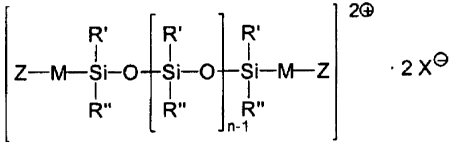Note: Descriptions are shown in the official language in which they were submitted.
CA 02320500 2000-09-18
RINSE AID COMPOSITIONS
Field of the Invention
The present invention relates to the use of diquaternary
polysiloxanes in detergent and rinse aid compositions for
machine dishwashing. The present invention further
relates to rinse aid compositions for machine dishwashing
and to commercial forms which provide detergent and rinse
aid in one product, and also to the processes for
preparing such rinse aid and detergent compositions.
Background of the Invention
Frequently, more stringent requirements are nowadays
imposed on machine-washed kitchen- and tableware than on
handwashed ware. For instance, even ware which has been
completely cleaned of food residues is not considered
flawless when, following machine dishwashing, it still
has whitish spots deriving from water hardness or other
mineral salts and originating from dried water droplets
for lack of wetting agent.
In order to obtain sparkling and spotless ware, rinse
aids are nowadays used successfully. The addition of
rinse aid at the end of the wash program ensures that the
water runs off from the ware as completely as possible,
so that at the end of the wash program the different
surfaces are free from residues and gleam immaculately.
The machine cleaning of kitchen- and tableware in
domestic dishwashing machines normally involves a prewash
cycle, a main wash cycle and a rinse cycle, interspersed
with intermediate wash cycles. With the majority of
machines, the prewash cycle may be selected for highly
soiled ware, but is selected by the user only in
exceptional cases, so that in the majority of machines a
main wash cycle, an intermediate wash cycle with clean
water, and a rinse cycle are conducted. The temperature
1
CA 02320500 2000-09-18
of the main wash cycle varies, according to machine type
and program step choice, between 40 and 65°C. In the
rinse cycle, rinse aid compositions are added from a
dosing tank within the machine, these rinse aids normally
comprising nonionic surfactants as their principal
constituent. Rinse aids of this kind are in liquid form
and have been widely described in the prior art. Their
primary object is to prevent lime spots and deposits on
the cleaned ware. Besides water and low-foaming nonionic
surfactants, these rinse aids often also include
hydrotropes, pH modifiers such as citric acid, or scale-
inhibiting polymers.
EP-B1 0 197 434 (Henkel) disdoses liquid rinse aids
comprising mixed ethers as nonionic surfactants . A large
number of different materials (glass, metal, silver,
plastic, porcelain) are cleaned in the dishwashing
machine. This diversity of materials must be wetted as
thoroughly as possible in the rinse cycle. Rinse aid
formulations comprising exclusively mixed ethers as the
surfactant component fail completely or adequately to
meet these requirements, so that the rinse-clean effect
or drying effect, especially in the case of plastics
surfaces, is unsatisfactory.
The reservoir tank within the dishwashing machine has to
be filled up at regular intervals with rinse aid, one
fill being sufficient for from 10 to 50 cycles, depending
on machine type. If the user forgets to fill up the tank,
then glasses, in particular, acquire unsightly lime spots
and deposits. In the prior art, therefore, there exist a
number of proposals for integrating a rinse aid into the
machine dishwashing detergent. These proposals are tied
to the commercial form of the compact tablet.
For instance, European Patent Application EP-A-0 851 024
(Unilever) describes two-layer detergent tablets whose
2
CA 02320500 2000-09-18
first layer comprises peroxy bleach, builder and enzyme
while the second layer comprises acidifiers, a continuous
medium having a melting point of between 55 and 70°C, and
scale inhibitors. It is intended that the high-melting
continuous medium will effect retarded release of the
acids) and scale inhibitors) to produce a rinse aid
effect. This document makes no mention of powder-form
machine dishwashing compositions or rinse aid systems
comprising surfactant.
The prior German Patent Application DE 198 51 426.3
(Henkel KGaA) describes a process for producing
multiphase detergent tablets which comprises compressing
a particulate premix into tablets which have a
depression, which is subsequently filled with a
separately prepared melt suspension or melt emulsion
comprising a coating substance and one or more active
substances suspended or dispersed therein.
Summary of the Invention
One commercial form which may be used both separately as
a rinse aid in solid form to be dosed by the user, and as
an admix component to pulverulent machine dishwashing
compositions is described in the prior German Patent
Application DE 199 14 364.1 (Henkel KGaA).
It is an object of the present invention to provide new
rinse aids which in terms of their performance properties
provide results at least equal to those of customary
commercial rinse aids and which, furthermore, bring
additional performance advantages. The new rinse aids
ought to be suitable for use both as conventional rinse
aid compositions and in the form of combination products
and ought to develop their advantageous properties
irrespective of the type of formulation. Not least, the
new rinse aid compositions should also be suitable for
use in conventional machine dishwashing detergents, i.e.,
3
CA 02320500 2000-09-18
in the form of an additive component, too, the
compositions ought to permit performance advantages.
It has now been found that the use of diquaternary
polysiloxanes in machine dishwashing detergents brings
about advantageous effects. It is particularly
advantageous if the diquaternary polysiloxanes are
employed in the rinse cycle.
In a first embodiment, therefore, the present invention
provides for the use of diquaternary polysiloxanes in
machine dishwashing compositions.
The sole addition of these compounds to detergents
results in ware items treated with such compositions
becoming much cleaner in subsequent washes than ware
items washed with conventional compositions. This effect
is independent of whether the machine dishwashing
compositions are in liquid, powder, or tablet form.
An additional positive effect occurring is a shortening
of the drying time of the ware items treated with the
detergent, i.e., after the wash program has run, the user
is able to take the ware from the machine, and use it
again, sooner.
The invention is notable for an enhanced "cleanability"
of the treated substrates on subsequent washes and for a
considerable reduction in the drying time relative to
comparable compositions without the use of diquaternary
polysiloxane. In addition to this primary effect of the
invention, the diquaternary polysiloxane, as expected,
displays the foam-suppressing activity inherent in the
silicones. Surprisingly, moreover, liquid compositions
which comprise diquaternary polysiloxanes display a
cleaning power which is not only not adversely affected
by the diquaternary polysiloxane but indeed is usually
4
CA 02320500 2000-09-18
increased, and also an increased low-temperature
stability in all cases.
In the context of the teaching of the invention, drying
time generally has its literal meaning; that is, it is
the time which elapses until a ware surface treated in a
dishwashing machine has dried, but in particular is the
time which elapses until 90% of a surface treated with a
detergent or rinse aid composition in concentrated or
dilute form has dried.
Diquaternary polysiloxanes for the purposes of the
invention are polyorganosiloxanes (i.e., silicones)
having two quaternized organic ammonium groups, i.e., two
quaternary nitrogen atoms, which each carry four organic
radicals and are attached by each of these four radicals
to one silicon atom of the polyorganosiloxane. In
accordance with the invention, diquaternary polysiloxanes
are used individually or as mixtures of different
diquaternary polysiloxanes in the composition or process.
It is particularly advantageous if the diquaternary
polysiloxanes are present in the last cycle, i.e., in the
rinse cycle. In this way, the advantageous action is not
weakened by subsequent cycles. The invention therefore
additionally provides for the use of diquaternary
polysiloxanes in the rinse cycle during machine
dishwashing.
Detailed Description of the Invention
The diquaternary polysiloxanes used in accordance with
the invention preferably comprise compounds of the
formula I,
5
CA 02320500 2000-09-18
2O
R' R' R'
Z-M-Si-O Si-O Si-M-Z ' 2 X~ (I)
R" R" R"
n-1
in which Z is a quaternized nitrogen center,
R' and R" independently of one another are a
C1_4 alkyl radical or an aryl radical,
M is a divalent hydrocarbon radical with at
least 4 carbon atoms, which preferably has
at least one hydroxyl group and may be
interrupted by one or more oxygen atoms
and/or groups of the type -C(O)-, -C(O)0-
or -C (O) N-,
n is a number from 1 to 201, and
X- is an organic or inorganic anion,
as are described, for example, in DE 37 19 086 C1 and
EP 0 294 642 B1.
Particularly preferred diquaternary polysiloxanes used
are the diquaternary poly(dimethylsiloxanes) of the
formula II,
CH3 CH3 CH3 2 0
Z-M-Si0~ Si0- SI-M-Z . 2 X~ (II)
CH3 CH3 n-~ CH3
in which Z is the radical,
R~ R4 0
-N ~-RZ Or -N 0-(CH j R6-~R~
2x
~3 R5 R9 Rio
w~
or -N
~N
6
CA 02320500 2000-09-18
R1, Rz , R3 , R4 , RS , R' , R9 and R1° independent ly
of one another are C1_zz alkyl or Cz_zz alkylene
radicals without or with one or more hydroxyl
groups or radicals -CHz-aryl, preferably at
least one of the radicals R1, Rz and R3 having
at least 10 carbon atoms or one of the radicals
R1, Rz and R3 being a benzyl radical,
R6 is an oxygen atom or a group -N (Ra) , R8
being a C1_4 alkyl or hydroxyalkyl radical
or hydrogen,
M is a divalent hydrocarbon radical with at
least 4 carbon atoms, which preferably has
at least one hydroxyl group and may be
interrupted by one or more oxygen atoms
and/or groups of the type -C(O)-, -C(O)O-
or -C (O) N-,
n is a number from 1 to 201 and
X- is an organic or inorganic anion.
These are, in particular, diquaternary poly(dimethyl-
siloxanes) of the formula III,
2p
CH3 CH3 CH3 CH3
R-N~ Si-O Si-M-N~ R ~ 2 XO {III)
I I I I
CH3 CH3 CH3 CH3
n
in which R is a C6_zz alkyl or alkylene radical,
especially a stearyl radical,
M is a spacer of the formula
CH2CH (OH) CH20 (CHz) 3, the connectivity
N+-M-Si of the spacer corresponding to
N+-CH2CH (OH) CH20 (CHz) 3-Si,
n is a number from 1 to 100, especially 10,
30 or 50, and
X- is an organic or inorganic anion,
preferably an acetate ion.
7
CA 02320500 2000-09-18
Examples of anions that are suitable in accordance with
the invention, in addition to acetate ions, include
chloride ions, bromide ions, hydrogen sulfate ions, and
sulfate ions.
The diquaternary poly(dimethylsiloxanes) of the formula
III which are particularly preferred in accordance with
the invention, with stearyl radicals R, acetate ions X-
and values for n of 10, 30 and 50, respectively, are
available as Tegopren~ 6920, Tegopren~ 6922 and
Tegopren~ 6924 from Th. Goldschmidt AG.
Further examples of diquaternary polysiloxanes of the
formula I to III that are suitable in accordance with the
invention may be found in DE 37 19 086 C1 and
EP 0 294 642 B1.
The use in accordance with the invention may be simply
actualized by incorporating the aforementioned substances
into liquid, powder or tablet detergents or into rinse
aid compositions. Machine dishwashing compositions
comprising diquaternized polysiloxanes are likewise
provided by the present invention.
In preferred embodiments of the present invention, the
diquaternized polysiloxanes are employed in the rinse
cycle of machine dishwashing. Preferred further
embodiments of the present invention therefore relate to
rinse aid compositions for machine dishwashing and to
combination forms which combine detergent and rinse aid
compositions with one another, the latter being offered
in particular in powder or tablet form.
The present invention therefore additionally and
preferably provides rinse aid compositions for machine
dishwashing which comprise diquaternary polysiloxanes.
8
CA 02320500 2000-09-18
As described above, such rinse aid compositions are
placed by the user into the reservoir tank of the
dishwashing machine, from where it is dosed automatically
into the rinse cycle . Depending on the dosing volume and
capacity of the tank, one tank fill is normally
sufficient for from 10 to 50 rinse cycles.
In analogy to the preferred use (see above), preference
is also given to rinse aid compositions of the invention
comprising one or more diquaternary polysiloxanes of the
formula I,
R' R' R'
Z-M-Si-O Si-O Si-M-Z ~ 2 X~ (I)
R" R" R"
n-1
in which Z is a quaternized nitrogen center,
R' and R" independently of one another are a
C1_4 alkyl radical or an aryl radical,
M is a divalent hydrocarbon radical with at
least 4 carbon atoms, which preferably has
at least one hydroxyl group and may be
interrupted by one or more oxygen atoms
and/or groups of the type -C (O) -, -C (O) O
or -C(O)N-,
n is a number from 1 to 201, and
X- is an organic or inorganic anion,
preference being given to rinse aid compositions
comprising one or more diquaternary poly(dimethyl-
siloxanes) of the formula II,
CH3 CH3 - CH3 2 ~
Z-M-Si0- Si0- SI-M-Z . 2 X ~ (II)
CH3 CH3 n-~ CH3
9
CA 02320500 2000-09-18
in which Z is the radical,
R~ R4 0
-H ~-R2 Or -N ~-~CN2 jXR6-~R~
~3 RS R,o
Rs
v,~
or -N' \N
Rl , RZ , R3 , R4 , RS , R' , R9 and Rl° independent ly of one
another are Cl_22 alkyl or CZ_22 alkylene radicals without
or with one or more hydroxyl groups or radicals
-CH2-aryl, preferably at least one of the radicals R1, Rz
and R3 having at least 10 carbon atoms or one of the
radicals R1, R2 and R3 being a benzyl radical,
R6 is an oxygen atom or a group -N (Re) , Re being a Cl_4
alkyl or hydroxyalkyl radical or hydrogen,
M is a divalent hydrocarbon radical with at least 4
carbon atoms, which preferably has at least one
hydroxyl group and may be interrupted by one or more
oxygen atoms and/or groups of the type -C(O)-,
-C (O) O- or -C (O) N-,
n is a number from 1 to 201 and
X- is an organic or inorganic anion,
and particular preference being given to those
compositions comprising one or more diquaternary
poly(dimethylsiloxanes) of the formula III,
2p
CH3 CH3 CH3 CH3
R-N- Si-O Si-M-N~ R ~ 2 X~ ~IU~
CH3 CH3 CH3 CH3
n
in which R is a C6_22 alkyl or alkylene radical,
especially a stearyl radical,
M is a spacer of the formula
CHZCH ( OH ) CHZO ( CH2 ) 3 ,
CA 02320500 2000-09-18
n is a number from 1 to 100, especially 10,
30 or 50, and
X- is an organic or inorganic anion,
preferably an acetate ion.
The amount of one or more diquaternary polysiloxanes in
the compositions of the invention may vary depending on
the intended application and the desired product
performance, with preferred rinse aid compositions of the
invention comprising the diquaternary polysiloxane(s) in
amounts of from 0.001 to 20% by weight, preferably from
0.01 to 10% by weight, with particular preference from
0.1 to 5% by weight, and in particular from 0.15 to 2.5%
by weight, based in each case on the rinse aid
composition.
The compositions of the invention may, furthermore, as
their surfactant component comprise anionic, nonionic,
cationic and/or amphoteric surfactants, nonionic
surfactants being preferred on account of their foaming
capacity.
Anionic surfactants used are, for example, those of the
sulfonate and sulfate type. Preferred surfactants of the
sulfonate type are C9_13 alkylbenzenesulfonates,
olefinsulfonates, i.e., mixtures of alkenesulfonates and
hydroxyalkanesulfonates, and also disulfonates, as are
obtained, for example, from Cla-is monoolefins having a
terminal or internal double bond by sulfonating with
gaseous sulfur trioxide followed by alkaline or acidic
hydrolysis of the sulfonation products. Also suitable are
alkanesulfonates, which are obtained from Clz-la alkanes,
for example, by sulfochlorination or sulfoxidation with
subsequent hydrolysis or neutralization, respectively.
Likewise suitable, in addition, are the esters of a-sulfo
fatty acids (ester sulfonates), e.g., the a-sulfonated
11
CA 02320500 2000-09-18
methyl esters of hydrogenated coconut, palm kernel or
tallow fatty acids.
Further suitable anionic surfactants are sulfated fatty
acid glycerol esters. Fatty acid glycerol esters are the
monoesters, diesters and triesters, and mixtures thereof,
as obtained in the preparation by esterification of a
monoglycerol with from 1 to 3 mol of fatty acid or in the
transesterification of triglycerides with from 0.3 to 2
mol of glycerol. Preferred sulfated fatty acid glycerol
esters are the sulfation products of saturated fatty
acids having 6 to 22 carbon atoms, examples being those
of caproic acid, caprylic acid, capric acid, myristic
acid, lauric acid, palmitic acid, stearic acid, or
behenic acid.
