Note: Descriptions are shown in the official language in which they were submitted.
CA 02320728 2006-11-02
ABSOR8ABLE COPOLYMERS AND
$URGICAL ARTICLES FABRICATED THEREFROM
TECHNICAL FIELD
Absorbable copoly,nie.rs of randomly polymer:i.zec). glycolide
and caprolactone are described. Pr.ocesses for making the
copolymers c-ind s>>rgi.ca]. articles in7de totally or in par.'t from
such copolymers, including sutures, are also described.
BACKGROUND
Bioabsorbable surgical devices made from copolymers
dertived from glycolide and epsilon-caprolactone are known in the
art. Such bioabsor_bable surgical devices include surgical
sutures.
A de<:ir.able ch.arGcteri; t=ic of a bioabsorbable suture is
its ability to exhibit and maintain dc:sired tensile properties
for a predetermined time period ' o.llowed by rapid absorption of
the suture mass (hereinafter "mass
los_" . }
Synthetic absozbable sutures are ;:nown in the art.
Absorbable multifi.lament sutures such as *DEXON sutures (made
from glycolide homopolymer and commercial..ly ava;_? zb7.e from Davis
& Geck, Danburv, Connecticut), *VICRYL sutures (made from a
eopolymer of glycolide and lactide and commercially available
from Ethicon, Inc.. So;nmerville, New Jersey) , and *pOLYSORB
sutures (also n!1de f_om a copolyme:- or glyco],ide anci 7.actide ancl
coi'nmercial].y availlbze from United States Surgical Corporation,
39 Norwalk, Connecticut) are knoiv-n in t,ae industry as snorC term
absorbable sutures. The classification short term absor_bable
sutures generally.refers to surgical sutures which retain at
least about 20 percer:t of their origi.nal strength ac three weeks
aftc'r ].rnpIaI-Itation, w.i.t'-) Che JU1.1.11.C' bc:i.ng r_c~;cne.i ~l.l.y
absorbed in the body within about GO to 90 clays port
implantation.
*trade-mark
-l-
CA 02320728 2006-11-02
Long "irezm absorbable sutures are generally classified
as sutures capable of retaining at least about 20 percent of
their original strength for six or more weeks after
implantation, with Lhe suture mass being essentially absorbed in
1~he body withi.n about 1.80 days post :i.mnl.r1ntal.:i.on. For example,
*PDS II sutures (commercially available from Ethicon, Inc.,
Sommer_vi:1 J.e, New .7n.rsey) , are synthetic absorb-bl1-1 monof'ilament
sutures ChZt reportedly retain at ].cast zbout 20 to 30 pcrcent-
of its originsl sLrength six weeks. after implantation. I-Iowever.,
18 PDS II reportedly exhibits minim2l mass loss until 90 days afL-er
i--ipllntat.ion with the suture mass boin7 essentially zab:.orbed in
thc body about 180 cia,Is after implantation. *MAXON suture
(commerci,ally available from Davis & Geclc, Danbury, C'onncc:ticut)
.is lnother, abcorbab].c synthetic mono:filarncnt t;hat: ronort:cdly
generally fi.t:: th:i:: ah:;orption profile.
Most recenL-1y, United States Surgical Corporation has
introduced *BIOSYN monofilament sutures which exhibit good
flexibility, hlndling characteristics, knot strenqth and
absorption characteristics similar to those of presently
available shoz;t term absorbable multi_:EilamenL suture=:.
Another zttei,.pt to provide an acceraLable synt+het:i.c
absorbabl.c monofi.1,.Zmer,L sutures resulted in *MONOCRYL, a suture
fabricated from an absorbable block copol),-mer containg glycolide
and caprolactone, commierciaily available from Ethicon, Inc. .
T-Iowevcr, no s vntheLic absorbable :nono:Cilament sutures
ex:i,st Lod<<y which approximate the strength retention, mass 7.oss,
and modulus of sutures ccmmonly referred to in the art as
"catgut" or "gut" suL=ure,:. .Il: is well known in the ar.i- that the
term gut sut+ur_c+ refers Co a collagen based suture of any Cype or
origin oLten fabricated from the mammal ] an intestir.Cs, ~:uch as
the serosal, lzyer of bovine intestines or the submucosaJ. fibrous
lzyer of layer sheep intestines. GuL sutures exhib:.t the unique
combinlti.on of two week strength retention Znd zibout 75 c3ay mass
los<: whi.a.c cnairtt_aiTiinq accept=able modulus and LensiJ.e strength;
55 aT1C1 ~hll.'-', 'r1) e i=I1CJC?.y used in qynec"oioJ:i.cal nurgerV.
