrasonography. Also, as will be
discussed herein, sphincter mapping and treatment apparatus 10 can include
visualization capability including, but not limited to, a viewing scope, an
expanded eyepiece, fiber optics, video imaging and the like.
Referring to FIG. 2 , shaft 18 is configured to be coupled to expandable
mapping assembly 20 and has sufficient length to position expandable mapping
assembly 20 in the LES and/or stomach using a transoral approach. Typical
lengths for shaft 18 include, but are not limited to, a range of 40-180 cm. In
various embodiments, shaft 18 is flexible, articulated and steerable and can
contain fiber optics (including illumination and imaging fibers), fluid and
gas
paths, and sensor and electronic cabling. In one embodiment, shaft 18 can be a
multi-lumen catheter, as is well known to those skilled in the art.
-10-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
In another embodiment of the invention, an introducing member 21, also
called an introducer, is used to introduce sphincter mapping and treatment
apparatus 10 into the LES. Introducer 21 can also function as a sheath for
expandable mapping assembly 20 to keep it in a nondeployed or contracted state
during introduction into the LES. In various embodiments, introducer 21 is
flexible, articulated and steerable, and contains a continuous lumen of
sufficient
diameter to allow the advancement of sphincter mapping and treatment
apparatus 10 within. Typical diameters for introducer 21 include 0.1 to 2
inches, while typical length include 40-180 cm. Suitable materials for
introducer 21 include wire-reinforced plastic tubing as is well known to those
skilled in the art.
Referring now to FIG. 3, the flexible elongate shaft 18 is circular in
cross section and has proximal and distal extremities (also called ends) 30
and
32. Shaft 18 may also be coupled at its proximal end 32 to a proximal fitting
34, also called a handle, used by the physician to manipulate sphincter
mapping
and treatment apparatus 10 to reach treatment site 12. Shaft 18 may have one
or
more shaft lumens 36, that extend the full length of shaft 18, or part way
from
shaft proximal end 30 to shaft distal end 32. Shaft lumens 36 may be used as
paths for catheters, guide wires, pull wires, insulated wires and cabling,
fluid
and optical fibers. Shaft lumens 36 are connected to and/or accessed by
connections 38, also called connector 38, on or adjacent to proximal fitting
34.
Connections 38 can include luer-lock, swage and other mechanical varieties
well known to those skilled in the art. Connections 38 can also include
electrical connections 38' which can include lemo connectors, nucro connectors
and other electrical varieties well known to those skilled in the art.
Additionally, connectors 38 can include opto-electronic connections 38" which
allow optical and electronic coupling of optical fibers and/or viewing scopes
to
illuminating sources, eye pieces and video monitors. In various embodiments,
shaft 18 may stop at the proximal extremity 40 of expandable mapping
assembly 20 or extend to, or past, the distal extremity 42 of expandable
-11-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
mapping assembly 20. Suitable materials for shaft 18 include, but are not
limited to, polyethylenes, polyurethanes and other medical plastics known to
those skilled in the art.
Referring now to FIG. 4, in one embodiment of the present invention,
expandable mapping assembly 20 comprises one or more elongated arms 44 that
are joined at their proximal arm ends 46 and distal arm ends 48 to form a
basket
assembly 50. Proximal arm end 46 is attached to a supporting structure, which
can be the distal end 32 of shaft 18 or a proximal cap 51. Likewise, distal
arm
end 48 is also attached to a supporting structure which can be a distal basket
cap
52 or shaft 18. Attached arms 44 may form a variety of geometric shapes
including, but not limited to, curved, rectangular, trapezoidal and
triangular.
Arms 44 can have a variety of cross sectional geometries including, but not
limited to, circular, rectangular and crescent-shaped. Also, arms 44 are of a
sufficient number (two or more), and have sufficient spring force ( 0.01 to
0.5
lbs. force ) so as to collectively exert adequate force on sphincter wail 26
to
sufficiently open and efface the folds of sphincter 16 to allow treatment with
sphincter mapping and treatment apparatus 10, while preventing henniation of
sphincter wall 26 into the spaces 53 between arms 44. Suitable materials for
arms 44 include, but are not limited to, spring steel, stainless steel,
superelastic
shape memory metals such as nitinol or wire-reinforced plastic tubing as is
well
known to those skilled in the art.
