Note: Descriptions are shown in the official language in which they were submitted.
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
MUSHROOM SPAWN SUPPLEMENT
The present invention relates to the art of mushroom cultivation and
specifically pertains to an improved mushroom spawn-supplement that
efficiently
inoculates the mushroom substrate and provides an improved nutrient source for
promoting mushroom growth.
The commercial production of mushrooms (Agaricus bisporus) involves a
series of steps, including compost preparation, compost pasteurization,
inoculating
the compost with the mushroom fungus (spawning), incubation to allow thorough
colonization of the compost with mushroom mycelia, top dressing the compost
with
moistened peat moss (casing), and controlling the environment to promote the
development of mature mushrooms. The mushroom growing process is described
in detail in several publications (for example, Chang & liayes, 1978; Flegg et
al.,
1985; Chang & Miles, 1989; Van Griensven, 1988).
Mushroom spawn is used to inoculate the nutritive substrate (compost).
Virtually all spawn now used is based on a grain substrate. The technology for
making grain based mushroom spawn was first taught by Sinden (U.S. Patent No.
1,869,517). Spawn is generally made from sterilized grain that is inoculated
with
pure cultures of the desired mushroom strain. Mushroom spawn can be prepared
by several methods. In one method, dry grain (rye, millet, wheat, sorghum, or
other grain), water, CaC03, and (optionally) CaSO4 are placed in suitable
containers
and capped with lids that allow passage of air and steam but do not allow the
passage of microbes that would contaminate the finished product. Containers
are
subject to steam sterilization for times and temperatures suitable to render
the
mixtures commercially sterile. Following cooling, the grain mixture is
inoculated
with a starter culture of the desired mushroom strain, and incubated under
permissive conditions for approximately 14 days. Containers are shaken at
specific
intervals to promote even colonization of the mycelium throughout the mixture.
1
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Following complete colonization of the hydrated, sterile grain with the
mushroom
fungus, the spawn can be used immediately to inoculate mushroom compost. The
mixtures can also be transferred to plastic bags and refrigerated in
anticipation of
spawning at a future date.
Spawn properly prepared according to the above cited method has the
following characteristics: Approximately 48 to 50 wt W moisture, pH 6.6 to
7.2,
free flowing, even white color resulting from the heavy growth of the Agaricus
bispoms mycelium. Spawn is generally added to mushroom compost at a rate of 2-
4 ~ {fresh weight spawn/dry weight compost). Since rye spawn contains about
1.15
Rb nitrogen (Kjeldahl) on a fresh weight basis {about 2.3 96 on a dry weight
basis),
and also contains carbohydrate and lipid, spawn contributes some nutrients to
the
mushroom substrate.
Properly prepared mushroom spawn is resistant to contamination by foreign
microorganisms. The heavy growth of the mushroom mycelium on the grain
particles excludes the growth of many competitor microorganisms. Even when
spawn is added to mushroom compost, which contains high levels of bacteria and
molds, properly prepared spawn does not show overt growth of foreign
microorganisms (Fdliott, 1985). This is in part due to the exclusionary effect
of the
heavy growth of the Agaricur bispoms mycelium and in part due to the
"selectivity"
of properly prepared mushroom compost.
An alternate method of spawn production involves bulk cooking of grain in
large kettles. Grain and water mixtures are heated to hydrate the grain. After
draining excess water, the hydrated grain is mixed with CaC03 and CaS04,
filled
into bottles or heat resistant plastic bags, sterilized, cooled, inoculated
with starter
cultures of the desired mushroom strain, and incubated to allow colonization
of the
grain with the mycelium.
Another method of spawn production involves placing grain, water, CaC03,
and CaSO, into steam jacketed mixers. Mixtures are cooked, sterilized, cooled,
and
inoculated in the mixers. The inoculated sterile grain is aseptically
transferred to
sterile plastic bags that are ventilated to allow passage of air while
maintaining
2
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
sterility. Following mycelial growth, spawn can be shipped to mushroom
production facilities with minimal further handling of the product.
V~allY all spawn used to inoculate mushroom compost is made using rye,
millet, wheat, sorghum, or other grain substrate. Fritsche (1978) describes a
formula reported by Lemke (1971) for spawn on a perlite substrate. The formula
is as follows: perlite (1450 g), wheat bran (1650 g), CaS04 ~ 2 H20 (200 g),
CaC03
(50 g), water (6650 ml). The pH after sterilization is 6.2 to 6.4. This
formula is
calculated to contain 1.10 to 1.34 9b nitrogen on a dry weight basis (assuming
a
typical nitrogen content of wheat bran of 2.24 to 2.7296).
Stoller (U.S. patent No. 3,828,470) teaches that mushroom mycelium will
not grow on feedstuffs such as cottonseed meal, soybean meal, etc., when used
alone as an autoclaved substrate. Stoller also teaches spawn in which the
cereal
substrate has been diluted with an inorganic material containing calcium
carbonate
or an organic flocculating agent. Nitrogen contents are generally low. For
example, Stoller's example 16 is estimated to contain about 0.22 % nitrogen.
Stoller's example 18 is estimated to contain about 0.7~ nitrogen. Stoller also
teaches that a fine, granular or powdery spawn is preferable to the large,
whole
grain particles of grain spawn. This is generally due to the number of "points
of
inoculum" per unit weight of spawn.
Romaine (U.S. Patent No. 4,803,800) teaches production of mushroom
casing spawn by encapsulation of nutrients in a hydrogel polymer. Casing spawn
is used to inoculate the mushroom casing layer rather than the compost. Use of
casing spawn speeds fruiting. Nitrogen contents in the Romaine casing spawn
are
generally low. For example, Romaine teaches total nutrient levels of 2 to 6 96
(wt/vol of formula). Assuming the use of 10096 protein as the nutrient source,
total
nitrogen would be about 0.96 % . Some of Romaine's formulas contain perlite,
~niculite, soy grits, or similar materials at about 2 to 696 (wt/vol) of the
formula
as texturizing agents.
Dahlberg & l:.aPolt (U.S. Patent No. 5,503,647) teach the development of
a mushroom casing spawn prepared from nutritionally inert particles (calcined
earth,
vermiculite, perlite, etc) amended with nutrients. The casing spawn is
formulated
3
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
with low nitrogen contents (generally less than 19b) to allow inoculation of
the
mushroom casing layer with Agaricus bisporus mycelium without promoting the
growth of pests and pathogens. Dahlberg & LaPolt also teach that high levels
of
proteinaceous ingredients such as soybean fines, etc. are inhibitory to
Agaricus
bisporus growth. Generally, nitrogen levels above about 2 9b in a casing spawn
formula result in reduced growth of Agaricus bisporus mycelium. This casing
spawn formulation is also proposed as a substrate for inoculation of spawn
during
its preparation.
Many mushroom growers add nutrient supplements to the mushroom
compost at the time of s~wning or casing. Because of the danger of spreading
diseases, especially at tray-type mushroom farms, most mushroom gmwers add
supplements at spawning. Addition of such supplements usually results in an
increase in mushroom yield. Nutrient supplements generally consist of
proteinaceous materials such as cracked soybean particles, soybean meal, corn
gluten, feather meal, and similar materials. For example, in Hughes et al.
{U.S.
Patent No. 3,560,190), a dry formulation based on a combination of cottonseed
meal and cottonseed oil is disclosed as a suitable supplement.
Nutrient supplementation, however, is susceptible to some undesirable
effects. One problem that has been encountered is excessive bed heating,
apparently
caused by the ready availability of the nutrient source to the highly active
microbial
mushroom culture. Temperature excursions above 35 ° C (95 °F),
sufficient to
significantly deplete, if not completely destroy the mushroom mycelia have
been
observed. Another problem is encountered when adding the supplement to the
compost at the titre of spawning. In many cases, other microorganisms,
primarily
molds, preexisting in the compost, introduced with the supplement, or
introduced
via airborne contamination, compete with the mushroom mycelium for the added
nutrients. This reduces the availability of the supplement for its intended
purpose,
and often hinders the development of the mushroom mycelium.
Recognizing these problems, Carroll et al. (U.S. Patent No. 3,942,969)
provides a supplement suitable for addition to the compost at the time of
spawning,
4
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
in which the release of the nutrient is delayed. The supplement comprises a
denatured protein source, including protein derived from cottonseed, soybean,
and
peanuts. As disclosed, the denaturing can be accomplished by heat treating or
by
treatment with alkalies, acids, or formaldehyde. Unfortunately, the potential
gains
in mushroom yields are disadvantageously oi~et by the economic penalty
associated
with the denaturation treatment. The potential health and environme~tat
hazards of
denaturing treatments such as formaldehyde is also a disadvantage.
Wu {U.S. Patent No. 4,534,781) teaches an improved nutrient supplement
comprising a particulate nutrient, such as a cracked soybean particle, coated
with
a hydrophobic material that is not readily assimiiable by competing
microorganisms
in the compost. A further improvement in this technology was taught by Wu &
Bretzloff (U.S. Patent No. 4,617,047) in which the protein containing nutrient
is
dated with a hydrophobic material and a mold inhibitory composition. Again,
the
potential gains in mushroom yield are disadvantageously offset by the cost
associated with the antimicrobial treatments. The cost and potential health
and
environmental hazards of the mold inhibitory treatments are also a
disadvantage.
Razz et al. (Eur. Pat. Publ. 0 0290 236) teaches another nutrient supplement
for mushroom cultivation, prepared by coating protc~ rich particles with a
hydrophilic carbohydrate. This coating also retards the release of nitrogen
into the
medium. Pratt et al. (U.S.. patent No. 4,764,199) teach a mushroom growing
supplement prepared from acidic corn gluten meal treated with aqueous
formaldehyde while maintaining the meal in a free flowing condition.
