Note: Descriptions are shown in the official language in which they were submitted.
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-1-
Title: METHOD AND APPARATUS FOR MEASURING PROTEINS
GELD OF INVENTION
This invention relates to immunoturbidimetry and spectrophotometric analysis
of
plasma for proteins.
BACKGROUND OF INVENTION
Clinical laboratory tests are routinely performed on serum or plasma of whole
blood. In a routine assay, red blood cells are separated from plasma by
centrifugation, or
red blood cells and various plasma proteins are separated from serum by
clotting prior to
centrifugation.
Haemoglobin (Hb), bilirubin (BR), biliverdin (BV), and light-scattering
substances like lipid particles are typical substances which will interfere
with and affect
spectrophotometric and other blood analytical measurements. Such substances
are
referred to generally, and in this specification as interferents. Elevated BR
and BV
referred to as bilirubinemia and biliverdinemia respectively can be due to
disease states,
increased lipid particles in the blood also known as lipemia, can be due to
disease states
and dietary conditions; elevated Hb in the blood known as haemoglobinemia can
be due to
disease states and as a result of sample handling.
Many tests conducted on plasma or serum samples employ a series of reactions
which terminate after the generation of chromophores which facilitate
detection by
spectrophotometric measurements at one or two wavelengths. Measurement of the
quantity of interferents in a sample prior to conducting such tests is
important in providing
meaningful and accurate test results. In fact if a sample is sufficiently
contaminated with
interferents, tests are normally not conducted as the results will not be
reliable.
Current methods used for detecting haemoglobinemia, bilirubinemia and lipemia
or turbidity utilize visual inspection of the sample with or without
comparison to a color
chart. Visual inspection is sometimes employed on a retrospective basis where
there is a
disagreement between test results and clinical status of the patient in order
to help
explain such discrepancies.
Pre-test screening of samples by visual inspection is semi-quantitative at
best, and
highly subjective and may not provide sufficient quality assurance as required
for some
tests. Furthermore, visual inspection of samples is a time consuming, rate
limiting process.
Consequently, state-of-the-art blood analyzers in fully and semi automated
laboratories
do not employ visual inspection of samples.
Other methods used to assess the amount of contamination of a sample, i.e.,
sample integrity, employ direct spectrophotometric measurement of a diluted
sample in a
special cuvette. In order to obtain a measurement of the sample of the plasma
or serum,
sample tubes must be uncapped, a portion of the sample taken and diluted prior
to
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-2-
measurement. Both of these steps are time consuming and require disposable
cuvettes.
An apparatus used for measuring sample integrity can also be used to measure
plasma proteins, e.g., Immunoglobulin A (IgA), (32-microglobulin and C-
reactive protein
(CRP). To do so, an antibody reagent is required for each protein, and a
37°C incubation
chamber. This method of analysis is called immunoturbidimetry because the
specific
antibody reagent forms immunocomplexes with the corresponding protein, when
present in
the sample. The immunocomplexes scatter light in various directions depending
on the
size distribution of the immunocomplexes or particles; turbidity in a sample
is a result of
scattered light and the absorbance increase is inversely proportional to
wavelength. It
must be understood that the use of the term absorbance includes "true
absorbance" and the
effect of light loss by any other means; the detector in the spectrometer
measures the
light transmitted through the sample, and absorbance is calculated as the
negative log of
transmittance. Therefore, any light which does not reach the detector, e.g.,
due to
scattering caused by turbidity, will be interpreted as absorbed light.
For proteins in low concentrations, e.g., in the order of mg/L, the turbidity
created
by immunocomplexes is very small and are usually measured in one of two ways:
1)
Measurement of light scattered in the forward direction on an instrument
called a
nephelometer, which is like a spectrophotometer that measures light propagated
at an
acute angle to the incident light. Such a method would require a separate
instrument
which would increase the cost per test; 2) Measurement of "absorbance" at
340nm by a
spectrophotometer. In the prior art which uses absorbance measurements, the
absorbance
at 340nm at zero time is subtracted from the absorbance at 340nm after
incubation at
approximately 37°C for approximately five minutes, in order to remove
the effect of
sample interferents. This approach cannot be used for the near infrared (NIR)
and
adjacent visible wavelengths where the light-scattering caused by the
immunocomplexes
is very small.
It is desirable to use an apparatus designed for measuring plasma and serum
interferents to perform immunoturbidimetric measurements. This feature allows
tests
which are not available on general chemistry analyzers, to become available,
and at the
same time the apparatus can provide a screening system for serum and plasma
interferents.
The present invention uses a novel wavelength range and method to subtract
endogenous sample turbidity and the effect of other interferents. The present
invention
uses a disposable dispensing tip in a novel way both as a reaction and
incubation chamber,
as well as a cuvette. The use of a disposable dispensing tip as a reaction
chamber and
cuvette allows this invention to be integrated into a chemistry analyzer, or
built as a
stand-alone instrument for measuring serum and plasma interferents as well as
plasma
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-3-
proteins. This invention is particularly relevant to chemistry analyzers which
do not
already possess similar optical hardware as described for this invention,
which could
facilitate the measurement of serum and plasma interferents, and plasma
proteins. By
integrating such optical capabilities in the chemistry analyzer, the current
test menu can
be expanded by offering immunoturbidimetric measurements.