Preferred alk(en)yl sulfates are the alkali metal salts,
and especially the sodium salts, of the sulfuric
monoesters of Clz-C18 fatty alcohols, examples being those
of coconut fatty alcohol, tallow fatty alcohol, lauryl,
myristyl, cetyl or stearyl alcohol, or of Clo-Czo oxo
alcohols, and those monoesters of secondary alcohols of
these chain lengths. Preference is also given to
alk(en)yl sulfates of said chain length which contain a
synthetic straight-chain alkyl radical prepared on a
petrochemical basis, these sulfates possessing
degradation properties similar to those of the
corresponding compounds based on fatty-chemical raw
materials. From a detergents standpoint, the Clz-Cis alkyl
sulfates and Clz-Cls alkyl sulfates, and also C14-Cls alkyl
sulfates, are preferred. In addition, 2,3-alkyl sulfates,
which may be obtained as commercial products from the
Shell Oil Company under the name DAN~, are suitable
anionic surfactants.
Also suitable are the sulfuric monoesters of the
straight-chain or branched C~_zl alcohols ethoxylated with
12
CA 02320500 2000-09-18
from 1 to 6 mol of ethylene oxide, such as 2-methyl-
branched C9_11 alcohols containing on average 3.5 mol of
ethylene oxide (EO) or Cla-la fatty alcohols containing
from 1 to 4 EO. Because of their high foaming behavior
they are used in detergents only in relatively small
amounts, for example, in amounts of from 1 to 5% by
weight.
Further suitable anionic surfactants include the salts of
alkylsulfosuccinic acid, which are also referred to as
sulfosuccinates or as sulfosuccinic esters and which
constitute monoesters and/or diesters of sulfosuccinic
acid with alcohols, preferably fatty alcohols and
especially ethoxylated fatty alcohols. Preferred
sulfosuccinates comprise Ca_la fatty alcohol radicals or
mixtures thereof. Especially preferred sulfosuccinates
contain a fatty alcohol radical derived from ethoxylated
fatty alcohols which themselves represent nonionic
surfactants (for description, see below). Particular
preference is given in turn to sulfosuccinates whose
fatty alcohol radicals are derived from ethoxylated fatty
alcohols having a narrowed homolog distribution.
Similarly, it is also possible to use alk(en)ylsuccinic
acid having preferably 8 to 18 carbon atoms in the
alk(en)yl chain, or salts thereof.
Further suitable anionic surfactants are, in particular,
soaps. Suitable soaps include saturated fatty acid soaps,
such as the salts of lauric acid, myristic acid, palmitic
acid, stearic acid, hydrogenated erucic acid and behenic
acid, and, in particular, mixtures of soaps derived from
natural fatty acids, e.g., coconut, palm kernel or tallow
fatty acids.
The anionic surfactants, including the soaps, may be
present in the form of their sodium, potassium or
ammonium salts and also as soluble salts of organic
13
CA 02320500 2000-09-18
bases, such as mono-, di- or triethanolamine. Preferably,
the anionic surfactants are in the form of their sodium
or potassium salts, in particular in the form of the
sodium salts.
Nonionic surfactants used are preferably alkoxylated,
advantageously ethoxylated, especially primary, alcohols
having preferably 8 to 18 carbon atoms and on average
from 1 to 12 mol of ethylene oxide (EO) per mole of
alcohol, in which the alcohol radical may be linear or,
preferably, methyl-branched in position 2 and/or may
comprise linear and methyl-branched radicals in a
mixture, as are commonly present in oxo alcohol radicals.
In particular, however, preference is given to alcohol
ethoxylates containing linear radicals from alcohols of
natural origin having 12 to 18 carbon atoms, e.g., from
coconut, palm, tallow fatty or oleyl alcohol and on
average from 2 to 8 EO per mole of alcohol. Preferred
ethoxylated alcohols include, for example, Clz-14 alcohols
containing 3 EO or 4 EO, C9_11 alcohol containing 7 EO,
Ci3-is alcohols containing 3 EO, 5 EO, 7 EO or 8 EO, Clz-is
alcohols containing 3 EO, 5 EO or 7 EO, and mixtures
thereof, such as mixtures of Clz-14 alcohol containing 3 EO
and Clz-la alcohol containing 5 EO. The stated degrees of
ethoxylation represent statistical mean values, which for
a specific product may be an integer or a fraction.
Preferred alcohol ethoxylates have a narrowed homolog
distribution (narrow range ethoxylates, NREs). In
addition to these nonionic surfactants it is also
possible to use fatty alcohols containing more than 12
EO. Examples thereof are tallow fatty alcohol containing
14 EO, 25 EO, 30 EO or 40 EO.
As further nonionic surfactants, furthermore, use may
also be made of alkyl glycosides of the general formula
RO(G)X, where R is a primary straight-chain or methyl-
branched aliphatic radical, especially an aliphatic
14
CA 02320500 2000-09-18
radical methyl-branched in position 2, containing 8 to
22, preferably 12 to 18, carbon atoms, and G is the
symbol representing a glycose unit having 5 or 6 carbon
atoms, preferably glucose. The degree of oligomerization,
x, which indicates the distribution of monoglycosides and
oligoglycosides, is any desired number between 1 and 10;
preferably, x is from 1.2 to 1.4.
A further class of nonionic surfactants used with
preference, which are used either as sole nonionic
surfactant or in combination with other nonionic
surfactants, are alkoxylated, preferably ethoxylated, or
ethoxylated and propoxylated, fatty acid alkyl esters,
preferably having 1 to 4 carbon atoms in the alkyl chain.
Nonionic surfactants of the amine oxide type, examples
being N-cocoalkyl-N,N-dimethylamine oxide and
N-tallowalkyl-N,N-dihydroxyethylamine oxide, and of the
fatty acid alkanolamide type, may also be suitable. The
amount of these nonionic surfactants is preferably not
more than that of the ethoxylated fatty alcohols, in
particular not more than half thereof.
Further suitable surfactants are polyhydroxy fatty acid
amides of the formula (IV)
R1
R-CO-N-[Z] (IV)
where RCO is an aliphatic acyl radical having 6 to 22
carbon atoms, R1 is hydrogen or an alkyl or hydroxyalkyl
radical having 1 to 4 carbon atoms, and [Z] is a linear
or branched polyhydroxyalkyl radical having 3 to 10
carbon atoms and from 3 to 10 hydroxyl groups. The
polyhydroxy fatty acid amides are known substances which
are customarily obtainable by reductive amination of a
CA 02320500 2000-09-18
reducing sugar with ammonia, an alkylamine or an
alkanolamine, and subsequent acylation with a fatty acid,
a fatty acid alkyl ester or a fatty acid chloride.
The group of the polyhydroxy fatty acid amides also
includes compounds of the formula (V)
R 1 _~_R2
~-co-~-~z~
where R is a linear or branched alkyl or alkenyl radical
having 7 to 12 carbon atoms, R1 is a linear, branched or
cyclic alkyl radical or an aryl radical having 2 to 8
carbon atoms and Rz is a linear, branched or cyclic alkyl
radical or an aryl radical or an oxyalkyl radical having
1 to 8 carbon atoms, preference being given to Cl_4 alkyl
radicals or phenyl radicals, and [Z] is a linear
polyhydroxyalkyl radical whose alkyl chain is substituted
by at least two hydroxyl groups, or alkoxylated,
preferably ethoxylated or propoxylated, derivatives of
said radical.
[Z] is preferably obtained by reductive amination of a
reduced sugar, e.g., glucose, fructose, maltose, lactose,
galactose, mannose or xylose. The N-alkoxy- or N-aryloxy-
substituted compounds may be converted to the desired
polyhydroxy fatty acid amides by reaction with fatty acid
methyl esters in the presence of an alkoxide as catalyst.
Preferred surfactants used are low-foaming nonionic
surfactants. With particular preference, the detergent
components of the invention for machine dishwashing
comprise nonionic surfactants, especially nonionic
surfactants from the group of the alkoxylated alcohols.
16
CA 02320500 2000-09-18
Nonionic surfactants used are preferably alkoxylated,
advantageously ethoxylated, especially primary, alcohols
having preferably 8 to 18 carbon atoms and on average
from 1 to 12 mol of ethylene oxide (EO) per mole of
alcohol, in which the alcohol radical may be linear or,
preferably, methyl-branched in position 2 and/or may
comprise linear and methyl-branched radicals in a
mixture, as are commonly present in oxo alcohol radicals.
In particular, however, preference is given to alcohol
ethoxylates containing linear radicals from alcohols of
natural origin having 12 to 18 carbon atoms, e.g., from
coconut, palm, tallow fatty or oleyl alcohol and on
average from 2 to 8 EO per mole of alcohol. Preferred
ethoxylated alcohols include, for example, Clz-14 alcohols
containing 3 EO or 4 EO, C9_11 alcohol containing 7 EO,
Ci3-is alcohols containing 3 EO, 5 EO, 7 EO or 8 EO, Clz-is
alcohols containing 3 EO, 5 EO or 7 EO, and mixtures
thereof, such as mixtures of Clz-14 alcohol containing 3 EO
and Clz-le alcohol containing 5 EO. The stated degrees of
ethoxylation represent statistical mean values, which for
a specific product may be an integer or a fraction.
Preferred alcohol ethoxylates have a narrowed homolog
distribution (narrow range ethoxylates, NREs). In
addition to these nonionic surfactants it is also
possible to use fatty alcohols containing more than 12
EO. Examples thereof are tallow fatty alcohol containing
14 EO, 25 EO, 30 EO or 40 EO.
Especially preferred rinse aid compositions of the
invention are those that comprise a nonionic surfactant
having a melting point above room temperature.
Accordingly, preferred rinse aid compositions comprise
nonionic surfactants) having a melting point above 20°C,
preferably above 25°C, with particular preference between
25 and 60°C, and in particular between 26.6 and 43.3°C.
17
CA 02320500 2000-09-18
Suitable nonionic surfactants having melting or softening
points within the stated temperature range are, for
example, low-foaming nonionic surfactants which may be
solid or highly viscous at room temperature. If nonionic
surfactants which are highly viscous at room temperature
are used, then it is preferred that they have a viscosity
above 20 Pas, preferably above 35 Pas, and in particular
above 40 Pas. Also preferred are nonionic surfactants
which possess a waxlike consistency at room temperature.
Preferred nonionic surfactants for use that are solid at
room temperature originate from the groups of alkoxylated
nonionic surfactants, especially the ethoxylated primary
alcohols, and mixtures of these surfactants with
surfactants of more complex construction such as
polyoxypropylene/polyoxyethylene/ polyoxypropylene
(PO/EO/PO) surfactants. Such (PO/EO/PO) nonionic
surfactants are notable, furthermore, for good foam
control.
In one preferred embodiment of the present invention, the
nonionic surfactant having a melting point above room
temperature is an ethoxylated nonionic surfactant
originating from the reaction of a monohydroxy alkanol or
alkylphenol having 6 to 20 carbon atoms with preferably
at least 12 mol, with particular preference at least 15
mol, in particular at least 20 mol, of ethylene oxide per
mole of alcohol or alkylphenol, respectively.
A particularly preferred nonionic surfactant for use that
is solid at room temperature is obtained from a straight-
chain fatty alcohol having 16 to 20 carbon atoms (Cls-ao
alcohol), preferably a C18 alcohol, and at least 12 mol,
preferably at least 15 mol, and in particular at least 20
mol of ethylene oxide. Of these, the so-called "narrow
range ethoxylates" (see above) are particularly
preferred.
18
CA 02320500 2000-09-18
Accordingly, particularly preferred rinse aid
compositions of the invention comprise ethoxylated
nonionic surfactant (s) obtained from C6_zo mono-
hydroxyalkanols or C6_zo alkylphenols or C16-20 fatty
alcohols and more than 12 mol, preferably more than
mol, and in particular more than 20 mol, of ethylene
oxide per mole of alcohol.
10 The nonionic surfactant which is solid at room
temperature preferably further possesses propylene oxide
units in the molecule. Preferably, such PO units account
for up to 25% by weight, with particular preference up to
20% by weight, and in particular up to 15% by weight, of
15 the overall molecular mass of the nonionic surfactant.
Particularly preferred nonionic surfactants are
ethoxylated monohydroxy alkanols or alkylphenols, which
additionally comprise polyoxyethylene-polyoxypropylene
block copolymer units. The alcohol or alkylphenol moiety
of such nonionic surfactant molecules in this case makes
up preferably more than 30% by weight, with particular
preference more than 50% by weight, and in particular
more than 70% by weight, of the overall molar mass of
such nonionic surfactants. Preferred rinse aid
compositions comprise ethoxylated and propoxylated
nonionic surfactants wherein the propylene oxide units in
the molecule account for up to 25% by weight, preferably
up to 20% by weight, and in particular up to 15% by
weight, of the overall molecular mass of the nonionic
surfactant.
Further nonionic surfactants whose use is particularly
preferred, with melting points above room temperature,
contain from 40 to 70% of a polyoxypropylene/
polyoxyethylene/polyoxypropylene block polymer blend
which comprises 75% by weight of an inverted block
copolymer of polyoxyethylene and polyoxypropylene
19
CA 02320500 2000-09-18
containing 17 mol of ethylene oxide and 44 mol of
propylene oxide and 25% by weight of a block copolymer of
polyoxyethylene and polyoxypropylene, initiated with
trimethylolpropane and containing 24 mol of ethylene
oxide and 99 mol of propylene oxide per mole of
trimethylolpropane.
Nonionic surfactants which may be used with particular
preference are, for example, obtainable under the name
Poly Tergent~ SLF-18 from Olin Chemicals.
A further preferred rinse aid composition of the
invention comprises nonionic surfactants of the formula
R10 [CHZCH (CH3) O] X [CH2CH20] y [CH2CH (OH) R2]
in which R1 is a linear or branched aliphatic hydrocarbon
radical having 4 to 18 carbon atoms, or mixtures thereof,
R2 is a linear or branched hydrocarbon radical having 2
to 26 carbon atoms, or mixtures thereof, and x is between
0.5 and 1.5, and y is at least 15.
Further nonionic surfactants which may be used with
preference are the endgroup-capped poly(oxyalkylated)
nonionic surfactants of the formula
R10 [ CHzCH ( R3 ) O ] X [ CHZ ] kCH ( OH ) [ CH2 ] ~ ORz
in which R1 arid RZ are linear or branched, saturated or
unsaturated, aliphatic or aromatic hydrocarbon radicals
having 1 to 3 0 carbon atoms , R3 i s H or a methyl , ethyl ,
n-propyl, isopropyl, n-butyl, 2-butyl or 2-methyl-2-butyl
radical, x is between 1 and 30, k and j are between 1 and
12, preferably between 1 and 5. Where x >_ 2, each R3 in
the above formula may be different. R1 and RZ are
preferably linear or branched, saturated or unsaturated,
aliphatic or aromatic hydrocarbon radicals having 6 to 22
CA 02320500 2000-09-18
carbon atoms, radicals having 8 to 18 carbon atoms being
particularly preferred. For the radical R3, H, -CH3 or
CHZCH3 are particularly preferred. Particularly preferred
values for x lie within the range from 1 to 20, in
particular from 6 to 15.
As described above, each R3 in the above formula may be
different if x >_ 2. By this means it is possible to vary
the alkylene oxide unit in the square brackets. If x, for
example, is 3, the radical R3 may be selected in order to
form ethylene oxide (R3 - H), or propylene oxide (R3 -
CH3) units, which may be added on to one another in any
sequence, examples being (EO)(PO)(EO), (EO)(EO)(PO),
(EO) (EO) (EO) , (PO) (EO) (PO) , (PO) (PO) (EO) and
(PO)(PO)(PO). The value of 3 for x has been chosen by way
of example in this case and it is entirely possible for
it to be larger, the scope for variation increasing as
the values of x go up and embracing, for example, a large
number of (EO) groups, combined with a small number of
(PO) groups, or vice versa.
Particularly preferred endgroup-capped poly(oxyalkylated)
alcohols of the above formula have values of k = 1 and j
- 1, thereby simplifying the above formula to
R10 [CHzCH (R3) O] XCHzCH (OH) CH20R2
In the last-mentioned formula, Rl, Rz and R3 are as
defined above and x stands for numbers from 1 to 30,
preferably from 1 to 20, and in particular from 6 to 18.
Particular preference is given to surfactants wherein the
radicals R1 and RZ have 9 to 14 carbon atoms, R3 is H, and
x adopts values from 6 to 15.
Summarizing the last-mentioned statements, preference is
given to rinse aid compositions of the invention
21
CA 02320500 2000-09-18
comprising endgroup-capped poly(oxyalkylated) nonionic
surfactants of the formula
R10 [CHZCH (R3) O] X [CHZ] kCH (OH) [CHz] ~ORZ
in which R1 and R2 are linear or branched, saturated or
unsaturated, aliphatic or aromatic hydrocarbon radicals
having 1 to 30 carbon atoms, R3 is H or a methyl, ethyl,
n-propyl, isopropyl, n-butyl, 2-butyl or 2-methyl-2-butyl
radical, x is between 1 and 30, k and j are between 1 and
12, preferably between 1 and 5, particular preference
being given to surfactants of the type
R10 [ CH2CH ( R3 ) O ] XCHZ CH ( OH ) CH20R2
where x is from 1 to 30, preferably from 1 to 20, and in
particular from 6 to 18.
