Note: Descriptions are shown in the official language in which they were submitted.
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
INHIBITORS OF PHOSPHOLIPASE ENZYMES
Background of the Invention
The present invention relates to chemical inhibitors of the activity of
various
phospholipase enzymes, particularly phospholipase AZ enzymes.
Leukotrienes and prostaglandins are important mediators of inflammation, each
of
which classes contributes to the development of an inflammatory response in a
different way.
Leukotrienes recruit inflammatory cells such as neutrophils to an inflamed
site, promote the
extravasation of these cells and stimulate release of superoxide and proteases
which damage the
tissue. Leukotrienes also play a pathophysiological role in the
hypersensitivity experienced by
asthmatics [See, e.g. B. Samuelson et al., Science, x:1171-76 (1987)].
Prostaglandins
enhance inflammation by increasing blood flow and therefore infiltration of
leukocytes to
inflamed sites. Prostaglandins also potentiate the pain response induced by
stimuli.
Prostaglandins and leukotrienes are unstable and are not stored in cells, but
are instead
synthesized [W. L. Smith, Biochem. J., x:315-324 (1989)] from arachidonic acid
in
response to stimuli. Prostaglandins are produced from arachidonic acid by the
action of COX-
1 and COX- 2 enzymes. Arachidonic acid is also the substrate for the distinct
enzyme pathway
leading to the production of leukotrienes.
Arachidonic acid which is fed into these two distinct inflammatory pathways is
released
from the sn-2 position of membrane phospholipids by phospholipase A., enzymes
(hereinafter
PLAZ). The reaction catalyzed by PLAz is believed to represent the rate-
limiting step in the
process of lipid mediated biosynthesis and the production of inflammatory
prostaglandins and
leukotrienes. When the phospholipid substrate of PLA2 is of the phosphotidyl
choline class
with an ether linkage in the sn-1 position, the lysophospholipid produced is
the immediate
precursor of platelet activating factor (hereafter called PAF), another potent
mediator of
inflammation [S.I. Wasserman, Hospital Practice, 15:49-58 ( 1988)].
Most anti-inflammatory therapies have focussed on preventing production of
either
prostglandins or leukotrienes from these distinct pathways, but not on all of
them. For
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
example, ibuprofen, aspirin, and indomethacin are all NSAIDs which inhibit the
production of
prostaglandins by COX-1/COX-2, but have no effect on the inflammatory
production of
leukotrienes from arachidonic acid in the other pathways. Conversely, zileuton
inhibits only
the pathway of conversion of arachidonic acid to leukotriense, without
affecting the production
of prostaglandins. None of these widely-used anti-inflammatory agents affects
the production
of PAF.
Consequently the direct inhibition of the activity of PLAZ has been suggested
as a
useful mechanism for a therapeutic agent, i.e., to interfere with the
inflammatory response.
[See, e.g., J. Chang et al, Biochem. Pharmacol., xø:2429-2436 ( 1987)].
A family of PLAz enzymes characterized by the presence of a secretion signal
sequenced and ultimately secreted from the cell have been sequenced and
structurally defined.
These secreted PLAZs have an approximately 14 kD molecular weight and contain
seven
disulfide bonds which are necessary for activity. These PLAZs are found in
large quantities in
mammalian pancreas, bee venom, and various snake venom. [See, e.g., references
13-15 in
Chang et al, cited above; and E. A. Dennis, Drug Devel. Res., 10:205-220 (
1987).] However,
the pancreatic enzyme is believed to serve a digestive function and, as such,
should not be
important in the production of the inflammatory mediators whose production
must be tightly
regulated.
The primary structure of the first human non-pancreatic PLAz has been
determined.
This non-pancreatic PLAZ is found in platelets, synovial fluid, and spleen and
is also a secreted
enzyme. This enzyme is a member of the aforementioned family. [See, J. J.
Seilhamer et al,
J. Biol. Chem., ,x:5335-5338 (1989); R. M. Kramer et al, J. Biol. Chem.,
x:5768-5775
( 1989); and A. Kando et al, Biochem. Biophvs. Res. Comm., x:42-48 ( 1989)].
However,
it is doubtful that this enzyme is important in the synthesis of
prostaglandins, leukotrienes and
PAF, since the non-pancreatic PLAz is an extracellular protein which would be
difficult to
regulate, and the next enzymes in the biosynthetic pathways for these
compounds are
intracellular proteins. Moreover, there is evidence that PLAZ is regulated by
protein kinase C
and G proteins [R. Burch and J. Axelrod, Proc. Natl. Acad. Sci. U.S.A.,
84:6374-6378
( 1989)] which are cytosolic proteins which must act on intracellular
proteins. It would be
impossible for the non-pancreatic PLAz to function in the cytosol, since the
high reduction
potential would reduce the disulfide bonds and inactivate the enzyme.
A murine PLA2 has been identified in the murine macrophage cell line,
designated
RAW 264.7. A specific activity of 2 mols/min/mg, resistant to reducing
conditions, was
2
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
reported to be associated with the approximately 60 kD molecule. However, this
protein was
not purified to homogeneity. [See, C. C. Leslie et aI, Biochem. Bionhys.
Acta., 9_øx:476-492
( 1988)]. The references cited above are incorporated by reference herein for
information
pertaining to the function of the phospholipase enzymes, particularly PLA2.
A cytosolic phospholipase AZ (hereinafter "cPLA2") has also been identified
and
cloned. See, U.S. Patent Nos. 5,322,776 and 5,354,677, which are incorporated
herein by
reference as if fully set forth. The enzyme of these patents is an
intracellular PLAZ enzyme,
purified from its natural source or otherwise produced in purified form, which
functions
intracellularly to produce arachidonic acid in response to inflammatory
stimuli.
It is now desirable to identify pharmaceutically useful compounds which
inhibit the
actions of these phospholipase enzymes for use in treating or preventing
inflammatory
conditions, particularly where inhibition of production of prostaglandins,
leukotrienes and PAF
are all desired results. There remains a need in the art for an identification
of such anti-
inflammatory agents for therapeutic use in a variety of disease states.
Numerous pieces of evidence have supported the centrat role of cPLA., in lipid
mediator biosynthesis: cPLAz is the only enzyme which is highly selective for
phospholipids
containing arachidonic acid in the sn-2 position (Clark et al., 1991, 1995;
Hanel & Gelb,
1993); activation of cPLA2 or its increased expression have been linked with
increased
leukotriene and prostaglandin synthesis (Lin et al., 1992a, 1992b, 1993); and
following
activation, cPLA2 translocates to the nuclear membrane, where it is co-
localized with the
cyclooxygenase and lipoxygenase that metabolize arachidonate to prostaglandins
and
leukotrienes (Schievella et al., 1995; Glover et al., 1995). Although these
data are compelling,
the most definitive evidence for the central role of cPLA2 in eicosanoid and
PAF production
came from mice made deficient in cPLA2 through homologous recombination
(Uozumi et al.,
1997; Bonventre et al., 1997). Peritoneal macrophages derived from these
animals failed to
make leukotrienes, prostaglandins, or PAF. The cPLA2 deficient mice have also
been
informative of the role of cPLA2 in disease, since these mice are resistant to
bronchial
hyperreactivity in an anaphylaxis model used to mimic asthma (Uozumi et al.,
1997). Thus,
despite the size of the phospholipase AZ superfamily, cPLA2 is essential for
prostaglandin,
leukotriene, and PAF production.
Clark, J. D., Lin, L.-L., Kriz, R. W., Ramesha, C. S., Sultzman, L. A., Lin,
A. Y.,
Milona, N., and Knopf, J. L. ( 1991 ). A novel arachidonic acid-selective
cytosolic PLAz
contains a Caz+-dependent translocation domain with homology to PKC and GAP.
Cell 65,
3
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
1043-1051. Hanel, A. M., and Gelb, M. H. (1993). Processive interfacial
catalysis by
mammalian 85-kilodalton phospholipase AZ enzymes on product-containing
vesicles:
application to the determination of substrate preferences. Biochemistry 32,
5949-5958.
Lin, L.-L., Lin, A. Y., and DeWitt, D. L. (1992a) IL-1 _ induces the
accumulation of
cPLA2 and the release of PGEZ in human fibroblasts. J. Biol. Chem. 267, 23451-
23454. Lin,
L.-L., Lin, A. Y., and Knopf, J. L. ( 1992b) Cytosolic phospholipase A, is
coupled to
hormonally regulated release of arachidonic acid. Proc. Natl. Acad. Sci. USA
89, 6147-61 S 1.
Lin, L.-L., Wartmann, M., Lin, A. Y., Knopf, J. L., Seth, A., and Davis, R. J.
(1993)
cPLA2 is phosphorylated and activated by MAP kinase. Cell 72, 269-278.
Glover, S., de Carvalho, M., Bayburt, T., Jonas, M., Chi, E., Leslie, E., and
Gelb,
M. ( I995) Translocation of the 85-kDa phospholipase AZ from cytosol to the
nuclear envelope
in rat basophilic leukemia cells stimulated with calcium ionophore or
IgE/antigen. J. Biol.
Chem. 270, 15359-15367. Schievella, A. R., Regier, M. K., Smith, W. L., and
Lin, L.-L.
( 1995). Calcium-mediated translocation of cytosolic phospholipase AZ to the
nuclear envelope
and endoplasmic reticulum. J. Biol. Chem. 270, 30749-30754.
Uozumi, N., Kume, K., Nagase, T., Nakatani, N., Ishii, S., Tashiro, F.,
Komagata,
Y., Maki, K., Ikuta, K., Ouchi, Y., Miyazaki, J-i., & Shimizu, T. ( 1997).
Role of cytosolic
phospholipase Az in allergic response and parturition. Nature 390, 618-622.
Bonventre, J. V.,
Huang, Z., Reza Taheri, M., O'Leary, E., Li, E., Moskowitz, M. A., and
Sapirstein, A.
( 1997) Reduced fertility and postischaemic brain injury in mice deficient in
cytosolic
phospholipase AZ. Nature 390, 622-625.
Summary of the Invention
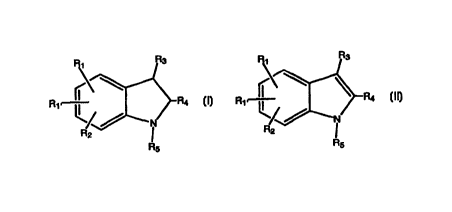
Compounds of this invention have the following formulae:
R1 R1 R4
or
4
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
wherein:
R, and R,. are independently selected from H, halogen, -CF,, -OH, -C,-C,o
alkyl,
preferably -C,-C6 alkyl, -S-C,-C,o alkyl, preferably -S-C,-C6 alkyl, C,-C,o
alkoxy, preferably
C,-C6 alkoxy, -CN, -NOZ, -NHZ, phenyl, -O-phenyl, -S-phenyl, benzyl, -O-
benzyl, -S-
benzyl; or a ring moiety of the groups a), b) or c), below, directly bonded to
the indole ring or
bonded to the indole ring by a -S-, -O- or -(CHZ)~- bridge;
a) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, isothiazole, isoxazole, pyrrolidine, pyrroline, imidazolidine,
pyrazolidine, pyrazole,
pyrazoline, imidazole, tetrazole, oxathiazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NH2, -
CN, -CF3; or
b) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyran, pyridine,
pyrazine, pyrimidine,
pyridazine, piperidine, piperazine, tetrazine, thiazine, thiadizine, oxazine,
or morpholine, the
six-membered heterocyclic ring being optionally substituted by from 1 to 3
substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -NO2, -NH2, -CN, -CF3 or -OH; or
c) a bicyclic ring moiety optionally containing from 1 to 3 ring heteroatoms
selected from N, S or O including, but not limited to benzofuran, chromene,
indole, isoindole,
indoline, isoindoline, napthalene, purine, indolizine, indazole, quinoline,
isoquinoline,
quinolizine, quinazoline, cinnoline, phthalazine, or napthyridine, the
bicyclic ring moiety being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NH" -CN, -
CF3 or -OH; or
d) a moiety of the formulae:
(s Is
R
R~ ~ R ~O~ R ~S~
Z ~ Z
5
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rs
N O~ ~O Rs
/O~ ~ ~S~ N N
IZI R~~ ~ R~~ ~ Rs
> >
Z
~R~
Is
R~...S/N~
or O O ;
ZisOorS;
R6 is selected from the relevant members of the group H, -CF3, C,-C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, phenyl, -O-
phenyl, -S-
phenyl, benzyl, -O-benzyl, or -S-benzyl, the phenyl and benzyl rings of these
groups being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NOZ, -
NHZ, -CN, -
CF3, or -OH;
R7 is selected from the relevant members of the group -OH, -CF3, C,-C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NHZ, -(CHZ)~-
NH2, -NH-
(C,-C6 alkyl), -N-(C,-C6 alkyl)2, -(CHZ}"NH-(C,-C6 alkyl), -(CHZ)~ N-(C,-C6
alkyl)2,
phenyl, -O-phenyl, benzyl, or -O-benzyl; or
a) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, isothiazole, isoxazole, pyrrolidine, pyrroline, imidazolidine,
pyrazolidine, pyrazole,
pyrazoline, imidazole, tetrazole, oxathiazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NH2, -
CN, or -CF3;
or
b) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyran, pyridine,
pyrazine, pyrimidine,
6
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
pyridazine, piperidine, piperazine, tetrazine, thiazine, thiadizine, oxazine,
or morpholine, the
six-membered heterocyclic ring being optionally substituted by from 1 to 3
substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -NO" -NHz, -CN, -CF3 or -OH; or
IO c) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, indolizine,
indazole, quinoline, isoquinoline, quinolizine, quinazoline, cinnoline,
phthalazine, or
napthyridine, the bicyclic ring moiety being optionally substituted by from I
to 3 substituents
IS selected from halogen, C,-C,a alkyl, preferably C,-C6 alkyl, C,-C,a alkoxy,
preferably C,-C6
alkoxy, -CHO, -NO2, -NH2, -CN, -CF3 or -OH;
n is an integer from 0 to 3;
20 RZ is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl,
preferably C,-
C6 alkyl, C,-C,a alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -NO2, -NH2, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)2, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
R3 is selected from -COOH, -C(O)-COOH, -(CH2)~ C(O)-COOH, -(CHZ)p COOH,
25 -CH=CH-COOH, -(CHZ)~ tetrazole,
O~~S~
N~~(Ci-Cs lower alkyl) or
O /S~ N~
/ \N N
S
N" C -C lower al 1
( ~ s kY )~ or
0
OH
-P---OH
O
7
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
or a moiety selected from the formulae -L'-M';
wherein L' is a bridging or linking moiety selected from a chemical bond, -
(CHZ)~ , -S-, -O-,
-C(O)-, -(CHZ)"-C(O)-, -(CHz)"C(O)-(CHZ)", -(CHZ)~ O-(CHZ)"-,-(CHZ)n S-(CHZ)~
,
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CHZ)~-, -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-N(R6)-(CHZ)n
,
-C(Z)-NH-SO~-, or -C(Z)-NH-SO~-(CHZ)~ ;
M' is selected from the group of -COOH, -(CHz)o COOH, -(CHZ)n C(O)-COOH,
tetrazole,
Rs
~R9 Rs
-N N
0 , ~ ,
S~~ 8 O' Rs N'/Re
/ . ~R / . ~ /.
s \ ~ Rs /' ~ Rs
Rlo Rto Rio
S
O
O
N~ P-OH \S'
O R~ t ~ ~ 0 ~ R9 ~ ~ \OH
R8
N_I~~Rs
Rio
or
Rg, in each appearance, is independently selected from H, -COOH, -(CHZ)o COOH,
-
(CHZ)~ C(O)-COOH, tetrazole,
8
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
O
P-OH \S'
O ~or ~ OH
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)~ COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -O-(CH2)~-COOH, -O-CH2 C=C-
COOH,
-O-C=C-CHz COOH, -NH(C,-C6 alkyl), -N(C,-C6 alkyl)2, -N-C(O)-(CHZ)~ COOH. -N-
SO~-
(CH2)~ COOH, -C(O)-N-(CHZ)n COOH;
R,o is selected from the group of H, halogen, -CF3, -OH, -(CHZ)~-COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -O-(C,-C6 alkyl)-(OH)~, -
NH(C,-C6
alkyl), -N(C,-C6 alkyl)2, -N-C(O)-N-(C,-C6 alkyl)-(OH)2,
Re Ra
O O\ ~ I~ R9 I~~Rs
~N~S \
\Z/
R$
I ~/Rs
Z/
R$ Re
N_I~~Rs I~~Rs
~- (CH2)~ , ~ (CH2)~
O %S O O jS,O
"N ~(C~-Cs lower alkyl "N ~(C~-C6 lower haloalkyl;
,
9
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
isp ~N
N S ,Ra
i
S ~ ~ ~Z/~~ / 'Rs
N (C1-C6 lower alkyl)
Ra N Ra N Ra
i ~ i
/\~ /'Rs ~ /\~ /'Rs ~ (CH2) /~~ / 'Rs
Z Z n
> >
S Ra Ra
i i
~- (CH2) /~ / 'Rs -,--- (CH2) /~ / 'Rs
or ;
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloalkyl, -CF3, -COOH, -
(CHZ)~
COOH, -(CHZ)~ C(O)-COOH,
Ra Ra
I ~~Rs I ~~Rs
- (CH2)n
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', Rg, Rg, R,o, and/or R" shall contain at least
one acidic moiety
selected from or containing a carboxylic acid, a tetrazole, or a moiety of the
formulae:
-QP~-OH ~S~
n ~ ~ ~ /S
0 , OH or N
O
/ 'N/S O NON
g ~ O
N~(C~-Ca lower alkyl;.
n is an integer from 0 to 3;
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R,, is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3-C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -LZ-M2:
Lz indicates a linking or bridging group of the formulae -(CHZ)o , -S-, -O-,
-C(O)-, -(CH2)~ C(O)-, -(CHZ)n C(O)-(CHZ)", -(CHz}o-O-(CHZ)~ , or -(CHz)n-S-
(CHz)~-,
C(O)C(O)X;
where X is O or N
M2 is selected from:
a) the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NO2, -NHZ, -CN, or -CF3; or
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, isothiazole, isoxazole, pyrrolidine, pyrroline, irnidazolidine,
pyrazolidine, pyrazole,
pyrazoline, imidazole, tetrazole, oxathiazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NH2, -
CN, or -CF3;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyran, pyridine,
pyrazine, pyrimidine,
pyridazine, piperidine, piperazine, tetrazine, thiazine, thiadizine, oxazine,
or morpholine, the
six-membered heterocyclic ring being optionally substituted by from 1 to 3
substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -N02, -NH2, -CN, -CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, indolizine,
indazole, quinoline, isoquinoline, quinolizine, quinazoline, cinnoline,
phthalazine, or
napthyridine, the bicyclic ring moiety being optionally substituted by from 1
to 3 substituents
11
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -NO" -NH2, -CN, -CF3 or -OH;
RS is selected from C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CH~)~ C3-C,o
cycloaIkyl,
-(CHZ)o S-(CHZ)~ C3 C,o cycloalkyl, -(CHZ)~ O-(CHZ)~ C3-C,o cycloalkyl, or the
groups of:
a) -(CH2)~ phenyl-O-phenyl, -(CHZ)"phenyl-CHZ phenyl, -(CHZ)~ O-phenyl-
CHZ phenyl, -(CHZ)~ phenyl-(O-CHZ phenyl)2, -CH2-phenyl-C(O)-benzothiazole or
a moiety
of the formulae:
O
Y ~(CH2)~ ~(CH2)~ /Y
O/ , Y , S ,
Y
O~ (CH2)n~Y ~ ~(CH2)~/~, '
~(CH~~S/ (CH2)~Y ~ /(CH2)~ / (CH2),.,~
O Y
/ (CH2)n
or ( ~ Y
wherein n is an integer from 0 to 3, preferably 1 to 3, more preferably 1 to
2,
Y is C3-C6 cycloalkyl, phenyl, biphenyl, each optionally substituted by from 1
to 3
groups selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o
alkoxy, preferably
C,-C6 alkoxy, -NO2, -NHZ, -CN, or -CF3; or
a) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, isothiazole, isoxazole, pyrrolidine, pyrroline, imidazolidine,
pyrazolidine, pyrazole,
12
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
pyrazoline, imidazole, tetrazole, oxathiazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NOZ, -NHS, -
CN, -CF3, or
by one phenyl ring, the phenyl ring being optionally substituted by by from 1
to 3 substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -NO" -NHZ, -CN, -CF3; or
b) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyran, pyridine,
pyrazine, pyrimidine,
pyridazine, piperidine, piperazine, tetrazine, thiazine, thiadizine, oxazine,
or morpholine, the
six-membered heterocyclic ring being optionally substituted by from 1 to 3
substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -NO2, -NHZ, -CN, -CF3 or -OH; or
c) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, indolizine,
indazole, quinoline, isoquinoline, quinolizine, quinazoline, cinnoline,
phthalazine, or
napthyridine, the bicyclic ring moiety being optionally substituted by from 1
to 3 substituents
selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-C,o alkoxy,
preferably C,-C6
alkoxy, -CHO, -NOZ, -NHz, -CN, -CF3 or -OH;
d) a moiety of the formulae -(CHZ)~ A, -(CH2)~-S-A, or -(CHz)~ O-A, wherein A
is the moiety:
~C
B
wherein
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, -CF3 or -(CHz)~ CF3;
B and C are independently selected from phenyl, pyridinyl, pyrimidinyl, furyl,
thienyl
or pyrrolyl groups, each optionally substituted by from 1 to 3, preferably 1
to 2, substituents
selected from H, halogen, -CN, -CHO, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -
NH, , -N(C,-
C6)2, -NH(C,-C6), -N-C(O)-(C,-C6), -NOZ, or by a 5- or 6-membered heterocyclic
or
heteroaromatic ring containing 1 or 2 heteroatoms selected from O, N or S,
such as, for
example, morpholino;
13
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
or a pharmaceutically acceptable salt thereof.
One group of compounds within this invention are those in which the indole or
indoline
2-position (R,~) is substituted only by hydrogen and the substituents at the
other indole or
indoline positions are as described above.
Another R3 is -L'-M', wherein L' is as defined above, more preferably wherein
L' is a chemical bond, and M' is the moiety:
Ry
and R9 is as defined in the broad genus above.
Another group of this invention comprises compounds in which RZ and R4 are
hydrogen and the groups at R,, R'. , R3, and Rs are as defined above. Within
this group are
two further preferred groups. In the first, R, is in the indole or indoline 5
position and in the
second R, is in the indole or indoline 6 position.
In a further preferred group herein, R, is in the indole or indoline 5-
position and is
benzyloxy, RZ and R4 are hydrogen and R3 and Rs are as defined above.
