Note: Descriptions are shown in the official language in which they were submitted.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
CATALYST COMPOUNDS WTrH BETA-DIIMINATE ANIONIC LIGANDS AND PROCESSES FOR
POLYMERIZING
OLEFINS
FIELD OF THE INVE11'TION
The present invention relates to catalyst systems, processes for making
such catalysts, intermediates for such catalysts. and olefin polymerization
processes using such catalysts.
BACKGROUND OF THE INVENTION
Olefin polymers are useful as plastics for packaging materials, molded
items. films, etc., and as elastomers for molded goods, industrial belts of
various types. tires, adhesives. and other uses. It has been well known in the
art that the structures of olefin polymers, and hence their properties and
capability of use, are highly dependent on the catalyst used during their
15 synthesis. Therefore, as the potential applications for polymers have
changed
and developed over the past years so too has the need for new and more
catalyst systems and improved polymerization processes utilizing such
catalysts become necessary.
2o SUMMARY OF THE INVENTION
In accordance with the present invention, there is provided a novel
catalyst system for the polymerization of olefins, said catalyst system
including a transition metal complex of a (3-diiminate bidentate ligand.
There is also provided in accordance with the present invention a novel
25 catalyst compound component for the polymerization of olefins, said
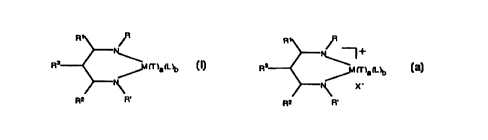
compound being represented by Formula (I), as follows:
(I)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
R R
N N -f-
~7 ~-
a ~b
X
\R ~~y \
R
wherein
R and R' independently represent a hydrogen atom, or a
substituted or unsubstituted, branched or unbranched hydrocarbyl or
organosilyl radical;
R', R2, and R3 independently represent a hydrogen atom, or a
substituted or unsubstituted, branched or unbranched hydrocarbyl
radical; and
to M represents a group LIIB, IVB, VB, VIB, VIIB or VIII
transition metal;
each T independently represents a univalent anionic ligand such
as a hydrogen atom, or a substituted or unsubstituted hydrocarbyl,
halogeno, aryloxido, arylorganosilyl, alkylorganosilyl, amido,
15 arylamido, phosphido, or arylphosphido group, or two T groups may
together or other anionic ligands such as an alkylidene or a
cyclometallated hydrocarbyl radical;
each L independently represents a sigma donor stabilizing
ligand or one L together with one T may together represent a second ~3-
20 diiminate ligand represented by Formula (TI) (below);
X, which is optional, represents a relatively weakly coordinated
anion; and
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
3
a = an integer from 0 to 4 inclusive, b = an integer 0 to 4
inclusive, provided a+b <_ 4.
Further provided in accordance with the present invention is a novel
5 process for the polymerization of olefins. The process provides for the
polymerization of one or more olefins in the presence of a homogeneous
catalyst comprising a catalyst represented by Formula (>] or a heterogeneous
catalyst system comprising a Formula (1] catalyst and one or more co-
catalysts.
10 The present invention also provides for a novel process of making a
catalyst component represented by Formula ()) by contacting a group I>IB,
NB, VB, VIB, V1ZB or VIB transition metal containing compound with a
compound containing a (3-diiminate ligand represented by the following
Formula (II), in particular a compound represented by Formula (>~ (below):
15 B
/R
R3
R'
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
4
R
N
'
R3 ~
m
N
R2 R'
wherein
5 R, R', R~, R2 and R~ have the same meanings stated above; and
m represents a group that is readily displaced by a transition
metal, for example hydrogen or a group comprising a group IA or IIA
metal.
to BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 depicts the crystal structure of the (Ph)2nacnacTiCl2(THF)2, prepared
in
Example 1 A.
Fig. 2 depicts the crystal structure of the (Ph)2nacnacVCl2(THF)2, prepared in
Example 1B.
15 Fig. 3 depicts the crystal structure of the (Ph)2nacnacCrCl2(THF)2,
prepared in
Example 1C.
Fig. 4 depicts the crystal structure of the (Ph)2nacnacVMe2, prepared in
Example 3A.
Fig. 5 depicts the crystal structure of the
20 (Ph)ZnacnacVMe(Et20)(THF)[B(C6H3(CF3)2)a], prepared in Example SA
(BArF anion not depicted).
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
Fig. 6 depicts the crystal structure of the ((Ph)~nacnac)2Cr, prepared in
Example 6.
DETAILED DESCRIPTION OF THE INVENTION
5 Herein certain terms are used to define certain chemical groups and
compounds. These terms are defined below.
"alkyl metal" or "metal alkyl" refer to a compound having an alkyl
radical bound directly to a metal. For example, an alkyl metal (or metal
alkyl)
would include alkyl aluminum (or aluminum alkyl).
l0 "group IA, IIA, IIB, IIIA, IBB, IVB, VB, VIB, VIIB or VIII" refers to
the metals within the respective group number of the Periodic Table of the
Elements (CRC Handbook of Chemistry and Physics, 78'h ed. 1997-1998).
For example, group IVB would include titanium, zirconium, etc. and group
VIII would include palladium, platinum, cobalt, etc.
"hydrocarbyl" refers to a univalent group containing only carbon and
hydrogen. If not otherwise stated, hydrocarbyl as used herein preferably
contains 1 to about 30 carbon atoms.
"linear a-olefin" refers to an olefin, defined below, wherein R~°
represents a hydrogen atom or an n-alkyl. If not otherwise stated, linear a-
olefin as used herein preferably contains 2 to about 12 carbon atoms.
"olefin" refers to a compound of formula CH2=CHR~°, wherein R~°
represents a hydrogen atom or n-alkyl or branched alkyl, preferably hydrogen
or n-alkyl.
"organosilyl" refers to a univalent group containing at least one carbon
to silicon bond. One example is trimethylsilylmethyl.
"Polymerization" refers to a process that produces polymers,
copolymers, terpolymers, etc. that generally have a degree of polymerization
of
at least about 20 or more. However, the process is also useful to produce
oligomers of a lower degree of polymerization.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
6
"saturated hydrocarbyl" refers to a hydrocarbyl radical that is free from
double or triple bonds, also referred to as unsaturated bonds. Examples of
such groups include alkyl and cycloalkyl.
"substituted hydrocarbyl" refers to a hydrocarbyl radical that contains
one or more substituent groups.
"transition metals" refers generally to the group 1118, NB, VB, VIB,
VIIB or VIII transition metals. If not otherwise stated, transition metals as
used herein preferably includes the group IVB, VB, or VIB transition metals.
"unsaturated hydrocarbyl" refers to a hydrocarbyl radical that contains
to one or more double or triple bonds. Examples of such groups include
olefinic,
acetylenic, or aromatic groups.
"unsubstituted hydrocarbyl" refers to a hydrocarbyl radical that
contains no substituent groups.
The present invention concerns catalysts and polymerization processes
for olefins in the presence of various homogenous transition metal catalysts
complexed with at least one (3-diiminate bidentate ligand or a catalyst
systems
comprising at least one such transition metal catalyst with one or more co-
catalysts. The (3-diiminate ligand may be represented by Formula (II), as
2o follows:
11
R
R3
R'
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
__
wherein
R, R', R~, R'' and R3 have the meanings stated above; and
said transition metal also has bound to it a ligand that may be
displaced by said olefin or added to said olefin.
The following Reaction Scheme 1 details one way of synthesizing a (3-
diiminate precursor compound corresponding to the (3-diiminate monoanionic
ligand, represented by Formula (II). This synthesis reaction is further
1o discussed in the journal articles by S.G. McGeachin Canadian J. of Chem.
v.46, pp.l X03-1912 (1968) and T. Potesil and H. Potesilova, J. of
Chromatogr., v.312, pp. 387- 393 ( 1984), the disclosures of which are hereby
incorporated by reference. It will be appreciated from this series of
transformations that the ~i-diimine compound can readily be prepared with
different groups on each of the nitrogen atoms by utilizing two different
substituted amines in the reaction sequence. By analogy to the familiar "acac"
nickname for the acetylacetonato moiety, the nickname "nacnac" will be used
herein to refer to the 2,4-pentane diiminato moiety, represented by Formula
(II). For example, the hydrogen or lithium bridged diimine structures in the
last two steps of Reaction Scheme 1 may be represented herein as nacnacH
and nacnacLi, respectively. The nacnac terminology used herein may further
include a prefix indicating the type of radical group present in the R and R'
positions, for instance, "Me" to represent methyl or "Ph" to represent phenyl
(e.g., (Ph)(Me)nacnacH or (Ph)ZnacnacH).
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
RNH NR O 1) Et30+BF4- NRH NR'H +
2
2) R'NH2 \
Base
R\N/~~N~R~ R\N~H,.N~R,
_MeLi
Reaction Scheme 1: Synthesis of (3-Diiminates ((R)(R')nacnacLi)
The catalyst compound of the present invention may be prepared in a
variety of ways, using techniques and, in addition to the novel ~i-diimine
compounds and corresponding monoanionic (3-diiminate ligands, known
precursors for the cationic and anionic portions of the catalyst compound. The
catalyst compound of the present invention may be formed either beforehand
to or in situ (i.e., in the vessel in which the polymerization is to take
place).
The ~3-diimine compounds of Formula (111), which may serve as
precursors for the monoanionic bidentate ligand, represented by Formula (II),
can be reacted with a transition metal compound to form a catalyst compound,
15 as represented by Formula (I), that is useful for the polymerization of
olefins.
In a preferred form of the (3-diimine compounds of Formula (11T), the hydrogen
or metal containing group represented by m includes hydrogen or a group IA
metal, in particular, lithium, sodium or potassium.
2o Useful transition metal containing compounds for forming such
catalyst compounds include those which comprise a group IffB, IVB, VB,
V1B, VIIB or VIII transition metal having ligands that may be displaced by the
monoanionic bidentate ligand derived from the ~3-diimine precursor
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
compound. Particularly suitable transition metal containing compounds
include transition metal salts having ligands, in addition and/or including
those
represented by T and L of Formula (>], that are readily displaceable by the
ligand derived from the diimine precursor compound under conditions that do
not adversely affect either the transition metal compound or ligand adducts
thereof. These transition metal salts include transition metal halides (such
as
dichloride, trichloride or tetrachloride, with trichioride being preferred),
transition metal carboxylates (such as acetates), transition metal alkoxides
(such as methoxides), or transition metal sulfonates (such as triflates or
l0 tosylates). Typically, these catalyst may be formed in the presence of a
suitable solvent. Suitable solvents include Lewis bases such ethers,
thioethers,
amines or nitrites with diethylether and tetrahydrofuran being preferred. In
addition, a metal alkyl including, in particular, metal alkyls having a group
IA,
IIA or IIIA metal such as lithium alkyls (such as alkyl methyl lithium, ethyl
15 lithium, n-propyl and/or i-propyl lithium, n-butyl, or t-butyl lithium),
aluminum alkyls, preferably including aluminum trialkyls (such as trimethyl
aluminum, triethyl aluminum, triisobutylaluminum or trioctyl aluminum),
Grignard reagants and the like may be simultaneously reacted with the other
reactants to form the desired catalyst compound. Alternatively, a compound
2o comprising the (3-diiminate ligand, such as those represented by Formula
(n,
can be subsequently reacted with such metal alkyls to form the desired
catalyst
compound or a compound of Formula ()7 can be reacted in situ and/or in the
presence of an olefin to provide a catalyst having the desired activity.
25 With respect to the catalyst compounds represented by Formula (n,
above, the relatively weakly coordinated anion X, when present, may be any
suitable anion known for this purpose. Suitable anions are often bulky anions,
particularly those that deiocalize their negative charge. X, in Formula (n,
preferably represents tetrakis [3,5-bis(trifluoromethyl)phenyl)borate (herein
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/Oi86_3
referred to as BArF-), (phenyl)4B~, (C6F5)4B-, (CH3){C6F5)3B-, PF6-, BF4-,
SbF6-
trifluoromethanesulfonate ( herein referred to as triflate or OTf), and p-
toluenesulfonate (herein referred to as tosylate or OTs-). Preferred weakly
coordinating anions include BArF and (C6F5)4B. Catalyst compounds of
Formula (I) wherein the weakly coordinated anion is present may be made by
further reacting a compound of Formula (I) having at least one alkyl group,
with about one equivalent of a strong acid, the conjugate base of which is a
non-coordinating anion such as noted for X above, in the presence of a
suitable
solvent. Suitable solvents include, for example, methylene chloride, hexane,
10 benzene, toluene, chlorobenzene, diethyl ether and the like.
The substituent groups represented by R, R', R~, R2 and R~ should be
selected so that they do not substantially interfere or impede the particular
type
of polymerization reaction for which the catalyst is designed. Whether a
particular group is likely to interfere can initially be judged by one skilled
in
the art based on the parameters of the process where the catalyst will be
employed. For instance, in polymerization processes where an alkyl aluminum
compound is used, catalyst containing an active (relatively acidic) hydrogen
atom, such as hydroxyl or carboxyl may not be suitable because of the known
2o reaction between alkyl aluminum compounds and such active hydrogen
containing groups (but such polymerization processes may still be possible if
enough "extra" alkyl aluminum compound is added to react with these
groups). However, in very similar polymerization processes where alkyl
aluminum compounds are not present, these groups containing active hydrogen
may be present. An important factor to consider in determining the operability
of compounds containing any particular functional group are the effect of the
group on the coordination of the metal atom, and side reactions of such a
group with other process ingredients, such as those noted above. Therefore, of
course, the further away from the metal atom the functional group is, the less
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
il
likely it is to influence, say, a polymerization. If there is doubt as to
whether a
particular functional group, in a particular position, will affect a reaction,
simple minimal experimentation will provide the requisite answer.
In a preferred form of Formulas (I), R and R' independently represent
a hydrogen atom, or an alkyl, aryl, alkylaryl, arylorganosilyl, or
alkylorganosilyl radical. Preferably, R and R' will independently include such
radicals wherein the carbon atom, directly bound to the nitrogen, has at least
two carbon atoms bound thereto, for example, isopropyl, phenyl, 2,6-
to isopropylphenyl, 2,6-dimethylphenyl, 2,6-diethylphenyl, 4-methylphenyl,
2,4,6-trimethylphenyl or 2-t-butylphenyl. R', R2, and R~ independently
represent a hydrogen atom or a hydrocarbyl radical, preferably a hydrogen
atom or an alkyl radical having 1-6 carbon atoms, and more preferably a
hydrogen atom or methyl radical. M represents a group IVB, VB or VIB
15 transition metal, preferably, chromium, vanadium or titanium. These
variables
defining the preferred forms of the compounds represented by Formula (>7 are
equally applicable, when present, to the preferred forms of the (3-diiminate
ligand and ~3-diiminate compound represented by Formula (II) & ()~,
respectively.
Exemplary hydrocarbyl groups for T, in Formula (I), include methyl,
ethyl, propyl, butyl, amyl, isoamyl, hexyl, iso-butyl, heptyl, octyl, nonyl,
decyl,
cetyl, 2-ethylhexyl, phenyl and the like, with methyl being preferred.
Exemplary halogeno groups for T include chloro, bromo, fluoro, and iodo,
with chloro being preferred. Exemplary alkoxido and aryloxido groups for T
include methoxido, ethoxido, phenoxido and substituted phenoxido's.
Exemplary amido groups for T include dimethylamido, diethylamido,
methylethylamido, di-t-butylamido, diisopropylamido and the like. Exemplary
arylamido groups for T include diphenylamido and other substituted phenyl
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
12
amido's. Exemplary phosphido groups for T include diphenylphosphido,
dicyclohexylphosphido, diethylphosphido, dimethylphosphido and the like.