Romaine & Marlowe (LT.S. Patent Nos. 5,291,685 and 5,427,592) teach
another nutrient supplement for mushroom cultivation in which intact seeds,
such
a rapeseed, or other small oilseed are heat treated, such as at 90.5°C
(195°~ for
24 hours. The heat treatment prevents sprouting and provides a delayed release
mechanism for seed nutrients. The Romaine & Marlowe supplement is used at
fairly high rates of between 7 and 14 ~ of the dry weight of the compost
All prior art for mushroom supplements involves treating nutrients with heat
or chemicals to reduce the availability of the nutrients to competing
microorganisms
in the compost. In all cases, treatments represent a significant portion of
the cost
5
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
of the supplement. In the case of chemical treatments of the supplements,
ingredients such as formaldehyde and various pesticides represent potential
health
and environmental hazards, and the practicality of using such agents may be
reduced
due to regulatory issues. The development of mushroom supplements without
using
such chemicals is highly desirable.
Brini & Sartor (European patent Application EP 0 700 884 Al) teach a
mixture of a water retaining-dispersing agent (e.g., peat), a buffer, a
protein
containing component (e.g. soybean meal), a growth promoting component (e.g.
corn gluten and/or corn starch), and water. The mixture is sterilized,
inoculated
IO with the mushroom fungus, and used to spawn mushroom compost. The
formulation inoculates the mushroom beds and adds protein, while eliminating
residual antimicrobial substances and suppressing the growth of antagonistic
molds.
Moisture contents of the mixtures are typically 54 to 60 3b . Protein contents
of the
mixtures are 4 to 20 wt ~ protein (0.64 to 3.2 wt % nitrogen). Mixtures
typically
contain 7.4 to 15.2 wt ~ (1.18 to 2.43 wt ~ nitrogen). Use of the mixtures as
mushroom spawn is asserted to allow the faster growth of the mushroom and
prevent the growth of molds. However, routine experimentation has shown that
the
mixtures taught by Brim & Sartor tend to form clumps, resulting in incomplete
sterilization and areas within the mixtures that are not completely colonized
by the
Agaricus bisporus mycelium. The failure to achieve sterilization results in an
ec~omic loss, while a poorly colonized mixture can allow the growth of
competitor
molds and bactera in the compost, causing high compost temperatures and
reducing
mushroom yield.
~gy pF~ ,
It is an object of the present invention to provide a mushroom spawn-
supplement to inoculate mushroom compost and provide performance at least
equivalent to existing mushroom spawn formulas in mushroom yield and time to
achieve full colonization of the substrate.
It is a further object of this invention to pmvide a formulated mushroom
spawn with small particles in order to maximize the number of points of
inoculum
5
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
in the mushroom substrate and to reduce the time for full colonization of the
substrate.
It is a further object of this invention to provide a formulated mushroom
spawn with a high content of nutrients to reduce or eliminate the need to
separately
add a mushroom nutrient supplement.
It is a fiuther object of this invention to provide supplementary nutrients to
the mushroom substrate without a resultant detrimental increase in compost
temperature.
It is yet another object of this invention to provide supplementary nutrients
to the mushroom substrate without the need to treat the nutrients with
pesticides,
denaturants, or other chemical or physical treatments to eliminate the growth
of
competing microorganisms.
It is yet another object of this invention to provide a formulated mushroom
spawn that reduces the risks of sterilization failure and incomplete
colonization of
the mixtures by improving aeration of the mixtures and reducing the formation
of
clumps.
These and other objects are met by the present invention which comprises
an improved mushroom spawn-supplement which is formulated with mixtures of:
(a) proteinaceous ingredients such as corn gluten, feather meal, cracked
soybeans,
soybean meal, cottonseed meal, or other ingredient to provide a high nutrient
content; (b) paper pellets to provide multiple points of inoculum and water
holding
capacity; (c) particulate materials such as calcined earth, vermiculite,
perlite, or
similar material to provide multiple points of inoculum, water holding
capacity,
aeration of mixtures, density, and a free flowing character to the mixtures,
(d)
calcium carbonate (CaCO~ to neutralize pH, and (e) water. The spawn-supplement
optionally contains (f) gypsum (CaSO,, ~ 2 H20) to reduce clumping.
The spawn-supplement may optionally contain a fraction of grain (i.e., rye,
millet, wheat) as used in the prior art. Oleaginous ingredients such as
various
vegetable oils may be added to increase the total nutrient content of the
spawn-
supplement. The proteinaceous and oleaginous components of the spawn-
7
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
svpplt can be combined by using ingredients such as whole cracked soybeans
which contain both protein and oil.
Mushroom spawn-supplement according to the invention contains (on a dry
weight basis): about 5 to 80 wt % proteinaceous ingredient, about 2 to 30 wt %
paper pellets, about 5 to 60 wt % particulate material, about 1 to 12 wt %
CaC03,
and optionally about 1 to 10 wt % CaS04 (gypsum). Water is added to between 40
and 54 % . If used, grain is added at about 1 to 50 wt % (dry weight basis).
Mud ~ sterilized, inoculated, and incubated in any suitable manner within the
skill of the art.
Mushroom spawn-supplement is generally used to inoculate mushroom
compost at rates between about 1 and 8 wt % (fresh weight supplement/dry
weight
compost). When prepared and used as disclosed herein, mushroom spawn-
supplement reduces the time to achieve full colonization of the mushroom
compost
and provides unexpected increases in mushroom yield and production efficiency.
Mushroom spawn-supplement used at 4 to 5 wt % supports a mushroom yield at
least equivalent to the use of 3 wt % rye spawn and 4 wt % traditional
mushroom
supplement. Use of traditional mushroom supplements in addition to the spawn
suPpleme~t may further improve mushroom yield. Addition of small amounts
(i.e.,
2%) of traditional supplements generally do not contribute significantly to
compost
heating.
The spawn-supplement of the present invention provides a fully functional
formulated mushroom spawn and mushroom supplement in a single composition.
Because the spawn-supplement as disclosed is heavily colonized with Agar~cus
bisponts mycelium, most foreign microorganisms cannot grow well on the
material.
Therefore, the invention also provides a mushroom supplement containing no
pesticides, denaturants, or other chemical or physical treatments to control
the
growth or competing microorganisms and avoids deleterious increases in compost
temperature. The invention unexpectedly reduces the frequency and severity of
°green mold" disease caused by virulent strains of TYichoderma
harzianum.
The spawn-supplement of the present invention as disclosed differs from
perlite spawn as taught by Lemke (1971) and Fritsche (1978) in that the
nutrient
8
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
content of spawn-supplement, especially the protein nitrogen content, is
maumized.
Typical nitrogen contents of spawn-supplement are approximately four-fold to
five-
fold higher than of perlite spawn. During the course of investigations leading
to the
development of spawn-supplement, many formulations were developed that
represent functional "non-grain spawns." The generally low nutrient contents
of the
non-grain spawns require that traditional mushroom supplements be added to the
compost to achieve maximum mushroom yields.
The spawn-supplement of the present invention as disclosed differs from the
mixtures taught by Brim & Sartor in that nitrogen contents are substantially
higher,
moisture contents are substantially lower, and the invention is less subject
to
sterilization failure and clumping of the finished product. The latter
difference is
due to the presence of particulate ingredients that improve steam penetration
during
sterilization. The particulate materials also provide better aeration of the
mixtures
during mycelial growth, reducing "dead spots" due to clumping or excessive
moisture of parts of the mixtures,
It is to be understood that both the foregoing general. description and the
following detailed description are exemplary and explanatory only and are not
restrictive of the present invention as claimed.
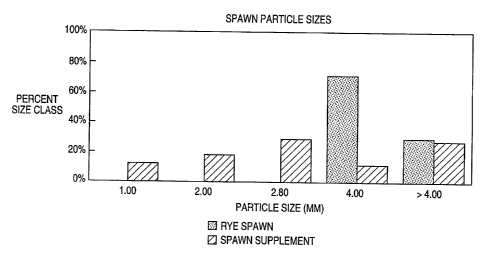
Figure 1 compares the particle size distribution for rye spawn and spawn-
supplement (formula 83).
Figure 2 shows the growth rate of Agaricus bisporus mycelium in compost
inoculated with rye spawn or a spawn-supplement.
Figure 3 shows the effects on spawn run compost temperature of a standard
rye spawn plus S44 supplement and a spawn-supplement (formula 83).
Figure 4 shows the effects on spawn run compost temperature of a standard
rye spawn plus S44 supplement and a spawn-supplement (formula 80).
Figure 5 shows the effects on spawn run compost temperature of a standard
rye spawn plus S44 supplement and a spawn-supplement (formulas 68 and 7$).
9
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Figure 6 shows the effects on spawn run compost temperature of a standard
rye spawn plus S41 supplement and varying rates of spawn-supplement formula
80.
Figure 7 shows the effects on spawn run compost temperature of a standard
rye spawn plus 496 S41 supplement, spawn-supplement formula 68, spawn-
s supplement formula 80, and spawn-supplement formula 80 plus 2 96 S41
supplement.