Among the more preferred compounds of this invention are those of the
following
formulae:
or
wherein:
14
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R, is selected from H, halogen, -CF3, -OH, -C,-C,o alkyl, preferably -C,-C6
alkyl, -S-
C,-C,o alkyl, preferably -S-C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6
alkoxy, -CN, -NO2,
-NH2, phenyl, -O-phenyl, -S-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety
of the
formulae:
(s Is Is
R N~ ./N N\ ~O N~
R~ ~ ~O\ R~
O , O , R~ , O ,
is
O S//O Is R~S/N'~
R ~ \ R~~N' O O
or
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NO2, -NH2, -
CN, -CF3, or -
OH;
R, is selected from -OH, -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -
N-(C,-
C6 alkyl)2, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, -O-phenyl, benzyl, -O-
benzyl, pyrazolyl
and thiazolyl, the rings of these groups being optionally substituted by from
1 to 3 substituents
selected from halogen, -CN, C,-C6 alkyl, C,-C6 alkoxy, -N02, -NH2, -CF3, or -
OH;
RZ is selected from H, halogen, -CF3, -OH, -C,-C,o alkyl, preferably -C,-C6
alkyl, C,-
C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -N02, -NH2, -NH-C,-C6 alkyl, -
N(C,-C6
alkyl)2, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
R3 is selected from -COOH, -C(O)-COOH, -(CHZ)o C(O)-COOH, -(CHZ)o COON,
-CH=CH-COOH, -(CHZ)~ tetrazole,
15
CA 02322162 2000-08-24
WO 99143654 PCT/US99/03898
N~~(Ci-Cs lower alkyl) or
~(C~-Cs lower alkyl)
, or
0
(I pH
o OH II
or a moiety selected from the formulae -L'-M';
wherein L' is a bridging or linking moiety selected from a chemical bond, -
(CHZ)~ , -S-, -O-,
-C(O)-, -(CH2)~ C(O)-, -(CHZ)~ C(O)-(CHZ)~ , -(CHZ)o O-(CHZ)o ,-(CH2)~ S-
(CHZ)n ,
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CH2)~ , -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-N(R6)-(CH2)~
,
-C(Z)-NH-SOZ , or -C(Z)-NH-SOZ (CHZ)o ;
M' is selected from the group of -COOH, -(CH2)~ COOH, -(CHZ)~-C(O)-COOH,
tetrazole,
8 ~B R9
''R9
Re~
-N N
~ Rio Rio
> > >
16
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rs S~~Ra O'/Ra N '/Ra
Ra
~ ~R / . ~R
HN N ~~ ~ s ~ s ~\ ~ Rs
, Rlo , Rio , Rio
S Ra
0 ~I~~Rs
N~ -~P-OH S ~ \R
O Rig _ ~ O _ ~ OOH or ~ to
Rg, in each appearance, is independently selected from H, -COOH, -(CHZ)~ COOH,
-
(CHZ)~ C(O)-COOH, tetrazole,
P--OH ~S~
O ~or ~ OH
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)~-COON,
-(CHZ)p C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), or -N(C,-C6
alkyl)2;
R,o is selected from the group of H, halogen, -CF3, -OH, -(CH2)~ COON,
-(CH2)o C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2,
Ra Ra Ra
~S/~ I~~Rs N I~~Rs (~~Rs
N \ \
'Z/ \ Z/
Ra Ra
N I~~Rs I~~Rs
1- (CH2)/ ~ (CH2}~
17
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S Re
i
~Z/\~ /~Rs
(C~-Cs lower alkyl)
Re N ERs N Rs
i i
/\~ /ERs ~ /'~ /~Rs ~ (CH2) ~~' ~~Rs
Z Z
, ,
g R8 R8
i i
1- (CH2) ~~~ ~~Rs ~ (CH2) ~~~ ~~Rs
or
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloalkyl, -CF3, -COOH, -
(CHZ)"
COOH, -(CHZ)n C(O)-COOH,
Rg Rg
N I~~Rs I~~Rs
- (CH2)n
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', R8, Rg, R,o, andJor R" shall contain at least
one acidic moiety
selected from or containing a carboxylic acid, a tetrazole, or a moiety of the
formulae:
P-OH O\S' O\
n ~ \ ~ /S\
O ~ OH or N ;
n is an integer from 0 to 3;
R~ is selected from H, -CF3, C,-C6 lower alkyl, C,-Cb lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3-C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -LZ-M2:
18
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
L'- indicates a linking or bridging group of the formulae -(CHZ)~-, -S-, -O-,
-C(O)-, -(CHZ)~ C(O)-, -(CHZ)~ C(O)-(CHz)~ , -(CHZ)rt O-(CHZ)~ , or -(CHZ)~ S-
(CHZ)~-;
MZ is selected from the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloallcyl, phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being
optionally
substituted by from 1 to 3 substituents selected from halogen, C,-Coo alkyl,
preferably C,-C6
alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NOZ, -NHz, -CN, or -CF3; or
a) a five-membered heterocyciic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, or tetrazole, the five-membered heterocyclic ring being
optionally
substituted by from 1 to 3 substituents selected from halogen, C,-C,o alkyl,
preferably C,-C6
alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NOZ, -NHZ, -CN, or -CF3; or
b) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to pyridine, pyrimidine,
piperidine,
piperazine, or morpholine, the six-membered heterocyclic ring being optionally
substituted by
from 1 to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6
alkyl, C,-C,o
alkoxy, preferably C,-C6 alkoxy, -CHO, -NOZ, -NHZ, -CN, -CF3 or -OH; or
c) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S' or O including,
but not limited to
benzofuran, indole, indoline, napthalene, purine, or quinoline, the bicyclic
ring moiety being
optionally substituted by from i to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NHS, -CN, -
CF3 or -OH;
Rs is selected from C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHZ)~ C3-C,o
cycloalkyl,
-(CHZ)~ S-(CHZ)o C3 C,o cycloalkyl, -(CHZ)n O-(CH2)~ C3-C,o cycloalkyl, or the
groups of:
a) -(CH2)~ phenyl-O-phenyl, -(CHZ)"phenyl-CHZ phenyl, -(CH2)~ O-phenyl-
CHZ phenyl, -(CHZ)~ phenyl-(O-CHZ phenyl)z, -CH2 phenyl-C(O)-benzothiazole or
a moiety
of the formulae:
O O
~Y ~O~Y ~(CH2)r,~Y ~(Cti2)r,~s/Y
, . > >
19
CA 02322162 2000-08-24
WO 99/43b54 PCT/US99/03898
O~(Cl"~2)n~Y ~(C~"~2)r~O/Y ~(CH2)rt~s~ (C~"~2)r't~Y
~(CH2)~O/ (CH2)~Y
wherein n is an integer from 0 to 3, preferably 1 to 3, more preferably 1 to
2, Y is C3-C5
cycloalkyl, phenyl, benzyl, napthyl, pyridinyl, quinolyl, furyl, thienyl,
pyrrolyl, benzothiazole
and pyrimidinyl, the rings of these groups being optionally substituted by
from 1 to 3
substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -
CN, -NH2, -
NOZ or a five membered heterocyclic ring containing one heteroatom selected
from N, S, or O,
preferably S or O; or
b) a moiety of the formulae -(CH2)~ A, -(CHZ)~ S-A, or -(CHZ)~ O-A, wherein A
is the moiety:
~C
B
wherein
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, -CF3 or -(CHZ)~ CF3;
B and C are independently selected from phenyl, pyridinyl, pyrimidinyl, furyl,
thienyl
or pyrrolyl groups, each optionally substituted by from 1 to 3, preferably 1
to 2, substituents
selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NHZ or -N02;
or a pharmaceutically acceptable salt thereof.
One group of compounds within this invention are those in which the indole or
indoline
2-position (R4) is substituted only by hydrogen and the substituents at the
other indole or
indoline positions are as described above.
In an another preferred group of this invention R, is in the indole or
indoline 5 or 6
position and is cyclopentylcarboxamide or cyclopentyloxycarbonylamino, R2 and
R~ are
hydrogen, and R3 and RS are as defined above.
20
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
A further preferred group of this invention consists of R, and R,at the indole
or
indoline ~ and or 6 position and are each selected from the group consisting
of C,-C6alkoxy,
cyano, sulfonyl and halo, RZ and R, are hydrogen, and R3 and RS are as defined
above.
Another group of this invention comprises compounds in which Rz and R4 are
hydrogen and the groups at R,, R3, and R3 are as defined above. Within this
group are two
further preferred groups. In the first, R~ is in the indole or indoline 5
position and in the second
R, is in the indole or indoline 6 position.
In a further preferred group herein, R, is in the indole or indoline S-
position and is
benzyloxy, RZ and R4 are hydrogen and R3 and RS are as defined above.
A preferred group of compounds of this invention have the following formulae:
or
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NOZ, -
NHz,
CN, phenyl, -O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the
formulae:
Is Is
O S~ Is R~S/N~ R N~
R /O~ R ~ ~ R / N'~ // O
~ , ~ , ~ , O O , O ,
(s Is
N N~ O N~
R~~ ~ R ~
O , or O ;
21
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S R6 is selected from H, Ci-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl,
-O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NH2, -NOz, -
CF3, or -OH;
R., is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -N-
(C,-C6
alkyl)2, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, -O-phenyl, benzyl, -O-
benzyl, pyrazolyl
and thiazolyl, the rings of these groups being optionally substituted by from
1 to 3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NH2, -NOZ, -CF3, or -OH;
R~ is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl, preferably
C,-
C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -NOZ, -NHS, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)2, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
R3 is selected from -COOH, -C(O)-COOH, -(CHZ)~ C(O)-COOH, -(CHZ)n COOH,
-CH=CH-COOH, -(CHz)n tetrazole,
S
N~ ~(C~-Cs lower alkyl) or
N/S~ ~N
S
N" C -C lower al 1
( ~ s kY )
0
Q II pH
~yP-OH
Or
or a moiety selected from the formulae -L'-M';
wherein L' is a bridging or linking moiety selected from a chemical bond, -
(CHZ)n-, -S-, -O-,
-C(O)-, -(CHZ)~ C(O)-, -(CHZ)n-C(O)-(CHZ)~ , -(CHZ)~-O-(CHZ)~-,-(CHZ)~ S-
(CH,)~ ,
22
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CHZ)~-, -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-1V(Rs)'(CH,)~-
,
-C(Z)-NH-S02-, or -C(Z)-NH-SO,-(CHZ)~ ;
M' is selected from the group of -COOH, -(CHZ)~-COOH, -(CHZ)~-C(O)-COOH,
tetrazole,
Is
_Rs Rs
Ra~~
-N N
0 .J
Rs S~~Ra O'/Ra N ~ a
Ra~~
HN N /\ ~~Rs /~ ~~Rs /\ ~~Rs
%/ ~ I
R10 R10 R10
s > > >
Ra
O _~~~Rs
°w°
OH S \
O N\R11 ~ ~ O ~ ~ OOH or ~~ Rio
R8, in each appearance, is independently selected from H, -COOH, -(CHZ)~ COOH,
-
(CHZ)~ C(O)-COOH, tetrazole,
O
P-OH S-
O ~or ~ OH ;
R.~ is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)~ COON,
-(CH2)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2;
R,o is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CHZ)n COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2,
23
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rs R8
~S~ I~~Rs N I~~Rs
/ \ \
N \
'Z/
,
Ra
I~~Rs
/ \
or
\N
N
S
N"(C1-C6 lower alkyl)
O O.. .O O O.. .O
~ S ~ S
"N ~ ~(C~-C6 lower alkyl: "N ~ ~(C~-Cs lower haloalkyl; .
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloalkyl, -CF3, -COOH, -
(CH2}~-
COOH, -(CHZ)n C(O)-COOH,
R8 R8
N I~~Rs I~~Rs
\ - (CH2)n / \
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', R8, Rg, R,o, and/or R" shall contain at least
one acidic moiety
selected from or containing a carboxylic acid, a tetrazole, or a moiety of the
formulae:
O
P-OH \S~
a ~ ~ ~ ~S~
O ~ OH or N ;
n is an integer from 0 to 3;
24
CA 02322162 2000-08-24
WO 99/43654 PCT/I3S99/03898
R~ is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3-C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -L2-M2:
LZ indicates a linking or bridging group of the formulae -(CHZ)n , -S-, -O-,
-C(O)-, -(CHZ)p C(O)-, -(CHZ}"-C(O)-(CHZ)o , -(CHZ)~ O-(CHZ)~ , or -(CHZ)"-S-
(CHZ)";
Mz is selected from:
a) the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NO2, -NH2, -CN, or -CF3; or
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, pyrazole, or tetrazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
Clo alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NOZ, -NH2, -
CN, or -CF;;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyridine, pyrazine,
pyrimidine,
piperidine, piperazine, thiazine, or morpholine, the six-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NOz, -
NH2, -CN,
CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, quinoline
or isoquinoline, the bicyclic ring moiety being optionally substituted by from
1 to 3
substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-
C,o alkoxy,
preferably C,-C6 alkoxy, -CHO, -NO2, -NH2, -CN, -CF3 or -OH;
RS is selected from C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHZ)~ C3-C5
cycloalkyl,
-(CHz)o S-(CHZ)"C3-C5 cycloalkyl, -(CHZ)n O-(CHZ}~ C3-C5 cycloalkyl, or the
groups of:
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
a) -(CHZ)n phenyl-O-phenyl, -(CHz)~ phenyl-CHz phenyl, -(CHZ)~ O-phenyl-
CHZ phenyl, -(CHZ)~ phenyl-(O-CHZ phenyl)2, -CHZ phenyl-C(O)-benzothiazole or
a moiety
of the formulae:
Y COY ~~CH~~Y /~CH2)~S/Y
> > > >
O~ ~CHp)n~Y yCE"~2)~O/Y yCH2)rr~s/ (CH2)~Y
> > >
~(CH2)r,~o/ (CH~,.~Y
wherein n is an integer from 0 to 3, preferably 1 to 3, more preferably 1 to
2, Y is C3-CS
cycloalkyl, phenyl, benzyl, napthyl, pyridinyl, quinolyl, furyl, thienyl,
pyrrolyl benzothiazoie
or pyrimidinyl, the rings of these groups being optionally substituted by from
1 to 3
substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -
NO2, -NHZ or
a five membered heterocyclic ring containing one heteroatom selected from N,
S, or O,
preferably S or O; or
b) a moiety of the formulae -(CHZ)~ A, -(CHZ)n S-A, or -(CHZ)p O-A, wherein A
is the moiety:
D
C
B
wherein
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHZ)n CF3 or -CF3;
B and C are independently selected from phenyl, pyridinyl, pyrimidinyl, furyl,
thienyl
or pyrrolyl groups, each optionally substituted by from 1 to 3, preferably 1
to 2, substituents
selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NHZ or -NO2;
or a pharmaceutically acceptable salt thereof.
A preferred group among the compounds above are those in which the R,
substitution
is at the indole or indoline ring's 5-position and the other substituents are
as defined above.
Another preferred group of this invention are those of the formulae:
26
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
I 6
/N\ /N\
R
or O
Ra
or
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NO" -
NH2,
phenyl, -O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the formulae:
is
O~S O ~s R~S/N\
R ~~ R~/ ' R~~ N ~ O \O
> > >
Is ~s Is
R N\ /N N\ /O N\
R~ ~ R~
O , O , or O
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NO2, -CF3, or -
OH;
R., is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -N-
(C,-C6
alkyl)2, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, -O-phenyl, benzyl, -O-
benzyl, pyrazolyl or
thiazolyl, the rings of these groups being optionally substituted by from 1 to
3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NH2, -NO2, -CF3, or -OH;
R, is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl, preferably
C,-
C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -NO2, -NH2, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)Z, -N-SO~ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
27
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R3 is selected from -COOH, -C(O)-COOH, -(CHz)~ C(O)-COOH, -(CHZ)~-COOH,
-CH=CH-COOH, -(CHZ)~ tetrazole,
O~,
N~~(C~-C6 lower alkyl) or
"(C1-C6 lower alkyl)
or
0
II pH
-'P-OH II
O , or o
or a moiety selected from the formulae -L'-M';
wherein L' is a bridging or linking moiety selected from a chemical bond, -
(CHZ)o , -S-, -O-,
-C(O)-, -(CHZ)a C(O)-, -(CHZ)~ C(O)-(CHZ)a , -(CHZ)o O-(CHZ)p ,-(CHZ)n S-
(CHZ)n ,
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CHZ)", -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-N(R6)-(CHZ)p-,
-C(Z)-NH-SOZ , or -C(Z)-NH-SOZ (CHZ)~ ;
M' is selected from the group of -COOH, -(CHZ)n COOH, -(CH2)~ C(O}-COOH,
tetrazole,
-N N
> > ,
28
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rs S~~ s O/R8 N~ s
Re
~R / ~ ~R
HN N ~~ ~ s ~ ~ s ~' ~ Rs
Rlo Rlo Rio
, , ,
S Rs
O _~~~Rs
Q v
N~ -~P-OH S / ~R
O R1~ ' ~ O - ~ OOH or ~ ~o
Rg, in each appearance, is independently selected from H, -COOH, -(CHz)o-COOH,
-
(CHz)~ C(O)-COOH, tetrazole,
P-OH
O ~or ~ OH
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHz)~ COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)z;
Rto is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CHz)~ COOH,
-(CHz)"C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)z,
Rs Re
~S~ I~~Rs N ~~~Rs
N \
\Z/
RB
~~Rs
~z~
or
29
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
/S~
N N
S
N~(C~-Cs lower alkyl)
,
O %S ,O O O~S ;O
"N ~(C~-Cs lower alkyl; or "N ~ ~(C~-Cs lower haloalkyl; .
> >
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloallcyl, -CF3, -COOH, -
(CHZ)a
COOH, -(CHZ)n C(O)-COOH,
R8 R8
I~~Rs I~~Rs
_ (CH2)n
_
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', R8, R.~, R,o, and/or R" shall contain at least
one acidic moiety
selected from or containing a carboxylic acid, a tetrazole, or a moiety of the
formulae:
OH ~S~
y.
O , OH or N ;
n is an integer from 0 to 3;
R,, is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3-Coo cycloalkyl, -CHO, halogen, or a moiety of the
formula -LZ-M2:
L2 indicates a linking or bridging group of the formulae -(CHZ)o , -S-, -O-,
-C(O)-, -(CHZ)"C(O)-, -(CH2)~ C(O)-(CHZ)~ , -(CHz)o-O-(CHZ)p , or -(CHZ)o-S-
(CHZ)~ ;
MZ is selected from:
a) the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NO2, -NH2, -CN, or -CF3; or
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, pyrazole, or tetrazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NH2, -
CN, or -CF3;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyridine, pyrazine,
pyrimidine,
piperidine, piperazine, thiazine, or morpholine, the six-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NH2, -CN, -
CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, quinoline
or isoquinoline, the bicyclic ring moiety being optionally substituted by from
1 to 3
substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-
C,o alkoxy,
preferably C,-C6 alkoxy, -CHO, -NO2, -NH2, -CN, -CF3 or -OH;
R5 is selected from C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHz)n-C3-Cs
cycloalkyl
or --(CHZ)~ A, -(CHZ)n S-A, or -(CHZ}~ O-A wherein A is selected from
, ,
31
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
, ,
Rt2
J
R , , or
;
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, or -CF3;
R~Z is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, or -CF3;
32
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
or a pharmaceutically acceptable salt thereof.
Other compounds of this invention have the following formulae:
R
or
Rs
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NOZ, -
NHZ,
phenyl, -O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the formulas:
~s ~s ~s
R N~ /N N~ /O
R~ ~ ~O~ R~
O , O , R~ ~ O
Rs
R~S
R~ \ ~ R~ ~ or O O
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NH2, -NO2, -
CF3, or -OH;
R~ is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -N-(Cl-
C6
alkyl}Z, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, pyrazolyl, thiazolyl, -O-
phenyl, benzyl or -
O-benzyl, the rings of these groups being optionally substituted by from 1 to
3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NHZ, -NOZ, -CF3, or -OH;
RZ is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl, preferably
C,-
C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -NO2, -NH2, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)z, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
33
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S R3 is selected from -COOH, -C(O)-COOH, -(CHZ)~ C(O)-COOH, -(CHZ)~ COOH,
-CH=CH-COOH, -(CHZ)~ tetrazole,
O~, Jl~
N~~(Ct-C6 lower alkyl) or
N/S~ N\N
S
N" C -C lower al I
( t s kY ), or
0
off
-~P-off
~ O , or
or a moiety selected from the formulae -L'-M' or L2M2;
L' is a bridging or linking moiety selected from a chenucal bond, -(CH2)~ , -S-
, -O-,
-C(O)-, -(CHZ)n C(O)-, -(CH2)o C(O)-(CHZ)o , -(CH2)n O-(CHZ)o ,-(CH2)n
S=(CHZ)o ,
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CHZ)o , -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-N(R6)-(CHZ)p
,
-C(Z)-NH-SOZ , or -C(Z)-NH-SOz (CHZ)o ;
M' is selected from the group of -COOH, -(CHZ)"-COOH, -(CHZ)"C(O)-COOH,
tetrazole, '
Rs Rs S~~Re O/ s
RB~~
HN N ~~ / R9 ~~ % Rs
N ~ _l J
Rto Rto
34
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
N '' 8 S
O
/~ WRs
I N~ -P--OH
Rio , O R» , ~ O
R8
N ~~~Rs
W
S
Rlo
OH or
LZ is a bridging or linking moiety selected from a chemical bond -S-, -O-,
-C(O)-, -(CHz)n-C(O)-, -(CHZ)~-C(O)-(CHZ)~ , -(CHZ)~-O-(CH2)~ , -(CHZ)~ S-
(CH2)~-,
-C(Z)-N(R6)-, -C(Z)-N(R6)-(CHZ)n-, -C(O)-C(Z)-N(R6)-, -C(O)-C(Z)-N(R6)-(CHZ)~
,
-C(Z)-NH-SOZ-, or -C(Z)-NH-SOZ-(CHZ)4 ;
MZ is the moiety
Rs
IS
Rg, in each appearance, is independently selected from H, -COOH, -(CHz)n-COOH,
-
(CHZ)~ C(O)-COOH, tetrazole,
O
-P-OH \S'
O ~or ~ OOH
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)o COOH,
-(CH2)n C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)z;
R,o is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CH2)~-COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)Z,
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R8 R$ R8
~S~ (~~Rs ~I~~Rs ~~~Rs
/
N
OT
~N
S
N~(C~-Cs lower alkyl)
O
O~ ,O
S ~ OoS .O
N ~ ~(C~-C6 lower alkyl "N ~ ~(C1-Cs lower haloaikyl; .