Exemplary alkylidene anionic ligands, for two T groups taken together,
include methylidene, ethylidene and propylidene.
Each L in the above Formula (n can represent any suitable electron
donor iigand. Suitable ligands include those containing an atom, such as
oxygen, nitrogen, phosphorous or sulfur, which has a non-bonded electron
pair. Examples of these ligands include, but are not limited to, ethers,
amines,
to phosphines and thioethers. Ethers such as tetrahydrofuran (THF) and amines
such as pyridine are preferred, with THF being particularly preferred.
In a preferred form of the catalytic compound represented by Formula
(n, a and b independently represent integers from 0 to 3, inclusive. More
preferably, a and b independently represent either 0 or 2. It will be
appreciated
that when Formula (1) is meant to characterize a mixture of two or more
catalytic compounds whereby a and b represent an average of the wand b
values of the catalytic compounds, a and b may independently represent any
number from 0 to 4, including 1.2 to 1.8.
The polymerization reaction using the catalyst of the present invention
may be carried out with a catalyst compound represented by Formula (I) either
by itself, referred to as a homogenous catalyst system, or with one or more co-
catalysts. The catalyst and or co-catalysts may initially be in a solid state
or in
solution. The olefin and/or olefins may be in the gas or liquid state
(including
gas dissolved in a solvent). A liquid, which may or may not be a solvent for
any or all of the reactants and/or products may also be present. Suitable
liquids include alkanes, cycloalkanes, halogenated alkanes and cycloalkanes,
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
13
and ethers. Solvents that are especially useful include methylene chloride,
hexane, toluene, dichlorobenzene, and benzene.
Co-catalysts useful in the practice of the present invention are group
5 IIA, I)B, IBA and II>B metal alkyls having at least one alkyl group,
preferably
an alkyl group having 1 to 8 carbon atoms, bonded to the metal. Suitable
metal alkyls include dialkyl magnesium, dialkyl zinc, trialkyl boranes,
triarylboranes and aluminum alkyls. Suitable aluminum alkyls include
trialkylaluminums (such as trimethylaluminum, triethylaluminum,
1o triisobutylaluminum, and trioctylaluminum). Trialkylaluminums with alkyl
groups of four carbons or greater are preferred. Other aluminum alkyls useful
in the practice of the present invention include alkylaluminum alkoxides (such
as diethylaluminum ethoxide and ethylaluminum diethoxide), and
alkylaluminum halides (such as diethylaluminum chloride, diethylaluminum
15 bromide, diethylaluminum iodide, diethylaluminum fluoride, ethyl aluminum
dichloride, ethyl aluminum dibromide, ethyl aluminum diiodide, ethyl
aluminum difluoride and ethyl aluminum sesquichloride). Suitable
triarylboranes include those that are fluorine substituted (such as
tripentafluorophenyl borane).
Other suitable aluminum alkyls are aluminoxanes including those
represented by the general formula (R"-Al-O)~ for the cyclic form and R"(R"-
Al-O)~ Al(R")2 for the linear form. In these formulas, R" independently
represents an alkyl group (such as methyl, isopropyl, butyl and the like)
preferably with more than two carbon atoms, more preferably with 3-5 carbon
atoms, and n is an integer, preferably from about I to about 20. Most
preferably, R includes a methyl or isobutyl group. Mixtures of linear and
cyclic aluminoxanes useful in this invention include, but are not limited to,
ethyl aluminoxanes, isobutyl aluminoxane, and methyl aluminoxane.
CA 02321419 2000-08-14
WO 99/41290 PCT/tJS99/0186_3
14
The preferred metal alkyl co-catalysts generally include aluminoxanes
and trialkylaluminum. When a co-catalyst is used, the mole ratio of the metal
alkyl co-catalyst to catalyst should be from about 1:1 to about 1000:1. The
preferred mole ratio being from about 10:1 to about 200:1.
The catalyst system of the present invention may be used in either
slurry or gas phase polymerization processes. After catalysts have been
formed, the polymerization reaction is conducted by intermixing the monomer
to charge with a catalytic amount of the catalyst at a temperature and at a
pressure
sufficient to initiate the polymerization reaction. If desired, an organic
solvent
may be used as a diluent and to facilitate materials handling. The
polymerization reaction is carried out at temperatures of from about -
100°C
up to about 200°C, depending on the operating pressure, the pressure of
the
entire monomer charge, the particular catalyst being used, and its
concentration. Preferably, the temperature is from about 20°C to about
135°C.
The pressure can be any pressure sufficient to initiate the polymerization of
the
monomer charge. For instance, the pressure may range from atmospheric up
to about 1000 prig. As a general rule, a pressure of about 20 to about 800
psig
is preferred.
When the catalyst is used in a slurry-type process, an inert solvent
medium is used. The solvent should be one which is inert to all other
components and products of the reaction system, and be stable at the reaction
conditions being used. It is not necessary, however, that the inert organic
solvent medium also serve as a solvent for the polymer produced. The inert
organic solvents which may be used include saturated aliphatic hydrocarbons
(such as hexane, heptane, pentane, isopentane, isooctane, purified kerosene
and the like), saturated halogenated alkanes (such as dichloromethane,
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
choloroform and the like) saturated cycloaliphatic hydrocarbons (such as
cyclohexane, cyclopentane, dimethylcyclopentane, and the like), aromatic
hydrocarbons (such as benzene, toluene, xylene and the like). Particularly
preferred solvents are dichloromethane, toluene, cyclohexane, hexane, benzene
5 and heptane.
When the catalyst is used in a gas phase process, it may be suspended
in a fluidized bed with, e.g., ethylene. Temperature, pressure and ethylene
flow rates are adjusted so as to maintain acceptable fluidization of the
catalyst
to particles and resultant polymer particles.
The catalyst of the present invention may be employed on a solid
catalyst support (as opposed to just being added as a solid or in solution),
for
instance on silica gel or any other suitable catalyst support that does not
15 adversely affect the performance of the catalyst. By supported is meant
that
the catalyst may simply be carried physically on the surface of the solid
support, may be adsorbed, absorbed, or carried by the support by other means.
Preferred olefins and cycloolefins in the polymerization include at least
2o one or more of the following monomers: ethylene, propylene, I-butene,
cyclopentene, I-hexene; with ethylene and mixtures of ethylene with
propylene and/or I-hexene being more preferred. Ethylene alone is especially
preferred. Oligomers may also be used, with or without a co-monomer. As
may be desired, more than one monomer may be employed in which case a
copolymer will be the likely product obtained. However, depending on the
reactants employed and the given reaction conditions, polymerization may not
always occur.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
16
EXAMPLES
The following examples are given as particular embodiments of the
invention and to demonstrate the practice and advantages thereof. It is
understood that the examples are given by way of illustration and are not
intended to limit the specification or the claims that follow in any manner.
In preparation of the following catalysts, all manipulations were
performed under vacuum using glove box or Schlenk techniques. The
chemicals are commercially available from sources such as Strem Chemical
Co. and Aldrich Chemical Co. Methylaluminoxane (MAO), unless otherwise
noted, was used as a 10 wt.% in toluene solution. Methyl lithium (MeLi) was
used as a 1.4M solution or as a solid obtained by evaporation of the solvent.
Aniline and Aniline-ds (C6DSNH2) was freshly distilled just prior to use.
Trimethylsilylmethyllithium was supplied as a 1.OM solution in pentane and
crystallized as a white crystalline solid from solution at -30°C prior
to use.
Benzene-d6 (C6D6) and Tetrahydrofuran-d8 (THF-dA) were pre-dried with Na
and stored under vacuum over a Na/K alloy prior to use. Pyridine-ds (pyr-ds)
and Dichloromethane-d2 (CD2C12) were dried with CaH2 and vacuum distilled
onto pre-activated 4 A molecular sieves prior to use. Pentane, Diethylether,
Tetrahydrofuran (THF), and Hexamethyldisiloxane (HMDS) were dried over
Na/benzophenone prior to use.
Trichloro tris(tetrahydrofuran) vanadium (VCl3(THF)3) and Trichloro
tris(tetrahydrofuran) titanium (TiCI~(THF)3) are prepared from the
corresponding metal trichloride (TiCl3 and VCl3, respectively) by reaction
with
anhydrous tetrahydrofuran as noted in the article by Manzer, L.E. Inorganic
Synthesis Vol. XXI, pp. 135-140, John Wiley & Sons (1982) the complete
disclosure of which is hereby incorporated by reference.
CA 02321419 2000-08-14
WO 99/41290 PC1'/US99/0186_3
17
Trichloro tris(tetrahydrofuran) chromium (CrCI~(THF)~) is prepared by
converting anhydrous chromium trichloride into its tetrahydrofuranate by
continuous extraction with anhydrous tetrahydrofuran of its solid form
admixed with catalytic amounts of zinc dust as noted in the article by Herwig,
W.; Zeiss, H.H, Journal of Organic Chemistry Vol. 23, p. 1404 ( 1958) ) the
complete disclosure of which is hereby incorporated by reference.
The crystalline oxonium acid [(3,5-(CF3)ZC6H3)4B]~[H(OEt2)2]+ is
to synthesized by exposing a solution of Na[(3,5-(CF3)ZC6H3)4B] in ether to
HCl
and isolating the [(3,5-(CF3)ZC~H3)4B]-[H(OEt2)2J+. This synthesis is
discussed in the article by Brookhart, M.; Grant, B.; Volpe, A.F.,
Organometallics Vol. I I, No. 11, pp. 3920-3922 (1992).
Analytical Procedures:
NMR spectra were recorded using one or more of the following
spectrometers Bruker AM-250, WM-250 or 400; chemical shifts were
referenced to the residual proton resonance of the deuterated solvent
indicated.
Fourier Transform Infra Red spectra were recorded on a Mattson Alpha
Centauri spectrometer with a resolution of 4 cm-~.
UV-VIS spectra were recorded using a Bruins Omega 20
spectrophotometer and a Beckman DU 640 spectrometer.
Mass spectra were obtained from the University of Delaware Mass
Spectrometry Facility.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
18
Elemental analyses were performed either by Oneida Research
Services, Whiteboro, NY 13492 or Schwarzkopf Microanalytical Laboratory,
Woodside, N.Y. 11377. Note: the elemental analyses for Examples 1 A and 1 B
did not fit the calculated values, presumably, due to the loss of the
coordinated
solvents resulting from vigorous pumping under high vacuum.
Room temperature magnetic susceptibilities were determined using a
Johnson-Matthey Magnetic Susceptibility Balance which utilizes a
modification of the Gouy method. The molar magnetic susceptibility was
1o corrected for diamagnetism using Pascal constants and the effective
magnetic
moment (l.leff) was calculated from the expression:
Neer = 2.828 (Txm)'~'
where T is the temperature in Kelvin and xm is the molar magnetic
susceptibility corrected for diamagnetism.
Preparation of 2-N-Phenylamino-4-N'-Phenylimino-2-Pentene,
(Ph)~nacnacH
In a flask, re-distilled aniline was mixed with a molar equivalent (eq.)
of 2,4-pentanedione and benzene were mixed. The mixture was boiled on an
oil-bath and the distilled-off azeotropic mixture (benzene-water) was replaced
with benzene until all water was separated. Then, the surplus solvent was
removed by distillation. The crude product was re-distilled in vacuo (boiling
point approx.75°C) to give a crystalline substance which was re-
crystallized
from n-hexane to yield fine yellowish crystals of 2-N-phenylamido-2'-penten-
4-one.
These 2-N-phenylamido-2'-penten-4-one yellow crystals were then
used to prepare (Ph)ZnacnacH in accordance with the Reaction Scheme 1
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
19
(above). A molar eq. of triethyloxonium tetrafluoroborate in dichloromethane
was added dropwise to the 2-N-phenylamido-2'-penten-4-one in the same
solvent. The mixture was allowed to stand for 30 minutes. Then, 1 molar eq.
of aniline in dichloromethane was added. After I hour the solvent was
removed completely in vacuo and the residual oil was dissolved in hot ethyl
acetate and the 2-N-phenylamino-2'-penten-4-phenylimmonium
tetrafluoroborate product was allowed to crystallize.
The free base of the ligand, (Ph)2nacnacH was prepared from the
cationic salt, 2-N-phenylarnino-2'-penten-4-phenylimmonium
tetrafluoroborate. An equimolar reaction with potassium hydride (KH)
(optionally MeLi} resulted in about a 98% yield of yellow neutral,
deprotonated, (Ph)2nacnacH crystals. Alternatively, a metal salt, for instance
(Ph)2nacnacLi (or (Ph)2nacnacK), could have been formed from the 2-N-
phenylamino-2'-penten-4-phenylimmonium tetrafluoroborate cation salt and
two equivalents of MeLi (or KH). Deuterated versions of these compounds
are formed by substituting aniline-ds for unlabeled aniline.
EXAMPLE lA
Preparation of 2,4-pentane di(N-phenyl)iminato dichloro bis-
tetrahydrofuran titanium, (Ph)2nacnacTiCl2(THF)Z and the
Corresponding Compound with the Deuterated Ligand, (Ph-ds)2nacnac:
1.50 g (6.0 mmoles) of (Ph)2nacnacH was dissolved in 50 ml of THF
and cooled to -30°C. 1 equivalent (132 mg) of MeLi was slowly added as
a
solid with stirring. The resulting (Ph)2nacnacLi was added dropwise over a
three hours period, to a cooled 2.223 g (6.0 mmole) solution of TiCl3(THF)3 in
150 ml of THF. The color of the solution changed from sky blue to brown,
then to dark brown. The reaction mixture was concentrated to 50 ml, and
cooled to -30°C for crystallization. A dark brown microcrystalline
powder
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
was isolated by filtration and washed with cold THF several times. After
drying under vacuum, 2.92 g (95°lo yield) of (Ph)2nacnacTiCl2(THF)2 as
microcrystalline brown compound was isolated.
5 The resulting compounds were analytically tested and the results are
shown in Tables lA.l-3. The single crystal X-ray diffraction results are shown
in Fig. 1.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
21
TABLE lA.l
ANALYTICAL DATA FOR
{Ph)ZnacnacTiCl2(THF)i
(Ph-ds)ZnacnacTiCl2(THF)2
~H 103.1 89.5 11.4 6.25 3.65 3.27 1.61
NMR (6H,6)(lH,b} (2H) (4H) (8H) (2H) (8H)
(CDzCIz)e: 27.07 12.79 8.07 3.95 3.31 *** ***
8 (6H) ( (4H) (4H) (2H)
m 1
~H H)
NMR
(THF-dg)':
b
(
m
~H 108.7685.80 *** *** *** *** ***
NMR (6H) (1H)
(THF-dg)':
t
8
m
'H 16.1 15.4 6.1 *** *** *** ***
NMR (4D) (2D) -
(THF)': (4D)
8
m
IR 3053m 2971 2928m 2876m 1591 1534si
- s m 483s
KBr
:
(cm
1):
1447m 1359s 1290m 1263s 1185w 1066w919m
840s 761m 709s 518w 447w *** ***
IR 2969m 2928m 2890m 2876m 2271 1575m1530m
- w
KBrb:
(cm
):
1432m 1369vs 1298vs 1250w 1147w lOlsm875m
852m 824w 769w 557m 464w *** ***
Mass 367 [M+ 311.8
Spectrometry: ( - (3.
m/z 100.0) (THF)2] I
(% )
[M+
-
CI(THF)z]
Mass 377.03 342.06
Spectrometry: ( (3.4)
t 100.0) [M+
M/z [M+ -
(% - Cl(THF)2]
(THF)Z]
UV-vis 517 445
(THF) (493.6 (2383.9
': M'~ M-~
E crri crri
i) )
2.0(std.
dev.
