Note: Descriptions are shown in the official language in which they were submitted.
CA 02320300 2000-08-10
WO 99/39647 PG"f/US99/02332
APPARATUS AND METHOD FOR INHIBTIZNG RESTENOSIS
BY APPLYING ULTRASOUND ENERGY TOGETHER WITH DRUGS
CROSS REFERENCE TO RELATED APPLICATIONS
This application is a Continuation-in-Part of pending application Serial
No. 08/700,064, filed August 19, 1996.
BACKGROUND OF THE INVENTION
The present invention relates generally to a method for inhibiting
restenosis in mammals by compromising the migration, viability and/or
adherence of
smooth muscle cells within a mammalian blood vessel. In particular, the method
of
1o the invention is useful in the prevention of restenosis following
angioplasty or other
invasive cardiovascular therapies.
Coronary artery disease is a major cause of morbidity and mortality in
the Western world. The disease is typically manifested in intravascular
stenosis
(narrowing) or occlusion (blockage) due to atherosclerotic plaque.
Percutaneous
transluminal coronary balloon angioplasty (PTCA) is widely used as the primary
treatment for arteriosclerosis involving stenosis.
Unfortunately, a significant fraction of the patients who have undergone
coronary balloon angioplasty develop restenosis at the treated site within 3-6
months
after the procedure. Austin G. E., Ratliff M. B., and Holman J. suggest that
intimal
2o proliferation of smooth muscle cells may account for the observed recurrent
coronary
artery stenosis after percutaneous transluminal coronary angioplasty in "J.
Am. Coll.
Cardiol." (1985) 6:369-375.
The limitations of balloon angioplasty stimulated an explosion of new
angioplasty techniques, including laser, stents, thermal, and arthrectomy
devices.
2s However, these new devices also suffer from the same serious drawback of
the
previous method of treatment, primarily due to their inability to damage the
atherosclerotic lesion without incurring injury to the arterial wall. The
resulting injury
repair process, which includes migration of smooth muscle cells to the cite of
the
injury, then leads to restenosis.
CA 02320300 2000-08-10
WO 99/39647 PCTlUS99/02332
Thus, restenosis following successful balloon angioplasty or other new
intervention techniques, remains a major problem limiting the long term
efficacy of
angioplasty procedures. In up to 50% of treated patients, a clinically
significant
restenosis develops.
s Ultrasound angioplasty is a relatively new transcatheter technology
developed for arterial recanalization and is described, for example, in U.S.
Patent No.
4,870,953 to DonMicheal et al. In this method, generally, the clots in the
coronary
arteries are lysed by application of high-power, low-frequency ultrasound
(ULS)
delivered by a dedicated device. Studies have suggested that ultrasound energy
at
levels known to induce effective thrombus lysis appear to be minimally harmful
to a
blood vessel wall. For example, the ultrasound power level required to ablate
thrombi
is about 1/40 of that required to induce arterial wall damage. Rosenschein U.,
Bernstein J. J., DiSegni E., Kaplinsky E., Bernheim J., and Rozenszsajn L.
describe
experimental procedures and results of ultrasonic angioplasty leading to
disruption of
1s atherosclerotic plaques and thrombi and arterial recanalization in "J. Am.
Coll.
Cardiol." (1990) 15:711-717. The selective ablation of thrombi by ultrasound
makes
this technology a potentially effective and safe clinical method to treat
patients with
coronary thrombosis, including heart attacks.
All vascular interventional techniques (e.g. PTCA, stent installation,
lasers) are associated with intimal and medial damage of the treated blood
vessel
which leads to platelet adhesion, aggregation and the release of an array of
mitogens.
The mitogens stimulate migration of smooth muscle cells (SMC) from the media
to
the intima and across the internal elastic lamina, followed by their
proliferation,
extracellular matrix production, neointima formation and the resulting
restenosis of the
2s treated artery.
Thus, vascular injuries induce the synthesis of multiple factors which
induce SMC migration and proliferation leading to neointimal formation which
is
manifested clinically and angiographicaily as restenosis. Ross R. describes
this
pathogenesis of atherosclerosis in "Nature" (1993} 362:801-809. The effect of
2
CA 02320300 2000-08-10
WO 99!39647 PCT/US99/02332
therapeutic ultrasound on SMC functions (primarily migration, adhesion and
proliferation or viability) which play a role in the restenotic process has
generally not
as yet been researched excepting the work done by applicant herein.
To date, the strategies to treat restenosis were targeted mainly at
s inhibiting SMC proliferation or viability. For example, WO 94/07529
describes the
use of a vascular smooth muscle binding protein which binds in a specific
manner to
the cell membrane of a vascular SMC thus inhibiting the activity of the cell.
U.S.
Patent No. 5,472,985 issued to Grainger, describes the use of TGF-beta
activators and
production stimulators to inhibit the pathological proliferation of vascular
SMC. All
1o these strategies have failed to date to demonstrate clinical e~cacy in
preventing
restenosis. Due to the high incidence of restenosis after non-surgical
recanalization of
occluded and stenotic arteries, an effective method to prevent this
complication can be
expected to find wide application.
Fahnestock M., Rimier U. G., Yamakawi R. M., Ross P., and Edmonds
~s P. D. studied the effect of in vitro ultrasound exposure on neuroblastoma
cell
membrane. As reported in "Ultrasound Med. Biol." (1989) 15:133-144, they found
that high frequency ultrasound on cell lines affects cell permeability which
is
dependent on the integrity of the cell membrane. After low frequency
ultrasound
treatment, a transient decrease in cell proliferation of cancer cells, without
significant
20 changes in cell cycle distribution and cell alteration of intracellular
adhesion, has also
been observed by Nicolai H., Steinbach P., Knuechel-Clarke R., Grimm D.,
Roessler
W., Wieland and Hofstaedter W. F. They report that proliferation of tumor
spheroids
may be reduced after shock-wave treatment in "3. Cancer Res. Clin. Oncol: ' (
1994)
120:43 8-441.
2s In a presentation at the 1995 Conference of the European Society of
Cardiology (Amsterdam, August 20-24), the applicant demonstrated that
ultrasound
induces inhibition of SMC migration and adhesion. No mention was made of the
effect of ultrasound on restenosis however. The applicant has also described
features
related to the invention herein in U. Rosenschein, A. Alter and L. A.