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloalkyl, -CF3, -COOH, -
(CHZ)n
COOH, -(CHZ)~-C(O)-COOH,
R8 R8
N-I~~Rs (~~Rs
- (CH2)n
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', LZ, M2, R8, R9, R,o, and/or R" shall contain at
least one
acidic moiety selected from or containing a carboxylic acid, a tetrazole, or a
moiety of the
formulae:
O
P-OH \S~
n ~ ~ ~ /S
O , OH or N \ .
n is an integer from 0 to 3;
R4 is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3-C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -L3-M3:
L3 indicates a linking or bridging group of the formulae -(CHZ)~ , -S-, -O-,
36
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
-C{O)-, -(CHZ)n-C(O)-, -(CHZ)~-C(O)-(CHZ)~-, -(CH2)n-O-(CHZ)~-, Or -(CHZ)~-S-
(CHZ}~-;
M; is selected from:
a} the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NOZ, -NHz, -CN, or -CF3; or
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, pyrazole, or tetrazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NHZ, -
CN, or -CF3;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyridine, pyrazine,
pyrimidine,
piperidine, piperazine, thiazine, or morpholine, the six-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,a alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NH2, -CN, -
CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, quinoline
or isoquinoline, the bicyclic ring moiety being optionally substituted by from
1 to 3
substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-
C,o alkoxy,
preferably C,-C6 alkoxy, -CHO, -NO2, -NHZ, -CN, -CF3 or -OH;
Rs is selected from C,-C6 lower alkyl, C,-C6 Lower alkoxy, -(CHz)n C3 C5
cycloalkyl,
-(CHZ)~ S-(CHz)~ C3 CS cycloalkyl, -(CHZ)p O-(CHZ)~ C3 CS cycloalkyl, or the
groups of:
a) -(CHZ)"phenyl-O-phenyl, -(CHZ)o phenyl-CHZ phenyl, -(CHZ)~ O-phenyl
CHz phenyl, -{CHZ)~ phenyl-(O-CHZ phenyl)2, -CH2 phenyl-C(O)-benzothiazole or
a moiety
of the formulae:
37
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
O
_Y O~Y ~(CH2)~Y ~(CH2)~S/Y
, , ,
O~(CH2)n~Y ~(Cl"~2)rr~0/Y ~(C~"~2)rr~S/ (CH2),~Y
> > >
/(CH2)r,~p~ (CH2),~Y
wherein n is an integer from 0 to 3, preferably 1 to 3, more preferably I to
2, Y is C3-CS
cycloalkyl, phenyl, benzyl, napthyl, pyridinyl, quinolyl, furyl, thienyl,
pyrrolyl,
benzothiazole, or pyrimidinyl, the rings of these groups being optionally
substituted by from 1
to 3 substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6
alkoxy, -NH2,
NOZ or a five membered heterocyclic ring containing one heteroatom selected
from N, S, or O,
preferably S or O; or
b) a moiety of the formulae -(CHZ)~-A, -(CHZ)~ S-A, or -(CHZ)~ O-A, wherein A
is the moiety:
D
C
B
wherein
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, -CF3 or -(CHZ)n CF3;
B and C are independently selected from phenyl, pyridinyl, pyrimidinyl, furyl,
thienyl
or pyrrolyl groups, each optionally substituted by from 1 to 3, preferably 1
to 2, substituents
selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NHz or -N02;
or a pharmaceutically acceptable salt thereof.
Another preferred group of this invention are those of the formulae:
38
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
or
R5
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NO2,
phenyl,
-O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the formulae:
~s ~s (s
R N\ /N N\ /~ N\
R' ~ R ~O~ R7
~ , ~ ,
Is
R~.S/N\
or O \O
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NHZ, -N02, -
CF3, or -OH;
R~ is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -N-(C,-
C6
alkyl)2, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, -O-phenyl, benzyl, -O-
benzyl, pyrazolyl
and thiazolyI, the rings of these groups being optionally substituted by from
1 to 3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NOz, -NH2, -CF3, or -OH;
RZ is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl, preferably
C,-
C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -NO2, -NH2, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)2, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
R3 is selected from -COOH, -C(O)-COOH, -(CH2)"C(O)-COOH, -(CHZ)"COOH,
-CH=CH-COOH, -(CHZ)"C(O)NS(O)(O)(C,-C6 lower alkyl), -(CHZ)NC(O)NS(O)(O)(C,-C6
lower haloalkyl),
39
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Ra
Rs Rs
Ra I-I Ra ~I N
~ Rs
N p I
~ ~J
, . Rio
R
O S'/ a O O~ a
i
N ~ ~ s ~ i ERs CH
N ~ ~ ( 2) OH
N/
Rio , Rio , O
R8
I~ Rs
(CH2)
N/ ~OH NH
O p O Rio
O S~ a O' Ra
O
N~ / Rs ~ ~ R
O H ~~~ ~~ ~~~ s
O
Rio , R1o , or
O
O ~~
-n(H2CT 'N~ ./Rs
N
p ~R11
Rg is selected from H, -COOH, -(CH2)o-COOH, -(CHZ)~ C(O)-COOH;
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)"COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2;
Rio is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CHZ)~ COOH,
-(CHz)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2,
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R8 Ra
O ~ /~ I ~ Rs
~ S ~ N I~~Rs
~N~
_O/
, or
(C1-C6 lower alkyl)
O /S O O O.S:O
"N ~(C~-Cs lower alkyl ~N / ~(C~-Cs Power haloalkyl; .
> >
R" is selected from H, C,-C6 lower alkyl, -CF3, -COOH, -(CHZ)n COOH,
-(CHZ}n C(O)-COOH, or
R8
I~~Rs
n is an integer from 0 to 3;
R4 is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o
cycloalkyl, -C,-C6 alkyl-C3 C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -LZ-MZ:
LZ indicates a linking or bridging group of the formulae -(CHZ)", -S-, -O-,
-C(O)-, -(CHZ}p C(O)-, -(CHZ)n C(O)-(CHz)a , -(CH2)~ O-(CHZ)n , or -(CHZ)n S-
(CH2)~ ;
MZ is selected from:
a) the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NOZ, -NHz, -CN, or -CF3; or
41
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, pyrazole, or tetrazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C~-C,o alkoxy, preferably C,-C6 alkoxy, -NO2, -NH2, -
CN, or -CF3;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyridine, pyrazine,
pyrimidine,
piperidine, piperazine, thiazine, or morpholine, the six-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,a alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NH2, -CN, -
CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, quinoline
or isoquinoline, the bicyclic ring moiety being optionally substituted by from
1 to 3
substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-
C,o alkoxy,
preferably C,-C6 alkoxy, -CHO, -NO2, -NH2, -CN, -CF3 or -OH;
R5 is selected from C,-Cb lower alkyl, C,-C6 lower alkoxy, -(CHZ)~ C3-CS
cycloalkyl
or --(CHZ)~ A, -(CHZ)o S-A, or -(CHZ)~ O-A wherein A is selected from:
,
42
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
, ,
\ D
\ N.
Ri2
N
N~ " R12
R~2
R > > or ;
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, or -CF3;
R,2 is H, C~-C6 lower alkyl, CI-C6 lower alkoxy, or -CF3;
or a pharmaceutically acceptable salt thereof.
The compounds of this invention have the following formulae:
43
CA 02322162 2000-08-24
WO 99/43654 PCT/I3S99/03898
Ra
or
R5
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, G,-C6 alkoxy, -NOz, -
NH2,
phenyl, -O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the formulae:
Is Is
R N\
R~ ~ R ~O~
O ~ O
Is
/O\/N\
R ~~
O
Rs
R~S/N\
R~/ ' R~/ N ' O ~O
Or ,
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NO2, -NH2, -
CF3, or -OH;
R~ is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl), -N-(C,-
Cb
alkyl)Z, pyridinyl, thienyl, furyl, pynrolyl, phenyl, pyrazolyl, thiazolyl, -O-
phenyl, benzyl or -
O-benzyl, the rings of these groups being optionally substituted by from 1 to
3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NO2, -NH2, -CF3, or -OH;
44
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rz is selected from H, halogen, -CN, -CHO, -CF3, -OH, C,-C,o alkyl, preferably
C,-
C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -CN, -N02, -NHS, -NH-
C,-C6
alkyl, -N(C,-C6 alkyl)z, -N-SOZ C,-C6 alkyl, or -SOZ C,-C6 alkyl;
R3 is selected from -COOH, -C(O)-COOH, -(CH2)n C(O)-COOH, -(CHZ)~ COOH,
-CH=CH-COOH, -(CHZ)~ tetrazole,
O
O.S
~N~ ~(C1-C6 lower alkyl) or
/S~ N~
N N
S
N" C -C lower al I
( t s kY ), or
0
Q ~~ OH
-~P-OH
O
or a moiety selected from the formulae -L'-M';
wherein L' is a bridging or linking moiety selected from a chemical bond, -
(CHZ)~ , -S-, -O-,
-C(O)-, -(CHZ)A C(O)-, -(CHZ)o C(O)-(CHZ)p-, -(CHz)~ O-(CH2)~ ,-(CHZ)~-S-
(CHZ)n ,
-C(Z}-N(R6)-, -C(Z)-N(R6)-(CHZ)", -C(O}-C(Z}-N(R6)-, -C(O}-C(Z)-N(R6)-(CHZ)~ ,
-C(Z)-NH-SOZ , or -C(Z)-NH-SOZ (CHZ)n ;
M' is selected from the group of -COOH, -(CHZ)"COOH, -(CHZ)~ C(O)-COOH,
tetrazole,
45
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rs
RB ~~
-Rs ,_..--Rs
-N N
S o , Rlo ,
R Rs S~~Ra O/R8 N ~ a
HN N /' ERs / ERs / ~ ~ R
/ s
Rio , Rio Rio
> >
S Ra
O N I'~~Rs
Q W
N~ -P-OH S
O Ri 1 , ~ O , ~ OOH or ~~ Rio
R8, in each appearance, is independently selected from H, -COOH, -(CHZ)n COOH,
-
(CHZ)~ C(O)-COOH, tetrazole,
O ~\
-~P-OH ~S~
i
O ~or ~ OOH
R9 is selected from H, halogen, -CF3, -OH, -COOH, -(CHZ)~ COOH,
-(CHz)o C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)z;
R,o is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CH2)~ COOH,
-(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)z,
46
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
O ~ ~ I~ Rs N I~ Rs
~N/S
_Z/
,
R8
~~~Rs
\Z/
or
/S ~
N N
S
N"(C1-C6 lower alkyl)
/S ~O ~O O'S ~O
N ~(C~-Cs lower alkyl; "N / ~(C~-Cs lower haloaikyi; .
R" is selected from H, C,-C6 lower alkyl, C,-C6 cycloalkyl, -CF3, -COOH, -
(CHZ)~
COOH, -(CHZ)n C(O)-COOH,
Ra Re
N~I~~Rs ~~~Rs
- (CH2)n
with a proviso that the complete moiety at the indole or indoline 3-position
created by
any combination of R3, L', M', Rg, Rg, R,o, and/or R" shall contain at least
one acidic moiety
selected from or containing a carboxylic acid, a tetrazole, or a moiety of the
formulae:
O
-P-OH \S~
n ~ ~ ~ /S
O , OH or N ~.
n is an integer from 0 to 3;
47
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
R4 is selected from H, -CFA, C,-C6 lower alkyl, C,-Cb lower alkoxy, C;-C,o
cycloalkyl, -C,-C6 alkyl-C3 C,o cycloalkyl, -CHO, halogen, or a moiety of the
formula -LZ-M2:
Lz indicates a linking or bridging group of the formulae -(CHZ)~-, -S-, -O-,
-C(O)-, -(CHZ)~ C(O)-, -(CHz)~-C(O)-(CHZ)n-, -(CHZ)n-O-(CHZ)~-, or -(CHZ)"S-
(CHZ)n , -
C(O)C(O)X;
where X is O or N,
MZ is selected from:
a) the group of C,-C6 lower alkyl, C,-C6 lower alkoxy, C3-C,o cycloalkyl,
phenyl or benzyl, the cycloalkyl, phenyl or benzyl rings being optionally
substituted by from 1
to 3 substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl,
C,-C,o alkoxy,
preferably C,-C6 alkoxy, -NO2, -NH2, -CN, or -CF3; or
b) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, pyrrolidine, pyrazole, or tetrazole, the five-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -NOZ, -NH2, -
CN, or -CF3;
or
c) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyridine, pyrazine,
pyrimidine,
piperidine, piperazine, thiazine, or morpholine, the six-membered heterocyclic
ring being
optionally substituted by from 1 to 3 substituents selected from halogen, C,-
C,o alkyl,
preferably C,-C6 alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -NO2, -
NH2, -CN, -
CF3 or -OH; or
d) a bicyclic ring moiety containing from 8 to 10 ring atoms and optionally
containing from 1 to 3 ring heteroatoms selected from N, S or O including, but
not limited to
benzofuran, chromene, indole, isoindole, indoline, isoindoline, napthalene,
purine, quinoline
or isoquinoline, the bicyciic ring moiety being optionally substituted by from
1 to 3
substituents selected from halogen, C,-C,o alkyl, preferably C,-C6 alkyl, C,-
C,o alkoxy,
preferably C,-C6 alkoxy, -CHO, -NOz, -NH2, -CN, -CF3 or -OH;
48
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
RS is selected from -(CHZ)n S-(CHZ)~ C3 CS cycloalkyl, -(CHZ)~ O-(CHZ)o-C3-CS
cycloalkyl, or the groups of:
a) -(CHZ)~ phenyl-O-phenyl, -(CHZ}~ phenyl-CHZ phenyl, -(CHZ)n O-phenyl
CHZ phenyl, -(CHz)~ phenyl-(O-CHz phenyl)Z, -CHZ phenyl-C(O)-benzothiazole or
a moiety
of the formulae:
O
Y ~O/Y ~(CH2)~S/Y
, ,
O
~O~(CH2)n~Y ~(CE"~2)r~0/Y ~(C~'d2)rr~s~ (C~..~2)r~
Y
/(CH2)~O~ (CH2>~Y
wherein n is an integer from 0 to 3, preferably 1 to 3, more preferably 1 to
2, Y is C3 CS
cycloalkyl, phenyl, benzyl, napthyl, pyridinyl, quinolyl, furyl, thienyl,
pyrrolyl,
benzothiazole or pyrimidinyl, the rings of these groups being optionally
substituted by from 1
to 3 substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6
alkoxy, -NO2, -
NHZ or a five membered heterocyclic ring containing one heteroatom selected
from N, S, or O,
preferably S or O; or
~ (CH2)~
b) a moiety of the formula Y wherein n is an integer from 0 to 3 ,
preferably 1 to 3, more preferably 1 to 2, Y is napthyl, pyridinyl, quinolyl,
furyl, thienyl,
pyn:olyl benzothiazole, or pyrimidinyl, the rings of these groups being
optionally substituted
by from 1 to 3 substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl,
C,-C6 alkoxy,
-NH2, -NOZOr a five membered heterocyclic ring containing one heteroatom
selected from N,
S, or O, preferably S or O; or
c) a moiety of the formulae -(CHZ}p-A, -(CHZ)~ S-A, or -(CHZ)"O-A, wherein A
is the moiety:
D
C
B
wherein
49
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
D is H, C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHZ)~-CFA or -CFA;
B and C are independently selected from phenyl, pyridinyl, pyrimidinyl, furyl,
thienyl
or pyrrolyl groups, each optionally substituted by from 1 to 3, preferably 1
to 2, substituents
selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NHz or -NOZ;
or a pharmaceutically acceptable salt thereof.
In a further preferred group within the subgenus above, R, is benzyloxy and
R4, R3
and RS are as defined above.
Yet another preferred group herein are the compounds of the formulae:
R1 R
R4
or
wherein:
R, is selected form H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NO2, -
NH2,
phenyl, -O-phenyl, benzyl, -O-benzyl, -S-benzyl or a moiety of the formulae:
fs
O ~ R~ /N\
R ~O~ R~~ ~ R~/ N
or O O ,
Is Is Is
R N\ /N N\ /O
R~ ~ R~
O , O ,or O ;
R6 is selected from H, C,-C6 alkyl, C,-C6 alkoxy, phenyl, -O-phenyl, benzyl, -
O-
benzyl, the phenyl and benzyl rings of these groups being optionally
substituted by from 1 to 3
substituents selected from halogen, Cl-C6 alkyl, C,-C6 alkoxy, -NH2, -N02, -
CF3, or -OH;
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S R~ is selected from -CF3, C,-C6 alkyl, C,-C6 alkoxy, -NH-(C,-C6 alkyl}, -N-
(C,-C6
alkyl)2, pyridinyl, thienyl, furyl, pyrrolyl, phenyl, -O-phenyl, benzyl, -O-
benzyl, pyrazolyl or
thiazolyl, the rings of these groups being optionally substituted by from 1 to
3 substituents
selected from halogen, C,-C6 alkyl, C,-C6 alkoxy, -NH2, -N02, -CF3, or -OH;
R3 is selected from -COOH, -C(O)-COOH, -(CHZ)~ C(O)-COOH, -(CHZ)~ COOH,
-CH=CH-COOH, -(CHz)nC(O)NS(O)(O)(C,-C6 lower alkyl), -(CHZ)NC(O)NS(O)(O)(C,-C6
lower haloalkyl),
R9
R9 N ~~I-
R
I /\N
N
R9 O S '/Rs
'N
Ra~ ~ _Rs
~N ~N~' / Rs
O i
1$ , 10 _ R10
O O ~Re
O
N ~\ ~~Rs (CH2) OH
Rio N
R8
(~ Rs
(CH2}
N~ OH NH
O ~ O Rio
51
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
O S /R8 O'/Rs
/O
,R ~
O H ~~~ s O ~H~~~~ Rs
Rlo Rio or
S O
O ,~ O'g' ~ ~~.,
-n(H2CT 'N/ ~'Rs
N
O ~R> >
> >
RB and R9 are independently selected from H, halogen, -CF3, -OH, -COOH, -
(CHz)o
COOH, -(CHZ)~ C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), or -
N(C,-C6
alkyl)2;
R,o is selected from the group of H, halogen, -CF3, -OH, -COOH, -(CHZ)n COOH,
-(CHZ)o C(O)-COOH, -C,-C6 alkyl, -O-C,-C6 alkyl, -NH(C,-C6 alkyl), -N(C,-C6
alkyl)2,
Rs Re
~S~ (~~Rs N I~~Rs
N
_O/
or
/S~
N N
S
N"(C1-Cs lowerai I
kY )
O /S O O O.S.O
"N ~(C1-Cs lower alkyl "N / ~(C~-C6 lower haloalkyi; .
> >
R" is selected from H, C~-C6 lower alkyl, -CF3, -COOH, -(CHZ)n COOH,
-(CHZ)~ C(O)-COOH, or
52
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Rg
~~~Rs
n is an integer from 0 to 3;
R4 is selected from H, -CF3, C,-C6 lower alkyl, C,-C6 lower alkoxy, or
halogen;
RS is selected from C,-C6 lower alkyl, C,-C6 lower alkoxy, -(CHZ)~-C3-CS
cycloalkyl
or the groups of:
a) -C(O)-O-(CHZ)~ C3-CS cycloalkyl, -(CH2)~ phenyl, -(CHZ)~ S-phenyl, -
(CHZ)~ phenyl-O-phenyl, -(CHZ)p phenyl-CHZ phenyl, -(CHZ)~-O-phenyl-CH2-
phenyl, -
(CHZ)n phenyl-(O-CHZ-phenyl)2, -C(O)-O-phenyl, -C(O)-O-benzyl, -C(O)-O-
pyridinyl,
C(O)-O-napthyl, -(CHZ)n-S-napthyl, -(CHZ)"S-pyridinyl, -(CHZ)o pyridinyl or -
(CHZ)~
napthyl, the phenyl, pyridinyl and napthyl rings of these groups being
optionally substituted by
from 1 to 3 substituents selected from H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-
C6 alkoxy,
NHZ, or -NOZ; or
b) a moiety of the formula -(CHZ)~ A, -(CHZ)"S-A, or -(CHZ)~ O-A, wherein A is
the moiety:
D
C
B
wherein
D is H, C,-C6 lower alkyl, C~-C6 lower alkoxy, or -CF3;
B and C are independently selected from phenyl, pyridinyl, furyl, thienyl or
pyrrolyl
groups, each optionally substituted by from 1 to 3, preferably 1 to 2,
substituents selected from
H, halogen, -CF3, -OH, -C,-C6 alkyl, C,-C6 alkoxy, -NH2, or -NO2;
or a pharmaceutically acceptable salt thereof.
Detailed Description of the Invention
53
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
As used herein, the terms "aryl" and "substituted aryl" are understood to
include
monocyclic, particularly including five- and six-membered monocyclic, aromatic
and
heteroaromatic ring moieties and bicyclic aromatic and heteroaromatic ring
moieties,
particularly including those having from 9 to 10 ring atoms. Among these aryl
groups are
understood to be phenyl rings, including those found in phenoxy, benzyl,
benzyloxy, biphenyl
and other such moieties. The aryl and heteroaryl groups of this invention also
include the
following:
a) a five-membered heterocyclic ring containing one or two ring heteroatoms
selected from N, S or O including, but not limited to, furan, pyrrole,
thiophene, imidazole,
pyrazole, isothiazole, isoxazole, pyrrolidine, pyrroline, imidazolidine,
pyrazolidine, pyrazole,
pyrazoline, imidazole, tetrazole, or oxathiazole; or
b) a six-membered heterocyclic ring containing one, two or three ring
heteroatoms
selected from N, S or O including, but not limited to, pyran, pyridine,
pyrazine, pyrimidine,
pyridazine, piperidine, piperazine, tetrazine, thiazine, thiadizine, oxazine,
or morpholine; or
c) a bicyclic ring moiety optionally containing from 1 to 3 ring heteroatoms
selected from N, S or O including, but not limited to benzofuran, chromene,
indole, isoindole,
indoline, isoindoline, napthalene, purine, indolizine, indazole, quinoline,
isoquinoline,
quinolizine, quinazoline, cinnoline, phthalazine, or napthyridine.
The "substituted aryl" groups of this invention include such moieties being
optionally
substituted by from 1 to 3 substituents selected from halogen, C,-C,o alkyl,
preferably C,-Cb
alkyl, C,-C,o alkoxy, preferably C,-C6 alkoxy, -CHO, -COOH or esters thereof, -
NOZ, -NH2,
-CN, -CF3 or -OH or combinations thereof, such as -CHZCF3, -NH(CH3), etc.
A preferred subset of these groups, optionally substituted as just described,
include
moieties formed from benzene, pyridine, napthylene or quinoline rings. A
further preferred
group includes those of furan, pyrrole, thiophene, pyrimidine, and morpholine
rings. A
preferred group of bicyclic aromatic groups includes benzofuran, indole,
napthalene, and
quinoline rings.
The alkyl, alkenyl and alkinyl groups referred to herein indicate such groups
having
from 1 to 10, preferably 1 to 6 carbon atoms, and may be straight, branched or
cyclic. Unless
indicated otherwise, it is preferred that these groups be straight or
branched. Halogens herein
are understood to include F, CI, Br and I.
54
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
As used herein, "phospholipase enzyme activity" means positive activity in an
assay for
metabolism of phospholipids (preferably one of the assays described in Example
116 below).
A compound has "phospholipase enzyme inhibiting activity" when it inhibits the
activity of a
phospholipase (preferably cPLA2) in any available assay (preferably an assay
described below
in Example 116 or Example 117) for enzyme activity. In preferred embodiments,
a compound
has ( 1 ) an ICso value of less than about 25 N.M, preferably less than about
6 ~.~M, in the
LysoPC assay; (2) an ICso value of less than about 50 E1M in the vesicle
assay; (3) an ICso
value of less than about 1 pNi in the PMN assay; (4) an ICS, value of less
than about 15 ErM in
the Coumarine assay; and/or (5) measurable activity (preferably at least about
5% reduction in
edema, more preferably at least about 10% reduction, more preferably at least
about 15%, most
preferably 20-30%) in the rat carrageenan-induced footpad edema test.
Compounds of the present invention are useful for inhibiting phospholipase
enzyme
(preferably cPLA2) activity and, therefore, are useful in "treating" (i.e.,
treating, preventing or
ameliorating) inflammatory or inflammation-related responses or conditions
(e.g., rheumatoid
arthritis, psoriasis, asthma, inflammatory bowel disease, and other diseases
mediated by
prostaglandins, leukotrienes or PAF) and other conditions, such as
osteoporosis, colitis,
myelogenous leukemia, diabetes, wasting and atherosclerosis.