1
,
294K)
Meltin 154
Pt. -
Range: 159C
C 2 H N O ! . ~ ::. . '!
TiCI : . .
'
Calculated:C 5$.69H N
(% 6.51 5.48
Measured:
%
Sam ie 1 C 56.86H N
5.89 3.77
Sam le 2 C 57.36H N
6.51 5
52
t adze lvotes: (Applicable for Tables lA-6)
t- indicates analytical results for corresponding catalyst
prepared using deuterated ligand, (Ph-ds)2nacnac(H).
a - indicates solvent used for NMR measurements.
b - indicates solvent used for IR measurements.
- indicates solvent used for UV-VIS measurements.
Letter designations after the numbers in the IR results
provide an indication of the strength of the designated
peak: vs = very strong, s = strong, m = medium, and w =
weak.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
22
Table 1.A-2 Interatomic Distances and Angles for
(Ph)2nacnacTiCl2(THF)2
(Note: the bond designations are with reference to FIG. 1 and the values noted
in parentheses
after the distances and angles represent the estimated standard deviation.)
Bond Distance l~) Bond Distance
(~)
Ti-N( 1 ) 2.086(3) C(4)-C(5) 1.366(6)
Ti-N(2) 2.088(3) C(5)-C(6) 1.392{5)
Ti-O(2) 2.209(3) C(7)-C(9) 1.386(5)
Ti-O( 1 ) 2.213(3) C(7)-C(8) 1.513(5)
Ti-Ci( 1 2.3888( 10) C(9)-C( 10) 1.415(5}
)
Ti-Cl(2) 2.4001 ( 10) C( 10)-C( 11 ) 1.525(5)
N( 1 )-C(7) 1.336(4) C( 12)-C( 13) 1.378(6)
N( 1 )-C( 1.439(5) C( 12)-C( 17) 1.386(5)
1 )
N(2)-C(10) 1.325(4) C(13)-C(14) 1.395(6)
N(2)-C(12) 1.439(5) C(14)-C(15) 1.379(6)
O( 1 )-C(21 1.440(6) C( I S)-C( 16) 1.354(7)
)
O( 1 )-C( I .458(5) C( 16)-C( 17) I .396(6)
18)
O(2)-C(22) 1.428(6) C( 18)-C( 19) 1.470(7)
O(2)-C(25) 1.475(5) C( 19)-C(20) 1.468(8}
C(1)-C(2) 1.378(5) C(20)-C(21) 1.514(8)
C(I)-C(6) 1.383(6) C(22)-C(23) 1.455(8)
C(2)-C(3) 1.392(6) C(23)-C(24) 1.463(9)
C(3)-C(4) 1.378(6) C(24)-C(25) 1.491
(7)
Bond Angle An Ig c (dee.)Bond Angle Angle
(deQ.)
N( 1 )-Ti-N(2)87.52(12) C(6)-C( 1 )-N( i 19.9(3)
1 )
N(I)-Ti-O(2)176.65(12) C(1)-C(2)-C(3) 120.8(4)
N(2)-Ti-O(2)95.73(9) C(4)-C(3)-C(2) 119.4(4)
N(1)-Ti-O(1)95.39(10) C(5)-C(4)-C(3) 120.3(4)
N(2)-Ti-O(1)175.83(13) C(4)-C(5)-C(6) 120.4(4)
O(2)-Ti-O( 81.40( I 1 C( 1 )-C(6)-C(5 120.0(4)
1 ) ) )
N( 1 )-Ti-Cl(192.48(9) N( 1 )-C(7)-C(9) 123.9(3)
)
N(2)-Ti-Cl(1)89.35(10) N(1)-C(7)-C(8) 120.6(3)
O(2)-Ti-CI(1)88.33(9) C(9)-C(7)-C(8) 115.5(3)
O(1)-Ti-Cl(1)87.55(8) C(7)-C(9)-C(10) 127.9(3)
N(1)-Ti-Cl(2)91.33(9) N(2)-C(10)-C(9) 124.1(3)
N(2)-Ti-CI(2)94.06( 10) N(2)-C( 10)-C( 120.3{3)
11 )
O(2)-Ti-CI(2)87.69(9) C(9)-C(10)-C(11) 115.6(3)
O(1)-Ti-C1(2)88.86(8) C(13)-C(12)-C(17)119.4(3)
Cl(1)-Ti-Cl(2)175.00(4) C(13)-C(12)-N(2) 119.7(3)
C(7)-N( 1 117.1 (3) C( 17)-C( 12)-N(2)120.9(4)
)-C( 1 )
C(7)-N(1)-Ti127.4(2) C(12)-C(13)-C(14)120.5(4)
C(1)-N(I)-Ti115.3(2) C(15)-C(14)-C(13)119.4(5)
C(10)-N(2)-C(12)116.8(3) C(16)-C(IS)-C(14)120.5(4)
C(10)-N(2)-Ti127.7(2) C(15)-C(16)-C(17)120.7(4)
C(12)-N(2)-Ti115.2(2) C(12)-C(17)-C(16)119.6(4)
C(21)-O(1)-C(18)106.5(3) O(1)-C(I8)-C(19) 107.4(4)
C(21)-O(1)-Ti126.5(3) C(20)-C(19)-C(18)108.0(4)
C(18)-O(1)-Ti126.7(3) C(19)-C(20)-C(21)104.9(5)
C(22)-O(2)-C(25)107.1(3) O(1)-C(21)-C(20) 106.4(4)
C(22)-O(2)-Ti127.4(3) O(2)-C(22)-C(23) 108.2(5)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
23
C(25)-O(2)-Ti 125.2(3) C(22)-C(23)-C(24) 107.1(5)
C(2)-C(1)-C(6) 119.1(3) C(23)-C(24)-C(25) 103.2(5)
C(2)-C(1)-N(1) 120.9(4) O(2)-C(25)-C(24) 106.0(5)
Table 1A.3 Structure Determination Summary for
(Ph)2nacnacTiClZ(THF)2
Crystal Data
Formula C25H33CI2N2O2T1
Formula Weight S 12.33
Crystal color red
to Crystal Size (mm) 0.35 x 0.25 x 0.14
Crystal System orthorhombic
Space Group Pna2,
Unit Cell Dimensions a = 19.5601 (8)A
b = 9.4959(4) A
c = 13.5555(5) A
a = 90
(3 = 90
y = 90
Volume 2517.8(2) ~3
2o Z 4
Density (calc.) 1.352 g/cm3
Absorption Coefficient 5.76 cm ~
F(000) 1076
Data collection
Diffractometer Used Siemens P4
Radiation MoKa (1 = 0.71073A)
Temperature 223(2) K
Monochromator Highly oriented graphite
crystal
28 Range (w) 4.16 to 56.66
Scan type Omega, Phi
Scan Range 0.3
Index Ranges -18 <h < 20
-12<k< 11
-18<1<17
Reflections Collected 9097
independent Reflections 4584 (R;", = 3.41 %)
Observed Reflections 3916
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
24
Solution and Refinement
System Used SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
Quantity minimized S[w(Fo2 - F~2)2~IS[(WFp2)2~1/2
Hydrogen Atoms idealized contributions
Weighting Scheme W ~ = s2(F) + 0.0010
FZ
Final R Indices (obs. data)R = 4. I 5%, wR = 10.51
%
R Indices (all data) R = 5.34%, wR = 11.50%
to Goodness-of-Fit 1.272
Data-to-Parameter Ratio 15.8:1
Largest Difference Peak 0.355
Largest Difference Hole -0.249
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
EXAMPLE 1B
Preparation of 2,4-pentane di(N-phenyl)iminato dichloro bis-
5 tetrahydrofuran vanadium, (Ph)2nacnacVCl2(THF)2 and the
Corresponding Compound with the Deuterated Ligand, (Ph-ds)2nacnac:
3.72 mmole (0.93g) of (Ph)2nacnac(H) was dissolved in 50 ml of THF
and cooled to -30°C in a round bottom flask. 3.72 mmoles MeLi solution
was
10 added slowly and allowed to stir for two hours to give a yellow solution of
(Ph)2nacnacLi in THF with gas evolution. After the reaction mixture was
allowed to stir until no more bubbles were observed, it was cooled to -
30°C.
In a separate round bottom flask, 3.72 mmoles (1.37 g) of VC13(THF)~ was
dissolved in 150 ml of THF. The THF solution of (Ph)2nacnacLi was then
15 transferred to an addition funnel and added dropwise to the THF solution of
VC13(THF)3 over a three hour period. The color of the solution slowly
changed from dark red to purple brown and finally dark green. After stirring
overnight, the solvent was evaporated to dryness. The resulting brown solid,
was extracted with toluene, by trituration in ether. The brown solution was
20 then filtered and toluene was vacuum removed. The solid was dissolved in
THF which turned dark green. The THF solution was cooled to -30°C
for
crystallization. Dark green microcrystalline powder was isolated by filtration
and washed with cold THF several times. After drying under vacuum, 1.05 g
(55 % yield) of (Ph)2nacnacVCl2(THF)2 was isolated.
The resulting compounds were analytically tested and the results are
shown in Tables 1B.1-3. The single crystal X-ray diffraction results are shown
in Fig. 2.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186.~
26
TABLE 1B.1
ANALYTICAL DATA FOR
(Ph)ZnacnacVCl2(THF)2
(Ph-~ds)~nacnacVCh(THF)~
'H NMR (CDZCI2)':111.5299.81 86.72 25-8 8.49 5.55 1.83
8 ( m) (2H,b)(2H,b) (1 (4H,vb) ( ( (8H)
H,b) I 11
H) H,b)
'H NMR (THF-dg)':107.5986.92 12.22 6.23 *** *** ***
8 ( m (6H) (IH) (4H) (6H)
'H NMR (THF-da)':108.7685.80 *** ***- *** *** ***
t (6H) (IH)
b m
'H NMR (THF)':12.1 6.2 *** *** *** *** ***
$ ( m (4D) (6D)
IR - KBr: cni 3053m 3031m 2968s 2927m 2879m 1590m 1532s
)
1485s 1435m 1430m 1368m 1319s 1066w _
1021s
924s 784w 779m 524w
920m 708s
87Ss
844s
IR - KBr: (ctxi2968m 2927m 2876m 2269w 1561 1528vs 1430m
): m
1382vs1318vs 1242m 1021s 924w 870m 8S4m
811w 779w 556m 457m *** *** ***
Mass Spectrometry:369.99 299.04
m/z 9b (37) {32)
[M+ [M+
- -
(THF)z] CI(THF)z]
Mass Spectrometry:380.02 345.05
t ( (2.54)
m/z % 100.0) [M'
[M+ -
- CI(THF)z]
(THF)z]
UV-vis (THF)':598(1,318.4 474(1,404.2 350(10,57
7~,""x(E) M''crri') M-'crri') 4.7
M'
~cm
~)
3.2(std.
dev.
1),
(294K
Meltin Pt. 162
Range: -
164C
Cases N O VCI r, :: y ; ,, .
. . :
;
,
Calculated: C 58.26 H 6.45 N 5.44
(%)
Measured: (%) C 57.81 H 6.37 N 3.77
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
27
Table 1B.2
Interatomic
Distances
and Angles
for
(Ph)ZnacnacVCl2(THF)Z
(Note: the
bond designations
are with
reference
to FIG.
2 and the
values
noted in
parentheses
after the
distances
and angles
represent
the estimated
standard
deviation.)
Bond Distance (t~) Bond Distance
(~)
V(I)-N(1) 2.023(2) C(6)-C(11) 1.382(4)
V(1 )-N(2).2.030(2) C(7)-C(8) 1.379(S)
V( I )-O( 2.208(2) C(8)-C(9) 1.366(S)
1 )
V(1)-O(2) 2.221(2) C(9)-C(10) 1.393(4)
V ( I )-CI(2.3592(6) C( 10)-C( I 1 1.389(3)
1 ) )
V( I )-Cl(2)2.3646(6) C( 12)-C( 13) 1.383(4)
O( I )-C(211.445(4) C( 12)-C( 17) 1.385(4)
)
O(1)-C(18) 1.456(4) C(13)-C(14) 1.397(4)
O(2)-C(2S) 1.447(4) C(14)-C(IS) 1.382(4)
O(2)-C(22) 1.480(4) C( 1 S)-C( 16) 1.386(4)
N( 1 )-C( 1.345(3) C( 16)-C( 17) 1.393(4)
I )
N( I )-C( 1.430(3) C( 18 )-C( 19) 1.506(5
11 ) )
N(2)-C(3) 1.337(3) C(19)-C(20) 1.471(6)
N(2)-C( 1.431 (3) C(20)-C(21 ) 1.488(5)
17)
C(I)-C(2) 1.400(3) C(22)-C(23) 1.518(S)
C( 1 )-C(4)I.S23(4) C(23)-C(24') 1.39(2)
C(2)-C{3) I .395(4) C(23)-C(24) 1.SS9(13)
C(3)-C(S) 1.509(3) C(24)-C(2S) 1.383(
10)
C(6)-C(7) 1.380(4) C(24')-C(25) 1.597(12)
Bond Anele An~le (dee.) Bond Anele An~le (dee.)