Referring now to FIG. 4B, a plurality of spaced apart mapping electrodes
22 are carned by each arm 44 for engaging sphincter wall 26 and are
electrically
coupled by a conductor 23 to a multiplexer chip 25 for transmitting signals
sensed thereby to controller 24 via electrical connections 38'. Various
geometric patterns for placement of mapping electrodes 22 on basket assembly
50 or expandable mapping assembly 20 are disclosed later herein. Multiplexor
chip 25 transmits only a selected one of the electrode signals at a time to
the
controller 24, subject to switching signals that controller 24 generates. The
switching signals of controller 24 serve to multiplex the electrode signals
-12-
CA 02320105 2000-08-08
WO 99/42045 PGT/US99/03764
through electrical connector 38'. This reduces the number of electrical
pathways
required through shaft lumen 36. In various embodiments, conductor 23 can be
an insulated lead wire as is well known to those skilled in the art.
In various embodiments, expandable mapping assembly 20 or basket
assembly 50 may also be coupled to one or more energy delivery devices 88,
also called electrodes, coupled to power source 56. Energy delivery devices 88
are used to delivery energy to treatment site 12 to produce lesions 14.
Expandable mapping assembly 20 is further configured to facilitate the
positioning of energy delivery devices 88, to a selectable depth in a
sphincter
wall 26 or adjoining anatomical structure. In one embodiment mapping
electrodes 22 can also be used as energy delivery devices.
Referring to FIG. SA, arms 44 can have an outwardly bowed shaped
memory for expanding the basket assembly into engagement with sphincter wall
26 with the amount of bowing, or camber 54 being selectable from a range 0 to
2 inches from longitudinal axis 28 of basket assembly 50. For the case of a
curve-shaped arm 44', expanded arms 44' are circumferentially and
symmetrically spaced-apart.
In another embodiment shown in FIG SB, an expansion device 55, which
can be a balloon, is coupled to an interior or exterior of basket assembly 50.
Balloon SS is also coupled to and inflated by shaft lumen 36 using gas or
liquid.
In various other embodiments (not shown), arms 44 may be asymmetrically
spaced and/or distributed on an arc less than 360°. Also, arms 44 may
be
preshaped at time of manufacture or shaped by the physician.
Referring now to FIG. 6A, arms 44 may also be solid or hollow with a
continuous lumen 58 that may be coupled with shaft lumens 36. These coupled
lumens provide a path for the delivery of a fluid or electrode delivery member
60 from shaft 18 to any point on expandable mapping assembly 20. In various
embodiments electrode delivery member 60 can be an insulated wire, an
insulated guide wire, a plastic-coated stainless steel hypotube with internal
wiring or a plastic catheter with internal wiring, all of which are known to
those
-13-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
skilled in the art. As shown in FIG. 6B, arms 44 may also have a partially
open
channel 62, also called a track 62, that functions as a guide track for
electrode
delivery member 60. Referring back to FIG. 6A, arms 44 may have one or more
apertures 64 at any point along their length that permit the controlled
placement
of electrodes 88 at or into sphincter wall 26. Referring now to FIG. 7,
apertures
64 may have stepped sections 66 or tapered sections 68 in all or part of their
length, that are used to control the penetration depth of electrodes 88 into
sphincter wall 26. Referring back to FIG. 6A, apertures 64 in combination with
arm lumens 58 and shaft lumens 36 may be used for the delivery of cooling
solution 70 or electrolytic solution 72 to treatment site 12 as described
herein.
Additionally, arms 44 can also carry a plurality of longitudinally or radially
spaced apart radiopaque and or echogenic markers or traces, not shown in the
drawings, formed of suitable materials to permit viewing of basket assembly 50
via fluoroscopy or ultrasonography. Suitable radiopaque materials include
platinum or gold, while suitable echogenic materials include gas filled micro-
particles as described in US Patent No 's 5,688,490 and 5,205,287. Arms 44
may also be color-coded to facilitate their identification via visual medical
imaging methods and equipment, such as endoscopic methods, which are well
known to those skilled in the art.
In another embodiment of the present invention, a radial supporting
member 74 is attached to two or more arms 44. Radial supporting member 74,
also called a strut, can be attached to arms 44 along a circumference of
basket
assembly 50 as shown in FIG. 8. Apertures 64 can extend through radial
supporting member 74 in one or more places. Radial supporting member 74
serves the following functions: i} facilitates opening and effacement of the
folds
of sphincter 16, ii) enhances contact of apertures 64 with sphincter wall 26;
and,
iii) reduces or prevents the tendency of arms 44 to bunch up. The cross
sectional geometry of radial supporting member 74 can be rectangular or
circular, though it will be appreciated that other geometries are equally
suitable.
-14-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
In one embodiment shown in FIG. 9, arms 44 are attached to distal
basket cap 52 that in turn, moves freely over shaft 18, but is stopped
distally by
shaft cap 78. One or more pull wires 80 are attached to distal basket cap 52
and
also to a movable fitting 82 in proximal fitting 34 of sphincter mapping and
treatment apparatus 10. When pull wire 80 is pulled back by movable fitting
82,
the camber 54 of basket assembly 50 increases to 54', increasing the force and
the amount of contact applied by basket assembly 50 to sphincter wall 26 or an
adjoining structure. Basket assembly 50 can also be deflected from side to
side
using deflection mechanism 84. This allows the physician to remotely point and
steer the basket assembly within the body. In one embodiment shown in FIG.
