Note: Descriptions are shown in the official language in which they were submitted.
CA 02320445 2000-08-09
WO 99140422 PCT/US99/02777
A VALVELESS GAS CHROMATOGRAPHIC SYSTEM HTITH PULSED
INJECTION AND TEMPERATURE PROGRAMMED ELUTION
CROSS-REFERENCE TO RELATED APPLICATIONS
The following patent application is based on and
claims the benefit of U.S. Provisional Patent Application
Serial No. 60/074,195 filed February 10, 1998.
DESCRIPTION
Field of the Invention
The present invention relates generally to detection
and analysis of gaseous components and more particularly
to a valueless system using gas chromatography with
pulsed injection and temperature programmed elution.
Background of the Invention
Gas chromatography is an established analytical
technique for separating the components of a gaseous
mixture as the mixture flows through a tubular column.
There are many different ways of injecting the initial
sample into the column and performing the separation.
For example, one known method of carrying out the
separation in open tubular columns is shown in Figures 1a
and 1b. In this method, valve 1 admits a small volume of
the sample flowing through the loop 2 into the column 3
when the valve is switched from the sampling position
shown in Figure 1a to the injection position shown in
'Figure lb. This volume of sample is then carried down
CA 02320445 2000-08-09
WO 99/40422 PCT/US99l02777
the column by a flow of carrier gas through the port 4
and 5 of the valve and separated into its components when
it interacts with the column wall coated with the
appropriate separating medium. The net result is that
the components exit the column as separate volumes at
different times. The time between the injection and the
exit of a component is called its retention time. The
components are detected by an appropriate detection
system, for example, an electron capture detector (ECD)
or a thermal conductivity detector. The signal generated
by the detector (the chromatogram) can then be plotted
out for analysis.
The speed at which analysis takes place in this
system is dependent on several factors including the type
and length of the column, its temperature and the
velocity of the carrier gas in the column. In general
total analysis times are in the order of minutes to
hours. Sample preparation and injection can take several
minutes to hours depending on the nature of the sample.
Thus for real time analysis this process needs to be
speeded up considerably.
Real time analysis is highly desirable when using
the technique of gas chromatography for quickly detecting
and identifying compounds contained in narcotics and
explosives. Then sampling and detection systems based on
gas chromatography can be used for checking suspicious
objects which could contain explosive devices or
controlled drugs and narcotics. Such devices are useful
at border crossings and airports for identifying and
preventing drug smuggling or terrorist activity.
Therefore, it is also highly desirable to make such
detection system portable and operable in real time.
-2-
CA 02320445 2000-08-09
WO 99140422 PCT/US99/02777
Moreover, it is also desirable to make such systems
battery operable. Gas chromatography of drug or
explosive samples require that the sampling separation
systems work at high temperatures typically in the region
of 100 to 300 degrees Celsius. Presently, there are no
energy efficient or portable GC-IMS devices which can
operate in the high temperature regime for analyzing
drugs or explosives because power requirements for gas
chromatography systems usually preclude battery operated
portable systems of practical size and weight.
Therefore, it is also highly desirable to have a gas
chromatography system with minimum power consumption
without sacrificing performance.
Summary of the Invention
The present invention provides a novel design and
method of operation for a pulsed high speed sampling and
gas chromatographic (GC) separation system which is
capable of sampling and analyzing particles and vapors
containing drug and explosive residues in less than
twenty seconds and which at the same time consume very
little power. The speed and power savings provided by
the present invention uses a heat-on-demand (HOD)
technology explained below.
There are several important advantages for using the
pulsed analysis technique in simple, portable, low power
GC-IMS sample gathering and analytical system as
disclosed by the present invention. Because of the
pulsed nature of the system, power consumption takes
place only when the system is analyzing, greatly
increasing the overall energy efficiency of the system
-3- _
CA 02320445 2000-08-09
WO 99/40422 PCT/US99/02777
compared to static systems where the components are
always maintained at high operating temperatures. This
makes its use practical in hand-held analytical devices
using batteries as power sources.
Moreover, the pulsed heating sequence avoids the use
of valves to switch a sample packet into the column as is
done in static high temperature systems, making the
system simpler and more reliable.