Instead of the aforementioned surfactants, or in
conjunction with them, it is also possible to use
cationic and/or amphoteric surfactants. In summary,
preference is given to rinse aid compositions of the
invention comprising surfactant(s), preferably nonionic
surfactant (s) , and especially nonionic surfactant (s) from
the group of the alkoxylated alcohols, in amounts of from
0.1 to 40°s by weight, preferably from 0.5 to 30% by
weight, with particular preference from 1 to 20% by
weight, and in particular from 2 t.o 15~ by weight, based
in each case on the rinse aid composition.
Nonaqueous solvents which may be used in the compositions
of the invention come, for example, from the group of
monohydric or polyhydric alcohols, alkanolamines or
glycol ethers, provided they are miscible with water in
the stated concentration range. The solvents are
preferably selected from the ethanol, n- or i-propanol,
butanols, glycol, propane- or butanediol, glycerol,
22
CA 02320500 2000-09-18
diglycol, propyl- or butyldiglycol, hexylene glycol,
ethylene glycol methyl ether, ethylene glycol ethyl
ether, ethylene glycol propyl ether, ethylene glycol
mono-n-butyl ether, diethylene glycol methyl ether,
diethylene glycol ethyl ether, propylene glycol methyl,
ethyl or propyl ether, dipropylene glycol methyl or ethyl
ether, methoxy-, ethoxy- or butoxytriglycol,
1-butoxyethoxy-2-propanol, 3-methyl-3-methoxybutanol,
propylene glycol t-butyl ether, and mixtures of these
solvents groups, so that preferred rinse aid compositions
are those comprising nonaqueous solvent(s), preferably
ethanol, n-propanol, i-propanol, 1-butanol, 2-butanol,
glycol, propanediol, butanediol, glycerol, diglycol,
propyl diglycol, butyl diglycol, hexylene glycol,
ethylene glycol methyl ether, ethylene glycol ethyl
ether, ethylene glycol propyl ether, ethylene glycol
mono-n-butyl ether, diethylene glycol methyl ether,
diethylene glycol ethyl ether, propylene glycol methyl,
ethyl or propyl ether, dipropylene glycol methyl or ethyl
ether, methoxy-, ethoxy- or butoxytriglycol,
1-butoxyethoxy-2-propanol, 3-methyl-3-methoxybutanol,
propylene glycol t-butyl ether, and mixtures of these
solvents.
The rinse aid compositions of the present invention may
further comprise hydrotropes. The effect of adding such
substances is that a substance of poor solubility becomes
soluble in water in the presence of the hydrotrope, which
is not itself a solvent. Substances which bring about
such an improvement in solubility are known as
hydrotropes or hydrotropic agents. Typical hydrotropes,
for example, in the formulation of liquid laundry
detergents or cleaning products, are xylene-sulfonate and
cumenesulfonate. Other substances, e.g., urea or
N-methylacetamide, raise the solubility through a
structure-disrupting effect, where the water structure in
23
CA 02320500 2000-09-18
the environment of the hydrophobic group of a poorly
soluble substance is broken down.
Rinse aid compositions that are preferred in the context
of the present invention comprise solubilizers,
preferably aromatic sulfonates of the formula
R~
R3~~ \~SO3 X+
R~'
in which each of the radicals Rl, R2, R3, R4 and Rs
independently of one another is selected from H or a Cl_s
alkyl or alkenyl radical and X is a cation.
Preferred substituents R1, Rz, R3, R4 and Rs are selected
independently of one another from H or a methyl, ethyl,
n-propyl, iso-propyl, n-butyl, iso-butyl, tert-butyl,
n-pentyl, iso-pentyl or neo-pentyl radical. In general,
at least three of said radicals R1 to Rs are hydrogen
atoms, preference being given to aromatic sulfonates in
which three or four substituents on the aromatic ring are
hydrogen atoms. The remaining radical or the remaining
two radicals may adopt any position with respect to the
sulfonate group and to one another. In the case of
monosubstituted compounds of the formula I, it is
preferred if the radical R3 is an alkyl radical while R1,
Rz, R4 and Rs are H (para-substitution) .
Particularly preferred aromatic sulfonates in the context
of the present invention are toluene-, cumene- or
xylenesulfonate.
24
CA 02320500 2000-09-18
Of the two industrially available toluenesulfonates
(ortho- and para-toluenesulfonate), the para isomer is
preferred in the context of the present invention. With
the cumenesulfonates, as well, the para-
isopropylbenzenesulfonate is the preferred compound.
Since xylene is used industrially usually as an isomer
mixture, the industrially available xylenesulfonate is
also a mixture of a plurality of compounds which result
from the sulfonation of ortho-, meta- and para-xylene.
Predominant in these isomer mixtures are the compounds in
which, in each case, the following radicals in the above
general formula are methyl groups (all other radicals are
H) : R1 and R2, R1 and R4, R1 and R3, and R1 and R5. In the
case of the xylenesulfonates, accordingly, there is
preferably at least one methyl group ortho to the
sulfonate group.
X in the general formula indicated above is a ration, for
example, an alkali metal ration such as sodium or
potassium. X may alternatively be the charge-equivalent
fraction of a polyvalent ration, for example, Mg2+/2 or
A13+/3, preference among the aforementioned rations being
given to sodium.
In accordance with the invention, the sulfonates are used
preferably in amounts of from 0.2 to 10% by weight, more
preferably from 0.3 to 5% by weight, and in particular
from 0.5 to 3% by weight, based in each case on the rinse
aid composition.
Acidifiers as well may be added to the rinse aid
compositions of the invention in order to lower the pH of
the liquor in the rinse cycle. Appropriate compounds here
include both organic and inorganic acids, provided they
are compatible with the other ingredients. For reasons of
protecting the consumer and of safe handling, the solid
CA 02320500 2000-09-18
mono-, oligo- and polycarboxylic acids may be used in
particular. Preferred in turn from this group are citric
acid, tartaric acid, succinic acid, malonic acid, adipic
acid, malefic acid, fumaric acid, oxalic acid, and
polyacrylic acid. Organic sulfonic acids such as
amidosulfonic acid may likewise be used. A commercially
available compound which is likewise preferred for use as
an acidifier in the context of the present invention is
Sokalan~ DCS (trademark of BASF), a mixture of succinic
acid (max. 31% by weight), glutaric acid (max. 50~ by
weight) and adipic acid (max. 33~ by weight). Rinse aid
compositions comprising acidifiers, preferably organic
acids, with particular preference adipic acid, amido-
sulfonic acid, succinic acid, citric acid, fumaric acid,
malefic acid, malonic acid, oxalic acid, and tartaric
acid, and also mixtures of these acids, are preferred
embodiments of the present invention.
In addition, the rinse aid compositions of the invention
may preferably include one or more substances from the
groups of the soil release polymers, the dyes, and the
fragrances. These are described in detail later on below.
The rinse aid compositions described above are suitable
for dosing via the reservoir tank of standard commercial
dishwashing machines. The use of the diquaternary
polysiloxanes that is preferred in accordance with the
invention may, however, also be actualized by providing
dissolution-retarded forms which are metered into the
dishwashing machine by the user before the beginning of
the wash, but which do not release the active substances
until the rinse cycle. This has the advantage that the
user need only add one product, instead of two as
previously.
The present invention further provides, in addition, a
particulate rinse aid for machine dishwashing, comprising
26
CA 02320500 2000-09-18
a) from 0 to 65% by weight of one or more support
materials,
b) from 30 to 70% by weight of coating substance (s)
having a melting point of more than 50°C,
c) from 0 to 65% by weight of fatty substance(s),
d) from 0 to 50% by weight of further active
substances and/or auxiliaries, and
e) from 0.1 to 70% by weight of diquaternary
polysiloxane.
Suitable support materials a) are all substances which
are solid at room temperature. Normally, the substances
selected will be those which develop an additional
activity in the wash, builders being particularly
appropriate. In preferred particulate rinse aids
containing support materials, the support materials
present comprise substances from the group of the water-
soluble detergent ingredients, preferably carbonates,
hydrogen carbonates, sulfates, phosphates, and the
organic oligocarboxylic acids that are solid at room
temperature, in amounts of from 35 to 60% by weight,
preferably from 40 to 55% by weight, and in particular
from 45 to 50% by weight, based in each case on the
particle weight.
The abovementioned preferred support materials are
described in detail later on below.
The particulate rinse aids may also be formulated without
support materials and thus consist only of ingredients b)
to e) . Such components are generally produced by shaping
a melted mixture of the ingredients. Rinse aid particles
containing support material may be obtained, for example,
by spraying or pouring such a melt onto the support
materials.
27
CA 02320500 2000-09-18
The production processes are described later on below;
there now follows a description of the ingredients b) to
d), the amounts relating to rinse aid particles without
support material. If support-material-based particles are
to be produced, the data below refer to the composition
of the mixture/melt that is applied to the supports, the
fraction in the particle resulting from the fractions of
mixture and of support material in the particle.
The coating substances used in the rinse aid compositions
of the invention are subject to a variety of
requirements, relating on the one hand to the melting
behavior or, respectively, solidification behavior but on
the other hand also to the material properties of the
coating in the solidified state, i.e., in the rinse aid
particles. Since the rinse aid particles are to be
durably protected against ambient influences in transit
or storage, the coating substance must possess a high
stability with respect, for example, to impacts occurring
in the course of packaging or transport. The coating
substance should, therefore, have either at least
partially elastic or at least plastic properties, in
order to react by elastic or plastic deformation to any
impact that does occur, and not to become crushed. The
coating substance should have a melting range
(solidification range) situated within a temperature
range in which the active ingredients to be coated are
not exposed to any excessive thermal load. On the other
hand, however, the melting range must be sufficiently
high still to offer effective protection for the enclosed
active substances at least at slightly elevated
temperature. In accordance with the invention, the
coating substances have a melting point above 30°C.
It has proven advantageous for the coating substance not
to exhibit a sharply defined melting point, as
encountered commonly with pure, crystalline substances,
28
CA 02320500 2000-09-18
but instead to have a melting range which covers, in some
cases, several degrees Celsius.
The coating substance preferably has a melting range
which lies between about 45°C and about 75°C. In the
present case that means that the melting range occurs
within the stated temperature interval, and does not
denote the width of the melting range. The width of the
melting range is preferably at least 1°C, more preferably
from about 2 to about 3°C.
The abovementioned properties are in general possessed by
what are called waxes. The term "waxes" is applied to a
range of natural or synthetic substances which melt
without decomposition, generally at above 40°C, and are
of comparatively low viscosity, without stringing, even
at just a little above the melting point. They have a
highly temperature-dependent consistency and solubility.
According to their origin, the waxes are divided into
three groups: the natural waxes, chemically modified
waxes, and the synthetic waxes.
The natural waxes include, for example, plant waxes such
as candelilla wax, carnauba wax, Japan wax, esparto grass
wax, cork wax, guaruma wax, rice germ oil wax, sugar cane
wax, ouricury wax, or montan wax, animal waxes such as
beeswax, shellac wax, spermaceti, lanolin (wool wax), or
uropygial grease, mineral waxes such as ceresin or
ozokerite (earth wax), or petrochemical waxes such as
petrolatum, paraffin waxes or microcrystalline waxes.
The chemically modified waxes include, for example, hard
waxes such as montan ester waxes, sassol waxes, or
hydrogenated jojoba waxes.
29
CA 02320500 2000-09-18
By synthetic waxes are meant, in general, polyalkylene
waxes or polyalkylene glycol waxes. As coating material
it is also possible to use compounds from other classes
of substance which meet the stated requirements in terms
of softening point. Examples of synthetic compounds which
have proven suitable are higher esters of phthalic acid,
especially dicyclohexyl phthalate, which is available
commercially under the name Unimoll~ 66 (Bayer AG). Also
suitable are synthetically prepared waxes from lower
carboxylic acids and fatty alcohols, an example being
dimyristyl tartrate, which is available under the name
Cosmacol~ ETLP (Condea). Conversely, synthetic or
partially synthetic esters of lower alcohols with fatty
acids from natural sources may also be used. This class
of substance includes, for example, Tegin~ 90
(Goldschmidt), a glyceryl monostearate palmitate. Shellac
as well, for example, Schellack-KPS-Dreiring-SP (Kalkhoff
GmbH), may be used according to the invention as a
coating material.
Likewise counted among the waxes in the context of the
present invention are, for example, the so-called wax
alcohols. Wax alcohols are relatively high molecular
mass, water-insoluble fatty alcohols having in general
from about 22 to 40 carbon atoms. The wax alcohols occur,
for example, in the form of wax esters of relatively high
molecular mass fatty acids (wax acids) as a principal
constituent of many natural waxes. Examples of wax
alcohols are lignoceryl alcohol (1-tetracosanol), cetyl
alcohol, myristyl alcohol, and melissyl alcohol. The
coating of the particulate solids coated in accordance
with the invention may, if desired, also include wool wax
alcohols, by which are meant triterpenoid and steroid
alcohols, an example being lanolin, which is available
under the commercial designation Argowax~ (Parentier &
Co.), for example. Likewise possible for use, at least
proportionally, as a constituent of the coating are, in
CA 02320500 2000-09-18
the context of the present invention, fatty acid glycerol
esters or fatty acid alkanolamides, and also, if desired,
water-insoluble or only sparingly water-soluble
polyalkylene glycol compounds.
Preferably, the coating substance particles present in
the rinse aid of the invention comprises a predominant
paraffin wax fraction. That means that at least 50% by
weight of the total coating substances present,
preferably more, consist of paraffin wax. Particularly
suitable are paraffin wax contents (based on total
coating substance) of approximately 60% by weight,
approximately 70% by weight or approximately 80% by
weight, with even higher proportions, of, for example,
more than 90% by weight being particularly preferred. In
one particular embodiment of the invention, the total
amount of the coating substance used consists exclusively
of paraffin wax.
Relative to the other, natural waxes mentioned, paraffin
waxes have the advantage in the context of the present
invention that in an alkaline detergent environment there
is no hydrolysis of the waxes (as is to be expected, for
example, with the wax esters), since paraffin wax
contains no hydrolyzable groups.
Paraffin waxes consist primarily of alkanes, with small
fractions of isoalkanes and cycloalkanes. The paraffin
for use in accordance with the invention preferably
contains essentially no constituents having a melting
point above 70°C, with particular preference above 60°C.
Below this melting temperature, in the detergent liquor,
fractions of high-melting alkanes in the paraffin may
leave unwanted wax residues on the surfaces to be cleaned
or on the ware to be cleaned. Wax residues of this kind
lead in general to an unattractive appearance of the
cleaned surface and should therefore be avoided.
31
CA 02320500 2000-09-18
Preferred particulate rinse aids comprise as coating
substance at least one paraffin wax having a melting
range of from 50°C to 60°C.
Preferably, the amount of alkanes, isoalkanes and
cycloalkanes which are solid at ambient temperature
(generally from about 10 to about 30°C) in the paraffin
wax used is as high as possible. The larger the amount of
solid wax constituents in a wax at room temperature, the
more useful that wax is in the context of the present
invention. As the proportion of solid wax constituents
increases, there is an increase in the resistance of the
rinse aid particles to impacts or friction against other
surfaces, resulting in a longer-lasting protection of the
particles of active substances. High proportions of oils
or liquid wax constituents may cause weakening of the
particles as a result of which pores are opened and the
active substances are exposed to the ambient influences
mentioned at the outset.
In addition to paraffin, the coating substance may
further comprise one or more of the abovementioned waxes
or waxlike substances as main ingredient. In principle,
the mixture forming the coating substance should be such
that the rinse aid particles are at least substantially
water-insoluble. At a temperature of about 30°C, the
solubility in water should not exceed about 10 mg/1 and
preferably should be below 5 mg/1.
In any case, however, the coating should preferably have
as low a solubility in water as possible, even in water
at elevated temperature, in order as far as possible to
avoid temperature-independent release of the active
substances.
32
CA 02320500 2000-09-18
The principle described above is used for the delayed
release of ingredients at a particular point in time in
the cleaning operation and may be employed with
particular advantage if washing is carried out in the
main wash cycle at a relatively low temperature (for
example, 55°C), so that the active substance is not
released from the rinse aid particles until the rinse
cycle at higher temperatures (approximately 70°C).
Preferred particulate rinse aids comprise as coating
substance one or more substances having a melting range
of between 40°C and 75°C in amounts of from 6 to 30% by
weight, preferably from 7.5 to 25% by weight and in
particular from 10 to 20% by weight, based in each case
on the particle weight, particulate rinse aids being
preferred which comprise as ingredient b) one or more
substances having a melting range of between 50 and
100°C, preferably between 52.5 and 80°C, and in
particular between 55 and 75°C, paraffin waxes having a
melting range of from 50°C to 65°C and/or substances from
the group of the polyethylene glycols (PEGs) and/or
polypropylene glycols (PPGs) being preferred coating
substances.