10, deflection mechanism 84 includes a second pull wire 80' attached to shaft
cap 78 and also to a movable slide 86 integral to proximal fitting 34.
Turning now to a discussion of energy delivery, suitable power sources
56 and electrodes 88 that can be employed in one or more embodiments of the
invention include: (i) a radio-frequency (RF) source coupled to an RF
electrode,
(ii) a coherent source of light coupled to an optical fiber, (iii) an
incoherent light
source coupled to an optical fiber, (iv) a heated fluid coupled to a catheter
with a
closed channel configured to receive the heated fluid, (v) a heated fluid
coupled
to a catheter with an open channel configured to receive the heated fluid,
(vi) a
cooled fluid coupled to a catheter with a closed channel configured to receive
the cooled fluid, (vii) a cooled fluid coupled to a catheter with an open
channel
configured to receive the cooled fluid, (viii) a cryogenic fluid, (ix) a
resistive
heating source, (x) a microwave source providing energy from 915 MHz to 2.45
GHz and coupled to a microwave antenna, (xi) an ultrasound power source
coupled to an ultrasound emitter, wherein the ultrasound power source produces
energy in the range of 300 KHZ to 3 GHz, or (xii) a microwave source. For
ease of discussion for the remainder of this application, the power source
utilized is an RF source and electrode 88 is one or more RF electrodes 88.
However, all of the other herein mentioned power sources and mapping
-15-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
electrodes are equally applicable to sphincter mapping and treatment apparatus
10.
For the case of RF energy, RF electrode 88 may operated in either
bipolar or monopolar mode with a ground pad electrode. In a monopolar mode
of delivering RF energy, a single electrode 88 is used in combination with an
indifferent electrode patch that is applied to the body to form the other
electrical
contact and complete an electrical circuit. Bipolar operation is possible when
two or more RF electrodes 88 are used. Multiple RF electrodes 88 may be used.
These electrodes may be cooled as described herein. RF electrodes 88 can be
attached to electrode delivery member 60 by the use of soldering methods which
are well known to those skilled in the art. Suitable solders include Megabond
Solder supplied by the Megatrode Corporation (Milwaukee, Wisconsin).
Suitable electrolytic solutions 72 include saline, solutions of calcium
salts, potassium salts, and the like. Electrolytic solutions 72 enhance the
electrical conductivity of the targeted tissue at the treatment site 12. When
a
highly conductive fluid such as electrolytic solution 72 is infused into
tissue the
electrical resistance of the infused tissue is reduced, in turn, increasing
the
electrical conductivity of the infused tissue. As a result, there will be
little
tendency for tissue surrounding electrode 88 to desiccate (a condition
described
herein that increases the electrical resistance of tissue) resulting in a
large
increase in the capacity of the tissue to carry RF energy. Referring to FIG.
11, a
zone of tissue which has been heavily infused with a concentrated electrolytic
solution 72 can become so conductive as to actually act as an enhanced
electrode 88'. The effect of enhanced electrode 88' is to increase the amount
of
current that can be conducted to the treatment site 12, making it possible to
heat
a much greater volume of tissue in a given time period.
Also when the power source is RF, power source 56, which will now be
referred to as RF power source 56, may have multiple channels, delivering
separately modulated power to each electrode 88. This reduces preferential
heating that occurs when more energy is delivered to a zone of greater
-16-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
conductivity and less heating occurs around RF electrodes 88 which are placed
into less conductive tissue. If the level of tissue hydration or the blood
infusion
rate in the tissue is uniform, a single channel RF power source 56 may be used
to provide power for generation of lesions 14 relatively uniform in size.
RF electrodes 88 can have a variety of shapes and sizes. Possible shapes
include, but are not limited to, circular, rectangular, conical and pyramidal.
Electrode surfaces can be smooth or textured and concave or convex. The
conductive surface area of electrode 88 can range from 0.1 mm2 to 100 cm2. It
will be appreciated that other geometries and surface areas may be equally
suitable.