*trade-mark
-2-
CA 02320728 2006-11-02
It would be advantageous to provide a synthetic
absorbable suture which exhibits physical properties similar
to the gut suture.
U.S. Patent No. 4,700,704 to Jamiolkowski does not
teach that sutures can be fabricated from random copolymers
of glycolide and epsilon-caprolactone, and more specifically
from random copolymers containing from 20 to 35 weight
percent epsilon-caprolactone and from 65 to 80 weight
percent glycolide. Moreover, Jamiolkowski reports that
sutures fabricated from glycolide/epsilon-caprolactone
copolymers containing over 35% caprolactone under are not
orientable to a dimensionally stable fiber. Jamiolkowski
further reports that some sutures fabricated from glycolide/
epsilon-caprolactone copolymers containing 15% caprolactone
are also not orientable to a dimensionally stable fiber.
Furthermore, Jamiolkowski also reports the undesirable
combination of low modulus and low tensile strength for the
glycolide/epsilon-caprolactone copolymers which he was able
to fabricate into sutures.
Therefore, it would be unexpected that sutures made
from random copolymer of glycolide and epsilon-captrolactone
would provide the strength retention and mass loss
characteristics approximating those of gut sutures while
maintaining an acceptable modulus and tensile strength.
SUMMARY
It has now surprisingly been found that absorbable
surgical articles formed from a random copolymer of
glycolide and caprolactone exhibit strength retention, mass
loss and modulus similar to that of gut sutures.
Preferably, the copolymers used in forming surgical articles
include between about 25 and about 32 weight percent of
hydroxy caproic acid ester units and between 75 and 68
weight percent of glycolic acid ester units.
-3-
CA 02320728 2006-11-02
In accordance with an embodiment of the present
invention there is provided a suture fabricated from a
random copolymer comprising from about 68 to about 75 weight
percent glycolide and about 25 to about 32 weight percent
epsilon-caprolactone, the suture exhibiting two week
strength retention, mass loss of about 50% in 32 hours as
measured in Sorenson's buffer solution at 80 C and a modulus
ranging from about 150 kpsi to about 250 kpsi and a knot
pull strength of about 1.7 to about 2.8 kg.
A further embodiment of the present invention provides
for the use of such sutures for suturing a wound.
In a preferred embodiment the suture is a size 3/0
suture and exhibits a mass loss of about 50% after 32 hours
in Sorenson's buffer solution at 80 C.
According to a particularly preferred embodiment, the
suture is a size 3/0 suture and exhibits a mass loss of
about 30% after 72 hours in Sorenson's buffer solution at
80 C.
In yet another preferred embodiment, the suture is a
size 3/0 suture and exhibits a mass loss of about 12% after
120 hours in Sorenson's buffer solution at 80 C.
30
-3a-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
In pa.rticul.arly useful embodiments, the random
copolymers can be spun into fibers. The fibers can be
advantageously fabricated into either monofilament or
muJ.tifilament sutures having physica]. properties similar to
those of gut sutures.
In addition, a process of making such synthetic
absorbable monofilament sutures from the above described
caprolactone/glyco).ide random copolymers has been found. The
process, for a given size suture, comprises the operations of
18 extruding the random caprolactone/glycolide copolymer at an
extrusion temperAture of from about 70 C to about 21.5 C to
provide a monofilament fiber, passing the solidified
monofi].ament through water (or other suitabJ.e liquid medium)
quench bath at a temperature of from about 15 C to about 25
C or through in air (or, other suitable gaseous medium) at from
about 15 C to about 25 C, stretching the monofilament through
a ccrie:c air ovenn at an overal}. stretch rar.io of r?'c71fl a}out
7:1 to about 14:1 to provide a stretched monofilament. In a
particularly useful embodiment, the monofilament is stretched
28 through three air ovens by four godet stations. The first air
ovcn is maintained at ambient temperature, whereas the second
air oven is heated to a temperature above the crystalization
temperature of the glycolide /epsilon caprolactone copolymer
at about 80 C to about-. 110 C , and the third air oven is
set at about 85 C to about 120 C. The draw ratio between
the first and ,econd godet station ranges between about 5:1. to
about 8:1. The draw ratio between the second and third godet
station ranges between about 1.3:1 to about 1.8:1. The draw
ratio between the third and fourth godet station ranges
38 between about 1.04:1 to about 1.06:1. The stuture then may
be annealed with or without relaxation at a temperature of
from about A0 C to about 120 C to provide the fini ,hec3 :;uture.