Rozenszajn, in
3
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Endoluminal Stentin~~, (Sigwart, U., Ed.) W. B. Saunders Co. Ltd. (1996) pgs.
129-
133, the contents of which are incorporated herein by reference.
Until now, therapeutic ultrasound has been used mainly for recanalized
arteries occluded by clots. None of the modalities currently employed for
arterial
recanalization is used to modify SMC biology and prevent restenosis.
SUMMARY OF THE INVENTION
Generally speaking, in accordance with the invention, a method and an
apparatus and method for inhibiting restenosis by applying ultrasonic energy
in
combination with drug therapy is provided which inhibits the migration,
viability and
adherence of smooth muscle cells in a blood vessel. The method includes
treating the
smooth muscle cells with one or more anticytoskeleton agents, such as
cytochalasin B
or colchisine, and irradiating the cells with ultrasonic energy, such as in an
amount
effective for compromising the migration, viability or adhesion of the smooth
muscle
cells. The apparatus and method are particularly well suited for the
prevention of
~5 restenosis associated with smooth muscle cell migration, viability and
adherence in a
blood vessel following vascular intervention, such as a balloon angioplasty.
It is an object of the present invention to provide an apparatus and
method for inhibiting restenosis.
It is another object of the present invention to provide an apparatus and
2o method for preventing restenosis of a blood vessel following angioplasty.
It is a further object of the present invention to provide a system which
can be used to prevent restenosis following intervention in coronary disease
patients.
Still other objects and advantages of the invention will in part be obvious
and will in part be apparent from the specifications and drawings.
25 The invention accordingly comprises the several steps and the relation of
one or more of such steps with respect to each of the others, and the
apparatus
embodying features of construction, combinations of elements and arrangement
of
parts which are adapted to effect such steps, all as exemplified in the
following
detailed disclosure, and the scope of the invention will be indicated in the
claims.
4
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention will be better understood with reference to the
following detailed description taken in connection with the accompanying
drawings,
in which:
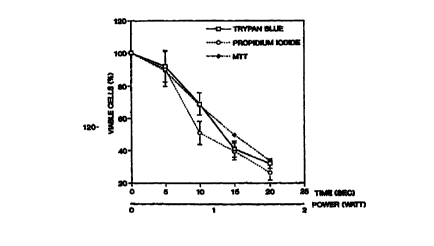
Fig. 1 is a graph illustrating the viability of SMC as a function of
exposure to ultrasound (ultrasound power x time of sonication) at a frequency
of 20
kHz;
Figs. 2a and 2b are representative, light microscope views of SMC that
migrated through the 0.5 ~tm pore filter membrane of the chamber before (Fig.
2a) and
to after (Fig. 2b) exposure to ultrasound. The stain is May-Grunwald Giemsa;
Fig. 3 is a bar graph illustrating the migration capacity of sonicated SMC
evaluated by their ability to cross a filter membrane;
Figs. 4a and 4b are graphs illustrating the time course of the change in
adhesion capacity of sonicated SMC. The adherence capacity was quantified 6
times
1s during the first 3 hours (Fig. 4a) and every 24 hours thereafter for 120
hours (Fig. 4b);
Figs. Sa-Sc are scanning electron microscope views of SMC
morphology. Fig. Sa shows an unsonicated cell. trigs. Sb and Sc show SMC after
exposure to ultrasound;
Figs. 6a-6c show actin filaments of SMC which were stained using
2o antibodies against a-SM actin and examined by indirect immunofluorescence
technique. Fig. 6a shows the cytoskeletal organization of SMC with the
uninterrupted
a-actin filaments; Fig. 6b shows the SMC 0.2 hours after ultrasound; and Fig.
6c
shows the SMC 24 hours after ultrasound;
Figs. 7a and 7b are photomicrographs of SMC after ULS which were
25 stained with anti-tyrosine tubulin (Fig. 7a) or anti-vimentin (Fig. 7b) and
examined by
indirect immunofluorescence technique;
Fig. 8 is a graphic representation of the spectrum of sound. Frequency
(in kHz) is displayed on the X axis and intensity (in Dynes/cm2) on the Y
axis;
S
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Fig. 9 is a schematic illustration of an invasive ultrasonic device in
accordance with one embodiment of the system of the invention;
Fig. 10 is a graph showing the combined effect of therapeutic ultrasound
and cytochalasin B on SMC migratory capacity. The data is the average values
of
s triplicate assay from 3 experiments. Values are expressed as mean + SD.
Statistical
analysis was performed by a paired t-test using Statview software.
Statistically
significant differences compared to the control are marked with asterisks
(*p<0.05,
**p<0.01); and
Fig. 11 is a graph showing the combined effect of therapeutic ultrasound
and cytochalasin B on SMC adhesive capacity. The data is the average values of
triplicate assay from 3 experiments. Values are expressed as mean ~ SD.
Differences
were considered significant for a p value of 0.05 or less and marked with
asterisks.
(*p<0.05, **p<0.01).
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
~s Vascular smooth muscle cells (SMC) play a fundamental role in
atherosclerotic legion formation and restenosis. Vascular injury induces
synthesis of
multiple factors, and mitogens, which ultimately induce SMC migration,
followed by
SMC proliferation leading to intimal hyerplasia. For example, a significant
fraction of
patients ( up to 50%) who have undergone coronary balloon angioplasty develop
2o restenosis. Restenosis is due primarily to the vascular injury induced by
percutaneous
balloon angioplasty which leads to migration and adherence of SMC in to the
intima
of the blood vessel, followed by excessive proliferation and formation of
occlusive
neo-intimal.
The applicant has studied the effects of therapeutic ultrasound on the
2s migration, viability and adherence functions of smooth muscle cells, which
play a role
in the restenotic process. Therefore, the applicant has studied the structural
and
functional changes associated with the application of ultrasound on smooth
muscle
cells. Initially, in vitro testing was done to determine the parameters and
results of
ultrasound radiation of smooth muscle cells.