N(1)-V(1)-N(2)90.94(9) N(2)-C(3)-C(2) 123.5(2)
N(1)-V(1)-O(2)94.15(7) N(2)-C(3)-C(S) 120.6(2)
N(2)-V(I)-O(2)174.89(9) C(2)-C(3)-C(S) 115.8(2)
N(1)-V(1)-O(1)174.75(9) C(7)-C(6)-C(11) 120.9(3)
N(2)-V(1)-O(1)93.45(7) C(8)-C(7)-C(6) 120.0(3)
O(2)-V(I)-O(1)81.44(8) C(9)-C(8)-C(7) 119.8(3)
N(1)-V(1)-Cl(I)89.68(7) C(8)-C(9)-C(10) 120.?(3)
N(2)-V(1)-CI(1)91.35(7) C(I1)-C(10)-C(9) 119.7(3)
O(2)-V(1)-CI(1)88.24(7) C(6)-C(11)-C(10) 118.9(3)
O(1)-V(1)-Cl(I)87.33(6) C(6)-C(11)-N(1) 120.2(2)
N(1)-V(1)-Cl(2)93.78(7) C(10)-C(I1)-N(1) 120.8(2)
N(2)-V(1)-CI(2)92.22(7) C(13)-C(12)-C(17)121.0(3)
O(2)-V ( 87.90(7) C( 12)-C( 13)-C( 119.6(3)
1 )-Cl(2) 14)
O(I)-V(1)-CI(2)88.93(6) C(1S)-C(14)-C(13)119,8(3)
Cl(I)-V(1)-CI(2)174.99(3) C(14)-C(IS)-C(16)120.2(3)
C(21)-O(I)-C(18)106.5(2) C(15)-C(16)-C(17)120.4(3)
C(21)-O(1)-V(1)126.8(2) C(12)-C(17)-C(16)119.0(3)
C(18)-O(1)-V(1)125.8(2) C(12)-C(17)-N(2) 120.3(2)
C(25)-O(2)-C(22)108.2(3) C( 16)-C( 17)-N(2)120.7(2)
C(2S)-O(2)-V(126.5(2) O( 1 )-C( 18)-C(19)l OS.9(3)
1 )
C(22)-O(2)-V(1)125.0(2) C(20)-C(19)-C(18)106.0(3)
C(1)-N(1)-C(I1)116.4(2) C(19)-C(20)-C(21)107.0(3)
C(1)-N(1)-V(1)126.2(2) O(1)-C(21)-C(20) 107.2(3)
C( I 1 )-N(117.1 (2) O(2)-C(22)-C(23) 104.8(3)
1 )-V(
1 )
C(3)-N(2)-C(17)117.6(2) C(24')-C(23)-C(22)102.0(7)
C(3)-N(2)-V(1)125.8(2) C(22)-C(23)-C(24)105.5(5)
C(17)-N(2)-V(116.4(2) C(25)-C(24)-C(23)106.9(7)
1 )
N(1)-C(1)-C(2)123.3(2) C(23)-C(24')-C(25)104.7(6)
N(1)-C(1)-C(4)120.3(2) C(24)-C(25)-O(2) 110.7(S)
C(2)-C(1)-C(4)116.4(2) O(2)-C(25)-C(24')102.3(7)
C(3)-C(2)-C(I)128.8(2)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
28
Table 1B.3 Structure Determination Summary for
(Ph)=nacnacVCh(THF)Z
Crystal Data
Formula C2SH33Cl2N2O2V
Formula weight 515.37
Crystal color black block
Crystal Size (mm) 0.35 x 0.25 x 0.14
Crystal System orthorhombic
1o Space Group Pna2,
Unit Cell Dimensions a = 19.5215(4) A
b = 9.5341 (2) A
c = 13.4898(3) A
a = 90
t5 ~i = 90
y = 90
Volume 2510.72(9) A
Z 4
Density {calc.) 1.363 g/cm~
2o Absorption Coefficient 6.32 cm~i
F(000)
1080
Data collection
Diffractometer Siemens P4
25 Radiation MoKa (1 = 0.71073A)
Temperature 218(2) K
Monochromator Highly oriented graphite
crystal
28 Range (w) 4.18 to 56.56
30 Scan type Omega, Phi
Scan Range 0.3
Index Ranges -25 <h < 12
-10<k< 12
-17 < 1 < 17
35 Reflections Collected 9021
Independent Reflections 5585 (R;"~ = 2.04%)
Observed Reflections 4948
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
29
Solution and Refinement
System Used SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
Quantity minimized S[w(Fo2 - F~2)2]/S[(wFo2)2~u'-
Hydrogen Atoms Idealized contributions
Weighting Scheme w-~ = s2(F) + 0.0010 F2
Final R Indices (obs. data) R = 3.75%, wR = 8.23%
R Indices (all data) R = 4.67%, wR = 9.77%
Goodness-of-Fit 1.390
Data-to-Parameter Ratio 18.6:1
Largest Difference Peak 0.301
Largest Difference Hole -0.240
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
EXAMPLE 1C
Preparation of 2,4-pentane di(N-phenyl)iminato dichloro bis-
5 tetrahydrofuran chromium, (Ph)2nacnacCrCl2(THF)2 and the
Corresponding Compound with the Deuterated Ligand, (Ph-ds)2nacnac:
2.40 mmole (0.6 g,) of (Ph)2nacnacH was dissolved in 50 ml of THF and
cooled to -30°C. 2.40 mmoles (53 mg} of MeLi was slowly added as a
solid
10 with stirring. The THF solution of (Ph)2nacnacLi prepared in-situ was then
slowly added over a three hour period to a slurry of 2.40 mmoles (900 mg,) of
CrCl3(THF)~ in 150 ml of THF. The color of the solution changed from a
purple to red brown. After stirring at room temperature overnight, the
reaction
mixture was concentrated to 50 ml and cooled to -30°C for
crystallization. A
15 brown microcrystalline powder was isolated by filtration. After washing
with
cold THF several times and drying under vacuum, 2.09 mmoles ( 1.08 g, 87%
yield) of the resulting (Ph)2nacnacCrCl2(THF)2 compound was isolated.
The resulting compounds were analytically tested and the results are
2o shown in Tables 1 C.1-3. The single crystal X-ray diffraction results are
shown
in Fig. 3.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
31
TABLE 1C.1
ANALYTICAL DATA FOR
(Ph)2nacnacCrCl2(THF)2
(Ph-ds)2nacnacCrCl2(THF)Z
'H NMR (CD~CIz)a:111.5299.81 86.72 25-8 8.49 5.55 1.83
8 ( m) (2H,b)(2H,b) (lH,b) (4H,vb) (1H) (llH,b) (8H)
'H NMR (THF-d8)15.46 6.19 12.22 6.23 *** *** ***
: (6H) (4H) (4H) (6H)
b ( m)
'H NMR (THF) 16.1 15.4 6.1 *** *** *** ***
a: (4D) (2D) (4D)
t
8 ( m)
IR - KBrb: 3050w 3017m 2966s 2928s 2883s1590w 155Svs
(cm ):
1530vs1484vs 1448vs 1387vs 1263s1200s1065 1017s
w
921m 871m 848s 764m 710s 662w 526m 477w
IR - KBr : 2967m 2926m 2878m 2269w1550m1528vs 1453m
(cm-'):
t
1381vs 1320vs 1157m 1016m969m 869m 855m
811w 753m 558m 427m *** *** ***
UV-vis (THF)':598(1,318.4 474(1,404.2 350(10,574.7
ax(E) M~'crri') M- M-
cm cni
) )
Mass Spectrometry:370.76 335.81 300.86(6.01
m/z (%) (41.24) (41.60) )
[M+ M+ [M+
- - -
(THF) CI(THF Clz(THF)
)
Mass Spectrometry:381.02 346.05(46.83) 311.09(5.45)
t m/z (%) ( [M+ [M+
19.41 - -
) CI(THF Clz(THF)
[M+ ] ]
-
(THF)z]
UV-vis (THF)':527(572.2 419(8,232.5 400(5,711.7
~az(E) M''crri M~ M-'cni')
~) cm
)
4.1(std.
dev.
1),
~
(294K)
Melting Pt. 174
Range: -
180C
CzSH 3N OzCrCI
Calculated: C: J2S.14 H 6.44 N 5.42
(%)
Measured: (%) C 57.95 H b.81 N 5.51
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
32
Table 1C.2 Interatomic Distances and Angles for
(Ph)znacnacCrCl2(TIiF)
(Note: the
bond designations
are with
reference
to FIG.
3 and the
values
noted in
parentheses
after the eviation.)
distances
and angles
represent
the estimated
standard
d
Bond Distance (A) Bond Distance
(A)
Cr(1 )-N( 2.032(6) C(6)-C(7) 1.398(7)
1 )
Cr( 1 )-N(2)2.033(5) C(7)-C(8) 1.415(8)
Cr( 1 )-O(2)2.141 (5) C(8)-C(9) 1.347(9)
Cr( 1 )-O( 2.144(5 ) C(9)-C( 10) 1.402(8)
I )
Cr( 1 )-Cl(2.3392( 12) C( 10)-C( I 1 1.415(7)
1 ) )
Cr( 1 )-Cl(2)2.3532( 12) C( 12)-C( 17 1.369(7)
)
O( 1 )-C(18)1.462(8) C( 12)-C( 13) 1.406(8)
O(1)-C(21) 1.464(8) C(13)-C(14) 1.383(8)
O(2)-C(2S) 1.459(8) C(14)-C(15) 1.393(8)
O(2)-C(22) 1.473(8) C(IS)-C(16) 1.374(8)
N( 1 )-C( 1.31 S(8) C( 16)-C( 17) 1.424(7)
1 )
N(1)-C(11) 1.459(7) C(18)-C(19) 1.526(9)
N(2)-C(3) 1.323(8) C(19)-C(20) 1.519(11)
N(2)-C(17) 1.453(7) C(20)-C(21) 1.492(10)
C( 1 )-C(2)I .431 (7) C(22)-C(23 ) 1.497( I
0)
C(1)-C(4) 1.537(8) C(23)-C(24') 1.40(3)
C(2)-C(3) 1.412(8) C(23)-C(24) 1.58(2)
C(3)-C(5) 1.525(8) C(24)-C(25) 1.47(2)
C(6)-C(11) 1.377(8) C(24')-C(25) 1.50(3)
Bond Anele An Ig_e (dee Bond Angle Anale (den.)
)
N( 1 )-Cr(191.7(2) N(2)-C(3)-C(2) 124.7(5)
)-N(2)
N( 1 )-Cr( 93.4(2) N(2)-C(3)-C(5) I 21.2(6)
1 )-O(2)
N(2)-Cr(1)-O(2)174.8(2) C(2)-C(3)-C(5) 114.1(6)
N( 1 )-Cr( 176.0{2) C( 11 )-C(6)-C(7)I 21.7(5)
1 )-O(
1 )
N(2)-Cr(1)-O(1)92.1(2) C(6)-C(7)-C(8) 118.0(5)
O(2)-Cr(I)-O(1)82.7(2) C(9)-C(8)-C(7) 120.5(6)
N(1)-Cr(I)-Cl(1)88.5(2) C(8)-C(9)-C(10) 122.2(6)
N(2)-Cr(I)-Cl(1)89.5(2) C(9)-C(10)-C(11)118.0(6)
O(2)-Cr( 90.1 (2) C(6)-C( 1 I )-C(1 19.6(5)
I )-Cl( 10)
1 )
O(1)-Cr(I)-CI(1)90.28(13) C(6)-C(II)-N(I) 121.1(5)
N(1)-Cr(1)-Cl(2)90.6(2) C(10)-C(11)-N(1)119.1(5)
N(2)-Cr(1)-Cl(2)90.1(2) C(17)-C(12)-C(13)120.8{S)
O(2)-Cr(1)-Cl(2)90.3(2) C(14)-C(13)-C(12)119.8(5)
O(1)-Cr(1)-Cl(2)90.71(13) C(13)-C(14)-C(15)119.5(6)
Cl(1)-Cr(1)-C1(2)178.95(11) C(16)-C(15)-C(14)121.3(5)
C(l8)-O(1)-C(2l)106.3(6) C(15)-C(16)-C(17)119.3{5)
C(18)-O(1)-Cr(1)126.5(4) C(12)-C(17)-C(16)119.3(5)
C(21)-O(1)-Cr(1)125.8(4) C(12)-C(17)-N(2)121.6(5)
C(25)-O(2)-C(22)107.4(6) C(16)-C(17)-N(2)119.0(5)
C(25)-O(2)-Cr(1)126.9(4) O(1)-C(18)-C(19)105.9(6)
C(22)-O(2)-Cr(I)125.2(5) C(20)-C(19)-C(18)103.8(6)
C(1)-N(1)-C(ll)117.1(5) C(21)-C(20)-C(19)107.4(6)
C( 1 )-N( 125.7(4) O( I )-C(21 )-C(20)106.8(6)
1 )-Cr(
1 )
C(I1)-N(1)-Cr(I)116.8(4) O(2)-C(22)-C(23)105.8(7)
C(3)-N(2)-C(17)117.7(5) C(24')-C(23)-C(22)100.4(12)
C(3)-N(2)-Cr(1)125.3(4) C(22)-C(23)-C(24)109.4(9)
C(17)-N(2)-Cr(1)117.0(4) C(25)-C(24)-C(23)101.3(13)
N(1)-C(1)-C(2)124.6(6) C(23)-C(24')-C(25)109(2)
N(I)-C(I)-C(4)121.2(5) C(24)-C(25)-O(2)112.3(10)
C(2)-C(1)-C(4)114.2(6) O(2)-C(25)-C(24')103(2)
C(3)-C(2)-C(1)126.9(6)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
33
Table 1C.3 Structure Determination Summary for
(Ph)ZnacnacCrCl2(THF)
Crystal Data
Empirical Formula Cz5H33C12N202Cr
Color; Habit brown plate
Crystal Size (mm) 0.35 x 0.35 x 0.12
Crystal System orthorhombic
Space Group Pna2,
Unit Cell Dimensions a = 19.6220(4) t~
b = 9.597 I (2) ~
c = 13.4509(3) ~
a = 90
(3=90
t 5 'y = 90
Volume 2533.00(9) A3
Z 4
Formula Weight S 16.43
Density (calc.) 1.354 g/cm~
Absorption Coefficient 6.87 cm's
F(000) 1084
Data collection
Diffractometer Used Siemens P4/CCD
Radiation MoKa {I = 0.71073A)
Temperature 218(2) K
Monochromator Highly oriented graphite
crystal
28 Range (w) 4.16 to 56.12
3o Scan type Omega, Phi
Scan With 0.3
Index Ranges -24 <h < 25
-12<k< 12
-16 < 1 < 13
Reflections Collected 9160
Independent Reflections 4228 (R;"t = 13.02%)
Observed Reflections 2408
CA 02321419 2000-08-14
WO 99/41290 PCT/1.JS99/01863
3a
Solution and Refinement
System Used SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
s Quantity minimized S[w(Fo2 -
F~2)2]/S [(wFo2)2) iiz
Hydrogen Atoms Idealized contributions
Weighting Scheme w-~ = s2(F) + 0.0010 F2
Final R Indices (obs. data) R = 5.35%, wR = 11.14%
to R Indices (all data) R =10.56%, wR = 16.45%
Goodness-of-Fit 0.808
Data-to-Parameter Ratio 14.2: I
Largest Difference Peak 0.375
Largest Difference Hole -0.880
15
EXAMPLE 2A
Preparation of 2,4-pentane di(N-phenyl)iminato chloro methyl vanadium,
(Ph)2nacnacV(Cl)(Me):
0.97 mmoles (0.500 g) of (Ph)2nacnacVCl2(THF)2, reference Example
1B, was dissolved in 150 ml of THF and the solution was cooled to -
30°C.
0.97 mmoles of MeLi in ether was slowly added to the suspension of
{Ph)2nacnacVCl2(THF)2 in THF which caused a color change from dark green
to dark red brown. After stirring for 5 hours, the reaction mixture was
evaporated to dryness. The residual THF was removed by trituration in ether.
The brown solid was then extracted with ether and filtered to remove LiCI.
The ether solution was concentrated and cooled to -30°C for
crystallization. A
microcrystalline brown powder was filtered and washed with cold pentane.
3o After drying under vacuum, 0.202 g (59 % yield} of (Ph)2nacnacV(Cl)(Me)
was isolated. The resulting compound was analytically tested and the results
are shown in Table 2A.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
35
TABLE 2A
ANALYTICAL DATA FOR
(Ph),nacnacV(CIl(Mel
~H NMR (C6D6)a:8.70 -3.90 *** *** *** *** ***
8 ( m) (6H,vb)(2H,vb)
IR - KBrb: 3057w 3029w2992w 2959w 2918w 2848wI592m
(cm- )
1530s 1510m1483s 1447m 1427m 1371s1339s
1299m 1262m1184w 1071w 1023m 1023s997m
937w 918w 854w 799w 754s 700s SlSm
Mass Spectrometry:350 335( 299(6)
m/z (%) ( I 100) [M'
S) [M+ -
M'" - CH~CL]
CH
]
UV-vis (Et20)541 435
': (4.27x (
x(e) 10 I
M-~ .42x
crri 10
~ ) M''
cm
)
ff 3.0(std.
dev.