-4-
CA 02320728 2000-08-21
PCTIUS99/04242
WO 99/43364
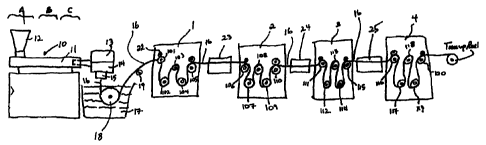
t':i.g. 1 is a schematic illustration of an apparal.t.t~ which
:its :,ua.LAl.AL! Lo.17 martuf<ict-uriang of monofi.lament= ::utur.e:-, c7
i.:.:c:].c~;ed
herein; .1i0
Vi.(J. 2 i:> tt pctrypective view of a suture attac:ltud t:c) Ft
neeclle; .
3A-3C illustrate the form.ation of the lcuot:. wtii.ch wa3
employed in in the loop pull test used in Example 2.
pZLCRjPTION- _T-, r_ b E~I~Q~IN11-
1e .[t: ha; been found that glycolide and epsiloncaprolzctone
monomers can advantageously be combined to form a randorn copolymer
usefu.l. :i.n f:or,ming s:ur.cli.c,ll, ar.ticJ.e, having ~-.trengt:h
r.ct:rnt.iorr,
inztss 1o:.;:., and modul.uw chlractera.st:ics similar to or. -,uner7.or to
gut sutu.r.=c:~:.
'I'l~c.= r.,in<lr.,ni c':catxo.i.yniQJ: can he u:':.i.ttc,l c:cativotit:iona'1
techniqc.tca::.. x'or extimple, monomers can be dried, mixed in o
re.xct:.ioit vt+:::,c;=i wJ.t.=.li . cn itii.tJ.ator (e:i.thc:r a :,a.ttc~le;
or
multif=unc.ti.ona3. ina.t:i.ator) and a suitable polymerization cztnlyst-
and heated at temperatures from about 170'C to about 200'C for a
period of t-ime ranging from about 10 hours to about 30 hours.
The copol.ymer h,_ repeating units derived from g;l.ycolide
randomly combi.necl with repeating units derived from caprolactone.
Repeating units derived from glycolide comprise between ccboue 25
tind aboia-. .i:? wci Jht: purc:c;nt of the copol.ymer. and pr.ef:er.ztb7.y
cabout
wc:igl=tt: pe:rcent of caprolactone and abouL 70 weight percent of
gylcoa.idc.'. copolymers of caprolactone and glycolide havi.nr! an
inher.ent_ vi.:;co:~it,y of from about 1.0 to about 1.8 hc/c7 meca:.uxed at
30 30'C und ..tt: a, concetrtration of 0.25 g/dl in chloroLor.rn or. ilr'I1'
rnay
generally be used.
r,utriotu i~ol,ul.ynu:r::: c:;jri br_ (ormcc3 :i.nt:o
ii::iml my kiic,w>> t.%.~c.:1õnitauc:, .:uc:lt ..t:.:, J.ur u:~.anq)J.c:.
extrusion, tnoldinct and/or solvent ca::ting. The copo1.yinc:r:.: t:.act be
uwoca .a.luttc:, 1-Aetuf.ed wit:li other absorbablc composit5.on::., or i.n
combi.riation with non-absorbable componen.t:;. A wide vca.r.:i.ct;y nf
c::LttI be iitanu,Eactured trom the copolymers;
-5-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
described A"erein. These include but are not limited to clips
and other fasteners, staples, sutures, pins, screws, prosthetic
devices, wound dressings, drug delivery devices, anastomosis
rings, and other implantable devices. Fibers made Erom the
copolymers can be knitted, woveri or made into non-woven
materials with other fibers, either absorbable or nonabsorbable
to form fabrics, such as meshes and felts. Compositions
including the::,e random copolymer s can also be used as an
absorbable coating for surgical devices. Preferably, however,
18 the copolymers are spun into fibers to be used in making
sutures.
