Note: Descriptions are shown in the official language in which they were submitted.
CA 02321353 2000-09-28
BACKGROUND OF THE INVENTION
The present invention relates to wood preservatives,
processes for their preparation, and their use.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide
an aqueous wood preservative or a wood preservative which is
dilutable in water, which, compared with known aqueous wood
preservatives or known wood preservatives which are
dilutable in water, shows an improved penetration depth and
an improved efficacy against timber pests.
The above object is achieved according to the
present invention by a wood preservative which comprises a
complex composed of a cyclodextrin derivative comprising a
hydrophobic active ingredient.
The wood preservative according to the invention may
be present as a ready-to-use aqueous solution, such as, for
example, as an impregnating solution, glaze, color glaze or
primer.
- 1 -
CA 02321353 2000-09-28
Further, it may also be present in the form of an
aqueous concentrate which is brought to the use
concentration by the end user by adding water.
It may furthermore be present as a solid wood
preservative which is soluble in water.
The cyclodextrin derivatives in the wood
preservative according to the invention are preferably
selected from the group of the alkylated, hydroxyalkylated,
acylated, branched and sulfoalkyl-ether-substituted a-, a-
or Y-cyclodextrins.
The cyclodextrin derivatives are preferably selected
from the group of the alkylated a-, p- or Y-cyclodextrins
and hydroxyalkylated a-, ~- or Y-cyclodextrins.
Especially preferred are the cyclodextrin
derivatives which are selected from the group of the
alkylated a-, ~- or Y-cyclodextrins.
Particularly preferred are the cyclodextrin
derivatives which are selected from the group of the
methylated a-, ~- or Y-cyclodextrins.
- 2 -
CA 02321353 2000-09-28
The complexed hydrophobic active ingredient is
preferably a hydrophobic fungicide or a hydrophobic
insecticide.
The active ingredient which is particularly
preferably used is a mixture of various fungicides and/or
insecticides.
The fungicide is preferably a fungicide from the
group of the triazoles, tolylfluanid (N-
dichlorofluoromethylthio- N',N'-dimethyl-N-p-
tolylsulfamide), dichlorofluanide (N-dichlorofluoro-
methylthio-N',N'-dimethyl-N-phenylsulfamide), IPBC (3-
iodopropargyl N-butylcarbamate), isothiazolinones (N-
octylisothiazolin-3-one) and 4,5-dichloro-N-
octylisothiazolin-3-one.
It is particularly preferably a fungicide selected
from the group consisting of propiconazole (1-(2,4-
dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl)methyl)-iH-1,2,4-
triazole), tebuconazole ((RS)-1-(4-chlorophenyl)-4,4-
dimethyl-3-(1H-1,2,4-triazol-1-ylmethyl)pentan-3-ol),
azaconazole (1-(2,4-dichlorophenyl)-1,3-dioxolan-2-yl)-
methyl)-1H-1,2,4-triazole), fenbuconazole (4-(4-chloro-
- 3 -
CA 02321353 2000-09-28
phenyl)-2-phenyl-2-(iH-1,2,4-triazol-1-ylmethyl)-
butyronitrile) and triadimenol ((1 RS,2 RS;1 RS,2 SR)-1-(4-
chlorophenoxy)-3,3-dimethyl-1-(iH-1,2,4-triazol-1-yl)butan-
2-0l), or 4,5-dichloro-N-octylisothiazolin-3-one. It is
particularly preferably a fungicide selected from the group
consisting of propiconazole or tebuconazole.
The insecticide is preferably a synthetic pyrethroid
(such as, for example, cypermethrin, deltamethrin,
permethrin, alphamethrins), bifenthrin, fipronil, a
benzeneurea derivative (such as, for example, hexaflumuron,
teflubenzuron or flufenoxuron), a phosphoric ester (such as,
for example, phoxim, parathion, fenitrothion, trichlorphon
or dichlorphos), or a carbamate (such as, for example,
propoxur, pirimicarb or aldicarb).
It is especially preferably cypermethrin,
deltamethrin, permethrin or bifenthrin.