As a third ingredient, the detergent components of the
invention comprise one or more fatty substances, with
preferred detergent components comprising as ingredient
c) from 1 to 60, preferably from 5 to 55, with particular
preference from 10 to 50, and in particular from 20 to
45% by weight of fatty substance(s).
In the context of this specification, fatty substances c)
are substances which at standard temperature (20°C) are
liquid to solid and come from the group of the fatty
alcohols, fatty acids and fatty acid derivatives,
especially the fatty acid esters. Reaction products of
fatty alcohols with alkylene oxides, and the salts of
33
CA 02320500 2000-09-18
fatty acids, are included for the purposes of the present
specification among the surfactants (see above) and are
not fatty substances in the sense of the invention. Fatty
substances which may be used with preference in
accordance with the invention are fatty alcohols and
fatty alcohol mixtures, fatty acids and fatty acid
mixtures, fatty acid esters with alkanols and/or diols
and/or polyols, fatty acid amides, fatty amines, etc.
Preferred detergent components comprise as ingredient c)
one or more substances from the groups of the fatty
alcohols, fatty acids, and fatty esters.
Fatty alcohols used are, for example, the alcohols
obtainable from natural fats and oils: 1-hexanol (caproyl
alcohol), 1-heptanol (enanthyl alcohol), 1-octanol
(capryl alcohol), 1-nonanol (pelargonyl alcohol), 1-
decanol (capric alcohol), 1-undecanol, 10-undecen-1-ol,
1-dodecanol (lauryl alcohol), 1-tridecanol, 1-
tetradecanol (myristyl alcohol), 1-pentadecanol, 1-
hexadecanol (cetyl alcohol), 1-heptadecanol,
1-octadecanol (stearyl alcohol), 9-cis-octadecen-1-of
(oleyl alcohol), 9-trans-octadecen-1-of (elaidyl
alcohol), 9-cis-octadecene-1,12-diol (ricinolyl alcohol),
all-cis-9,12-octadecadien-1-of (linoleyl alcohol), all-
cis-9,12,15-octadecatrien-1-of (linolenyl alcohol), 1-
nonadecanol, 1-eicosanol (arachidyl alcohol), 9-cis-
eicosen-1-of (gadoleyl alcohol), 5,8,11,14-eicosatetraen-
1-0l, 1-heneicosanol, 1-docosanol (behenyl alcohol), 13-
cis-docosen-1-of (erucyl alcohol), 13-trans-docosen-1-of
(brassidyl alcohol), and mixtures of these alcohols. In
accordance with the invention, guerbet alcohols and oxo
alcohols, for example, C13-is oxo alcohols or mixtures of
Clz-la alcohols with Clz-14 alcohols can also be used
without problems as fatty substances. However, it is of
course also possible to use alcohol mixtures, for example
those such as the Cls-la alcohols prepared by Ziegler
34
CA 02320500 2000-09-18
ethylene polymerization. Specific examples of alcohols
which may be used as component c) are the alcohols
already mentioned above and also lauryl alcohol, palmityl
alcohol and stearyl alcohol, and mixtures thereof.
Particularly preferred detergent components of the
invention comprise as ingredient c) one or more Clo-ao
fatty alcohols, preferably Clz-z4 fatty alcohols, with
particular preference 1-hexadecanol, 1-octadecanol,
9-cis-octadecen-1-ol, all-cis-9,12-octadecadien-1-ol,
all-cis-9,12,15-octadecatrien-1-ol, 1-docosanol, and
mixtures thereof.
As ingredient c) it is also possible to use fatty acids.
Industrially, these are obtained primarily from natural
fats and oils by hydrolysis. Whereas the alkaline
saponification, conducted as long ago as the last
century, led directly to the alkali metal salts (soaps),
nowadays only water is used industrially to cleave the
fats into glycerol and the free fatty acids. Examples of
processes employed industrially are cleavage in an
autoclave or continuous high-pressure cleavage.
Carboxylic acids which may be used as fatty substances in
the context of the present invention are, for example,
hexanoic acid (caproic acid), heptanoic acid (enanthic
acid), octanoic acid (caprylic acid), nonanoic acid
(pelargonic acid), decanoic acid (capric acid),
undecanoic acid etc. Preference is given in the context
of the present invention to the use of fatty acids such
as dodecanoic acid (lauric acid), tetradecanoic acid
(myristic acid), hexadecanoic acid (palmitic acid),
octadecanoic acid (stearic acid), eicosanoic acid
(arachidic acid), docosanoic acid (behenic acid),
tetracosanoic acid (lignoceric acid), hexacosanoic acid
(cerotic acid), triacontanoic acid (melissic acid) and
also the unsaturated species 9c-hexadecenoic acid
(palmitoleic acid), 6c-octadecenoic acid (petroselinic
CA 02320500 2000-09-18
acid), 6t-octadecenoic acid (petroselaidic acid), 9c-
octadecenoic acid (oleic acid), 9t-octadecenoic acid
(elaidic acid), 9c,12c-octadecadienoic acid (linoleic
acid), 9t,12t-octadecadienoic acid (linolaidic acid), and
9c,12c,15c-octadecatrienoic acid (linolenic acid). Also
possible for use, of course, are tridecanoic acid,
pentadecanoic acid, margaric acid, nonadecanoic acid,
erucic acid, eleostearic acid, and arachidonic acid. For
reasons of cost it is preferred to use not the pure
species but rather technical-grade mixtures of the
individual acids as obtainable from fat cleavage. Such
mixtures are, for example, coconut oil fatty acid
(approximately 6% by weight Ca, 6% by weight Clo, 48% by
weight C12, 18% by weight C14, 10% by weight C16, 2% by
weight Cla, 8% by weight Cla,, 1% by weight Cla~~) , palm
kernel oil fatty acid (approximately 4% by weight Ca, 5%
by weight Clo, 50% by weight C12, 15% by weight C14, 7% by
weight C16, 2% by weight Cla, 15% by weight Cla, , 1% by
weight C18"), tallow fatty acid (approximately 3% by
weight C14, 26% by weight C16, 2% by weight Cls~, 2% by
weight C1~, 17% by weight Cla, 44% by weight Cla,, 3% by
weight Cla", 1% by weight C18~ , , ) , hardened tallow fatty
acid (approximately 2% by weight C14, 28% by weight Cls,
2% by weight Cl~, 63% by weight Cla, 1% by weight Cla~ ) ,
technical-grade oleic acid (approximately 1% by weight
C12, 3% by weight C14, 5% by weight C16, 6% by weight Cls~.
1% by weight Cl-,, 2% by weight Cla, 70% by weight Cla,, 10%
by weight Cla~~, 0 . 5% by weight Cla~ ~ ~ ) , technical-grade
palmitic/stearic acid (approximately 1% by weight C12, 2%
by weight C14, 45% by weight C16, 2% by weight Cl-,, 47% by
weight C18, 1% by weight Cla~ ) , and soybean oil fatty acid
(approximately 2% by weight C14, 15% by weight C16, 5% by
weight C18, 25% by weight Cla~, 45% by weight Cla~~, 7% by
weight Cla ~ ~ ~ ) .
As fatty acid esters, use may be made of the esters of
fatty acids with alkanols, diols or polyols, fatty acid
36
CA 02320500 2000-09-18
polyol esters being preferred. Suitable fatty acid polyol
esters include monoesters and diesters of fatty acids
with certain polyols. The fatty acids that are esterified
with the polyols are preferably saturated or unsaturated
fatty acids of 12 to 18 carbon atoms, examples being
lauric acid, myristic acid, palmitic acid, and stearic
acid, preference being given to the use of the fatty acid
mixtures obtained industrially, for example, the acid
mixtures derived from coconut oil, palm kernel oil or
tallow fat. In particular, acids or mixture of acids
having 16 to 18 carbon atoms, such as tallow fatty acid,
for example, are suitable for esterification with the
polyhydric alcohols. In the context of the present
invention, suitable polyols for esterification with the
aforementioned fatty acids include sorbitol,
trimethylolpropane, neopentyl glycol, ethylene glycol,
polyethylene glycols, glycerol, and polyglycerols.
Preferred embodiments of the present invention provide
for the polyol esterified with fatty acids) to be
glycerol. Accordingly, preference is given to detergent
components of the invention comprising as ingredient c)
one or more fatty substances from the group consisting of
fatty alcohols and fatty acid glycerides. Particularly
preferred detergent components comprise as component c) a
fatty substance from the group consisting of the fatty
alcohols and fatty acid monoglycerides. Examples of such
fatty substances used with preference are glyceryl
monostearate and glyceryl monopalmitate.
The particulate rinse aids of the invention may
preferably comprise as ingredient d) further active
substances and/or auxiliaries from the groups of the
surfactants, bleaches, bleach activators, soil release
polymers, enzymes, silver protectants, complexing agents,
dyes and fragrances in amounts of from 0 to 50~ by
weight, preferably from 2.5 to 45~ by weight, with
37
CA 02320500 2000-09-18
particular preference from 5 to 40% by weight, and in
particular from 10 to 30% by weight.
Particular preference is given in this case to the use of
the surfactants described earlier on above, so that
preferred particulate rinse aids further comprise as
ingredient d) surfactant(s), preferably nonionic
surfactant(s), with particular preference those from the
group of the alkoxylated alcohols, in amounts of from 5
to 47.5% by weight, preferably from 10 to 45% by weight,
with particular preference from 15 to 42.5% by weight,
and in particular from 20 to 40% by weight, based in each
case on the particulate rinse aid.
The other detergent ingredients which may be incorporated
as component d) into the rinse aids of the invention are
described in detail later on below.
As ingredient e), the particulate rinse aids of the
invention comprise diquaternary polysiloxanes, the
abovementioned remarks concerning preferred species
continuing to apply in their entirety. In order to avoid
redundancy, therefore, reference is made to the formulae
given above. Preferred particulate rinse aids comprise
diquaternary polysiloxanes, preferably diquaternary
polysiloxanes of the formula I, with particular
preference diquaternary poly(dimethyl-siloxanes) of the
formula II, and in particular diquaternary
poly(dimethylsiloxanes) of the formula III, in amounts of
from 0.5 to 60% by weight, preferably from 1 to 50% by
weight, with particular preference from 2.5 to 40% by
weight, and in particular from 5 to 30% by weight, based
in each case on the particulate rinse aid.
The present invention additionally provides a process for
preparing particulate detergent components, which
comprises applying a melt comprising
38
CA 02320500 2000-09-18
a) from 30 to 70% by weight of coating substances)
having a melting point above 50°C,
b) from 0 to 65% by weight of fatty substance(s),
c) from 0 to 50% by weight of further active
substances and/or auxiliaries, and
d) from 0.1 to 70% by weight of diquaternary
polysiloxanes
to one or more support materials and shaping the mixture.
In this process variant, first of all a melt is prepared,
which may include further active substances and
auxiliaries. This melt is applied to a support material
and shaped as a mixture with said support material.
With the abovementioned preparation process for the rinse
aid particles of the invention, preferred process
variants are those wherein the meltable substance
accounts for from 25 to 85% by weight, preferably from 30
to 70% by weight, and in particular from 40 to 50% by
weight of the melt.
The application of the melt to the support material may
be conducted in all customary mixing equipment. The
shaping step for the mixture of melt and support material
is likewise not subject to any technical restriction, so
that here as well the skilled worker is able to select
from the processes customary to him or her. In the course
of experiments conducted by the applicant, processes
which have proven preferable are those wherein the
shaping takes place by granulating, compacting,
pelletizing, extruding, or tableting.
The process of the invention embraces the application of
melts comprising the ingredients a) to d) to support
materials. In principle, melt and support materials) may
be present in varying amounts in the resultant rinse aid
particles. In preferred processes, the mixture shaped
39
CA 02320500 2000-09-18
comprises from 5 to 50% by weight, preferably from 10 to
45% by weight, with particular preference from 15 to 40%
by weight, and in particular from 20 to 35% by weight of
a melt comprising the ingredients a) to d), and from 50
to 95% by weight, preferably from 55 to 90% by weight,
with particular preference from 60 to 85% by weight, and
in particular from 65 to 80% by weight, of support
material (s) .
Regarding the ingredients which are used in the process
of the invention and are processed to the support
material-based detergent components of the invention, the
comments made earlier on above apply analogously.
The detergent components of the invention may also be
formulated without support material, so that they consist
solely of the ingredients a) to d). In this case, for the
preparation of particulate detergent components of the
invention, prilling, pelletizing and flaking by means of
cooling rolls have proven particularly suitable.
The present invention therefore additionally provides, in
a first embodiment, a process for preparing prilled
detergent components, which comprises spraying a melt
comprising
a) from 30 to 70% by weight of coating substance (s)
having a melting point above 50°C,
b) from 0 to 65% by weight of fatty substance(s),
c) from 0 to 50% by weight of further active
substances and/or auxiliaries, and
d) from 0.1 to 70% by weight of diquaternary
polysiloxanes
into a cold gas stream.
The process of the invention, which is referred to for
short as prilling, comprises the production of granular
elements from meltable substances, the melt comprising
CA 02320500 2000-09-18
the ingredients a) to d) being sprayed in defined droplet
size at the top of a tower, solidifying in free fall, and
being obtained as prill granules at the base of the
tower.
As the cold gas stream it is possible in very general
terms to use all gases, the temperature of the gas being
below the melting temperature of the melt. In order to
avoid long falling sections, use is frequently made of
cooled gases, for example, supercooled air or even liquid
nitrogen, which is injected through a nozzle into the
spray towers.
The particle size of the resulting prills may be varied
by way of the choice of droplet size, with particle sizes
which are easy to realize technically lying within the
range from 0.5 to 2 mm, preferably around 1 mm.
An alternative process to prilling is pelletizing. A
further embodiment of the present invention therefore
envisages a process for preparing pelletized detergent
components, which comprises metering a melt comprising
a) from 30 to 70°s by weight of coating substance (s)
having a melting point above 50°C,
b) from 0 to 65~ by weight of fatty substance(s),
c) from 0 to 50s by weight of further active
substances and/or auxiliaries, and
d) from 0.1 to 70~ by weight of diquaternary
polysiloxanes
onto cooled pelletizing plates.
Pelletizing comprises the metering of the melt comprising
the ingredients a) to d) onto rotating, inclined plates
which have a temperature below the melting temperature of
the melt and are preferably cooled to below room
temperature. Here again, process variants may be
practiced in which the pelletizing plates are
41
CA 02320500 2000-09-18
supercooled. In this case, however, measures must be
taken to counter the condensation of atmospheric
moisture.
Pelletizing produces relatively large particles, which in
standard industrial processes have sizes of between 2 and
mm, preferably between 3 and 6 mm.
As an even more cost-effective variant for producing
10 particulate detergent components of the stated
composition from melts, the use of cooling rolls is
appropriate. A further subject of the present invention
is therefore a process for preparing particulate
detergent components, which comprises applying a melt
comprising
a) from 30 to 70% by weight of coating substance (s)
having a melting point above 50°C,
b) from 0 to 65% by weight of fatty substance(s),
c) from 0 to 50% by weight of further active
substances and/or auxiliaries, and
d) from 0.1 to 70% by weight of diquaternary
polysiloxanes
by spraying or otherwise to a cooling roll, scraping off
the solidified melt, and comminuting the scrapings if
necessary.
The use of cooling rolls permits ready establishment of
the desired particle size range, which in this process of
the invention may also be below 1 mm, for example from
200 to 700 ~,m.
The rinse aid particles of the invention may be given
directly to the consumer, who then doses them into the
detergent additionally as required. On the basis of this
additional dosing step, however, apart from the solid
supply form and the addition to the same dispenser, the
advantages relative to liquid rinse aid compositions
42
CA 02320500 2000-09-18
would be minimized. It is therefore preferred to admix
the rinse aid particles of the invention to particulate
machine dishwashing compositions.
The present invention therefore additionally provides a
particulate machine dishwashing composition comprising
builders and also, optionally, further ingredients from
the groups of the surfactants, enzymes, bleaches, bleach
activators, corrosion inhibitors, polymers, dyes, and
fragrances, which comprises a particulate rinse aid of
the invention in amounts of from 0.5 to 30% by weight,
preferably from 1 to 25% by weight, and in particular
from 5 to 15% by weight, based in each case on overall
composition.
The ingredients of the machine dishwashing compositions
are described hereinbelow. In some cases, they may also
be present as active substances or support materials in
the rinse aid particles of the invention.