In one embodiment, RF electrodes 88 can be in the shape of needles and
of sufficient sharpness and length to penetrate into the smooth muscle of the
esophageal wall, sphincter 16 or other anatomical structure. In this
embodiment
shown in FIG.s 12 and 13, needle electrodes 90 are attached to arms 44 and
have an insulating layer 92, covering an insulated segment 94 except for an
exposed segment 95. For purposes of this disclosure, an insulator or
insulation
layer is a barrier to either thermal, RF or electrical energy flow. Insulated
segment 94 is of sufficient length to extend into sphincter wall 26 and
minimize
the transmission of RF energy to a protected site 97 near or adjacent to
insulated
segment 94 (see FIG. 13). Typical lengths for insulated segment 94 include,
but
are not limited to, 1-4 mm. Suitable materials for needle electrodes 90
include,
but are not limited to, 304 stainless steel and other stainless steels known
to
those skilled in the art. Suitable materials for insulating layer 92 include,
but
are not limited to, polyimides and polyamides.
During introduction of sphincter mapping and treatment apparatus 10,
basket assembly 50 is in a contracted state. Once sphincter mapping and
treatment apparatus 10 is properly positioned at the treatment site 12, needle
electrodes 90 are deployed by expansion of basket assembly 50, resulting in
the
protrusion of needle electrodes 90 into the smooth muscle tissue of sphincter
wall 26 (refer to FIG. 14 ). The depth of needle penetration is selectable
from a
-17-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
range of 0.5 to 5 mm and is accomplished by indexing movable fitting 82 so as
to change the camber 54 of arm 44 in fixed increments that can be selectable
in
a range from 0.1 to 4 mms. Needle electrodes 90 are coupled to power source
56 via insulated wire 60.
In another embodiment of sphincter mapping and treatment apparatus 10
shown in FIG.15, needle electrodes 90 are advanced out of apertures 64 in
basket arms 44 into the smooth muscle of the esophageal wall or other
sphincter
16. In this case, needle electrodes 90 are electrically coupled to RF power
source 56 by electrode delivery member 60. In this embodiment, the depth of
needle penetration is selectable via means of stepped sections 66 or tapered
sections 68 located in apertures 64. Refernng to FIG.16, apertures 64 and
needle electrodes 90 are configured such that the penetration angle 96 (also
called an emergence angle 96) of needle electrode 90 into sphincter wall 26
remains sufficiently constant during the time needle electrode 90 is being
inserted into sphincter wall 26, such that there is no tearing or unnecessary
trauma to sphincter wall tissue. This is facilitated by the selection of the
following parameters and criteria: i) the emergence angle 96 of apertures 64
which can vary from 1 to 90 °, ii) the arc radius 98 of the curved
section 100 of
aperture 64 which can vary from 0.001 to 2 inch, iii) the amount of clearance
between the aperture inner diameter 102 and the needle electrode outside
diameter 103 which can very between 0.001" and 0.1 "; and, iv) use of a
lubricous coating on electrode delivery member 60 such as a Teflon ~ or other
coatings well known to those skilled in the art. Also in this embodiment,
insulated segment 94 can be in the form of an sleeve that may be adjustably
positioned at the exterior of needle electrode 90.
In another alternative embodiment shown in FIG. 17 , electrode delivery
member 60 with attached needle electrodes 90, can exit from shaft lumen 36 at
distal shaft end 32 and be positioned into contact with sphincter wall 26.
This
process may be facilitated by use of a hollow guiding member 101, known to
those skilled in the art as a guiding catheter, through which electrode
delivery
-18-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
member 60 is advanced. Guiding catheter 101 may also include stepped
sections 66 or tapered sections 68 at it distal end to control the depth of
penetration of needle electrode 90 into sphincter wall 26.
RF energy flowing through tissue causes heating of the tissue due to
absorption of the RF energy by the tissue and ohmic heating due to electrical
resistance of the tissue. This heating can cause injury to the affected cells
and
can be substantial enough to cause cell death, a phenomenon also known as cell
necrosis. For ease of discussion for the remainder of this application, cell
injury
will include all cellular effects resulting from the delivery of energy from
electrode 88 up to, and including, cell necrosis. Cell injury can be
accomplished
as a relatively simple medical procedure with local anesthesia. In one
embodiment, cell injury proceeds to a depth of approximately 1-4 mm from the
surface of the mucosal layer of sphincter 16 or that of an adjoining
anatomical
structure.
Referring now to FIGS 18A, 18B, 18C and 18D, mapping electrodes 22,
RF electrodes 88 and/or apertures 64 may be distributed in a variety of
patterns
along expandable mapping assembly 20 or basket assembly SO to facilitate
mapping and in order to produce a desired placement and pattern of lesions 14.
Typical electrode (both mapping and RF varieties) and aperture distribution
patterns include, but are not limited to, a radial distribution 104 (refer to
FIG.18A), a longitudinal distribution 105 (refer to FIG. 18B), a spiral
distribution 106 (refer to FIG. 18C) and a combination of longitudinal and
radial
distributions 107 (refer to FIG. 18D). It will be appreciated that other
combinations, patterns and geometries for electrode and aperture placement,
may also be suitable. These electrodes may be cooled as described hereafter.