The present invention encompasses both pharmaceutical compositions and
therapeutic
methods of treatment or use which employ compounds of the present invention.
Compounds of the present invention may be used in a pharmaceutical composition
when combined with a pharmaceutically acceptable carrier. Such a composition
may also
contain (in addition to a compound or compounds of the present invention and a
carrier)
diluents, fillers, salts, buffers, stabilizers, soIubilizers, and other
materials well known in the
art. The term "pharmaceutically acceptable" means a non-toxic material that
does not interfere
with the effectiveness of the biological activity of the active ingredient(s).
The characteristics
of the carrier will depend on the route of administration. The pharmaceutical
composition may
further contain other anti-inflammatory agents. Such additional factors and/or
agents may be
included in the pharmaceutical composition to produce a synergistic effect
with compounds of
the present invention, or to minimize side effects caused by the compound of
the present
invention.
The pharmaceutical composition of the invention may be in the form of a
liposome in
which compounds of the present invention are combined, in addition to other
pharmaceutically
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
acceptable Garners, with amphipathic agents such as lipids which exist in
aggregated form as
micelles, insoluble monolayers, liquid crystals, or lamellar layers in aqueous
solution. Suitable
lipids for liposomal formulation include, without limitation, monoglycerides,
diglycerides,
sulfatides, lysolecithin, phospholipids, saponin, bile acids, and the like.
Preparation of such
liposomal formulations is within the level of skill in the art, as disclosed,
for example, in U.S.
Patent No. 4,235,871; U.S. Patent No. 4,501,728; U.S. Patent No. 4,837,028;
and U.S.
Patent No. 4,737,323, all of which are incorporated herein by reference.
As used herein, the term "therapeutically effective amount" means the total
amount of
each active component of the pharmaceutical composition or method that is
sufficient to show a
meaningful patient benefit, i.e., treatment, healing, prevention or
amelioration of an
inflammatory response or condition, or an increase in rate of treatment,
healing, prevention or
amelioration of such conditions. When applied to an individual active
ingredient, administered
alone, the term refers to that ingredient alone. When applied to a
combination, the term refers
to combined amounts of the active ingredients that result in the therapeutic
effect, whether
administered in combination, serially or simultaneously.
In practicing the method of treatment or use of the present invention, a
therapeutically
effective amount of a compound of the present invention is administered to a
mammal having a
condition to be treated. Compounds of the present invention may be
administered in
accordance with the method of the invention either alone or in combination
with other therapies
such as treatments employing other anti-inflammatory agents, cytokines,
lymphokines or other
hematopoietic factors. When co-administered with one or more other anti-
inflammatory
agents, cytokines, lymphokines or other hematopoietic factors, compounds of
the present
invention may be administered either simultaneously with the other anti-
inflammatory agent(s),
cytokine(s), lymphokine(s), other hematopoietic factor(s), thrombolytic or
anti-thrombotic
factors, or sequentially. If administered sequentially, the attending
physician will decide on the
appropriate sequence of administering compounds of the present invention in
combination with
other anti-inflammatory agent(s), cytokine(s), lymphokine(s), other
hematopoietic factor(s),
thrombolytic or anti-thrombotic factors.
Administration of compounds of the present invention used in the
pharmaceutical
composition or to practice the method of the present invention can be carried
out in a variety of
conventional ways, such as oral ingestion, inhalation, or cutaneous,
subcutaneous, or
intravenous injection.
56
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
When a therapeutically effective amount of compounds of the present invention
is
administered orally, compounds of the present invention will be in the form of
a tablet,
capsule, powder, solution or elixir. When administered in tablet form, the
pharmaceutical
composition of the invention may additionally contain a solid carrier such as
a gelatin or an
adjuvant. The tablet, capsule, and powder contain from about 5 to 95% compound
of the
present invention, and preferably from about 25 to 90% compound of the present
invention.
When administered in liquid form, a liquid carrier such as water, petroleum,
oils of animal or
plant origin such as peanut oil, mineral oil, soybean oil, or sesame oil, or
synthetic oils may be
added. The liquid form of the pharmaceutical composition may further contain
physiological
saline solution, dextrose or other saccharide solution, or glycols such as
ethylene glycol,
propylene glycol or polyethylene glycol. When administered in liquid form, the
pharmaceutical
composition contains from about 0.5 to 90% by weight of compound of the
present invention,
and preferably from about 1 to 50% compound of the present invention.
When a therapeutically effective amount of compounds of the present invention
is
administered by intravenous, cutaneous or subcutaneous injection, compounds of
the present
invention will be in the form of a pyrogen-free, parenterally acceptable
aqueous solution. The
preparation of such parenterally acceptable protein solutions, having due
regard to pH,
isotonicity, stability, and the like, is within the skill in the art. A
preferred pharmaceutical
composition for intravenous, cutaneous, or subcutaneous injection should
contain, in addition
to compounds of the present invention, an isotonic vehicle such as Sodium
Chloride Injection,
Ringer's Injection, Dextrose Injection, Dextrose and Sodium Chloride
Injection, Lactated
Ringer's Injection, or other vehicle as known in the art. The pharmaceutical
composition of the
present invention may also contain stabilizers, preservatives, buffers,
antioxidants, or other
additives known to those of skill in the art.
The amount of compounds) of the present invention in the pharmaceutical
composition
of the present invention will depend upon the nature and severity of the
condition being treated,
and on the nature of prior treatments which the patient has undergone.
Ultimately, the
attending physician will decide the amount of compound of the present
invention with which to
treat each individual patient. Initially, the attending physician will
administer low doses of
compound of the present invention and observe the patient's response. Larger
doses of
compounds of the present invention may be administered until the optimal
therapeutic effect is
obtained for the patient, and at that point the dosage is not increased
further. It is contemplated
that the various pharmaceutical compositions used to practice the method of
the present
invention should contain about 0.1 pg to about 100 mg (preferably about .1 mg
to about 50
57
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
mg, more preferably about 1 mg to about 2 mg) of compound of the present
invention per kg
body weight.
The duration of intravenous therapy using the pharmaceutical composition of
the
present invention will vary, depending on the severity of the disease being
treated and the
condition and potential idiosyncratic response of each individual patient. It
is contemplated that
the duration of each application of the compounds of the present invention
will be in the range
of 12 to 24 hours of continuous intravenous administration. Ultimately the
attending physician
will decide on the appropriate duration of intravenous therapy using the
pharmaceutical
composition of the present invention.
Compounds of the present invention invention can be made according to the
methods
and examples described below. Synthesis of preferred compounds of the present
invention are
described in the examples below.
Method A
The indole may be alkylated at the c-3 position with the appropriate alkyl
bromide and
treatment with a lewis acid such as silver(I)oxide or silver tetrafluoroborate
in a solvent such as
dioxane or THF at elevated temperatures of 50 °C - 100 °C.
Alternatively it may be alkylated in
a two step procedure by treatment of the indole with n-BuLi in a solvent such
as THF or ether
followed by ZnCl2 and then concentrated and treated with the appropriate
alkylating agent in a
variety of solvents such as THF, ether, toluene or benzene. The indole
nitrogen may then be
alkylated by treatment with a strong base such as sodium
bis(trimethylsilyl)amide, n-BuLi,
sodium hydride or potassium hydride in a solvent such as DMF, DMSO or THF
followed by
exposure to the appropriate alkyl halide. The ester can be hydrolyzed under
basic conditions
with sodium hydroxide in water and methanol and THF. Alternatively it may be
cleaved by
treatment with sodium thiomethoxide in a solvent such as THF or DMF at
elevated
temperatures (50 °C - 100 °C). The product acid by be coupled to
a sulfonamide by the agency
of a variety of coupling reagents such as DCC, EDCI or carbonyl diimidazole in
a solvent such
as THF, methylene chloride, dichloroethane or DMF in the presence of a base
such as triethyI
amine and/or N, N-dimethyl pyridine. In the case of Rl = nitro the nitro group
can be reduced
by exposure to Pt/C in the presence of hydrogen in a solvent such as methanol,
ethyl acetate or
THF. The resulting amine can be acylated or sulfonylated by exposure to the
appropriate agent
in the presence of a base such as triethyl amine, sodium bicarbonate or
pyridine in a biphasic
solvent system such as methylene chloride:water ( 1:1 ) or THF:water ( 1:1 )
or a monophasic
organic solvent such as methylene chloride, THF or DMF with triethylamine. The
resulting
58
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S acid may then be hydrolyzed and modified as described above. Also in the
case R 1 = Br, it
may be replaced with the copper salt of the desired nucleophile such as
thiomethoxide,
methoxide or sulphinic acid.
Method A
Ag(I)O R ~ COZMe
Ri..-I \ Dioxane ~ ~ NaH
60 °C r ~ DMF
H R ~ COZMe R~-I ~> R3-Br
N
H
Br
NaOH
MeOH
THF
R
Pt/C CHZCIz
Hz EDCI
THF DMAP
R4SOZNHz
COZMe
R
59
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
RSCI ~
H20 H v NaOH
NaHC03 Rsi ~ MeOH
CH2C12 ~~ THF
CHZC12
EDCI
DMAP
~R4 R4S02NH2
'O
Ri R2 R3 Ra R5 R6
~yl, vitro, halogen H, Me0 ~i 1, alkenyl, aryl ~.yl alkyl ureamate methoxgen,
5,6-methylenedioxy heterocyclic ~kyl amide y
methoxy aryl amide
sulfonamide
Method B
The indoleglyoxalyl chloride may be reacted with the desired amino ester in a
biphasic system
with methylene chloride and saturated sodium bicarbonate or in a monophasic
system with a
solvent such as methylene chloride, ethyl acetate or THF and a base such as
triethylamine,
Hunigs base or pyridine. The indole nitrogen may then be alkylated with a
variety of alkylating
reagents in a solvent such as DMF, DMSO or THF and a base such as sodium
hydride, n-BuLi
or potassium bis(trimethylsilyl)amide. The ester may then be hydrolyzed with
sodium
hydroxide or lithium hydroxide in a solvent system such as water:methanol:THF.
CA 02322162 2000-08-24
WO 99/43654 PC'T/US99103898
Method B
1
O CH2C12 \ I NaH
\ Sat'd bicarb \ \O ~ DMF
/ \
/ ~ O R~Br
HZ \ I H
O
NaOH \ I
\ I MeOH OH
THF \ O
\ \ ~O O ~ ~ / O
/ R~ R1
alkyl, aryl
Method C
The 3-carboxyindole is elaborated via reductive amination by allowing the
aldehyde to
condense with an amino ester in a solvent such as methylene chloride or
dichloromethane with
or without acetic acid. The resulting imine is reduced in-situ with a reducing
agent such as
sodium borohydride, sodium cyanoborohydride or sodium triacetoxyborohydride.
The acid is
then prepared by hydrolysis of the resulting ester with sodium hydroxide or
lithium hydroxide
in a solvent system such as water:methanol:THF.
61
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Method C
1)NaBH(OAc)3
acetic acid
CH2C1CH2Cl
:
2)NaOH OR t
MeOH
THF
OR t
OR ~ H~ / ' N
ORt
R1 = alkyl
N
R'
CN O
OH
OR t H
..1~5
Method D
5-benzyloxyindole may be treated with a base such at methyl or ethyl grignard
and acylated at
the 3-position with ethyloxychloride in a suitable solvent such at ether or
THF. The indole
nitrogen may then be alkylated with a benzylbromide by the action of a base
such as sodium
hydride or n-butyllithium in a solvent such a THF or DMF. The ester is then
hydrolysed under
basic conditions with sodium hydroxide or tetrabutylammonium hydroxide in a
suitable solvent
system such at water:MeOH:THF. Coupling of the appropriate aminoester may then
be effected
by the use of a coupling agent such as DCC or EDCI in a solvent such as
methylenechloride,
THF or DMF. The target acid may the be revealed by hydrolysis of the ester
under the same
conditions discussed above.
62
CA 02322162 2000-08-24
WO 99!43654 PCT/US99/03898
Method D
1/
1 )EtMgBr
Et2O l )NaH DMF
2)ethyloxychlori Z)R ~Br
K'
NaOH
THF, MeOH
THF
MeOH'
Method E
EDC1, DMF
aminoester
R~ =aryl
RZ =H, aryl, C02H
Indole-3-acetic acid was alkylated with an appropriate alkyl bromide which was
then subjected
to Suzuki coupling conditions using Pd(PPh3)4 as a catalyst in a mixed solvent
(ethanol-
benzene-water) at elevated temperature to give the 1-alkyl-5-substituted
indole.
63
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
Method E
Br ~ COOH Br ~COOH R' COOH
/~. ~ RsBr / N, R B(OH)z /
'Pr2NEt Rs (C6Hs)aPd Rs
I II Na2C03 III
Rs R1
Br-~-
1/
i
CF3
\ \
I\
i
Method F
Alkylation of the nitrogen atom of I with a suitable base such a sodium
hydride or potassium
carbonate and an alkyl halide gave the aldehyde II. The aldehyde could be
transformed to the
thiazolidinedione III using a base such as piperdine and isolated with an acid
such as acetic
acid. Deprotonation with a suitable base such as sodium hydride and alkylation
on the nitrogen
atom of the thiazolidinedione with selected electrophiles such as alkyl or
benzyl halides
provided compounds such as IV.
64
CA 02322162 2000-08-24
WO 99/43654 PCT/EJS99/03898
Method F
0 0
NaH, DMF ~ \
/ H / ~ ~ / ~ /
0
0
~o
piperidine
NaH
R-Br
DMF
R ~COZH or
$ / COZH
Method G
The nitro-indole I was converted to the unsaturated ester via a Horner-Wittig
reaction with
trimethoxyphosphonoacetate in a suitable solvent such.as tetrahydrofuran.
Reduction of the
nitro group of II can be accomplished via hydrogenation with palladium on
carbon in the
presence of hydrogen and acylation of the resulting amine under Schotten-
Bowmann
conditions to give amides such as III. Saponification of the ester function
gave the acid-indole
IV.
65
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
Method G
trimethoxyphosphonoacetate
1 ) HZ, Pd-C, THF
2) NaHC03 Aqueous
CHZC12
C1
NaOH
THF
MeOH
Method H
5-Chloro-2-methylindole could be reductively alkylated at the 3-position with
a suitable
aldehyde in the presence of an acid such as trifluoroacetic acid and a
reducing agent such as
triethylsilane in a suitable solvent such as methylene chloride to give the
ester II. The nitrogen
atom could be alkylated by treatment with a suitable base such as sodium
hydride and diphenyl
bromo methane and the resulting compound III could be saponified to give IV.
66
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Method H
Ci ~ ~ ~ OHC ~ ~ COZMe / COZMe
~ '~ Me
C1
-Me
I TFA, Et3SiH, CHyCIz O°C ~ H II
NaH
R-Br
NaOH
MeOH
THF
Method I
The starting indole is C3 functionalized by either reaction of DMF/POC13 or by
reacting the
magnesium salt of the indole with methyl oxalyl chloride. The resulting esters
and aldehydes
were then Nalkylated by treating the salt of the indole, generated by treating
the indole with a
strong base, with a variety of alkyl halides. In th case of the aldehydes,
when r' is a nitro
group, the nitro is reduced to the amine using Pt/C and H2 or copper
acetate/sodium
borohydride and then acylated usind various acid chlorides, isocyanates,
chloroformates or
reductively alkylated using aldehydes and sodium triacetoxyborohydride. These
aldehydes
could then be oxidised to the desired acid which could be coupled to an amino
alkyl or aryl
esters by an EDCI coupling method or by first transforming the acid into the
acid chloride
under the action of oxalyl chloride and the reacting this with an amino alkyl
or aryl ester. These
were then hydrolyzed to yield the final product. The esters generated above
could be treated in
a similar fashion. The ester could hydrolyzed and then coupled to an amino
alkyl or aryl esters
by an EDCI coupling method or by first transforming the acid into the acid
chloride under the
action of oxalyl chloride and the reacting this with an amino alkyl or aryl
ester. These were then
hydrolyzed to yield the final product.
67
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Method I(a)
H H
,
R / I \ DMF/POCl3 / \ NaH/DMF /
R' \ ~ R X R,-
H H \ N
R"
R'=NOy
I ) HZ/Pt OH
2)R"""COC! / \
R'-
3) NAHP04/tBuOH \ ~ N
NaO2C1 R~~
1 ) C1COCOC1/NR"'COzR""
or
EDCI/NR"'COZR""
2) NaOH/THF/MeOH
O
~R~''~
N pH
/
R'
\ N
1
R"
Method I(b)
o-
/
R'- I \ I) R""'MgX/CH30CCOC1 / O NaH/DMF
\ H R' \ I ~ R"~
N
H
R'=N02 H
\ I ) HZ/Pt O
/ \ 'O 2)R' ""COCI /
R' ~ ~ 3)NaOHft'HF/MeOH R' I \
N
R~~ \ R"
1) C1COCOC1/NR"'COZR""
or
EDC1/NR"'C02R""
R'
2) NaOH/fHF/MeOH
Method J
The starting amine was treated with various sulfonyl chlorides in the presence
of pyridiine and
then the excess sulfonylchloride was scavenged by adding a polymer bound
amine. The
68
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
desired products where then hydrolyzed using sodium hydroxide in THF/MeOH and
the
reaction was aidified using IR-120 resin to yield the desired products.
Method J
H R
1 ) NaOH/THF/MeOH
2) Amberlite IR120
Method K
l) RSOzCI/Pyridine
NHy
2) ~NHz
R
The starting indole was bis alkylated by the addition of a strong base such as
sodium hydride
and then an alkylating agent such as an alkyl or aryl halide followed by the
hydrolysis of the
resulting ester with sodium hydroxide in THF/MeOH. The acid was then coupled
with an alkyl
or aryl amino ester and then hydrolyzed to yield the desired acid.
Method K
COZH COZH
R' / I ~ 1 ) NaH/R"X _"X _ R' /
y;~ 2) NaOH/THF/MeOH ~ N
H 1
R"
1 ) C1COCOCI/NR"'C02R""
R, ~ I ~ ~-OH
EDCI/NR"'COZR"" ~ O
2) NaOHITHF/MeOH
R"
ss
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
exam lp a 1
4-f (5-I f f cvclopentyloxylcarbon~llamino)-1-progyl-1 H-indol-3-vl)meth~l-3-
methoxybenzoic acid
Step 1 - To a solution of 5-nitro indole (21.24 g, 131 mmol} in dioxane (128
mL) in a
reaction vessel wrapped in aluminum foil is added silver(I)oxide (30.34 g, 131
mmoL, 1.5 eq)
and methyl 4-(bromomethyl)-3-methoxy-benzoate (34 g, 131 mmol) and the mixture
is brought
to 60 °C and stirred 20 h. The reaction is cooled, filtered through
celite, taken up in ethyl
acetate {500 mL), washed with brine (2 X 50 mL), dried (MgS04) and filtered.
The crude
material was purified by silica chromatography ( 15% ethyl acetate / hexanes)
to afford the
desired product (5.8 g, 55%).
Step 2 - The C3-alkylated indole ( 1.5 g, 4.4 mmol) was dissolved with 15 mL
THF. In
a separate flask, NaH (185 g, 4.61 mmol) was suspended with 25 mL THF at 0
°C. The
solution of starting material was cannulated into the NaH suspension, giving a
deep red
solution. This was then allowed to stir at room temperature for 10 minutes. 1-
iodopropane was
added (0.47 mL, 1.1 mmol) and the reaction was allowed to pr°ceed
overnight at room
temperature. As the reaction was not complete (TLC) and additional 0.5 mL of 1-
iodopropane
was added and the reaction continued for another 3 h. There was no change in
the TLC and the
reaction was poured into cold 1 N HCl and extracted with CHZC12 (3 X 75 mL).
The combined
organic layers were dried over MgS04, filtered and evaporated to yield the
crude N-alkylated
nitroindole. The crude material was absorbed onto silica and loaded onto a
silica gel column.
The column was eluted with 100% CH2C12 to give the pure yellow N-alkylated
nitroindole
(0.96 g, 57%).
Step 3 - The N-alkylated nitroindole (0.95 g) was dissolved with 40 mL
anhydrous
THF. The system was purged with argon. To the clear, yellow solution, Pt/C
(0.462 g) was
added. The argon was then removed by evacuation and hydrogen was introduced to
the
system. The reaction was stirred 6.5 h. The hydrogen was evacuated and argon
was then
purged through the system. The reaction mixture was filtered through celite
with THF. The
solvent was removed by rotary evaporation to give the crude amine as a dark
oil.
Chromatography (5% ethyl acetate/CH2C12) afforded the desired product (0.7 g,
80%)
Step 4 - The amine from above (0.7 g) was dissolved in 40 mL CHZCl2. 4-
methylmorpholine (0.3 mL, 3.0 mmol) and cyclopentyl chloroformate (383 mg,
2.57 mmol)
were then added to give a yellow/orange solution. The reaction was allowed to
proceed at room
temperature for 3 h. The reaction mixture was acidified with 1 N HCl and the
mixture was
extracted with 50 mL CHZC12. The combined organic phases were washed with
brine, dried
over MgS04, filtered and concentrated to give the crude carbamate. The crude
product was
absorbed onto silica gel and loaded onto a silica gel column. The column was
eluted with 100%
CHZCIz to afford the desired product (0.87 g, 39%) as a yellow foam.
CA 02322162 2000-08-24
WO 99/43654 PCTlUS99/03898
Step 5 - The carbamate (0.831 g) was dissolved with hydrolysis solution (2:1:1
THF:MeOH:2N NaOH) and the reaction was allowed to proceed for 5.25 h. The
reaction was
acidified to pH 2 with 2N HCl and extracted with CHZCl2. The organic layer was
washed with
water and brine. The combined organic layers were then dried over MgS04,
filtered and
evaporated to yield the crude acid, which was recrystallized from CHZC12 to
afford the title
compound (0.575 g, 71 %) as pink crystals.
MS: m/z (M-1) 449
Example 2
Cyclopentyl N-~3-f2-method-4-(~(f(2-methylpheny)sulfonyllamino}carbonyl)
Benz lx 1-1-nronvl-1H-indol-5-vl]~carbamate
Step 1 - The intermediate 5-vitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example 1, step 3,
using the above
intermediate.
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acylating agent.
Step 4 - The title compound is prepared as in Example 1, step 5, using the
above intermediate.
example 3
4-I(1-benzhydr_yl-5lff(cyclonentvloxy]carbonyllaminol-1H-indol~3-
yllmethyl]-3-methoxvbenzoic acid
Step 1 - The intermediate 5-vitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example 1, step 3,
using the above
intermediate.
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acylating agent.
Step 4 - The title compound is prepared as in Example 1, step 5, using the
above intermediate.
Example 4
4-~ f 5-1 f (cyclopentyloxylcarbonyllamino}-1-(2-na~hthylmetl~l)-1H-indol-3-
yllmethyll-3-methoxybenzoic acid
Step 1 - The intermediate 5-vitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example 1, step 3,
using the above
intermediate.
71
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acylating agent.
Step 4 - The title compound is prepared as in Example 1, step 5, using the
above intermediate.
MS : m/z (M-1 ) 547
Example 5
4-1 f 5-1 f (cvclouentvloxv)carbonvllaminol-1-(cvcIoprowlmethYl)-1H-indol-3-
yllmethyl}-3-methoxybenzoic acid
Step 1 - The intermediate 5-nitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example l, step 3,
using the above
intermediate.