1 ),
B (294K)
Melting Pt. 132
Range: - 134C
C18H20N2VC1
Calculated: I C: 61.64 I H J. /J I N -/.yy I I 1.:
Measured: (%) ~ C 61.53 ~ H 5.74 ~ N 7.98 ~ ~ ~ ]
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
36
EXAMPLE 2B
Preparation of 2,4-pentane di(N-phenyl)iminato chloro
trimethylsilylmethyl vanadium, (Ph)2nacnacV(Cl)(CH2Si{CH3)3)~
0.58 mmoles (300 mg) of (Ph)ZnacnacVCl2(THF)2, reference Example
1B, was dissolved in 50 ml of diethylether and the solution was cooled to
-30°C. 0.58 mmoles of LiCH2Si(CH3)3) in a diethylether solution was
added
1o slowly to the (Ph)2nacnacVCl2(THF)2 in diethylether solution which clouded
the brown solution without an observable color change. After stirring
overnight at room temperature, the reaction mixture was evaporated to
dryness. The dark brown oil was dissolved in pentane and evaporated to
dryness twice to remove residual THF. The solid was then extracted with
pentane and filtered to remove LiCI. The solvent was again evaporated and
the dark red brown oil was dissolved in a minimum amount of HMDS with
some drops of pentane and cooled to -30°C for crystallization. 124 mg
(505
yield) of dark brown (Ph)2nacnacV(Cl)(CH2Si(CH3)~ crystals were isolated
after two crystallization extraction cycles. The crystals were analytically
tested
2o and the results are shown in Table 2B.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
37
TABLE 2B
ANALYTICAL DATA FOR
s (Ph)2nacnacV(CI)(CHzSi(CH;)s)
'H NMR (C6D6)a:8.69 -1.40 3.16 *** *** *** ***
b ( m) (6H) (9H) (2H)
IR - KBr : 30s6w 3026w
(cm- ) 2999w
29s6m
2922m
28sOw
1593m
1532s 1510m 1485s1447m 1431m 1371vs 1299m 1251m
1071 1022m 998m848s 757m 701 488w 471
w s w
Mass Spectrometry:422(7) 335(57)
m/z (%) [M+] [M+-
CHZSi(CH3)sl
LJV-vis (Et20)':s41 629
az(~) (266 (421
M~' M''
cm'') crri')
3.0(std.
dev.
1 ),
(294K)
Melting Pt. 2s0-2s4C
Range:
CZ,H gN CIVSi
EXAMPLE 3A
1o Preparation of 2,4-pentane di{N-phenyl)iminato dimethyl vanadium,
{Ph)2nacnacVMe2:
5.0 mmoles (2.58g,) of (Ph)2nacnacVCl2(THF)2, reference Example
1 B, was suspended in 150m1 of THF and cooled to -30°. 11.0 mmoles (2.2
15 equiv.) of MeLi was added slowly causing a color change from dark green to
dark brown. After stirring for 5 hours, the reaction mixture was evaporated to
dryness. The brown solid was dissolved in diethylether and evaporated to
dryness twice to remove residual THF. The solid was then extracted with
diethlyether and filtered to remove LiCI. The solution was concentrated to 30
2o ml of ether and cooled to -30°C for crystallization. 1.397 g of
brown cubic
(Ph)2nacnacVMe2 crystals containing chloride impurity of
(Ph)2nacnacV(CI)(Me) were isolated after vacuum drying. The content of the
chloride impurity ranges from 18 to 50%.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/018b3
38
The resulting compounds were analytically tested and the results are
shown in Tables 3A.1-3. The single crystal X-ray diffraction results are shown
in Fig. 4.
TABLE 3A.1
ANALYTICAL DATA FOR
(Phl~nacnacVMe~
~H NMR (C6D6)':8.68 -4.99 *** *** *** *** ***
8 m) (6H) (2H)
IR - KBr : 3053m 3031m 2968s 2927m 2879m 1590m 1532s
(crri ~)
1485s 1435m 1430m 1368m 1319s 1066w 1021s
924w 784w 779m 708s
920m 524w
875s
844s
Mass Spectrometry:330 315(22.29) 300(29.24)
(23.4)
m/z (lo [M+] [M+ [M+
- -
CHI] 2CH~]
UV-vis (Et20) 597 446
'~: (194.4 (334
M-~ M-
cm's) cm'
)
~nax(E)
3.1(std.
dev.
1),
B
(294K)
Meliir~g Pt. 125-129C
Rangc:
C,aHasN 'J .. .:' : .. ;: ....
. ' . .
.:: ..
~aicmaieo: ~ ion l: O%.U2S ri /.UL. N tS.4tS
Measured: (%) C 64.?6 H 6.36 N 8.29
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
39
Table 3A.2 Interatomic Distances and Angles for (Ph)ZnacnacVMe2
(Note: the bond designations are with reference to FIG. 4 and the values noted
in parentheses
after the distances and angles represent the estimated standard deviation.)
Bond Distance ltd) Bond Distance
(A)
V-N( 1 ) 1.963(3) C(7)-C(8) 1.398(5)
V-N(2) 1.972(3) C(7)-C(12) 1.415(6)
V-C(1) 2.080(4) C(8)-C(9) 1.387(6)
V-C 2.126(4) C(9)-C(10) 1.385(7)
N( 1 )-C(2)1.353(5 ) C( 10)-C{ 11 ) 1.396(6)
N( 1 )-C( 1.448(5) C( 11 )-C(12) 1.378(6)
12)
N(2)-C(4) I .335(5) C( 13)-C( 18) 1.388(6)
N(2)-C(18) 1.433(5) C{13)-C(14) 1.409(6)
C(2)-C(3) 1.405(5) C(14)-C(15) 1.377(7)
C(2)-C(5) 1.523(5) C(IS)-C(16) 1.382(7)
C(3)-C(4) 1.416(5) C(16)-C(17) 1.388(6)
c(4)-c(6) 1.531 (5) C( 17)-C( 18) 1.383(6)
Bond Anr~leAngle (dee.) Bond Angle An~le
(dee.)
N(1)-V-N(2)92.11(13) C(3)-C(4)-C(6) 117.6(3)
N( 1 )-V-C(11 I .1 (2) C(8)-C(7)-C( 12) 120.0(4)
1 )
N(2)-V-C(1)115.1(2) C(9)-C(8)-C(7) 120.4(4)
N(1)-V-C 114.72(14) C(10)-C(9)-C(8) 119.4(4)
N(2)-V-C 108.4(2) C(9)-C(10)-C(11) 120.5(4)
C(1)-V-C II3.6(2) C(12)-C(11)-C(10)120.9(4)
C(2)-N(1)-C(12)120.4(3) C(11)-C(12)-C(7) 118.6(4)
C{2)-N(I)-V127.6(3) C(II)-C(12)-N(1) 123.5(4)
C(12)-N(1)-V111.8(2) C(7)-C(12)-N(1) 117.6(3)
C(4)-N(2)-C(18)119.2(3) C(18)-C(13)-C(14)119.6(4)
C(4)-N(2)-V127.2(3) C(15)-C(14)-C(13)119.7(4)
C(18)-N(2)-V113.5(2) C(14)-C(15)-C(16)120.3(4)
N(1)-C(2)-C(3)122.0(3) C(15)-C(16)-C(17)120.4(5)
N(I)-C(2)-C(5)120.9(3) C(18)-C(17)-C(16)119.9(4)
C(3)-C(2)-C(5)117.1(3) C(17)-C(18)-C(13)120.1(4)
C(2)-C(3)-C(4)127.8(4) C(17)-C(18)-N(2) 120.2(4)
N(2)-C(4)-C(3)122.9(3) C(13)-C(18)-N(2) 119.5(4)
N(2)-C(4)-C(6)119.4(3)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
Table 3A.3 Structure Determination Summary for (Ph)ZnacnacVMe~
Crystal Data
Formula C,9H23N2V
Formula weight 330.13
5 Crystal color brown block
Crystal Size (mm) 0.40 x 0.20 x 0.10
Crystal System monoclinic
Space Group P2i/n
Unit Cell Dimensions a = 8.6616(2) ~r
to b = 15.9937(4) A
0
c = 13.2812(1) A
a = 90
(3 = 93.317(2)
'y = 90
15 Volume 1836.78(8) /~;
Z 4
Density (calc.) 1.220 g/cm~
Absorption Coefficient 6.05 cm
F(000)
712
Data collection
Diffraetometer Siemens P4
Radiation MoKa (1= 0.71073.)
Temperature 295(2) K
Monoehromator Highly oriented graphite
crystal
29 Range (w) 3.98 to 56.30
Scan type Omega, Phi
Scan Range 0.3
3o Index Ranges -11 <h < 10
18<k<21
-17 < 1 < 16
Reflections Collected 7272
Independent Reflections 3894 (R;"~ = 4.00%)
Observed Reflections
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
41
Solution and Refinement
System Used Siemens SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
s Quantity minimized S[w(F~2 - F~Z)2]/S[(wF
2)2]1/2
Hydrogen Atoms Idealized contributions
Weighting Scheme w-~ = s2(F) + 0.0010
F2
Final R Indices (obs. R =6.01 %, wR = 15.52%
data)
R Indices (all data) R = 10.22%, wR =
19.76%
Goodness-of-Fit 1.167
Data-to-Parameter Ratio 19.37:1
Largest Difference Peak 0.488
Largest Difference Hole -0.492
CA 02321419 2000-08-14
WO 99/41290 PCT/US99I0186.~
42
EXAMPLE 3B
Preparation of 2,4-pentane di(N-phenyl)iminato bis-trimethyl silylmethyl
vanadium, (Ph)ZnacnacV(CHZSi(CH3)3)z:
1.94 mmoles ( 1.00 g) of (Ph)ZnacnacVCl2(THF)2, reference Example
1B, was dissolved in 150 ml of THF and the solution was cooled to -
30°C.
3.88 moles (0.366 g) of LiCH2Si(CH3)3 crystals were slowly added to the THF
to solution which caused a color change from dark green to dark red brown.
After stirring at room temperature for 4 hours, the reaction mixture was
evaporated to dryness. By trituration in pentane, the residual THF was
removed. A dark brown solid was extracted with pentane and filtered to
remove LiCI. After the solvent was removed from the filtrate, the resulting
brown oil was dissolved in HMDS and cooled to -30°C. No solid was
isolated. However, 0.11 g (65 % yield) of a dark brown oil, i.e.,
(Ph)2nacnacV(CH2Si(CH3)3)Z, was isolated by evaporation of the solvent. The
oil was analyzed and the results are shown in Table 3B.
TABLE 3B
ANALYTICAL DATA FOR
(PhhnacnacVlCH~Si(CH~)z)~
'H NMR (CbDb): 8.56 -0.92 -3.69 *** *** *** ***
b ( m (6H) (i8H) (2H)
IR - neat : 306iw 3031w 2816w 1592m
cm' 2948s
2889m
2862w
1528s 1510s 1483vs 1447s1429m 1362vs 1262m1241s
1184w 1069w 1025m 935w884vs 845vs 750s 700vs
Mass Spectrometry:474.32(4.1 386.22(58.47) 300(24.56)
m/z ) [M+- [M+-
(%) [M''] SiMe4] 2CH2Si(CH3)sl
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
43
EXAMPLE 4
Preparation of 2,4-pentane di(N-phenyl)iminato (OTf)Z bis-
tetrahydrofuran vanadium, (Ph)ZnacnacV(OTf)2(THF)Z
0.52 mmoles {270 mg,)(Ph)2nacnacVCl2(THF)2, reference Example
1B, were dissolved in 40 ml of THF. 1.04 mmoles (280 mg) of AgOTf was
added as a solid to the THF solution. After stirring overnight, the solution
was filtered to remove AgCI. The dark green solution was concentrated and
cooled to -30°C for crystallization. 340 mg (87% yield) of dark green
(Ph)2nacnacV(OTf)2(THF)2 crystals were isolated. The crystals were analyzed
and the results are shown in Table 4.
TABLE 4
ANALYTICAL DATA FOR
(Ph)znacnacV(OTf)2(THF)2
~H NMR (THF-dg)126.94 15.91 3.28 *** *** *** ***
a: (6h,vb)(4H,vb)(4H)
8 ( m)
IR - KBr : 3061w 3031w 2985w 2933w 2907w 1592w1540m
(ctri )
1487s 1447w 1434w 1339vs 1236vs 1201vs1014s
928w 850m 844s 764w 710m 632s 524w
Mass Spectrometry:597.98(6.4) 465.02(54.3)
m/z (%) [M' M+
- 2THF] -
SOZCF,,2THF)
UV-vis (THF)':598(1,318.4 474(1,404.2 350(10,574.7
ax(E) M-~crti M-~crri M-~cm-~)
~) ~)
rr 3.2(std.
dev.
1),
B (294K)
Melting Pt. 160
Range: - 163C
Cz~H33N O
F S V
Calculated: C 43.66 H 4.48 N 3.77
(%) ~. '~ ''
Measured: (%) C 42.53 H4.59 N3.87
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
44
EXAMPLE 5A
Preparation of 2,4-pentane di(N-phenyl)iminato methyl diethylether
s tetrahydrofuran vanadium tetrakis-(3,5-bis-trifluoromethyl-
phenyl)borate, [(Ph)2nacnacVMe(Et20)(THF)][B(C6H3(CF3)2)al~
0.30 mmoles ( 100 mg) (Ph)2nacnacVMe2, reference Example 3A, was
dissolved in 20 ml of diethylether and cooled to -30°C. In a separate
flask, .30
to mmoles (310 mg) of H(Et20)2[B(C6H3(CF3)2)al was dissolved in 10 ml of
diethylether and cooled to -30.C. The diethylether solution of
H(Et2O)2[B(C6H3(CF3)2)4] was slowly added by pipette to the cold solution of
(Ph)2nacnacVMe2. With gas evolution, the color of the solution turned to
slightly darker brown. 0.21 mmoles (278 mg, 69 °lo yield) of orange
ts [(Ph)2nacnacVMe(Et20)(THF)][B(C6H3(CF3)2)a) crystals were isolated from a
concentrated diethylether solution containing a several drops of THF that was
cooled to -30°C.
The resulting compounds were analytically tested and the results are
shown in Tables SA.1-3. The single crystal X-ray diffraction results are shown
2o in Fig. 5.
TABLE SA.1
ANALYTICAL DATA FOR
25 ffPh)2nacnacVMe(Et20)(THF)1(B(C~H3(CF3)2)al
~H NMR (CDZC12)121.4 90.5 8.68 7.73 7.57 0.90 -2.46
: (6H,vb)(lH,vb) (4H,vb) (8H) (4H) (8H,b) (2H,vb)
8( m
IR - KBr : 3087w 2982w 2936w 2907w 1610w ISSSm 1486m
(cni
1450w 1429w 1356vs 1284vs 1158vs, b 1020m
1133vs,b
938s 897s 795w 758w 710s 700s 679s
670s 525w 496w *** *** *** ***
LTV-vis (EtzO)739(222 609(334 475(
': M crri M' 1350
ax{) ) cm' M'
) cm-
)
3.4(std.
dev.
I),
B {294K
Melting Pt. 95-97C
Ran e:
CsaHsoN O F
qVB
Calculated: (%) C 53.02 H 3.84 N 2.13
Measured: (%) C 49.89 H 3.28 N 2.16
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
45
Table SA.2 Interatomic Distances and Angles for the Cation of
[(Ph)znacnacVMe(EtzO)(THF)][B(C6H3(CF3)z)4]
(Note: the bond designations are with reference to FIG. 5 and the values noted
in parentheses
after the distances and angles represent the estimated standard deviation.)
Bond Distance (~) Bond Distance
(~)
V-N(2) 1.94(2) C(8)-C(9) 1.39(4)
V-N( 1 ) 2.00(2) C( 10)-C( 11 ) I .38(3)
V-O( I ) 2.029( I 3) C( 10)-C( 14) I .50(3)
V-C(1) 2.09(2) C(ll)-C(12) 1.53(3)
V-O(2) 2.16(2) C( 12)-C( I 3) 1.54(3)
O(1)-C(4) 1.45(3) C(15)-C(16) 1.44(3)
O( 1 )-C(3) 1.49(3) C( 15)-C(20) 1.37(3)
O(2)-C(6) 1.42(3) C( 16)-C( 17) I .37(3)
O(2)-C(9) 1.41 (2) C( 17)-C( 18) 1.32(3)
N(1)-C(I2} 1.26(3) C(18)-C(19) 1.49(3)
N( 1 )-C(20)1.46(2) C(I9)-C(20) 1.42(3)
N(2)-C( 10) 1.33(2) C(21 )-C(22) 1.41 (2)
N(2)-C(26) 1.51(2) C(21)-C(26) 1.41(2)
C(2)-C(3) 1.74(4) C(22)-C(23) 1.36(2)
C(4)-C(5) 1.26(4) C(23)-C(24) 1.35(3)
C(6)-C(7) 1.34(3) C(24)-C(25) 1.23(2)
C(7)-C(8) I .64(5) c(2s)-c(26) 1.50(2)
Bond Anele An lg a (deg;}Bond An~le An le (dee.)
