Note: Descriptions are shown in the official language in which they were submitted.
CA 02322053 2000-10-23
77430-8D
1
NOVEL NUCLEOSIDES
This application is a divisional of copending
Canadian application 2,278,158 stemming from a PCT application
PCT/US98/00634 filed on January 13, 1998.
FIELD OF THE INVENTION
The present invention relates to the field of
nucleosides.
BACKGROUND OF THE INVENTION
Mammalian immune systems contain two major classes of
lymphocytes: B lymphocytes (B cells), which originate in the
bone marrow; and T lymphocytes (T cells) which originate in the
thymus, B cells are largely responsible for humoral immunity
(i.e., antibody production), while T cells are largely
responsible for cell-mediated immunity.
T cells are generally considered to fall into two
subclasses, helper T cells and cytotoxic T cells. Helper T
cells activate other lymphocytes, including B cells and
cytotoxic T cells, and macrophages, by releasing soluble
protein mediators called cytokines which are involved in cell-
mediated immunity. As used herein, lymphokines are a subset of
cytokines.
Helper T cells are also generally considered to fall
into two subclasses, Thl and Th2. Thl cells (also known as
Type 1 cells) produce interleukin 2 (IL-2), tumor necrosis
factor (TNFa) and interferon gamma (IFNy), and are responsible
primarily for cell-mediated immunity such as delayed type
hypersensitivity and antiviral immunity. In contrast, Th2
cells (also known as Type 2 cells) produce interleukins, IL-4,
IL-5, IL-6, IL-9, IL-10 and IL-13, and are primarily involved
in assisting humoral immune responses such as those seen in
CA 02322053 2000-10-23
77430-8D
2
response to allergens, e.g. IgE and IgG4 antibody isotype
switching (Mosmann, 1989, Annu Rev Immunol, 7:145-173).
As used herein, the terms Thl and Th2 "responses" are
meant to include the entire range of effects resulting from
induction of Thl and Th2 lymphocytes, respectively. Among
other things, such responses include variation in production of
the corresponding cytokines through transcription, translation,
secretion and possibly other mechanisms, increased
proliferation of the corresponding lymphocytes, and other
effects associated with increased production of cytokines,
including motility effects.
The mechanisms by which nucleosides and other
compounds selectively modulate Thl and Th2 responses relative
to each other are still unclear. One possibility contemplated
by the present inventors is that effective nucleosides alter
the pool of guanosine triphosphate (GTP), which in turn affects
the rate at which cytokines are produced. In this theory,
relatively large variations in available GTP are sufficient to
affect concentrations of both Thl and Th2 cytokines, while
relatively smaller variations in available GTP tend to affect
concentrations of Thl and Th2 cytokines to different extents.
These discoveries are especially significant because
modern treatment strategies for many of the above-listed
diseases have either limited effectiveness, significant side
effects, or both. Treatment of autoimmune disease, for
example, is frequently limited to palliative measures, removal
of toxic antibodies (as in myasthenia gravis), and
administration of hazardous drugs including corticosteroids,
chloroquine derivatives, and antimetabolic or antitumor drugs,
and drugs such as cyclosporines which target immune system
cells.
CA 02322053 2000-10-23
77430-8D
3
SUMMARY OF THE INVENTION
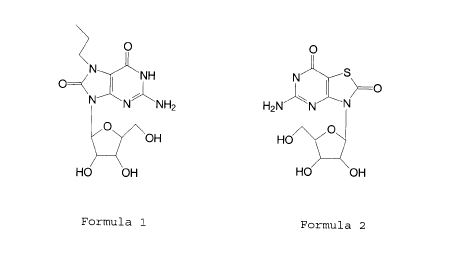
This application relates to novel nucleosides.
Nucleosides contemplated are those nucleosides corresponding to
Formulas 1 and 2.
O
O
N S
O N NH ~ ~N~O
H2N N
N N~ NH2
O
HO
OH
HO OH
HO OH
Formula 1 Formula 2
DETAILED DESCRIPTION
Where the following terms are used in this
specification, they are used as defined below.
The terms "a" and "(3" indicate the specific
stereochemical configuration of a substituent at an asymmetric
carbon atom in a chemical structure as drawn.