Accordingly, the present invention provides an apparatus for determining the
concentration of one or more plasma proteins in a sample by
immunoturbidimetry, said
apparatus comprising:
a blood analyzer;
a disposable dispensing tip;
means for sealing a first end of the disposable dispensing tip;
a second tip capable of being inserted into an open second end of the
disposable
dispensing tip for adding one or more reagents to the disposable dispensing
tip;
a heated cavity for receiving the sample in the disposable dispensing tip of
the analyzer;
means for transferring the disposable dispensing tip into and out of the
heated
cavity;
a radiation source for emitting a beam of radiation;
means for directing the radiation onto the sample in the disposable dispensing
tip;
a sensor responsive to receipt of the radiation; and
means for correlating said concentration of the one or more proteins in the
sample to
a sensor response from the sample. Preferably the means for sealing is a vice
and the
radiation source means, means for directing said radiation onto said sample,
and sensor
are contained in a spectrophotometer. More preferably the beam of radiation is
near
infrared and adjacent visible region light and has wavelengths from about
475nm to about
910nm.
An apparatus of the invention for the correlation referred to above
incorporates
calibration algorithms in respect of IgA, (i2-microglobuiin and C-reactive
protein (CRP)
respectively which are:
a . mg/L IgA = - a(Xnm) + b(Y nm) - c
where a, b and c are coefficients of the first derivative of absorbances at
the wavelengths
X and Y; {Xnm) is the first derivative of the absorbance at wavelength X;
(Ynm) is the
first derivative of the absorbance at wavelength Y; preferably a = 3327100-
3327120, b =
484250-484290 and c = 70-85, more preferably a = 3327114.33, b = 484270.80 and
c = 77.3;
where X is about 780-800 nm, and Y is about 820-830 nm, preferably X is about
789 nm and Y
is about 825 nm
b. mg/L (32-microglobulin = a(Xnm) + b(Ynm) + c
where a, b and c are coefficients of the first derivative of absorbances at
wavelengths X
and Y; (Xnm) is the first derivative of the absorbance at wavelength X; (Ynm)
is the first
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-4-
derivative of the absorbance at wavelength Y; preferably a = -33640-33660, b =
36550-
36560 and c = 2-3, more preferably a = -33648.79, b = 36556.81 and c = 2.3;
where X is about
545-550 nm and Y is about 825-835 nm, preferably X is about 548 nm and Y is
about 829 nm;
c. mg/L CRP = a(Xnm) + b(Ynm) + c
where a, b and c are coefficients of the first derivative of absorbances at
wavelengths X
and Y; (Xnm) is the first derivative of the absorbance at wavelength X; (Ynm)
is the first
derivative of the absorbance at wavelength Y; preferably a = (-1813675)-(-
1813685), b =
1808670-1808680 and c = 9.5-10, more preferably a = -1813682.71, b =1808677.58
and c = 9.8;
where X is about 655-665 run and Y is about 675-685 nm, preferably X is about
661 nm and Y
is about 679 nm.
In another aspect the invention, there is provided a method for determining
the
concentration of one or more plasma proteins in a sample by immunoturbidimetry
in a
blood analyzer, the method comprising:
filling a disposable dispensing tip with the sample;
sealing a first end of the tip with means for sealing;
adding a reagent to an open second end of the disposable dispensing tip with a
second tip capable of being inserted into the open end;
placing the disposable dispensing tip into a heated cavity;
radiating the sample in the disposable dispensing tip with a source which
emits a
beam of radiation;
sensing the radiation having passed through the sample;
correlating the concentration of said one or more proteins in said sample to
the
sensor response from the sample. The disposable dispensing tip which contains
the
reagent or reagents and sample may be removed from the heated cavity prior to
being
subjected to radiation. The preferred means for sealing is a vice. The method
also
contemplates that the beam of radiation is near infrared and adjacent visible
region
light, preferably the near infrared and adjacent visible region light has
wavelengths
from about 475nm to about 910nm.
Concerning this method the correlation referred to above incorporates
calibration
algorithms in respect of IgA, (32-microglobulin and C-reactive protein (CRP)
respectively
which are:
a . mg/L IgA = - a(Xnm) + b(Y nm) - c
where a, b and c are coefficients of the first derivative of absorbances at
the wavelengths
X and Y; (Xnm) is the first derivative of the absorbance at wavelength X;
(Ynm} is the
first derivative of the absorbance at wavelength Y; preferably a = 3327100-
3327120, b =
484250-484290 and c = 70-85, more preferably a = 3327114.33, b = 484270.80 and
c = 77.3;
where X is about 780-800 nm, and Y is about 820-830 nm, preferably X is about
789 nm and Y
is about 825 nm
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-5-
b. mg/L (32-microglobulin = a(Xnm) + b(Ynm) + c
where a, b and c are coefficients of the first derivative of absorbances at
wavelengths X
and Y; (Xnm) is the first derivative of the absorbance at wavelength X; (Ynxn)
is the first
derivative of the absorbance at wavelength Y; preferably a = -33640-33660, b =
36550-
36560 and c = 2-3, more preferably a = -33648.79, b = 36556.81 and c = 2.3;
where X is about
545-550 nm and Y is about 825-835 nm, preferably X is about 548 nm and Y is
about 829 nm;
c. mg/L CRP = a(Xnm) + b(Ynm) + c
where a, b and c are coefficients of the first derivative of absorbances at
wavelengths X
and Y; (Xnm) is the first derivative of the absorbance at wavelength X; (Ynm)
is the first
derivative of the absorbance at wavelength Y; preferably a = (-1813675)-(-
1813685), b =
1808670-1808680 and c = 9.5-10, more preferably a = -1813682.71, b =
1808677.58 and c = 9.8;
where X is about 655-665 nm and Y is about 675-685 nm, preferably X is about
661 nm and Y
is about 679 nm.