N(2)-V-N( 89.1 (6) C(6)-C(7)-C(8) 97(2)
1 )
N(2)-V-O(1) 134.4(6) C(9)-C(8)-C(7) 106(2)
N(I)-V-O(I) 89.6(6) C(8)-C(9)-O(2) 110(2)
N(2)-V-C(I) 95.7(10) N(2)-C(10)-C(11) 120(2)
N(1)-V-C(I) 91.9(9) N(2)-C(10)-C(14) 119(2)
O(I)-V-C(1) 129.9(10) C(11)-C(10)-C(14)120(2)
N(2)-V-O(2) 98.0(6) C(10)-C(11)-C(12)129(2)
N(1)-V-O{2) 171.0{6) N(1)-C(12)-C(13) 126(2)
O( 1 )-V-O(2)81.4(5) N( 1 )-C( I 2)-C(116(2)
11 )
C(1)-V-O(2) 92.9(9) C(13)-C(12)-C(11)117(2)
C(4)-O(1)-C(3)109(2) C(16)-C(15)-C(20)118(3)
C(4)-O(1)-V 118.3(13) C(17)-C(16)-C(IS)123(2)
C(3)-O(1)-V 131(2) C(16)-C(17)-C(18)122(2)
C(6)-O(2)-C(9)105(2) C(19)-C(18)-C(17)118(2)
C(6)-O(2)-V 122.7(12) C(18)-C(19)-C(20)119(2)
C(9)-O(2)-V 132.3(13) C(19)-C(20)-C(15)120(2)
C(12)-N(1)-C(20)111(2) C(19)-C(20)-N(1) 119(2)
C(12)-N(1)-V132(2) C(15)-C(20)-N(1) 121(2)
C(20)-N(1)-V116.4(12) C(22)-C(21)-C(26)119(2)
C(10)-N(2)-C(26)112.5(14) C(23)-C(22)-C(21)119(2)
C(10)-N(2)-V131.3(14) C(22)-C(23)-C(24)119(2)
C(26)-N(2)-V116.0(11) C(25)-C(24)-C(23)125(2)
O(1)-C(3)-C(2)106(2) C(26)-C(25)-C(24)120(2)
C(5)-C(4)-O(1)128(3) C(25)-C(26)-C(21)115(2)
C(7)-C(6)-O(2)117(2) C(25)-C(26)-N(2) 126.0(14)
C(21)-C(26)-N(2) 119(2)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
46
Tabte SA.3 Structure Determination Summary for
[(Ph)ZnacnacVMe(Et20)(THF)J[B(C6H3(CF3)a)41
Crystal Data
Formula CsaHsoBFz4N2O2V
Formula Weight 1324.75
Crystal color Orange-brown block
Crystal Size (mm) 0.05 x 0.20 x 0.40
l0 Crystal System monoclinic
Space Group Cc
Unit Cell Dimensions a = 20.1893(11) ~
b = 15.6580( 11 ) ~A
c = 20.1295(13) ~
a = 90
(3 = 106.893(2)
y = 90
Volume 6088.8(7) A
Z 4
Density (calc.) 1.445 g/cm3
Absorption Coefficient 2.79 cm ~
F(000) 2688
Data collection
Diffractometer Siemens P4
Radiation MoKa (1= 0.71073A)
Temperature 293(2) K
Monochromator Highly oriented graphite
crystal
3o 20 Range (w) 3.34 to 56.46
Scan type Omega, Phi
Scan Range 0,3
Index Ranges -25 <h < 26
0<k<20
-25 < 1 < 26
Reflections Collected 8943
Independent Reflections 17489 (R;"~ = 10.94%)
Observed Reflections 4631
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3_
47
Solution and Refinement
System Used SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
Quantity minimized S[w(Foz - F~2)2~IS[(WFp2)2]1/2
Hydrogen Atoms Idealized contributions
Weighting Scheme w-~ = s2(F) + 0.0010
Final R Indices (obs. data) R = 14.8%, wR = 30.9%
R Indices (all data) R = 24.9%, wR = 37.5%
Goodness-of Fit 1.800
Data-to-Parameter Ratio 11.1:1
Largest Difference Peak 2.565
Largest Difference Hole -0.531
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
48
EXAMPLE SB
Preparation of 2,4-pentane di(N-phenyl)iminato methyl bis-diethylether
vanadium tetrakis-(3,5-bistrifluoromethylphenyl)borate,
[(Ph)ZnacnacVMe(Et20)2][B(C6H3(CF3)i)a]:
Before the reaction, the drybox was flushed for 30 minutes in an
attempt to remove all of the THF from the inert atmosphere. The same reaction
sequence and the same reactant quantities as in Example SA were followed for
the synthesis of (Ph)2nacnacVMe(Et20)2[B(C6H3(CF3)2)4]. Orange crystals of
were isolated in moderate yield (195 mg, 46 % yield) from a concentrated
diethylether solution cooled to -30°C, and based on the analytical data
shown
in Table SB the product is believed to contain
[(Ph)ZnacnacVMe(Et20)2][B(C6H3(CF3)2)4].
TABLE 5B
ANALYTICAL DATA F4R
f (Phl~nacnacVMelFt.,Wl.,lfRl('WT..II'F..1_1.7
'H NMR (C6D6)123.95 7.60 4.39 -16.75' ***. *** ***
e: ' '
b ( m) (6H,vb)(4H) (6H) (lH,vb)
IR- KBr : 3081w 2977w2936w 2907w 2882w 1610w 1437w
(cm' )
1355vs 1279vsI126m 1027w 945w 887m 839m
798w 771w 744w 713m 682m 448w ***
UV-vis (Et20)':776(266 629(421 M' 469(1400
M~'crri') ctrl M~'crri
) )
a"~ax(E)
rr 3.1(std.
dev.
I),
B (294K)
Melting Pt. 89-93C
Range:
CsH NOF4VB ;r
~.aicmaiea: io ~: ~~.4ts n 3.y~ N 2.! 1
Measured: (% C 49.41 H 3.47 N 2.11
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
49
EXAMPLE 6
Preparation of bis-( 2,4-pentane di(N-phenyl)iminato) chromium(II),
((Ph)Znacnac)zCr:
Preparation 1: Reaction of 2-N-phenylamino-4-N'-phenylimino-3-pentenyl
dichloro bis-tetrahydrofuran chromium, (Ph)~nacnacCrCl2(THF)2, with MeLi:
l0 1.16 mmoles (600 mg) of (Ph)2nacnacCrCh(THF)2, reference Example
1C, was dissolved in THF and cooled to -30°C. 2 molar eq. of a MeLi
solution was added dropwise to the (Ph)2nacnacCrCl2(THF)2 solution. Upon
addition of the MeLi solution, the suspension rapidly turned to brown. After
the reaction mixture was allowed to stir at room temperature for 4 hours, the
1S solution was evaporated to dryness. THF was removed by trituration in
ether.
The resulting solid was extracted with ether and filtered to remove LiCI. The
black-green filtrate was then concentrated and cooled to -30°C for
crystallization. 210 mg (35% yield) of ((Ph)Znacnac)2Cr black green crystals
of were isolated.
The resulting compounds were analytically tested and the results are
shown in Tables 6A.1-3. The single crystal X-ray diffraction results are shown
in Fig. 6.
Preparation 2: Reaction of 2-N-Phenylamino-4-N'-Phenylimino-2-Pentene,
(Ph}2nacnacH, with MeLi and CrCl2:
16 mmoles (4.0 g) of (Ph)2nacnac(H) was dissolved in THF and cooled
to -30°C. 16 mmoles (352 mg) MeLi was added to the THF solution of
(Ph)2nacnacH. Using an addition funnel, the THF - (Ph)2nacnacLi solution
was added dropwise, over a one hour period, to 8 mmoles {983 mg) of CrCl2
in a THF solution. The reaction mixture was then allowed to stir at room
temperature overnight. The solvent was removed to dryness and extracted
with diethylether. The extract was then filtered to remove LiCI. 7.09 mmoles
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
50
(3.7 g, 84% yield) of black ((Ph)Znacnac)~Cr crystals were isolated from a
concentrated diethylether solution that was cooled to -30°C.
TABLE 6.1
ANALYTICAL DATA FOR
((Ph)Znacnac)2Cr
'H NMR (C6D6)a:123.957.60 4.39 -16.75 *** *** ***
8 ( m (6H,vb)(4H) (6H) (
1
H,vb)
IR - KBr : 3055w 3029w 2922w 2879m1591w 1540s 1515m
ctti ~)
1482s 1449m 1382vs 1276w1264w 1021 866w
m
842w 752w 700m *** *** *** ***
Mass Spectrometry:550.22(100) 301.05(45.75)
m/z (%) [M+] [M+
-
C
1
~H
N
UV-vis (EtzO)':348(shoulder) ***
7,,",a"(E) (10,444.5
M~~crri
~)
5.1
(std.
dev.
1
,
,~
(294K)
Meltin Point: 220C
C14H 4N4Cr
Calculated: % C 74.15 H 6.23 N 10.18
Measured: (%) C 72.42 H 6.30 N 10.01
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
51
Table 6.2 Interatomic Distances and Angles for ((Ph)Znacnac)ZCr
(Note: the bond designations are with reference to FIG. 6 and the values noted
in parentheses after the
distances and angles represent the estimated standard deviation.)
Bond Distance (A) Bond Distance (A)
Cr-N(2) 2.049(4) C( 12)-C( 17) 1.381 (6)
Cr-N(3) 2.055(4) C(12)-C(13) 1.406(7)
Cr-N( 1 ) 2.058(3) C( 13)-C( 14) I .375(9)
Cr-N(4) 2.069(3) C( 14)-C( I S) 1.353(9)
N(1)-C(2) 1.336(5) C(15)-C(16) 1.392(7)
N(l)-C(11) 1.451(5) C(16)-C(17) 1.380(6)
N(2)-C(4) 1.314(5) C(18)-C(19) 1.522(6)
N(2)-C(17) 1.432(5) C(19)-C(20) 1.389(6)
N(3)-C( 19) 1.323(5) C(20)-C(21 ) 1.381 (6)
N(3)-C(28) 1.431 (5) C(21 )-C(22) 1.505(6)
N(4)-C(21 ) 1.339(5) C(23)-C(24) 1.378(7)
N(4)-C(34) 1.424(5) C(23)-C(28) 1.385(6)
C(1)-C(2) 1.515(6) C(24)-C(25) 1.372(9)
C(2)-C(3) 1.401(6) C(25)-C(26) 1.359(8)
C(3)-C(4) 1.406(6) C(26)-C(27) 1.376(7)
C(4)-C(5) 1.513(6) C(27)-C(28) 1.393(6)
C(6)-C(7) 1.377(6) C(29)-C(30) 1.377(6)
C(6)-C( 11 ) 1.379(6) C(29)-C(34) 1.398(6)
C(7)-C(8) 1.379(8) C(30)-C(31 ) 1.388(7)
C(8)-C(9) 1.363(7) C(31 )-C(32) 1.387(7)
C(9)-C(10) 1.378(7) C(32)-C(33) 1.383(7)
C( 10)-C( 1 I l .380(6) C(33)-C(34) 1.398(6)
)
Bond Anele An Ig a (dee.)Bond Angle An Ig-a
(deQ.)
N(2)-Cr-N(3) 149.78(14) C(10)-C(11)-N(1)121.3(4)
N(2)-Cr-N(1) 88.47(14) C(17)-C(12)-C(13)120.0(5)
N(3)-Cr-N(1) 105.49(14) C(14)-C(13)-C(12)119.8(5)
N(2)-Cr-N(4) 100.69(14) C(15)-C(14)-C(13)119.7(5)
N(3)-Cr-N(4) 89.35(14) C(14)-C(15)-C(16)121.4(6)
N(I)-Cr-N(4) 132.88(14) C(17)-C(16)-C(15)119.7(5)
C(2)-N(1)-C(11) 117.3(3) C(12)-C(1?)-C(16)119.3{4)
C(2)-N(1)-Cr 121.6(3) C(12)-C(17)-N(2)123.3(4)
C(11)-N(1)-Cr 119.2(3) C(16)-C(17)-N(2)117.2(4)
C(4)-N(2)-C(17) 122.9(4) N(3)-C(19)-C(20)123.6(4)
C(4)-N(2)-Cr 126.2(3) N(3)-C(19)-C(18)119.3(4)
C{17)-N(2)-Cr 110.9(3) C(20)-C(19)-C(18)117.2(4)
C(19)-N(3)-C(28)119.9(4) C(21)-C(20)-C(19)128.7(4)
C( 19)-N(3)-Cr 125.5(3) N(4)-C(21 )-C(20)123.8(4)
C(28)-N(3)-Cr 114.1(3) N(4)-C(21)-C(22)120.1(4)
C(21)-N(4)-C(34)118.9(4) C(20)-C(21)-C(22)116.0(4)
C(2l)-N(4)-Cr 123.1(3) C(24)-C(23)-C(28)120.0(5)
C(34)-N(4)-Cr 117.0(3) C(25)-C(24)-C(23)120.7(5)
N(1)-C(2)-C(3) 123.8(4) C(26)-C(25)-C(24)119.2(5)
N(1)-C(2)-C(I) 119.7(4) C(25)-C(26)-C(27)121.7(6)
C(3)-C(2)-C(1) 116.5(4) C(26)-C(27)-C(28)119.3(5)
C(2)-C(3)-C(4) 128.0(4) C(23)-C(28)-C(27)119.1(4)
N(2)-C(4)-C(3) 121.9(4) C(23)-C(28)-N(3)118.8(4)
N(2)-C(4)-C(5) 121.5(4) C(27)-C(28)-N(3)122.1(4)
C(3)-C(4)-C(5) 116.6(4) C(30)-C(29)-C(34)121.2(4)
C(7)-C(6)-C(11) 121.1(5) C(29)-C(30)-C(31)120.3(4)
C(6)-C(7)-C(8) 119.6(5) C(30)-C(31)-C(32)119.5(5)
C(9)-C(8)-C(7) 119.7(5) C(33)-C(32)-C(31)119.8(5)
C(8)-C(9)-C(10) 120.8(5) C(32)-C(33)-C(34)121.5(4)
C(9)-C(10)-C(11)120.2(5) C(33)-C(34)-C(29)117.6(4)
C(6)-C(I1)-C(10)118.6(4) C(33)-C(34)-N(4)122.2(4)
C(6)-C(I1)-N(1) 120.0(4) C(29)-C(34)-N(4)120.1(4)
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/0186_3
52
Table 6.3 Structure Determination Summary for((Ph)2nacnac)2Cr
C~stal Data
Empirical Formula C~4H~4N4Cr
Formula Weight 550.65
Crystal color Dark green plates
Crystal Size (mm) 0.40 x 0.40 x 0.06
Crystal System triclinic
1o Space Group PI
0
Unit Cell Dimensions a = 10.54650(10) A
b = 11.4442(2) A
0
c = 13.8021 (2) A
a =87.5203(9)
~3 = 72.7442(8)
y = 65.33
Volume 1439.44(5) ~3
Z 2
Density (calc.) 1.270 g/cm3
2o Absorption Coefficient 4.27 cm-~
F(000)
580
Data collection
Diffractometer Used Siemens P4
Radiation MoKoc (1= 0.710730
Temperature 218(2) K
Monochromator Highly oriented graphite
crystal
28 Range (w) 3.10 to 56.60
Scan type Omega, Phi
Scan Range 0.3
Index Ranges -13 <h < 13
-14<k< 14
0<1<18
Reflections Collected 6231
Independent Reflections 6231 (R;"~ = 0.0000%)
Observed Reflections 4039
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
53
Solution and Refinement
System Used SHELXTL (5.03)
Solution Direct Methods
Refinement Method Full-Matrix Least-Squares
Quantity minimized S[w(Fo2 - F~2)2)/S[(wFoz)z~vz
Hydrogen Atoms Idealized contributions
Weighting Scheme w-~ = s2(F) + 0.0010
Final R Indices (obs. data)R = 8.87%, wR = 21.76%
R Indices (all data) R = 11.77%, wR = 24.49%
to Goodness-of-Fit 1.100
Data-to-Parameter Ratio 17.7:1
Largest Difference Peak 0.892
Largest Difference Hole -0.812
20
Polymerization Experiments
Method For Determining MW. M~, M~. & M~
The MW, weight average molecular weight, M", number average
molecular weight, weighted to the low end of the material, MZ, average
weighted to the high end of the material, and MP, the peak position molecular
weight for the polymer samples are determined using Size Exclusion
Chromatography (SEC) columns. SEC columns separate a polymer solution
into fractions based on their 3-dimensional molecular size (hydrodynamic
volume - Hv). These fractions are detected by a refractive index (RI) detector
which responds linearly to the concentration of homogenous polymers. The
molecular weight distribution (MWD) is then determined as the linear
equivalent molecular weight relative to a linear calibration polyethylene (PE)
standard (Chevron 9640). For high density PE (HDPE), the molecular weights
determined can be considered an absolute quantity. For low density PE
(LDPE), the average molecular weight (MW) is underestimated proportionately
to the additional weight of branches along the backbone. For samples
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
54
containing a fairly consistent amount of branching, molecular weight
distributions can be compared on a relative scale to each other.