Multifilament sutures of the present invention may be
made by methods known in the art. Braid constructions such as
those disclosed and claimed in U.S. Patent No.'s 5,059,213 and
5,019,093 are suitable for the multifilament suture of the
present invention.
Fi,g. 1 substantially ii].uctrateC the extruding,
quenching and stretching operations of the monofilament
manufacturing operation herein. Extruder unit 10 is of a known
or converit;ional type and is equipped with controls i:or
regulating the temperature of barrel 11 in various zones
thereof, e.g., progressively higher temperatures in three
consecutive zoncs A, B and C along the length of the barrel.
Pellets or powder of resins of the present invention are
introduced to the extruder through hopper 3.2. Any of the above
described copoxymers which are useful for the formatiori of
fibers can be used herein.
Motor-driven metering pump 13 delivers melt extruded
resin at a constant rate to spin pack 14 and thereaftPr through
spi.nncret 15 pos:ce;:;sing one or more orifices of desired diameter
to provide a molten monofilament 16 which then enters quench
bath 17, e.g., containing water, where the monofilament
solidifies. The distance monofilament 16 travels after emerging
froin spa.nnereL- 15 to Lhe point where iL enL-ers quench bath 17,
i.e., the air gap, can vary and can advantageously be from about
-6-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
0.5 to about 100 cm and preferab].y from about 1 to about 20 cm.
If desired, a chimney (not shown), or shield, can be provided to
isolate monofilament 16 from contact with air currents which
might otherwise affect the cooling of the monofilament in an
unpredictable manner. in general, barrel zone A of the extruder
can be maintained at a temperature of from about 170 C to 215 C,
zone B at from about 1.70 C to 23.5 C and zone C at from about
1'70"C ro about 915"C. Additiona7. temperature puraihc:Cers
inclucle: metering pump block 13 at from about 170 C to about
18 215 C, spinneret 15 at from about 170 C to about 225"C and
quench bath at from about 15 C to about 40 C.
Monofi].ament 16 is passed through quench bath 17
around driven roller 18-and over idle roller 19. optionally, a
wiper (not shown) may remove excess water from the monofilament
as it is reinoved from quench bath 3.7. On exiting the quench bath
the monofilament is passed through first godet station 1, which
is equiped with five individual godets, i.e. godets 101, 102,
103, 104 and 105. Upon entering godet station 1, monofi.lament 16
is wrapped around a first godet 101 provided with nip roll 22 to
prevent slippage which might otherwise result from the
subsequent stretching operation; and subsequently passed over
godet 3.01, under godet 102, over godet 103, under godet 104, and
over godet 105 to godet station 2, containing godets 106, 107,
108, 109, and 3.10, where it is wrapped over godet 106, under
godet 107, over godet 108, under godet 109, and over, godet 110..
Monofilament 16 passing from godet station 1 to godet station 2
is drawn through air oven 23 at a temperature ranging form about
20 C to about 30"C by the godets of godet station 2 which rotate
at speeds faster than the speed of the godet station 1 to
provide the desired draw ratio, which is from about 5:1 to about
10 :]. and preferably from about 6:1 to about 8:1, to eifect the
molecular orientation of the copolymer from which it is
fabricated and thereby increase its tensile strencrrh.
Following the initial draw at ambient temperature,
monofilament 16 i: then subjected. to a second and a third.
-7-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
drawing operation. Monofilament 16 is subsequently drawn from
godet 105 through air oven 24, which in mai.ntai.ned nt: from about
fio"C to ..ibotit: 7.].0"C:, t:n godet ::t:Zt.i.ail 3 r;otll::la.ty5.riji
gotlut.:.~ 111.
112, 113, 114, and 3.15 where it is wrapped over godet 111, under
godet 112, over godet 113, under godet 3.14, and over qodet 115.
Godel: : tation :") :spin:; faster than godet: station 2 to provide the
desired draw ratio, which is from about 1.3:1 to about 1.8:1.