6
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Bovine aortic SMC (BASMC) were prepared by the explant technique
and cultured on 100 x 20 mm tissue culture dishes (Falcon, Oxanard, CA) in
Dulbecco-modified Eagle's medium (DMEM) supplemented with 10% heat-
inactivated calf serum (CS) (5% fetal calf serum and 5% newborn calf serum), L-
glutamine (0.3 mg/ml), penicillin 100 (~g/ml), streptomycin ( 100 ~,g/ml) and
amphotericin (0.25 ~g/ml) (Biological Industries, Beit Haemek, Israel). For
experiments, cells at passage levels 9 to 17 were replicate-plated into 100 mm
culture
dishes, refed every third day and used at confluence. All cell growth was
conducted at
37°C in a fully humidified incubator containing 10% C02 in air.
to Cultures were passaged immediately before full confluence by brief
exposure to PBS containing trypsin (0.5 mg/ml) and ethylene-diamine-
tetraacetic acid
(EDTA) (0.5 mmoles/L) at 37 ° C and grown to confluence in DMEM
containing 10%
CS. Cell growth was observed under an inverted phase contrast microscope (Carl
Zeiss Oberkochen, Germany) with a magnification of 200x. The cells were
~s characterized as SMC by morphologic criteria as well as by expression of
smooth
muscle a-actin in accordance with the procedure of Powell R. J., Cronenwett J.
L.,
Filliner M.F., and Wagner R.J., as outlined in their study on the effect of
endothelial
cells and transforming growth factor-B 1 on cultured vascular smooth muscle
cell
growth patterns in "J. Vasc. Surg." (1994) 20:787-94.
2o SMC of confluent cultures were trypsinized, and thereafter were
suspended, 2.5 x 106/5 ml DMEM, in a 16 x 125 mm culture tube (Corning-
Staffordshire, UK). They were then exposed to ULS using a sonicator (Vibra
Cell,
Sonic Materials Inc., Danbury, CT). The sonicator consists of a resonant
length (90
mm) of a vertically suspended, thin titanium probe (diameter 2 mm), which
resonates
25 at a frequency of 20 kHz and variable power levels. To prevent aerosol
formation
during sonication, the depth of immersion of the probe is adjusted so that the
meniscus
of the liquid comes in contact with the ultrasonic wire at a displacement
node.
Each of the following experiments were done in triplicate and repeated
at least 4 times. Data is typically expressed as mean t SD. The student's t-
test was
7
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/OZ332
used in all experiments. For the experiments on adherence, analysis of
variance was
also performed. A p value s 0.05 was considered to be significant.
F;xample 1 - Viability Experiment
The viability of sonicated SMC was tested by propidium iodide (PI)
staining, trypan blue exclusion and tetrazolium (MTT) methods. In the PI
staining
method, SMC (1 x 106) were suspended in PBS containing sodium azide (0.3
mg/ml)
and PI (0.5 ~,g/ml) (Sigma, St. Louis, MO). The cells were analyzed for
viability
within 30 minutes after adding the PI solution using an Epics-Profile II flow
cytometer
(Coulter Corp., Luton, LTK) measuring red fluorescence with band pass filters
> 650
nm.
In the MTT assay, aliquots of SMC ( 100 ~L) were transferred to flat-
bottomed 96 mierowell plates. MTT (Sigma, St. Louis, MO) was dissolved in DMSO
(Sigma, St. Louis, MO) and diluted with PBS to a final concentration of 5
mg/ml; 10
~,L MTT were added to each well. The plates were incubated at 37 ° C
for 2 hours and
1s 100 ~,L of 0.04N HCl in isopropanol were added to each well. The viability
of cells
was evaluated in an ELISA reader (Kontron SLT-210) using a 550 nm filter.
In the trypan blue exclusion assay, aliquots of SMC were removed and
the dye (0.4% in PBS) was added to the cell suspension. The extent of dye
uptake
being indicative of cell damage, viable cells were counted using a
hemocytometer.
2o Each experiment was performed with a given number of unseparated
viable cells after sonication. The same number of unsonicated cells was used
as
controls.
Referring to Fig. 1, it can be seen that excellent correlation between
ultrasound dose (ultrasound power x time of sonication) and SMC viability was
2s observed using the PI (r = 0.98649), MTT (r = 0.9890) and trypan blue (r =
0.98582)
methods over a range of sonication times (1-20 sec) and power (0-2 watts). The
LD50
of the ULS was established as a sonication dose of 1.5 watts for 15 sec. (22.5
watt
sec) at a frequency of 20 kHz and was used in all experiments.
8
CA 02320300 2000-08-10
WO 99139647 PCT/US99/02332
Example 2 - Migration Experiment
The migration capacity of the sonicated BASMC was assayed in a 48-
well microchemotaxis chamber (Neuro Probe, Inc., Cabin John, MD). Subconfluent
SMC were detached from 100 mm culture dishes by incubation with trypsin-EDTA.
To avoid excessive exposure to trypsin, the incubation time was usually
restricted to 3
minutes. The cells were washed once in DMEM, resuspended in DMEM-0.5% CS
and then sonicated. The number of sonicated SMC was adjusted to 106 viable
cells/ml
in DMEM-0.5% CS.
In the migration assay, the bottom wells contained DMEM
supplemented with either 0.5% CS or 10% CS, which acted as chemoattractant.
Fifty
~,L of the sonicated SMC samples were placed in the upper wells of the
chamber. A 5
~m pore size polyvinyl pyrolidone-free polycarbonate filter membrane (Neuro
Probe,
Inc.) was placed above the lower wells. The chamber was assembled and
incubated at
37 ° C in a humidified atmosphere of 10% C02 in air for 90 minutes. The
filter was
~5 then removed and the vital SMC that had migrated through the filter
membrane were
stained with May-Grunwald Giemsa (Sigma, St. Louis, MO). The filter was then
mounted on a glass slide. Cells that had migrated across the filter were
counted using
a light microscope with a micrometered eye piece {Carl Zeiss, Oberkochen,
Germany)
and a magnification of 320X.
2o The migration ability was tested 0.2 hours, 2 hours and 24 hours after
sonication and expressed as the average number of migrating SMC/field, counted
in 9
fields, in triplicate wells. Non-sonicated SMC served as controls. AlI the p
values
were computed relative to the control.
Examination under a light microscope of non-sonicated SMC that had
25 migrated across the filter membrane revealed that the cell had a flattened
shape and
that its membrane exhibited the prominent lamellipodia characteristic of
migrating
cells. The unsonicated SMC migrated across the filter membrane in large
numbers
{Fig. 2a). Sonicated SMC had lesser asymmetry in the architecture of their
membrane.