As disclosed, the present inv~tion comprises a formulated mushroom spawn
with sufficiently high nutrient content that addition of supplementary
nutrient
formulations (i.e., mushroom supplement) is unnecessary. Mixtures of
proteinaceous ingredients (corn gluten, soybean meal, feather meal, wheat
bran,
etc.) and/or oleaginous ingredients (cracked soybeans, soybean fines, soybean
oil,
sunflower oil, cracked sunflowers, corn oil, etc.), paper pellets, particulate
materials to improve water holding capacity and aerate the mixture (calcined
earth,
vermiculite, perlite, ~c.), CaC03, CaS04 2 HZO (optional), and water are
prepared,
steam sterilized, inoculated with starter cultures of Agaricus bisporus, and
incubated
at permissive conditions. After incubation to allow colonization of the spawn-
supplement by the Agaricus bisporus mycelium, the spawn-supplement is used to
inoculate mushroom compost in a manner equivalent to the prior art for
mushroom
spawn and mushroom supplement. Some experimental data suggest that addition
of small amounts of traditional mushroom supplements may further enhance
mushroom yields. Additional mushroom supplements may be added in any suitable
amount, typically 1 to 6 wt ~ preferably about 2 wt 96 additional mushroom
supplements
Spawn-supplement formulas can have a nitrogen content as low as 1 ~ (dry
weight) nitrogen. A typical and prefen~ed spawn-supplement formula of the
present
invention (see example 1) contains at least about 3.59b, more preferably about
6.0
to 6.5 ~ (dry weight) nitrogen (Kjeldahl), although formulas with higher or
lower
nitrogen contents can be prepared. This preferred nitrogen content is
substantially
higher than the approximately 2.3 96 (dry weight) nitrogen present in rye
spawn and
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
substantially higher than the 0.64 to 3.2 ~ nitrogen typically present in the
Brini &
Sartor formulations. Currently available mushroom supplements typically
contain
5.6 to 9.6 ~ (dry weight) nitrogen. Spawn-supplement, as disclosed,
unexpectedly
supports mushroom yields equivalent to or higher than those obtained with
higher
levels of grain spawn and supplement. For example, the spawn-supplement
formulas described in the examples give the same yield when used at 4 to 5 %
as rye
spawn at 3 ~ plus 4 96 S41 or S44 supplements. The example 1 spawn-supplement
formula delivers about the same total nitrogen to the compost as the standard
rye
grain plus supplement combination. The example 2 spawn-supplement delivers
less
than half of the nitrogen of the standard rye grain plus supplement
combination.
The spawn-supplement formulas in example 3 (formulas 68 and 78) deliver about
half of the nitrogen as the standard rye spawn plus supplement combination:
While the phenomenon is not fully understood, and speculation should not
limit the scope of the claims, it is believed that the rapid colonization of
the compost
resulting from the use of spawn-supplement allows the Agaricus bisporus
mycelium
to benefit more from the nutrients than the slower colonization of grain spawn
and
supplement. That is, rapid colonization allows the Agaricus bisporus mycelium
to
absorb the nutrients. With a standard grain spawn and supplement combination,
the
competing microorganisms in the compost utilize the nutrients to the detriment
of
Agaricus bisporus.
Pointc of ino ~l gym: Spawn-supplement as disclosed contains significantly
more particles per unit weight than grain spawn. Rye spawn typically contains
about 1,500 to 2000 kernels per 100 g (at 5096 moisture content). Rye spawn
has
?99b of the particles between 3 and 4 mm in average size and 29°~ of
the particles
greater than 4 mm (Figure 1). Millet spawn typically contains about 10,000
particles per 100 g (at 46 to 48~ moisture). The Brini and Sartor formulation
contains about 9,000 particles per 100 g (at moisture contents in excess of
54~).
The present invention preferably contains at least 10,000 particles per 100 g,
more
preferably at least 25,000, even more preferably at least about 40,000. There
is no
upper limit contemplated. It is believed that amounts up to 100,000 or more
can
provide a functional spawn-supplement. Spawn-supplement (example 1 formula)
11
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
is estimated to contain over 42,000 particles per 100 g (at 48'~ moisture
content).
It is difficult to accurately estimate the total number of particles because
of their
small size and large number. About 3096 of the particles are smaller than 2.0
mm,
and about 12 R6 are smaller than 1.0 mm. The large increase in the number of
points
of inoculum results from the use of ingredients with low bulk densities and
fine
Lextures. The small particles are fully colonized with Agaricus bisporus
mycelium.
When mined with the compost, they efficiently inoculate the mushroom
substrate.
Because of the larger number, the average distance between spawn-supplement
particles is smaller than with rye spawn. Given that Agaricus bisporus has a
fixed
linear gmwth rate, the distance the mycelium must grow to reach confluence is
reduced. As a result, the time to achieve confluent growth through the compost
is
also reduced. Completion of spawn run is generally defined as achievement of
heavy, confluent gmwth in the compost. Use of spawn-supplement therefore
reduces the total spawn run time. Figure 1 demonstrates the particle size
distribution of the spawn-supplement of the present invention and rye spawn.
Completion of spawn run is subjectively determined. In various tests of
spawn-supplement on a research scale and on commercial mushroom farms, spawn
run was pec~eived to be complete within 10 days with the spawn-supplement of
the
present inv~tion. In contrast, a grain spawn and supplement combination
generally
requires 13-15 days to achieve a similar level of growth. Therefore, the use
of
spawn-supplement can reduce spawn run time by about 3 to 5 days.
In an attempt to objectively measure spawn run time, batches of compost
were inoculated with varying levels (3 to 796) of a standard rye grain spawn
with
or without supplementation with S41 mushroom supplement or varying Levels of
spawn-supplement formula 83 (see example 1) with or without S41 mushroom
supplement. At daily intervals, the color of the surface of the compost was
measured using a Minolta color meter. Color was expressed on a "delta E"
scale,
where a smaller number represents a whiter color. Uninoculated compost
typically
has a delta E value of about 75 arbitrary units. A standard 3 ~ spawning rate
with
rye spawn results in a delta E of 57 arbitrary units after 13 days of spawn
run.
Therefore, a delta E of 57 was taken as a color value representing a completed
12
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
spawn run. The time required for other experimental treatments to reach a
delta E
of 57 was calculated from daily color determinations. The results of this test
are
summarized in Figure 2.
While use of 3 % rye spawn results in a 13 day spawn run, increasing the
spawning rate to 7 % rye spawn provides a complete spawn run in about 12 days.
Use of 3 % spawn-supplement formula 83 results in a completed spawn run in
about
days. Increasing the spawning rate to 7% formula 83 spawn-supplement
provides a completed spawn run in about 8 days. A linear regression analysis
shows
that with rye spawn, each additional percentage point in the spawning rate
results
10 in a 0.45 day decrease in spawn run time (R2 correlation coefficient =
0.919). The
same analysis shows that for each additional percentage point of spawning with
spawn-supplement formula 83, spawn run time is shortened by 0.67 days (RZ
correlation coefficient = 0.856). Clearly, spawn-supplement results in a
faster
spawn run than the use of rye spawn, which has larger particles and fewer
points of
inoculum. There is no clear effect of addition of supplementary nutrients on
spawn
run time, although some data show an increase in mushroom yield with further
supplementation.
A shortenend spawn run is advantageous for many mushroom growers,
particularly those employing the bed (or shelf) and bag methods of growing
mushrooms. A faster spawn run reduces the time between the beginning of a
mushroom crop and the appearance of the first mushrooms. Reducing this
unproductive period improves the efficiency of a mushroom farm. By shortening
the sewn run time, more crops per year can be grown in a given space,
resulting
in an overall mushroom yield increase for a facility. A shorter total cropping
period
also reduces the time available for pests and pathogens to become established.
Short
cropping cycles are associated with a reduction in diseases and pests on
mushroom
farms.
TemneTatnre effect/ awn-c dement recictc microbi 1~~; ~ spa~_
supplement, all particles are heavily colonized with Agaricus bisporus
mycelium.
This heavy mycelial growth is believed to effectively reduce or prevent the
growth
of competing microorganisms on the particles. The reduction or prevention of
13
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
growth by competitors minimizes the compost temperature increases often
associated
with the use of mushroom supplements. Agaricus bisporus has an optimum
temperature for growth of about 25 to 26°C. Growth is progressively
reduced
above about 28°C, and virtually absent above about 35°C.
Agaricus bisporus is
S physiologically incapable of causing dangerously high temperatures in the
compost,
since its growth rate and associated rate of metabolism are reduced above its
tempesat<lre optimum. In contrast, many of the competitor microorganisms
present
in mushroom compost are cable of growing at elevated temperatures. Indeed, the
composting process selects for thermotolerant and thermophilic microorganisms
(Fermor et al. 1985). If provided with available nutrients, the metabolic heat
from
these microorganisms would increase the compost temperature to levels that
would
be dangerous to Agaricus bisporus.
To demonstrate the effect of heavy Agaricus bisporus mycelial growth on
competitor microorganisms, the following experiment was conducted. Spawn-
supplement formula 83 was prepared and sterilized as usual. One replicate was
inoculated with Agaricus bisporus and allowed to grow for 14 days. A second
replicate was uninocuhtted and held under sterile conditions for 14 days. A
rye
spawn formula was prepared as usual, with one replicate inoculated and another
left
uninoculated. In addition, cracked soybeans with a supplement coating (i.e.
S41)
and without a coating (untreated soybeans) were obtained. S41 and untreated
cracked soybeans were moistened with sterile deionized water. Sterility was
relaxed
on day 0 of this test, and all materials were placed in nonsterile petri
dishes and
maintained at 25°C, ca. 80~ relative humidity. Materials were inspected
daily for
evidence of mold growth and bacterial contamination. Results of this test are
summarized in Table 1.
14
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Table 1.
TREATMENT MICROBIAL GROWTH
..-_"_
RYE SPAWN NO GROWTH IN 9 DAYS
RYE SUBSTRATE MOLD AND BACTERIA, 4 DAYS
FORMULA 83 SPAWN-SUPPLEMENT NO GROWTH IN 9 DAYS
FORMULA 83 SUBSTRATE MOLD AND BACTERIA, 4 DAYS
S41 SUPPLEMENT MOLD IN 6 DAYS
CRACKED SOYBEANS, NO COATINGMOLD IN 3 DAYS
This test clearly shows that colonization of a nutrient substrate by the
Agaricus bisporus mycelium exerts a protective effect against attack by molds
and
bacteria. A similar antimierobial effect is seen when spawn and spawn-
supplement
is added to compost. As shown in several examples, spawn-supplements do not
support mold growth in compost. In contrast, other commercially available
supplements eventually support the growth of mold and other microorganisms.
