Note: Descriptions are shown in the official language in which they were submitted.
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
ARTICLES OF 1~L~NU~FACTURE AND METHODS
FOR STA.I1~TING BIOMOLECULES
Background of the Invention
Electrophoresis techniques have become principal tools for characterizing
biomolecules. The method is based on the fact that macromolecules such as
DNA, RNA and proteins possess a charge and can therefore move in an electric
field through sieving materials such as agarose or polyacrylamide. The
application of electrophoretic techniques has required the development of
chemical indicators for use in visualizing separated macromolecules. One such
indicator used to visualize DNA is ethidium bromide (EtBr). The disadvantage
of this widely used stain is that it is a potent mutagen. The potential
personal
hazard of directly contacting such a solution and the environmental hazard of
pouring it or other hazardous chemicals down the drain has resulted in a need
for a better and safer method of preparing, using and disposing of such toxic
solutions.
A procedure using entrapped stain in gels provides a vast improvement
for the containment of dyes in gels (Chirikjian and Collier, U.S. Patent No.
5,776,684). However, these gels have several disadvantages which include
stability, storage, sensitivity to cold temperatures and relative fragility.
Furthermore, a lack of manufacturability increases the cost of these gels
precluding their wide spread use. It is apparent, therefore, that an apparatus
which economically provides a means of safely staining biomolecules is needed.
Summary of the Invention
It is therefore an object of the present invention to provide a readily
manufacturable apparatus capable of safely staining or labeling biomolecules.
In accomplishing this and other objects of the invention, there is
provided, in accordance with one aspect of the present invention, an article
of
-1-
CA 02321100 2000-08-17
WO 99/42620 PC'T/US99/03704
manufacture comprising a flexible backing and a dry coating adhered to a
surface of the backing, wherein the coating comprises an indicator that stains
or
labels a biomolecule. In preferred embodiments of the present invention, the
backing promotes good contact with a gel containing a biomolecule, is
5 impermeable, allows the coating solution to be evenly spread and dried and
is
easily dispensable. In other preferred embodiments, the coating solution is
selected from the group consisting of starch, glue, gum, flour, agarose or
polyacrylamide, or mixtures of two or more of these. In still other preferred
embodiments; the indicator is selected from the group consisting of stains,
dyes
or labeled probes.
In accordance with another aspect of the present invention, there is
provided a method for staining or labeling biomolecules, comprising applying
an
article of manufacture comprising a flexible backing and a dry coating adhered
to a surface of the backing, wherein the coating comprises an indicator that
stains or labels a biomolecule, to a gel or support containing the
biomolecules
for a sufficient period of time to allow diffusion to the biomolecules.
In accordance with yet another aspect of the present invention, there is
provided a kit suitable for use in a method for staining or labeling
biomolecules,
the kit comprising (A) an article of manufacture comprising a flexible backing
20 and a dry coating adhered to a surface of the backing, wherein the coating
comprises an indicator that stains or labels a biomolecule; (B) reagents to
effect
staining or labeling of the biomolecules; and (C) instruments to effect
staining or
labeling of the biomolecules.
Other objects, features and advantages of the present invention will
25 become apparent from the following detailed description. It should be
understood, however, that the detailed description and the specific examples,
. while indicating preferred embodiments of the invention, are given by way of
illustration only, since various changes and modifications within the spirit
and
scope of the invention will become apparent to those skilled in the art from
this
30 detailed description.
-2-
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
Brief Description of the Drawing
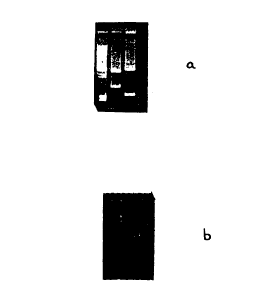
Figure 1. Demonstration of EtBr staining of DNA using two types
coated backings. Figure 1(a) shows an agarose gel stained with a backing
comprising White Uncoated Paper #100. Figure 1(b) shows an agarose gel
stained with a backing comprising Prevail Paper #125. The gels were stained
for
2-3 minutes and then placed on a Short Wave UV Transilluminator for
visualization of the DNA bands and for obtaining photographs.