9
CA 02320300 2000-08-10
qrp 9g~39~q~ PCTNS99/02332
Few cells were able to cross the filter membrane and those that did were round
in
shape with very small lamellipodia (Fig. 2b).
The migration capacity of sonicated SMC was reduced both with or
without the use of chemoattractant (10% CS) (Fig. 3). Twelve minutes after SMC
s were exposed to ultrasound, in the presence of chemoattractant, their
motility
decreased 2.4 fold (72 t 5 vs. 175 t 16 cells/field, p=0.0001).
Two hours after exposure to ultrasound, the inhibition of cell motility
decreased partially, to a 1.3 fold reduction in migration (121 t 2 vs. 175 t
16
cells/field, p--0.0004). Cell migration remained significantly diminished 24
hours
after ultrasound (130 t 24 vs. 175 t 16, p = 0.001).
Example 3 - Adhesion Experiment
The assay for SMC adherence capacity was a modification of the
method described by Dartsch P. C., Voisard R., Bauriedel G., Hofling B., and
Betz E.
in a study on characteristics and cytoskeletal organization of cultured smooth
muscle
~s cells from human primary stenosing and restenosing in lesion, reported in
"Arteriosclerosis" (1990) 10:62-75. After sonication, viable SMC were
centrifuged at
200 g for 10 minutes. The pelleted cells were suspended in Waymouth, HB 752/1
and
Ham's F-12 medium (2:1, v/v) and seeded in 0.2 ml aliquots of 5x104 cells/ml
in flat-
bottomed, 96 microwell plates (Nunc, Denmark) either untreated or precoated
with
2o fibronectin (25 ng/mm2) (Biological Industries, Beit Haemek, Israel). The
SMC were
incubated at 37 ° C and the adherence capacity of the SMC was analyzed
frequently
during the first 3 hours after sonication. The adherence capacity was fiuther
followed
once every 24 hours for 5 days. At each data collection point along the time
axis, the
supernatant was discarded and the non-adherent cells removed by gently washing
the
2s wells twice with PBS. The adherent cells were counted using an inverted
phase
contrast microscope with a micrometered eye piece (Carl Zeiss, Oberkochen,
Germany) and a magnification of 200X. The adherence capacity was expressed as
the
average number of adhering SMC/field, in 3 fields of triplicate wells. Non-
sonicated
SMC served as controls.
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
The adhesion of cultured SMC exposed to ultrasound was significantly
reduced in all the assays performed after sonication (Fig. 4). When tested on
fibronectin-coated surfaces, a S.S fold decrease was recorded 3 hours after
sonication
(29 t 3 vs. 160 t 20 cells/field, p=0.0001). On plastic surfaces, a 6.S fold
decrease
(12.6 t 2.S vs. 83 t 8 celUfield, p=0.0001) was noted. Analysis of variance
shows
that a significant decrease in adhesive capacity was attained (p=0.0001) at
each time
point, from 1 S minutes to 180 minutes (Fig. 4a). The reduced adhesive
capacity was
evident for up to 120 hours after exposure to ultrasound (Fig. 4b).
Example 4
1o The incorporation of 3H-thymidine by sonicated SMC synchronized to
the quiescent state (Go) by serum deprivation (O.S% CS), then stimulated to
enter the
cell cycle by serum repletion (10% CS), was measured and compared to non-
sonicated
cells.
SMC obtained from confluent cultures were cultured for 48 hours in the
presence of O.S% CS. The cells were well suspended 2.Sx104/mL DMEM-O.S% CS,
treated with ultrasound, and 12 minutes or I20 minutes after sonication,
seeded in
round-bottomed, 96 well microplates (Nunc, Denmark), lOS cells/well, in 0.2 ml
medium containing either O.S% or 10% CS and 3H-thymidine (3 ~Ci/mL). After 2
hours, the cells were harvested onto filters by a cell harvester (Linca, Tel
Aviv, Israel)
2o and radioactivity determined by liquid scintillator spectroscopy (1600 TR,
Packard,
CT).
SMC obtained from confluent cultures were well suspended
(2.Sx1()4mL), sonicated and seeded in flat-bottomed, 96 micmwell plate (0.2
mUwell)
in DMEM-10% CS and cultured at 37°C in a humidified atmosphere of 10%
CS in air
2s for 48 hours. The culture medium was replaced by DMEM-O.S% CS for 48 hours
and
the SMC synchronized to the quiescent state. After 24 hours, 3H-thymidine
(Nuclear
Research Center, Negev, Israel) (3 ~,Ci/mL) was added for an additional 18
hours.
11
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Example 5
In a different experiment, the sonicated cells were seeded directly in
DMEM-0.5% CS, cultured for 48 hours, and the procedure completed as described
above.
s The extent of 3H-thymidine incorporation was determined by aspirating
the medium, subjecting the cultures to 10 washes with PBS, and extracting the
3H-
thymidine in the cells with 0.2 ml of 0.2N NaOH/well. A volume of 0.1 ml of
the
extracted solution was added to 3 ml scintillation liquid/vial (Quicksafe, A.
Zinsser,
Germany) and the radioactivity counted in a liquid scintillation analyzer (
1600 TR,
1o Packard, CT).
3H-thymidine incorporation, reflecting DNA synthesis, was expressed as
the stimulation index. This index was defined as the ratio of the mean CPM of
sonicated, stimulated (10% CS) SMC to the mean CPM of sonicated, non-
stimulated
(0.5% CS) cells. Non-sonicated SMC, activated or not activated by CS, served
as
~s controls.
In the short-term cultures, the SMC were in suspension during the entire
incubation with 3H-thymidine. The proliferative capacities of SMC seeded for
12
minutes or for 120 minutes after sonication were similar to those of the
unsonicated
control cells. The stimulation indices were 3.2 t 0.7 vs. 3.29 t 0.7 p=NS and
3.19 t
20 0.5 vs. 3.25 t 0.5 p=NS, respectively.
In the long-term cultures, the 3H-thymidine was not significantly
different whether the SMC were sonicated before initiation of culture with 10%
CS or
before the starvation step with 0.5% CS. The stimulation indices were 3.26 t
0.8 vs.