The present invention also provides a method for determining the concentration
of
plasma protein IgA, ~i2-microglobulin or C-reactive protein in a plasma sample
by
immunoturbidimetry in a blood analyzer, said method comprising:
aspirating a small volume of plasma into a disposable dispensing tip;
further aspirating the small sample in the sample tip to pull the sample away
from the
lower end of the tip;
sealing the lower end of the tip with means for sealing the tip without
trapping air
below the sample in the tip;
adding an antibody reagent to the disposable dispensing tip with a second
dispensing
tip, the second tip is capable of being inserted into the open end of the
disposable
dispensing tip;
heating the disposable dispensing tip in a heating cavity;
radiating the sample in a disposable dispensing tip in a spectrophotometer;
and
correlating the concentration of the IgA, (32-microglobulin or C-reactive
protein in the
sample to a sensor response from the sample. Preferably
the temperature of the heating cavity is 37°C and the tip is maintained
in a heating
cavity for 2 minutes. More preferably, the plasma sample is 5 ~.1. In a
preferred
embodiment the antibody reagent is about 60 ~.1 of antibody selected from the
group
consisting of: antibody reactive to IgA; antibody reactive to (32
microglobulin; and
antibody reactive to C reactive protein.
DESCRIPTION OF THE DRAB
Figure 1 is a perspective view of a system incorporating an apparatus of the
present invention for analyzing sample integrity and measuring a variety of
proteins;
Figure 2 is a schematic representation of elements of the apparatus
of Figure 1;
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-6-
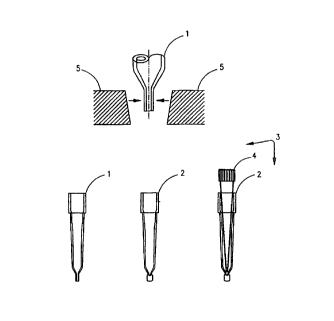
Figure 3 is a perspective view of two disposable dispensing tips and jaws of a
small
vice used to squeeze the lower end of the tip, for the purpose of sealing;
Figure 4 is a graphic representation of the absorbance spectra of variable
amounts
of IgA, zero time after incubation with antibodies against IgA, at
37°C, in the dispensing
tip of an analyzer. The concentration of IgA is shown in the figure;
Figure 5 is a graphic representation of the absorbance spectra of variable
amounts
of IgA, 2 minutes after incubation with antibodies against IgA, at
37°C, in the dispensing
tips of an analyzer. The concentration of IgA is shown in the figure;
Figure 6 is a graphic representation of a linear regression fit for data used
for the
development of an IgA calibration algorithm for samples in dispensing tips of
an
analyzer, with IgA in units of milligrams per litre on the abscissa and
ordinant axes;
Figure 7 is a graphic representation of a linear regression fit for data in
respect of
predicted IgA concentration for samples not used in the calibration process,
for samples in
dispensing tips of an analyzer, with IgA in units of milligrams per litre on
the abscissa
and ordinant axes;
Figure 8 is a graphic representation of a linear regression fit for data used
for the
development of a (32-microglobulin calibration algorithm for samples in
dispensing tips of
an analyzer, with ~i2-microglobulin in units of milligrams per litre on the
abscissa and
ordinant axes;
Figure 9 is a graphic representation of a linear regression fit for data used
for the
development of a C-reactive protein calibration algorithm for samples in
dispensing tips
of an analyzer, with C-reactive protein in units of milligrams per litre on
the abscissa and
ordinant axes;
Figure 10 is a graphical representation of the percent error in IgA prediction
caused by endogenous turbidity created by intralipid, with and without
subtraction of the
1st derivative of the absorbance at zero time.