The most important ingredients of machine dishwashing
compositions are builders. The machine dishwashing
detergents of the invention may comprise all of the
builders commonly used in detergents, i.e., in
particular, zeolites, silicates, carbonates, organic
cobuilders, and - where there are no ecological
prejudices against their use - the phosphates as well.
The builders mentioned below are all suitable as support
materials for the rinse aid particles of the invention,
as set out earlier on above.
Suitable crystalline, layered sodium silicates possess
the general formula NaMSiX02X+l~yHz~, where M is sodium or
hydrogen, x is a number from 1.9 to 4, y is a number from
0 to 20, and preferred values for x are 2, 3 or 4.
Crystalline phyllosilicates of this kind are described,
for example, in European Patent Application
43
CA 02320500 2000-09-18
EP-A-0 164 514. Preferred crystalline phyllosilicates of
the formula indicated are those in which M is sodium and
x adopts the value 2 or 3. In particular, both (3- and
8-sodium disilicates Na2Si205~yH20 are preferred, (3-sodium
disilicate, for example, being obtainable by the process
described in International Patent Application WO-A-
91/08171.
It is also possible to use amorphous sodium silicates
having an NazO:Si02 modulus of from 1:2 to 1:3.3,
preferably from 1:2 to 1:2.8, and in particular from 1:2
to 1:2.6, which are dissolution-retarded and have
secondary washing properties. The retardation of
dissolution relative to conventional amorphous sodium
silicates may have been brought about in a variety of
ways - for example, by surface treatment, compounding,
compacting, or overdrying. In the context of this
invention, the term "amorphous" also embraces "X-ray-
amorphous". This means that in X-ray diffraction
experiments the silicates do not yield the sharp X-ray
reflections typical of crystalline substances but instead
yield at best one or more maxima of the scattered X-
radiation, having a width of several degree units of the
diffraction angle. However, even particularly good
builder properties may result if the silicate particles
in electron diffraction experiments yield vague or even
sharp diffraction maxima. The interpretation of this is
that the products have microcrystalline regions with a
size of from 10 to several hundred nm, values up to max.
50 nm and in particular up to max. 20 nm being preferred.
So-called X-ray-amorphous silicates of this kind, which
likewise possess retarded dissolution relative to the
conventional waterglasses, are described, for example, in
German Patent Application DE-A-44 00 024. Particular
preference is given to compacted amorphous silicates,
compounded amorphous silicates, and overdried X-ray-
amorphous silicates.
44
CA 02320500 2000-09-18
The finely crystalline, synthetic zeolite used,
containing bound water, is preferably zeolite A and/or P.
A particularly preferred zeolite P is Zeolite MAP~
(commercial product from Crosfield). Also suitable,
however, are zeolite X and also mixtures of A, X and/or
P. Another product available commercially and able to be
used with preference in the context of the present
invention, for example, is a cocrystallizate of zeolite X
and zeolite A (approximately 80% by weight zeolite X),
which is sold by CONDEA Augusta S.p.A. under the brand
name VEGOBOND AX~ and may be described by the formula
nNa20~ (1-n) KzO~A1203~ (2-2 .5) Si02~ (3 .5-5.5) HzO.
Suitable zeolites have an average particle size of less
than 10 ~.m (volume distribution; measurement method:
Coulter counter) and contain preferably from 18 to 22% by
weight, in particular from 20 to 22% by weight, of bound
water.
Of course, the widely known phosphates may also be used
as builder substances provided such a use is not to be
avoided on ecological grounds . Among the large number of
commercially available phosphates, the alkali metal
phosphates, with particular preference being given to
pentasodium and pentapotassium triphosphate (sodium and
potassium tripolyphosphate, respectively), possess the
greatest importance in the detergents industry.
Alkali metal phosphates is the collective term for the
alkali metal (especially sodium and potassium) salts of
the various phosphoric acids, among which metaphosphoric
acids (HP03)n and orthophosphoric acid H3P04, in addition
to higher-molecular-mass representatives, may be
distinguished. The phosphates combine a number of
advantages: they act as alkali carriers, prevent
CA 02320500 2000-09-18
limescale deposits on machine components, and lime
encrustations on fabrics, and additionally contribute to
cleaning performance.
Sodium dihydrogen phosphate, NaH2P04, exists as the
dehydrate (density 1.91 g cm-3, melting point 60°) and as
the monohydrate (density 2.04 g cm-3). Both salts are
white powders of very ready solubility in water which
lose the water of crystallization on heating and undergo
conversion at 200°C into the weakly acidic diphosphate
(disodium dihydrogen diphosphate, Na2H2P20~) and at a
higher temperature into sodium trimetaphosphate (Na3P309)
and Maddrell's salt (see below). NaHzP04 reacts
acidically; it is formed if phosphoric acid is adjusted
to a pH of 4.5 using sodium hydroxide solution and the
slurry is sprayed. Potassium dihydrogen phosphate
(primary or monobasic potassium phosphate, potassium
biphosphate, PDP) , KH2PO4, is a white salt with a density
of 2.33 g cm-3, has a melting point of 253° [decomposition
with formation of potassium polyphosphate (KP03)X], and is
readily soluble in water.
Disodium hydrogen phosphate (secondary sodium phosphate),
Na2HP04, is a colorless, crystalline salt which is very
readily soluble in water. It exists in anhydrous form and
with 2 mol (density 2.066 g cm-3, water loss at 95°), 7
mol (density 1.68 g cm-3, melting point 48° with loss of 5
H20), and 12 mol (density 1.52 g cm-3, melting point 35°
with loss of 5 Hz0) of water, becomes anhydrous at 100°,
and if heated more intensely undergoes transition to the
diphosphate Na4P20~. Disodium hydrogen phosphate is
prepared by neutralizing phosphoric acid with sodium
carbonate solution using phenolphthalein as indicator.
Dipotassium hydrogen phosphate (secondary or dibasic
potassium phosphate), K2HP04, is an amorphous white salt
which is readily soluble in water.
46
CA 02320500 2000-09-18
Trisodium phosphate, tertiary sodium phosphate, Na3P04,
exists as colorless crystals which as the dodecahydrate
have a density of 1.62 g cm-3 and a melting point of
73-76°C (decomposition), as the decahydrate
(corresponding to 19-20% P205) have a melting point of
100°C, and in anhydrous form (corresponding to 39-40%
PZOS) have a density of 2.536 g cm-3. Trisodium phosphate
is readily soluble in water, with an alkaline reaction,
and is prepared by evaporative concentration of a
solution of precisely 1 mol of disodium phosphate and
1 mol of NaOH. Tripotassium phosphate (tertiary or
tribasic potassium phosphate), K3P04, is a white,
deliquescent, granular powder of density 2.56 g cm-3, has
a melting point of 1340°, and is readily soluble in water
with an alkaline reaction. It is produced, for example,
when Thomas slag is heated with charcoal and potassium
sulfate. Despite the relatively high price, the more
readily soluble and therefore highly active potassium
phosphates are frequently preferred in the detergents
industry over the corresponding sodium compounds.
Tetrasodium diphosphate (sodium pyrophosphate), Na4P20-,,
exists in anhydrous form (density 2.534 g cm-3, melting
point 988°, 880° also reported) and as the decahydrate
(density 1.815-1.836 g cm-3, melting point 94° with loss
of water). Both substances are colorless crystals which
dissolve in water with an alkaline reaction. Na4P20~ is
formed when disodium phosphate is heated to > 200° or by
reacting phosphoric acid with sodium carbonate in
stoichiometric ratio and dewatering the solution by
spraying. The decahydrate complexes heavy metal salts and
water hardeners and therefore reduces the hardness of the
water. Potassium diphosphate (potassium pyrophosphate),
K4P20~, exists in the form of the trihydrate and is a
colorless, hygroscopic powder of density 2.33 g cm-3 which
is soluble in water, the pH of the 1% strength solution
at 25° being 10.4.
47
CA 02320500 2000-09-18
Condensation of NaH2P04 or of KH2P04 gives rise to higher-
molecular-mass sodium and potassium phosphates, among
which it is possible to distinguish cyclic
representatives, the sodium and potassium metaphos-
phates, and catenated types, the sodium and potassium
polyphosphates. For the latter in particular a large
number of names are in use: fused or calcined phosphates,
Graham's salt, Kurrol's and Maddrell's salt. All higher
sodium and potassium phosphates are referred to
collectively as condensed phosphates.
The industrially important pentasodium triphosphate,
Na5P301o (sodium tripolyphosphate), is a nonhygroscopic,
white, water-soluble salt which is anhydrous or
crystallizes with 6 H20 and has the general formula Na0-
[P (O) (ONa) -O] n-Na where n - 3 . About 17 g of the
anhydrous salt dissolve in 100 g of water at room
temperature, about 20 g at 60°, around 32 g at 100°;
after heating the solution at 100°C for two hours, about
8% orthophosphate and 15% diphosphate are produced by
hydrolysis. For the preparation of pentasodium
triphosphate, phosphoric acid is reacted with sodium
carbonate solution or sodium hydroxide solution in
stoichiometric ratio and the solution is dewatered by
spraying. In a similar way to Graham's salt and sodium
diphosphate, pentasodium triphosphate dissolves numerous
insoluble metal compounds (including lime soaps, etc.).
Pentapotassium triphosphate, KSP301o (potassium
tripolyphosphate), is commercialized, for example, in the
form of a 50% strength by weight solution (> 23% P205, 25%
K20). The potassium polyphosphates find broad application
in the detergents industry. There also exist sodium
potassium tripolyphosphates, which may likewise be used
for the purposes of the present invention. These are
formed, for example, when sodium trimetaphosphate is
hydrolyzed with KOH:
48
CA 02320500 2000-09-18
(NaP03) 3 + 2 KOH ~ Na3KzP301o + H20
They can be used in accordance with the invention in
precisely the same way as sodium tripolyphosphate,
potassium tripolyphosphate, or mixtures of these two;
mixtures of sodium tripolyphosphate and sodium potassium
tripolyphosphate, or mixtures of potassium
tripolyphosphate and sodium potassium tripolyphosphate,
or mixtures of sodium tripolyphosphate and potassium
tripolyphosphate and sodium potassium tripolyphospate,
may also be used in accordance with the invention.
Organic cobuilders which may be used in the machine
dishwashing compositions of the invention are, in
particular, polycarboxylates/polycarboxylic acids,
polymeric polycarboxylates, aspartic acid, polyacetals,
dextrins, further organic cobuilders (see below), and
phosphonates. These classes of substance are described
below.
Organic builder substances which may be used are, for
example, the polycarboxylic acids usable in the form of
their sodium salts, the term polycarboxylic acids meaning
those carboxylic acids which carry more than one acid
function. Examples of these are citric acid, adipic acid,
succinic acid, glutaric acid, malic acid, tartaric acid,
malefic acid, fumaric acid, sugar acids, amino carboxylic
acids, nitrilotriacetic acid (NTA), provided such use is
not objectionable on ecological grounds, and also
mixtures thereof. Preferred salts are the salts of the
polycarboxylic acids such as citric acid, adipic acid,
succinic acid, glutaric acid, tartaric acid, sugar acids,
and mixtures thereof.
The acids per se may also be used. In addition to their
builder effect, the acids typically also possess the
49
CA 02320500 2000-09-18
property of an acidifying component and thus also serve
to establish a lower and milder pH of detergents. In this
context, mention may be made in particular of citric
acid, succinic acid, glutaric acid, adipic acid, gluconic
acid, and any desired mixtures thereof.
Also suitable as builders are polymeric poly-
carboxylates; these are, for example, the alkali metal
salts of polyacrylic acid or of polymethacrylic acid,
examples being those having a relative molecular mass of
from 500 to 70,000 g/mol.
The molecular masses reported for polymeric poly-
carboxylates, for the purposes of this document, are
weight-average molecular masses, MW, of the respective
acid form, determined basically by means of gel
permeation chromatography (GPC) using a W detector. The
measurement was made against an external polyacrylic acid
standard, which owing to its structural similarity to the
polymers under investigation provides realistic molecular
weight values. These figures differ markedly from the
molecular weight values obtained using poly-
styrenesulfonic acids as the standard. The molecular
masses measured against polystyrenesulfonic acids are
generally much higher than the molecular masses reported
in this document.
Suitable polymers are, in particular, polyacrylates,
which preferably have a molecular mass of from 2000 to
20,000 g/mol. Owing to their superior solubility,
preference in this group may be given in turn to the
short-chain polyacrylates, which have molecular masses of
from 2000 to 10,000 g/mol, and with particular preference
from 3000 to 5000 g/mol.
Also suitable are copolymeric polycarboxylates,
especially those of acrylic acid with methacrylic acid
CA 02320500 2000-09-18
and of acrylic acid or methacrylic acid with malefic acid.
Copolymers which have been found particularly suitable
are those of acrylic acid with malefic acid which contain
from 50 to 90% by weight of acrylic acid and from 50 to
10% by weight of malefic acid. Their relative molecular
mass, based on free acids, is generally from 2000 to
70,000 g/mol, preferably from 20,000 to 50,000 g/mol, and
in particular from 30,000 to 40,000 g/mol.
The (co)polymeric polycarboxylates can be used either as
powders or as aqueous solutions. The (co)polymeric
polycarboxylate content of the compositions is preferably
from 0.5 to 20% by weight, in particular from 3 to 10% by
weight.
In order to improve the solubility in water, the polymers
may also contain allylsulfonic acids, such as
allyloxybenzenesulfonic acid and methallylsulfonic acid,
for example, as monomers.
Particular preference is also given to biodegradable
polymers comprising more than two different monomer
units, examples being those comprising, as monomers,
salts of acrylic acid and of malefic acid, and also vinyl
alcohol or vinyl alcohol derivatives, or those
comprising, as monomers, salts of acrylic acid and of
2-alkylallylsulfonic acid, and also sugar derivatives.
Further preferred copolymers are those whose monomers are
preferably acrolein ans acrylic acid salts, and,
respectively acrolein and vinyl acetate.
Similarly, further preferred builder substances that may
be mentioned include polymeric amino dicarboxylic acids,
their salts or their precursor substances. Particular
preference is given to polyaspartic acids and their salts
51
CA 02320500 2000-09-18
and derivatives, which have not only cobuilder properties
but also a bleach-stabilizing action.
Further suitable builder substances are polyacetals,
which may be obtained by reacting dialdehydes with polyol
carboxylic acids having 5 to 7 carbon atoms and at least
3 hydroxyl groups. Preferred polyacetals are obtained
from dialdehydes such as glyoxal, glutaraldehyde,
terephthalaldehyde and mixtures thereof and from polyol
carboxylic acids such as gluconic acid and/or
glucoheptonic acid.
Further suitable organic builder substances are dextrins,
examples being oligomers and polymers of carbohydrates,
which may be obtained by partial hydrolysis of starches.
The hydrolysis can be conducted by customary processes,
for example, acid-catalyzed or enzyme-catalyzed
processes. The hydrolysis products preferably have
average molecular masses in the range from 400 to 500,000
g/mol. Preference is given here to a polysaccharide
having a dextrose equivalent (DE) in the range from 0.5
to 40, in particular from 2 to 30, DE being a common
measure of the reducing effect of a polysaccharide in
comparison to dextrose, which possesses a DE of 100. It
is possible to use both maltodextrins having a DE of
between 3 and 20 and dried glucose syrups having a DE of
between 20 and 37, and also so-called yellow dextrins and
white dextrins having higher molecular masses, in the
range from 2000 to 30,000 g/mol.
The oxidized derivatives of such dextrins comprise their
products of reaction with oxidizing agents which are able
to oxidize at least one alcohol function of the
saccharide ring to the carboxylic acid function. A
product oxidized at C6 of the saccharide ring may be
particularly advantageous.
52
CA 02320500 2000-09-18
Oxydisuccinates and other derivatives of disuccinates,
preferably ethylenediamine disuccinate, are further
suitable cobuilders. Ethylenediamine N,N'-disuccinate
(EDDS) is used preferably in the form of its sodium or
magnesium salts. Further preference in this context is
given to glycerol disuccinates and glycerol trisuccinates
as well. Suitable use amounts in formulations containing
zeolite and/or silicate are from 3 to 15% by weight.
Examples of further useful organic cobuilders are
acetylated hydroxy carboxylic acids and their salts,
which may also, if desired, be present in lactone form
and which contain at least 4 carbon atoms, at least one
hydroxyl group, and not more than two acid groups.
A further class of substance having cobuilder properties
is represented by the phosphonates. The phosphonates in
question are, in particular, hydroxyalkane- and
aminoalkanephosphonates. Among the
hydroxyalkanephosphonates, 1-hydroxyethane-1,1-diphos-
phonate (HEDP) is of particular importance as a
cobuilder. It is used preferably as the sodium salt, the
disodium salt being neutral and the tetrasodium salt
giving an alkaline (pH 9) reaction. Suitable
aminoalkanephosphonates are preferably ethylenediamine-
tetramethylenephosphonate (EDTMP), diethylenetriamine-
pentamethylenephosphonate (DTPMP), and their higher
homologs. They are used preferably in the form of the
neutrally reacting sodium salts, e.g., as the hexasodium
salt of EDTMP or as the hepta- and octa-sodium salt of
DTPMP. As a builder in this case, preference is given to
using HEDP from the class of the phosphonates.