Monof:iJ;.unet,t, !.G a.cs UiGri drawn .Croin godct 115 t-hrouJh air oven
25, which is maintained at from about 85 C to about 120 C, by
18 godet station 4, conLaining godets 116, 117 118, 13.9, and 120
where it is wrapped over godet 116, under godet, 117, over godet
118, under godet 3.19, and over godet 120. Godet station 4 spins
ta::Cer ttittn godet station 3 to provide the desired draw ratio,
which is from about 1.05:1 to about 1.06:1. It should be
understood that the godet arrangements in each of godPt;. stations
1, 2, 3, and 4, respectively should not be limited to the above
described arrangement and that each godet station may have any
suitable godet arrangement.
In an alternative operation for sutures for smaller
28 size sutures, sizes 4/0 to 8/0, monofi7.ament 16 is only passed
through godet stations 1 and 2 and not subjected to any further
stetching operations.
Annealing of the suture also may be accomplished with
or without shrinkage of the suture. In carrying out the
annealing operation, the desired length of suture may be wound
around a creeJ, and the creel placed in a heatxnc7 c.ahi.nel; under
nitrogen flow maintained at the desired temperature, e.g. about
70'C to about 120'C, as described in U.S. Patent No. 3,630,205.
nfter ft suit:able pera.od of residency in the heating c:crbinet,
e.g., for up to about 18 hours or so, the suture will have
undergone essent=inlly no shrinkage. 11s shown in U.S. Patent No.
3,630,205, the creel may be rotated within the heatinci cabinet
in or.cier to i.rir;u.re unif=or.m heating of t_he monof:il.amrr,t= or the
c:abiri4t ,nrxy be uf t:hc circulating hoL air lype in whieh case
uniform ?teati.nq c,f th(-- monofilamc~nt wi 1 7. he: iir.hi.cvrcl wii'licm1.:
the
-8-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
need to rotgte the creel. Thereafter, the creel with its
annealed suture is removed from the heating cabinet and when
returned to room temperature, the suture is removed from the
creel, conveniently by cutting the wound monofiilamQnt at
opposite ends of the creel. The annealed sutures, optionally
attached to surgical needles, are then ready to be pAckaged and
sterilized.
Alternatively, the suture may be annealed on line with or
without relaxation. For relaxatiori, the fourth godet station
1A rotates at a slower speed than the third godet station thus
relieving tension on the filament.
The suture disclosed herein, suture 101, may be
attached to a surgica], needle 100 as shown in Fig. 2 by methods
well known in the art. Wounds may be sutured by pas}ing the
needled suture through tissue to create wound closure. The
needle preferably is then removed from the suture and the
suture ti.cd.
it is further within the scope of this invention to
incorporate one or more medico-surgically useful substances
into the present invention, e.g., those which acce].erate or
benef ic: i,a].ly inodil:y the healing proce:;4; when par. tic:l,us are
applied to a surgical repair site. So, for example, the suture
can carry a therapeutic agent which will be deposited at the
repair site. The t'herapeutic agent can be chosen f.or its
antimicrobial properties, capability for promoting repair or
reconstruction and/or new tissue growth. Antimicrobial agents
such as broad spectrum antibiotic (gentamycin sulfate,
erythromycin or derivatized glycopeptides) which are slowly
released into the tissue can be applied in this manner to aid
in combating clinical and sub-clinical infections in a tissue
repair site. To promote repair and/or tissue growth, one or
several growth promoting factors can be introduced into the
sutures, e.g.,.fibroblast growth factor, bone growth 1=actor,
c:pidurinttl growLh f'rzctor, platelet derived growth factor,
macrophage derived growth factor, alveolar derived growth
-g..
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
factor, monocyte derived growth factor, magainin, and so forth.
Some therapeutic indications are: glycerol with tissue or
kidney plasminogen activator to cause thrombosis, superoxide
dimutase to scavenge tissue damaging free radicals, tumor
necrosis factor for cancer therapy or colony sl=imulati.ng factor
and interferon, interleukin-2 or other lymphokine to enhance
the immune system.
It is contemplated that it may be desirable to dye
the sutures of the present invention in order to increase
i9 visibility of the suture in the surgica], field. Dyes known to
be suitable for incorporation in sutures can be used. Such
dyes include but are not limited to carbon black, bone black,
D&C Green No. 6, and D&C Violet No. 2 as described in the
hzndbook of U.S. Colorants for Food, Drugs and Cosmetics by
i5 Daniel M. Marrion (1979). Preferably, sutures in accordance
with the invention are dyed by adding up to about a few
percent and preferably about 0.2% dye, such as D&C Violet No.