Detailed Description of the Preferred Embodiments
The present invention provides an article of manufacture and methods for
safely staining or labeling biomolecules. The stable, resilient and pliable
article
can be used repeatedly in a method for staining biomolecules in gels and
membranes without directly contacting toxic staining solutions and without
handling delicate gels which contain such stains. The process eliminates the
environmental hazard resulting from the disposal of toxic stains since the
backing with the stain can be disposed of in a solid chemical waste container.
In
addition, the process reduces the amount of stain to be used and eliminates
the
personal hazards associated with staining biomolecules since the operator will
no
longer have direct contact with the stain itself. Furthermore, the article is
easily
manufactured which reduces the cost of the article and ensures its
accessibility in
the marketplace.
In accordance with one aspect of the present invention, an article of
manufacture is provided which comprises a flexible backing and a dry coating
adhered to a surface of the backing, wherein the coating comprises an
indicator
that stains or labels a biomolecule. In preferred embodiments of the present
invention, the backing is flexible, promotes good contact with a support or
gel
containing a biomolecule, is impermeable and allows the coating solution to be
evenly spread and dried. The backing can be composed of cellulose-based
compounds, such as various papers, for example wall paper, cloth' or vinyl, 3M
WhatmanTM paper, vinyl paper, cloth paper, prepasted paper, to name a few.
The backing can be any wood, preferably light and strong as Balsam of about
1/16-1/8 of an inch in thickness, any glass such as borosilicate of about 50
mm,
-3-
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
any cloth, cotton, rayon, tafetta, mesh, wool, synthetic polymers such as
nylon,
acetate, mixtures of cotton and acetate, polypropylene or polyethylene,
polycarbonate, acrylic, cellophane acetate, Gelbond (FMC Bioproducts, Maine)
to name a few, and any sponge. In another embodiment, the backing is
comprised of two layers, consisting of a permeable layer and an impermeable
layer.
In a preferred embodiment of the present invention, the coating solution
comprises an adhering solution and an indicator capable of staining or
labeling a
biomolecule. Any solution which adheres to the backing and is preferably
amenable to drying and rewetting, and does not degrade the biomolecule to be
stained or the gel or support which retains the biomolecule to be stained can
be
utilized. Most preferred are adhering solutions comprised of modified starches
and their derivatives. Starch solutions can be modified in a variety of ways,
including digestion with amylase. Other examples of adhering solutions
include,
but are not limited to, water-based glues, flour, agarose, polyacrylamide and
its
derivatives, and any mixture there of. Examples of glues include, but are not
limited to, wall-paper glue, paper glue and multiple function glue. Gums, such
as locust bean gum, also can be used. Adhering solutions containing silica or
diatomaceous earth also are advantageous.
In preferred embodiments, one or more indicators are entrapped in the
adhering solution. In one embodiment, the desired indicator is mixed with the
coating solution at varying amounts depending on the sensitivity of the stain.
' The indicator can be in the form of a solution. For example, the indicator
can
be dissolved in water or a buffer. Suitable buffers can comprise mixtures of
aqueous and organic solutions or comprise only organic solutions, such as
isopropanoI for ethidium bromide. Alternatively, the indicator can be mixed
into the coating solution as a powder. In another embodiment, the stain is
spread onto a backing onto which a coating solution has been dried and which
is
capable of being rewetted again and redried.
A variety of indicators can be utilized individually or in combination in
the present invention to stain or label biomolecules. Preferred indicators
include, but are not limited to, stains, dyes and labeled probes. Examples of
acceptable stains and dyes include, but are not limited to, ethidium bromide,
CA 02321100 2000-08-17
WO 99/42620 PCTNS99/03704
YoYo (Molecular Probes, Inc., Boulder, CO), Toto (Molecular Probes, inc.
Boulder, CO), Stains-All (Sigma), SYBR Green ITM, SYBR Green IIT"~, SYBR
GoldTM (Molecular Probes), methylene blue, crystal violet, methyl green,
pyronin y, thionin, basic blue 66, basic red 49, indoine blue, safranin O,
Janus
5 green B, rile blue, pinacyanol, basic yellow 11, Coomassie Brilliant Blue 8-
250, Hoechst 3325$ and Hoechst 33342. In one embodiment, the release of
such stains from the surface of the backing can be controlled by alterations
in
pH.