Furthermore, the aminoalkanephosphonates possess a
pronounced heavy metal binding capacity. Accordingly, and
especially if the compositions also contain bleach, it
may be preferred to use aminoalkanephosphonates,
53
CA 02320500 2000-09-18
especially DTPMP, or to use mixtures of said
phosphonates.
Furthermore, all compounds capable of forming complexes
with alkaline earth metal ions may be used as cobuilders.
Important ingredients of detergents in addition to the
builders are, in particular, substances from the groups
of the surfactants (see above), bleaches, bleach
activators, enzymes, polymers, fragrances, and dyes.
Important representatives from the aforementioned classes
of substance are described below.
Among the compounds used as bleaches which yield H202 in
water, particular importance is possessed by sodium
perborate tetrahydrate and sodium perborate monohydrate.
Examples of further bleaches which may be used are sodium
percarbonate, peroxy pyrophosphates, citrate perhydrates,
and also H202-donating peracidic salts or peracids, such
as perbenzoates, peroxophthalates, diperazelaic acid,
phthaloimino peracid, or diperdodecanedioic acid.
Detergents of the invention may also comprise bleaches
from the group of organic bleaches. Typical organic
bleaches are the diacyl peroxides, such as dibenzoyl
peroxide, for example. Further typical organic bleaches
are the peroxy acids, particular examples being the alkyl
peroxy acids and the aryl peroxy acids. Preferred
representatives are (a) peroxybenzoic acid and its ring-
substituted derivatives, such as alkylperoxybenzoic
acids, but also peroxy-a-naphthoic acid and magnesium
monoperphthalate, (b) aliphatic or substituted aliphatic
peroxy acids, such as peroxylauric acid, peroxystearic
acid, s-phthalimidoperoxy caproic acid
[phthaloiminoperoxyhexanoic acid (PAP)], o-
carboxybenzamidoperoxycaproic acid, N-
nonenylamidoperadipic acid and N-nonenylamido-
persuccinates, and (c) aliphatic and araliphatic peroxy
54
CA 02320500 2000-09-18
dicarboxylic acids, such as 1,12-diperoxydecane-
dicarboxylic acid, 1,9-diperoxyazelaic acid, diperoxy-
sebacic acid, diperoxybrassylic acid, the diperoxy-
phthalic acids, 2-decyldiperoxybutane-1,4-dioic acid and
N,N-terephthaloyldi(6-aminopercaproic acid) may also be
used.
Bleaches in the detergents of the invention for machine
dishwashing may also be substances which release chlorine
or bromine. Among the suitable chlorine- or bromine-
releasing materials examples include heterocyclic N-
bromoamides and N-chloroamides, examples being
trichloroisocyanuric acid, tribromoisocyanuric acid,
dibromoisocyanuric acid and/or dichloroisocyanuric acid
(DICA) and/or salts thereof with cations such as
potassium and sodium. Hydantoin compounds, such as 1,3-
dichloro-5,5-dimethylhydantoin, are likewise suitable.
In order to achieve an "after-bleaching" effect in the
rinse cycle, the abovementioned bleaches may also be
introduced into the machine dishwashing compositions of
the invention in whole or in part by way of the rinse aid
particles.
Bleach activators, which boost the action of the
bleaches, were mentioned earlier on above as a possible
ingredient of the rinse aid particles. Known bleach
activators are compounds containing one or more N-aryl
and/or O-acyl groups, such as substances from the class
of the anhydrides, esters, imides and acylated imidazoles
or oximes. Examples are tetraacetylethylenediamine TAED,
tetraacetylmethylene-diamine TAMD, and
tetraacetylhexylenediamine TAHD, and also
pentaacetylglucose PAG, 1,5-diacetyl-2,2-dioxohexahydro-
1,3,5-triazine DADHT, and isatoic anhydride ISA.
CA 02320500 2000-09-18
Bleach activators which may be used are compounds which
under perhydrolysis conditions give rise to aliphatic
peroxo carboxylic acids having preferably 1 to 10 carbon
atoms, in particular 2 to 4 carbon atoms, and/or
substituted or unsubstituted perbenzoic acid. Suitable
substances are those which carry O-acyl and/or N-acyl
groups of the stated number of carbon atoms, and/or
substituted or unsubstituted benzoyl groups. Preference
is given to polyacylated alkylenediamines, especially
tetraacetylethylenediamine (TAED), acylated triazine
derivatives, especially 1,5-diacetyl-2,4-dioxohexahydro-
1,3,5-triazine (DADHT), acylated glycolurils, especially
tetraacetylglycoluril (TAGU), N-acylimides, especially N-
nonanoylsuccinimide (NOSI), acylated phenolsulfonates,
especially n-nonanoyl- or isononanoyloxybenzenesulfonate
(n- or iso-NOBS), carboxylic anhydrides, especially
phthalic anhydride, acylated polyhydric alcohols,
especially triacetin, ethylene glycol diacetate, 2,5-
diacetoxy-2,5-dihydrofuran, N-
methylmorpholiniumacetonitrile methyl sulfate (MMA), and
the enol esters known from German Patent Applications DE
196 16 693 and DE 196 16 767, and also acetylated
sorbitol and mannitol and/or mixtures thereof (SORMAN),
acylated sugar derivatives, especially pentaacetylglucose
(PAG), pentaacetylfructose, tetraacetylxylose and
octaacetyllactose, and acetylated, optionally N-alkylated
glucamine and gluconolactone, and/or N-acylated lactams,
for example, N-benzoylcaprolactam. Hydrophilically
substituted acylacetals and acyllactams are likewise used
with preference. Combinations of conventional bleach
activators may also be used.
In addition to the conventional bleach activators, or
instead of them, it is also possible to incorporate what
are known as bleaching catalysts into the rinse aid
particles. These substances are bleach-boosting
transition metal salts or transition metal complexes such
56
CA 02320500 2000-09-18
as, for example, Mn-, Fe-, Co-, Ru- or Mo-salen complexes
or -carbonyl complexes. Other bleaching catalysts which
can be used include Mn, Fe, Co, Ru, Mo, Ti, V and Cu
complexes with N-containing tripod ligands, and also Co-,
Fe-, Cu- and Ru-ammine complexes.
Preference is given to the use of bleach activators from
the group of polyacylated alkylenediamines, especially
tetraacetylethylenediamine (TAED), N-acyl imides,
especially N-nonanoylsuccinimide (NOSI), acylated
phenolsulfonates, especially n-nonanoyl- or
isononanoyloxybenzenesulfonate (n- or iso-NOBS), N-
methylmorpholiniumacetonitrile methyl sulfate (MMA),
preferably in amounts of up to 10% by weight, in
particular from 0.1% by weight to 8% by weight, more
particularly from 2 to 8% by weight, and with particular
preference from 2 to 6% by weight, based on the overall
composition.
Bleach-boosting transition metal complexes, especially
those with the central atoms Mn, Fe, Co, Cu, Mo, V, Ti
and/or Ru, preferably selected from the group of
manganese and/or cobalt salts and/or complexes, with
particular preference from cobalt ammine complexes,
cobalt acetato complexes, cobalt carbonyl complexes, the
chlorides of cobalt or manganese, and manganese sulfate,
are used in customary amounts, preferably in an amount of
up to 5% by weight, in particular from 0.0025% by weight
to 1% by weight, and with particular preference from
0.01% by weight to 0.25% by weight, based in each case on
the overall composition. In specific cases, however, it
is also possible to use a greater amount of bleach
activator.
Suitable enzymes in the detergents of the invention
include in particular those from the classes of the
hydrolases such as the proteases, esterases, lipases or
57
CA 02320500 2000-09-18
lipolytic enzymes, amylases, glycosyl hydrolases, and
mixtures of said enzymes. All of these hydrolases
contribute to removing stains, such as proteinaceous,
fatty or starchy marks. For bleaching, it is also
possible to use oxidoreductases. Especially suitable
enzymatic active substances are those obtained from
bacterial strains or fungi such as Bacillus subtilis,
Bacillus licheniformis, Streptomyces griseus, Coprinus
cinereus and Humicola insolens, and also from genetically
modified variants thereof. Preference is given to the use
of proteases of the subtilisin type, and especially to
proteases obtained from Bacillus lentus. Of particular
interest in this context are enzyme mixtures, examples
being those of protease and amylase or protease and
lipase or lipolytic enzymes, or of protease, amylase and
lipase or lipolytic enzymes, or protease, lipase or
lipolytic enzymes, but especially protease and/or lipase-
containing mixtures or mixtures with lipolytic enzymes.
Examples of such lipolytic enzymes are the known
cutinases. Peroxidases or oxidases have also proven
suitable in some cases. The suitable amylases include, in
particular, alpha-amylases, iso-amylases, pullulanases,
and pectinases.
The enzymes may be adsorbed on carrier substances or
embedded in coating substances in order to protect them
against premature decomposition. The proportion of the
enzymes, enzyme mixtures or enzyme granules may be, for
example, from about 0.1 to 5% by weight, preferably from
0.5 to about 4.5% by weight.
Dyes and fragrances may be added to the machine
dishwashing compositions of the invention in order to
enhance the esthetic appeal of the products which are
formed and to provide the consumer with not only the
performance but also a visually and sensorially "typical
and unmistakable" product. As perfume oils and/or
58
CA 02320500 2000-09-18
fragrances it is possible to use individual odorant
compounds, examples being the synthetic products of the
ester, ether, aldehyde, ketone, alcohol, and hydrocarbon
types. Odorant compounds of the ester type are, for
example, benzyl acetate, phenoxyethyl isobutyrate, p-
tert-butylcyclohexyl acetate, linalyl acetate,
dimethylbenzylcarbinyl acetate, phenylethyl acetate,
linalyl benzoate, benzyl formate, ethyl
methylphenylglycinate, allyl cyclo-hexylpropionate,
styrallyl propionate, and benzyl salicylate. The ethers
include, for example, benzyl ethyl ether; the aldehydes
include, for example, the linear alkanals having 8-18
carbon atoms, citral, citronellal,
citronellyloxyacetaldehyde, cyclamen aldehyde,
hydroxycitronellal, lilial and bourgeonal; the ketones
include, for example, the ionones, a-isomethylionone and
methyl cedryl ketone; the alcohols include anethole,
citronellol, eugenol, geraniol, linalool, phenylethyl
alcohol, and terpineol; the hydrocarbons include
primarily the terpenes such as limonene and pinene.
Preference, however, is given to the use of mixtures of
different odorants, which together produce an appealing
fragrance note. Such perfume oils may also contain
natural odorant mixtures, as obtainable from plant
sources, examples being pine oil, citrus oil, jasmine
oil, patchouli oil, rose oil or ylang-ylang oil. Likewise
suitable are clary sage oil, camomile oil, clove oil,
balm oil, mint oil, cinnamon leaf oil, lime blossom oil,
juniperberry oil, vetiver oil, olibanum oil, galbanum oil
and labdanum oil, and also orange blossom oil, neroli
oil, orange peel oil, and sandalwood oil.
The fragrances may be incorporated directly into the
detergents of the invention; alternatively, it may be
advantageous to apply the fragrances to supports, which
strengthen the adherence of the perfume to the laundry
and, by slowing the release of fragrance, provide for
59
CA 02320500 2000-09-18
long-lasting fragrance of the textiles. Materials which
have become established as such supports are, for
example, cyclodextrins, it being possible in addition for
the cyclodextrin-perfume complexes to be additionally
coated with further auxiliaries. Incorporating the
fragrances into the rinse aid particles of the invention
is also possible, and results in a fragrance sensation
when the machine is opened (see above).
In order to enhance the esthetic appeal of the
compositions produced in accordance with the invention,
they (or parts thereof) may be colored with appropriate
dyes. Preferred dyes, whose selection presents no
difficulty whatsoever to the skilled worker, possess a
high level of storage stability and insensitivity to the
other ingredients of the compositions or to light and
possess no pronounced affinity for the substrates to be
treated with the compositions, such as glass, ceramic, or
plasticware, so as not to stain them.
The detergents of the invention may include corrosion
inhibitors for protecting the ware or the machine, with
special importance in the field of machine dishwashing
being possessed, in particular, by silver protectants.
The known substances of the prior art may be used. In
general it is possible to use, in particular, silver
protectants selected from the group consisting of
triazoles, benzotriazoles, bisbenzotriazoles, amino-
triazoles, alkylaminotriazoles, and transition metal
salts or transition metal complexes. Particular
preference is given to the use of benzotriazole and/or
alkylaminotriazole. Frequently encountered in cleaning
formulations, furthermore, are agents containing active
chlorine, which may significantly reduce corrosion of the
silver surface. In chlorine-free cleaners, use is made in
particular of oxygen-containing and nitrogen-containing
organic redox-active compounds, such as divalent and
CA 02320500 2000-09-18
trivalent phenols, e.g. hydroquinone, pyrocatechol,
hydroxyhydroquinone, gallic acid, phloroglucinol,
pyrogallol, and derivatives of these classes of compound.
Inorganic compounds in the form of salts and complexes,
such as salts of the metals Mn, Ti, Zr, Hf, V, Co and Ce,
also find frequent application. Preference is given in
this context to the transition metal salts selected from
the group consisting of manganese and/or cobalt salts
and/or complexes, with particular preference cobalt
ammine complexes, cobalt acetato complexes, cobalt
carbonyl complexes, the chlorides of cobalt or of
manganese and manganese sulfate. Similarly, zinc
compounds may be used to prevent corrosion on the ware.
In terms of their composition, the rinse aid particles of
the invention may be designed so that they dissolve to a
minor extent, if at all, in the main wash cycle (and also
in optional prewash cycles). This ensures that the active
substances are not released until the rinse cycle, where
they develop their action. In addition to this chemical
formulation, a physical formulation may be necessary
depending on the type of dishwashing machine, so that the
rinse aid particles are not pumped off when the water is
changed in the machine and hence are no longer available
for the rinse cycle. Standard domestic dishwashing
machines, upstream of the detergent-liquor pump, which
pumps the water or cleaning solution from the machine
after the individual cleaning cycles, comprise a sieve
insert, intended to prevent clogging of the pump by food
residues. If the user cleans heavily soiled kitchen- and
tableware, then this sieve insert requires regular
cleaning, which is a simple operation owing to the ease
of access and removability. The rinse aid particles of
the invention, then, are preferably designed in terms of
their size and shape such that they do not pass through
the sieve insert of the dishwashing machine even after
the cleaning cycle, i . a . , after exposure to agitation in
61
CA 02320500 2000-09-18
the machine and to the detergent solution. This ensures
that rinse aid particles are present in the dishwashing
machine in the rinse cycle, these rinse aid particles
releasing the active substances) under the action of the
warmer water and so bringing the desired rinse effect.
Particulate machine dishwashing compositions that are
preferred in the context of the present invention are
those wherein the particulate rinse aid has particle
sizes of between 1 and 40 mm, preferably between 1.5 and
30 mm, and in particular between 2 and 20 mm.
In the dishwashing compositions of the invention, the
rinse aid particles, having the sizes stated above, may
project from the matrix of the other particulate
ingredients; alternatively, the other particles may
likewise have sizes within the stated range, so that,
overall, a detergent is formulated that comprises large
detergent particles and rinse aid particles. Especially
if the rinse aid particles of the invention are colored,
i.e., have red, blue, green, or yellow color, for
example, it is advantageous for the appearance of the
product, i.e. of the overall detergent, if the rinse aid
particles are visibly larger than the matrix comprising
the particles of the other ingredients of the detergent.
Here, preference is given to inventive particulate
machine dishwashing compositions which (without taking
into account the rinse aid particles) have particle sizes
of between 100 and 3000 ~,m, preferably between 300 and
2500 Vim, and in particular between 400 and 2000 ~,m.
As well as coloring the rinse aid particles, the visual
attractiveness of such compositions may also be enhanced
by contrasting coloration of the powder matrix or by the
shape of the rinse aid particles. Since it is possible to
use technically uncomplicated techniques to produce the
rinse aid particles, it is readily possible to offer them
in a wide variety of shapes. In addition to the particle
62
CA 02320500 2000-09-18
shape which approximates to the spherical form, for
example, cylindrical or cuboid particles may be produced
and used. Other geometric shapes as well may be realized.
Specific product designs may include, for example, star-
s shaped rinse aid particles. It is also possible without
problems to produce disks and shapes with plants and
animal bodies as their base, examples being tree, flower,
blossom, sheep, fish, etc. Interesting visual attractions
may also be created in this way by producing the rinse
l0 aid particles in the form of a stylized glass, in order
to underscore visually the clear-rinse effect in the
product as well. No limits are placed on the imagination
in this context.