As discussed above, the present invention provides apparatus and a method for
performing immunoturbidimetric measurements on an apparatus used for measuring
plasma and serum interferents. This feature allows tests which are not
available on
general chemistry analyzers, to become available, and at the same time the
apparatus
can provide a screening system for serum and plasma interferents. The
apparatus for
measuring serum and plasma interferents comprises a housing for receiving a
sample; a
radiation source; a sensor; a means for optically connecting the radiation
source with the
sensor along a sample path through the housing and along a reference path
which
bypasses the sample; a means for selectively passing a beam from the sample
path and
from the reference path to the sensor; and a means for correlating a sensor
response, from
the sample path relative to a sensor response from the reference path, to a
quantity of a
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-7_
known substance in said sample. The sample housing can be an integral part of
the
conveyor system as shown in Figure 1, or the housing can have a cavity for
receiving a
sample and a lid for selectively opening and closing the cavity, also shown in
Figure 1. A
cover may not be necessary in an automated system, where the dispenser stem,
when
inserted into the dispensing tip, can provide sufficient light shielding, and
further
because of the strategic location of the shutters, the subtraction of dark
current from both
the sample and the reference light measurements, can effectively eliminate the
effect of
room light. The radiation source is for emitting a beam of radiation, and the
sensor is
responsive to receipt of radiation. In order to perform immunoturbidimetry
using an
existing apparatus, a means for sealing the lower end of the dispensing tip as
required. In
a preferred embodiment the means for sealing is a small vice. A preferred
example of a
dispensing tip is the disposable tip used by the VitrosTM analyzer
manufactured by
Johnson and Johnson.
The apparatus further comprises a quartz-tungsten-halogen lamp capable of
emitting a near infrared, and adjacent visible region light beam having
wavelengths from
475nm to 910nm and a bifurcated fibre-optic cable for splitting the light beam
from the
quartz-tungsten-halogen lamp into a sample path beam for travel along a sample
path
and a reference path beam for travel along a reference path. This apparatus
further
comprises a shutter for selectively blocking the sample path light beam which
travels
along the sample path and the reference path light beam which travels along
the
reference path, as well as optical fibre bundles for transmitting the sample
path light
beam through a sample enclosed in the housing, and optical fibre bundles for
transmitting
the sample path light beam from the sample to a second bifurcated fibre-optic
cable,
where the beam from the sample path and the beam from the reference path
converge
into a single fibre-optic cable. It is understood that any means for excluding
from the
sample, light other than that from the radiation source of the apparatus, is
within the
scope of this invention. Also, if dark current, i.e., sensor response when
sensor is not
exposed to the instrument light, is subtracted from both the reference and
sample
measurements, the room light impinging on the detector can be effectively
subtracted
without affecting the instrument performance significantly.
Preferably, the bottom end of the dispensing tip is sealed by flattening
between
the jaws of a small vice, after a sample is aspirated into said tip.
Preferably the
dispensing tip is disposable and more preferably the tip of an analyzer is
used as a
reaction and incubation chamber after the tip is sealed with the sample
inside, and the
same sealed dispensing tip is used as a cuvette.
Analytes, such as proteins, preferably Immunoglobulin A (IgA), ~i2-
microglobulin and C-reactive protein (CRP), can be measured on the apparatus
through
the use of reagents, eg. antibodies, by the process of immunoturbidimetry.
Each plasma
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
_g_
protein requires a specific antibody, and the specificity of each test can be
increased by
subtracting the first derivative of the absorbance at zero time from the first
derivative of
absorbance after approximately 2 minutes at 37°C, at single or multiple
wavelengths. It
will be understood that optimum incubation time and temperature may vary for
different
plasma proteins.
Only 5~L of sample and 60~L of antibody reagent is required. It will be
understood
that optimum sample and reagent volumes may vary for different proteins.
In another aspect of the invention, the same dispensing tip used to aspirate
5~1L of
sample is sealed at the lower end by increasing the vacuum on the tip by an
equivalent of
4~.L. It will be understood that deviations from this volume are within the
scope of this
invention, particularly when other disposable tips are used. The extra vacuum
equivalent
to an aspiration of 4~1L, is sufficient to pull the fluid away from the lower
end of the tip
which is within the grasp of the jaws of a small vice without trapping air
below the S~.L
of sample, and without trapping sample below or within the seal.
According to a preferred embodiment, the jaws are slightly nonparallel as
shown
in Figure 3, and will therefore force upwards any residual fluid which is in
the grasp of
the jaws. This aspect of the invention assists in reducing any loss of any
part of the
sample.
In practising the invention, an antibody reagent is mixed with the sample by
injecting 60~L of antibody reagent into 5~.L of a sample. Preferably, 60uL of
antibody
reagent is in a narrow pipette tip, e.g., as shown as 4 in Figure 3, which can
reach the
bottom of the sealed tip, allowing enough space to facilitate proper
dispensing of the
antibody reagent. More preferably, narrow tips such as shown as 4 in Figure 3
are 960
Envirotips~ manufactured by Eppendorf, but any similar tip may be used. It is
desirable
that the ratio of antibody reagent volume to sample volume facilitates
adequate mixing
of sample and reagent. In carrying out the invention it is preferable if the
ejection of the
antibody reagent is such that only the fluid is ejected and no air is injected
into the
reaction chamber.
Zero-time absorbance measurement is triggered after antibody reagent is
dispensed into a sealed tip of the invention, and the zero-time measurement is
performed
with the dispensing stem attached to the tip.
According to one embodiment of the invention, the tip holder has a sliding lid
which closes after antibody reagent is dispensed.
In another aspect of the invention, because of the location of the shutters in
the
lamp assembly the subtraction of dark current from both the sample and the
reference
light measurements, can effectively eliminate the effect of room light.