An aqueous wood preservative formulation according
to the,invention comprises water and, preferably, 0:01-2% by
weight of hydrophobic active ingredient and, preferably, 1-
20% by weight of cyclodextrin derivative, but preferably
0.5-1.5% by weight of hydrophobic active ingredient and 3-
- 4 -
CA 02321353 2000-09-28
16% by weight of cyclodextrin derivative. The balance up to
100% by weight is water. All percents by weight are based
upon the total weight of the aqueous wood preservative
formulation.
An aqueous wood preservative concentrate according
to the invention comprises water and, preferably, 1-5% by
weight of hydrophobic active ingredient and, preferably, 5-
70% by weight of cyclodextrin derivative, especially
preferably 2-6% by weight of hydrophobic active ingredient
and 20-50% by weight of cyclodextrin derivative. The
balance up to 100% by weight is water. All percents by
weight are based upon the total weight of the aqueous wood
preservative concentrate.
A solid wood preservative which is soluble in water
comprises, preferably, 2-20% by weight of hydrophobic active
ingredient and 50-98% by weight of cyclodextrin derivative,
especially preferably 5-10% by weight of hydrophobic active
ingredient and 70-95% by weight of cyclodextrin derivative.
The wood preservative according to the invention may
furthermore comprise the following in the use concentrations
- 5 -
CA 02321353 2000-09-28
which are customary in each case (as a rule, approximately
0.001-5% by weight):
Bactericides, for example chloromethyliso-
thiazolinone, methylisothiazolinone, aldehydes (for
example 0.001%-0.2% by weight).
Boron compounds such as, for example, boric
acid, borax.
Binders such as, for example, dispersible and
emulsifiable synthetic resins (for example,
polyvinyl acetate, polyester resin, acrylates,
silicone resin).
Wetters.
Small amounts of solubilizers (approximately
0.01-2% by weight): alcohols, glycols, glycol
ethers.
Anticorrosives.
Thickeners.
W stabilizers.
Desiccants.
Colorants.
Pigments.
pH regulators.
- 6 -
CA 02321353 2000-09-28
However, the above-described aqueous CD complexes on
their own are also effective as wood-preservatives. The
invention therefore also relates to the use of complexes of
a CD derivative and a hydrophobic active ingredient as wood
preservative, complexes of the already-mentioned derivatives
with the already-mentioned hydrophobic active ingredients
preferably being used.
The cyclodextrin/active ingredient complexes are
produced by processes which are known per se, such as, for
example, those described in J. Sejtli, "Cyclodextrin
Technology", Kluwer Academic Publishers, 1988, p. 86 et seq.
For example, the cyclodextrin/active ingredient
complexes may be prepared by stirring or shaking aqueous
solutions of cyclodextrin derivatives with the active
ingredient or the solutions of the active ingredient at
temperatures of 10-80°C. Customary stirrers or dispersers
are used for stirring. If appropriate, cosolvents such as,
for example, lower alcohols, acetone, glycols and ethyl
acetate may be employed in this process.
In a further method of preparing the complexes, the
cyclodextrin derivatives are made into a paste with water in
CA 02321353 2000-09-28
a weight ratio of 6:4 to 9:1 and the paste is kneaded
together with the active ingredient for approximately 10-200
minutes at 20°-80°C. The paste can be dried, for example in
vacuo, to give solid wood preservatives according to the
invention. The paste may furthermore be diluted with water
to the desired application time.
Moreover, the cyclodextrin derivative and the active
ingredient may be dissolved in a joint organic solvent, such
as, for example, C1-C6-alcohols, C3-C6-ketones, ethyl
acetate, methyl acetate or glycols. The solvent may
subsequently be eliminated by drying, for example in vacuo.
This gives, as a rule, solids which can be dissolved in
water.
If the liquid wood preservatives according to the
invention are dried to obtain a solid wood preservative
according to the invention, conventional methods such as,
for example, spray-drying or drying in vacuo are employed.
,The wood preservatives in solid form can be
processed for example by granulating, pelletizing,
compacting, sieving, screening, tableting or packaging in
water-soluble films.
_ g _
CA 02321353 2000-09-28
Moreover, the invention relates to a method of
preparing an aqueous wood preservative, wherein a solid wood
preservative comprising a complex of a hydrophobic active
ingredient and a cyclodextrin derivative is dissolved in
water.
The invention furthermore relates to a method of
protecting wood and timber materials, wherein wood or a
timber material is treated with an aqueous formulation
comprising a hydrophobic active ingredient and a
cyclodextrin derivative, and subsequently dried.