FIG. 19 is a flow chart illustrating one embodiment of the procedure for
using sphincter mapping and treatment apparatus 10. In this embodiment,
sphincter mapping and treatment apparatus 10 is first introduced into the
esophagus under local anesthesia. Sphincter mapping and treatment apparatus
10 can be introduced into the esophagus by itself or through a lumen in an
-19-
CA 02320105 2000-08-08
WO 99/42045 PC"f/US99/03764
endoscope (not shown), such as disclosed in U.S. Patents Nos. 5,448,990 and
5,275,608, incorporated herein by reference, or similar esophageal access
device
known to those skilled in the art. Basket assembly 50 is expanded as described
herein. This serves to temporarily dilate the LES or sufficiently to efface a
portion of or all of the folds of the LES. In an alternative embodiment,
esophageal dilation and subsequent LES fold effacement can be accomplished
by insufflation of the esophagus (a known technique) using gas introduced into
the esophagus through shaft lumen 36, or an endoscope or similar esophageal
access device as described above. Once treatment is completed, basket
assembly 50 is returned to its predeployed or contracted state and sphincter
mapping and treatment apparatus 10 is withdrawn from the esophagus. This
results in the LES returning to approximately its pretreatment state and
diameter. It will be appreciated that the above procedure is applicable in
whole
or part to the treatment of other sphincters in the body.
As discussed previously, controller 24 and electropotential map 27 are
used to by the physician to diagnose abnormalities and pathologies within_
sphincter 16 and adjoining structures. More specifically, they are used to
identify electrical events that include depolarization, contraction and
repolarization. This information is used by the physician to determine target
treatment sites 12 in the LES or adjoining anatomical structures that are
acting
as foci 108 or pathways 109 for aberrant electrical signals 111 causing
abnormal
or otherwise inappropriate relaxation of the smooth muscle of the LES or other
sphincter 16 (Refer to FIG. 20). These targeted treatment sites 12 are then
treated as described herein so as to create lesions 14 which disrupt, block or
otherwise prevent the generation and transmission of sufficient abezzant
electrical signals 111 to cause relaxation of the LES ur other sphincter wall
26.
A variety of other diagnostic methods can be employed as an adjunct to
surface mapping of sphincter wall 26. These method include, but are not
limited to, the following: (i) visualization of the interior surface of the
esophagus via an endoscope or other viewing apparatus inserted into the
-20-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
esophagus, (ii) visualization of the interior morphology of the esophageal
wall
using ultrasonography to establish a baseline for the tissue to be treated;
and,
(iii) impedance measurement to determine the electrical conductivity between
the esophageal mucosal layers and sphincter mapping and treatment apparatus
10.
In the treatment phase of the procedure, the delivery of energy to
treatment site 12 can be conducted under feedback control, manually or by a
combination of both. Feedback control (described herein) enables sphincter
mapping and treatment apparatus 10 to be positioned and retained in the
esophagus during treatment with minimal attention by the physician. RF
electrodes 88 can be multiplexed in order to treat the entire targeted
treatment
site 12 or only a portion thereof. Feedback can be included and is achieved by
the use of one or more of the following methods: (i) visualization, (ii)
impedance measurement, (iii) ultrasonography, (iv) temperature measurement;
and, (v) sphincter contractile force measurement via manometry. The feedback
mechanism permits the selected on-off switching of different RF electrodes 88
in a desired pattern, which can be sequential from one electrode 88 to an
adjacent electrode 88, or can jump around between non-adjacent RF electrodes
88. Individual RF electrodes 88 are multiplexed and volumetrically controlled
by controller 23'.
The area and magnitude of cell injury in the LES or sphincter 16 can
vary. However, it is desirable to deliver sufFlcient energy to the targeted
treatment site 12 to be able to achieve tissue temperatures in the range of 55-
95 °
C and produce lesions 14 at depths ranging from 1-4 mm from the interior
surface of the LES or sphincter wall 26. Typical energies delivered to the
esophageal wall include, but are not limited to, a range between 100 and
50,000
joules per electrode 88. It is also desirable to deliver sufficient energy
such that
the resulting lesions 14 have a su~cient magnitude and area of cell injury to
cause an infiltration of lesion 14 by fibroblasts 110, myofibroblasts 112,
macrophages 114 and other cells involved in the tissue healing process (refer
to
-zl-
CA 02320105 2000-08-08
WO 99/42045 PGTNS99/03764
FIG. 21 ). As shown in FIG. 22, these cells cause a contraction of tissue
around
lesion 14, decreasing its volume and, or altering the biomechanical properties
at
lesion 14 so as to result in a tightening of LES or sphincter 16. These
changes
are reflected in transformed lesion 14' shown in FIG. 19B. The diameter of
lesions 14 can vary between 0.1 to 4 mm. It is preferable that lesions 14 are
less
than 4 mm in diameter in order to reduce the risk of thermal damage to the
mucosal layer. In one embodiment, a 2 mm diameter lesion 14 centered in the
wall of the smooth muscle provides a 1 mm buffer zone to prevent damage to
the mucosa, submucosa and adventitia, while still allowing for cell
infiltration
and subsequent sphincter tightening on approximately 50% of the thickness of
the wall of the smooth muscle (refer to FIG. 23).