2 to the xesin prior to extrusion.
In order that those skilled in the art may be better
20 able to practice the compositions and methods described herein,
the following example is given as an illustration of the
preparation of random copolymers as well as of the preparation
and superior characteristics of sutures made from the random
copolymers. It should be noted that the invention is not
25 limited to the specific details embodS.ed in the examn.los and
further that all ratios or parts recited are by weight, unless
otherwise indicated.
EXAMPLE 1
38 Dry glycolide (4200 grams) and undistilled epsilon-
caprolactone were added to a reactor aJ.ong with 0.35 grams of
dxstil.l.c d tf.lnnous octoate and 3 grams of 1,6 hexaneclioJ.. The
mixture was dried for about 48 hours with agitation under flow
of nit:.r.ogun. '!'he rcactor temperaCure was then set at 100'C.
35 When the temperature of the reactants reached 100'C the
-10-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
temperature- was maintained for about 15 minutes at w}iich point
the temperature of the reactants was raised'to about 1506C and
the reaction vessel heated for about an addi.tional. 15 minutes.
The temperature of the reactants was then raised to about 190'C
and polymerization conducted with stirring under a nitrogen
attnosphero for about 18 hours. The reaction product is then
isolated, comniinuted, and treated to remove residual reactants
using known techniques. The treatment to remove residual
reactants occurs at 130'C for 48 hours under vaccuum.
ie
Table I below sets forth typical conditions for
extruding, stretching of size 3/0 sutures in accordance with
this invention. All of the monofilament sutures were fabricated
from the resin of Example 1.
-11-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
TAT3LE I
CONDITIONS OF MANUFACTURING VARIOUS SIZES
OF MONOFILAMENT OF THE PRESF=NT INVENTION
Ex ip1e 1
St ire Size 3/0
P~- :)ce ,s Conditi.on:: Extriision
extr.2der screw, rpm ?
putn. rpm 15.4
dr- en roller, mpm 2.7
br~ -zel temp., C, zone A 183
b:.:rel temp., C, zone B 186
b:.rrnl temp., C, zone C ].R9
clamp temp., 'C, 1.88
adaDter temp., 'C 189
put=p temp., 'C 196
b?_.)ck temp., 'C 190
barrel melt temp., 'C 192
pump melt temp., 'C 191
spinneret melt temp., 'C 194
barrel pressure, psi 1040
pump pressure, psi 1000
spir.nerer pressure, psi 1400
purr.n size, cc per revolution 0.16
diameter of spinneret, orifices, mm 1.2
no. of spinneret orifices 1
quer:,,h bath temp., 'C 20
Stretching (Orienting) Operation
. arn g
draw bath tenip., 'C ambient
first godet station, mpm 2.9
second godet, mpm 20.8
thi.rd. godc+l. inpin 34.6
fourth godet station,mpm 36.2
-1Z-
CA 02320728 2006-11-02
first oven temp, 'C 28
second oven Cemp,'C 85
third oven t.emp, "C 90
overall draw ratio 12.57:1
Annealing OperaL=ion
1;X~.tmr~7.e 7annealing temp., "C FO'C
time (hrs.) 6
The phy:;:.cal pr.ope.rti.e~; of i,nc and l:hc,-,
pr. c~C:cdux<~:: c:mp i oyed L'or their measuTetnCnt. are seC .Lorth in
Table ii, as follows:
TASrjI? '1'I
PROCEDURES FOR MEASURING PHYSICAL PROPERTIES
OF MCNOT'ILANENT SUTURES OF THE PRESENT ZNVENTION
Phys.i.cn]. Pr oF>r_ rty Te2; L P:: ac:c.dur. c
knot-pull strength, kg *U.S.P. XXI, tensile strength,
sutures (881)
straic7ht--pull strength, kg *ASTM D-2256, Instron Corporation
elongation, v ASTM D-2256
tensile strength, kg/m.m2 ASTM D-2250", Instron Corporation
Series IX Automated Materials
Testing Syste:n 1.03A
Young's ?-Iodulus *Instron Merlin Software version
2000 Serie: IX calc:ulation 15.3
(commercially avz:i.lable from
Tnstron Cor_porat_i.on)
Table III below sets .f-orth the physical properties of
18 l:I:e size 3/0 ,;utux'e of the rDre~=cnt inve~n-. ~on.