Treatment is accomplished by means of methods known
per se such as by means of atomizing, painting on, spraying
or impregnating methods such as dipping, immersing, and the
pressure, vacuum and double-vacuum methods.
A wood preservative formulation according to the
invention exhibits unexpected advantages compared with known
formulations which can be diluted with water. In the
formulations according to the invention, lower active
ingredient limits (determined as specified in DIN EN 113)
have been found than in known formulations which are
dilutable in water, both after thermal treatment and after
_ g _
CA 02321353 2000-09-28
leaching. In particular the lower limits after leaching with
water are entirely unexpected since a person skilled in the
art would expect that the active ingredients would be more
rapidly leached, due to the solubilization with
cyclodextrins, thus leading to higher limits.
BRIEF DESCRIPTION OF THE DRAWINGS
Other objects and features of the present invention will
become apparent from the following detailed description
considered in connection with the accompanying drawings
which disclose several embodiments of the present
invention. It should be understood, however, that the
drawings are designed for the purpose of illustration only
and not as a definition of the limits of the invention.
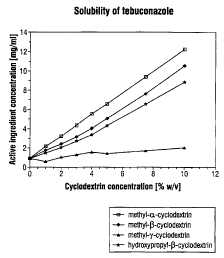
In the drawings, FIG. 1 shows that tebuconazole can
be solubilized in water with the aid of methylated or
hydroxypropylated cyclodextrins;
FIG. 2 shows that propiconazole can be solubilized
in water with the aid of methylated cyclodextrins; and
- 10 -
CA 02321353 2000-09-28
FIG. 3 shows that bifenthrin can be solubilized in
water with the aid of methylated cyclodextrins.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
EXAMPLES
The examples which follow are intended to illustrate
the invention in greater detail. All percents are by weight
unless specified otherwise.
EXAMPLE 1: Determination of the phase solubility
diactrams of tebuconazole with various cyclodextrin
derivatives
Among the cyclodextrin derivatives to be tested
(products of blacker-Chemie GmbH), quantities corresponding
to 10.0 g of anhydrous substance are measured into a 100 ml
volumetric flask and made up to the mark with 0.1-molar
phosphate buffer (KZHPOQ/KHZPOQ) . By diluting this 10
strength (w/v) stock solution, solutions are prepared whose
concentrations range from 1% to 10% (w/v). Pure 0.1-molar
phosphate buffer is used as comparison. 1-ml aliquots of the
cyclodextrin derivative solution are treated with approx.
30 mg of tebuconazole and shaken for 48 hours at room
- 11 -
CA 02321353 2000-09-28
temperature. The samples are subsequently subjected to
microfiltration (0.2 ~.m filter). The tebuconazole
concentration in the clear filtrates is determined by HPLC.
The results are shown in FIG. 1. It can be seen
that tebuconazole can be solubilized in water with the aid
of methylated or hydroxypropylated cyclodextrins
EXAMPLE 2: Determination of the phase solubility
diactrams of propiconazole with various c~clodextrin
derivatives
The experiments were carried out analogously to
Example 1 using propiconazole.
The results are shown in FIG. 2. It can be seen
that propiconazole can be solubilized in water with the aid
of methylated cyclodextrins; and
EXAMPLE 3: Determination of the phase solubility
diacrrams of bifenthrin with various cyclodextrin derivatives
The experiments were carried out analogously to
Example 1 using bifenthrin.
- 12 -
CA 02321353 2000-09-28
The results are shown in FIG. 3. It can be seen
that bifenthrin can be solubilized in water with the aid of
methylated cyclodextrins.
EXAMPLE 4: Preparation of a wood,preservative
formulation A (fundicidal wood preservationZ
55 g of anhydrous methyl-a-cyclodextrin are
dissolved in 945 ml of water. After addition of 3.0 g of
tebuconazole and 3.0 g of propiconazole, the solution is
warmed to 50°C, and the active ingredients are dissolved in
the course of 3 hours with the aid of a dispenser. This
gives a clear solution which constitutes a ready-to-use wood
preservative formulation with an active ingredient content
of 0.6% by weight.