15 If the detergents of the invention are formulated as a
powder mixture, then - especially if there are large
differences between the size of rinse aid particles and
detergent matrix - on the one hand partial separation may
occur when the pack is shaken, and on the other hand
20 dosing may be different in two successive washing
operations, since the user does not always automatically
dose equal quantities of the detergent and rinse aid
particles. If it is desired technically to use an
identical quantity for each washing operation, this can
25 be realized by the packaging - familiar to the skilled
worker - of the compositions of the invention in water-
soluble film bags. The present invention also provides
particulate machine dishwashing compositions wherein one
dose unit is welded in a water-soluble film bag.
By this means, the user need only insert a bag,
containing for example a detergent powder and a plurality
of visually distinctive rinse aid particles, into the
dispenser of his or her dishwashing machine. This
embodiment of the present invention is therefore a
visually attractive alternative to conventional detergent
tablets.
63
CA 02320500 2000-09-18
The detergents of the invention may be produced in a
manner known per se. A process for producing pulverulent
machine dishwashing compositions with clear-rinse effect,
in which a conventional pulverulent machine dishwashing
composition is mixed with rinse aid particles of the
invention, is therefore also provided by the present
invention.
The desired retention, described earlier on above, of the
rinse aid particles in the machine even when the water is
changed may be effected not only by the abovementioned
enlargement of the rinse aid particles but also by a
reduction in the size of the holes in the sieve insert.
In this way, it is possible to formulate machine
dishwashing compositions having a uniform average
particle size of less than, for example, from 4 to 12 mm.
For this purpose, a sieve insert which replaces or covers
the insert present in the machine is added to the product
of the invention wherein the rinse aid particles also
have relatively small particle sizes. The present
invention therefore additionally provides a kit of parts
comprising a pulverulent machine dishwashing composition
of the invention and a sieve insert for domestic
dishwashing machines.
As already mentioned, the inventive combination of
composition and sieve insert makes it possible to
formulate compositions in which the rinse aid particles
also have relatively small particle sizes. Kits of parts
in accordance with the invention wherein the particle
sizes of the machine dishwashing composition (including
the rinse aid particles) is in the range from 400 to
2500 Vim, preferably from 500 to 1600 Vim, and in
particular from 600 to 1200 Vim, are preferred.
64
CA 02320500 2000-09-18
In order to prevent clogging of the added sieve insert by
residues of soil, the chosen mesh size or hole size
should not be too small. Here, preference is given to
kits of parts in accordance with the invention wherein
the mesh size or hole size of the sieve insert is from 1
to 4 mm and the rinse aid particles are larger than this
mesh size or hole size of the sieve insert.
The kit of parts in accordance with the invention is not
restricted to the particular form of the sieve insert at
which said insert replaces or covers the insert present
in the machine. In accordance with the invention it is
also possible, and preferred, to enclose with the kit of
parts a sieve insert having the form of a basket, which
may be suspended in a known manner in the dishwashing
machine - on the cutlery basket, for example. In this
way, a sieve insert thus designed replaces the dispenser
compartment, i.e., the user doses the machine dishwashing
composition of the invention directly into this sieve
insert, which acts in the manner described above in the
washing and rinse cycle.
The advantages associated with the invention may also be
utilized when the composition is in the form of the
compact tablet. The present invention therefore
additionally provides a detergent tablet for machine
dishwashing, comprising builders and also, optionally,
further detergent ingredients, which comprises
diquaternary polysiloxanes, preferably diquaternary
polysiloxanes of the formula I, with particular
preference diquaternary poly(dimethylsiloxanes) of the
formula II, and especially diquaternary poly(dimethyl-
siloxanes) of the formula III, in amounts of from 0.5 to
60% by weight, preferably from 1 to 50% by weight, with
particular preference from 2.5 to 40% by weight, and in
particular from 5 to 30% by weight, based in each case on
the tablet weight.
CA 02320500 2000-09-18
Here again, in respect of preferred embodiments,
reference may be made to the above remarks. As already
mentioned, the introduction of the diquaternary
polysiloxanes into the rinse cycle is particularly
preferred, so that preferred detergent tablets are those
comprising the diquaternary polysiloxanes in dissolution-
retarded form.
Particular preference is given in this context not only
to a retarded dissolution but also to a controlled
release based not on slower solubility but, instead, on a
release controlled by external circumstances. The above-
described rinse aid particles may also be formulated as a
tablet phase and in tablets as well offer the possibility
of temperature-controlled release of the diquaternary
polysiloxanes.
The present invention thus further provides multiphase
detergent tablets for machine dishwashing, comprising
builders and also, optionally, further detergent
ingredients, wherein at least one phase comprises
a) from 0 to 65% by weight of one or more carrier
materials,
b) from 30 to 70% by weight of coating substances)
having a melting point above 50°C
c) from 0 to 65% by weight of fatty substance(s),
d) from 0 to 50% by weight of further active
substances and/or auxiliaries, and
e) from 0.1 to 70% by weight of diquaternary
polysiloxanes.
Entirely in analogy to the above remarks relating to the
particulate rinse aid, the same embodiments (amount,
nature and formulation of the ingredients, etc.) are also
preferred in the case of the tablets of the invention.
66
CA 02320500 2000-09-18
In the context of the present invention, the individual
phases of the tablet may have different three-dimensional
forms. The simplest embodiment is that of two-layer or
multilayer tablets, each layer of the tablet constituting
one phase. In accordance with the invention, however, it
is also possible to prepare multiphase tablets in which
individual phases have the form of inclusions into
(an)other phase(s). In addition to so-called "ring-core"
tablets, possible examples include laminated tablets or
combinations of the stated embodiments. Examples of
multiphase tablets can be found in the figures of
EP-A-0055 100 (Jeyes), which describes toilet cleaning
blocks. The most widespread three-dimensional form in the
art at present for multiphase tablets is the two-layer or
multilayer tablet. In the context of the present
invention, therefore, it is preferred for the phases of
the tablet to have the form of layers and for the tablet
to have 2, 3 or 4 phases.
The tablets of the invention may take on any geometric
form whatsoever, with particular preference being given
to concave, convex, biconcave, biconvex, cubic,
tetragonal, orthorhombic, cylindrical, spherical,
cylinder-segmentlike, discoid, tetrahedral, dodecahedral,
octahedral, conical, pyramidal, ellipsoid, pentagonal-,
heptagonal- and octagonal-prismatic, and rhombohedral
forms. It is also possible to realize completely
irregular outlines such as arrow or animal forms, trees,
clouds, etc. If the tablets of the invention have corners
and edges, these are preferably rounded off. As an
additional visual differentiation, an embodiment having
rounded corners and beveled (chamfered) edges is
preferred.
Instead of the layer structure, it is also possible to
prepare tablets which comprise the detergent component of
the invention in the form of other phases. Here, it has
67
CA 02320500 2000-09-18
been found suitable to prepare base tablets which have
one or more cavities, and to insert the melt comprising
ingredients a) to d) of the detergent component of the
invention into the cavity and allow it to solidify
therein. This preparation process produces preferred
multiphase detergent tablets comprising a base tablet,
which has a cavity, and a part present at least partly in
the cavity.
The cavity in the compressed part of such tablets of the
invention may have any form whatsoever. It may go right
through the tablet, i.e., have an opening on different
sides, for example, at the top and bottom side, of the
tablet; alternatively, it may be a cavity which does not
go through the entire tablet, and whose opening is
visible only on one tablet side. The form of the cavity
may also be chosen freely within wide limits. For reasons
of process economy, continuous holes whose openings are
located on opposite faces of the tablets, and depressions
having an opening at one tablet side, have become
established. In preferred detergent tablets, the cavity
has the form of a continuous hole whose openings are
located on two opposite tablet surfaces. The form of a
continuous hole of this kind may be chosen freely,
preference being given to tablets wherein the continuous
hole has circular, ellipsoid, triangular, rectangular,
square, pentagonal, hexagonal, heptagonal or octagonal
horizontal sections. It is also possible to realize
completely irregular hole shapes, such as arrow or animal
forms, trees, clouds, etc. As with the tablets,
preference is given, in the case of angular holes, to
those having rounded corners and edges or having rounded
corners and chamfered edges.
The abovementioned geometric embodiments may be combined
with one another as desired. For instance, it is just as
possible to prepare tablets having a rectangular or
68
CA 02320500 2000-09-18
square outline and circular holes as it is to prepare
circular tablets having octagonal holes, there being no
limits on the diversity of possible combinations. For
reasons of process economy and the esthetic perception of
the user, hole-type tablets particularly preferred are
those wherein the tablet outline and the hole cross
section have the same geometric shape, examples being
tablets having a square outline and a square hole made
centrally therein. Particular preference is given in this
context to annular tablets, i.e., circular tablets with a
circular hole.
If the aforementioned principle of the hole open at two
opposite tablet sides is reduced to an opening,
depression tablets are obtained. Detergent tablets of the
invention wherein the cavity has the form of a depression
are likewise preferred. With this embodiment, as with the
"hole tablets", the tablets of the invention may take on
any geometric form whatsoever, with particular preference
being given to concave, convex, biconcave, biconvex,
cubic, tetragonal, orthorhombic, cylindrical, spherical,
cylinder-segmentlike, discoid, tetrahedral, dodecahedral,
octahedral, conical, pyramidal, ellipsoid, pentagonal-,
heptagonal- and octagonal-prismatic, and rhombohedral
forms. It is also possible to realize completely
irregular outlines such as arrow or animal forms, trees,
clouds, etc. If the tablet has corners and edges, these
are preferably rounded off. As additional visual
differentiation, an embodiment having rounded corners and
beveled (chamfered) edges is preferred.
The form of the depression may also be chosen freely,
preference being given to tablets in which at least one
depression may take on a concave, convex, cubic,
tetragonal, orthorhombic, cylindrical, spherical,
cylinder-segmentlike, discoid, tetrahedral, dodecahedral,
octahedral, conical, pyramidal, ellipsoid, pentagonal-,
69
CA 02320500 2000-09-18
heptagonal- and octagonal-prismatic, or rhombohedral
form. It is also possible to realize completely irregular
depression forms, such as arrow or animal forms, trees,
clouds, etc. As with the tablets, depressions having
rounded corners and edges or having rounded corners and
chamfered edges are preferred.
In the case set out above, the part present at least
partially in the cavity consists solely of ingredients a)
to d) of the detergent components. It is, however, also
possible to introduce support material-based detergent
components into the cavity (cavities). For reasons of
process economy, however, preference is given to
multiphase detergent tablets wherein the part present in
the cavity comprises
a) from 0 to 10% by weight, preferably from 0 to
7.5% by weight, and in particular from 0 to 5% by
weight, of one or more carrier materials,
b) from 30 to 70% by weight, preferably from 35 to
65% by weight, and in particular from 40 to 60%
by weight, of coating substances) having a
melting point above 50°C,
c) from 0 to 65% by weight, preferably from 10 to
60% by weight, and in particular from 20 to 50%
by weight, of fatty substance(s),
d) from 0 to 50% by weight, preferably from 5 to 45%
by weight, and in particular from 10 to 40% by
weight, of further additive substances and/or
auxiliaries, and
e) from 0.1 to 70% by weight, preferably from 1 to
50% by weight, and in particular from 5 to 40% by
weight, of diquaternary polysiloxanes.
The size of the depression or continuous hole in
comparison to the total tablet is guided by the desired
end use of the tablets. Depending on with how much
further active substance the remaining void volume is to
CA 02320500 2000-09-18
be filled, and on whether a smaller or larger amount of
detergent component is to be present, the size of the
cavity may vary. Irrespective of the end use, in
preferred detergent tablets the volume ratio of
compressed part ("base tablet") to detergent component is
from 2:1 to 100:1, preferably from 3:1 to 80:1, with
particular preference from 4:1 to 50:1, and in particular
from 5:1 to 30:1.
Besides the stated volume ratio, it is also possible to
state a mass ratio of the two parts, the two values
correlating to one another by way of the densities of the
base tablet and, respectively, of the detergent
component. Irrespective of the density of the individual
parts, preference is given to detergent tablets of the
invention wherein the weight ratio of base tablet to
detergent component is from 1:1 to 100:1, preferably from
2:1 to 80:1, with particular preference from 3:1 to 50:1,
and in particular from 4:1 to 30:1.
Analogous details may also be given for the surfaces
visible in each case of the base tablet and,
respectively, of the detergent component. Here,
preference is given to detergent tablets wherein the
outwardly visible surface area of the detergent component
accounts for from 1 to 25%, preferably from 2 to 20%,
with particular preference from 3 to 15%, and in
particular from 4 to 10%, of the total surface area of
the tablet.
The detergent component and the base tablet are
preferably colored so as to be visually distinguishable.
In addition to visual differentiation, performance
advantages may be obtained by virtue of different
solubilities of the different regions of the tablet.
Detergent tablets in which the detergent component
dissolves more rapidly than the base tablet are preferred
71
CA 02320500 2000-09-18
in accordance with the invention. By incorporating
certain constituents, firstly, it is possible to
accelerate specifically the solubility of the detergent
component; secondly, the release of certain ingredients
from the detergent component may lead to advantages in
the washing or cleaning process.
Preference is also given, of course, to detergent tablets
of the invention wherein the detergent component
dissolves later in the wash program than the base tablet.
Performance advantages from this retarded release may be
achieved, for example, by using a slower-dissolving
detergent component to release active substances) only
in later cycles. Thus in the case of machine dishwashing,
for example, it can be ensured by means of slower-
dissolving detergent components that further active
substances) is (are) available in the rinse cycle. By
means of additional substances such as nonionic
surfactants, acidifiers, soil release polymers, etc., it
is possible in this way to enhance the rinse results. The
incorporation of perfume is also readily possible; by
means of its retarded release it is possible in the case
of dishwashing machines to eliminate the "alkali odor"
when the machine is opened, which is a frequent
occurrence. In relation to the detergent components of
the invention, the acidifier, soil release polymer, etc.
ingredients are in this case ingredients d).
In preferred embodiments of the present invention the
base tablet possesses a high specific weight. The
invention prefers detergent tablets wherein the base
tablet has a density of more than 1000 g dm-3, preferably
more than 1025 g dm-3, with particular preference more
than 1050 g dm-3, and in particular more than 1100 g dm-3.
In order to facilitate the disintegration of highly
compacted tablets, it is possible to incorporate
disintegration aids, known as tablet disintegrants, into
72
CA 02320500 2000-09-18
the tablets in order to reduce the disintegration times.
Tablet disintegrants, or disintegration accelerators, are
understood in accordance with Rompp (9th Edition, Vol. 6,
p. 4440) and Voigt "Lehrbuch der pharmazeutischen
Technologie" [Textbook of pharmaceutical technology] (6th
Edition, 1987, pp. 182-184) to be auxiliaries which
ensure the rapid disintegration of tablets in water or
gastric fluid and the release of the drugs in absorbable
form.
These substances increase in volume on ingress of water,
with on the one hand an increase in the intrinsic volume
(swelling) and on the other hand, by way of the release
of gases, the generation of a pressure which causes the
tablets to disintegrate into smaller particles. Examples
of established disintegration aids are carbonate/citric
acid systems, with the use of other organic acids also
being possible. Examples of swelling disintegration aids
are synthetic polymers such as polyvinylpyrrolidone (PVP)
or natural polymers and/or modified natural substances
such as cellulose and starch and their derivatives,
alginates, or casein derivatives.
Preferred detergent tablets contain from 0.5 to 10% by
weight, preferably from 3 to 7% by weight, and in
particular from 4 to 6% by weight, of one or more
disintegration aids, based in each case on the tablet
weight. If only the base tablet comprises disintegration
aids, then these figures are based only on the weight of
the base tablet. If disintegration aids are incorporated
into the detergent components of the invention, they
count as ingredient d).
Preferred disintegrants used in the context of the
present invention are cellulose-based disintegrants and
so preferred detergent tablets comprise a cellulose-based
disintegrant of this kind in amounts from 0.5 to 10% by
73
CA 02320500 2000-09-18
weight, preferably from 3 to 7~ by weight, and in
particular from 4 to 6~ by weight. Pure cellulose has the
formal empirical composition (C6H1o05) n and, considered
formally, is a /3-1,4-polyacetal of cellobiose, which
itself is constructed of two molecules of glucose.
Suitable celluloses consist of from about 500 to 5000
glucose units and, accordingly, have average molecular
masses of from 50,000 to 500,000. Cellulose-based
disintegrants which can be used also include, in the
context of the present invention, cellulose derivatives
obtainable by polymer-analogous reactions from cellulose.