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acylating agent.
Step 4 - The title compound is prepared as in Example 1, step 5, using the
above intermediate.
MS: m/z (M-1 ) 461
Exam In a 6
4-~ f 5-1 f (cyclopentyloxylcarbonyllamino}-1-(4-Ryridinylmethyl)-1H-indol-3-
yllmeth~}-3-methoxybenzoic acid
Step 1 - The intermediate 5-nitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example 1, step 3,
using the above
intermediate.
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acyladng agent.
Step 4 - The title compound is prepared as in Example 1, step S, using the
above intermediate.
Exam Ip a 7
4-f (5-if (cyclopent~y)carbonyliaminol-1-isopropyl-1H-indol-3yllmethyll-
3-methoxybenzoic acid
Step 1 - The intermediate 5-nitro indole is prepared as in Example l, step 2,
using the
appropriate alkylating agent.
Step 2 - The intermediate 5-amino indole is prepared as in Example 1, step 3,
using the above
intermediate.
Step 3 - The intermediate carbamate is prepared as in Example 1, step 4, using
the appropriate
acylating agent.
72
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 4 - The title compound is prepared as in Example 1, step 5, using the
above intermediate.
MS: m/z (M-1) 449
Example 8
4-f~l-cyclo~ntvl-5-{f(cyclopentyloxy)carbonyllamino]~-1H-indol-3-
yl)methyll-3-methoxybenzoic acid
Step 1 - The intermediate 5-vitro indole is prepared as in Example 1, step 2,
using the
appropriate alkylating agent.
Step 2
The intermediate 5-amino indole is prepared as in Example 1, step 3, using the
above
intermediate.
Step 3
The intermediate carbamate is prepared as in Example l, step 4, using the
appropriate acylating
agent.
Step 4
The title compound is prepared as in Example 1, step 5, using the above
intermediate.
MS: m/z (M-1) 475
Example 9
4-[(1-benzhydryl-5-~[f (butylamino)carbonyllaminol-1H-indol-3-vl]~meth~ll-3-
methoxpbenzoic acid
The intermediate 5-vitro indole is prepared as in Example l, step 2, using the
appropriate alkylating agent and the intermediate 5-amino indole is prepared
as in Example 1,
step 3, using the 5-vitro indole intermediate. The intermediate urea is
prepared as in Example
1, step 4, using the appropriate acylating agent. The title compound is
prepared as in Example
1, step 5, using the urea intermediate.
MS: m/z (M-1) 560
Example 10
4-(~1-benzhydrvl-5-f (methylsulfonyl)aminol-1 H-indol-3-yl )~methyll-3-
methoxybenzoic acid
The intermediate 5-vitro indole is prepared as in Example 1, step 2, using the
appropriate alkylating agent followed by preparation of the intermediate 5-
amino indole as in
Example 1, step 3, using the 5-vitro indole. The intermediate sulfonamide is
next prepared as
in Example 1, step 4, using the appropriate acylating agent. The title
compound is then
prepared as in Example 1, step 5, using the sulfonamide intermediate. MS: m/z
(M-1) 539
73
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 11
4-f{1-benzhydryl-5-flcyclopentylcarbonyl)aminol-1H-indol-3-ylJ~meth, l~l-3-
methoxybenzoic acid
The intermediate 5-vitro indole is prepared as in Example l, step 2, using the
appropriate alkylating agent and intermediate 5-amino indole is prepared as in
Example 1, step
3, using this S-vitro indole intermediate. The corresponding intermediate
amide is then
prepared as in Example 1, step 4, using the appropriate acylating agent. The
final title
compound is prepared as in Example 1, step 5, using this amide intermediate.
MS: m/z (M-1)
557
Example 12
4-f (1-benzhydryl-5-vitro-1H-indol-3-yl)methyll-3-methoxybenzoic acid
The intermediate 5-vitro indole is prepared as in Example l, step 2, using the
appropriate alkylating agent and the title compound is prepared as in Example
1, step 5, using
this intenmediate. MS: m/z (M-1) 657
Exmaple 13
4-f(1-benzhydryl-5-bromo-1H-indol-3-yl)methyll-3-methoxybenzoic acid
The intermediate 5-bromo indole is prepared as in Example 1, step 1, using the
appropriate indole and as in Example 1, step 2, using the appropriate
alkylating agent. The
title compound is then prepared as in Example 1, step 5, using the above
intermediate. MS: m/z
(M-1} 526
Example 14
4-f(1-benzhydryl-5-fluoro-1H-indol-3~1)methyll-3-methoxybenzoic acid
The intermediate 5-fluoro indole is prepared as in Example 1, step 1, using
the
appropriate indole and as in Example l, step 2, using the appropriate
alkylating agent. The
title compound is prepared as in Example 1, step 5, using the above
intermediate. MS: m/z (M-
1 ) 464
Example IS
4-f(1-benzhydryl-5-methyl-1H-indol-3-yl)methyll-3-methoxybenzoic acid
The intermediate 5-methyl indole is prepared as in Example 1, step 1, using
the
appropriate indole and as in Example 1, step 2, using the appropriate
alkylating agent. The title
compound is then prepared as in Example 1, step 5, using the above
intermediate. MS: m/z (M-
1 ) 460
74
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 16
4-f (5-benzhvdrvl-SH-f 1.31dioxolof 4.5-flindol-7-yl)methyll-3-methoxybenzoic
acid
The intermediate 5,6-methylenedioxy indole is prepared as in Example 1, step
1, using
the appropriate indole and as in Example 1, step 2, using the appropriate
alkylating agent. The
title compound is then prepared as in Example 1, step 5, using the above
intermediate. MS: m1z
(M-1 ) 490
Exam lp a 17
4-f(1-benzhydryl-5-cyano-1H-indol-3-yl)methyl]-3-methoxybenzoic acid
Step 1
To the intermediate from Example 13, step 2 (0.25 g, 0.46 mmol), in DMF ( 1
mL) is added
CuCN (0.05g, 1.2 eq} and the reaction mixture is stirred at 145 °C
overnight and then cooled.
To the cooled reaction mixture is added FeCl3 (0.09 g, 1.2 eq). The reaction
mixture is stirred
5 min, taken up in ethyl acetate (30 mL), washed with brine (3 X 10 mL), dried
(MgS04),
filtered and concentrated. The product was purified by silica chromatography
(20% ethyl
acetate/hexanes) to afford the intermediate ester (0.2 g, 89%) as a colorless
oil.
Step 2
To the intermediate ester (0.2 0.41 mmol) in DMF (2 mL) is added sodium
thiomethoxide (0.1
g, 3.4 eq) and the reaction mixture is stirred at 90 °C for 10 min. The
reaction is cooled,
poured into ethyl acetate (5 mL), washed with sodium biphosphate ( 1 X 2 mL),
brine (2 X 2
mL), dried (MgS04), filtered and concentrated. Purification by silica
chromatography ( 1 %
acetic acid, 25% ethyl acetate/hexanes) afforded the title compound (0.114 g,
59%) as a
colorless amorphous powder. MS: m/z (M-1) 471
Example 18
4-{f 1-benzhydryl-5-(met~ylsulfonyl)-1H-indol-3-vllmethyl)'~-3-
methoxybenzoic acid
Step 1
To the intermediate from Example 13, step 3 ( 1 g, 1.9 mmol), in a solution of
THF (2 mL) and
methanol (2 mL) is added sodium hydroxide (0.41 mL, 4.63 M, 1 eq). The mixture
is stirred
for 20 min and then concentrated. The residual water is chased off by the
addition of toluene
and it's removal (3 X) a white powder (1 g, 100%).
Step 2
To the sodium salt prepared above (0.88 g, 1.6 mmol) in DMF (3 mL) is added
methanesulfmic acid, sodium salt (0.72 g, 4.4 eq) and CuI (0.74 g, 2.4 eq).
The reaction
mixture is stirred at 130 °C overnight, cooled, taken up in ethyl
acetate (50 mL) and acetic acid
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
(10 mL), filtered (celite), washed with brine (4 X 10 mL), dried (MgS04),
filtered and
concentrated. Silica chromatography ( 1 % acetic acid, 25% ethyl
acetate/hexanes - 1 % acetic
acid, 50% ethyl acetate/hexanes) afforded the title compound (0.2 g, 24%) as a
colorless
amorphous solid. MS: m/z (M-1} 524
Exam lv a 19
Cvclopentyl N-d 1-benzhydryl-3-f 2-methox~-4-~,{f l2-
methylphenylysulfo~~]amino]carbonyl benzyl]-1H-indol-5-~}carbamate
To the product of Example 3, step 4 (0.5 g, 0.87 mmol), in CHZCIz (4 mL) is
added EDCI
(0.2 g, 1.0 mmol, 1.2 eq), DMAP (0.011 g, 0.087 mmol, 0.1 eq) and ortho-
toluene
sulfonamide. The reaction is stirred overnight at room temperature, taken up
in ethyl acetate
(50 mL), washed with sodium biphosphate ( 1 X 10 mL), brine (2 X 10 mL), dried
(MgS04),
filtered and concentrated. Silica chromatography (1% acetic acid, 25% ethyl
acetate/hexanes)
afforded the title compound (0.4 g, 63%) as a colorless solid.
Exam lp a 20
Cyclopentyl N-~(3-f2-methoxy-4-(~[[(2-methy_lphenyl)sulfo~llamino}
carbo~llbenz 1~1-1-propyl-1H-indol-5-yl}carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 1, step 5, and the appropriate sulfonamide.
Example 21
Cyclopentyl N-I1-(cyclopro~ylmethyll-3-f 2-methoxv-4-(I f (2-
methylphenyl)sulfonyllamino~carbonyl}benzvl]-1H-indol-S-vllcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 5, step 4, and the appropriate sulfonamide. MS: zn/z (M-1) 614
Example 22
Cyclouentyl N-f3-f2-methoxy-4-(~(f(2-meth~phenyl)sulfonyllamino}carbonyl)
benzKll-1-(4-pyridinylmethyl)-1H-indol-5-yllcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 6, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 651
76
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 23
Cyclopentyl N-f3-f2-methoxy-~i((2-
methylphenyl)sulfonyllamino}carbonyl)benzyll-1-(2-naphthylmethyl)-1H-
indol-5-y_Ijcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 4, step 4, and the appropriate sulfonamide. MS: m/z (M-1 } 700
Exam Ip a 24
Cyclonentyl N-11-isoRropyl-3-f2-methoxy-4-(lf(2-
methylphenyl)sulfonyllamino}carbon)benzyll-1H-indol-5-yllcarbamate
1 S The title compound is prepared as illustrated in Example 19 starting with
the product of
Example 7, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 602
Example 25
C~pentyl N-11-cyclopent~~2-methox~-4-(f f (2-methylnhen~l)
~»lfonyllaminolcarbonyl)benzyll-1H-indol-5-yl)~carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 8, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 628
Example 26
Cyclopent~{1-benzhydryl-3-f2-method-4-
(1f(trifluoromethyl)sulfo~llamino~carbonyl)benzyll-1H-indol-5-yl}carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 704
Example 27
cyclopentyl N-f 1-benzhydryl-3-(2~methox
~ffmethvlsulfonvl)aminolcarbonyl}benzyl)-1H-indol-5-vllcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide. MS: m/z (M-1) 650
77
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Exam lp a 28
cyclopentyl N-!I-benzhydrvl-3-f4-(~j(2-
chlorophenyl)sulfonyllamino}carbonyl)-2-methoxybenzyll-IH-indol-5-
yllcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide.
Example 29
cyclopentyl N-(3-,(4-f((f5-(acetylimino)-4-methyl-4.5-dihydro-1.3.4-
thiadiazol-2-yllsulfonyl}amino)carbonyll-2-methoxybenzyl}-I-benzhydryl-
1H-indol-5-yl)carba-mate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide.
Exam lu a 30
cyclopentyl N-~1-benzhydryl-3-14-f (~( f 5-(dimethylamino~-1-
nay t hvllsulfonvllamino)carbonvll-2-methoxvbenzvl l-1H-indol-5-
yl)carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide.
Example 31
cyclopentyl N-[1-benzhydryl-3-(4-~(f(benzylsulfonyl)aminolcarbonyl}-2-
methoxvbenzyl)-1H-indol-5-yllcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 726
Exam lp a 32
cyclopentyl N-il-benzhydryl-3-f4-({f(2i4-dimethyl-1.3-thiazol-5-
Y_llsulfonyllamino carbonyl)-2-methoxybenzyll-1H-indol-5-yl}carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 747
78
CA 02322162 2000-08-24
WO 99/43654 PGT/US99/03898
Example 33
cy~clopent~~-benzhydrvl-3-f 4-(,( f 13,5-dimethyl-4-
isoxazolyl)sulfonyllamino carbonyl)-2-methoxybenzyl]-1H-indol-5-
yl ]'rcarbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide. MS: m/z (M-1 ) 731
example 34
cyclopentyl N-(3-~4-f(!f5-(acetylamino,~-1s3.4-thiadiazol-2-
yllsulfonvl lamino)carbonyll-2-metho ~benzvll-1-benzhvdryl-1H-indol 5
yl)carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide.
Exam Ip a 35
cy~o~entyl N-(I-benzhydryl-3-~2-methoxy-4-f(lf4-(3-methyl S oxo 4,5
dih~dro-1H-p_yrazol-1-yl)phenyllsulfonyllamino)carbon Ily benz~rll 1H indol
5-~)carbamate
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 3, step 4, and the appropriate sulfonamide.
Example 36
N-t4-f (1-benzhydrvl-5-vitro-1H-indol-3-yl)methyll-3-methoxybenzoyl J~-2-
me ylbenzenesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 12, step 2, and the appropriate sulfonamide. MS: m/z (M-1 ) 644
Example 37
N-~4-[(I-benzhydryl-5-vitro-1H-indol-3-yl)methyll-3-
~nethoxybenzoyl}(trifluoro)methanesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 12, step 2, and the appropriate sulfonamide. MS: m/z (M-1 ) 622
79
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 38
N-14-f(1-benzhvdrvl-5-bromo-1H indol 3 yl)methyll 3 methoxybenzovl} 2
methylbenzenesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 13, step 2, and the appropriate sulfonamide. MS: m/z (M-1 ) 679
Example 39
N-d4-fll-benzhvdryl-5-bromo-1H indol 3 yl)methyll 3
methoxvbenzovll(trifluoro)methanesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 13, step 2, and the appropriate sulfonamide.
MS: m/z (M-1 ) 657
Examale 4_0
N-11-benzhvdrvl-3-f2-methoxy-4 ((f(trifluoromethyl)s~yllaminol
_carbonvllbenzvll-1H-indol-5-yllcyclopentanecarboxamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 11, step 4, and the appropriate sulfonamide.
MS: m/z (M-1 ) 688
Example 41
N-f4-!11-benzhvdrvl-5-f(methvlsulfonyllaminol 1H indol 3 ~l methyl) 3
methoxvbenzovll(trifluoro)methanesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 10, step 4, and the appropriate sulfonamide.
MS: m/z (M-1 ) 670
Exam Ip a 42
N-(4-f(1-benzhvdrvl-5-ff(butylamino)carbonyllamino} 1H indol 3
vl)methvll-3-methoxvbenzoy_l ~(trifluorolmethanesulfonamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 9, step 4, and the appropriate sulfonamide.
MS: m/z (M-1 ) 691
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 43
N-i 1-benzh~rdryl-3-(2-methox~-4~,~f (2-methylphenyl)sulfonyllaminol
carbonyl)benzyl]-1H-indol-5-vl~cyclopentanecarboxamide
The title compound is prepared as illustrated in Example 19 starting with the
product of
Example 11, step 4, and the appropriate sulfonamide.
MS: m/z (M-1 ) 710
Example 44
4-(~5-f(cyclopentylcarbonyl)aminol-1-fnhenyll2-pvridinyl)methyll 1H indol
3-yl~methYl_1-3-methoxxbenzoic acid
Step 1
The intermediate 5-amino indole is prepared as in Example 1, step 3.
Step 2
The intermediate sulfonamide is prepared as in Example 1, step 4, using the
appropriate
acylating agent.
Step 3
The intermediate acid is prepared as in Example 1, step S, using the above
intermediate.
Step 4
The title compound is prepared as illustrated in Example 19 starting with the
intermediate above
and the appropriate sulfonamide.
MS: m/z (M-1 ) 738
Example 4~
N-f 4-(~ 1-benzhvdrvl-5-f lbenzylsulfonyllaminol-1H-indol-3-yl ~methyl)~3-
~hoxybenzoyll (trifluorolmethanesulfonamide
Step 1
The intermediate 5-amino indole is prepared as in Example l, step 3.
Step 2
The intermediate sulfonamide is prepared as in Example 1, step 4, using the
appropriate
acyladng agent.
Step 3
The intermediate acid is prepared as in Example 1, step 5, using the above
intermediate.
Step 4
The title compound is prepared as illustrated in Example 19 starting with the
intermediate above
and the appropriate sulfonamide.
MS: m/z (M-1 ) 746
81
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 46
N-~[1-benzh~dryl-3-f2-methox -4-( [(trifluoromethyllsulfonyllamino}
carbonyl)benzylj-IH-indol-5-yl)'~-3-thiophenecarboxamide
Step 1
The intermediate 5-amino indole is prepared as in Example 1, step 3.
Step 2
The intermediate amide is prepared as in Example 1, step 4, using the
appropriate acylating
agent.
Step 3
The intermediate acid is prepared as in Example 1, step 5, using the above
intermediate.
Step 4
The title compound is prepared as illustrated in Example 19 starting with the
intermediate above
and the appropriate sulfonamide.
MS: m/z (M-1 ) 702
Example 49
benzyl N-{1-benzhydryl-3-f2-methoxy-4-
(~( f (trifluo~rometh~)sulfonyllamino}carbonyl)benz1r11-1H-indol-5-yl
lcarbamate
Step 1
The intermediate 5-amino indole is prepared as in Example 1, step 3.
Step 2
The intermediate carbamate is prepared as in Example 1, step 4, using the
appropriate acylating
agent.
Step 3
The intermediate acid is prepared as in Example 1, step 5, using the above
intermediate.
Step 4
The title compound is prepared as illustrated in Example 19 starting with the
intermediate above
and the appropriate sulfonamide.
MS: m/z (M-1 ) 726
Example 50
4-f(1-benzhydryl-5-nitro-1H-indol-3-vl)methyllbenzoic acid
Step 1
The intermediate 3-alkylated 5-nitroindole is prepared as illustrated in
Example 1, step
1, using the appropriate alkylating agent.
82
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 2
The intermediate 3-alkylated 5-nitroindole is N-alkylated as illustrated in
Example 3, step 1.
Step 3
The title compound is prepared as illustrated in Example 1, step 5.
MS: m/z (M-1 ) 461
Exam~,le 51
4-f(1-benzhydryl-S-bromo-1H-indol-3-yl)methyllbenzoic acid
Step 1
The intermediate 3-alkylated 5-bromoindole is prepared as illustrated in
Example 13,
step 1, using the appropriate alkylating agent.
Step 2
The intermediate 3-alkylated 5-nitroindole is N-alkylated as illustrated in
Example 13, step 2.
Step 3
The title compound is prepared as illustrated in Example 13, step 3.
MS: m/z (M-1 ) 494
Example 52
4-f (1-benzhydryl-5-{f (cyclopentyloxy)carbonyllamino}-1H-indol-3-
yllmeth,~l]benzoic acid
Step 1
Starting with the material prepared in Example 50, step 2, the desired
intermediate is
prepared as illustrated in Example 3, step 2.
Step 2
The intermediate carbamate is prepared from the above intermediate as
illustrated in Example 3,
step 3.
Step 3
The title compound is prepared from the above intermediate as illustrated in
Example 3, step 4.
MS: m/z (M-1 ) 543
Example 53
~clopentyl N-~(1-benzhydryl-3-f4-(ff(2-methylphenyllsulfonyllaminol
carbonyl_lbenzxl]-1H-indol-5-yl}carbamate
The title compound is prepared from the product of Example 52, step 3, as
illustrated in
Example 19. MS: m/z (M-1 ) 697
83
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 54
cyclopentyl N-~(1-benzhydryl-3-f4-({f(trifluorometh~l)sulfonyllaminol
carbonyl)benzyll-1H-indol-5-yl)carbamate
The title compound is prepared from the product of Example 52, step 3, as
illustrated in
Example 26. MS: m/z (M-1 ) 674
Example 55
N-~4-f ll-benzhydryl-5-vitro-1H-indol-3-yl)methxllbenzoyll
(trifluoro)methanesulfonamide
The title compound is prepared from the product of Example 55, step 3, as
illustrated in
Example 26. MS: m/z (M-1) 592
Exam lu a 56
N-14-f (1-benzhydryl-5-vitro-1H-indol-3-yl)methy_1]benzoyl}-2-
methylbenzenesulfonamide
The title compound is prepared from the product of Example 55, step 3, as
illustrated in
Example 19. MS: m/z (M-1 ) 614
Example 57
N-~(4-f (1-benzhydryl-5-bromo-1H-indol-3-yl)met~llbenzoyl}-2-
methylbenzenesulfonamide
The title compound is prepared from the product of Example 51, step 3, as
illustrated in
Example 38. MS: m/z (M-1 ) 649
Example 58
N-14-f (1-benzhydryl-5-bromo-1H-indol-3-vl)meth~llbenzoyll
(trifluoro)methanesulfonamide
The title compound is prepared from the product of Example 51 step 3 as
illustrated in
Example 39. MS: m/z (M-1 ) 627
Example 59
3-(12-f 1-(4-benzvlbenzvl)-1H-indol-3-vll-2-oxoacet ~amino)benzoic acid
Step 1 - To a solution of methyl 3-aminobenzoate (2.4 g, 16.0 mmol) in CHzCIz
(50
mL) and saturated sodium bicarbonate (50 mL) at 5 °C is added 3-
indolylglyoxalyl chloride
(3.0 g, 14.4 mmol). The reaction is stirred to room temperature over 2 h,
taken up in ethyl
acetate (200 mL), washed with brine (3 X 50 mL), dried (MgS04), filtered and
concentrated.
84
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Crystallization of the crude material afforded the desired intermediate (2.7
g, 58%) as a
colorless solid.
Step 2 - To a solution of the above intermediate (0.3 g, 0.93 mmol) in DMF (
1.5 mL)
at 0 °C is added potassium bis(trimethylsilyl)amide (0.41 g, 2.06
mmol). After the reaction is
stirred at room temperature 30 min 4-benzylbenzyl bromide (0.27 g, 1.03 mmol)
is added.
The reaction is stirred 3 h, taken up in ethyl acetate ( 10 mL), washed with
brine (3 X 2 mL),
dried (MgS04), filtered and concentrated. Radial silica chromatography (2 mm,
10% - 35%
ethyl acetate/hexanes) afforded the desired intermediate (0.19 g, 41%) as a
colorless oil.