From a diagnostic standpoint, it is desirable to image the interior surface
and wall of the LES or other sphincter 16, including the size and position of
created lesions 14. It is desirable to create a map of these structures which
can
be inputed to controller 23' and used to direct the delivery of energy to
treatment site 12. Referring to FIG. 24, this can be accomplished through the
use of ultrasonography (a known procedure) which involves the use of an
ultrasound power source 116 coupled to one or more ultrasound transducers 118
that are positioned on expandable mapping assembly 20 or basket assembly 50.
An output is associated with ultrasound power source 116.
Each ultrasound transducer 118 can include a piezoelectric crystal 120
mounted on a backing material 122 that is in turn, attached to expandable
mapping assembly 20 or basket assembly 50. An ultrasound lens 124,
fabricated on an electrically insulating material 126, is mounted over
piezoelectric crystal 120. Piezoelectric crystal 120 is connected by
electrical
leads 128 to ultrasound power source 116. Each ultrasound transducer 118
transmits ultrasound energy into adjacent tissue. Ultrasound transducers 118
can be in the form of an imaging probe such as Model 21362, manufactured and
sold by Hewlett Packard Company, Palo Alto, California. In one embodiment,
two ultrasound transducers 118 are positioned on opposite sides of expandable
-22-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
mapping assembly 20 or basket assembly 50 to create an image depicting the
size and position of lesion 14 in selected sphincter 16.
It is desirable that lesions 14 are predominantly located in the smooth
muscle layer of selected sphincter 16 at the depths ranging from 1 to 4 mm
from
the interior surface of sphincter wall 26. However, lesions 14 can vary both
in
number and position within sphincter wall 26. It may be desirable to produce a
pattern of multiple lesions 14 within the sphincter smooth muscle tissue in
order
to obtain a selected degree of tightening of the LES or other sphincter 16.
Typical lesion patterns shown in FIGS 25A-D include, but are not limited to,
(i)
a concentric circle of lesions 14 all at fixed depth in the smooth muscle
layer
evenly spaced along the radial axis of sphincter 16, (ii) a wavy or folded
circle
of lesions 14 at varying depths in the smooth muscle layer evenly spaced along
the radial axis of sphincter 16, (iii) lesions 14 randomly distributed at
varying
depths in the smooth muscle, but evenly spaced in a radial direction; and,
(iv) an
eccentric pattern of lesions 14 in one or more radial locations in the smooth
muscle wall. Accordingly, the depth of RF and thermal energy penetration
sphincter 16 is controlled and selectable. The selective application of energy
to
sphincter 16 may be the even penetration of RF energy to the entire targeted
treatment site 12, a portion of it, or applying different amounts of RF energy
to
different sites depending on the condition of sphincter 16. If desired, the
area of
cell injury can be substantially the same for every treatment event.
Referring to FIG. 26, it may be desirable to cool all or a portion of the
area near the electrode-tissue interface 130 before, during or after the
delivery of
energy in order to reduce the degree and area of cell injury. Specifically,
the use
of cooling preserves the mucosal layers of sphincter wall 26 and protects, or
otherwise reduces the degree of cell damage to cooled zone 132 in the vicinity
of lesion 14. Refernng now to FIG. 27, this can be accomplished through the
use of cooling solution 70 that is delivered by apertures 64 which is in fluid
communication with shaft lumen 36 that is, in tum, in fluid communication with
-23-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99103764
fluid reservoir 134 and a control unit 136, whose operation is described
herein,
that controls the delivery of the fluid.
Similarly, it may also be desirable to cool all or a portion of the electrode
88. The rapid delivery of heat through electrode 88, may result in the build
up
of charred biological matter on electrode 88 (from contact with tissue and
fluids
e.g., blood) that impedes the flow of both thermal and electrical energy from
electrode 88 to adjacent tissue and causes an electrical impedance rise beyond
a
cutoff value set on RF power source 56. A similar situation may result from
the
desiccation of tissue adjacent to electrode 88. Cooling of the electrode 88
can
be accomplished by cooling solution 70 that is delivered by apertures 64 as
described previously. Referring now to FIG. 28, electrode 88 may also be
cooled via a fluid channel 138 in electrode 88 that is in fluid communication
with fluid reservoir 134 and control unit 136.