Samples of the polymer are ground up to a 20 mesh size. 8mg +/-
0.2mg are weighed into a 4 ml vial with three separate preparations per
measurement. 4mL of TCB (with SOOppm antioxidant to prevent molecular
decomposition) is added with an automatic solvent dispenser to each vial.
Samples are dissolved in a oven for 4 Hours at 180°C. The vials are
shaken 3
times over this 4 hour period. The sample are then tested using a Waters 150C
1o Chromatography System equipped with 3 Mixed A + 1 SOA Polymer
Laboratories (UK) Columns. The measurements are conducted under the
following conditions:
Concentration: 2mg/mL
Injection Volume: 400uL
Flowrate: 1 mlJmin
Column and Injector Compartment Temperature: 150C
Run Time: 1 Hour
The Method of Calculation is: Weight fraction of polymer is weighted against
2o molecular weight with Flow Rate Correction employed by referencing flow
rate marker peak. The ViscoTek:TriSec Software Conventional Calibration
Module ver. 3.00 is used to report M", MW, MZ, MP, and D average for the
three separate preparations.
Method For Determining Short Chain Branchine (SCB)
Samples were analyzed on a Varian Unity+ NMR spectrometer at a
magnetic field of 7 Tesla with a lOmm broadband probe tuned for C-13.
Approximately O.Sg of sample was placed in a lOmm NMR tube and filled
with 3m1 of a 3:1 1,2,4-trichlorobenzene / deuterated benzene mixture. The
3o sample is warmed to 130°C and allowed to dissolve until a clear
solution is
formed. When bubbles and voids in the viscous solution have been
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
eliminated, the sample is ready for analysis. The sample is placed in the bore
of the NMR magnet and heated to 130°C. The sample is allowed to come to
thermal equilibrium and stabilize for 5 minutes. The sample is deuterium
locked on to the deuterated benzene signal for magnet field stability and the
5 sample's magnetic field is shimmed to reduce magnetic field inhomogeneities
in order to increase resolution and the signal to noise ratio. The sample is
pulsed every 5.9 seconds (0.9s acquisition time and Ss recycle delay for
relaxation) for 2500 total transients making a total experiment time of 4
hours. The recorded free induction decay is Fourier transformed to yield the
l0 NMR spectrum. The spectrum is then phased and baseline corrected. The
short chain branching content is determined using specific resonances that are
characteristic and unique to each type of short chain branch (methyl through
hexyl and longer). The ratio of the integrals of each characteristic resonance
with the resonance for the polymer backbone (27.8 to 31.5 ppm) is taken and
15 the ratio is reported as short chain branches per 1000 carbons. Low
molecular
weight carbon content is determined by the ratio of the integral of the
characteristic resonance at 114 ppm to the integral of the polymer backbone.
Method For Determinintr Melting Point (Peak DSC M.P.)
20 Samples were analyzed on a Perkin-Elmer DSC7 differential scanning
calorimeter with an intercooler attachment. The sample size of approximately
lOmg was placed in an aluminum pan and an aluminum lid was crimped on.
The sample is heated twice, the first time to eliminate thermal history and
the
second time where the DSC sample measurement is recorded. The sample is
25 heated from 0°C the first time to 170°C at 20°C/min,
held for 5 minutes at
170°C, then control cooled at 10°Clmin to 0°C. The sample
is held at 0°C for
1 minute then reheated at 20°C/min to 170°C. This second heating
scan is
recorded. The peaks are used to determine the melting points which generally
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
56
appear between 90 to 140°C. The area under the curve is considered to
be the
heat of fusion of the polyethylene copolymer.
The following polymerization that resulted in formation of oligimers
were characterized using an HP 5890 Gas Chromatograph fitted with a FID
Detector, Helium Carrier Chrompack Column: WCOT ulti-metal lOM
X0.53MM coating HT SIMDIST CBDF=0.15 UM. At the following
conditions: 120°C x Imin x 10°C/min x 150°C x 0 min and
RampA:
6.0°C/min x 350°C x Omin.
EXAMPLE 7
Polymerization of Ethylene in a NMR Tube Reaction in the Presence Of
in-situ (Ph)ZnacnacVMe2[B(C6F5)3l:
Preparation I (in CDaCI~:
0.0453 mmoles ( 15 mg) of (Ph)ZnacnacVMe2, reference Example 3A,
and 0.0453 mmoles (23 mg) of B(C6F5)3 were transferred to an NMR tube
reactor and CD2Cl2 was vacuum transferred. Ethylene (@ 1 atm.) was charged
to the NMR tube and it was closed with a Teflon tap. After 5 minutes, a ~H
NMR spectrum was recorded. Only the ethylene resonance (b 5.40 ppm) and a
new peak 8 1.7 ppm was added to the original spectrum, reported in example
3A above. After 10 minutes, white particles of polyethylene had precipitated
out of the solution. One more charge of ethylene (@ 1 atm) was added to the
NMR tube and allowed to react for three hours. Another'H NMR reading was
taken and the spectrum had all the resonances associated with the catalyst
while the ethylene monomer peak had nearly disappeared.
Preparation 2 (in C6D6~:
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
57
Polymerization of ethylene was also tried in an NMR tube with
4.53x 10-5 moles ( 15 mg) of, reference Example 3A, and 4.53x 105 moles (23
mg) of B(C6F5);. Upon condensing C6D6, (Ph)2nacnacVMe2 and B(C6F5)~
reacted to give a brown black oil which was not soluble in C6D6. After one day
at room temperature, the ethylene monomer peak had decreased to approx.
40% (integrated to C6D6 peak) ' H NMR (C6D6): broad peaks from 2.4 to 0.6
ppm.
EXAMPLE 8
to
Polymerization Of Ethylene in a Parr Reactor in the Presence of
(Ph)2nacnacVMe2 and Cocatalyst in CH2Cl2:
0.151 mmoles (50 mg) of (Ph)2nacnacVMe2, reference Example 3a,
and 0.1 S 1 mmoles of B(C6F5)3 (77 mg) were dissolved in 100 ml of CH2Cl2
and the solution was placed in a Parr reactor. Ethylene (@700 psig) was
charged to the reactor. After about five minutes, the ethylene supply was
closed because the temperature of the reactor had reached 120°C. The
pressure decreased steadily with stirring. After stirring for several hours,
the
reactor was opened to atmosphere. Dark colored polymer was found in blocks.
30
The blocks were washed with a methanol/HCl mixture and deionized (D~
water and then dried in a vacuum oven at 60°C overnight. 5.2 grams of
polymer were collected. Polymer analysis: MW = 547,700; MW/M~= 2.06; Peak
DSC M.P.: 134.2°C.
EXAMPLE 9
Polymerization Of Propylene in a NMR Tube Reactor in the Presence of
(Ph)ZnacnacVMe2 and Cocatalyst in CDZCl2:
4.53x10-5 moles(15 mg) of (Ph)2nacnacVMe2, reference Example 3a,
and 4.53x10-S moles (23mg) of B(C6F5)3 were dissolved in CDZC12 and placed
in a NMR tube reactor. Propylene (@ 1 atm.) was then charged to the NMR
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
58
to
tube reactor. When a ~H NMR spectrum was recorded after 10 minutes, there
was no indication of reaction. 'H NMR spectra obtained after several hours at
room temperature and after heating to 60°C overnight, continued to show
no
reaction.
EXAMPLE 10
Polymerization of 1-Hexene in a NMR Tube Reactor in the Presence of
(Ph)2nacnacVMeZ and Cocatalyst in CD2CI2:
(Ph)2nacnacVMe2[B(C6F5)3] was prepared as described above,
reference Example 9. 1-hexene (pre-dried over Na/K alloy) was vacuum
transferred to a NMR tube reactor. A'H NMR spectrum was recorded after 10
minutes and then the NMR tube was heated to 60°C overnight. Analysis of
the resulting product indicated formation of oligomers, mostly dimers to
hexamers, including branched oligomers.
EXAMPLE 11
2o Copolymerization of Ethylene and 1-Hexene in a Parr Reactor in the
Presence of (Ph)2nacnacVMe2 and Cocatalyst in CH2Cl2:
0.151 mmoles (50 mg) of (Ph)ZnacnacVMe2, reference Example 3a,
and 0.151 mmoles of B(C6F5)3 (77 mg) were dissolved in a solvent mixture of
60 ml of CH2C12 and 30 ml of 1-hexene. The solution was placed in a Pan
reactor. Ethylene (@800 psig) was charged to the reactor. Then the ethylene
supply was closed because the temperature of the reactor reached nearly
120°C. With stirring, the pressure decreased steadily. After stirring
for several
hours, the reactor was opened and blocks of polymer were observed. The
blocks were washed with a methanol/HCl mixture and deionized (Dn water
and then dried in a vacuum oven at 60°C overnight. 5.2 grams of polymer
were collected. Polymer analysis: MW = 1,136,000; MW/M" = 2.42; Peak DSC
M.P: 131.4°C;. ~~C NMR: no side chains were indicated.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
59
EXAMPLE 12
Polymerization of Ethylene in a NMR Tube Reactor in the Presence of
[(Ph)2nacnacVMe(Et20)(THF)][B(C6H~(CF3)z)a] in CDZCl2:
1.88x I 0-5 moles (25 mg)
[(Ph)2nacnacVMe(Et20)(TH)~][B(C6H3(CF3)z)a], reference Example 5A, in
to CD2CI2 was introduced into a NMR tube reactor. Ethylene (@ 1 atm.) was
charged into the NMR tube and the Teflon tab was closed. After 15 minutes, a
fine precipitate of polyethylene was visible and a ~H NMR spectrum recorded
trace amounts of free diethylether peaks along with an intense ethylene
monomer peak at 8 5.40 ppm. After allowing the reaction to stand overnight
is at room temperature, several particles of polymer were visually observed
and
25
~H NMR spectrum recorded free diethylether resonances and trace amounts of
unreacted ethylene monomer. This reaction was run an additional two times
substituting propylene (@ 1 atm.) and I-hexene for ethylene and no
polymerization was observed even with heating.
EXAMPLE 13
Polymerization of Ethylene in a Parr Reactor in the Presence of
[(Ph)2nacnacVMe(Et20)(THF)][B(C6H3(CF3)2)4] in CH2Cl2:
0.045 mmoles (60mg)
[(Ph)2nacnacVMe(Et20)(THF)][B(C6H3(CF3)2)al, reference Example 5A,
dissolved in 100 ml of CH2C12 was introduced into a Pan reactor. Ethylene
(@700 psig) was supplied to the reactor. Once the reactor was pressurized, the
3o ethylene supply was shutoff. With stirring, the ethylene pressure decreased
slowly. After stirring for several hours, the reactor was opened to
atmosphere.
After stirring for several hours, the reactor was opened and blocks of polymer
were observed. The blocks were washed with a methanol/HCl mixture and
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/018b3
deionized (DI) water and then dried in a vacuum oven at 60°C overnight.
2.5
g of polymer were collected. Polymer analysis: MW = 450,600; M",/M~ = 1.96;
and Peak DSC M.P: 135.9°C.
5 EXAMPLE 14
Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)ZnacnacVCl2(THF)2 and Cocatalyst in CH2CIZ:
l0 0.776 mmoles (40mg) of (Ph}2nacnacVCl2(THF)2, reference Example
1B, was dissolved in 100 ml of dry CH2C12 in a Parr reactor. The color of the
solution turned brown. Upon addition of 7.3g (approx. 100 molar eq.) of
MAO, 10 wt.°lo solution in toluene, the color of the solution
instantaneously
changed to dark red brown. Ethylene (@400 psig) was supplied to the reactor.
15 Once the reactor was pressurized, the ethylene supply was shutoff. Pressure
decreased slowly with stirring. After stirring for several hours, the reactor
was
opened and blocks of polymer were observed. The blocks were washed with a
methanol/HCl mixture and deionized (Dn water and then dried in a vacuum
oven at 60°C overnight. 4.6 g of polymer were collected. Polymer
analysis:
20 MW = 350,032, M,~/M~ = 10.84.
EXAMPLE 15
25 Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)2nacnacVCl2(THF)2 and Cocatalyst in Toluene:
1.20 x 10-5 moles (8mg) of (Ph)2nacnacVCl2(THF)2, reference
Example 1B, was dissolved in 50 ml of toluene in a Parr reactor and 1.2 g
30 (approx. 100 molar eq.) of MAO, 10 wt.% solution in toluene, was added to
the solution. Polymerization was performed under a constant pressure of
ethylene (@ 300 psig) for 1 hour, after which the ethylene supply was closed.
The reaction was allowed to continue for an additional 1/2 hour to monitor
further ethylene uptake. During the 1/2 hour, the ethylene pressure decreased
CA 02321419 2000-08-14
WO 99/41290 PCT/EJS99/01863
61
by 50 psi. The reaction temperature was maintained under 60°C by
cooling
water circulation system. The resulting product was washed with a
methanol/HCI mixture and deionized (DI) water and then dried in a vacuum
oven at 60°C overnight. 4.7 g of white polyethylene polymer was
collected.