Step 3 - The ester obtained in step 2 was treated with sodium hydroxide (2 mL,
5 M) in
THF (5 mL) and MeOH (2 mL). The reaction was stirred overnight, taken up in
ethyl acetate
(50 mL), washed with sodium biphosphate (I X 10 mL), brine (2 X 10 mL), dried
(MgS04),
filtered and concentrated. Trituration of the material in ethyl acetate with
hexanes afforded the
title compound (0.105 g, 60%) as a colorless solid. MS: m/z (M-1 ) 487
Example 60
3-(12-f 1-f4~13.5-bis tri~luoromethyl)phenoxy]methyl}benzyll-1H-indol-3-
yll-2-oxoacetyl}aminolbenzoic acid
The intermediate prepared in Example 59, step l, was N-1 alkylated with the
appropriate reagent using the procedure described in Example 59, step 2.
Step 2
The product ester was hydrolyzed as described in Example 59, step 3.
MS: m/z (M-1 ) 639
Example 61
3-a~(2-(1-benzhydryl-1H-indol-3-yll-2-oxoacetvllamino)benzoic acid
The intermediate prepared in Example 59, step 1, was N-1 alkylated with the
appropriate reagent using the procedure described in Example 59, step 2.
Step 2
The product ester was hydrolyzed as described in Example 59, step 3.
MS: m/z (M-1 ) 473
85
CA 02322162 2000-08-24
WO 99/4365a PGT/US99/03898
Example 62
3J-~2-{ 1-f 3-(4-benzylphenoxy)propyll-1H-indol-3-yl{-2-
oxoacetyllaminolbenzoic acid
Step 1
The intermediate prepared in Example 59, step 1, was N-1 alkylated with the
appropriate
reagent using the procedure described in Example 59, step 2.
Step 2
The product ester was hydrolyzed as described in Example 59, step 3.
MS: m/z (M-1 ) 531
Exam In a 63
3-f (2-{ 1-f 3.4-bis(benzyloxylbenzyll-1H-indol-3-yl )~-2-
oxoacetyllaminolbenzoic acid
Step 1
The intermediate prepared in Example 59, step 1, was N-1 alkylated with the
appropriate
reagent using the procedure described in Example 59, step 2.
Step 2
The product ester was hydrolyzed as described in Example 59, step 3.
MS: m/z (M-1 ) 609
Example 64
3-f(2-11-f2-lbenzylsulfonyl)benzyl~-1H-indol-3-yl)~-2-
oxoacetyllaminolbenzoic acid
Step 1
The intermediate prepared in Example 59, step 1, was N-1 alkylated with the
appropriate
reagent using the procedure described in Example 59, step 2.
Step 2
The product ester was hydrolyzed as described in Example 59, step 3.
MS: m/z (M-1 ) 551
Exam lp a 65
3-((11-benzh~~l-5-f (cyclopentylcarbonvllaminol-1H-indol-3-
yl)~meth~laminolbenzoic acid
Step 1
To a solution of the aldehyde prepared in Example 114, step 3 (0.3 g, 0.7
mmol) in
dichloroethane (2 mL) and DMF ( 1 mL) is added methyl 3-amino benzoate (0.113
g, 0.735
86
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/o3898
mmol, 1.05 eq) and acetic acid (0.13 mL, 2.1 mmol, 3 eq). After stirnng 30 min
sodium
triacetoxyborohydride (0.18 g, 0.84 mmol, 1.2 eq) is added and the reaction is
allowed to stir
an additional 4 h after which it is taken up in ethyl acetate (20 mL), washed
with saturated
sodium bicarbonate ( 1 X 10 mL), brine (2 X 5 mL), dried (MgS04), filtered and
concentrated.
Silica chromatography (30% ethyl acetate/hexanes) afforded the desired
intermediate (0.24 g,
60%) as a colorless oil.
Step 2
The product ester was hydrolyzed as described in Example 59 step 3 to give the
title compound
(0.11 g, 55%). MS: m/z (M-1) 542
Example 66
2-f4-(l l-benzhydryl-5-f (cyclopentylcarbonYl_lamino]-1H-indol-3-
yllmeth~~piperazinolacetic acid
The title compound was prepared as described in Example 65 using the
appropriate
amine. MS: m/z (M-1 ) 549
Example 67
2-[1-l,] 1-benzhvdryl-5-f (cyclopentylcarbonyl)aminol-1H-indol-3-yl )~methylZ
3-oxo-2-piuerazinyllacetic acid
The title compound was prepared as described in Example 65 using the
appropriate
amine. MS: m/z (M-1 ) 563
Example b8
2-[~,j -benzhydryl-5-j(cyclopentylcarbonyllaminol-1H-indol-3-
yl]methyllaminol-3~hydroxypropanoic acid
The title compound was prepared as described in Example 65 using the
appropriate
amine. MS: m/z (M-1 ) 510
Example 69
2-( 1-(4-benzvlbenzvl)-5-(benzvloxv)-1H-indol-3-vll-2-oxoaceticacid
Step 1 - Ethylmagnesium bromide (3M in ether, 57 mL) was diluted in ether (50
mL).
5-Benzyloxyindole (12.7 g) dissolved in ether (150 mL) was added to the
Grignard solution at
- 78 °C. After 1.25 h, ethyloxalyl chloride ( 17.12 g) was added. The
reaction was stirred 15
min, quenched with saturated sodium bicarbonate, taken up in ethyl acetate and
washed with
water, dried (MgS04), filtered and concentrated. The resulting solid was
triturated with ethanol
87
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
and stirred for 1 h. The desired product (5.75 g, 31 %) was isolated as a
yellow solid and used
without further purification.
Step 2 - To the above indole in DMF at 0 °C was added sodium hydride
(0.4 g, 60%
dispersion in oil). After warming to room temperature, 4-benzylbenzylbromide
(2.2 g) was
added and the mixture was stirred overnight. As the reaction was not yet done
(TLC) additional
I O 4-benzylbenzylbromide ( 1.0 g) was added and the reaction stirred for 2.5
h. The reaction was
taken up in ethyl acetate and washed with water, dried (MgS04), filtered and
concentrated.
Chromatography (20% ethyl acetate/hexanes) afforded the desired compound (3.1
g 90%).
Step 3 - The above ester was placed in a solution of NaOH (2N):THF:MeOH
(1:2:1)
and stirred overnight at room temperature. The reaction was acidified with 6 N
HCI and the
product extracted with ethyl acetate. The organic layers were dried (MgS04),
filtered and
concentrated. The solid was triturated with ethanol and stirred for 1 h. The
solid was filtered
and dried affording the title compound ( 1.85 g) as a yellow solid. MS: m/z (M-
1 ) 474
Example 70
2-{5-(benz~loxY -) 1-f2.4-bisltrifluoromethyl)benzyll-1H-indol-3-yl~-2-
oxoacetic acid
The indole prepared in Example 69, step 1, was alkylated with the appropriate
alkyl
bromide and hydrolyzed as described in Example 69, steps 2 and 3.
MS: m/z (M-1 ) 520
Examgle 71
3-(~2-f 1-(4-benzylbenzyll-5-(benzyloxyl-1H-indol-3-vll-2-
oxoacetyllamino~benzoic acid
Step 1 - To a solution of the acid from Example 69, step 3, (0.810 g) in THF
(28 mL)
was added CDI. The reaction was stirred 30 min and then ethyl 3-aminobenzoate
(0.330 g)
was added and the reaction was stirred overnight. The reaction mixture was
taken up in ethyl
acetate and washed with water, dried (MgS04}, filtered and concentrated. The
crude material
was triturated with ethanol and stirred for 1 h, filtered and dried. The
desired product (0.76 g,
75%) was isolated as a yellow solid.
Step 2 - The above ester was dissolved in NaOH (2N):THF:MeOH (1:2:1) and
stirred
4h. The mixture was acidified with 6 N HCl and extracted with ethyl acetate.
The combined
organic layers were dried (MgS04), filtered and concentrated. The crude solid
was triturated
with ethanol/hexane to afford the title compound (0.48 g, 69%) as a yellow
solid.
88
CA 02322162 2000-08-24
WO 99/43654 PCTIUS99/03898
Example 72
5-f(2-IS ~benzxlox~)-1-f2.4-bis(trifluoromethyl)benzvll-1H-indol-3-X1JE-2-
oxoacetyllaminolisophthalic acid
The alkylated indole from Example 70 was coupled to the appropriate amino acid
and
hydrolyzed as illustrated in Example 71, steps 1 and 2.
MS: m/z (M-1 ) 683
Example 73
3-L(2-f 5-(benz~y_L1-[2,4-bis(trifluoromethyl)benzyll-1H-indol-3-
oxoacetyllaminolbenzoic acid
The alkylated indole from Example 70 was coupled to the appropriate amino acid
and
hydrolyzed as illustrated in Example 71, steps 1 and 2.
MS: m/z (M-1 ) 639
Exam Ip a 74
5-(I2-f 1-(4-benzylbenz 1~(benz~y)-1H-indol-3-yll-2-oxoacetyllaminol-
2-f(5-chloro-3-pyridinylloxylbenzoic acid
The alkylated indole from Example 69 was coupled to the appropriate amino acid
and
hydrolyzed as illustrated in Example 71, steps 1 and 2.
Example 75
5-f (2-~5-(benzyloxy,)-1-f 2.,4-bisltrifluoromethvllbenzyll-1H-indol-3-yl1-2-
Qxoacetylaminol-2-f (5-chloro-3-pyridinylloxylbenzoic acid
The alkylated indole from Example 70 was coupled to the appropriate amino acid
and
hydrolyzed as illustrated in Example 71, steps 1 and 2.
Example 76
2-fl-l4-benzylbenzyll-5- benzyloxy)-1H-indol-3-yll-N-f3-llfl4-
meth~rlnhenyllsulfonyl]aminolcarbonyl)phenyll-2-oxoacetamide
To the acid obtained in Example 71 (0.1 g) in CHZC12 ( 10 mL) is added THF ( 5
mL) to
help dissolve the compound. EDCI (0.045 g) and DMAP (0.02 g) was added and the
mixture
was stirred a room temperature of 1 h. p-Toluenesulfonamide (0.04 g} was added
and the
reaction was stirred overnight. The reaction mixture was take up in ethyl
acetate and washed
with water, dried (MgS04), filtered and concentrated. Chromatography (7%
MeOH/CHZC12)
afforded the title compound (0.045 g, 40%) as a yellow solid. MS: m/z (M-1 )
746
89
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 77
2-f5-bromo-1-(cyclopropylmethyl)-1H-indol-3-yllacetic acid
To 5-bromoindole-3-acetic acid (890 mg, 3.5 mmol) in 1-methyl-2-pyrrolidinone
(12
mL) at 0 °C were added'Pr2NEt (21 mmol) and bromomethylcyclopropane
(10.5 mmol). The
reaction mixture was heated at 50 °C for 19 h before partitioning
between diethyl ether and ice
water. After adjusting the pH to 3, the aqueous layer was extracted with
diethyl ether. The
organic layers were combined, washed with NaH2P04, dried over MgS04 and
evaporated to
dryness. Purification on silica gel column ( 30% EtOAc in hexane) yielded 927
mg (86 %
yield) of the product.
Exam In a 78
2-fl-(~cloprog, I~methyl)-5-(2-thienyll-1H-indol-3-yllacetic acid
To a sealed tube containing 2-[5-bromo-1-(cyclopropylmethyl)-1H-indol-3-
yl]acetic
acid ( 100 mg, 0.32 mmol), 2-thiopheneboronic acid ( 124 mg, 0.97 mmol),
(C6H5)4Pd (37 mg,
0.032 mmol), NazC03 (2.6 mmol) in a mixture of benzene/EtOH/H20 (5/1/3, 4.5
mL) was
heated at 85 °C for 19 h. The mixture was poured onto diethyl ether and
adjusted to pH 3
before extracting with diethyl ether. The mixture was washed with NaH2P04,
dried over
MgS04 and evaporated to give the crude product which was purified on silica
gel column
33% EtOAc in hexane with 1 % HCOOH) to give 79 mg (78% yield) of the product.
Exam lp a 79
2-j_1-(cvclopropylmeth~rl)-5-f3-(trifluoromethyl)phenyll-1H-indol-3-yl]~acetic
acid
The title compound was prepared according to the procedure described in
Example 78
except that 3-(trifluoromethyl)phenylboronic acid was used.
Example 80
2-j5-(1-benzofuran-2-yl)-1-benzyl-1H-indol-3-vllacetic acid
The title compound was prepared according to the procedure described in
Example 78
except that 2-[5-bromo-1-benzyl-1H-indol-3-yl]acetic acid and benzo[b]furan-2-
boronic acid
were used.
Example 81
2-(1-benzvl-5-5-ghenyl-1H-indol-3-yllacetic acid
The title compound was prepared according to the procedure described in
Example 78
except that 2-[5-bromo-1-benzyl-1H-indol-3-yl]acetic acid and phenylboronic
acid were used.
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 82A
5-((E)-{ 1-[3-(3-benzylphenoxy)propyl]-1H-indol-3-yl}methylidene)-1,3-
thiazolane-2,4-dione
to 1
The procedure in Example 22 was followed using 3-formyl indole (0.4g,
2.8mrno1),
sodium hydride (0.102g, 3.Ommo1) and the iodide (0.97g, 2.8mmo1) in DMF (
lOml). Flash
chromatography (Hex/EtOAc, 1/1) gave 0.86g (84%) of the desired intermediate.
Ste~2_
The intermediate from step 1 (0.8 g, 2.2 mmol) and 2.4-thiazolidinedione
(0.25, g, 2.2
mmol) was dissolved in toluene (5 mL). Piperidine (0.064 mL, 0.6 mmol) and
acetic acid
(0.012 mL) were added and the mixture was heated to reflux for 2h. The
reaction was allowed
to cool to rt, water was added and the aqueous layer was extracted with ethyl
acetate. The
organic layer was washed with water, brine , dried (MgS04), filtered and
concentrated. Flash
chromatography (hexane/ ethyl acetate , 3/2) afforded the title compound
(0.345 g (33%) as an
orange solid.
Example 82 B
4-{ [5-((E)-{ 1-[3-(3-benzylphenoxy)propyl]-1H-indol-3-yl }methylidene)-2,4-
dioxo-1,3-thiazolan-3-yl]methyl}benzoic acid
The procedure in Example 22 steps 1 and 2 were followed to give 0.14g (47% for
2
steps) of the title compound as a yellow powder.
Example 82 C
2-[5-((E)-{1-[3-(3-benzylphenoxy)propyl]-1H-indol-3-yl}methylidene)-2,4-
dioxo-1,3-thiazolan-3-yl]acetic acid
The procedure in Example 22 steps 1 and 2 were followed to give 0.107g (42%
for 2
steps) of the title compound as a yellow powder.
Example 83
3-{1-[3-(3-benzylphenoxy)propyl]-1H-indol-3-yl}propanoic acid
The procedure in Example 22 step 1_ was followed except 2 eq. of sodium
hydride was
used and 0.142g (65%) of the title compound was isolated as a white oily
solid.
91
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 8 4
3-{1-benzhydryl-5-I(cyclopentylcarbonyllaminol-1H-indol-3-yl nropanoic
to 1
To a solution of the aldehyde from Example 114, step 1 ( 1.Og, 2.8mmo1) in
toluene
(20m1) was added carbomethoxyethylidene triphenylphosphorane (0.98g, 2.9mmo1).
The
mixture was heated overnight at reflux and then concentrated. The residue was
dissolved in
CH2C12 and silica gel was added. The mixture was concentrated and the
resulting solid was
purified by flash chromatography (Hex/EtOAc, 3/1). Compound ~0, l.Olg (88%)
was isolated
as a yellow solid.
to 2
To a solution of the above intermediate (0.1 g, 0.24mmo1) in THF ( l Oml), was
added
platinum on activated carbon (5% Pt, O.OSg, 50 wt%). Hydrogen gas was bubbled
into the
suspension for 2min, the vessel was sealed tightly and the reaction was
stirred overnight at rt.
Argon gas was then bubbled through the reaction for l5min before the mixture
was filtered
through a pad of Celite. The pad was washed with EtOAc and the filtrate was
concentrated.
The residue was dissolved in CHZCIZ (Sml). Aqueous saturated NaHC03 (3ml) was
added,
followed by cyclopentanecarbonyl chloride (0.036m1). The biphasic mixture was
stirred for 2h
at rt and diluted with CHZC12. The organic layer was washed with water and
brine, dried and
concentrated to a white solid. Recrystallization from EtOAc/Hex gave O.llg
(95%) of the
desired intermediate as a white solid.
Step 3
Hydrolysis of the above ester with NaOH ( 1 N, 2 mL) in THF (2mL) and MeoOH (2
mL)
followed by recrystallization from hot EtOAc afforded 0.054g (50%) of the
title compound as a
white solid.
Exam~t]~ 8 5
N-(1-benzhydryl-3-{3-[(methylsulfonyl)amino]-3-oxopropyl}-1H-indol-5-
yl)cyclopentanecarboxamide
To a solution of the acid from Example 84 step 3 (O.lg, 0.22mmol) in THF (Sml)
was
added methanesulfonamide (0.027g, 0.28mmo1), EDCI (0.54g, 0.28mmo1) and DMAP
(0.012g, O.lmmol). The mixture was heated at 50°C overnight then
diluted with EtOAc,
washed with water and brine, dried and concentrated. Flash chromatography
(Hex/EtOAc,
1/1) gave O.lg (87%) of the title compound as a white solid.
92
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
S Example 86 A
(E)-3-{1-benzhydryl-5-[(cyclopentylcarbonyl)amino]~1H-indol-3-yl}-2-
propenoic acid
Step 1 The same procedure as Example 84 step 2 was used to prepare the desired
intermediate from the nitroindole (Example 114 step 11.
Step 2 The procedures in Example 84, step l and 3 were used to prepare the
title
compound from the above intermediate.
Example 86 B
N-(1-benzhydryl-3-{(E)-3-[(methylsulfonyi)amino]-3-oxo-1-propenyl}-1H-
1S indol-5-yl)cyclopentanecarboxamide
The acid from Example 86A was used to prepare the title compound according to
the
procedure in example 8S.
Example 87A
(E)-3-{1-benzhydryl-5-nitro-1H-indol-3-yl}-2-propenoic acid
The ester from Example 84 step 1 was saponified according to the procedure in
Example 84
step 3 and recrystallization from hot EtOAc afforded O.ISSg (90%) of the title
compound as a
white solid.
2S Example 87B
N-((E)-3-{ 1-benzhydryl-5-vitro-1H-indol-3-yl }-2-
propenoyl)methanesulfonamide
The procedure in Example 8S was used to prepare the title compound from the
product of
Example 87A.
Example 8 8
4-f(1-benzhydry-5-chloro-2-methyl-1H-indol-3-yllmethyllbenzoic acid
Step 1 To an ice-cold (0°C) solution of trifluoroacetic acid (1.7m1,
lSmmol) and triethylsilane
(4.8m1, 30mmol) in CHzCl2 (20mL) was added a solution of S-chloro-2-
methylindole ( 1.66g,
3S lOmmol) and methyl 4-formylbenzoate (1.8g, l lmmol) in CHZC12 (SOmL) over a
period of S
min. The resulting homogeneous solution was stirred at 0°C for lh and
rt for 2h, at which
time EtOAc ( 1 SOmL) and aqueous sodium bicarbonate (to pH=8) was added. The
organic
layer was washed with water and brine, dried over MgS04 and concentrated.
Flash
chromatography (Hex/EtOAc, 4/1) gave 1.98g (63%} of desired intermediate as a
light-tan
solid.
93
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 2 Sodium hydride (0.2g, Smmol) was washed with dry hexanes (3x10m1) and
then
suspended in DMF (6mL) and cooled to 0°C. A solution of the above
intermediate ( 1.57g,
Smmol) in DMF (4mL) was dropwise at 0°C and the resulting mixture was
stirred for 30min at
which time the diphenylbromomethane ( 1.24g, Smmol) was added. The mixture was
allowed
to reach rt and stirred for an additional 48h. EtOAc (30mL) was added followed
by aqueous
NaHZPO,, solution ( l Oml). The organic layer was washed with water and brine,
dried and
concentrated. Flash chromatography (Hex/EtOAc, 7/1) provided 0.98g (41%) of
the desired
intermediate as a ivory foam.
to 3
The above intermediate was saponified according to the procedure in Example 84
step 3. Flash
chromatography (EtOAc) provided 0.3g (89%) of the title compound as a tan
crystalline solid.
MS: m/z (M-1 ) 464
Example 8 9
4-]fl-benzhydry -ll S-({(4-(trifluoromethyl)phenyllsulfonvl)amino)-1H-indol-3-
xl_]methyl}-3-methoxybenzoic acid
Step 1 - The intermediate from Example 3 step 2 ( leq) (see scheme #) was
weighed in
to a flask along with the 4-trilflouromethylbenzene sulfonyl chloride ( 1.2
eq) and then they
were flushed with nitrogen, taken up in dichloroethane (O.IS M) and then
pyridine was added
( 1.2 eq) at which time the reaction was left to stir overnight and then
worked up by the addition
of the polymer bound amine ( Parlow, J.J, Mischke, D. A., Woodard, S.S.J. Org.
Chem.
1997, 62, 55908-5919) (1.6g/Immol) and the resulting slurry was stirred a
minimum of 15
minutes and then it was filtered and washed with dichloroethane and the
dichloroethane
solution was dried and concentrated to yield 98% of the desired product with
high purity.
Step 2 - The crude material from stepl was dissolved THF/MeOH (2.5/1) and then
4N
NaOH was added ( 3 eq) and the reaction was stirred until complete hydrolysis
was observed
by TLC. At this point the reaction quenched with enough amberlite it 120 to
make the solution
acidic and then the resin was filtered off and rinsed and the desired product
was obtained in
94% yield by drying and concentrating the solution. MS: m/z (M-1 ) 669
xample 9 0
4-}f5-l~(f2-facet~lamino)-4-methyl-1,3-thiazol-5-yllsulfonyl}aminol-1-
benzhydryl-1H-indol-3-yllmethYl_}-3-methoxybenzoic acid
Step l: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded 76%
of the title compound after chromatographic purification.
94
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 2: An analogous proceedure to step 2 for Example 89 above yielded 83% of
the desired
product. MS: m/z (M-1 ) 679
Example 91
4-f ll-benzhydryl-5-{f (4-chloro-3-nitrophenyl)sulfonyllamino}-1H-indol-3-
~)imethyll-3-methoxybenzoic acid
Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded
100% of the title compound.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 54% of the
desired product
after chromatographic purification. MS: m/z (M-1 ) 681
Example 9 2
4- fll-benzhvdrvl-5-f f(dimethvlamino)sulfonvllaminol-.1H-indQl-3-vl~methvll-
3 - Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded
49% of the title compound after chromatographic purif canon.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 100% of the
desired
product. MS: m/z (M-1 ) 568
Example 9 3
4-{,[ 1-benzhydryl-5-l{ f 4-(trifluoromethoxy,)phenyllsulfonvl lamino)-1H-
indol-
3- vllmethvll-3-methoxvbenzoic ~d
Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded
100% of the title compound.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 100% of the
desired
product. MS: m/z (M-1 ) 685
Exam lp a 9 4
f(1-benzhydryl-5-~j(2-methylphenvllsulfonvllaminol-1H-indol-3-
yl)methxll-3-methoxybenzoic acid
Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded 56%
of the title compound after chromatographic purification.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 82% of the
desired
product. MS: m/z (M-1 ) 615
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 9 5
4-f ( 1-benzhydryl-5-1 f (5-chloro-1,3-dimethyl-1H-~yrazol-4-
vllsulfonvllaminoi-1H-indol-3-vl)methvll-3-methoxvbenzoic acid
Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded
100% of the title compound.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 96% of the
desired product.