3.18 t 0.9 p=NS and 3.3 t 0.7 vs. 3.3 f 0.6 p=NS, respectively.
2s The data regarding DNA biosynthesis of SMC, as reflected by
radioactive thymidine incorporation, indicate that the proliferative capacity
of these
cells was not significantly affected by sonication, whether the cells were
stimulated or
not.
12
CA 02320300 2000-08-10
WO 99139647 PCT/US99/02332
The components of sonicated SMC cytoskeleton were examined by an
immunofluorescent method. Actin fibers were examined by staining for a-SM
actin,
intermediate filaments for vimentin, microtubules for tyrosinated alpha
tubulin and
focal contacts for vinculin. SMC were cultured at 37°C in 10% C02 in
air on a
s culture chamber slide (2 x 103 to 5 x 103 cells/10 mm2) (Nuns, Denmark).
Before full
confluence, the cells were sonicated and the cytoskeletal components examined.
Non-
sonicated SMC served as controls under the same experimental conditions.
Cells were gently washed with PBS, then fixed with 3% para-
formaldehyde for 10 minutes. The SMC were permeabilized with 0.5% triton-x100
in
1o PBS for 2 minutes at room temperature, then washed 3 times with PBS, 5
minutes
each time. The cells were subsequently incubated 60 minutes at room
temperature
with the first antibody, mouse anti-a-smooth muscle actin (BioMakor, Rehovot,
Israel), or mouse anti-vimentin, both diluted 1:100 with PBS, or mouse anti-
vinculin
(Immuno Sigma, St. Louis, MO), diluted 1:200, or anti-tyrosine tubulin
(BioMakor,
t s Rehovot, Israel) diluted 1:400.
After 3 washes with PBS, the cells were incubated with the fluorescein-
conjugated second antibody, rat anti-mouse (Jackson, Immunoresearch Lab., PA)
diluted 1:100 in PBS containing 1 mM MgCl2 and 0.1 mM CaCl2, and incubated for
30 minutes at room temperature. The cells were washed with PBS and the wall of
the
2o chamber slide removed. The slide was embedded in 90% glycerol, cover slips
were
mounted and sealed with nail polish. Structural changes in the cytoskeleton
were
examined using a fluorescent microscope (AH3-RFCA-Venox AHBT3, Olympus,
Japan).
Phase-contrast microscopy (Fig. 5) showed that isolated SMC in
2s suspension were rounded in shape. Within a few hours of attachment to the
culture
plates, either base plastic or fibronectin-coated, they began to elongate and
to assume
an extended configuration. Later, they spread and assumed a flattened shape,
resulting
in increased cell size and surface area, until finally confluence, with the
characteristic
SMC growth pattern of hill and valley formation, was attained. Microscopic
13
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
examination of sonicated cells revealed changes in their morphology. Most of
the
cells were dispersed, the suspension consisted predominantly of single cells,
some
rounded in shape. Scanning electron microscopy showed that the surface of
untreated
cells was flat with lamellipodia and relatively thin, spike-like protuberances
(Fig. Sa).
s After exposure to ultrasound, a change in cell morphology was noted: fewer
cells
exhibited lamellipodia (24% vs. 44% cells), zeiotic crowns appeared (Fig. Sb),
large
areas of membrane collapse were observed in 36% of the sonicated SMC (Fig.
Sc),
and extensive bleb formation was observed on the surface of the sonicated
cells.
Figs. 6a-c show the actin fibers which were examined by an
immunofluorescence technique. The effect of sonication was manifested by rapid
alteration of the normally well-organized, long, linear arrangement of the
actin fibers
(Fig. 6a). The intensity of stain of the stress fibers decreased with the
simultaneous
appearance of diffuse cytoplasmic fluorescence (Fig. 6b). After 24 hours in
culture,
partial reorganization of actin fibers could be detected in the sonicated SMC
(Fig. 6c).
1s However, residual cytoplasmic fluorescence suggested the presence of non-
assembled
actin. Distribution of focal contacts in the sonicated cells was also altered:
vinculin
concentration decreased in most cells as reflected by diminished stain
intensity and the
absence of the discrete characteristic punctate pattern at the termini of
actin fibers at
the periphery of the cells.
2o Figs. 7a and 7b indicate no alteration of intermediate filaments and
microtubule organization after sonication. Vimentin and tyrosine tubulin were
organized in a filamentous, well-defined network originating in the nucleus,
spreading
throughout the cytoplasm and terminating near the periphery of the cell.
Example 6
25 To evaluate signal transduction in sonicated SMC, the applicant
investigated the phospholipase-C (PLC) pathway by triggering the receptor with
bradykinin and measuring the accumulation of inositol phosphates (IPA, IP2,
and IP3).
With minor modifications, the method was based on techniques
previously described by Steinberg S. F., and Alter A. in a study on enhanced
receptor-
14
CA 02320300 2000-08-10
WO 99/39647 PCTNS99/02332
dependent inositol phosphate accumulation in hypoxic myocytes, in "Ann. J.
Physiol."
(1993) 265:H691-H698. Briefly, viable SMC (2 x 105 cells/well) were seeded in
6
well culture plates {Corning, Staffordshire, LTK), precoated with 25 ng/mm2
fibronectin (Biological Industries, Beit Haemek, Israel) and incubated at 37
° C in 10%
C02 in air with 5 ~i/ml myo-[2-3H]inositol (NEN, Boston, MA) for 72 hours
during
which they reach confluency. After incubation, sonication was performed
according
to protocol and the SMC washed with HEPES-buffered saline (pH 7.4) and
incubated
at room temperature for 2 hours with DMEM containing 10 mM LiCI (Sigma, St.
Louis, MO). Experiments were conducted 2 hours after sonication, using 10-6 M
bradykinin stimulation (Sigma, St. Louis, MO), for 2 minutes. An experiment
was
terminated by the addition of ice-cold methanol, lipids were extracted, phases
separated and the inositol phosphates in the aqueous phase fractionated by
Dowex
anion chromatography (Bio-Rad, Richmond, CA). Radiolabeled IP samples after
fractionation were analyzed (10 ml scintillation liquid/vial: Quicksafe A,
Zinsser,
Germany) in a liquid scintillation analyzer (1600 TR, Packard, CT), and the
separation
of inositol mono (IPA), di (IP2) and tri (IP3) phosphates verified using
standards of
tritiated inositol phosphates. Bradykinin-stimulated and non-stimulated SMC
were
studied. Bradykinin-stimulation was expressed as fold stimulation: the ratio
of the
mean CPM of stimulated SMC to the mean of non-stimulated cells. Non-sonicated
2o SMC served as controls.