Preferably the
sample chamber is shielded from light but is not required to be completely
light-tight; a
cover may not be necessary in an automated system, where the dispenser stem,
when
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-9-
inserted into the dispensing tip, can provide sufficient light shielding, even
when dark
current is not subtracted.
In another embodiment of the invention, the spectrometer can be run in single-
beam mode.
In another aspect of an alternative embodiment of the invention, zero-time
measurement is used as the reference scan when the spectrometer is run in the
single-beam
mode. Preferably the rate of change of the first derivative of absorbance is
monitored
during the first 15 seconds, to forecast if a high-dose hook effect will
occur.
Immunoturbidimetric measurements are performed using multiple wavelengths in
the visible and NIR electromagnetic radiation.
A method of the invention provides for measuring the concentration of a series
of
proteins in a sample by recording the absorbance spectrum of the sample before
and after
incubation with antibodies specific to each protein. Preferably the effect of
interferents
in a sample can be minimized by virtue of the wavelength range used, i.e., NIR
and
adjacent visible radiation. More preferably the remaining effect of
interferents can be
substantially removed by subtracting the first derivative of absorbance at
zero time from
the first derivative of absorbance after a two-minute incubation at
37°C. It will be
understood that other times and incubation temperatures can be used.
The effect of small air bubbles on absorbance is minimized by using the first
derivative of absorbance. It will be understood that any higher order of
derivative of
absorbance may also be used, eg., second derivative of absorbance.
A system incorporating the apparatus of the present invention is generally
illustrated in Figure 1. The apparatus 10 generally comprises a spectrometer
14 optically
coupled to, or communicating with a sample held on the conveyor 94 through
fibre optic
bundles 44 and 46, installed in a cover 92, or a sample holder 98 with a cover
100.
Apparatus 10 is mounted or installed adjacent to an automated conveyor 94
which carries
a plurality of sample tubes, e.g. 86 and 88. Because samples are presented in
variable tube
sizes, there may be a gap between the walls of the tube and the ends of fibres
44 and 46,
focusing lenses 96 are attached to the ends of the fibres. Sample holder 98 is
designed for
a disposable dispensing tip. Cover 92 acts as a light shield and also provides
a restraint
for the fibres 44 and 46, against any movement.
Cover 100 in Figure 1 also acts as a light shield for the apparatus. The
dispensing
stem of an analyzer and the tip holder can act as a light shield, with the tip
holder
designed deep enough to accommodate the stem of the analyzer dispenser.
Neither the
tip holder and cover 100, nor cover 92 are intended to provide a light-tight
sample
chamber. Sample presentation on a conveyor line 94 in Figure 1 is only
relevant to the
analysis of sample integrity functionality of the spectrometer. For the
present invention,
a sample is presented to the optical apparatus in a tip holder 98 in Figure 1.
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-10-
For the measurement of proteins by immunoturbidimetry, a separate sample
holder such as that illustrated (98) is required, and is imbedded in a heated
block. In a
preferred embodiment, 5~.L of plasma is aspirated in a dispensing tip, as
shown as 1 in
Figure 3. Extra vacuum, equivalent to an aspiration of 4itL is applied to the
sample to
pull the fluid away from the lower end of the tip which is within the grasp of
the jaws as
shown in Figure 3. Different volumes can be used, it being understood that the
objective is
to have the sample removed far enough from the tip and to allow for sealing.
The extra
vacuum must be sufficient to pull the fluid away from the lower end of the
tip, without
trapping air below the 5~L of sample. The same dispensing tip used to aspirate
5pL of
sample is sealed after the sample is aspirated into said tip. 'The end of the
dispensing tip
is sealed underneath the 5uL of sample by squeezing between the jaws of a
small vice,
shown as 5 in Figure 3. The sealed tip with a flattened lower end is shown as
2 in Figure 3.
It will be understood that although Figure 3 shows a VitrosT"' tip as 1 and 2,
other
disposable tips can be used and deviations from 4~.L are within the scope of
this
invention, particularly when other disposable tips are used. The jaws 5 in
Figure 3 are
slightly nonparallel, and will therefore force upwards, any residual fluid
which is in the
grasp of the jaws. 'This aspect of the invention precludes loss of any part or
the S~,L of the
sample.
In this invention, analytes are measured on the apparatus through the use of
reagents by the process of immunoturbidimetry. Each protein requires a
specific antibody,
and the specificity of each test can be increased by subtracting the first
derivative of the
absorbance at zero time from the first derivative of absorbance after
approximately 2
minutes at 37°C, at a single or multiple wavelengths. It will be
understood that optimum
incubation time and temperature could vary for different proteins.
60p.L of antibody reagent is aspirated from a bottle into a narrow pipette tip
shown as 4 in Figure 3. In a preferred embodiment, narrow tips shown as 4 in
Figure 3 are
960 Envirotips~ manufactured by Eppendorf, but any similar tip which can reach
the
bottom of the sealed tip may be used. The Eppendorf tip or its equivalent must
be allowed
to reach the bottom of the Vitros tip or its equivalent, with just enough
space between the
ejection port and the 5ltL of sample, to facilitate proper dispensing of the
antibody
reagent. The antibody reagent is mixed with the sample by injecting the 6011L
of antibody
reagent into the S~L of sample. Little or no air should be injected into the
sample. This
can be accomplished by injecting the 601tL or less of the antibody reagent, as
long as the
volume is dispensed in a precise manner. It will be understood that further
mixing can be
achieved by reaspirating and redispensing the reaction mixture.