As shown in FIG. 29, one or more sensors 140 may be positioned
adjacent to or on electrode 88 for sensing the temperature of sphincter tissue
at
treatment site 12. More specifically, sensors 140 permit accurate
determination
of the surface temperature of sphincter wall 26 at electrode-tissue interface
130.
This information can be used to regulate both the delivery of energy and
cooling
solution 70 to the interior surface of sphincter wall 26. In various
embodiments,
sensors 140 can be positioned at any position on expandable mapping assembly
20 or basket assembly 50. Suitable sensors that may be used for sensor 140
include: thermocouples, fiber optics, resistive wires, thermocouple IR
detcctors,
and the like. Suitable thermocouples for sensor 140 include: T type with
copper
constantene, J type, E type and K types as are well known those skilled in the
art.
Temperature data from sensors 140 are fed back to control unit 136 and
through an algorithm which is stored within a microprocessor memory of
control unit 136. Instructions are sent to an electronically controlled
micropump (not shown) to deliver fluid through the fluid lines at the
appropriate
-24-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
flow rate and duration to provide control temperature at the electrode-tissue
interface 130 (refer to FIG. 27).
The reservoir of control unit 136 may have the ability to control the
temperature of the cooling solution 70 by either cooling the fluid or heating
the
fluid. Alternatively, a fluid reservoir 134 of sufficient size may be used in
which the cooling solution 70 is introduced at a temperature at or near that
of
the normal body temperature. Using a thermally insulated reservoir 142,
adequate control of the tissue temperature may be accomplished without need of
refrigeration or heating of the cooling solution 70. Cooling solution 70 flow
is
controlled by control unit 136 or another feedback control system (described
herein) to provide temperature control at the electrode-tissue interface 130.
A second diagnostic phase may be included after the treatment is
completed. This provides an indication of LES tightening treatment success,
and whether or not a second phase of treatment, to all or only a portion of
the
esophagus, now or at some later time, should be conducted. The second
diagnostic phase is accomplished through one or more of the following
methods: (i) visualization, (ii) measuring impedance, (iii) ultrasonography,
(iv) temperature measurement, or (v) measurement of LES tension and
contractile force via manometry.
In one embodiment, sphincter mapping and treatment apparatus 10 is
coupled to an open or closed loop feedback system. Referring now to FIG. 30,
an open or closed loop feedback system couples sensor 346 to energy source
392. In this embodiment, electrode 314 is one or more RF electrodes 314.
The temperature of the tissue, or of RF electrode 314 is monitored, and
the output power of energy source 392 adjusted accordingly. The physician can,
if desired, override the closed or open loop system. A microprocessor 394 can
be included and incorporated in the closed or open loop system to switch power
on and off, as well as modulate the power. The closed loop system utilizes
microprocessor 394 to serve as a controller, monitor the temperature, adjust
the
RF power, analyze the result, refeed the result, and then modulate the power.
-25-
CA 02320105 2000-08-08
WO 99/42045 PGTNS99/03764
With the use of sensor 346 and the feedback control system a tissue
adjacent to RF electrode 314 can be maintained at a desired temperature for a
selected period of time without causing a shut down of the power circuit to
electrode 314 due to the development of excessive electrical impedance at
electrode 314 or adjacent tissue as is discussed herein. Each RF electrode 314
is
connected to resources which generate an independent output. The output
maintains a selected energy at RF electrode 314 for a selected length of time.
Current delivered through RF electrode 314 is measured by current
sensor 396. Voltage is measured by voltage sensor 398. Impedance and power
are then calculated at power and impedance calculation device 400. These
values can then be displayed at user interface and display 402. Signals
representative of power and impedance values are received by a controller 404.
A control signal is generated by controller 404 that is proportional to the
difference between an actual measured value, and a desired value. The control
signal is used by power circuits 406 to adjust the power output in an
appropriate
amount in order to maintain the desired power delivered at respective RF
electrodes 314.
In a similar manner, temperatures detected at sensor 346 provide
feedback for maintaining a selected power. Temperature at sensor 346 is used
as a safety means to intemlpt the delivery of energy when maximum pre-set
temperatures are exceeded. The actual temperatures are measured at
temperature measurement device 408, and the temperatures are displayed at user
interface and display 402. A control signal is generated by controller 404
that is
proportional to the difference between an actual measured temperature and a
desired temperature. The control signal is used by power circuits 406 to
adjust
the power output in an appropriate amount in order to maintain the desired
temperature delivered at the sensor 346. A multiplexer can be included to
measure current, voltage and temperature, at the sensor 346, and energy can be
delivered to RF electrode 314 in monopolar or bipolar fashion.