*trade-mark
-13-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
TAME III
Physical Property Example 1
diameter (mm) .29R
knot-pull strength (kg) 2.66
Young's Modulus (kpsi) 7.70
Elongation % 22
Tensile Strength (kpsi) 102.2
As the data in Tables III illustrates, the suture
made of the copolymer provided herein shows a desii=ed physical
properties, such as modulus and tensile strength.
Example 2
~~~~nL='~L'~iIrr
18 Monofilament sutures manufactured in accordance
with the above described process using the copolymer of
Example 1 were tested for in vitro strength retonta.on. In
vitro loop-pull strength retention is indicative of in vivo
strength retention. The in vitro strength retention of the
suture was tested as follows:
To sitf1i7latE in vivo conditions, l:he SU l:ure r:r.lilipl '4' z werc
stored in a container filled with Sorenson's buffer solution
at 37"C. After various periods of time, the suture samples
were t.Ilon removed f=rom the contaaner to test their loop-pull
strength as follows. A knotted loop was formed.in a test
suture in three steps as shown in FIGS. 3A - 3C. As shown in
stFp 1 of of i~tG 3A , each suture was given tl douta'Le throw
(left over right ) around a 2 cm diamater cylinder. In Stop
2, the free ends of the suture were set in a single throw
throw (right over left) onto the initial throw of rct-c-p 1.
Fiu<<lly, , in ;::t;ep 3, another double throw (;lof t ovor right)
was set onto the single throw of Step 2 to complete the knot.
-14-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
The free ends of the suture were cut to approximately 0.5
inches and the loop was carefully erased f:roin the cyJ. i.nder. .
Testing of the loop was carried out useing an Instron
Corporation (Canton, Mass.) Tensile Tester Model No. 4307,
operated with a crosshead speed of. 51 mm/min and equipped with
flat grips, each having a pin over which the loop is
posi t ioned .
The results of the tests are presented in Table IV
hereinbelow. In the strength retention data reported in Table
l8 IY, Tn represents the time elapsed in weeks since Che sample
was placed in the solution, with n zepresenti.ng thc number of
weeks.
TABLE ?V
PMtCWiMl; or IN VITRO STRrdaC7'{t rJsPnINf'~.1)
COMMSI7'ION Tl R'2 T3 T4 T6 T11 Z'10 'ri
r=.xnrtrra: T 44 71 0 -
EXAMPLE 3
IN VITRO MASS LOSS
MonoFi.lament sutures manufactured in accordance with the
above described process using the copolymer of Example 1 were
tested for in vitro mass retention. In vitro mass retention
str.cnc7tn is inc3ica1.i.ve of in vivo mas:: retention. The in vitro
strength retention of the suture was tested as follows:
To simulate in vivo conditions, the suture samples were
stored in a container filled with Sorenc:on's buff.r:r %:o].ution
at 80'C. After various periods of, time, the sutur.e ~amples
were then removed from the container filterred, rinsed with
distiioled water and dried for about 6 hours at about 40'C
under vaccum and subsequently weighed.
The results of the tests are presented in Tablc V
herrinbc'low. Tn Lhe strength reteiition data report:ec1 in Table
V, Tn represents the time elapsed in hours since t:he sample
- 1 5-
CA 02320728 2000-08-21
WO 99/43364 PCT/US99/04242
was placed in the solution, with n representing thc number of
hours. It is well known in the art that one hour of immersion
in the the container filled with Sorenson's buffer solution at
8o'C approximates about one week of invivo mass loss. For
comparison purposes, the same cests were conducted on Monoczyl
sutures.
All comparattive tests were performed on size 3/0 suLures.
TABLE
ie
MCT1Urf3L OF z1V vI7'RO MAS,r, RCTnYNM
COME'o.SITION T2 T2 T3 I'4 T6 TH T10 Tl:
[JNMPT.Ii T 92.79 66.35 51 37.73 34.3]. 29.35 26.97 23.:
Mnllocryl 74,86 74.79 fifi.A3 47.95 47.G3 35,31 32.1/1 27.3
Modifications and variations of the compositions and
processes disclosed herein are possible in liQht of the above
teachings. It is therefore to be understood that changes may be
made in particular embodiments described which are within the
fuI], intended scopo of the invention as defined by the claims.
-16-