The disposable dispensing tip of an analyzer is used as a reaction and
incubation
chamber after the tip is sealed with the sample inside; it is also used as a
cuvette.
Although Figure 1 only shows one tip holder 98, a preferred embodiment
contains two tip
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-11-
holders 98; one used for measurement of interferents and the other for protein
measurement. It will be understood that one tip holder can be used for both
applications,
and the single tip holder is heated for the benefit of the protein
measurement, without
affecting the interferent measurements, since the dwell time for the
interferent
measurement is only one second. When two separate tip holders are installed,
they are
connected through a bifurcated optical fibre, to the sample optical fibre 44
in Figure 1.
Two new shutters must be installed external to the lamp assembly 20 in Figures
1 and 2.
The new shutters allow light to be directed only to the tip holder which is
functional.
Zero-time absorbance measurement is triggered after the antibody reagent is
dispensed, with the dispensing stem attached to the tip. In another embodiment
of the
invention, the tip holder has a sliding lid which closes after the antibody
reagent is
dispensed, and after the dispensing stem releases the tip. The effect of
interferents can be
substantially removed by subtracting the first derivative of the absorbance at
zero time
from the first derivative of absorbance after a two-minute incubation at
37°C. It will be
understood that other times and incubation temperatures can be used. In this
design, the
sample holder functions as both the incubator and the optical read station. It
will be
understood that the incubation can occur in a separate chamber, where the
incubated
sample can be aspirated into a disposable dispensing tip, which is
subsequently placed in
the tip holder 98 as shown in Figure 1. If a separate incubation chamber is
used, the same
read station or tip holder 98, as shown in Figure 1, can be used for both
interferent and
protein measurements. If a combined incubator-read station is used, then a
separate tip
holder is required for measuring interferents, and a separate set of optical
fibres and
shutters are required to supply and receive radiation to and from the
"incubator-read
station". If it is desired to have the dispensing stem remain with the
dispensing tip, a
second dispensing stem, can be added to the apparatus.
Sample fibres 44 and 46 direct radiation from a light source to and from the
sample respectively, and allow the bulk of the instrumentation to be placed
remotely
from the samples. Multiple optical fibres 46 and 48 are the strands of a
bifurcated optical
fibre which collect radiation alternately from the sample 44 and reference
optical fibre
66, and combines into one multiple optical fibre 54 which communicates with a
spectrometer 14. Reference fibre 66 is joined to a strand 48 of the bifurcated
fibre by a
coupling 52. The coupling 52 can be chosen to provide sufficient attenuation
of the
reference beam, where the detector is optimally integrated over a short period
of time.
Fibre 66 is a single flbre and fibre 44 can be a single or multiple fibres,
depending on the
light throughput required.
Referring to Figure 1, the apparatus 10 includes a spectrometer 14, a central
processing unit 16, a power supply 18, a lamp assembly module 20 and a sample
holder 92
and 94, or 98.
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-12-
Referring to Figure 2, the lamp assembly module 20 employs a light source 62.
Preferably the light source is a 20-watt quartz-tungsten-halogen lamp, but
other wattage
lamps can be employed. The input power supply is alternating current, but the
output to
the light source is a stabilized direct current. Attached to the lamp is a
photodiode 80,
which monitors lamp output. Spectral output from light source 62 is a broad
band covering
visible and NIR regions. Although the NIR region of the electromagnetic
spectrum is
generally considered to be the interval extending from 650nm to 2700nm, the
nominal
wavelength range of the preferred embodiment is from 475nm to 910nm, which is
referred
to as the "near infrared and adjacent visible region". A beam of radiation
from the light
source 62 is directed through a band-pass filter 64 and a shaping filter 69 in
the
spectrometer 14. The band-pass filter is required to reduce unwanted radiation
outside of
475-910nm. The shaping filter 69 is required to "flatten" the detection
system's optical
response. The beam of radiation from filter 64 is transmitted through a
bifurcated optical
mufti-fibre bundle 60 to provide sample and reference beams. Bifurcated bundle
60
provides random sampling of lamp radiation to supply the sample and reference
beams
via two arms of 60, 80 and 82 respectively. In a preferred embodiment, a
balanced
emerging radiation is provided to the photo diode array {PDA) detector 78,
from both the
sample and reference paths, where the radiation through 80 and 82 are 99% and
1%
respectively. With shutter 58 closed and shutter 56 open, radiation is
channeled through
optical fibre 44 to the sample, and the radiation transmitted through the
sample in
multiple-labeled tube or plastic dispensing tip and is received by fibre 46,
which returns
collected radiation to the spectrometer 14.