Advantageously, the system of the present invention
may operate as one integrated system for sample
gathering, analysis, and data presentation, thus, making
it an ideal portable real-time analytical instrument for
many applications, including drug and explosive checks
and searches at border points, airports, etc., and also
for air quality monitoring.
Furthermore, the present invention may be used with
an ion mobility spectrometer (IMS) device as a second
analyzer. Using the IMS greatly increases the overall
selectivity and sensitivity of the instrument without
adversely affecting its performance or energy efficiency.
Further features and advantages of the present
invention as well as the structure and operation of
various embodiments of the present invention are
described in detail below with reference to the
accompanying drawings. In the drawings, like reference
numbers indicate identical or functionally similar
elements.
Brief Description of the Drawings
Preferred embodiments of the present invention will
now be described, by way of example only, with reference
-4-
CA 02320445 2000-08-09
WO 99/40422 PCT/US99/02777
to the accompanying drawings in which:
Figures 1a and 1b illustrate an example of
separating the components of a gaseous mixture as the
mixture flows through a tubular column as known in the
prior art systems;
Figure 2a illustrates a schematic diagram of the
valueless gas chromatographic system of the present
invention; and
Figure 2b illustrates a graphical representation of
the heating and cooling sequence of the present
invention.
Detailed Description of the
Preferred Embodiment of the Invention
In the preferred embodiment, the valueless gas
chromatographic system of the present invention is an
integrated sampling and analysis device. Such
integration with an analysis device makes it possible to
use the system as a portable, hand-held device. A
description of the hand-held device which integrates the
system of the present invention can be found in the
related and commonly owned PCT Application No.
PCT/US98/22092 entitled A.SAMPLE TRAPPING ION MOBILITY
SPECTROMETER FOR PORTABLE MOLECULAR DETECTION, filed on
October 20, 1998, the description of which is fully
incorporated herein by reference thereto.
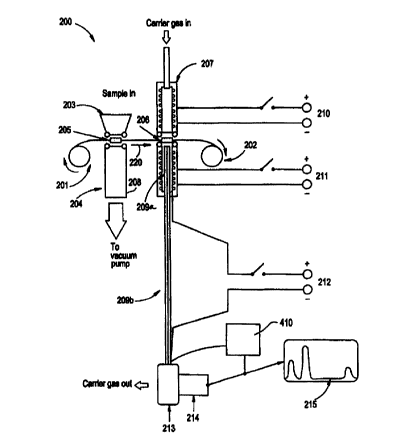
Figure 2a shows a schematic diagram of the system of
the present invention. The system may be divided into a
sampling section and analysis section. The system
includes a sample trap having a ribbon about half an inch
wide wound on bobbins 201 and 202 and passing between the
-5-
CA 02320445 2000-08-09
WO 99/40422 PCTNS99/02777
sampling and analysis sections. The material used to
make the ribbon may be a metallic mesh typically of size
400 or more or other porous type which allows air to pass
through freely but traps small particles and vapors. The
vapor trapping or collecting ability may be increased by
coating the ribbon with an absorbing media known in the
art for use in absorbing the desired molecules of
interest.
A nozzle 203 and a pump tube 204 are cylindrical
entities with soft 0 ring seals at the ends that are
closer to the ribbon. When the machine is in the
sampling mode, 203 and 204 form a tight seal on portion
205 of the ribbon. A vacuum pump attached to the pump
tube 204 sucks vapors and/or particles through the
sampling nozzle 203 onto the sampling area 205 of the
ribbon. After a predetermined time duration of sampling,
e.g., few seconds of sampling, the nozzle 203 and the
pump tube 204 are moved away from the ribbon to break the
seal. The moving process is accomplished with the aid of
electric motors controlled by a computer 410. After the
seal is broken, the ribbon is moved in the direction
shown by the arrow 220 to the location at 206. The
movement of the ribbon is also accomplished with the aid
of electric motors and position sensors which stop the
motors after positioning the sample.