SMC were cultured in 24-well culture dishes and sonication performed
before full confluence of the cultures. Ten minutes after sonication, the
cultures were
washed gently with PBS, fixed in situ with 2.5% glutaraldehyde and 2%
paraformaldehyde in PBS for 2 hours at 4°C. The fixed cells were washed
in PBS,
post-fixed with 1% osmium tetroxide in 0.1 mole/L cacodylate buffer (pH 7.3)
and
dehydrated through a graded ethanol series. Cells were dried with liquid C02
at the
critical point, coated with gold by sputtering in a vacuum evaporator. The
morphology of two hundred cells was studied by scanning electron microscope
(Jeol
SEM 840, Tokyo, Japan).
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Sonication did not erect inositol phosphate accumulation, as measured
by activation of the a-adrenergic receptor-PLC pathway by triggering the a-
adrenergic
receptor with bradykinin. No change in the basal accumulation was apparent.
The
accumulation of inositol phosphates of sonicated SMC did not differ from that
of pon-
s sonicated cells 2 hours after sonication. The quantitative increases of
inositol
phosphates of bradykinin-stimulated cells after 2 minutes of stimulation, were
similar
to those of the ultrasound-treated and the control groups: IP1 = 1.44 t 0.2
vs. 1.42 t
0.2 fold, p= NS; IP2 = 1.79 t 0.1 S vs. 1.8 t 0.14 fold, p = NS; IP3 = 1.52 t
0.2 vs.
1.44 t 0.2 fold, p = NS.
In summary, therapeutic LTLS, without the addition of drugs, causes
structural changes in SMC cytoskeleton, which create the basis for the altered
morphology and function of SMC.
From a therapeutic point of view, a useful strategy for the prevention of
restenosis is one which is targeted at disrupting the migration apparatus
(i.e.
~s cytoskeleton), thus leading to arrest of migration and adherence of SMC
after arterial
injury and thus, will prevent formation of neo-intima. Three biological
functions have
been identified by Casscells W., in a study on migration of smooth muscle and
endothelial cells reported in "Circulation" (1992) 86:723-729 as essential for
the
migration of SMC. They are: release of proteolytic agents, adherence to
extracellular
2o matrix, and the chemotactic response. The data presented herein suggests
the
unexpected results that ULS can inhibit the latter two of these SMC functions
by
virtue of its selective damage to actin fibers and the changes it effects on
focal
adhesion.
Although the above description relates to a specific ultrasonic energy
2s level, the invention is not restricted to the specific level or frequency
exemplified. In
general, the energy level is dependent on the power being above the cavitation
threshold of the relevant fluid, such as blood or an other relevant body fluid
such as
water.
16
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Example 7
With respect to the frequency, Fig. 8 roughly illustrates the frequency
location of several acoustic phenomena. In ultrasound thrombolysis,
frequencies at
the lower end of ultrasound (20-45 kHz, with high intensities) are employed.
In
s ultrasound imaging, higher frequencies, in the megahertz range, and low
intensities are
employed. Still, the cavitation effect will be present with any ultrasound
frequency in
intensities above the maximum sound pressure in the medium, such as blood or
water
(the cavitation threshold). In general, the lowest possible frequency will be
chosen,
depending on system-dependent limitations (e.g. length of probe, invasive vs.
non-
1o invasive method, etc.). Preferably, the frequency will be in the range of 1
S-250 kHz.
To compensate for multiple-variable changes in the cavitation threshold,
it is desirable to choose the power level with the help of an ultrasound
imaging system
such as described by Rosenschein U., Rozenszsajn L. A., Kraus L., Maraboe C.
C.,
Walkins J. F., Rose E. A., Cannon J. P., and Weinstein J. S. in their work on
ultrasonic
is angioplasty in totally occluded peripheral arteries reported in
"Circulation" { 1991 )
83:1976-1986. Thus, the area to be treated can be imaged by ULS while the
power
level is progressively increased. When the cavitation threshold is reached,
microbubbles appear on the ULS-imaging screen. At this point, power can be
reduced
to a level which will maintain cavitation. This will be the preferable power
level and
2o frequency to be used for treating the target area. Another method involves
the use of
an external acoustic emission detection device, such as an amplified
microphone type-
device, which detects acoustic emissions which are characteristic of
cavitation
bubbles. It has been found that once cavitation is detected, the power level
can be
reduced to a level sufficient to maintain cavitation, but less than that
needed to initiate
2s cavitation. Thus, the doctor or clinician can customize the optimal ULS
dose level for
each condition.
An especially important aspect of the method of the invention is in the
prevention of restenosis in a blood vessel of a mammal following angioplasty.
As
stated above, restenosis is associated with the migration of SMC in the blood
vessel.
17
CA 02320300 2000-08-10
WO 99/39647 PCTNS99/02332
By reducing or inhibiting migration of SMC as a result of the ULS treatment
described
herein, the likelihood of restenosis occurring following vascular intervention
is
decreased.
The therapeutic use of ULS for recanalization of occluded arteries has
been described in the literature. The use of ULS for the prophylaxis of
restenosis
following angioplasty would typically be done subsequent to such procedure.
The
ultrasonic energy may be transmitted to the occluded or stenotic artery either
directly
by the insertion of an ultrasonic probe into the blood vessel, or in a non-
invasive
manner. The following non-limiting examples illustrate certain therapeutic
methods in
to accordance with the invention.
Embodiment 1: Invasive ultrasonic treatment
A device suitable for the invasive application of ultrasonic energy in
accordance with the invention could be composed of the following elements
shown in
the embodiment of Fig. 9. A power generator 20, supplies the restenosis
inhibiting
~5 system 10 with the electrical energy needed to produce ultrasonic energy.