The sample and reference beams enter arms 46 and 48 respectively of a
bifurcated
optical mufti-fibre bundle which combine in fibre 54 and are focused
alternately onto a
slit 70, by a focusing lens 68 and a shaping filter 69. Emerging radiation is
collimated by
lens 72 before the beam is directed to grating 74 which is a dispersing
element which
separates out component wavelengths in a preferred embodiment dichromated
gelatin is
used as the grating material. Component wavelengths are focused by a lens 76,
onto the
PDA 78. Each element or pixel of the PDA is set to receive and collect a
predetermined
wavelength. In a preferred embodiment the PDA comprises 256 pixels. The pixels
are
rectangular in shape to optimize the amount of optical radiation detected.
Spectrometer 14 is preferably a "dual-beam-in-time" spectrometer with fixed
integration time for the reference beam and a choice of integration for the
sample beam.
Because the sample is only shielded from light, but is not in a light-tight
holder, sample
and reference dark scans can be subtracted from sample and reference light
scans
respectively; sample and reference dark scans are performed at the same
integration times
used for the respective light scans. In a preferred embodiment, the reference
scan is
performed at 13 milliseconds, and the sample scan is performed in 20
milliseconds; the
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-13-
maximum ADC value obtained at 20 milliseconds for a particular sample, is used
to
determine a new integration time up to 2600 milliseconds, such that saturation
of the
detector at any pixel does not occur. The maximum time allowed for any sample
depends
on the required speed of sample screening. Also, multiple scans can be
averaged to
minimize noise, but for interferent and protein measurements, the number of
scans
averaged must not require more than 1 second.
When in use, each pixel or wavelength portion is measured approximately
simultaneously during a particular scan. Optical radiation falling on each
sensor element
is integrated for a specified time and individual pixels or wavelengths are
samples
sequentially by a 16 bit analog-to-digital convertor or ADC.
Although the present embodiment details use of a PDA, any alternative means
which achieves the same result is within the scope of the present invention.
For example
a filter-wheel system may be used. In carrying out measurements each analyte
uses from
one to three wavelengths or pixels. Given that the first derivative of
absorbance with
respect to measurements with the PDA is the difference between the absorbance
at two
adjacent pixels, the first derivative of absorbance at one wavelength with a
filter-wheel
system will require absorbances measured with two different narrow band-pass
filters. It
will be readily understood by those skilled in the art that the filters do not
need to be
assembled on a rotating wheel, but that any structure which achieves the
result of a
narrow band-pass filtration of absorbed radiation is within the scope of the
present
invention.
The PDA integrates the optical radiation over a specified time and converts
the
optical signal to a time multiplexed analog electronic signal called scan
where absorbance
is calculated as:
Absorbance = log (Reference;/Sample measurements) +
log (TTM/TTR)
where References = reference pixel s readings;
Sample measurements = sample measurement pixel s reading;
TTM = Integration time measurements;
ITR - integration time reference;
ana
s = the particular pixel in the PDA.
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-14-
In respect of these calculations, absorbance can also equal log (Reference -
reference dark
measurement} /{sample measurement - sample dark measurement}) + log (ITM/ITR)
Depending upon the amount of light shielding provided by the apparatus and the
criticality of timing, the measurement of a reference dark and sample dark
values may or
may not be undertaken. The electronic signal is proportional to the time that
the sensor
integrates the optical signal. The electronic signal is amplified by analog
electronic
amplifiers and converted to a digital signal by an analog-to-digital converter
or ADC.
The digital information from the converter is interpreted for data analysis by
a
microprocessor which is in turn connected via an RS232 connector to a computer
84. The
results of the data analysis can be shown on an output device such as a
display and on a
printer.
The first part of the process for generating a calibration curve is to store
spectral
data for the calibration set. The calibration algorithm for each protein must
be installed
in a microprocessor so that when an unknown sample is tested for a particular
protein the
result is quickly produced in order to calculate the quantity of any protein
present, any one
of several different methods, all of which are within the scope of this
invention, may be
used.
A preferred method is to calculate the first derivative of certain portions of
the
spectra in respect of the particular protein being measured. It is also
possible to calculate
the second, or third derivatives, and such calculations are within the scope
of this
invention. However, each step of taking differences to calculate those
derivatives is more
time consuming and introduces more noise.
In practice, an optimal combination of first derivatives of at least two
portions of
a spectrum generated from a scan for a particular protein are used to
calculate protein
concentration. The precise approach used depends on the protein being
measured.
With respect to generating a calibration curve for IgA, 5~.L of each
calibrator was
aspirated in a Vitros dispensing tip using an Eppendorf pipette. The pipette
setting was
changed from 5~L to 9~.L; this extra vacuum allowed the sample to be drawn
away from
the end of the tip which is within the grasp of the vice shown in Figure 3. In
order to
prevent the fluid from leaking out, the bottom end of the dispensing tip was
sealed by
squeezing it with a pair of pliers. The tip with the fluid was placed in the
heated tip
holder, shown as 98 in Figure 1. Using a second pipette, 60wL of antibody
reagent was
added to the sample, with the lower end of the Eppendorf pipette tip almost in
contact
with the sample, as shown as 3 in Figure 3. The Eppendorf tip must reach as
far down as
possible, without restricting the flow of the antibody reagent. Immediately
after the
antibody reagent is added, the absorbance spectrum was recorded as the zero-
time
measurement. Two minutes later, a second absorbance spectrum was recorded.