EXAMPLE 5: Preparation of a wood preservative
formulation B (fungicidal wood preservation)
80 g of anhydrous methyl-~i-cyclodextrin are
dissolved in 920 ml of water. After addition of 2.O g of
tebuconazole, 2.0 g of propiconazole and 2.0 g of IPBC, the
mixture is stirred for approximately 8 hours at 30°C. This
- 13 -
CA 02321353 2000-09-28
gives a clear solution which constitutes a ready-to-use wood
preservative formulation.
EXAMPLE 6: Preparation of a wood preservative
formulation C linsecticidal wood preservatio ~i
100 mg of cypermethrin are dissolved in 100 ml of a
4% strength (w/w) aqueous solution of methyl-(3-cyclodextrin.
The pure solution constitutes a ready-to-use wood
preservation formulation.
Wooden cubes which have been impregnated with this
solution are not attacked by termites.
EXAMPLE 7: Preparation of a wood preservation
formulation D Lfunqicidal and insecticidal wood
preservation)
15 g of anhydrous methyl-(3-cyclodextrin are
dissolved in 985 ml of water. After addition of 0.6 g of
tebuconazole, 0.9 g of propiconazole and 0.06 g of
bifenthrin, the mixture is stirred for approximately
20 hours at 25°C. This gives a clear solution which
constitutes a ready-to-use wood preservation formulation.
- 14 -
CA 02321353 2000-09-28
Compared with untreated timber materials, timber materials
which have been impregnated with this solution are attacked
less by wood-discoloring, wood-destroying fungi and wood-
destroying insects (Hylotrupes bajulus).
EXAMPLE 8: Preparation of a wood preservation
formulation concentrate
22 g of methyl-a-cyclodextrin are dissolved in 85 g
of water. After addition of 0.5 g of tebuconazole, 1.0 g of
propiconazole and 0.08 g of bifenthrin, the mixture is
stirred for approximately 20 hours at 40°C. This gives a
clear solution which constitutes a wood preservation
formulation concentrate. Dilution with water 1/10 (v/v)
gives a clear, ready-to-use wood preservative formulation.
EXAMPLE 9: Spra~r-drying of a wood preservative
formulation
The solution of Example 4 is dried in a Niro
laboratory dryer with the following parameters.
Dry air temperature: 210°C
Exhaust air temperature: 105°C
Type of atomization: 2-substance nozzle
- 15 -
CA 02321353 2000-09-28
The resulting powder has a residual moisture of 1.5%
by weight. The active ingredient content was determined by
HPLC: 4.81% by weight of tebuconazole, and 4.72% by weight
of propiconazole.
The powder can be redissolved in water. At suitable
concentration, this gives a ready-to-use wood preservative.
EXAMPLE 10: Use of wood preservative against insects
Wooden test pieces are fully impregnated with a
solution of 1 g of cypermethrin and 25 g of methyl-p-
cyclodextrin in 74 ml of water. The wooden test pieces
treated thus were subjected to a test as specified in DIN
EN22 (Hylotrupes bajulus). The mortality rate of the larvae
was 87%.
EXAMPLE 11: Depth of penetration
Wooden test pieces (spruce: 250 x 60 x 40 mm) were
treated in an immersion bath with formulation A (according
to the invention) or formulation B (comparative example). To
test the depth of penetration of the formulation, test
specimens of approximate thickness 5 mm (5 x 60 x 40 mm)
- 16 -
CA 02321353 2000-09-28
were sawn out at right angles to the longitudinal axis of
the wooden test pieces after the latter had dried. The test
specimens were sterilized by gamma-irradiation, and, after
incubation with the blueing fungus (Aureobasidium
pullulans), incubated for 3 weeks at 23°C and an atmospheric
humidity of 65%. No fungal growth was observed in the
penetration zone of the formulation, while the untreated
core was stained by the fungus.
TABLE 1 gives the composition of formulations A and
B (Form. A and Form. B) and the parameters determined on the
treated wood.
TABLE l:
Composition OF UAI DOP
(kg/ Ik9/ I
m' m'
Form. A 0.25% propiconazole
(Invention) 0.25% IPBC
2.5% methyl-a-cyclodextrin 28.5 0.143 4
97% water
Form. ~ 0.35% propiconazole
(Comparison) 0.35% TPBC
0.5% ethoxylated nonylphenol20.8 0.146 2
6.5% Berol 278
- 17 -
CA 02321353 2000-09-28
1% N-methylpyrrolidone
91.3% water
OF - Uptake of formulation of the wooden test
pieces
UAI = Uptake of active ingredients
DOP = Depth of penetration
Despite a comparable uptake of active ingredients,
the formulation according to the invention exhibits deeper
penetration into the wood.