The term "aryl" refers to a monovalent unsaturated
aromatic carbocyclic radical having a single ring (e. g.,
phenyl) or two condensed rings (e.g., naphthyl), which can
optionally be substituted with hydroxyl, lower alkyl, chloro,
and/or cyano.
The term "enantiomers" refers to a pair of
steroisomers that are non-superimposable mirror images of each
other. A mixture of a pair of enantiomers, in a 1:1 ratio, is
a "racemic" mixture.
CA 02322053 2000-10-23
77430-8D
4
The term "heterocycle" refers to a monovalent
saturated or unsaturated carbocyclic radical having at least
one hetero atom, such as N, O or S, within the ring each
available position of which can be optionally substituted,
independently, with, e.g., hydroxy, oxo, amino, imino, lower
alkyl, bromo, chloro and/or cyano. Included within this class
of substituents are purines, pyrimidines.
The term "isomers" refers to different compounds that
have the same formula. "Stereoisomers" are isomers that differ
only in the way the atoms are arranged in space.
The term "L-configuration" is used throughout the
present invention to describe the chemical configuration of the
ribofuranosyl moiety of the compounds that is linked to the
nucleobases. The L-configuration of the sugar moiety of
compounds of the present invention contrasts with the
D-configuration of ribose sugar moieties of the naturally
occurring nucleosides such as cytidine, adenosine, thymidine,
guanosine and uridine.
The term "lower alkyl" refers to methyl, ethyl,
n-propyl, isopropyl, n-butyl, t-butyl, I-butyl or n-hexyl.
This term is further exemplified to a cyclic, branched or
straight chain from one to six carbon atoms.
The term "monocyclic" refers to a monovalent
saturated carbocyclic radical having at least one hetero atom,
such as O, N, S, Se or P, within the ring, each available
position of which can be optionally substituted, independently,
with a sugar moiety or any other groups like bromo, chloro
and/or cyano, so that the monocyclic ring system eventually
aromatized.
The term "nucleoside" refers to a compound composed
of any pentose or modified pentose moiety attached to a
CA 02322053 2000-10-23
77430-8D
specific position of a heterocycle or to the natural position
of a purine (9-position) or pyrimidine (1-position).
The term "C-nucleosides" is used throughout the
specification to describe the linkage type that is formed
5 between the ribose sugar moiety and the heterocyclic base. In
C-nucleosides, the linkage originates from a C-1 position of
the ribose sugar moiety and joins the carbon of the
heterocyclic base. The linkage that forms in C-nucleosides is
carbon-to-carbon type.
The term "D-nucleosides" refers to nucleoside
compounds that have a D-ribose sugar moiety (e. g., Adenosine).
The term "L-nucleosides" refers to nucleoside
compounds that have an L-ribose sugar moiety.
The term "N-nucleosides" is used throughout the
specification to describe the linkage type that is formed
between the ribose sugar moiety and the heterocyclic base. In
N-nucleosides, the linkage originates from the C-1 position of
the ribose sugar moiety and joins the nitrogen of the
heterocyclic base. The linkage that forms in N-nucleosides is
carbon to nitrogen type.
The term "nucleotide" refers to a phosphate ester
substituted on the 5-position of a nucleoside.
The term "Purine" refers to nitrogenous bicyclic
heterocycles depicted in Figures 1 and 2 herein.
Examples of compounds contemplated to be effective in
the invention are shown in Formula 1 and 2.
Formula 1 has the structure:
CA 02322053 2000-10-23
77430-8D
6
O
N NH
O
N N~ NH2
O
OH
HO OH
Formula 2 has the structure:
O
N S
N~O
H2N N
O
HO
HO OH
Administration
It is contemplated that compounds according to the
present invention will be administered in any appropriate
pharmaceutical formulation, and under any appropriate protocol.
Preferred monotherapeutic dosages and protocols for such drugs
are set forth in the PDR, or are at least available from the
manufacturer or distributor.