-26-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
Controller 404 can be a digital or analog controller, or a computer with
software. When controller 404 is a computer it can include a CPU coupled
through a system bus. This system can include a keyboard, a disk drive, or
other non-volatile memory systems, a display, and other peripherals, as are
known in the art. Also coupled to the bus is a program memory and a data
memory.
User interface and display 402 includes operator controls and a display.
Controller 404 can be coupled to imaging systems including, but not limited
to,
ultrasound, CT scanners, X-ray, MRI, mammographic X-ray and the like.
Further, direct visualization and tactile imaging can be utilized.
The output of current sensor 396 and voltage sensor 398 are used by
controller 404 to maintain a selected power level at RF electrode 314. The
amount of RF energy delivered controls the amount of power. A profile of the
power delivered to electrode 314 can be incorporated in controller 404 and a
preset amount of energy to be delivered may also be profiled.
Circuitry, software and feedback to controller 404 result in process
control, the maintenance of the selected power setting which is independent of
changes in voltage or current, and is used to change the following process
variables: (i) the selected power setting, (ii) the duty cycle (e.g., on-off
time),
(iii) bipolar or monopolar energy delivery; and, (iv) fluid delivery,
including
flow rate and pressure. These process variables are controlled and varied,
while
maintaining the desired delivery of power independent of changes in voltage or
current, based on temperatures monitored at sensor 346.
Referring now to FIG. 31, current sensor 396 and voltage sensor 398 are
connected to the input of an analog amplifier 410. Analog amplifier 410 can be
a conventional differential amplifier circuit for use with sensor 346. The
output
of analog amplifier 410 is sequentially connected by an analog multiplexer 412
to the input of A/D converter 414. The output of analog amplifier 410 is a
voltage which represents the respective sensed temperatures. Digitized
amplifier output voltages are supplied by A/D converter 414 to microprocessor
-27-
CA 02320105 2000-08-08
WO 99/42045 PCTNS99/03764
394. Microprocessor 394 may be a type 68HCII available from Motorola.
However, it will be appreciated that any suitable microprocessor or general
purpose digital or analog computer can be used to calculate impedance or
temperature.
Microprocessor 394 sequentially receives and stores digital
representations of impedance and temperature. Each digital value received by
microprocessor 394 corresponds to different temperatures and impedances.
Calculated power and impedance values can be indicated on user
interface and display 402. Alternatively, or in addition to the numerical
indication of power or impedance, calculated impedance and power values can
be compared by microprocessor 394 to power and impedance limits. When the
values exceed predetermined power or impedance values, a warning can be
given on user interface and display 402, and additionally, the delivery of
I'cF
energy can be reduced, modified or interrupted. A control signal from
microprocessor 394 can modify the power level supplied by energy source 392.
FIG. 32 illustrates a block diagram of a temperature and impedance-
feedback system that can be used to control the delivery of energy to tissue
site
416 by energy source 392 and the delivery of cooling solution 70 to electrode
314 and/or tissue site 416 by flow regulator 418. Energy is delivered to RF
electrode 314 by energy source 392, and applied to tissue site 416. A monitor
420 ascertains tissue impedance, based on the energy delivered to tissue, and
compares the measured impedance value to a set value. If the measured
impedance exceeds the set value, a disabling signal 422 is transmitted to
energy
source 392, ceasing further delivery of energy to RF electrode 314. If
measured
impedance is within acceptable limits, energy continues to be applied to the
tissue.
The control of cooling solution 70 to electrode 314 and/or tissue site 416
is done in the following manner. During the application of energy, temperature
measurement device 408 measures the temperature of tissue site 416 and/or RF
electrode 314. A comparator 424 receives a signal representative of the
-zg-
CA 02320105 2000-08-08
WO 99/42045 PCT/US99/03764
measured temperature and compares this value to a pre-set signal
representative
of the desired temperature. If the tissue temperature is too high, comparator
424
sends a signal to a flow regulator 418 (connected to an electronically
controlled
micropump, not shown) representing a need for an increased cooling solution
flow rate. If the measured temperature has not exceeded the desired
temperature, comparator 424 sends a signal to flow regulator 418 to maintain
the cooling solution flow rate at its existing level.
The foregoing description of a preferred embodiment of the invention
has been presented for purposes of illustration and description. It is not
intended to be exhaustive or to limit the invention to the precise forms
disclosed. Obviously, many modifications and variations will be apparent to
practitioners skilled in this art. It is intended that the scope of the
invention be
defined by the following claims and their equivalents.
What is claimed is:
-29-