The
absence of growth of competitor microorganisms results in lower peak compost
~mpduring spawn run. Lower compost temperatures are an advantage for
mushroom growers, since the deleterious effects of heat on Agaricus bisporus
growth are avoided. In addition, lower peak compost temperatures will result
in
cost savings by avoiding the need for air conditioning to reduce dangerously
high
compost temperatures. This is especially true during warm weather periods.
In addition to lower maximum compost temperatures, production data from
several commercial mushroom farms show that the compost temperature increases
that occur do so several days earlier with spawn-supplement than with a grain
spawn
plus supplement combination: Addition of rye spawn plus supplement typically
results in a maximum compost temperature at about 8 to 10 days after spawning.
In contrast, the maximum compost temperature observed with spawn-supplement
often occurs about 5 or 6 days after spawning. This change in the time of the
peak
compost temperature is of value to mushroom growers, since it avoids high
temperatures at and after casing. High compost temperatures can require
several
days to be brought under control. The time can be even longer during summer
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03Z54
months or on mushroom farms with marginal cooling capacity. Addition of a peat
moss casing layer to mushroom compost provides an insulating effect. If
compost
temperatures are even marginally high, the casing layer exacerbates the
effect, and
can result in dangerously high temperatures. By providing a maximum compost
temperature several days earlier, the use of spawn-supplement reduces the
possibility
that post-casing spawn run temperatures will reduce mushroom yield.
'' : A clear advantage of the spawn-
supplement formulas as disclosed is that the yield increases and protection
against
competitor microorganisms are achieved without the use of physical or chemical
treatments. Addition of chemicals such as formaldehyde or fungicides to
nutrient
supplement mixtures can result in substantial cost disadvantages. The
chemicals
used may represent safety or environmental hazards. As noted by Romaine &
Marlowe (U.S. Pat~t No. 5,427,592), future use of biohazardous chemicals in
the
mushroom industry is tenuous. Formaldehyde has been restricted by the U.S.
Environmental Protection Agency, and California now requires the routine
monitoring of workers handling one supplement for formaldehyde exposure. Heat
tn~nents of supplements are also costly. By including supplementary nutrients
in
a material that is already subject to steam heat to achieve sterilization,
substantial
cost advantages can be achieved over having larger quantities of two different
heat
treated materials (i.e., both spawn and supplement).
Protection apainct Trichoderma (~,~1 Mol,d), diceace; The worldwide
mushroom industry has recently been plagued by a virulent "Green Mold" disease
caused by ?)richode»na hanianum. Substantial losses in mushroom production,
with an attendant monetary loss, have been experienced in the U.S., Canada,
England, Ireland, and elsewhere. Tests have shown that the presence of soluble
carbohydrate due to grain spawns contributes to the growth of the virulent
Tricnoderma {Fletcher, 1997). Since the spawn-supplement formula as described
contains little starch or other readily available carbohydrate, the use of
this formula
was found to reduce the incidence and severity of the green mold disease.
Tests at
the Campbell's Fresh Prince Crossing mushroom farm (West Chicago, IL) and
other
commercial mushroom operations have shown that green mold disease is
16
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
substantially reduced when spawn-supplement formulas 80 or 83 are used to
spawn
mushroom beds (sae examples 2 and 7).
To further investigate the ability of spawn-supplement to resist Trichodernia
harzianum infection, the following experiment was conducted. Four large
sterile
screw-capped glass tubes were filled with either 30 g of rye spawn prepared
g to ~dand procedums or 30 g of spawn-supplement formula 83. In both
cases, the substrates were fully colonized with Agaricus bisporus strain M466.
A
single agar plug containing a sporulating culture of virulent Trt'choderma
harzianum
biotype TFI4 was placed on the surface of the substrates in each tube. Tubes
were
loosely capped to allow air exchange and incubated at room temperature for 6
days.
Three of the four tubes containing rye spawn showed vigorous growth of T.
harzianum within the six day period. No sporulation of the T. harlianum was
observed within that period. None of the four tubes containing spawn-
supplement
showed growth or sporulation of the virulent Trichoderma. In a parallel test,
all
tubes containing either rye spawn or spawn-supplement formula 83 that were not
heavily colonized with Agaricus bisporus supported the growth and sporulation
of
?~richoderma harzianum. It is clear from this test that fully colonized spawn-
supplement formula 83 resists the growth of the causative agent of "Green
Mold"
disease. Commercial mushroom production data further support the conclusion
that
the use of spawn-supplement helps to reduce or eliminate the incidence of
"Green
Mold" disease.
trient c ,rce; The p~cipal nutrient source is one that provides
high levels of protein nitrogen. While corn gluten is a favored principal
nutrient
source, other ingredients may be substituted successfully. Corn gluten meal is
the
dried residue from corn after the removal of the larger part of the starch and
germ,
and the separation of the bran by the process employed in the wet milling
manufacture of corn starch or syrup, or by enzymatic treatment of the
endosperm.
Corn gluten is water insoluble and hydrophilic, making it particularly
suitable for
use as a nutrient by a saprophytic fungus. Corn gluten is available from
several
sources, including Cargill, Inc. Corn gluten typically contains either 60 ~
protein
content (9. 6 ~ nitrogen) or 48 9o protein content {7. 68 96 nitrogen) . There
is no
17
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
apparent qualitative differeboe in perfonmanoe using either 60~ or 4896
protein corn
gluten. However, use of the 6096 protein corn gluten allows the addition of
higher
nitrogen contents to a given spawn-supplement formula.
Hydrolyzed feather meal is also a favored principal nutrient that can be used
alone or in combination with corn gluten or other nutrient source. Feather
meal is
tile product resulting from the treatment under pressure of clean,
undecomposed
feathers from slaughtered poultry. Feather meal typically contains 80-85'%
pmtein,
with over 75 9b of the crude protein in a digestible form. Feathers contain a
high
content of,keratin, a class of fibrous proteins found in vertebrate animals.
Because
of extensive cross linking of disulfide bonds, keratins are more resistant to
hydrolysis than most other proteins. This resistance to hydrolysis makes
keratin
suitable for use as a nutrient by a saprophytic fungus. Keratin can absorb and
hold
water, but is generally insoluble in water and organic solvents.
Other principal nutrient sources that have been used successfully in preparing
spawn-supplement are listed in Table 2.
18
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Table 2. Nutrient sources for spawn-supplement
NUTRIENT SOURCE ~ ~OG~
42.00
~T~ M~- 15.30
BLOOD MEAL 14.38
CORN GLUTEN 11.00
CONDENSED FISH SOLUBLES 9.68
DRIED ALGAE (SCENDESMUS) 8.14
PEANUT MEAL 8.00
SOYBEAN MEAL 7.68
YEAST SLUDGE 7.65
COTTONSEED MEAL 7.50
SAFFLOWER MEAL 7.31
CHEESE WHEY 7.31
SUNFLOWER MEAL 7.16
WHOLE CRACKED SOYBEANS 6.40
WHOLE SOYBEANS 6.40
CANOLA MEAL 6.06
LINSEED MEAL 5.98
DISTILLERS DRIED GRAIN 4.75
COTTONSEED WASTE 3.89
CORN STEEP LIQUOR 3.65
WHOLE CANOLA 3.52
'A 2.96
WHEAT BRAN 2.75
WHEAT FLOUR 2.71
CHICKEN LTITER 2.70
2.58
BONE MEAL 2.45
TURKEY LITTER 2.20
GRAPE PUMICE 2.03
S~~~ 1.84
R~ ~~ 1.83
P~~T ~~ 1.79
BARLEY FLOUR 1.76
SOYBEAN HULLS 1.62
GROUND CORN 1.53
BLUE CORN MEAL 1.48
CORN FLOUR 1.40
YELLOW CORN MEAL 1.26
COTTONSEED HULLS 0.64
CORN STARCH 0.11
19
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
The nutrients with the highest nitrogen contents are favored for use in
spawn-supplements, since they allow the highest possible total nitrogen
content in
the finished product. The nutrient sources generally contain protein nitrogen
and
may contain fats, oils, carbohydrates, and micronutrients. Persons skilled in
the art
could imagine many more possible nutrient sources. While an abundance of
experimental data show that pmtein nitrogen is a favored nutrient source for
Agaricus bisporus, other nutrients in the proper form and proportion could
easily
be defined by routine experimentation.
F~.~llels: Paper pellets preferably ate, but not limited to, a mixture of
53!& shredded paper (newsprint or bond paper), 22 ~b peat moss ( < 35 R&
moisture),
179b proteinac;eous material (soybean fines, etc.), 5.49b CaC03, and 1.6~
CaS04~2
HzO. The mixture is pelleted to a 3.2mm (1/8") diameter cylinders at 70 to
82°C
and an 18.2 lcg/hour feed rate. By assuring that the peat moss ingredient has
a
moisture of < 35 9b, the finished pellets have a moisture content of < 12 % ,
and
therefore do not support mold growth. The material typically has a nitrogen
content
of 1.5 to 1.6 % . Pelleting is done to improve the handling of the material.
The
pelleted material has a higher density and lower volume than unpelleted
material,
and is well mixed. Pellets are hammer milled such that about 80 9~ of the
resulting
fragments are between 4.75 mm and 0.85 mm in size. The pellets fall apart
after
being hydrated to provide a larger number of small particles and "points of
inoculum".
Earli~lat~mat~~l: A particulate material such as calcined earth, perlite,
vermiculite, or other ingredient is added to the spawn-supplement formula to
provide multiple points of inoculum, increase water holding capacity, aerate
the
mixtures, control the density of the mixture, and help to maintain a free
flowing
characteristic. Typical particulate ingredients include calcined earth,
vermiculite,
and perlite, but other particulate materials can be substituted successfully.