Of course, one of ordinary skill in the art will
recognize that a therapeutically effective amount will vary
with the infection or condition to be treated, its severity,
CA 02322053 2000-10-23
77430-8D
7
the treatment regimen to be employed, the pharmacokinetics of
the agent used, as well as the patient (animal or human)
treated. Thus, effective dosages may range from 1 mg/kg of
body weight, or less, to 25 mg/kg of body weight or more. This
dosage range generally produces effective blood level
concentrations of active compound ranging from about 0.04 to
about 100 micrograms/cc of blood in the patient. It is
contemplated, however, that appropriate patient-specific
regimens will be developed by administering a small amount, and
then increasing the amount until either the side effects become
unduly adverse, or the intended effect is achieved.
Administration of compounds according to the present
invention may take place orally, parenterally (including
subcutaneous injections, intravenous, intramuscularly, by
intrasternal injection or infusion techniques), by inhalation
spray, or rectally, topically and so forth, and in dosage unit
formulations containing conventional non-toxic pharmaceutically
acceptable carriers, adjuvants and vehicles.
It is contemplated that compounds according to the
present invention can be formulated in admixture with a
pharmaceutically acceptable carrier. For example, the
compounds of the present invention can be administered orally
as pharmacologically acceptable salts. Because the compounds
of the present invention are mostly water soluble, they can be
administered intravenously in physiological saline solution
(e. g., buffered to a pH of about 7.2 to 7.5). Conventional
buffers such as phosphates, bicarbonates or citrates can be
used for this purpose. Of course, one of the ordinary skill in
the art may modify the formulations within the teachings of the
specification to provide numerous formulations for a particular
route of administration without rendering the compositions of
the present invention unstable or compromising their
therapeutic activity. In particular, the modification of the
CA 02322053 2000-10-23
77430-8D
8
present compounds to render them more soluble in water or other
vehicle, for example, may be easily accomplished by minor
modifications (salt formulation, esterification, etc.) which
are well within the ordinary skill in the art. It is also well
within the ordinary skill of the art to modify the route of
administration and dosage regimen of a particular compound in
order to manage the pharmacokinetics of the present compounds
for maximum beneficial effect in patients.
In addition, compounds included in combinations
according to the present invention may be administered
separately or together, and when administered separately this
may occur in any order. The amounts of the active
ingredients) and pharmaceutically active agents) and the
relative timings of administration will be selected in order to
achieve the desired combined therapeutic effect.
Administration routes of compounds according to the
present invention may range from continuous (intravenous drip)
to several oral administrations per day (for example, Q.I.D.)
and may include oral, topical, parenteral, intramuscular,
intravenous, subcutaneous, transdermal (which may include a
penetration enhancement agent), buccal and suppository
administration, among other routes of administration.
To prepare therapies according to the present
invention, a therapeutically effective amount of a compound is
preferably intimately admixed with a pharmaceutically
acceptable carrier according to conventional pharmaceutical
compounding techniques to produce a dose. A carrier may take a
wide variety of forms depending on the form of preparation
desired for administration, e.g., oral or parenteral. In
preparing pharmaceutical compositions in oral dosage form, any
of the usual pharmaceutical media may be used. Thus, for
liquid oral preparations such as suspensions, elixirs and
CA 02322053 2000-10-23
77430-8D
9
solutions, suitable carriers and additives including water,
glycols, oils, alcohols, flavoring agents, preservatives,
coloring agents and the like may be used. For solid oral
preparations such as powders, tablets, capsules, and for solid
preparations such as suppositories, suitable carriers and
additives including starches, sugar carriers, such as dextrose,
mannitol, lactose and related carriers, diluents, granulating
agents, lubricants, binders, disintegrating agents and the like
may be used. If desired, the tablets or capsules may be
enteric-coated or sustained release by standard techniques.
For parenteral formulations, the carrier will usually
comprise sterile water or aqueous sodium chloride solution,
though other ingredients including those which aid dispersion
may be included. Of course, where sterile water is to be used
and maintained as sterile, the compositions and carriers must
also be sterilized. Injectable suspensions may also be
prepared, in which case appropriate liquid carriers, suspending
agents and the like may be employed.
It will also be appreciated that in general, the most
preferred uses according to the present invention are those in
which the active compounds are relatively less cytotoxic to the
non-target host cells and relatively more active against the
target.
While specific embodiments have been disclosed
herein, the scope of the invention is not limited except
through interpretation of the appended claims.