This was
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-15-
repeated for the 4 calibrators, and 5 independent samples used for validation
of the
developed calibration algorithm. The absorbance spectra for the calibrators
and
validation sample set are shown in Figures 4 and 5 respectively. The linear
regression fit
for the calibrators and validation sample set are shown in figures 6 and 7
respectively.
Similarly, calibration algorithms were developed ~i2-microglobulin and C-
reactive protein, and their linear regression fits are shown in Figures 8 and
9 respectively.
The antibody used for (i2-microglobulin is covalently coupled to polystyrene
beads in order
to and the antibody used for CRP was unenhanced, like the IgA antibodies.
These
antibodies are also available commercially. It must be understood that any
protein for
which specific antibodies are available, and where the concentration is
sufficient to
develop detectable immunocomplexes, can be measured by this invention.
Furthermore, for
proteins in relative low concentrations, the signals can be enhanced by
coupling
polystyrene beads to the antibody.
Due to the small absorbances which is expected at the wavelengths used, the
zero-time absorbance spectra obtained for IgA were observed to be in a random
order, as
shown in Figure 4, possible due to the presence of tiny air bubbles in the
fluid and
inconsistencies in the walls of the dispensing tip. However, after 2 minutes
at 37°C, both
the absorbances and the first derivative of the absorbance are proportional to
the
concentration of (32-microglobulin, as shown in Figure 5. The prior art
subtracts the zero
absorbance at around 340nm, from the absorbance at 340nm after the incubation
at
approximately 37°C for approximately 5 minutes, for the purpose of
removing the effects
of interferents in the sample. To those skilled in the art, the use of
dispensing tips along
with the prior art method to remove the effects of interferents cannot be use
for the
wavelength range as specified in this invention. The present invention uses a
new
approach for removing the effects of interferents, where the first derivative
of absorbance
is subtracted from the first derivative of absorbance after 2 minutes at
37°C at every
wavelength; the difference is then subjected to a statistical process of step-
wise linear
regression for the selection of optimal wavelengths. It will be understood
that for the
calculation of each first derivative of absorbance in the preferred
embodiment, requires
the raw absorbances at 9 pixels or wavelengths; if filters were used instead
of the PDA
used in this invention, 2 narrow band-pass filters would be required to
produce each first
derivative of absorbance. Therefore, even if a single first derivative of
absorbance is used
in the calibration algorithm, multiple wavelengths are necessary.
In respect of IgA, optimal results may be obtained by calculating the first
derivative of absorbance at wavelengths of approximately 789nm and 825nm. In
respect of
(32-microglobulin, optimal results may be obtained by calculating the first
derivative of
absorbance at wavelengths of approximately 548nm and 829nm. In respect of CRP,
optimal
results may be obtained by calculating the first derivative of absorbance at
wavelengths
CA 02323442 2000-09-08
WO 99/47261 PCT/CA99/00236
-16-
of approximately 661 nm and 679nm.
The calibration algorithm developed for IgA based on 4 calibrators is as
follows;
mgIL IgA = -3327114.33 (789nm) + 484270.80 (825nm) - 77.3 where (Xnm) is the
first
derivative of the absorbance at the wavelength specified.
The calibration algorithm developed for (32-microglobulin based on 7
calibrators is
as follows:
mg/L (i2-microglobulin = -33648.79 (548nm) + 36556.81 (829nm) + 2.3
where (Xnm) is the first derivative of the absorbance at the wavelength
specified.
The calibration algorithm developed for CRP based on 9 calibrators is as
follows:
mgL CRP = -1813682.71 (661nm) + 1808677.58 (679nm) + 9.8
where (Xnm) is the first derivative of the absorbance at the wavelength
specified.
It will be understood that several calibration algorithms can be developed for
each protein, using an apparatus described for measuring specimen integrity.
The protein measurements are based on the principle of immunoturbidimetry,
i.e.,
generation of antibody-antigen complexes or immunocomplexes which cause
turbidity.
The "absorbance" generated is due to light scattering caused by the
immunocomplexes,
therefore endogenous turbidity or true absorbances caused by interferents in
the sample
will falsely elevate the signals. To demonstrate how interferents are dealt
with, an
aqueous solution of 2 g/L IgA was mixed with IL to provide 4 different samples
with 1 g/L
IgA and variable amounts of IL, i.e. from O to 4 g/L. Separate algorithms were
developed
for IgA with and without zero time correction.
The error in the predicted results with and without zero time subtraction is
shown
in Figure 10. This invention is different from the current art because
multiple long
wavelengths are used, and because of the small absorbances caused by the
immunocomplexes at those wavelengths, endogenous interferents must be
compensated for.
This compensation cannot be performed using the raw absorbance due to the
effect of small
air bubbles and imprecise absorbance produce by disposable dispensing tips,
but can be
performed effectively by using the 1st derivative of the absorbance. As long
as the first
derivative of absorbance is employed, multiple wavelengths are necessary, even
if the
calibration algorithm uses a single first derivative of absorbance.
While the invention has been particularly shown and described with reference
to
preferred embodiments, it will be understood by those skilled in the art that
various
other changes in form, and detail may be made without departing from the scope
of the
invention.