Calcined earth is a clay based material that is subjected to a calcination
process. The clay is heated to a temperature below its melting point to bring
about
a state of thermal decomposition. The calcination process results in a porous
material that readily absorbs water. Depending on the particle size, calcined
earth
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
can absorb at least 1006 of its weight in water. Calcined earth is
commercially
available under the "Turface", "Oil Dri", and other brand names. Calcined
earth is
available in a range of particle sizes. Dry calcined earth has a density of
approximately 598 g/1 for the 8/16 mesh size. Various particle sizes affect
the
density of the finished spawn-supplement product, and therefore are useful in
formulating the product. The functional characteristics of calcined earth are
similar
regardless of the particle size. Smaller calcined earth particle sizes are
perceived
to be preferable in that they deliver more points of inoculum per unit weight.
Vermiculite is a hydrated magnesium-iron-aluminum silicate treated at high
temperatures to cause expansion. The material has a low density (97 to 109
g/1),
is water insoluble, and can absorb 200 to 500 ~ of its weight in water.
Perlite is a volcanic glass material that is heated to cause its expansion and
to improve its ability to hold moisture. It is typically used as a plant
growth
medium. It has a low density of about 109 g/1, and can absorb about 250 of its
weight in water.
The selection of the appropriate particulate material for the spawn-
supplement formula is based on desired final product density, particle sizes,
desired
number of particles (points of inoculum), cost, ease of handling and use, and
other
characteristics. The spawn application equipment used by most mushroom growers
is designed and optimized to deliver specific weights and volumes of grain
spawn.
High density materials such as calcined earth can be mixed with low density
materials such as vermiculite and perlite to closely approximate the density
of grain
spawn in the finished spawn-supplement formula.
One beneficial characteristic of the particulate materials used in spawn-
supplement formulae is that they generally contain pores, hollows, and a rough
texture. The Agaricus bisporus mycelium grows into these pores, and is
protected
from damage due to abrasion as spawn is shaken during preparation or
immediately
prior to its being added to compost. In grain spawn, virtually all mycelial
growth
is on the surface of the la~rnels. When abraded, the surface mycelia are
effectively
scrubbed off, exposing the surface of the grain to potential contamination by
competitor microorganisms. The protection from abrasion afforded by the rough
21
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
texture of the particulate material makes the spawn-supplement resistant to
the
deleterious effect of shaking and abrasion.
The texture of the particulate materials is also of value in that the pores
and
hollows allow good aeration of the mixtures and help to avoid clumping of
mixtures. Good aeration also helps in the sterilization process. Successful
steam
sterilization of a material requires that steam penetrate throughout the mass.
A
P~'lY ~~ ~~ cts the penetration of steam. Dense clumps of material
also restrict the penetration of steam. A failure of steam to penetrate the
mixture
results in cold spots that will not be successfully sterilized. The locally
unsterilized
areas of the mixtures reinoculate the substrate, resulting in contamination of
the
product. Sterilization failures are oflexl due to the presence of bacterial
spores, such
as Bacillus spp. Bacillus contamination renders spawn unsuitable for use.
On occasion, a dense clump of a mixture achieves commercial sterility, but
is not adequately colonized by the Agaricus bisporus mycelium due to poor
oxygen
penetration. Agaricus bisporus is a strictly aerobic fungus. Poor oxygen
availability in the center of a clump of unmixed material restricts the growth
of the
fungus in the clump. When the uncolonized clump is eventually blended with
mushroom compost, the nutrients can become available to the compost
microorganisms. The availability of the nutrients results in the growth of
competitor molds and high temperature in the compost. Inclusion of a
particulate
material (i.e., calcined earth) in the spawn-supplement formula reduces the
formation of clumps in the mixtures and allows better oxygen penetration in
the
clumps that do form.
ion n c' CaC03 is added to the spawn-supplement formula
to control pH through a buffering effect. Agaricus bisporus typically releases
organic acids during growth. Inclusion of CaC03 in the formula avoids a
significant
reduction in pH during growth, Typical, but not limiting amounts include about
1
to 12 ~ CaC03, more typically about 6 to 9 ~ . Spawn-supplement formulas
typically have a pH of about 7.2 immediately before being inoculated when made
with tap water. The pH of the finished product is typically about pH 6.7. The
exact content of CaC03 does not appear to be critical. The pH may range
between
22
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
6 and 7.8, but is preferably between 6.2 and 7.4, and more preferably about
6.4-
6.9.
CaS04 ~ 2 HZO (gypsum) may be added to the spawn-supplement formula
at approximately 7 to 8 ~ of the total dry weight. The CaS04 appears to coat
the
outside of the particles to avoid clumping and make any lumps that do form
easier
to break up. The CaS04 is an optional, but desirable, component of the
formula.
CaS04 and CaC03 may be premixed in a 1:1 mixture to simplify addition of the
ingredients.
~at~r/moicm n Pnr~ The optimum moisture content for spawn-
supplement is 489b moisture at the time of addition to compost. While rye and
millet spawns generally lose moisture during sterilization and growth, spawn-
supplement formulas do not lose a significant amount of moisture due to
evaporation. Therefore, most formulas are adjusted to 48 to S0~ moisture prior
to
sterilization. This moisture content allows vigorous growth of Agaricus
bisporus
mycelium on the substrate and optimum performance in the compost. This lower
moisture content also helps to prevent the formation of clumps and allows
better
oxygen penet<ation into the mixtures. This helps to prevent sterilization
failure and
uncolonized areas of the final product.
Spawn-supplement mixtures are prepared by placing dry ingredients in a
large mining container such as a paddle mixer, cement mixer, or other suitable
container in which the mixtures can be blended to obtain homogeneity.
Ingredients
are weighed, placed in the mixer, and mixed until thoroughly blended.
Sufficient
water' is added as a fine spray to bring the mixtures to approximately 48 ~6
moisture.
Additional mixing after the addition of water reduces any clumping that may
occur.
Polycarbonate jars (4.84 1 total capacity) are filled with 2.8 kg of the
hydrated
mixtures. This weight of a standard spawn-supplement formula (i.e., formula
83)
fills the jars to approximately 75 to 80~ of capacity. Some formulas are
denser
than formula 83. With denser formulas, the jars contain less total volume.
Jars are
filled either manually or with an automated jar filling machine. Jars are
capped
with lids containing a breathable filter element that allows the passage of
air and
23
CA 02320733 2000-08-21
WO 99/419b8 PCT/US98/03254
steam but prevents the passage of microorganisms that would contaminate the
finished product. The mixtures are steam sterilized at times and temperatures
needed to achieve commercial sterility. This is typically 124°C for 150
minutes.
Following sterilization, mixtures are cooled to less than ca. 27°C.
Jars are briefly
opened under aseptic conditions, and an inoculum is added. The inoculum may
consist of millet or rye grain colonized with a suitable strain of the
Agaricus
bisporus fungus, and is added to jars .at about 1.1 to 1.3 ~ (voUvol).
Mixtures may
also be inoculated with non-grain substrates colonized with Agaricus bisporus
mycelium (LT.S. Patent No. 5,503,647) at a similar inoculation rate.
Immediately
following inoculation, jars are briefly shaken in a modified commercial paint
shaker
to distribute the inoculum throughout the mixture and to break up any lumps
that
may have formed during sterilization. Jars are incubated at approximately 25
° C for
4 to 6 days, at which time they are again shaken to evenly distribute the
growing
mycelium. After an additional 4 to 6 day incubation at 25°C, the
mixtures are
evenly colonized with mushroom mycelium. The spawn-supplement can be used
immediately, or can be stored in the jars under refrigerated conditions (less
than 3.3
to 4.4°C). Alternatively, the contents of the jars can be transferred
to ventilated
plastic bags and stored pending use. Packaged mushroom spawn, including the
presently disclosed spawn-supplement, is typically stored at less than
5.5°C for
approximately 14 to 21 days to allow the "regrowth" of the mycelium and the
development of an even white color associated with heavy mycelial
colonization.
While the above description describes the method of spawn-supplement used
by the inventors, persons with ordinary skill vould easily prepare spawn-
supplement
formulas by other methods used for spawn production. These methods include,
but
are not limited to, the methods described above (Background of the Invention).
i TsP of S awn- ~ 1 m n
Spawn-supplement is used in a manner similar to standard grain spawn and
mushroom supplement combination. Details of use are inherent in the examples
cited, and are familiar to those skilled in the art of growing mushrooms.
24
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula 83
Corn Gluten (60~ protein) 30.2
g
Paper Pellets 14.5
g
Calcined Earth (8/16 mesh) 29.1
g
Feather Meal (15.4 ~ nitrogen) 17.4
g
CaC03 8.7 g
Wad 75.6
ml
'The nitrogen content of this formula is 6.39 9b . Spawn-supplement formula
83 was Prepared essentially as described above, and was stored at < 42
°F. In this
specific example, phase II mushroom compost was used. The compost was a
standard wheat straw/horse manure blend formula that had undergone a 22 day
phase I composting process and a 9 day phase II process. Compost (193 lb fresh
weight, equivalent to 72 lb dry weight at 63 ~ moisture) was filled into each
of 12
4' x 3' wooden trays (6 lb/ft2 dry weight). Trays were individually dumped
onto
a conveyor belt. Four trays were each inoculated with 982 g rye spawn, strain
M466 (3 ~ rate) and were amended with 1, 309 g S44 supplement (4 9~ rate; S44
is
heated, cracked soybeans treated with a hydrophobic coating and mold
inhibitory
composition). The spawn and supplem~t wec~e thoroughly mixed into the compost,
and compost was returned to the trays. Eight trays were spawned with 1,636 g
spawn-supplement formula 83 (listed as "SS83" in Table 3, equivalent to 5 ~
fresh
weight spawn-supplement to dry weight compost). Compost in all 12 trays was
hY~~IY ~mP, veered with polyethylene sheets to reduce moisture loss,
and placed in an environmentally controlled room. Humidity in the room was
maintained at 85%, and air temperature was controlled by a Fancom model 1060
mushroom computer in an attempt to maintain a 76°F compost temperature.