Polymer Polyethylene analysis: MW = 1,958,584; MW/M~ = 1.75; and Peak
DSC M.P : 135.4°C
COMPARATIVE EXAMPLE 1
Polymerization of Ethylene in a Parr Reactor in the Presence of
VCI3(TI-IF)3 and Cocatalyst inToluene:
1.20x 10-4 moles (6 mg) of VCI3(THF)~ was dissolved in 50 ml of
toluene in a Parr reactor. The color of the solution turned brown and
VC 13(THF)3 was somewhat soluble. Upon addition of 1.2g (approx. 100 molar
eq.) of MAO, 10 wt.% solution in toluene, the color of the solution
instantaneously changed to dark red brown. The reaction was allowed to
proceed for 1.5 hours under the constant pressure of ethylene (@ 300 psig),
after which the ethylene supply was closed to monitor ethylene uptake. A 0.5
hour after the ethylene supply was closed, the pressure had decreased by 130
psi. Over the course of the reaction, the reaction temperature was maintained
under 60°C with a water circulation pump. The resulting product was
washed
with a methanol/HCl mixture and deionized (DI) water and then dried in a
vacuum oven at 60°C overnight. 8.0 g of white polyethylene granules
were
collected. Polymer analysis: MW = 2,042,158; MW/M" = I.73 and Peak DSC
M.P: I 32.1 °C.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
62
EXAMPLE 16
Copolymerization of Ethylene and 1-Hexene in a Parr Reactor in the
presence of (Ph)ZnacnacVCl2(THF)2 And Cocatalyst in CH2C12:
7.76 x 10-5 moles (40 mg) of (Ph)2nacnacVCl2(THF)z, reference
Example IB, was dissolved in 100 ml of CH2C12 and 7.3g (approx. 100 molar
eq.) MAO, 10 wt.% solution in toluene, was added to the solution and placed
in a Parr reactor. 40 ml of dry I-hexene was added to the reaction mixture.
to Ethylene (@350 psig) was introduced into the reactor and the ethylene
supply
was closed. After one minute, the temperature had increased to 52°C.
The
temperature then decreased slowly and stayed at 45°C. The reactor was
stirred
for an hour. When the reactor was opened to the atmosphere, the entire reactor
was filled with white sticky polymer. The resulting product was washed with a
methanol/HCI mixture and deionized (DI) water and then dried in a vacuum
oven at 60°C overnight. 23.6 g of polymer were collected. Polymer
analysis:
MW =707,048; MW/M" = 212.33; and total carbons on side chain/1000 carbons
by ~ ~C NMR: 48.3.
2o EXAMPLE 17
Polymerization of 1-Hexene in the Presence of (Ph)2nacnacV(Me)Z and
Cocatalyst in CHZCl2:
6.04 x 10-5 moles (20 mg) of (Ph)2nacnacV(Me)2, reference Example
3A, was dissolved in 40 ml of dry CHZC12 in an ampoule. MAO (approx. 100
molar eq. in toluene) was added into the solution of (Ph)2nacnacV(Me)2 in the
ampoule and the ampoule was closed with a Teflon tap. After two cycles of
freeze/pump/thaw, approx. 5 ml of I-hexene was condensed into the ampoule.
3o When the reaction mixture was stirred at room temperature for two hours,
there was no apparent change in color or viscosity of the reaction mixture.
The oil bath temperature was elevated to 80°C and stirred
overnight. The
solution turned dark orange and became very viscous when cooled down to
CA 02321419 2000-08-14
WO 99/41290 PCTNS99/01863
63
room temperature. The ampoule was opened to atmosphere and a MeOH/HC1
solution was added to wash the resulting product. However, the dark orange
color was not removed. All the volatile species were removed by distillation
leaving a very dark brown oil. Polymer analysis: ~H NMR (CDC,~): broad
peaks at b 2.1, 1.3, 1.2, 0.8 ppm. Analysis of the resulting product indicated
formation of oligomers, mostly dimers to hexamers, which included branched
oligomers.
to
EXAMPLE 18
Polymerization of Ethylene in the Presence of (Ph)2nacnacTiCl2(THF)2
and Cocatalyst in CH2Cl2:
1 s 3.91 x 10-5 moles (20mg) of (Ph)ZnacnacTiCl2(THF)2, reference
Example lA, was dissolved in SO ml of CH2C12 in a 100 ml Schlenk flask
equipped with a stirring bar. 7.3g of MAO, 10 wt. % in toluene, was added to
the brown solution of (Ph)2nacnacTiCl2(THF)2 and Teflon stopper equipped
with a needle valve was attached. After two cycles of freeze/pump/thaw,
2o ethylene (@ I atm.) was introduced into the flask at room temperature.
Every
five minutes over a 1 hour period, the decrease in pressure was monitored. A
white powder of polyethylene ( 160 mg) was produced in one hour. Polymer
analysis: MW = 195,015, M,~,/M" = 21.11; and M.P by DSC=130.6°C
2s EXAMPLE 19
Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)2nacnacTiCl2(THF)2 and Cocatalyst in CHZCfz:
30 1.57 x 105 moles (8 mg) of (Ph)2nacnacTiCl2(THF)2, reference lA,
was dissolved in 50 ml of CH2CI2 and 1.2g (approx. 100 molar eq.) of MAO,
wt.% solution in toluene, was added to the solution and the solution was
placed into a Parr Reactor. The reaction was allowed to proceed for 1 hour
under the constant pressure of ethylene (@ 300 psig), after which the ethylene
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
64
supply was closed and the ethylene pressure decrease was monitored over the
next 0.5 hour. However, there was no further decrease in ethylene pressure.
The resulting product was washed with a methanol/HCl mixture and deionized
(DI) water and then dried in a vacuum oven at 60°C overnight. 6. I g of
white
polyethylene granules were isolated. Polymer analysis: MW = 685,963; MW/M"
= 30.9; and M.P by DSC=132.2°C'.
EXAMPLE 20
1o Copolymerization of Ethylene and 1-Hexene in a Parr Reactor in the
Presence of (Ph)ZnacnacTiCl2(THF)2 and Cocatalyst in CHZCI2:
3.90 x 10-5 moles (20mg) of (Ph)ZnacnacTiCl2(THF)2, reference
Example 1 A, and 8.0 g (approx. 200 molar eq.) MAO,10 wt.% solution in
toluene, were dissolved in the mixture of 1-hexene (30 ml) and CH2C12 (60
ml). This solution was placed in a Parr reactor. When ethylene ( @ 350 psig)
was introduced into the reactor, the temperature rapidly increased to
52°C
within a minute. The reaction was quenched after 25 minutes since the
temperature suddenly increased very rapidly to 140°C. When the reactor
was
opened to atmosphere, a light brown rubbery polymer was observed. . The
30
resulting product was washed with a methanol/HCl mixture and deionized (DI)
water and then dried in a vacuum oven at 60°C overnight. 12.5 g of
polymer
were collected. Polymer analysis: MW = 33,882; M,W/Mn = 218.51; and total
carbons on side chain/1000 carbons, by'3C NMR = 34.6.
EXAMPLE 21
Polymerization of 1-Hexene in the Presence of (Ph)2nacnacTiCl2(THF)2
and Cocatalyst in CHZC12:
The reaction was performed with 3.90x I 0-5 moles (20 mg) of
(Ph)2nacnacTiCl2(THF)2, reference Example lA, MAO (100 eq.) solution and
5 ml of I -hexene in 20 ml of CHIC 1 z in an ampoule sealed with a Teflon
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
stopper. After stirring for two hours at room temperature, the ampoule was
allowed to stir at 80°C overnight. The solution turned to dark orange
and
became very viscous when cooled down to room temperature. The ampoule
was opened to atmosphere. The resulting product was washed but the dark
5 orange color was not removed. All the volatile species were removed by
distillation to give a very dark brown oil. Analysis of the resulting product
indicated formation of oligomers, mostly dimers to hexamers, which included
branched oligomers.
to EXAMPLE 22
Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)2nacnacTiCl2(THF)2 and Cocatalyst in Toluene:
15 1.20 x 10-5 moles (8 mg) of (Ph)2nacnacTiCl2(THF)2, reference
Example lA, was dissolved in 50 ml of toluene in a Parr reactor. 1.2 g
(approx. 100 molar eq.) of MAO, 10 wt.% solution in toluene, was added to
the solution. The reaction temperature was kept below 60°C by
circulating
cooling water. The reaction proceeded for 1 hour under constant pressure
2o maintained by ethylene (@ 300 psig), after which the ethylene supply was
closed. The reaction was continued, with stirring, for another 0.5 hour to
monitor further ethylene uptake. During that 0.5 hour, the pressure decreased
by 100 psi. The resulting product was washed with a methanol/HCl mixture
and deionized (Dn water and then dried in a vacuum oven at 60°C
overnight.
25 10.5 g of white polyethylene granules were collected. Polymer analysis: MW
=
818,850; MW/M" = 28.58; and Peak DSC M.P: 134.2°C.
CA 02321419 2000-08-14
WO 99/41290 PCTNS99/01863
66
COMPARATIVE EXAMPLE 2
Polymerization of Ethylene in a Parr Reactor in the Presence of
TiCI~(THF)~, and Cocatalyst in Toluene:
1.20 x 10~ moles (6mg) of TiCl3(THF)~ was dissolved in 50 ml of
toluene in a Pan reactor. 1.2 g (approx. 100 molar eq.) of MAO, 10
wt.°lo
solution in toluene, was added to the TiCI~(THF)3 solution. Upon addition of
MAO, the color of the TiCl3(THF)3 solution changed instantaneously a darker
brown. The reaction temperature was kept under 60°C by circulating
cooling
water. The reaction proceeded for 1 hour under constant pressure maintained
by ethylene (@ 300 psig), after which the ethylene supply was closed. The
reaction was continued for another 0.5 hour during which the pressure
decreased only 25 psi. The resulting product was washed with a methanol/HCl
mixture and deionized (DI) water and then dried in a vacuum oven at
60°C
overnight. 3.4 g of white polymer granules were collected. Polymer analysis:
MW = 1,479,060; M",/M" = 153.12; Peak DSC M.P: 134.2°C.
EXAMPLE 23
Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)2nacnacCrCl2(THF)2 and Cocatalyst in CH2Cl2:
7.76 x 10-Smoies (40 mg) (Ph)ZnacnacCrCl2(THF)2, reference Example
1C, and 7.3 g (approx. 100 molar eq.) of MAO, 10 wt.°lo solution in
toluene,
were dissolved in 100 ml of CH2C 12 and the solution was placed in a Parr
reactor. Ethylene (@400 psig) was supplied to the reactor. Once the reactor
was pressurized, the ethylene supply was shutoff. With stirring, the
temperature increased slowly to a maximum of 110°C, after which is
decreased slowly. After stirring for 30 minutes, a white powder of
polyethylene was obtained. The resulting powder was washed with a
methanol/HCl mixture and deionized (DI) water and then dried in a vacuum
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
67
oven at 60°C overnight. 6.0 g of white polymer powder was collected.
Polymer analysis: MW = 48,613 and MW/M~ = 22.84.
EXAMPLE 24
Polymerization of Ethylene in a Parr Reactor in the Presence of
(Ph)ZnacnacCrCl2(THF)Z and Cocatalyst in Toluene:
l0 1.20 x 10-5moles (8 mg) (Ph)2nacnacCrCl2(THF)2, reference Example
1 C, arid 1.2 g (approx. 100 molar eq.) of MAO, 10 wt.% solution in toluene,
were dissolved in 50 ml of toluene in a Parr reactor. Ethylene (@300 psig)
was supplied to the reactor. After stirring for 1 hour under constant ethylene
pressure with cooling, the ethylene supply was closed. Over the course of the
following 45 minutes, the ethylene pressure decreased by 100 psi. The
resulting product was washed with a methanol/HCl mixture and deionized {D~
water and then dried in a vacuum oven at 60°C overnight. 10.5 g of a
white
solid polymer was isolated. Polymer analysis: MW: 1,157,039; MW/M": 72.81;
and Peak DSC M.P:134.9°C.
25
COMPARATIVE EXAMPLE 3
Polymerization of Ethylene in a Parr Reactor in the Presence of
CrCl3(THF)~, and Cocatalyst in Toluene:
1.20 x 10-4 moles (6mg) of CrCl3(THF)3 was dissolved in 50 ml of
toluene in a Parr reactor. 1.2 g (approx. 100 molar eq.) of MAO, 10 wt.%
solution in toluene, was added to the CrCl3(THF)~ solution. Upon addition of
MAO, the color of the solution was pale brown even after stirring for 10
minutes and solid CrCl3(THF)3 remained. The reaction proceeded for 1 hour
under constant pressure maintained by ethylene supplied @300 psig. The
ethylene supply was closed and there was no observable pressure decrease
over the next 30 minutes. The reaction temperature remained essentially
constant, around 23°C, throughout the reaction time, even without
cooling.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
68
The resulting product was washed with a methanol/HC1 mixture and deionized
(DI) water and then dried in a vacuum oven at 60°C overnight. 0.5 g of
polyethylene were obtained. Polymer analysis: MW = 1,319,817; MW/M" _
37.88; and Peak DSC M.P: 133.3°C.
EXAMPLE 25
Copolymerization of Ethylene and 1-Hexene in a Parr Reactor in the
1o Presence of (Ph)ZnacnacCrCl2(THF)Z and Cocatalyst in CH2C12:
The reaction was performed with 7.76 x 10-5 moles (40 mg) of
(Ph)2nacnacCrCl2(THF)2, reference Example IC, and 7.5 g (approx. 100 molar
eq.) of MAO, 10 wt.% solution in toluene, in a solvent mixture of 40 ml of 1-
hexene and 60 ml of CHIC 12 in a Parr reactor. Ethylene (@350 psig) was
supplied to the Parr reactor in accordance with the procedure set forth in
Example 22. 8.7 g of white polymer powder was collected. Polymer analysis:
MW = 9,659, MW/M":= 7, M.P. by DSC = 109.6 and 125.4°C and SCB=I6.
2o EXAMPLE 26
Copolymerization of Ethylene and Propylene in a Parr Reactor in the
Presence of (Ph)2nacnacCrCl2(THF)2 and Cocatalyst:
8mg (Ph)nacnacCrCl2(THF)2 and 100 molar equivalents of MAO solution was
dissolved in 50 ml of toluene in a Pan reactor. A gas mixture of ethylene and
propylene (@ 100 psig) was charged into the reactor. After stirring for three
hours the reaction was terminated. The resulting product was washed with a
methanol/HCI mixture and deionized (Dn water and then dried in a vacuum
oven at 60°C overnight. 250 mg of white rubbery polymer was isolated.
Polymer analysis: MW = 147,054, MW/M":= 82.14, M.P. by DSC = 95.1, I 13.8
and 125.1 °C, and SCB=9.69.
CA 02321419 2000-08-14
WO 99/41290 PCT/US99/01863
69
EXAMPLE 27
Polymerization of Propylene in a Parr Reactor in the Presence of
(Ph)2nacnacCrCl2(THF)2 and (:ocatalyst:
Attempts to polymerize propylene using reaction conditions similar to those
set forth resulted in a product that could not be readily characterized using
the
techniques employed herein. Additional test using the corresponding
Vanadium and Titanium catalyst equally provided a product that could not be
readily characterized.
EXAMPLE 28
Copolymerization of Ethylene and Propylene in a Parr Reactor in the
Presence of (Ph)ZnacnacVCl2(THF)2 and Cocatalyst:
8 mg. (Ph)2nacnacVCl2(THF)2 and 100 molar equivalents of MAO solution
was dissolved in 50 ml of toluene in a Parr reactor. A gas mixture gas of
ethylene and propylene (@ 100 psi) was charged into the reactor. After
stirring
for three hours the reaction was terminated. After washing and drying 800 mg
of white rubbery polymer was isolated. Polymer analysis: MW = 1,476,492
MW/M":= 3.94, M.P. by DSC = 117.4°C, and SCB=14.88.
Having described specific embodiments of the present invention, it will
be understood that many modifications thereof will readily appear or may be
suggested to those skilled in the art, and it is intended therefore that this
invention is limited only by the spirit and scope of the following claims.