An
ultrasound transducer 30 in the handpiece 40, consists of piezoelectric
elements (not
shown) that convert electrical energy into ultrasonic energy. An ultrasonic
transmission wire 50 is connected at its proximal end to the transducer and
has an
ultrasound tip 60 at the other end. The ultrasonic energy is transmitted as a
20 longitudinal vibration of transmission wire 50 which thereby directs
ultrasound energy
into the arterial system of a patient (not shown). Additional examples of
devices for
the invasive application of ultrasound into a body are set forth in the
following issued
U. S. patents and publications, the contents of which are incorporated herein
by
reference: 5,163,421, issued November 17, 1992; 5,269,297, issued December 14,
25 1993; 5,324,255; 4,474,180; and Julian Frederick, "Ultrasonic Engineering",
John
Wiley and Sons ( 1965).
The frequency level of ultrasound energy used is typically 20 kHz in
vitro, in vivo and in human peripheral arterial studies, and 20-45 kHz in
coronary
artery studies.
18
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Power is supplied by power generator 20. Peripheral arterial studies
were performed with a power, for example, of about 20 t 2 W in vivo, and of
about
12.0 t 0.9 W in humans. Coronary arterial studies are typically carried out
with a
power of about 18 t 2 W. System power is translated into longitudinal
displacement
s of ultrasound tip 60, measuring 150 t 25 ~,m in initial studies and 10-15 ~m
in
coronary artery application. The change of amplitude in coronary artery
application
resulted from the selection of thrombus, rather than atherosclerotic plaque,
as the
principal target of ablation in coronary arteries as the therapeutic target.
Suitable operating parameters will be determined depending on the
1o specific ultrasound system being used as well as on the target tissue. A
doctor or
clinician of ordinary skill in the art will know how to determine suitable
parameters.
Embodiment 2: Non-invasive ultrasonic treatment
Non-invasive ultrasound technology enables delivery of ultrasonic
energy from a source outside the human body to a specific internal location.
The
1s intensity level of the energy in the treatment has to be high enough to
create acoustic
transient cavitation at the locus of therapy.
In order to eliminate any risk in that respect, the energy is preferably
transmitted to the treatment area in such a way that the energy is focused
only at the
target location. Being unfocused along the way, minimal heat is created and no
risk is
2o involved. Either continuous wave or pulsed wave ULS can be used.
The device can be made compatible with ultrasound imaging systems by
the addition of a dedicated therapeutic ultrasound probe. The combined system
can
serve as both an imaging probe and a treatment probe, thereby transmitting the
required treatment energy to the selected target under ultrasound imaging. A
software
2s package can add the capability to support the visualization of the target,
and the
activation of the therapeutic energy transmission at the target.
The operation and handling of the device will also be similar to
ultrasound imaging systems, with the addition of activation of non-invasive
therapeutic ultrasound transmission. Examples of devices and operating
procedures
19
CA 02320300 2000-08-10
WO 99/39647 PGT/US99/02332
for the non-invasive application of ultrasound into a body are set forth in
U.S. Patent
No. 5,524,620, the contents of which are incorporated herein by reference.
A system for the prevention of restenosis in a blood vessel of a mammal
following angioplasty in accordance with the present invention may typically
include a
s therapeutic ultrasound probe, preferably containing therapeutic and imaging
capabilities. The therapeutic ultrasound element can be based on any method
for
focusing ultrasound (e.g., geometric, annular array, phase array). The system
will
typically also include a control unit for controlling the ultrasonic energy
output, which
may preferably include a monitor, similar to regular imaging monitors, and
more
io preferably, along with the software and hardware, suitable for operating
the combined
imaging and therapeutic transducer.
Drug delivery in accordance with the invention can be performed using a
local drug delivery system such a~s a stent-based or phonopheresis system
Example 8
15 Patients with anterior acute myocardial infarction were considered for
the study. Patients were determined to be eligible if they showed evidence of
anterior
AMI defined by ischemic chest pain for < 12 hours, accompanied by ST elevation
z 1
mm in Z 2 precordial leads. On angiography, there was thrombolysis in
myocardial
infarction (TIMI) grade flow 0 or 1 in the left anterior descending artery
(LAD).
2o An ultrasound thrombolysis device used was a 140 cm long solid
aluminum alloy probe, ensheathed in a plastic catheter and connected at its
proximal
end to a piezoelectric transducer available from Angiosonics Inc.,
Morrisville, North
Carolina. Ultrasonic energy is transmitted from the transducer as longitudinal
vibrations of the probe which directs the energy into the arterial system. The
last 18
25 cm of the device is a three-wire flexible segment with a 1.6 mm tip
designed to
optimize the thrombolytic effect of the ultrasound energy by promoting
cavitation.
The three wire flexible segment permits the use of a solid metal transmitter
for optimal
ultrasound transmission, while remaining flexible.
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
Power output at the handpiece was controlled by an integrated computer
designed to ensure constant output at the distal tip under variable loading
conditions
encountered during the procedure, and was set at about 18 watts.
The ultrasound probe was attached to a guidewire and advanced into the
s LAD under flouroscopy until the cavitation tip was positioned between about
1 to 2
mm past the proximal end of the occlusion. Sonication (at 18 watts) was
carried out to
ablate the clot. Therapeutic ultrasound induced normalization of perfusion,
and a
TIMI grade 3 was achieved with no adverse angiographic signs. At the end of
the
procedure, the probe was then used to irradiate the vascular intima over the
length of
the stenosis, thereby inhibiting the migration, viability and adherence of the
smooth
muscle cells in the lumen. Then, at a six-month follow up, seven of the 14
patients
studied underwent intravascular ultrasound (IVLJS) of the sonicated arterial
segment.
In six patients, IVLJS has shown minimal myo-intimal proliferation and in only
one
patient was any significant myo-intimal growth accompanied by lumen narrowing
1s observed. This data suggest the unexpected benefit that therapeutic
ultrasound in
accordance with the present invention is effective in preventing restenosis
following
vascularintervention.
It has been found that the combined treatment of SMC with ultrasound
and certain drugs, including anticytoskeletons such as those with anti-stress
fiber
2o and/or anti-microtubuli properties, leads to a further effect of reduction
of both SMC
adherence and migration abilities until almost a complete blockage of SMC
adherence
and migration abilities occurs. As described before, ultrasound treatment of
SMC
results in a decrease of cells migration and adhesion abilities. Treatment of
SMC with
anticytoskeleton agents, such as cytochalasin B or colchisine, induces a
similar
2s reduction of both SMC functions, as shown in Figs. 10 and 11.