Compost and air temperatures were recorded at 240 minute intervals with a data
acquisition system, at 255 minute intervals by the Fancom computer, and at
daily
intervals using mercury thermometers. Trays were inspected daily to assess the
growth of the Agaricus bisporus mycelium and for the presence of molds. After
15
days of spawn run, trays were top dressed with a 2° casing layer
consisting of
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Sunshine brand peat moss, CaC03, and water (to 85 ~ moisture). The casing
layer
was inoculated with 0.07 units/ftz casing spawn. A standard temperature regime
was maintained to promote mycelial growth into the casing layer, and trays
were
watered as needed. All trays were "flushed" by the introduction of fresh air
and
cooling to 66°F on day 6. Mushrooms were first harvested 15 days after
casing.
Mushroom yield data (lb/ft ) for this test are as follows:
Table 3. Yield data (in lb/ftz) for experiment 892.
SPAWN 1~' BREAK 2'''n BREAK 3'~ BREAK TOTAL
3 ~ RYFJ4 ~ 2. 69 1. 83 0.94 5.47 B
S44
5 ~ SS 83 2.44 2.26 1.39 6.10 A
Values with the same letter in the "total" column are not statistically
significant at
the 95 ~ confidence level.
The average yield using the formula 83 spawn supplement was 0.63 lb/ftz
higher than using the standard rye spawn plus soybean based supplement
formula.
This yield increase is statistically significant at the 95 ~ confidence level.
The
formula 83 spawn supplement also appears to provide a more desirable mushroom
production pattern. Mushroom production is typically highest in the first
break,
with lower yields in subsequent breaks. High first break yields are sometimes
associated with poorer mushroom quality because of problems with air
circulation
amund and between mushrooms and the resultant higher localized humidity. The
formula 83 spawn supplement provides a reduced first break yield, but
proportionally higher yields in the second and third breaks for an overall
yield
increase.
Mold growth on the standard rye spawn plus S44 supplement combination
was rated as medium to heavy. This combination can support the growth of
mucoraceous fungi as well as Penicillium, Aspergillus, and other common
airborne
molds. No mold growth was observed at any time in this trial on trays
inoculated
with the formula 83 spawn-supplement. The absence of mold growth on trays
26
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
inoculated with the formula 83 spawn-supplement is consistent with temperature
data for this trial. Temperature data for this trial are summarized in Figure
3.
The standard rye sewn plus supplement combination had a peak compost
temperature of 33.8°C on day 7 of spawn run. The spawn-supplement
formula in
this example had a maximum compost temperature of 32.5°C, also on day 7
of
spawn run. While maximum compost temperatures differ, the average compost
temperatures over the 14 day spawn run are similar (29.3 ° C for the
standard rye
spawn plus supplement, 29.2 for the spawn-supplement). The heat released by
the
spawn-supplement is spread over a longer period rather than being manifest as
temperature surges during spawn run. This helps to protect the Agaricus
bisporus
mycelium from the deleterious effects of high compost temperatures.
Formula 80
Corn Gluten (60 X protein) 30.3 g
Paper Pellets 22.4 g
Vermiculite 19.4 g
Calcined Farth 18.8 g
CaC03 9.1 g
Water 78.8 ml
The nitrogen content of this formula is 3.54 ~ . 1fie formula 80 spawn-
supplement was made in a manner essentially as described previously. Following
full colonization of the spawn-supplement, the material was packaged in
ventilated
polypropylene bags (20 lb/bag) and stored at 38°F for approximately 21
days. The
spawn-supplement was shipped to a standard "bed style" mushroom farm in which
phase I compost is filled into fixed beds or shelves. Phase II pasteurization
and
conditioning, spawn run, case holding, and cropping occur in the beds without
further moving the compost. This facility fills 6,830 ft of bed space per
house, and
typically fills the beds with fresh compost at 28 to 29 lb/ftz of bed space.
This
translates to approximately 7 to 8 lb/ftz dry weight after phase II
pasteurization and
conditioning. A block of 9 houses was used for this test (house numbers 51 to
59).
Seven houses used the standard mushroom growing conditions of 1,620 lb of rye
27
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
spawn (approximately 3 wt %; fresh weight spawn per dry weight compost) plus
1,600 lb of S44 supplement (approximately 2.9 wt %). Two houses were spawned
with the formula 80 spawn-supplement. House number 53 was spawned with 3,000
lb of the formula (approximately 6.3 wt %), while house 59 was spawned with
4,000 lb (approximately 7.3 wt %) of the formula. No supplement was used in
either house 53 or 59. All houses were substantially equal in terms of compost
quality and crap handling procedures. Rye spawn plus S44 supplement or formula
80 spawn-supplement were broadcast over the surface of the phase II compost. A
mushroom "digging machine" was used to mix the inocula into the compost in the
fixed beds. A digging machine is not unlike a garden tiller. All subsequent
mushroom cultivation steps (casing, flushing, etc.) are familiar to those
skilled in
the art of mushroom growing.
The seven houses of standard rye spawn plus S44 supplement gave an
average mushroom yield of 4.T7 lb/flz. This yield is somewhat lower than usual
for
this faality because of sporadic "green mold" (TYichodenrta hanianum biotype
TH4)
infections. The two houses spawned with the formula 80 spawn-supplement gave
an average yield of 5.13 lb/fti, for an average yield increase of 0.36 lb/ft Z
The
yield differences are not statistically significant due to the low number of
replicates
and unequal variances among the data. However, the 0.36 lb/fti yield increase
per
house would translate into an extra 2,880 lb of mushrooms per house. As noted
elsewhere, the preponderance of data show that the use of spawn-supplement
provides yields equal to or greater than the standard rye spawn plus
supplement
combinations. In this test, the increased yield is believed in part to be
associated
with a reduced incidence of green mold. House 53 had a total of 3 spots of
green
mold during the entire crop cycle. House 59 had a total of 6 spots of green
mold
during the crop. In ~ntrast, the remaining houses in this test block had an
average
of 84 green mold infection sites (range 40 to 139). The rapid colonization of
the
compost plus the absence of starchy nutrients appears to reduce the severity
of the
green mold disease.
Spawn run temperatures for houses 52 (rye spawn plus supplement) and 53
(spawn-supplement formula 80) are shown in Figure 4. Although this test was
28
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
conducted in July, 1997, house 53, which contained 3,000 Ib of formula 80
spawn
supplement, did not show excessive spawn run temperatures. The average compost
temperature during spawn run was 76.8°F, with a maximum temperature of
82°F.
Even with a mechanical problem on day 9 that caused an increase in spawn run
temperature, the crop was easy to control. Use of the standard rye spawn/S44
supplem~t combination resulted in an average compost temperature of
78.3°F and
a maximum temperature of 85.3 °F. Despite the higher spawning rate with
the
spawn-supplement formula, compost temperatures were lower.
Formula 68
Rye Grain 27.8 g
Corn Gluten (60 Y6 pmtein) 27. 8
g
Paper Pellets 27.8 g
Vermiculite 8.3 g
CaC03 8.3 g
Water 75 ml
Formula 78
Rye grain 23.1 g
Corn Gluten (609b protein) 17.0 g
Paper pellets 23.1 g
Wheat Bran 23.1 g
Vermiculite 6.9 g
CaCO, 6.9 g
Water 73.7 ml
The nitrog~ content of formula 68 is 4.169b, while the nitrogen content of
formula 78 is 3.53 ~ . Formulas 68 and 78 spawn-supplements were prepared
essentially as described previously. This trial was conducted at the same
facility as
described for example 1. Four trays were inoculated with 3 ~b rye spawn,
strain
M466, and were amended with 4 ~c S44 supplement (S44 is heated, cracked
soybeans treated with a hydrophobic coating and mold inhibitory composition).
Four trays were spawned with 4 9b spawn-supplement formula 68. Four trays were
inoculated with 4 ~Y spawn-supplement formula 78. Four trays were inoculated
with
49b spawn-supplement formula 78 and amended with 3 X S44 supplement. Standard
29
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
mushroom growing procedures were used throughout this test. Mushrooms were
first harvested 15 days after casing.
Mycelial was rated as good for formula 68, formula 78, and formula
78 plus S44 after 6 days. After 15 days of spawn run, spawn growth was rated
as
excelletlt for the same 3 treatments. Gmwth of the rye spawn/S44 trays were
rated
as fair to good after 15 days. Very light growth of a mucoraceous fungus was
observed on trays with rye spawn/S44 supplement and the formula 68 spawn
supplement after 4 days. No gmwth of mold was observed on the formula 78 and
formula 78/S44 spawn combinations at any time. One tray from each of
treatments
1, 2, and 4 were infected with Trichalerma, and were discarded, yield data for
this
trial are summarized in Table 4.
Table 4. Mushroom yield data (in lb/ftz).
SPAWN 1~ BREAK 2i''D BREAK3~ BEAK TOTAL
,~_..-.-
.
3 % RYE & 4 % 1.87 A 1. 34 A 1.02 A 4.23 A
S44 B
4% FORMULA 68 2.00 A 1.82 A 1.14 A 4.96 A
B
4 % FORMULA 78 2.11 A 1.69 A 1.16 A 4.96 A
4 % 78 & 3 % 1. 80 A 1. 61 A 0.79 B 4.20 A
S44
Columns with the same letter are not significantly different at the 95 %
confidence
level.