MS: m/z (M-1 ) 655
example 9 6
4-f(1-benzhvdrvl-5-(.f(3.5-dimethvl-4-i~zol sulfonvllamino~-1H-indol-3-
~rllmethy~l-3-methoxybenzoic acid
Step 1: Following step 1 for Example 89 using the appropriate sulfonyl
chloride yielded
100% of the title compound.
Step 2: An analogous proceedure to step 2 for Example 89 yielded 89% of the
desired product.
MS: m/z (M-1 ) 621
Example 9 7
C~rclopenty~-N-~(3-f4-(aminocarbon~l)-2-methoxybenzyll-1-benzhydryl-1H-
indol-5-yl}carbamate
The compound of Example 3 ( 1.0 eq) was dissolved in THF (O.15M) and then
carbonyl diimidizole (1.2 eq) was added and the reaction was stirred under NZ
for three hours
at which time ammonium hydroxide was added (3ml/g) and the reaction was
stirred overnight
when TIC analysis showed it was complete. To the reaction was added water and
ethyl acetate,
the layers were separated and the aqueous layer was extracted three times, the
combined
organic extracts were dried concenuated and chromatographed to yield 64% of
the desired
primary amide.
Exam lu a 9 8
~clopentyl N-{1-benzhydryl-3-f2-methoxy-4-(1H-1.2.3.4-tetraazol-5-
yl)ben~ll-1H-indol-5-vllcarbamate
Step 1 - To the compound of Example 97 ( 1.0 eq) under NZ was added CHZC12
(0.06M) and then (methoxycarbonylsulfamoyl)triethylammonium hydroxide inner
salt (5.0 eq)
portion wise over 5 hours and then the slurry was stirred overnight at which
time TLC analysis
indicated the reaction was complete so it was concentrated and chromatographed
to yield 78%
of the desired product.
96
CA 02322162 2000-08-24
WO 99/43654 PCTIUS99/03898
Step 2 - To the nitrite ( 1.0 eq) isolated in step 1 was add sodium azide (3
eq) and
triethyl amine hydrochloride ( 1.5 eq) and n-methyl-2-pryrrolidinone (O.OSm)
and then the
reaction was heated to reflux under an inert atmosphere for 2.5 hours when it
was poured into
ice and water that was then acidified to pH 2 and the product was filtered off
and then further
purified by preparative chromatography to yield the desired compound in 22%
yield. MS: m/z
{M-1 ) 597
E~cample 9 9
4-ff 1-benzhydryl-5-flc~~clopent,ylcarbonyllaminol-1H-indol-3-
yl}carbonyl)aminol-3-thiophenecarboxy is acid
step 1 To the indole acid ( 1.0 eq) was added the amine { 1.2 eq) the
dimethylaminopyridine
(10 mot %), 1-(3dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.5
eq) and then
DMF(0.3M) and the reaction was stirred under nitrogen for 24 hours at
40°C for 24 hours at
which time it was poured into 1/2 saturated ammonium chloride solution and
ethyl acetate and
then the layers were separated and the aqueous layer was extracted 3 times,
the combined
organic layers were washed with water 2X, dried, concentrated and
chromatographed to yield
38% of the amide.
Step 2 The ester from the previous step was dissolved in THF/MeOH (3:1) and
then 1N NaOH
(3.Oeq) was added and the reaction was stirred for until TLC analysis showed
that the reaction
was complete. The reaction was then concentrated, diluted with water,
acidified to pH 2 with
conc HCL, extracted with ethyl acetate 3X, the combined organics were dried
over magnesium
sulfate concentrated and purified via chromatography to yield the desired acid
in 64% yield.
Example 100
3-fill-benzhydryl-5-f(cyclo entylcarbonyl)aminol-1H-indol-3-
yl)carbonyl_)aminolbenzoic acid
Step 1: The acid (see scheme #) was coupled with the appropriate amino ester
following the
procedure in Example 99, step one, except the reaction was run at room
temperature and that
the procedure yielded 80% of the desired product isolated by recrystalization.
Step 2: The vitro ester from step one (1.0 eq) was weighed into a flask along
with 5% Platinum
on Carbon (40 wt%) and the vessel was sealed with a septum and evacuated and
flushed with
argon 3X, then freshly distilled THF is added and the reaction is evacuated 2X
and after the
second evacuation a balloon of hydrogen inserted into the septum. The reaction
is left under
atmospheric hydrogen for 16 hours at which time tlc analysis indicates
complete reduction and
the reaction is flushed with argon and then filtered through a bed of celite
and the catalyst is
97
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
washed exhaustively with ethyl acetate, the filtrate was dried and
concentrated and purified via
chromatography to deliver 71 % of the desired amine.
step 3: The amine ( 1.0 eq) was dissolved in dichloromethane (0.3M) and then
an equivalent
amount of saturated sodium bicarbonate was added and finally the acid chloride
introduced.
The biphasic reaction mixture was vigorously stirred until TLC analysis
indicated that the
reaction was complete (generally a few hours) and then the reaction was
diluted with
dichloromethane and water, the layers were separated, the aqueous layer was
extracted three
times with dichloromethane, the combined organic layers were dried,
concentrated and
chromatographed to yield the desired amide in 41 % yield.
Step 4:
According to step 2, Example 99, the ester was hydrolyzed to the acid and
yielded 71 % of the
final product. MS: m/z (M-1 ) 556
Example 101
3-L(~1-benzhvdr~l-5-f lcyclopentylcarbonyllaminol-1H-indol-3-
y_1]~carbonxllaminolpropanoic acid
step 1 To the final product in Example 114 (l.Oeq) in dichloromethane (O.1M)
at 0°C was
added oxallyl chloride (2.0 eq) and then a few drops of DMF. The reaction was
stirred a few
hours at room temperature and concentrated and azeotroped 2X with toluene and
placed on the
high vacuum for 2 hours before being used crude for the next step.
Step 2: To the acid chloride generated in step 1 was added dichloromethane
(O.1M) and then a
solution of alanine methyl ester ( 1.OSeq, free base) in dichloromethane (
1.OM) and then
triethylamine { 1.Seq)was added and the resulting mixture was stirred
overnight and worked up
by the addition of 1/2 saturated ammonium chloride, the layers were separated,
the aqueous
layer was extracted three times with dichloromethane, the combined organic
layers were dried
and concentrated and purified via chromatography to yield the desired amide.
Step 3: The ester from step 2 was hydrolyzed under the conditions outlined for
step 2, Example
99, to yield the desired acid.
Example 102
N-fl-benzhvdryl-3 j(f(2-methYl_phenyl)sulfonyllaminolcarbonyll-1H-indol-5-
ylicyclopentanecarboxamide
Step 1: The acid chloride (1.0 eq) synthesized in step 1, Example 101, was
weighed into a
flask along with o-tolylsulfonamide (l.Seq), DMAP (0.1 eq) and taken up in
dichloromethane
(O.1M) under nitrogen and then triethylamine (l.Seq) was added and the
resulting mixture was
stirred for 12 hours and then worked up by the addition of 1/2 saturated
ammonium chloride,
98
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
the layers were separated, the aqueous layer was extracted three times with
dichloromethane,
the combined organic layers were dried and concentrated and purified via
chromatography to
yield the desired acylsulfonamide in 52% yield.
~,~amnle 103
3-f (2-11-benzhydryl-5-(f cyciopentylcarbonyl)aminol-1H-indol-3-yl l-2-
oxoacetvllaminojpropanoic acid
Step 1: According to the general procedure in step 1, Example 101, using the
product from
Example 115 and the appropriate amino ester yielded the desired product in
100% yield.
Step 2: The ester from step 1 was hydrolyzed under the conditions outlined for
step 2, Example
99, to yield the desired acid. MS: m/z (M-1 ) 536
xample 104
3-(~,2-{ 1-benzhydryl-5-f (cyclopentylcarbonyllaminol-1H-indol-3-~l-2-
oxoace~rllami~plbenzoic acid
Step 1: According to the general procedure in step 1, Example 99, using the
product from
Example 115 and the appropriate amino ester yielded the desired product in
100% yield.
Step 2: The ester from step 1 was hydrolyzed under the conditions outlined for
step 2, Example
99, to yield the desired acid. MS: m/z (M-1 ) 584
Example 105
3-l12-f 1-(4-benzvlbenzvl)-5-(benzvloxvl-1H-indol-3-vllacetvllaminolbenzoic
Step 1
An oven dried flask was charged with 5-benzyloxy indole-3-acetic acid (1 eq)
(see scheme-1)
and anhydrous DMF (0.18 M) under nitrogen. Reaction mixture was then cooled to
0°C and to
this was added NaH (2.2eq, 60% dispersion in mineral oil}, stirred at
25°C for lh followed by
addition of a solution of the appropriate benzyl bromide (2.2eq, 40% purity)
(see scheme-1,
steps 5,6) in anhydrous DMF, stirred overnight. Workup with ethyl
acetate/water followed by
chromatographic purification afforded the desired product in 66% yield.
to
Dissolved the indole derivative from step 1(1 eq) (see scheme-1) in
THF/MeOH/Hz0 (3:1:1
0.094 M) and to this was added LiOH~H20 (1.2 eq), stirred at 25°C,
overnight. Workup with
ethyl acetate/water followed by chromatographic purification afforded the
desired product in
74% yield.
99
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Steo 33
To the acid from step 2 ( 1 eq) (see scheme-1 ) was added methyl 3-
aminobenzoate ( 1.05 eq),
EDCI (1.37 eq} and DMAP (0.2 eq) followed by anhydrous DMF (0.086M), stirred
at 25°C,
overnight. Workup with ethyl acetate/1N HCl followed by chromatographic
purification
afforded the desired product in 80% yield.
a 4
Dissolved the ester (1 eq) from step 3 (see scheme-1) in THF/MeOH/H20 (3:1:1
0.04 M) and
to this was added LiOH~H20 ( 1.2 eq), stirred at 25°C, overnight.
Workup with ethyl
acetateJlN HCI followed by trituration with CHZCIz/hexane (1:1) for O.Sh and
then
recrystallization from CHzCIz afforded the titled product in 97% yield. MS:
m/z (M-1 ) 579
Example 106
3-fi[2~5-(benzyloxY)-1-f2,4-bisltrifluoromethyl)benzyll-1H-indol-3-
y~~acetyllaminol benzoic acid
to 1
Following procedure in step 1 of example 105, scheme-1 and using the
appropriate benzyl
bromide afforded the desired product in 50% yield after chromatographic
purification.
Stgp Z
Following procedure in step 2 example 105, scheme-1 and using the appropriate
indole
derivative afforded the desired product in 67% yield after chromatographic
purification.
Sten 33
Following procedure in step 3 example 105, scheme-1 and using the appropriate
indole
derivative afforded the desired product in 75% yield after chromatographic
purification.
to 4
Following procedure in step 4 example 105, scheme-1 and using the appropriate
indole
afforded the desired product in 63% yield after chromatographic purification.
MS: m/z (M-1 ) 625
Example 107
5-fbenzyloxy)-1-f2,4-bis(trifluoromethyl)benzyll-2-methvi-1H-indole-3-
carboxylic acid
Step 1: The 5-Hydroxy-2-Methylindole-3-Carboxylate ( 1 eq) was combined with
benzyl
bromide ( 1.3 eq) and KzC03 (325 mesh, 1.3 eq) in CH3CN (0.1 M). The resulting
mixture
was heated to reflux for 2 h. An additional amount of benzyl bromide (0.2 eq)
and the heating
was continued for 2 h. The reaction was worked up by addition of water and
extraction with
CHzCl2. The organic extracts were washed with water, dried and concentrated.
Flash
100
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
chromatography provided the desired benzyl ether (63 % yield), as well as the
corresponding
N,O-bisbenzyl derivative (22 % yield).
Step 2: An ice cooled solution of the benzyl ether from step 1 ( 1 eq) in dry
DMF (0.25 M) was
treated with NaH (60 % in mineral oil, 1.1 eq). 2,4-Bis trifluoromethyl benzyl
bromide ( 1.1
eq) was added after 1 h and the resulting mixture was stirred at 25°C
for 2 h. Solvent was
evaporated under vacuo, the residue was dissolved in EtOAc, washed with water,
dried and
concentrated. The desired product was obtained in 77 % yield after
recrystallization from
hexane/CHCl3.
Step 3: The product from step 2 (1 eq) in THF/MeOH (3/1) was heated to reflux
with 1N
NaOH ( 12 eq). After 48 h the reaction was quenched with AcOH and solvent was
evaporated.
1 S The resulting product was recrystallized to afford crude material in 72 %
yield. Further
purification by flash chromatography followed by recrystallization provided
pure title
compound. MS: m/z (M-1 ) 506
Example 108
5-f({5-(benzYloxy)-1-f2.4-bis(trifluoromethyl)benzyll-2-methyl-1H-indol-3-
yl carbo vl)aminolisophthalic acid
Step 1: The acid prepared in step 3 ( 1 eq) of example 108 was reacted with
EDCI (2 eq) and
dimethyl 5-aminophthalate (5 eq) in THF (0.02 M) in the presence of DMAP (2
eq). The
reaction was heated to reflux for 48 h. EtOAdwater work up, followed by flash
chromatography produced the desired amide in 32 % yield.
Step 2: The material from step 1 ( 1 eq) was hydrolyzed by the action of
LiOH~H20 (2.2 eq) in
THF/MeOH/water (3/1/1, 0.07 M). After stirring at 25°C overnight, the
reaction mixture was
quenched with AcOH and solvent was evaporated. EtOAc/water work up and
trituration in
hexane/CH,C12 afforded the title compound in 82 % yield. MS: m/z (M-1 ) 669
Example 109
5-lbenzyloxy)-2-methyl-1-(2-naphthylmethyll-1H-indole-3-carboxylic acid
Step 1: An analogous procedure to step 2 example 108 using the main product of
step 1 above
and the appropriate bromide yielded the desired N-substituted indole in 7I %
yield after
recrystallization.
Step 2: The ester from step 2 above (1 eq) in THF/MeOH (3/1) was heated to
reflux with 4N
KOH (2 eq). After S days solvent was evaporated and the residue partitioned
between 1N HCl
and CHC13. The organic extract was washed, dried and concentrated. The title
compound was
obtained in 92 % yield after chromatographic purification and crystallization.
MS: mlz ( M -1 )
420
101
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 110
5-l ~ f 5-(benzyloxy)-2-methyl-1-l2-naphthXlmethyl)-1H-indol-3-
yllcarbonyllamino)isophthalic acid
Step l: The acid in Example 109 was converted in the corresponding amide
following an
analogous procedure to step 1 of Example 108. The product was contaminated
with the aniline
starting material which could only be partially removed by chromatography.
Step 2: Hydrolysis of the crude material following step 2 Example 108 provided
the title
compound after chromatographic purification (4 % yield in Example 109).
Example 111
1-benzyl-5-(benzyloxy)-2-methyl-1H-indole-3-carboxylic acid
Step 1: The minor product of step 1 ( 1 eq) Example 107 was dissolved in THF
(0.1 M).
KOH (2 eq) and 18-crown-6 (2 eq) were added and the resulting mixture was
heated to reflux
for 1.5 days. Work up as on step 2 Example 108 above provided the title
compound in 32 %
yield. MS: m/z (M-1 ) 370
~,xample 112
3-[(2-{5-(benzyloxy)-1-(4-chlorobenzyl)-2-[(2-naphthylsulfanyl)methyl)-1H-
indol-3-yl}-2-oxoacetyl)amino)benzoic acid
Step 1 The starting ethyl 5-benzyloxyindole-2-carboxylate (Scheme 21, step 1)
was treated
with LAH ( 1.3 eq) in THF (0.27 M) at 0 °C under nitrogen for 1 h.
Workup with NaOH and
water followed by concentration afforded crude product ( 100%).
Step 2 The crude alcohol from step 1 was dissolved in DMF (0.38 M), and
treated with t
butyldimethylsilyl chloride ( 1.16 eq) and imidazole ( 1.26 eq) at 25
°C for 1 d. Workup and
chromatographic purification afforded the pure product (93%).
Step 3 The silyl ether from step 2 was dissolved in methylene chloride (0.26
M), and treated
with BOC anhydride (1.24 eq), triethylamine (1.53 eq) and DMAP (0.21 eq) at 25
°C for 3 d.
Workup and chromatographic purification afforded the pure product (99%).
Step 4 The N-BOC silyl ether from step 3 was treated with acetic acid/ water/
THF
(3:1:1) (0.04 M) at 25 °C for 1 d. Workup and chromatographic
purification afforded the pure
product ( 100%).
Steps 5 The alcohol from step 4 was dissolved in methylene chloride (0.2 M),
and
under nitrogen at -40°C treated with triethylamine ( 1.33 eq), and
mesyl chloride ( 1.23 eq) for
1 h. In a separate dry flask was weighed naphthalene-2-thiol ( 1.31 eq), and
THF ( 1 M) was
added, followed by lithium hexamethyldisilazide (1N in THF, 1 eq) and this
mixture was
102
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
stirred at 25°C for 30 min. The resulting solution was then added
dropwise, over 30 minutes,
to the above mesylate solution, at -40°C. The reaction mixture was
allowed to warm to 25°C,
and stirred there for 4.5 h. Workup and chromatographic purification afforded
the BOC
thioether.
Step 6 The purified BOC thioether from step 5 was heated under nitrogen at 160
170°C for 1.25 h, and recrystallized from ethyl acetate and hexanes to
afford the free indole
thioether in 64% yield.
Step 7 The indole thioether from step 6 was dissolved in DMF (0.2 M), and
treated
with sodium hydride ( 1.1 eq) at 25°C for 45 min. 4-Chlorobenzyl
chloride ( 1.3 eq) and KI
(cat.) were added, and the mixture was stirred at 25°C for 3 d. Workup
(ethyl acetate/water)
and trituration (ethyl acetate/hexanes) afforded the pure product (52%).
Step 8: A solution of EtMgBr in ether (3 N, 2.17 eq) was cooled to - 70
°C. The product of
step 7 in scheme 21 ( I eq) in ether (0.55 M) was added and the reaction
mixture was stirred at -
70 °C for 2 h. After the addition of methyl oxalyl chloride (3 eq) in
ether ( 1.5 M) the reaction
was stirred at - 40 °C for 2 h, allowed to warm to 25 °C.
Quenched with sodium bicarbonate
EtOAc/water work up and crystallization from hexane/EtOAc the desired ketone.
Step 9 The ester from step 8 was hydrolyzed using the general method in step 2
example 108 to
yield the desired alpha keto acid.
Step 10 The indole thioether from step 9 was dissolved in dry methylene
chloride (0.05 M), and treated with oxalyl chloride (2.05 eq) at 0°C
for 1 h. In a separate dry
flask were weighed 3-aminobenzoic acid ( 10 eq) and triethylamine ( 1 S eq) in
methylene
chloride (0.5 M). The resulting solution was then added dropwise, at
0°C, and the mixture was
allowed to warm to 25°C overnight. Workup (methylene chloride/aqueous
HCl) and repeated
purification by chromatography afforded the pure title compound product.
Step 11 The product from step 9 was hydrolyzed using the procedure from step 2
Example I08
to yield the desired compound in 28%. MS: m/z (M-1 ) 709
Exam~,le 113
3-f f2-15-(benzvloxv)-1-methyl-2-f (2-nanhthvlsulfanvl)methvll-1H-indol-3-
3~~~-2-oxoacetyllaminolbenzoic acid
Step 1 Following step 4 of the above procedure using methyl iodide followed by
trituration (ethyl acetate/hexanes) afforded the pure product (72%).
103
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Step 2 An analogous procedure to step 5 through step 11 above yielded 58% of
the
title compound. MS: m/z (M-1 ) 599
Example 114 .
1-benzhydryl-5-f(cy~lopentylcarbonyllaminol-1H-indole-3-carboxylic acid
IO Step 1 5-nitroindole was alkylated as in Example 3 stepl with the
appropriate
bromide to yield the desired N-alkylated product.
Step 2 The indole from step 1 ( 1.Oeq) was dissolved in DMF (0.4M) and
treated with phosporous oxychloride (6.9 eq) at room temperature and then the
mixture was
stirred for 1 day at 80 C at which time the reaction was poured onto ice and
triturated with ethyl
acetate/hexanes, followed by workup with sodium bicarbonate%hloroform yielded
the C3
formylated product.
Step 3 The nitro indole from step 2 was reduced according to the procedure in
Example 100, step 2 to yield the amino aldehyde.
Step 4 The indole from step 3 was acylated according to the procedure from
Example 100, step 3.
Step 5 The indole from step 4 ( 1.0 eq), 2 methyl-2butene (45 eq),
sodium dihydrogen phosphate ( 11.6 eq). were dissolved in t-BuOH (0.2M), water
(0.2M) and
then sodium chlorite ( 11.6q) was added and the reaction was heated to 65 C
for 24 hours.The
reaction was diluted with water, extracted 3 times with ethyl acetate, dried
and concentrated and
then purified by chromatography to yield the title compound.
Example 115
2-11-benzhvdrvl-5-f (cvclopentvlcar~bo~vl)aminol-1 H-indol-3-vl1-2-oxoacetic
acid
Step 1 Following the procedure of Example 69, 5-niroindole was acylated in the
3-position
with ethylmagnesiumbromide and ethyloxalylchloride.
Step 2 The above intermediate was elaborated to the final product following
steps 2-5
of Example 114 to afford the title compound.
104
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 116
Table I reports data for the compounds described in the examples above in
cPLA2
inhibition assays (described below). In the data columns of Tables I and II,
assay results are
reported as a percent inhibition at the concentration specified.
Coumarin Assav
7-hydroxycoumarinyl 6-heptenoate was used as a monomeric substrate for cPLA2
as
reported previously (Huang, Z. et al., 1994, Nalytical Biochemistry 222, 110-
115). Inhibitors
were mixed with 200 pI. assay buffer (80 mM Heped, pH 7.5, 1 mM EDTA)
containing 60
~.~M 7-hydroxycoumarinyl 6-heptenoate. The reaction was initiated by adding 4
pg cPLA2 in
50 pL, assay buffer. Hydrolysis of the 7-hydroxycounarimyl 6-heptenoate ester
was monitored
in a fluorometer by exciting at 360 nm and monitoring emission at 460 nm.
Enzyme activity is
proportional to the increase in emission at 460 nm per minute. In the presence
of a cPLA2
inhibitor, the rate of increase is less.