It is believed that ultrasound causes impairment of the actin stress fibers
in SMC as demonstrated by immunofluorescence technique. It has been found that
cytochalasin B induces inhibition of SMC actin filaments polymerization
expressed by
damage of the cells adhesion and migration machinery. Based on the
experimental
21
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
results which follow, it was concluded that the combined biological effect of
ultrasound and cytochalasin B on SMC was found to be significantly higher than
each
one of the components by itself. This phenomenon may be explained by the fact
that
ultrasound interrupts existing stress fibers, while cytochalasin B prevents
the repair by
eliminating formation of new ones in the treated cells.
Example 9
Smooth muscle cells (SMC) of confluent cultures were trypsinized, and
thereafter well suspended, 2.5 x 106/5 mL DMEM, in a culture tube 16 x I25 mm
(Coming-Staffordshire, UK). They were then exposed to ULS using a sonicator
(Vibra Cell, Sonic Materials Inc., Danbury, CT). The sonicator consists of a
resonant
90 mm long, 2 mm diameter, vertically suspended, thin titanium probe, which
resonated at a frequency of 20 kHz and variable power levels. To prevent
aerosol
formation during sonication, the depth of immersion of the probe was adjusted
so that
the meniscus of the liquid came in contact with the ultrasonic wire at a
displacement
~s node. An excellent correlation (r = 0.98) between ultrasound dose
(ultrasound power
x time of sonication) and SMC viability was observed over a range of
sonication times
(I-20 sec) and power (0-2 watts). The LD50 of the ULS was established as an
ultrasound sonication dose of 1.5 watts for 15 seconds (22.5 watt~sec) at a
frequency
of 20 KHz.
1'he viability of sonicated SMC was tested by propidium iodide staining
and trypan blue exclusion methods. All the experiments were performed on SMC
which remained viable after sonication.
1. SMC Migration Capacities After Ll~rasound And Cytochalasin B
Treatment
The migration capacity of the treated aortic SMC was assayed in a 48-
well microchemotaxis chamber (Neuro Probe, Inc., Cabin John, MD). Subconfluent
SMC were detached from 100 mm culture dishes by incubation (3min) with trypsin-
EDTA. The cells were washed once in DMEM and resuspended 106/mL in DMEM-
0.5% CS containing cytochalasin B (Sigma) in concentration of 44~M. After 1
hour
22
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
of incubation, the cells were washed three times with DMEM. Sonication on
cytochalasin B treated cells or non-treated was followed as described in the
above
sonication protocol. The sonicated SMC number was adjusted to 106 viable
cells/ml in
DMEM-0.5%CS.
In the migration assay, the bottom wells contained DMEM
supplemented with 10% CS, which acted as chemoattractant. The SMC samples,
treated or non-treated, SO ~tL, were placed in the upper wells of the chamber.
A 5 gm
pore size polyvinyl pyrrolidone-free polycarbonate filter membrane (Neuro
Probe,
Inc.) was placed above the lower wells. The chamber was assembled and
incubated at
0 37°C in a humidified atmosphere of 10% C02 in air for 90 min. The
filter was then
removed and the SMC that had migrated through the filter membrane were stained
with MayGrunwald Giemsa (Sigma, St. Louis, MO). The filter was then mounted on
a
glass slide. Cells that had migrated across the filter were counted using a
light
microscope with a micrometered eye piece (Carl Zeiss, Oberkochen, Germany) and
a
15 magnification of 320X.
The migration capacity was expressed as the average number of
migrating SMC/field, counted in 9 fields, in triplicate wells. Nonsonicated
SMC
served as controls. As shown in Fig. 10, the combination of sonication and
cytochalasin B leads to a very significant reduction in cell migration
capability in
2o comparison to those obtained by ultrasound or cytochalasin B alone.
2. SMC Adherence Capacities After Ultrasound And Cytochalasin
B Treatment
For the assay for aortic SMC adherence capacity, cells were detached
from the culture dishes by trypsin-EDTA and the SMC were suspended 106/mL in
25 DMEM-0.5%CS containing cytochalasin B in a concentration of 22g,M.
Sonication of
cytochalasin B treated cells or non-treated cells was performed as described
in the
above sonication protocol. After sonication, SMC were centrifuged at 200 g for
10
min. The pelleted cells were suspended in Waymouth, HB 752/1 and Ham's F-12
medium (1:1, v/v) and seeded in 0.2 ml aliquots of 5x104 viable cells/mL in
flat-
23
CA 02320300 2000-08-10
WO 99/39647 PCT/US99/02332
bottomed fibronectin coated (25ng/cmZ), 96 microwell plates (Nunc, Denmark).
The
SMC were incubated at 37°C and the adherence capacity of the SMC was
analyzed at
15, 30, 60, 75 and 120 min. At each data collection point along the time axis,
the
supernatant was discarded and the nonadherent cells removed by gently washing
the
s wells twice with PBS. The adherent cells were counted using an inverted
phase
contrast microscope with a micrometered eye piece (Carl Zeiss, Oberkochen,
Germany) and a magnification of 200X. The adherence capacity was expressed as
the
average number of adhering SMC/field, in 3 fields of triplicate wells. Non-
sonicated
SMC served as controls. As shown in Fig. 10, the combination of sonication and
o cytochalasin B leads to a very significant reduction in cell migration
capability in
comparison to those obtained by ultrasound or cytochalasin B alone.
In vivo dosages would be effective at levels of similar order of
magnitude as the in vitro experiments. Tissue levels of colchicine would be in
the
nanogram range.
~s It will thus be seen that the objects set forth above, among those made
apparent from the preceding description, are efficiently attained and, since
certain
changes may be made in carrying out the above method and in the constructions
set
forth without departing from the spirit and scope of the invention, it is
intended that all
matter contained in the above description and shown in the accompanying
drawings
2o shall be interpreted as illustrative and not in a limiting sense.
It is also to be understood that the following claims are intended to cover
all of the generic and specific features of the invention herein described and
all
statements of the scope of the invention which, as a matter of language, might
be said
to fall therebetween.
2s
24