Although not statistically significant, the formula 68 spawn-supplement
combination yielded 0.73 lb/ftz more than the standard rye spawn plus standard
supplement combination. Also not statistically significant, the formula 78
spawn-
supplement also yielded 0.73 lb/ftz more than the standard rye spawn plus
standard
supplement combination. Interestingly, the formula 78 spawn-supplement gave a
reduced yield when amended with 4 % S44 supplement. Use of the 4 %
supplementation rate may add too much nutrient to the compost and result in an
inhibition of Agaricus bisporus growth or increase in the growth of competitor
microorganisms that could not be detected visually. Temperature data from this
test
are summarized in Figure 5. Spawn Run temperatures are consistently higher for
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
the rye spawn plus S44 supplement combination. Figure 5 shows the spawn run
temperatures.
Spawn-supplement formula 80 (as described in example 2) was prepared as
described for example 1. Approximately 3,000 lb of this spawn-supplement was
shipped to a "Dutch style" mushroom farm, in which phase II composting is
carried
out in bulk in pasteurization tunnels. Spawn is mixed with compost as it is
transported to bulk spawn run tunnels. Supplement is typically added to
compost
as it is being transported to fixed beds at the casing stage of the process.
Three
successive rooms comprised this test. Room 6 was spawned with 1,800 lb rye
spawn and supplemented (at casing) with 1,800 lb S44 supplement. Room 19 was
spawned with 2,920 lb spawn-supplement formula 80. Room 8 was spawned with
1,680 Ib rye spawn and supplemented (at casing) with 1,400 lb SF48 supplement.
The SF48 supplement consists of full fat soybean fines treated with an
antimicrobial
coating. Its performance is equivalent to S41 and 544. Note that although
these
rooms were spawned sequentially, the room numbers reflect the location on the
farm, not spawning order. Yield data for this trial are as follows:
Table 5. Mushroom yield data (lb/ft2)
,.-.. .. .
ROOM SPAWN l~ BREAK 2"' BREAK 3~ BREAK TOTAL
6 RYE/S44 3.16 1.61 ' .OS 4.82
19 SS 80 2.86 1.40 1.04 5.30
8 RYE/SF48 2.76 2.13 0.00* 4.89
*Third break in room 8 was lost due to "Bubble" disease caused by Verticillium
infection.
Although the mushroom yield increase in room 19 is not statistically
significant due to the low number of replicates, the yield of 5.30 lb/ftz is
substantially higher than in adjacent rooms. While detailed compost
temperature
data am not available for this test, mushroom farm personnel noted the absence
of
31
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
a "heat surge" in room 19. A peak in compost temperature is typically noted
around
days 8 to 10 of spawn run when a nutrient supplement is added to compost. This
surge was absent in room 19. This example also shows a very favorable mushroom
production pattern. Lower first break production is more than compensated by
the
increased second and third break yields.
Spawn-supplement formula 80 (see example 2) was prepared essentially as
described previously, and tested in a pilot plant trial as described in
example 1.
Four 4'x3' trays were spawned with 3 % rye spawn and supplemented with 4 ~ S41
supplement (treatment 1). Four trays were spawned with 5 ~ spawn-supplement
formula 80 (treatment 2). Four trays were spawned with 6°b spawn-
supplement
formula 80 (treatment 3). Four trays were spawned with 7~ spawn-supplement
formula 80 (treatment 4). Normal mushroom growing procedures were followed
throughout this trial. Yield data are summarized in Table 6.
Table 6. Mushroom yield data (ib/ft2)
- - ---- ,.
SPAWN I'' BREAK 2~ BREAK 3'~ BREAK TOTAL
_.-
RYE/S41 1.55 A 1.94 A 1.03 A 4.52 A
5 96 FORMULA 1.41 A 1. 68 A 1.07 A 4.16 A
80
6 ~ FORMULA 80 1.50 A 1. 84 A 0.99 A 4.33 A
7 9b FORMULA 1.53 A 1.90 A 1.14 A 4.57 A
80
Columns with the same letter are not significantly different at the 95 9~
confidence
level.
This trial shows that no statistically significant differences in mushroom
yield
occur with spawn-supplement levels between 5 and 7 °b of the dry weight
of the
compost. This and other trials suggest that the optimum spawning rate with
spawn
supplement is around 4 to 5 % . Temperature data from this trial are
summarized in
Figure b.
32
CA 02320733 2000-08-21
WO 99/41968 PCT1US98/03254
All spawn-supplement treatments showed lower spawn run compost
temperatures than the standard rye spawn plus supplement combination. Mold
growth (Mucor and Penicillium) was observed on trays spawned with rye spawn
plus
S41 supplement. No mold growth was observed on any trays inoculated with
spawn-supplement. Mold growth observations are consistent with temperature
data.
Spawn run was visually determined to be complete by day 10 of spawn run for
trays inoculated with spawn-supplement. Trays inoculated with rye spawn plus
supplement required about 15 days to complete spawn run.
Spawn-supplement formula 68 (example 3) and formula 80 (example 2) were
prepared essentially as described previously, and were tested for mushroom
yield
effects as described in example 1. Four trays were inoculated with 3 ~ rye
spawn
plus 4 ~ S41 supplement. Four trays were inoculated with 5 96 spawn-supplement
IS formula 68. Four trays were inoculated with 5 Y spawn-supplement formula
80.
Four trays were inoculated with 5 Rb spawn-supplement formula 80 and
supplemented with 2 ~ S41 supplement. Normal mushroom growing practices were
followed through out this test. Yield data are summarized in Table 7.
33
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Table 7. Mushroom yield data (lb/ft~
SPAWN 1" BREAK 2a 3'~ BREAK TOTAL
BREAK
3 % RYE/4 W S41 1.93 B 1.23 A 0.70 AB 3. 86
B
5 9& FORMULA 68 2.08 B 1.14 A 0.76 A 3.98
B
5 ~% FORMULA 80 2.06 B 1.19 A 0.57 B 3.83
B
5 Y FORMULA 2.68 A 1.19 A 0.60 B 4.46
80/2~%S41 A
Columns with the same letter are not significantly different at the 95 9&
confidence
level.
No significant differences in mushroom yield are observed between the
standard rye spawn plus supplement combination, spawn-supplement formula 68,
and spawn-supplement formula 80. However, when the spawn-supplement formula
80 is amended with 2 ~ S41 supplement, the mushroom yield is increased by 0.63
lb/ftz. Clearly the 5 ~ spawn-supplement plus 2 % S41 supplement combination
results in an improved productivity over unsupplemented spawn-supplement and
the
standard rye spawn plus 49b S41 supplement. Temperature data for this trial
show
that the highest spawn run compost temperatures were observed in trays with
rye
spawn and 4~b S41 supplement (Figure 6). Addition of 2~ S41 to the spawn-
supplement formula 80 trays did not result in a substantial increase in spawn
run
compost temperature compared with unsupplemented formula 80 trays. Mold
growth (Aspergillus) was observed on trays spawned with rye spawn and S41
supplement. A few spots of Neurospora mold were observed on trays spawned with
spawn-supplement formula 68. No other mold growth was observed on trays
inoculated with spawn-supplement. Spawn run for trays inoculated with formula
80
spawn-supplement was visually determined to be complete by day 12 of spawn
run.
Trays inoculated with formula 68 spawn-supplement completed spawn run in 14
days. Trays inoculated with rye spawn plus supplement required 14 days to
complete spawn run.
34
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Spawn-supplement formula 83 colonized with Agaricur bis~rus strain M473
was prepared as described previously and was shipped to a farm which has
experienced green mold disease. At the time of the test, each 3,200 ftz house
had
an average of 50 spots of green mold by the time of first break. In two houses
for
which data are available, house 1 showed one spot of green mold, while house 2
showed no green mold infection sites. Green mold infection is substantially
reduced
in houses spawned with spawn-supplement formula 83.