In another embodiment, the indicator comprises one or more probes
complementary to the biomolecule to be detected. Examples of a
complementary probe include, but are not limited to, a labeled synthetic
oligonucleotide, an antibody and antibody fragment. Such probes can be added
to the coating solution, or applied to the dried backing with the coating
solution
instead of, or in addition to, a stain. The probe can be labeled with a
15 radioactive label or a non-radioactive label such as biotin, alkaline
phosphatase,
and horseradish peroxidase, or a chemiluminescent reagent, among others. The
labeled probe can be added at a concentration of about 1 - 1000 pmol/mf,
preferably about 100 pmol/ml.
In another embodiment of the present invention, additional compositions
are added to the coating solution. Such compositions, for example tris
acetate,
polyethylene glycol and its derivatives, and triglycerol, can facilitate
staining or
can stabilize the stain or biomolecules. Incorporating a hybridization buffer
into
the coating solution is especially useful when the indicator comprises a
labeled
probe.
25 The coating solution with an indicator can be spread onto one or both
sides of the backing. Alternatively, the backing can be spread with the
coating
solution, dried, and then coated with an indicator solution. Coating and
indicator solutions can be applied onto the backing by rolling them with a
sponge roller or automated roller, or by brushing or spraying them onto the
backing. The coated backing can be air dried, oven dried, or dried in a
microwave oven.
In accordance with another aspect of the present invention, a method is
provided for staining biomolecules, comprising applying an article of
-5-
CA 02321100 2000-08-17
WO 99/42b20 PC'T/US99/03704
manufacture comprising a flexible backing and a dry coating adhered to a
surface of the backing, wherein the coating comprises an indicator that stains
or
labels a biomolecule, to a gel or support containing the biomolecules for a
sufficient period of time to allow diffusion to the biomolecules. Usually,
about
5 0.5 minute or longer of direct contact is required to allow the indicator to
diffuse to the biomolecules. Examples of biomolecules include, but are not
limited to, DNA, RNA and proteins.
In one embodiment, the present invention is used to stain or label
biomolecules contained in gels. A gel containing biomolecules can be made of
10 any sieving material such as agarose, starch, polyacrylamide synthetic
matrices,
or various blends of matrices. After the electrophoretic gel is stained, the
backing is peeled off, and can be disposed of in the solid chemical waste, or
reused. A fresh application of the desired stain may be necessary upon reuse.
This process prevents direct contact with toxic staining solutions, minimizes
the
15 volume of the staining solution utilized and saves the environment from any
hazards which such a staining solution may produce.
In another embodiment, the present invention is used to label
biomolecules immobilized on a support. Hybridization assays, such as
Southern, northern and western assays, utilize labeled probes to detect
specific
20 DNA, RNA or protein species immobilized onto membranes, such as nylon or
nitrocellulose. Such immobilized biomolecules may be detected using the
method described above. Instead of, or in addition to a stain, a probe, for
example a labeled synthetic oligonucleotide complementary to the biomolecule
to be detected, is added to the coating solution, or applied to the dried
backing
25 with the coating solution. As discussed above, the oligonucleotide can be
labeled with a radioactive label or a non-radioactive label such as biotin,
alkaline
phosphatase, horseradish peroxidase or a chemiluminescent reagent among
others. After transfer of the electrophoresed DNA onto a membrane, the
backing containing the labeled probe is applied to the membrane such that the
30 probe is in direct contact with the support containing the biomolecules to
be
detected and even transfer of the probe from the backing to the biomolecules
is
effected. The backing and the support are left in contact for several minutes
up
to eighteen hours or overnight. Detection of complementary binding between
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
the labeled probe and the immobilized biomolecule may be carried out by
methods known in the art.
In accordance with yet another aspect of the present invention, the
present invention provides a kit suitable for use in a method for staining or
labeling biomolecules, the kit comprising an article of manufacture comprising
a
flexible backing and a dry coating adhered to a surface of the backing,
wherein
the coating comprises an indicator that stains or labels a biomolecule,
reagents
to effect staining or labeling of the biomolecules, and instruments to effect
staining or labeling of the biomolecules. The kit can be an educational or
research kit, whereby other reagents for the preparation of the separation
gels
are included, as well as other reagents which facilitate detecting stained
biomolecules, for example buffers, glycerol solutions or enzymes.