EXAMPLE 12: Efficacy test: wood-discoloring fungi
Three formulations were compared.
Formulation C: Solution of the active ingredients in
Varsol 60 (hydrocarbons with a low aromatic content).
Formulation D: Emulsion of the active ingredients in
an aqueous solution of 0.6% ethoxylated nonylphenol, 6.5%
Berol 278 and 1.5% N-methylpyrrolidone.
Formulation E: Solution of the active ingredients in
4% methyl-~3-cyclodextrin in water.
- 18 -
CA 02321353 2000-09-28
Active ingredients in formulation: 1:1 mixture (w/w)
of propiconazole and IPBC.
Concentrations (total active ingredient's): 0.05%,
0.10%, 0.20%, 0.30% (w/w).
Wooden disks (pine; diameter: 50 mm, height: 15 mm)
are immersed into the wood preservative solution and dried
in the air. After drying, the disks are stored for 1 week at
80°C. After the disks had been placed on a suitable nutrient
medium, they are sterilized by gamma-irradiation and, after
incubation with the test fungi, incubated for 3 weeks at
23°C and an atmospheric humidity of 65%. .
TABLE 2 shows the results: (active ingredient
concentration at which no fungal growth on the test specimen
was observed).
TABLE 2:
Test fungus Form. C Form. D Form. E
(Comparison: (Comparison: ( I n v a n t
as i o n
in solvent) emulsion) formulated with
c clodextrins
- 19 -
r
CA 02321353 2000-09-28
Aureobasidium 0.20% 0.10% 0.05%
ullulans
The results show advantageous lower use
concentrations of the fungicides when using formulation C
according to the invention for the blueing fungus
(Aureobasidium pullulans).
EXAMPLE 13: Limits as specified in DIN EN 113 (wood-
destroying fungi)
Formulation 1: Formulation which is dilutable in
water, active ingredients are emulsified (comparative
examp 1 a )
0.6% Ethoxylated nonylphenol (emulsifier)
6.5% Berol 278 (emulsifier)
1.5% N-Methylpyrrolidone (cosolvent)
1.2% Propiconazole (active ingredient)
0.9% Tebuconazole (active ingredient)
0.5% IPBC (active ingredient)
88.8% Water
Formulation 2: Formulation which is dilutable in
water, solution of active ingredients in aqueous CD solution
(wood preservative according to the invention)
- 20 -
CA 02321353 2000-09-28
1.2% Propiconazole (active ingredient)
0.9% Tebuconazole (active ingredient)
0.5% IPBC (active ingredient)
25% Methyl-~i-cyclodextrin
72.4% Water .
Wooden test pieces of standardized dimensions (15 '
25 ' 50 mm) were fully impregnated by the vacuum method,
additionally subjected to a thermal treatment (1 week at
80°C) or leaching in water (72 hours under running water)
and exposed to the attack of wood-destroying Basidiomycetes.
After 16 weeks, the weight loss of the wooden test pieces
which is caused by the fungi is determined.
An acceptable efficacy is defined as an identified
weight loss of < 3%.
TABLE 3 shows the active ingredient concentration in
kg active ingredient/m3 wood at which the above criterion
was met.
TABLE 3: LIMITS - ACTIVE INGREDIENT CONCENTRATIONS
Com arison Com arison Invention Invention
Test fungus Form. 1 Form. 1 Form. 2 Form. 2
after after after after
thermal leaching thermal leaching
treatment treatment
Coniophora 4.4 kg/m' 6.6 kg/m' 3.65 kg/m' 6.3 kg/m'
- 21 -
CA 02321353 2000-09-28
uteana
C o r i 15.0 kg/m' 14.0 kg/m' 9. 6 kg/m' 9. 5 kg/m'
o 1 a a
versicolor
Accordingly, while a few embodiments of the present
invention have been shown and described, it is to be
understood that many changes and modifications may be made
thereunto without departing from the spirit and scope of the
invention as defined in the appended claims.
- 22 -