E$
Formula 83b Grams
Corn Gluten 30.2
Paper Pellets 14.5
Calcined Earth 29.1
Feather Meal 17.4
CaC03/CaS04 (1:1) 8.7
Wad' 75.6
~ Nitrogen (Calc) 6.39 %
% Moisture (Calc) 48.23
~
Formula 80b Grams
Corn Gluten 30.3
Paper Pellets 22.4
Vermiculite 19.4
Calcined Earth 18.8
CaC03/CaSO, (1:1) 9.1
Water 78.8
R~ Nitrogen (Calc) 3.54 ~
96 Moisture (Calc) 48.78
9~
35
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Formula 80c-2 Grams
Corn Gluten (60%) 8
Paper Pellets 33.6
Vermiculite 32.8
Calcined Earth 13.6
CaC03 12
Water 80
% Nitrogen (Calc) 1.38 %
% Moisture (Calc) 48.64
%
Formula 80c-16 Grams
Corn Gluten (60%) 63.5
Paper Pellets 13.3
Vermiculite 13.0
Calcined Earth 5.4
CaC03 4.8
Water 73.0
% Nitrogen (Calc) 7.01 %
% Moisture (Calc) 48.12
%
Formula 80d Grams
Corn Gluten (60%) 33.3
Paper Pellets 22.4
Vermiculite 18.2
Calcined Earth 17.0
CaC03 9.1
Water 78.8
% Nitrogen (Calc) 3. 87
%
% Moisture (Calc) 48.89
%
36
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Formula 80d-4 Grams
Corn Gluten (60 % ) 78.4
Paper Pellets 7.3
Vermiculite 5,g
Calcined Earth 5.5
CaC03 2.9
Water 72.5
% Nitrogen (Calc) 8.57 %
% Moisture (Calc) 48.37%
Formula 80d-4 Grams
Feather Meal (80%) 69.4
Paper Pellets 10.3
Vermiculite g,3
Calcined Earth 7.8
CaC03 4.2
Water ~,2
% Nitrogen (Calc) 10.10%
% Moisture (Calc) 48.01
%
Formula 80e-7 (P55) Grams
Linseed Meal 51.1
Paper Pellets 15.7
Vermiculite 13.2
Calcined Earth 13.6
CaC03 6.4
Water 74.5
% Nitrogen (Calc) 3.63 %
% Moisture (Calc) 48.66%
37
CA 02320733 2000-08-21
WO 99/41968 PCTNS98/03254
Formula 83 (P57) Grams
Corn Gluten 30.3
Paper Pellets 22.4
Calcined Earth 20.0
Feather Meal 18.2
CaC03 9.1
Water 78,8
~ Nitrogen (Calc) 6.58 %
96 Moisture (Calc) 48.789b
E~am pl~lZ
Formula 83-CS (P57) Grams
Cottonseed Waste 30.3
Paper Pellets 22.4
Calcined Earth 20.0
Feather Meal 18.2
CaC03 9.1
Water 78.8
96 Nitrogen (Calc) 4.69 96
96 Moisture (Calc) 48.78
96
Formula 83-s5 (P59) Grams
Whole Soybeans 51.1
Paper Pellets 15.7
Calcined Earth 14.0
Feather Meal 12.8
CaC03 6.4
Water 76.6
Nitrogen (Calc) 6.0396
Moisture (Calc) 48.74 ~
38
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula 83-c3 (P59) Grams
Cottonseed Meal 46.5
Paper Pellets 17.2
Calcined Earth 15.3
Feather Meal 14.0
CaC03 7.0
Water 76.7
% Nitrogen (Calc) 6.48 %
% Moisture (Calc) 48.66
%
Formula 83-c4 (P59) Grams
Ground Corn 54.5
Paper Pellets 13.5
Calcined Earth 12.0
Feather Meal 14.5
CaC03 5.5
Wad 76.4
% Nitrogen (Calc) 3.61 %
% Moisture (Calc) 48.74
%
Formula 83-sh2 (P61) Grams
Soybean Hulls 30.3
Paper Pellets 22:4
Calcined Earth 20.0
Feather Meal 18.2
CaC03 9.1
Water 78.8
% Nitrogen (Calc) 3.94
%
% Moisture (Calc) 48.78
%
39
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P69-1 Grams
Feather Meal 16.5
Corn Gluten 24.8
Calcined Earth 33.9
Paper Pellets 16.5
CaC03 8.3
Water 78.4
% Nitrogen (Calc) 5.58'%
% Moisture (Calc) 48.23 96
Formula P69-2 Grams
Feather Meal 24.8
Corn Gluten 16.5
Calcined Earth 33.9
Paper Pellets 16.5
CaC03 8.3
Water 78.4
X Nitrogen (Calc) 6.09 %
% Moisture {Calc) 48.239&
Formula P71-3 Grams
Peanut Hulls 53.6
Paper Pellets 8.9
Calcined Earth 17.9
Feather Meal 14.3
CaC03 5.4
Water 75.0
'i6 Nitrogen (Calc) 3.61 ~Y
96 Moisture (Calc) 48.12
96
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P71-4 Grams
Bone Meal 55.6
Paper Pellets 9.3
Calcined Earth 18.5
Feather Meal 11.1
CaC03 5.6
Water 75.9
% Nitrogen (Calc) 3.53 %
% Moisture (Calc) 48.48 %
Formula P73-w4 Grams
Wheat Flour 55.6
Paper Pellets 9.3
Calcined Earth 18.5
Feather Meal 11.1
CaC03 5.6
Water 75.9
% Nitrogen (Calc) 3.69 %
% Moisture (Calc) 48.48 %
Formula P73-cs4 Grams
Corn Starch 50.0
Paper Pellets 8.3
Calcined Earth 16.7
Feather Meal 20.0
CaC03 5.0
Water 76.7
% Nitrogen (Calc) 3.50%
% Moisture (Calc) 48.45 %
41
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P73-bf4 Grams
Barley Flour 53.6
Paper Pellets 8.9
Calcined Earth 17.9
Feather Meal 14.3
CaC03 5.4
Water 73.2
6 Nitrogen (Calc) 3.59 ~&
~ Moisture (Calc) 47.59
~
Formula P83-cf8 Grams
Corn Flour 62.5
Paper Pellets 6.3
Calcined Earth 12.5
Feather Meal 15.0
CaC03 3.8
Water 75.0
~ Nitrogen (Calc) 3.61 %
~ Moisture (Calc) 48.40%
Formula P75-yc4 Grams
Yellow Corn Meal 43.5
Paper Pellets 10.9
Calcined Earth 21.7
Feather Meal 17.4
CaC03 6.5
Water 78.0
~ Nitrogen (Calc) 3.69'
Moisture (Calc) 48.66 Ro
42
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P75-bc4 Grams
Blue Cornmeal 43.5
Paper Pellets 10.9
Calcined Earth 21.7
Feather Meal 17.4
CaC03 6.5
Water 78.0
~ Nitrogen (Calc) 3.79 96
96 Moisture (Calc) 48.669b
Formula P75-rf4 Grams
Rye Flour- 53.6
Paper Pellets 8,9
Calcined Earth 17.9
Feather Meal 14.3
CaC03 5.4
Water 75.0
'~ Nitrogen (Calc) 3.63 %
~ Moisture (Calc) 48.1296
Formula P75-pmt Grams
Peanut Meal 29.4
Paper Pellets 14.7
Calcined Earth 29.4
Feather Meal 17.6
CaC03 g, $
Water 79.4
96 Nitrogen (Catc) 5.72 96
R6 Moisture (Calc) 48.6296
43
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P87-rf Grams
Rye Flour 64.1
Paper Pellets 6.4
Calcined Earth 12.8
Feather Meal 12.8
CaC03 3.8
Water 74.4
96 Nitrogen (Calc) 3.581&
~ Moisture (Calc) 48.28 9b
Formula P87-wf Grams
Wheat Flour 63.4
Paper Pellets 7.6
Calcined Earth 15.2
Feather Meal 9.1
CaC03 4.6
Water 76.2
~% Nitrogen (Calc) 3.58 R&
~ Moisture (Calc) 48. 83
90
Formula P87-bf Grams
Barley Flour 64.1
Paper Pellets 6.4
Calcined Earth 12.8
Feather Meal 12.8
CaC03 3.8
Water 74.4
~ Nitrogen (Calc) 3.53 R
~ Moisture (Calc) 48.28
44
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P87-yc Grams
Yellow Corn Meal 51.7
Paper Pellets 8.6
Calcined Earth 17.2
Feather Meal 17, 2
CaC03 5.2
Water 75.9
~ Nitmgen (Calc) 3.76R&
R~ Moisture (Calc) 4$.29gb
Formula P87-be Grams
Blue Corn Meal 58.8
Paper Pellets 7.4
Calcined Earth 14.7
Feather Meal 14.7
CaC03 4.4
Water 76.5
Nitrogen (Calc) 3.57'
96 Moisture (Calc) 48.72 96
Formula P89-83b Grams
Feather Meal 20.0
Corn Gluten 30.0
20.0
Paper Pellets 20.0
CaC03 10.0
Water 77.9
96 Nitrogen (Calc) 6. 80
96
~ Moisture (Calc) 48.40
45
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Formula P89-83b-3 Grams
Feather Meal 20.0
Corn Gluten 30.0
Calcined Earth 20.0
Paper Pellets 16.6
CaC03 10.0
Enhanced Oat Fiber 3.4
Water 71.1
~ Nitrogen (Calc) 6.99 ~
~ Moisture (Calc) 48.24 %
Formula S41: greater than 95 % cracked soybeans with preservative coatings.
Cracked soybeans are dehulled, cracked, and screened to retain a ca. 30 mg
fraction. "Soybean fines" are what is left over after the cracked soybeans are
removed.
References:
Chang, S. T. & W. A. Hayes. 1978. The Biology and Cultivation of
Edible Mushrooms. Academic Press, New York. 819 pp.
Chang, S. T. & P. G. Miles. 1989. Edible Mushrooms and Their
Cultivation. CRC Press. Boca Raton, FL. 345 pp.
Elliott, T. 7. 1985. Spawn-making and Spawns. Chapter 8, Pages 131-
139, In: Flegg, P. B., D. M. Spencer, & D. A. Wood. The Biology and
Technology of the Cultivated Mushroom. John Wiley & Sons, Ltd. Chichester.
Fermor, T. R., P. E. Randle, & J. F. Smith. 1985. Compost as a Substrate
and its Preparation. Chapter 6, Pages 81-109, In: Flegg, P. B., D. M. Spencer,
&
D. A. Wood. The Biology and Technology of the Cultivated Mushroom. John
Wiley & Sons, Ltd. Chichester.
Flegg, P. B., D. M. Spencer, & D. A. Wood. 1985. The Biology and
Technology of the Cultivated Mushroom. John Wiley & Sons, Ltd. Chichester.
347 pp.
46
CA 02320733 2000-08-21
WO 99/41968 PCT/US98/03254
Fletcher, J. T. 1997. Mushroom Spawn and the Development of
Trichodernra Compost Mold. Mushroom News 45:6-11.
Fritsche, G. 1978. "Breeding Work." Chapter 10, pages 239-250, In:
Chang, S. T. & W. A. Hayes, Eds. "The Biology and Cultivation of Edible
Mushrooms." Academic Press, NY.
Lemke, G. 1971. Erfahrungen mit Perlite bei der Myzelanzucht and
Fruchtkorperproduktion des Kulturchampgnons Agaricus bisporus (Lge.) Sing.
Gartenbauwissenschaft 1:19-27.
Van Griensven, L. J. L. D. 1988. "The Cultivation of Mushrooms."
Darlington Mushroom Laboratories, Ltd. Russington, Sussex, England. S 15 pp.
It will be apparent to those skilled in the art that various modifications and
variations can be made in the methods and apparatus of the present invention
without departing from the spirit or scope of the invention. Thus, it is
intended that
the present invention cover the modifications and variations of this invention
provided they come within the. scope of the appended claims and their
equivalents.
47