Instruments which facilitate staining or detecting biomolecules can be
included in the kit. For example, a roller which can be used to promote
effective contact between the backing and the gel or support containing the
biomolecules can be included. Similarly, a cassette into which the backing and
the gel or support are placed and which promotes effective contact between the
two can be included in the kit. Additionally, a spray applicator which can be
used to apply reagents effecting staining can be included.
A kit for staining biomolecules may be packaged in a variety of ways.
When the kit contains a backing with a coating solution comprising ethidium
bromide for staining DNA or RNA, the coated backing must be stored such that
it is not exposed to light; since ethidium bromide is light sensitive: A
preferred
package for this embodiment is a foil pouch. It is contemplated that a
plurality
of backing strips can be stored in a roll such that the desired size of
backing can
be cut away either using scissors or using a sharp serrated metal edge adhered
to
the box in which the roll is stored. Additionally, the use of perforated paper
can
simplify the dispensing process. In these forms, it is preferable that the
coated
backing be covered with a liner which when peeled away exposes the coating
just prior to use so that the backings in the roll do not adhere to each
other.
Such a liner can be made from paper or other release liner. Alternatively, the
backing can be stacked in sheets, folded and stored in a dispenser for easy
removal of one sheet at a time. In another embodiment, the coating solution
can
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
be supplied in a separate container for application onto the backing when
needed. The coating solution can be supplied with or without stain, and the
staining solution or powder can be provided in yet another container.
Inasmuch as many changes could be made in the above constructions,
and many apparently different embodiments of the invention could be made
without departing from the scope thereof, it is intended that all matters
contained
in the above description shall be interpreted as illustrative and not in a
limiting
sense.
Finally, singular usage herein should be interpreted as denoting "one or
more." For example, the statement "an indicator can be spread onto one or
both sides of the backing" is meant to include the application of one or more
indicators, unless otherwise specified.
Described below are examples of the present invention which are
provided only for illustrative purposes, and not to limit the scope of the
present
invention.
Example 1. Preparation of an Article for Staining DNA Molecules
An adhering solution is prepared by forming a 10 % solution of modified
starch using warm, distilled water. An appropriate amount of ethidium bromide
20 is then added to the adhering solution, preferably to a final concentration
of
between 0.05 mg/ml and 0.2 mg/ml. The resulting solution is spread over one
side of a cellulose-based paper using a sponge roller and allowed to dry.
After
the coating solution has dried, the paper may be rolled up and stored until
use.
_g_
CA 02321100 2000-08-17
WO 99/42620 PCT/US99/03704
Example 2. Method for Staining DNA Molecules
Two restriction digests were performed on a plasmid DNA sample.
Each digest utilized approximately 1.5 p.g of DNA. The digested products were
fractionated, along with a ~,-Hind-III molecular ladder, on a 0. 8 % UltraSpec
5 AgaroseT"" (EDVOTEK cat. #605, Edvotek, West Bethesda, Maryland) in 1X
TAE electrophoresis buffer (20 mM Tris, 6 mM sodium acetate, 1 mM
Na2EDTA, pH7.8) at 30 ml per 7x7 cm gel bed. The gels were run at 120 volts
for 45 minutes and terminated when the tracking dye had migrated 4.5 cm from
the wells. An adhering solution of 50% modified starch was prepared as
10 described above. Ethidium bromide was added to a final concentration of
0.0625 mg/ml. The resulting coating solution was spread onto two types of
paper backing, White Uncoated Paper #100 and Prevail Paper #125, using a
sponge roller and allowed to air dry in the dark. Approximately 0.5 ml of the
coating solution was applied to each backing. Following fractionation, the
gels
15 were inverted, and the coated backing was placed on the gel such that the
ethidium bromide was in contact with the gel containing the DNA. The gels
were stained for 2-3 minutes and then placed on a Short Wave Ultraviolet
Transilluminator for visualization of the DNA bands. The results in Figure 1
show that the present invention provides a safe and effective means of
staining
20 DNA molecules contained in gels. Figure 1(a) is a photograph of a gel
stained
with the coated backing comprising White Uncoated Paper #100. Figure 1(b) is
a photograph of a gel stained with the coated backing comprising Prevail Paper
#125.
-9-