Table I
INHIBITION @
1 ~ 7 50
18 100
SO 170
3 50 5
51 6.25
50 .4
41 10
50 17.5
50 19
37 20
38 20
43 20
44 20
50 20
SO 20
50 22
50 23
50 23.5
105
CA 02322162 2000-08-24
WO 99J43654 PCT/US99/03898
50 24
_
39 100
50 5
S 1 6.25
4 SO -
50 11
50 5
50 lI
10 50 20
SO 20
50 30
50 33.5
50 40
50 45
11 42 10
50 12
52 12.5
36 20
SO 27.5
50 30
50 0
50 37
12 50 0.35
50 0.35
SO 0.38
50 0.38
50 0.38
50 0.39
50 0.4
50 0.4
50 0.4
106
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
50 0.44
50 _
r 0.45
64 0.5
86 1.25
13 50 0.39
50 0.4
50 0.48
5 0.55
50 0.6
0 0.65
50 0. 5
50 0.7
50 0.75
50 0.95
73 2.5
81 6.25
14 50 0.7
_
50 0.95
50 0.95
15 0.65
50 0. 5
50 _. _ 0.72
so 0.7
so .ss
90 6.25
16 50 0.125
61 0.125
71 0:125
50 .14
50 0.14
50 0.14
50 0.17
50 0.17
69 0.25
98 6.25
17 50 0.7
50 0.8
50 0.85
50 0.98
I 8 50 1.2
50 1.3
SO 1.9
SO 2
50 2
107
CA 02322162 2000-08-24
WO 99/43654 PGT/US99/03898
50 ~ 2
19 50 2.2
50 4.2
50 5.8
52 6.25
50 7.8
50 9
50 11
so l -. i
to
29 50 2.5
50 3.4
87 6
30 50 8
46 20
SO 21
SO 24
108
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
35 ~ 23 10
42 20
50 41
3 50 0.22
60 0.25
50 0.32
50 0.45
37 50 0.4
50 0.5
50 0.55
50 0.65
38 . 50 0.3
50 0.45
50 0. 7
0 0.59
50 0.6
50 0.6
50 .6
50 0.6
50 0.6
50 0.64
50 0.7
50 0.7
50 0.85
50 0.85
50 1
50 1
39 50 0.39
50 0.7
50 0.73
50 0.75
SO 0.75
50 0.8
SO 0.9
50 0.9
SO 1
50 1
109
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
50 1.2
50 1.3
50 1.6
40 50 2.5
__ _
55 2.5 ~~~
50 ~~~~~ 3
50 3.6
41 50 2.5
50 3.8
50 4.3
s0 s
42 50 2.2
50 3
50 3.8
44 50 1.65
50 1.7
50 1.75
50 1.9
50 2.1
71 2.5
97 6.25
4 ~ 50 1.75
50 1.8
50 1.9
50 2
50 2.1
74 2.5
46 50 2.2
67 2.5
50 2.7
50 3.5
50 4.5
49 50 1.5
50 1.8
50 2.3
50 50 0.8
50 0. 8
50 0.85
50 1.05
110
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
81 2.5
10
51 50 0.6
50 0.8
50 0.9
52 50 19
50 _
19
50 20
57 50 1.05
50 1.38
50 1.4
60 50 12.5
65 47 50
SO 72
_
50 80
111
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
66 50 ~~~ 70
15 200
19 200
69 50 12.5
71 69 6
_ - 1.5 -
50 - _. - 3 .5 -. -_
r. 50 3.8
72 SO 12.5
7 SO 4
20
112
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
84 22 ~ 100
50 265
5 350
86A 50 60
_
50 70
50 82
50 118
86B
87B 50 38
50 38
50 42.5
88 50 1.25
53 1.25
50 1.32
92 50 11
50 12.5
50 14.2
113
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
97 50 50
50 80
50 94
98 50 4.8
6.25
50 8.7
99 13 30
38 100
50 100
50 100
100 50 24
_
50 30
50 80
105 50 2.4
50 7
74 10
107 50 80
_
50 71
43 5
50 37
50 37
108 67 6.25
15 20
50 48
46 50
46 50
109 28 50
114
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
25 50
112 53 -[ 2.5 J
114 45 100
50 152
50 170
115 89 50
20 100
50 250
117 50 1.6
118 50 0.6
119 50 2.5
120 SO 1
121 20 1.
122 64 1.25
123 50 1.2
124 50 1.3
125 50 0.8
126 50 5.5
127 SO 1.1
128 50 0.9
129 50 1.1
130 50 2
131 SO 0.6
115
CA 02322162 2000-08-24
WO 99/43654 PC'T/US99/03898
10
I32 50 0.4
133 SO ~ 0.3
134 50 0.8
135 50 0.7 ~
13 50 0.4
137 _ - 50 - T -p.g_
I38 50 0.4 J
Compounds of the present invention were also tested for in vivo activity in a
rat paw
edema test according to the procedure described below. The results are
reported in Table II.
Rat Carrag~enan-Induced Footpad Edema Test
Each compound was suspended in 0.3m1 absolute ethanol, 0.1 ml Tween-80 and 2.0
ml Dulbecco's PBS (without calcium or magnesium). To this mixture, O.lml 1N
NaOH was
added. After solution was complete, additional amounts of PBS were added to
adjust the
concentration to 1 mg/ml. All compounds remained in solution. Compounds were
administered i.v. in a volume of 5 ml/kg to male Sprague Dawley rats at the
same time that
edema was induced by injection of O.OSmI of 1 % Type IV carrageenan into the
hind footpad.
Footpad volume was measured before dosing with compound and 3 hours after
dosing with
carageenan.
116
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Table II
Example ROUTE DOSE PERCENT
of (mg/Kg) INHIBTTION
ADMIN.
1 IV 5 2.51
IV 5 16. 1
2 IV 5 15.87
3 IV 5 10.38
PO 5 21.5
IV 5 22.84
IV 5 14.86
PO- 20 19.56
I .~ 5 T -_-10.38
20
4 IV 5 24.13
IV 5 4.95
5 IV 5 8.88
IV 5 24.28
IV 5 0.09
7 IV 5 -0. 5
8 IV -5.7
9 IV 5 4.46
10 IV 25.32
11 IV 5 13.98
12 PO 2 0.19
PO 10 -0.38
13 PO 2 25.99
PO 10 23.63
14 PO 2 ~ 11.53
PO 10 8.14
15 PO 2 7.05
PO 10 6.88
16 PO 2 3.8
PO 10 14.96
117
CA 02322162 2000-08-24
WO 99/43654 PCTNS99/03898
17 PO 2 19.29
PO 10 34.52
19 IV ~ 5 21.17
IV 5 13.32
IV 5 -0.09
21 N 5 16.18
N 5 19.01
N 5 8.66 J
22 N 5 9.22
N 5 4.14
23 N 5 15.71
N 5 14.45
IV 5 2.12
24 N 5 8.33
N 5 16.28
N 5 11.3
25 N 5 2.73
N 5 8.66
IV 5 16.02
26 5 25.31
27 N 5 6.48
28 N 5 0.29
30 ~ N 5 13.89
PO 2 _0.11
I to ~ 13.2s
37 PO 2 -7.94
PO 10 3.36
38 PO 2 15.44
PO 10 2 .32
39 PO 2 1.98
PO 10 -7.16
40 N 5 8.21
41 IV S 10.1
42 IV 5 7.72
118
CA 02322162 2000-08-24
15
25
WO 99143654 PCT/US99/03898
44 N 5 11.9 I
45 N 5 10.19
46 N 5 4.58
49 N 5 18.02 J
50 PO 2 ~ 5.44
PO 10 - ~ 12.34
51 PO 2 _ 3.23
PO 10 15.37
52 PO 2 .75
PO 10 3.33
53 PO 2 -1.81
PO 10 11.35
54 PO 2 2.47
PO 10 14.29
55 2 7.02
PO 10 21.51
PO 2 4.22
PO 10 9.34
~
57 PO 2 10.44
PO 10 20.68
58 PO 2 13.85
PO 10 9.96
59 N 2.9
61 N 5 18.33
63 N 5 19.59
5 N 5 2.84
66 IV~ S 25.34
67 N 5 10.78
8 N S -4.3
119
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
76 N S 14.84
80 N 5 l0.lg I
82B N 5 4.94 J
84 N 5 6.15-I
85 N S 7.13-I
86A N 5 7.4 J
87A PO ~~ 2 12.89
PO 10 25.44
87B PO 3 17.92
PO 10~ 31.4
89 PO 2 14.34
P 10 16.38
90 PO 2 -0.18
~_. ' P~ _I10 r 2.7-~
91 PO~ 2 r 13.5
PO 10 14.67
92 PO 2 27.3b
PO 10~ 21.34
93 PO 2 ~ -3.02
PO 10 9.91
94 PO 3 3.13
PO 10 4.46
PO 2 19.04
PO 10 27.45
95 PO 2 14.86
PO 10 23.19
9 PO 2 29.42
PO 10 21.99
97 N 5 21.31
98 N 5 18.39
99 PO 10 22.77
PO 2 24.51
120
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
100 PO 2 ~ 6.14
PO 10 20.7
101 PO 10 12.4s
PO 2 11.17
102 PO 2 2.s6
PO 10 8.48 - I
103 PO 10 17.31
PO 2 _~ _1 ~5 _
104 PO 2 14.49
PO 10 .O1
10s lv s l.sl
114 PO 2 12.15
PO 10 22.19
l l s PO 2 1.24
PO 10 18.46
Example 117
is 2-{4-fll-benzhydryl-6-chloro-1H-indol-3-yllmethyll-2.6-
dimethylnheno,~vlacetic acid
Step 1: To I-benzhydryl-6-chloro-1H-indole (I.0 eq) and methyl 2-(4-formyl-2,6-
dimethylphenoxy)acetate (0.6 eq) in CHZC12 (O.1M) at 0°C was added neat
triethysilane (3eq)
followed by triflouroacetic acid (3eq). After 10 minutes at 0°C the
reaction was warmed to
room temperature and stirred until the initially formed spot by TLC yields a
new spot. The
reaction was then quenched by the addition of saturated sodium bicarbonate,
diluted with
CHZC12 and washed with saturated sodium bicarbonate, water and brine, dried
over magnesium
sulfate and purified by column chromatography to yield 89% of the desired
product.
2s Step 2 The resulting ester was hydrolyzed as in example 1 step s to yield
the title compound
after trituration and/or column chromatography. m/z (M-1)s08.3
121
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 11$
2-14-[(1-benzhydryi-6-chloro-1H-indol-3-yl)methvll-3-
methoxyphenoxy)~acetic acid
Step l:This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole and
methyl 2-
(4-formyl-3-methoxyphenoxy)acetate according to the procedure in Example 117
Step 1.
Step 2: The ester intermediate was hydrolyzed according to step 2 Example 117
to yield the
title acid.
Example 119
2-;[4-f(1-benzhydryl-6-chloro-1H-indol-3-vl)methvllphenoxylacetic acid
Step I:This compound was prepared from the 1-benzhydryl-6-chloro-IH-indole and
methyl 2-
(4-formylphenoxy)acetate according to the procedure in Example 117 Step 1.
Step The ester intermediate was hydrolyzed according to step 2 Example 117 to
yield the title
acid.
Example 120
2-14-((1-benzhvdrvl-6-chloro-1H-indol-3-vl)methvll-3-chloronhenoxvlacetic
acid
Step I:This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole and
methyl 2-
(3-chloro-4-fonmylphenoxy)acetate according to the procedure in Example 1 I7
Step 1 in 70%
yield.
Step 2: The ester intermediate was hydrolyzed according to step 2 Example 117
to yield the
title acid.
Example 121
2-14-f (1-benzhydryl-6-chloro-1H-indol-3-yl)meth~r~l-2-
methoxyphenoxvlacetic acid
Step l:This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole and
methyl 2-
(4-formyl-2-methoxyphenoxy)acetate according to the procedure in Example 117
Step 1 in
71 % yield.
Step 2: The ester intermediate was hydrolyzed according to step 2 Example 117
yield the title
acid. m/z (M-1)510.2
122
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 122
(E)-4-~[4-f (1-benzhydryl-6-chloro-1H-indol-3-vl)methyllphenoxvl-2-butenoic
acid
Step 1: This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole
and (E)-4-(4-
formylphenoxy)-2-butenoate according to the procedure in Example 117 Step 1 in
91 % yield.
Step 2:The ester intermediate was hydrolyzed according to step 2 Example 117
to yield the title
acid. m/z (M-1)506.3
Example 123
4-~4-f (1-benzhydryl-6-chloro-1H-indol-3-vl)methvlianilino}-4-oxobutanooic
acid
Step I This intermediate compound was prepared from the 1-benzhydryl-6-chloro-
1H-indole
and 4-nitrobenzaldehyde according to the procedure in Example 117 Step 1 in
42% yield.
Step 2 -benzhydryl-6-chloro-3-(4-nitrobenzyl)-1H-indole was reduced by
dissolving in THF
(0.1 M), subjecting it to 1 atmosphere of hydrogen gas in the presence of
10%platinum on
carbon catalyst (25%w/w). When the starting material had all been converted to
a new spot by
TLC analysis the reaction was filtered and concentrated to yield the desired
intermediate in
nearly quantitative yield.
Step 3:To the intermediate above ( 1.0 eq) in CHZC12 (O.1M) at 0°C was
added triethylamine
( 1.5 eq) followed by 3-carbomethoxyproprionyl chloride( 1.5 eq). The reaction
was warmed to
room temperature, stirred until complete disappearance of starting material as
monitored by
TLC, and then worked by the addition of saturated sodium bicarbonate, dilution
with CHZC12,
and washing the organic layer with water, saturated sodium bicarbonate and
brine, dried,
concentration and purified by column chromatography to yield the desired
compound in 81 %
yield.
Step4: The ester from step 3 was then hydrolyzed according to step 2 Example
117 to yield the
tide acid. m/z (M-1)521.3
Example 124
sodium 3-{4-f f 1-benzhydryl-6-chloro-1H-indol-3;v1)methyllanilinol-3-
gxopro~anoic acid
Step 1 The intermediate from example 117, step 1 was acylated with methyl
malonyl chloride
according to the procedure for step 1 of Example 117 in 82 % yield.
Step 2 The ester was hydrolyzed according to step 2 for Example 123 to yield
the title
compound. m/z (M-1 )507.2
123
CA 02322162 2000-08-24
WO 99143654 PCT/US99/03898
Example 125
2-f 4-f (1-benzhyd~yl-6-chloro-1H-indol-3-yl)methyy~l~anilino~-2-oxoacetic
acid
Step 1 The intermediate from example 117, step 1 was acylated with methyl
oxalyl chloride
according to the procedure for step 1 of Example 117 in 67 % yield.
Step 2 The ester was hydrolyzed according to step 2 for Example 117 to yield
the title
compound. m/z (M-1)493.2
Example 126
2-f(1-benzhydryl-6-chloro-1H-indol-3-yl)methyllcyciopropanecarboxviic acid
Step 1: This intermediate compound was prepared from the 1-benzhydryl-6-chloro-
1H-indole
and ethyl 2-formyl-1-cyclopropanecarboxylate according to the procedure in
Example 117 Step
1 in 53% yield.
Step 2: The ester was hydrolyzed according to step 2 for Example 117 to yield
the title
compound in 93 % yield. m/z (M-1 ) 1414.2
Example 127
2-fll-benzh~r_dryl-6-chloro-5-fluoro-1H-indol-3-
yllmethyllc~eclopropanecarboxylic acid
Step 1: 6-chloro-5-flouroindole was N-alkylated with benzhydryl bromide
according to the
procedure in Example 69 step 2 to yield the target intermediate.
Step 2: The product from step 1 was C3 acylated with ethyl 2-formyl-1-
cyclopropanecarboxylate according to the procedure in Example 117 Step 1 in
53% yield.
Step 3: The ester was hydrolyzed according to step 2 for Example 117 to yield
the title
compound in 73 % yield. m/z (M-1)432.2
Example128
2-f ll-benzhydrvl-5.6-dichloro-1H-indol-3-YI_lmethyllcyclopropanecarboxylic
acid
Step 1: 5,6-dichloroindole was n alkylated with benzhydryl bromide according
to the procedure
in Example 69 step 2 to yield the target intermediate in 70% yield.
Step 2: The intermediate from step 1 was C3 acylated with ethyl 2-formyl-1-
cyclopropanecarboxylate according to the procedure in Example 117 Step 1 in
62% yield.
Step 3: The ester was hydrolyzed according to step 2 for Example 117 to yield
the title
compound in 73 % yield. m/z (M-1)448.2
124
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 129
2-(11-f bis(4-hvdroxyphenyl)methyll-6-chloro-1H-indol-3-
yl}methyllc~propanecarboxylic acid
Step 1: 6-chloroindole was C3 alkylated with ethyl 2-formyl-1-
cyclopropanecarboxylate
according to the procedure in Example 117 Step 1.
Step 2: The intermediate ffrom step 1 (2.0 eq.) was dissolved in THF (0.5 M)
and cooled to
-40°C and then triethylamine (2.0 eq) was added followed by
methanesulfonyl chloride (2.0
eq). The reaction was stirred at this temperature until TLC analysis indicated
no more starting
alcohol, and then it was cannulated directly into a mixture of the c3
alkylated indole from step
1 ( 1.0 eq) in DMF ( 1.0 M) at -20°C that had been stirred for 30
minutes at room temperature
with sodium hydride (4.0 eq of a 60% dispersion). The resulting mixture was
warmed to room
temperature overnight and quenched when the reaction was deemed complete by
the addition of
saturated ammonium chloride, diluted with ethyl acetate and washed with
saturated ammonium
chloride, saturated sodium bicarbonate and water (2X), dried, concentrated and
purified by
column chromatography.
Step 3: The intermediate from step 2 was dissolved in THF ( 1.OM) and treated
with a solution
of tetrabutylammonium flouride (2.5 eq) and stirred at room temperature until
TLC analysis
indicates that both siIyl ethers had been cleaved. The reaction was then
poured into saturated
ammonium chloride and extracted with ethyl acetate (3X), the combined organic
washed were
washed with water, brine, dried and concentrated and purified by column
chromatography to
yield the intermediate in 73 % yield.
Step 4: The ester from step 3 was hydrolyzed according to step 2 for Example
123 to yield the
title compound in 92% yield. m/z (M-1)447.12
Example 130
'4-f(1-benzhvdryl-6-chloro-1H-indol-3-yl)meth~ll-3-hydroxybenzoic acid
Step l:This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole and
4-
hydroxy-2-methoxybenzaldehyde according to the procedure in Example 117 Step
1.
Step 2: The ester was hydrolyzed according to step 2 for Example 117 to yield
the title
compound
Example 131
'4-f(1-benzhydryl-G-chloro-1H-indo~-:~yll-~,g~~ylL~~3-
h~droxypropoxy)benzoic acid
Step 1: The intermediate from Example 130, step 1, was dissolved in DMF
(1.OM), solid
potassium carbonate (3 eq) followed by 2-(3-bromopropoxy)tetrahydro-2H-pyran (
1.5 eq) was
added and the reaction was left to stir for 24 hours at room temperature. The
workup consisted
125
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
of diluting with half saturated ammonium chloride and ethyl acetate,
extracting aqueous layer
with ethyl acetate (2X), washing the organic layer with water (2X), drying,
concentration
followed by purification via column chromatography.
Step 2: The intermediate from step 1 was dissolved in THF (1.OM), treated with
glacial acetic
acid (2.0 eq) and heated at 45°C for 24 hours, at which time the
reaction was partitioned
between saturated sodium bicarbonate and ethyl acetate, the combined organic
layers where
washed with water (2X), dried, concentrated and purified by column
chromatography to yield
88% of the desired compound.
Step 3: The ester was hydrolyzed according to step 2 for Example 123 to yield
the title
compound. m/z (M-1)524.3
Example 132
'4-(( 1-f (4-aminophenyll(phen,yllmethvll-6-chloro-1H-indol-3-yl )~methy[,1-3-
methoxybenzoic acid
Step l:This compound was prepared from 6 chloroindole and methyl 2-(4-formyl-2-
methoxyphenoxy)acetate according to the procedure in Example 117 Step 1 in 61
% yield.
Step 2: The intermediate from step 1 was N-alkylated according to the
procedure for Example
129, step 2, with tert-butyl N-{4-[hydroxylphenyl)methyljphenyl)carbamate.
Step 3: The nitrogen protection was removed by heating the compound to
180°C to yield 45%
of the desired amino ester.
Step 4: The intermediate from step 3 was hydrolyzed following step 2 for
Example 117 to yield
the title compound in 78% yield. m/z (M-1)495.2
Example 133
'4-(16-chloro-1-f (4-methoxYphenyll(phenvllmethyll-1H-indol-3-yllmethvl)-3-
methoxybenzoic acid
Step 1: The intermediate from Example 132, step 1, (1.0 eq) was dissolved in
DMF (1.OM),
cooled to 0°C, and treated with sodium hydride ( 1.5 eq) and stirred
for 30 minutes to affect
deprotonation. The 1-[bromo(phenyl)methyl]-4-methoxybenzene ( I .5 eq), as a
solution in
DMF (2.OM), was added to the anion and the reaction was warmed to room
temperature, when
the reaction was deemed complete by TLC analysis it was partitioned between
ethyl acetate and
half saturated ammonium chloride, extracting the aqueous layer with ethyl
acetate (2X),
washing the organic layer with water (2X), drying, concentration followed by
purification via
column chromatography yielded the desired intermediate.
Step 2: The intermediate from step 1 was hydrolyzed following step 2 for
Example 117 to yield
the title compound. m/z (M-1)510.2
126
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 134
'4-((1-ibis(4-methoxyphenyl)methyl]-6-chloro-1H-indol-3-yl}methyl)-3-
methoxvbenzoic acid
Step 1: The intermediate from Example 132 was N-alkylated with 1-[bromo(4-
methoxypheny))methyl]-4-methoxybenzene according to the procedure described in
Example
133, step 1, to yield the desired intermediate.
Step 2: The intermediate from step I was hydrolyzed following step 2 for
Example 117 to
yield the title compound. m/z (M-1)540.3
Example 135
'4-(d6-chloro-1-f (2-mornholinophenyl)(phenyl)methyll-1H-indol-3-
IS yl~~methyl)-3-methox~benzoic acid
Step 1: The intermediate from Example 132 was N-alkylated according to the
procedure for
Example 129, step 2, with the appropriate electrophile.
Step 2: The intermediate form step 1 was hydrolyzed following step 2 for
Example 117 to yield
the title compound.
Example 136
4-(;6-chloro-1-f(2 4-dimethoxy-5-uvrimidinyl)~,phenyl)methyll-1H-indol-3-
yl_ methyl)-3-methoxybenzoic acid
Step I: The intermediate from Example 132 was N-alkylated according to the
procedure for
Example 129, step 2, with the appropriate electrophile to yield the desired
intermediate in 16%
yield.
Step 2: The intermediate from step 1 was hydrolyzed following step 2 for
Example 117 to yield
the title compound. m/z (M-1)542.3
Example 137
'4-f(1-benzhydrvl-6-chloro-1H-indol-3-yl)methvll-3-methoxvbenzoic acid
Step ):This compound was prepared from the 1-benzhydryl-6-chloro-1H-indole and
the
appropriate aldehyde according to the procedure in Example 117 Step I.
Step 2: The intermediate from step 1 was hydrolyzed following step 2 for
Example 117 to yield
the title compound. m/z (M- I )481.14
127
CA 02322162 2000-08-24
WO 99/43654 PCT/US99/03898
Example 13$
2-l~4-lll-benzhydryl-6-chloro-1H-in~dol-3-yl)methyl)-3-
methoxybenzoyllamino)acetic acid
Step 1: The intermediate from Example 137, step 2, treated with glycine ethyl
ester according
to the procedure in Example 76 to yield the desired ester.
Step 2: The intermediate from step 1 was hydrolyzed following step 2 for
Example 117 to yield
the title compound. m/z (M-1)537.2
All patents and literature references cited herein are incorporated as if
fully set forth
herein.
128