Once at position 206, the desorption port 207 and
the injection port 208 move towards the ribbon under
motor control and form an air-tight seal around 206. The
desorption port 207 is a cylindrical entity less than
1/4" in diameter, and may include a built-in electric
heater 210 to heat the gas passing through the ribbon to
a temperature of 200 Celsius or more within a few
-6-
CA 02320445 2000-08-09
WO 99/40422 PCT/US99/02777
seconds. A carrier gas flows into 207 and gets heated by
the heaters so that when the hot gas exits out of the
desorption port 207 and impinges on portion 206 of the
ribbon, it in turn heats the sample trapped in the ribbon
at 206. The rate of flow of the carrier gas is typically
about 50 to 200 cc/min.
At the time the desorption port 207 is hot, the
injection port 208 is also heated to the same temperature
using the same technique as for the desorption port 207
with the aid of electric heater 211. The injection port
208 has a more complex construction because it has the
gas chromatographic column 209 attached in a unique
manner. The column 209 in the preferred embodiment has a
metallic jacket which is directly heated by passing a
current through it from a controlled source 212. Portion
209a of the column 209 is inside the injection port 208
and portion 209b is outside the injection port 208. The
far end of 209b is connected to the detector 213. This
detector 213 is preferably an TMS detector. The carrier
gas flowing into the injection port 208
goes directly
into portion 209a of the column and thence into portion
209b. When the desorption port 207 and the injection
port 208 are heated, the column 209 is not heated. This
causes the vapors of the trapped sample at 206 which are
released by the hot carrier gas to move through portion
209a of the column and condense at the beginning of
portion 209b of the column.
Once the sample has settled down in the front end of
the column 209, the heaters 210 and 211 are switched off,
typically by a computer controller 410. The temperatures
of the heaters rapidly drop to ambient in a few seconds
because the ports 207, 208 are constructed with the
-7- _
CA 02320445 2000-08-09
WO 99/40422 PCT1US99/02777
minimum amount of heat capacity. The computer 410 senses
the temperature of the ports 207, 208 and when they have
reached an appropriate minimum value which is preferably
about 20 degrees Celsius above the ambient, the computer
410 turns on the heater 212. This causes the column
portions 209a, 209b to heat up rapidly from ambient to
more than 200 degrees Celsius in a few seconds. The rate
of this heating is controlled by the computer program.
Since there is a carrier gas flow in the column during
the heating cycle, the condensed compounds move down the
column and separate into the individual components and
exit into the IMS 213 at different times. The IMS
ionizes these packets of individual components in the
sample and further separate the components according to
their mobility in the drift gas flowing in the IMS. The
individual ion packets are then collected on an electrode
and amplified electronically by amplifier 214 for further
signal processing and display 215 using the computer 410.
It should be noted that detection devices other than IMS
may be used, e.g., by attaching a different detection
device at the end of the column 209.
The sequence of heating and cooling of the analysis
system is critical to the success of the device as a
programmed pulsed gas chromatographic system. A
graphical representation of the heating and cooling
sequence is shown in Figure 2b where the horizontal axis
is the time axis common to the three graphs. The three
separate vertical axes are the temperature axes. The
maximum values of the temperatures depend on the nature
of the compounds being analyzed, and are typically around
200 degrees Celsius for explosive and drug compounds.
The rate of rise and fall of the temperature programming
-8- _
CA 02320445 2000-08-09
WO 99/40422 PCT/US99/02777
of the column is in general constant, but can be changed
to follow a desired curve using the computer 410 to
control the duty cycle of the heater. As shown in Figure
2b, there is no delay between the heating cycles 240, 250
of the heaters in the ports 207, 208, but the column
heating starts as shown at 260 after these heaters have
cooled down, to achieve the desired effect described
above.
In addition, the heated portions have Iow heat
capacities and are designed to dissipate the heat
efficiently. Such a design is important for achieving
fast analysis times. With the system of the present
invention as described above, the ports 207, 208 and the
column 109 can be heated and cooled over the working
range in a few seconds.
While the invention has been particularly shown and
described with respect to a preferred embodiment thereof,
it will be understood by those skilled in the art that
the foregoing and other changes in form and details may
be made therein without departing from the spirit and
scope of the invention.
-9-