Such chemically modified celluloses include, for example,
products of esterifications and etherifications in which
hydroxyl hydrogen atoms have been substituted. However,
celluloses in which the hydroxyl groups have been
replaced by functional groups not attached by an oxygen
atom may also be used as cellulose derivatives. The group
of the cellulose derivatives embraces, for example,
alkali metal celluloses, carboxymethyl cellulose (CMC),
cellulose esters and cellulose ethers, and
aminocelluloses. Said cellulose derivatives are
preferably not used alone as cellulose-based
disintegrants but instead are used in a mixture with
cellulose. The cellulose derivative content of these
mixtures is preferably less than 50~ by weight, with
particular preference less than 20% by weight, based on
the cellulose-based disintegrant. The particularly
preferred cellulose-based disintegrant used is pure
cellulose, free from cellulose derivatives.
The cellulose used as disintegration aid is preferably
not used in finely divided form but instead is converted
into a coarser form, for example, by granulation or
compaction, before being admixed to the premixes intended
for compression. Detergent tablets comprising
disintegrants in granular or optionally cogranulated form
are described in German Patent Applications
74
CA 02320500 2000-09-18
DE 197 09 991 (Stefan Herzog) and DE 197 10 254 (Henkel)
and in International Patent Application W098/40463
(Henkel). These documents also provide further details on
the production of granulated, compacted or cogranulated
cellulose disintegrants. The particle sizes of such
disintegrants are usually above 200 Vim, preferably
between 300 and 1600 ~,m to the extent of at least 90% by
weight, and in particular between 400 and 1200 ~m to the
extent of at least 90% by weight. The abovementioned,
relatively coarse cellulose-based disintegration aids,
and those described in more detail in the cited
documents, are preferred for use as disintegration aids
in the context of the present invention and are available
commercially, for example, under the designation Arbocel~
TF-30-HG from the company Rettenmaier.
As a further cellulose-based disintegrant or as a
constituent of this component it is possible to use
microcrystalline cellulose. This microcrystalline
cellulose is obtained by partial hydrolysis of celluloses
under conditions which attack only the amorphous regions
(approximately 30% of the total cellulose mass) of the
celluloses and break them up completely but leave the
crystalline regions (approximately 70%) intact.
Subsequent deaggregation of the microfine celluloses
resulting from the hydrolysis yields the microcrystalline
celluloses, which have primary particle sizes of
approximately 5 ~m and can be compacted, for example, to
granules having an average particle size of 200 Vim.
Detergent tablets which are preferred in the context of
the present invention further comprise a disintegration
aid, preferably a cellulose-based disintegration aid,
preferably in granular, cogranulated or compacted form,
in amounts of from 0.5 to 10% by weight, preferably from
3 to 7% by weight, and in particular from 4 to 6% by
weight, based in each case on the tablet weight.
CA 02320500 2000-09-18
The detergent tablets of the invention may further
comprise, both in the base tablet and in the detergent
component, a gas-evolving effervescent system. Said gas-
evolving effervescent system may consist of a single
substance which on contact with water releases a gas.
Among these compounds mention may be made, in particular,
of magnesium peroxide, which on contact with water
releases oxygen. Normally, however, the gas-releasing
effervescent system consists in its turn of at least two
constituents which react with one another and, in so
doing, form gas. Although a multitude of systems which
release, for example, nitrogen, oxygen or hydrogen are
conceivable and practicable here, the effervescent system
used in the detergent tablets of the invention will be
selectable on the basis of both economic and
environmental considerations. Preferred effervescent
systems consist of alkali metal carbonate and/or alkali
metal hydrogen carbonate and of an acidifier apt to
release carbon dioxide from the alkali metal salts in
aqueous solution.
Among the alkali metal carbonates and/or alkali metal
hydrogen carbonates, the sodium and potassium salts are
much preferred over the other salts on grounds of cost.
It is of course not mandatory to use the pure alkali
metal carbonates or alkali metal hydrogen carbonates in
question; rather, mixtures of different carbonates and
hydrogen carbonates may be preferred from the standpoint
of wash technology.
In preferred detergent tablets, the effervescent system
used comprises from 2 to 20% by weight, preferably from 3
to 15% by weight, and in particular from 5 to 10% by
weight, of an alkali metal carbonate or alkali metal
hydrogen carbonate, and from 1 to 15, preferably from 2
76
CA 02320500 2000-09-18
to 12, and in particular from 3 to 10% by weight of an
- acidifier, based in each case on the total tablet.
As examples of acidifiers which release carbon dioxide
from the alkali metal salts in aqueous solution it is
possible to use boric acid and also alkali metal hydrogen
sulfates, alkali metal dihydrogen phosphates, and other
inorganic salts. Preference is given, however, to the use
of organic acidifiers, with citric acid being a
particularly preferred acidifier. However, it is also
possible, in particular, to use the other solid mono-,
oligo- and polycarboxylic acids. Preferred among this
group, in turn, are tartaric acid, succinic acid, malonic
acid, adipic acid, malefic acid, fumaric acid, oxalic
acid, and polyacrylic acid. Organic sulfonic acids such
as amidosulfonic acid may likewise be used. A
commercially available acidifier which is likewise
preferred for use in the context of the present invention
is Sokalan~ DCS (trademark of BASF), a mixture of
succinic acid (max. 31% by weight), glutaric acid (max.
50% by weight), and adipic acid (max. 33% by weight).
In the context of the present invention, preference is
given to detergent tablets where the acidifier used in
the effervescent system comprises a substance from the
group of the organic di-, tri- and oligocarboxylic acids,
or mixtures thereof.
Following production, the particulate detergents and/or
detergent tablets of the invention, and the novel
detergent components per se, may be packed, the use of
certain packaging systems having proven particularly
useful. The present invention additionally provides a
combination comprising (a) particulate detergents)
and/or (a) detergent tablets) of the invention and a
packaging system containing said detergent and/or said
detergent tablet(s), said packaging system having a
77
CA 02320500 2000-09-18
moisture vapor transmission rate of from 0.1 g/m2/day to
less than 20 g/m2/day if said packaging system is stored
at 23°C and a relative equilibrium humidity of 85%.
The packaging system of the combination of detergent
component and/or detergent and/or detergent tablets) and
packaging system has, in accordance with the invention, a
moisture vapor transmission rate of from 0.1 g/mz/day to
less than 20 g/m2/day when said packaging system is
stored at 23°C and a relative equilibrium humidity of
85%. These temperature and humidity conditions are the
test conditions specified in DIN Standard 53122, which
allows minimal deviations (23 ~ 1°C, 85 ~ 2% relative
humidity). The moisture vapor transmission rate of a
given packaging system or material may be determined in
accordance with further standard methods and is also
described, for example, in ASTM Standard E-96-53T ("Test
for measuring water vapor transmission of materials in
sheet form") and in TAPPI Standard T464 m-45 ("Water
vapor permeability of sheet materials at high temperature
and humidity"). The measurement principle of common
techniques is based on the water uptake of anhydrous
calcium chloride which is stored in a container in the
appropriate atmosphere, the container being closed at the
top face with the material to be tested. From the surface
area of the container closed with the material to be
tested (permeation area) , the weight gain of the calcium
chloride, and the exposure time, the moisture vapor
transmission rate may be calculated as follows:
~pR ~ _ 24 ~ 10000 , x
y ~g l m ' l 24h~
A
where A is the area of the material to be tested in cm2,
x is the weight gain of the calcium chloride in g, and y
is the exposure time in h.
78
CA 02320500 2000-09-18
The relative equilibrium humidity, often referred to as
"relative atmospheric humidity", is 85% at 23°C when the
moisture vapor transmission rate is measured in the
context of the present invention. The ability of air to
accommodate water vapor increases with temperature up to
a particular maximum content, the so-called saturation
content, and is specified in g/m3. For example, 1 m3 of
air at 17° is saturated with 14.4 g of water vapor; at a
temperature of 11°, saturation is reached with just 10 g
of water vapor. The relative atmospheric humidity is the
ratio, expressed as a percentage, of the actual water
vapor content to the saturation content at the prevailing
temperature. If, for example, air at 17° contains 12 g/m3
water vapor, then the relative atmospheric humidity (RH)
- (12/14.4)100 = 83%. If this air is cooled, then
saturation (100% RH) is reached at what is known as the
dew point (in the example: 14°), i.e., on further cooling
a precipitate is formed in the form of mist (dew). The
humidity is determined quantitatively using hygrometers
and psychrometers.
The relative equilibrium humidity of 85% at 23°C can be
established precisely, for example, in laboratory
chambers with humidity control, to +/-2% RH depending on
the type of apparatus. In addition, constant and well-
defined relative atmospheric humidities are formed in
closed systems at a given temperature over saturated
solutions of certain salts, these humidities deriving
from the phase equilibrium between water partial
pressure, saturated solution, and sediment.
The combinations of the invention may of course in turn
be packaged in secondary packaging, examples being
cardboard packaging or trays, there being no need to
impose further requirements on the secondary packaging.
The secondary packaging, accordingly, is possible but not
necessary.
79
CA 02320500 2000-09-18
Packaging systems which are preferred in the context of
the present invention have a moisture vapor transmission
rate of from 0.5 g/m2/day to less than 15 g/mz/day.
Depending on the embodiment of the invention, the
packaging system of the combination of the invention
contains a defined amount of novel detergent component, a
defined amount of a particulate detergent composition, or
one or more detergent tablets. In accordance with the
invention it is preferred either to design a tablet such
that it comprises one application unit of the detergent,
and to package this tablet individually, or to pack into
one packaging unit the number of tablets which totals one
application unit. In the case of an intended dose of 80 g
of detergent, therefore, it is possible in accordance
with the invention to produce and package individually
one detergent tablet weighing 80 g, but in accordance
with the invention it is also possible to package two
detergent tablets each weighing 40 g into one pack in
order to arrive at a combination in accordance with the
invention. This principle can of course be extended, so
that, in accordance with the invention, combinations may
also comprise three, four, five or even more detergent
tablets in one packaging unit. Of course, two or more
tablets in a pack may have different compositions. In
this way it is possible to separate certain components
spatially from one another in order, for example, to
avoid stability problems.
The packaging system of the combination of the invention
may consist of a very wide variety of materials and may
adopt any desired external forms. For reasons of economy
and of greater ease of processing, however, preference is
given to packaging systems in which the packaging
material has a low weight, is easy to process, and is
inexpensive. In combinations which are preferred in
CA 02320500 2000-09-18
accordance with the invention, the packaging system
consists of a bag or pouch of single-layer or laminated
paper and/or polymer film.
The detergent tablets may be filled unsorted, i.e. as a
loose heap, into a pouch made of said materials. On
esthetic grounds and for the purpose of sorting the
combinations into secondary packaging, however, it is
preferred to fill the detergent tablets individually, or
sorted into groups of two or more, into bags or pouches.
For individual application units of the detergent tablets
which are located in a bag or pouch, a term which has
become established in the art is that of the "flow pack".
Flow packs of this kind may optionally then - again,
preferably sorted - be packaged into outer packaging,
which underscores the compact commercial form of the
tablet.
The single-layer or laminated paper or polymer film bags
or pouches preferred for use as packaging systems may be
designed in a very wide variety of ways: for example, as
inflated pouches without a center seam or as pouches with
a center seam which are sealed by means of heat (heat
sealing), adhesives, or adhesive tapes. Single-layer
pouch and bag materials include the known papers, which
may if appropriate be impregnated, and also polymer
films, which may if appropriate be coextruded. Polymer
films that can be used as a packaging system in the
context of the present invention are specified, for
example, in Hans Domininghaus, "Die Kunststoffe and ihre
Eigenschaften", 3rd edition, VDI Verlag, Diisseldorf,
1988, page 193. Figure 111 shown therein also gives
indications of the water vapor permeability of the
materials mentioned.
Combinations which are particularly preferred in the
context of the present invention comprise as packaging
81
CA 02320500 2000-09-18
system a bag or pouch of single-layer or laminated
polymer film having a thickness of from 10 to 200 ~,m,
preferably from 20 to 100 ~,m, and in particular from 25
to 50 ~,m.
Although it is possible in addition to the abovementioned
films and papers also to use wax-coated papers in the
form of cardboard packaging as a packaging system for the
detergent tablets, it is preferred in the context of the
present invention for the packaging system not to
comprise any cardboard boxes made of wax-coated paper. In
the context of the present invention, the term "packaging
system" always relates to the primary packaging of the
detergent component, composition or tablets, i.e., to the
packaging whose inner face is in direct contact with the
detergent component, composition or tablet surface. No
requirements whatsoever are imposed on any optional
secondary packaging, so that all customary materials and
systems can be used in this case.
As already mentioned earlier on above, the detergent
components, detergent compositions, or detergent tablets
of the combination in accordance with the invention
comprise further ingredients of detergents, in varying
amounts, depending on their intended use. Independently
of the intended use of the compositions or tablets, it is
preferred in accordance with the invention for the
detergent compositions) or tablets) to have a relative
equilibrium humidity of less than 30~ at 35°C.
The relative equilibrium humidity of the detergent
compositions or tablets may be determined in accordance
with common methods, the following procedure having been
chosen in the context of the present investigations: a
water-impermeable 1 liter vessel with a lid which has a
closable opening for the introduction of samples was
filled with a total of 300 g of detergent tablets and
82
CA 02320500 2000-09-18
held at a constant 23°C for 24 h in order to ensure a
uniform temperature of vessel and substance. The water
vapor pressure in the space above the tablets can then be
determined using a hygrometer (Hygrotest 6100,
Testoterm Ltd, England). The water vapor pressure is then
measured every 10 minutes until two successive values
show no deviation (equilibrium humidity). The
abovementioned hygrometer permits direct display of the
recorded values in ~ relative humidity.
Likewise preferred are embodiments of the combination in
accordance with the invention wherein the packaging
system is of resealable configuration. Combinations
wherein the packaging system has a microperforation may
also be realized with preference in accordance with the
invention.
As mentioned earlier on above, detergent components,
detergent compositions or detergent tablets for machine
dishwashing may be prepared by the processes of the
invention. Accordingly, the present invention
additionally provides a method of cleaning kitchen- and
tableware in a dishwashing machine, which comprises
placing one or more particulate detergents and/or one or
more detergent tablets of the invention in the dispensing
compartment of the dishwashing machine and running a wash
program in the course of which the dispensing compartment
opens and the detergents) and/or tablets) is or are
dissolved.
With the cleaning method of the invention as well it is
possible to forego the dispensing compartment and to
place the detergent components and/or detergent
compositions or the tablets) of the invention, for
example, in the cutlery basket. Here again, of course,
the use of a dosing aid, for example, a basket insert
which is placed in the washing compartment, is possible
83
CA 02320500 2000-09-18
without problems. Accordingly, the present invention
further provides a method of cleaning kitchen- and
tableware in a dishwashing machine, which comprises
placing one or more particulate detergents of the
invention and/or one or more detergent tablets of the
invention, with or without a dosing aid, in the washing
compartment of the dishwashing machine and running a wash
program in the course of which the detergents) and/or
the tablets) is or are dissolved.
Examples:
Ware stained with standard stains (see below) was washed
using a standard commercial machine dishwashing
composition in powder form (dosage: 25 g), and in the
Comparative Example, V, a standard commercial rinse aid
was metered in from the reservoir tank of the machine. In
the inventive Example, E, the standard commercial rinse
aid of the comparative example was upgraded prior to use
by adding Tegopren~ 6922 (diquaternary
poly(dimethylsiloxane) of the formula III with stearyl
radicals R, acetate ions X- and a value for n of 30, from
Th. Goldschmidt AG). In both cases, the dosage of the
rinse aid was 5 ml per rinse cycle; in the inventive
example, E, 1 gram of Tegopren~ 6922 was introduced into
the rinse cycle by the rinse aid dosing. The machine used
was a Miele G 590 with a universal program; the water
hardness was 16°d [German hardness].
The ware washed in this way was stained again with
standard stains and washed again under the conditions
described above. After that, the cleaning performance of
the washes was evaluated on the basis of the evaluation
scale described below. The results of this evaluation are
indicated in the following table.
84
CA 02320500 2000-09-18
V E
Minced meat on glass 8.8 10.0
(baked on)
Minced meat on porcelain 8.5 10.0
(dried on)
Oat flakes 7.2 9.2
Milk (baked on) 7.4 9.3
The standard stains were applied by a standardized
procedure which is described in "Methoden zur Bestim~ung
der Reinigungsleistung von maschinellen
Geschirrspiilmitteln~~ [Methods of determining the cleaning
performance of machine dishwashing compositions] (Part A:
SOFW-Journal, vol. 124 11/98, pages 706 to 713; Part B:
SOFW-Journal, vol. 124 14/98, pages 1022 to 1034). Also
disclosed therein are the evaluation criteria in
accordance with which the evaluations listed in the above
table were made.
The table clearly shows that the ware treated in
accordance with the invention can be cleaned much better
in subsequent washes than the ware of the comparative
example.